Abstract
Catheter ablation (CA) of atrial fibrillation (AF) is the therapy of choice for the maintenance of sinus rhythm in patients with symptomatic AF. Time towards interventional treatment and peri-procedural management of patients undergoing AF ablation may vary in daily practice. The scope of this European Heart Rhythm Association (EHRA) survey was to report the current clinical practice regarding the management of patients undergoing AF ablation and physician’s adherence to the European Society of Cardiology Guidelines and the EHRA/HRS/ECAS expert consensus statement on the CA for AF. This physician-based survey was conducted among EHRA members, using an internet-based questionnaire developed by the EHRA Scientific Initiatives Committee. A total of 258 physicians participated in the survey. In patients with paroxysmal or persistent AF, 42 and 9% of the physicians would routinely perform AF ablation as first-line therapy respectively, whereas 71% of physicians would consider ablation as first-line therapy in patients with symptomatic AF and left ventricular ejection fraction <35%. Only 14% of the respondents manage cardiovascular risk factors in patients referred for CA using a dedicated AF risk factor management programme. Radiofrequency CA is the preferred technology for first-time AF (56%), followed by cryo-balloon CA (40%). This EHRA survey demonstrated a considerable variation in the management of patients undergoing AF ablation in routine practice and deviations between guideline recommendations and clinical practice.
Keywords: Catheter ablation, Atrial fibrillation, Physician-based survey, Clinical practice, Guidelines, EHRA survey
What’s new?
This is a structured survey reporting the clinical practice, regarding the contemporary management of patients undergoing catheter ablation of atrial fibrillation.
It reflects currently, how electrophysiologists implement in daily practice this increasingly utilized interventional treatment.
Introduction
Catheter ablation (CA) is the treatment of choice for patients with symptomatic, medically intractable atrial fibrillation (AF).1,2 Contemporary guidelines and recent position papers provide guidance on several aspects of the clinical management of patients undergoing AF ablation procedures.1–4 Nevertheless, divergent routines have been observed in clinical practice across Europe.5–9
The aim of this European Heart Rhythm Association (EHRA) survey was to report the current clinical practice regarding the management of patients undergoing AF ablation and the adherence of the treating physicians to the contemporary guidelines and expert consensus statement on CA of AF.1–4 We also aimed to investigate the implementation of novel imaging and ablation technologies and elucidate procedural aspects that have not yet been fully addressed in clinical trials.
Methods
This physician-based survey was conducted among EHRA members between 15 December 2019 and 20 January 2020, using an internet-based 25-item questionnaire developed by the EHRA Scientific Initiative Committee, to collect information about current practice in AF ablation among EHRA members.
Data were analysed using descriptive statistical methods. Categorical variables are presented numerically with absolute percentages (%).
Results
A total of 258 respondents from 36 countries (Albania, Austria, Belgium, Belarus, Bulgaria, Croatia, Czech Republic, Denmark, Egypt, Finland, France, Georgia, Germany, Greece, Hungary, Iceland, Ireland, Israel, Italy, Latvia, Malta, The Netherlands, Norway, Poland, Portugal, Romania, Russian Federation, San Marino, Serbia, Slovenia, Spain, Sweden, Switzerland, Turkey, Ukraine, and the UK) participated in the survey.
Regarding the number of yearly performed CA procedures, participants were affiliated with a wide range of electrophysiology (EP) volume centres: 24 respondents (9.3%) were affiliated with centres performing <50 AF ablation procedures per year, 52 (20.2%) to centres with 51–100 AF procedures per year, 80 (31%) were affiliated to centres performing 101–250 AF procedures per year, 60 (23.3%) to centres with 251–500 AF procedures per year, and 42 (16.3%) were affiliated to centres performing >500 CA for AF per year.
Patient selection
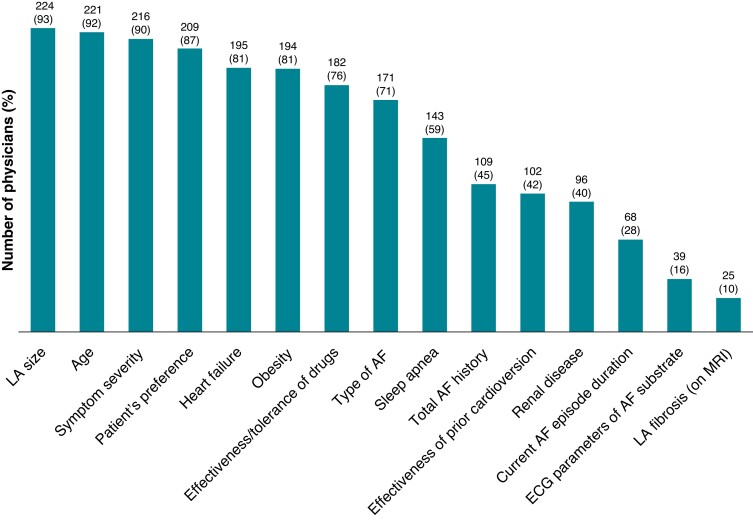
All physician-chosen qualifying criteria for interventional treatment of AF are shown in Figure 1 in order of importance. The 241 physicians who responded most commonly take into consideration the left atrial (LA) size (93%), the age (91.7%), the severity of AF symptoms (89.6%), the patient preference (86.7%), the coexistence of heart failure (HF) (80.9%), and the presence of obesity (80.5%); whereas the effectiveness of prior cardioversions (42.3%), the duration of the current AF episode (28.2%), and especially the presence of LA fibrosis on magnetic resonance imaging (MRI) (10.4%), were less frequently taken into account.
Figure 1.
Parameters considered when selecting patients for AF ablation (n = 241; multiple answers possible). AF, atrial fibrillation; ECG, electrocardiogram; LA, left atrial; MRI, magnetic resonance imaging.
First-line AF ablation would be routinely performed by 102 (42.3%) respondents in patients with symptomatic paroxysmal AF, in the absence of HF, or other co-morbidities, whereas 19 respondents (7.9%) would not perform first-line CA of paroxysmal AF. The remaining 120 (49.8%) would perform it only in selected patients (e.g. those aged <40 years) and/or upon specific patient request (respondents: n = 241).
First-line AF ablation would be routinely performed by 17 physicians (7%) in patients with persistent AF in the absence of HF, or other co-morbidities, in whom the rhythm control strategy is deemed feasible. Furthermore, 66 physicians (27.4%) would not perform first-line CA at all in the same category of patients with persistent AF. The remaining physicians would perform first-line AF ablation for persistent AF in selected cases only (e.g. age <40 years) with and/or upon specific patient request (respondents: n = 241).
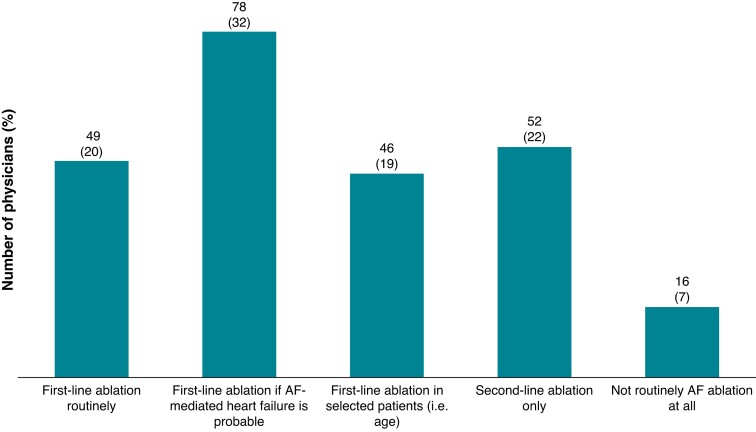
In patients with symptomatic AF, left ventricular ejection fraction (LVEF) < 35%, and an LA diameter <50 mm, first-line AF ablation is routinely and indiscriminately performed by 49 respondents (20.3%), whereas 78 (32.4%) and 46 (19.1%) responders are performing first-line CA, if HF is probably caused by AF and in selected patients, respectively. Fifty-two responders (21.6%) are performing CA for AF patients with HF as second-line therapy only. Finally, 16 responders (6.6%) would not routinely perform AF ablation in HF patients (respondents: n = 241) (Figure 2).
Figure 2.
Circumstances under which AF ablation is performed in patients with symptomatic AF, LVEF <35%, and a LA diameter <50 mm (n = 241). AF, atrial fibrillation; LA, left atrial; LVEF, left ventricular ejection fraction.
Peri-procedural management
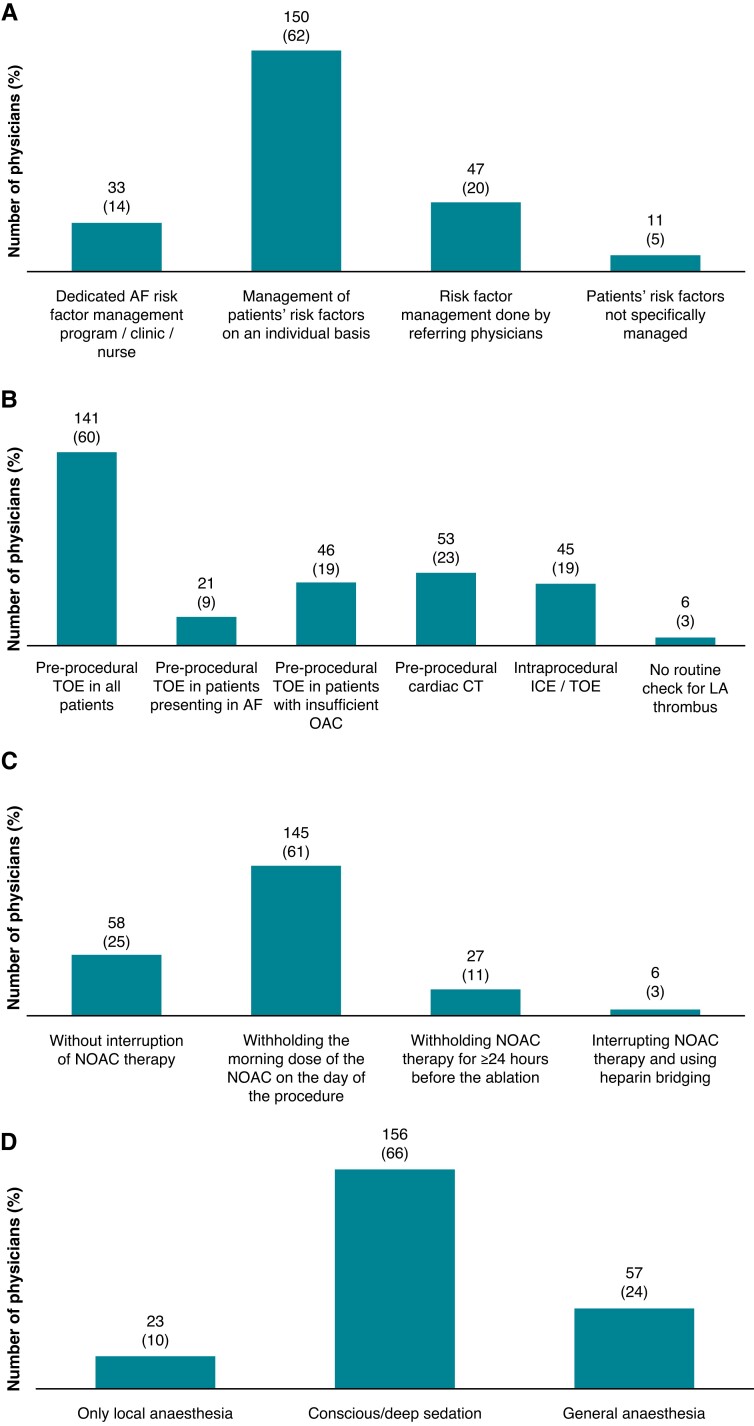
Only 33 participants (13.7%) managed cardiovascular risk factors in their AF patients referred for CA using a dedicated AF risk factor management programme (Figure 3A).
Figure 3.
Peri-procedural management of patients undergoing AF CA. (A) Management of cardiovascular risk factors in patients referred for AF ablation (n = 241). (B) Modality of left atrial thrombus exclusion prior/during AF ablation (n = 236; multiple answers possible). (C) Modality of DOAC interruption before AF ablation (n = 236). (D) Type of anaesthesia during AF ablation (n = 236). AF, atrial fibrillation; CA, catheter ablation; CT, computed tomography; DOAC, direct oral anticoagulant; ICE, intracardiac echocardiography; LA, left atrial; OAC, oral anticoagulation; TOE, transoesophageal echocardiography.
To exclude LA thrombus before/during CA, 141 respondents (59.8%) would perform a pre-procedural transoesophageal echocardiogram in all patients, whereas computed tomography (CT) and intracardiac echocardiography (ICE) would be used by 53 (22.5%) and 45 respondents (19.1%), respectively (Figure 3B). In patients receiving direct oral anticoagulation (DOAC), only 58 respondents (24.6%) would routinely perform CA without interruption of DOAC therapy, whereas 145 (61.4%) would perform the procedure after withholding the morning dose of DOAC on the day of the procedure (respondents: n = 236) (Figure 3C).
Regarding the peri-interventional modality of sedation during AF ablation, 156 respondents (66.1%) stated that they usually perform the procedure under conscious/deep sedation, 57 responders (24.2%) prefer general anaesthesia, whereas 9.7% use local anaesthesia only (Figure 3D). To prevent oesophageal injury during AF ablation, 48 physicians (20.3%) routinely use an oesophageal temperature probe, and 174 (73.6%) administer proton pump inhibitors in the following weeks after the procedure either routinely (138; 58.5%), or in selected patients only (36; 15.3%) (total respondents: n = 236; multiple answers possible).
Ablation techniques
As far as the guidance for the transseptal puncture (TSP) is concerned, 163 physicians (70.9%) use fluoroscopy only, whereas 44 (19.1%) would perform TSP assisted by transoesophageal echocardiography, and 31 (13.5%) guided by ICE. Regarding the number of TSPs for radiofrequency (RF) CA, 92 respondents (40%) would perform a single TSP only. Heparin management during TSP also varied: 45 physicians (19.6%) would administer heparin before performing the first TSP, 65 (28.2%) would administer part of the heparin dose before and the rest after successful TSP, and the remaining 120 respondents (52.2%) would administer heparin either after the completion of all TSPs, or directly after the first TSP (in case of double TSP) (respondents: n = 230; multiple answers possible).
The preferred initial CA approach for the treatment of AF was (RF, 129 respondents; 56%], whereas 93 respondents (40%) are opting for cryo-balloon CA. Eight physicians (4%) used different CA technologies, or implemented cryoablation in patients with paroxysmal AF and RF ablation in patients with persistent AF (respondents: n = 230).
In patients undergoing RF ablation, 111 respondents (48.3%) additionally use pre-procedural CT/MRI and 30 (13%) use peri-procedural ICE; 198 respondents (86%) use a contact force-guided catheter when performing RF ablation, but only 115 (50%) perform AF ablation with the support of a steerable sheath (respondents: n = 230; multiple answers possible).
Only 46 (20.4%) physicians would routinely perform a diagnostic EP study in all patients undergoing AF ablation, with an additional 16 (7.1%) doing so in specific situations (i.e. young patients; respondents: n = 225).
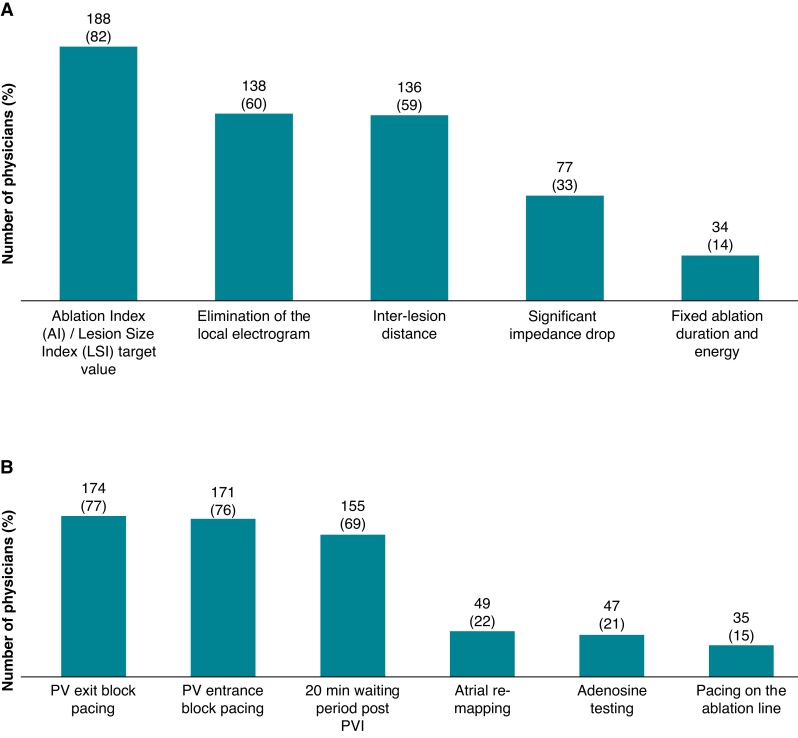
When performing cryo-balloon CA, 34 respondents (15.1%) would opt for double applications in all patients (respondents: n = 225). With regard to the endpoint for each RF ablation lesion, 188 (81.7%) are using integrated contact force target parameters, such as the Ablation Index or the Lesion Size Index for the RF lesion endpoint (Figure 4A). The responders to the survey are also employing a variety of EP manoeuvres to ensure pulmonary vein (PV) isolation after RF ablation, predominantly re-checking PVs after a 20 min waiting period post-PVI, PV entrance block pacing, and exit block pacing with few of them utilizing adenosine testing (Figure 4B).
Figure 4.
Ablation settings for RF ablation and confirmation of acute electrical PV isolation. (A) Endpoint for individual RF lesion formation during CA of AF (n = 230; multiple answers possible). (B) Technique used for the assessment of PV isolation during AF ablation (n = 225; multiple answers possible). CA, catheter ablation; PV, pulmonary vein; PVI, pulmonary vein isolation.
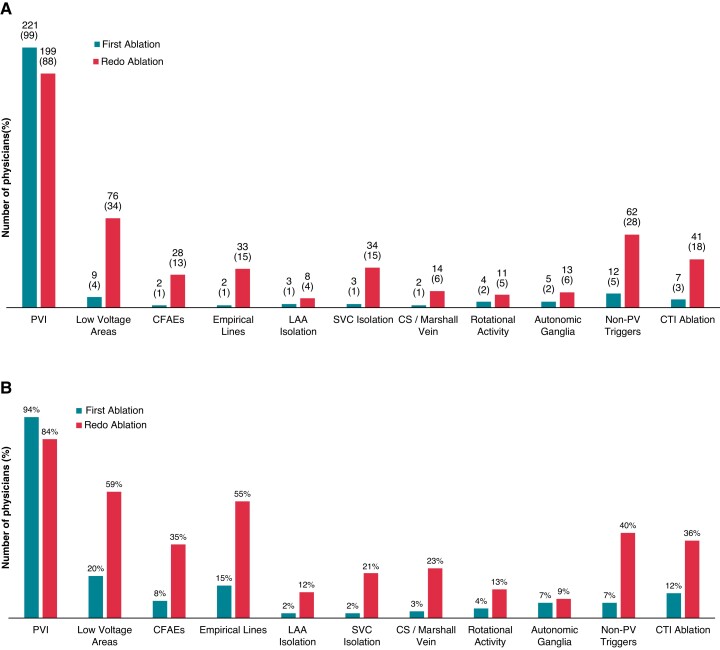
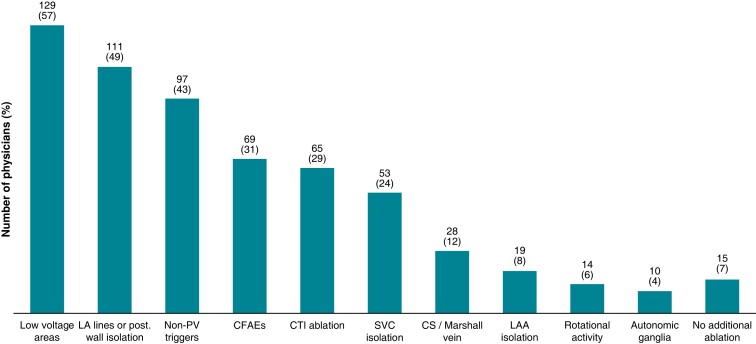
With regard to the endpoints of AF ablation, 221 (98.2%) and 210 respondents (93.3%) aim only for PV isolation in paroxysmal and persistent AF, respectively, for the first CA procedure. The ablation targets during the first ablation and re-do procedure differed according to the AF type, with low voltage area ablation being the most widely used substrate-based CA approach. Catheter ablation of rotational activity was rarely employed (Figure5A and B). In patients undergoing re-do ablation for AF, in the absence of PV reconnections at the beginning of the procedure, only 15 physicians (6.6%) would not perform any additional ablation. Two hundred and ten (93.3%) of the participating physicians would use diverse strategies, namely either LA low voltage area ablation, LA linear ablation, and/or posterior wall isolation; all three employed most commonly (Figure 6).
Figure 5.
Ablation targets during paroxysmal (A) and persistent (B) first-time and re-do procedures for CA of AF (n = 225; multiple answers possible). CA, catheter ablation; CFAE, complex fractionated atrial electrogram; CS, coronary sinus; CTI, cavotricuspid isthmus; LAA, left atrial appendage; PVI, pulmonary vein isolation; SVC, superior vena cava.
Figure 6.
Ablation targets in patients undergoing re-do ablation for AF, without any pulmonary vein reconnections at the beginning of the procedure (n = 225; multiple answers possible). AF, atrial fibrillation; CFAE, complex fractionated atrial electrogram; CS, coronary sinus; CTI, cavotricuspid isthmus; LAA, left atrial appendage; PVI, pulmonary vein isolation; SVC, superior vena cava.
Post-procedural management
Post-procedurally, 145 physicians (65.6%) would routinely prescribe Class I or III antiarrhythmic drugs for the first 1–3 months after AF ablation, 9 (4%) of whom would prolong antiarrhythmic therapy for ≥12 months, whereas 67 (30.3%) would not routinely prescribe antiarrhythmic drugs at all (respondents: n = 221).
The preferred screening modalities to check for AF recurrence in the first year after CA were 24 h Holter-electrocardiogram (ECG, 155; 70%), 12-lead ECG (129; 58.4%), and multi-day Holter-ECG (74; 33.5%). Interestingly, 40 respondents (18.1%) would use smartphone-based technology during follow-up, 26 (11.8%) would use implantable loop recorders, and four respondents (1.8%) would not use any modality at all to check for AF recurrence after CA (respondents: n = 221; multiple answers possible).
Regarding a re-do procedure, 187 physicians (84.6%) would perform repeat CA only for symptomatic AF recurrence after a 2- to 3-month blanking period. Another 17 respondents (7.7%) would consider re-do CA after 12 months, whereas 43 (19.5%) would perform re-do CA after a failed trial of antiarrhythmic drug therapy (respondents: n = 221; multiple answers possible).
Discussion
This EHRA physician-based survey reflects the clinical practice regarding the contemporary management of patients undergoing CA for AF and its procedural aspects. It also reports how electrophysiologists are currently implementing AF ablation in daily practice and if they adhere to the current guidelines and recommendations on this increasingly evolving interventional therapy.1–4
With regard to patient selection criteria for AF ablation, physicians still rely on traditional baseline parameters, such as LA size, the presence of obesity, and severity of symptoms. Only a minority of physicians consider an MRI-assessed LA fibrosis for patient selection, showing that this technology may not be readily available in the clinical setting.10
Integrated management for AF patients has received a Class I recommendation in the 2016 European Society of Cardiology (ESC) Guidelines for AF.1 However, only 13.7% of the physicians manage cardiovascular risk factors in patients referred for CA, using a dedicated AF risk factor management programme. Based on existing evidence, centres performing AF ablations should either implement a structured risk factor management programme to improve the overall outcome of CA or avoid the procedure.1,2,11 Furthermore, the 2020 ESC AF Guidelines recommend comprehensive risk factor management in patients considered for CA of AF.2
First-line CA for AF has a Class IIa and a Class IIb recommendation for treating symptomatic patients with paroxysmal and persistent AF, respectively, remaining unchanged over time.1,2,4 Currently, in clinical practice, 42.3% of the survey respondents routinely and another 59.8% occasionally perform CA for AF as first-line therapy in symptomatic patients with paroxysmal AF in the absence of HF, or other co-morbidities, when rhythm control is pursued. Interestingly, treating physicians seem more reluctant to perform AF ablation as first-line therapy in patients with symptomatic persistent AF in the absence of HF. Only 7% of the survey participants routinely perform first-line AF ablation in patients with persistent AF, aiming for rhythm control, whereas 27.3% would only perform it as second-line therapy. The remaining 65.6% of the responders would perform CA for AF as first-line therapy only upon patient request or under certain conditions.
Comparing the current survey findings to the previous EHRA ESS-PRAFA report on catheter AF ablation as the first-line treatment in symptomatic patients without structural heart disease, two interesting findings are noted: First, a significant increase from previously 11.2% to currently 42.3% preferring CA as a first-line treatment for paroxysmal AF—supported occasionally by additional 49.8% of the responders—was noted for this practice in patients with paroxysmal AF, although the class of recommendation remained unchanged. Currently, six randomized clinical trials utilizing either RF12–14 or cryothermal energy15–17 have demonstrated the superiority of CA for AF as a first-line treatment over medical therapy. Results from the ATTEST trial support this practice, as early AF ablation seems to delay disease progression from paroxysmal to persistent AF.18 Second and more interesting, a decrease from previously 14.7% to currently 7% in favour of direct CA as the first-line treatment for patients with persistent AF was noted. An additional 65.6% of the responders would consider first-line interventional treatment for persistent AF only under certain circumstances. Furthermore, 27.4% of the responders would consider it as second-line therapy only. Interventional treatment of persistent AF as first-line treatment currently has a Class IIa recommendation in the 2017 EHRA/HRS/ECAS expert consensus statement on CA of AF and a Class IIb recommendation in the 2020 ESC AF Guidelines.2,4 These results may be explained by the lack of randomized control trials for this group of patients. The SARA trial is the only multicentre randomized controlled study, which demonstrated the superiority of CA as a first-line treatment over medical therapy in patients with persistent AF. This trial, however, had to be terminated prematurely due to a lower-than-expected recruitment rate.19 These findings highlight the need for additional research in the interventional treatment of patients with persistent AF without concomitant structural heart disease.
Regarding patients with symptomatic AF and concomitant HF with reduced LVEF (<35%), in total, 71.8% of the physicians would opt for CA as first-line therapy in different scenarios. In clinical practice, two separate categories of patients with AF and HF should be noted. The first category is patients with AF-induced HF, in whom restoration and maintenance of sinus rhythm result in the normalization of LVEF.20 The recommendation for first-line CA of AF in these patients has been upgraded from Class IIaC to IB over time.2,4 The second category concerns patients with pre-existing HF with reduced LVEF, who develop AF and CA have a recommendation Class IIaB as a first-line therapy.2 For this subset of patients two studies, the AATAC and the CASTLE-AF, have assessed the impact of AF ablation on morbidity and mortality.21,22 Both studies showed a reduction in mortality and morbidity for those patients, however, the subgroup with severely reduced LVEF (<25%) in the CASTLE-AF, did not benefit from the interventional therapy. Despite the ongoing debate surrounding the limitations of this study and the data from the AMICA trial,23 the EP community seems to embrace this therapy option.24,25
Atrial fibrillation ablation constitutes an interventional procedure with an inherent risk for serious bleeding secondary to multiple femoral and TSPs, prolonged catheter manipulations, and energy applications to the vulnerable LA structure. Interestingly in patients on DOAC therapy, only 24.6% of the physicians would routinely perform AF ablation without interruption of DOAC therapy, despite recent studies and guidelines advocating AF ablation without interruption of DOAC therapy as a Class I recommendation.4,26
Compared with the previous EHRA ESS-PRAFA report on CA for AF,6,7 the number of patients in whom DOAC therapy is interrupted for ≥2 doses decreased significantly. This change in clinical practice is probably supported by data demonstrating the safety of performing AF ablation without interruption of DOAC therapy and a potentially increased bleeding rate in patients who undergo heparin bridging.3,26 The practice of performing AF ablation on uninterrupted anticoagulation therapy is also stated in recent guidelines and recommendations.1,2,4
With respect to currently available CA technology, the majority of physicians (56%) would preferably use irrigated RF ablation for first-time AF ablation, whereas 40% chose cryoablation. Compared with the EHRA ESS-PRAFA report on catheter AF ablation in 2015, a shift towards the single-shot device is noted; however, point-by-point RF ablation remains first in the preferences of treating physicians. This is a very interesting finding among treating electrophysiologists. Both ablation technologies have shown equal efficacy in reducing the AF burden. Nevertheless, electrical PV isolation using a cryothermal balloon catheter as the first approach has been shown to be faster, with greater procedural reproducibility and fewer rehospitalizations during follow-up, at the cost of longer fluoroscopy duration.27,28
Regarding the EP manoeuvres to ensure acute durable PV isolation after RF ablation, almost all respondents perform some kind of pacing or electroanatomic mapping to confirm electrical PV isolation, with PV entrance block pacing (Class I indication), and exit block pacing (Class IIb indication) being equally distributed.4 However, only 69% of physicians stick to the rule of a 20 min waiting period after PV isolation. On the one hand, this may be explained by a growing trust in new ablation features ensuring more durable lesions, such as contact force-guided catheters. On the other hand, the increasing workload in the EP labs requires further reduction of the procedural duration.29,30 The latter may also explain a rather low threshold in performing a baseline EP study to rule out concomitant supraventricular tachycardias prior to AF ablation.
Finally, regarding the general endpoints in AF ablation, most respondents always isolate PVs in paroxysmal and persistent AF, both in the first CA session and re-do procedure, showing that durable PV isolation remains the cornerstone of interventional therapy for rhythm control.4 Nevertheless, additional LA ablation targets during first and re-do CA vary significantly between paroxysmal and persistent AF. Our survey showed that ablation of low voltage areas and empirical lines and/or posterior wall isolation are still widely used (≥55% of the respondents), as well as complex fractionated atrial electrogram ablation (35%), despite limited evidence justifying such a practice.31–36 In contrast, the CA of rotational activity is performed only by a limited number of physicians even during re-do procedures for persistent AF, reflecting the limited availability of this technology, as well as the lack of evidence for its efficacy.34
Limitations
The present survey has limitations attributed to target respondents and questionnaire design. The survey included a limited number of selected physicians and participation was completely voluntary, therefore being prone to selection bias. However, it represents a timely snapshot, covering both acceptance of the recent guidelines and recommendations and the variations in current everyday practice regarding AF ablation.
Conclusions
This EHRA survey highlights several gaps between guideline recommendations and clinical practice, such as a low number of dedicated AF risk factor management programmes and significant heterogeneity in clinical practice and management of patients undergoing CA of AF. Furthermore, the decision to perform first-line AF ablation is mainly based on the type of AF.
Acknowledgements
The production of this document is under the responsibility of the Scientific Initiatives Committee of the European Heart Rhythm Association: T.S.P. (Chair), R.L. (Co-Chair), G.C., Gheorghe Andrei Dan, M.M.F., Malcolm Finlay, Estelle Gandjbakhch, K.E.I., Kristine Jubele, Deirdre A. Lane, Eloi Marijon, F.M., Frits Prinzen, and D.S. The production of this document is also under the responsibility of the Scientific Initiatives Committee of the European Heart Rhythm Association: Julian K.R. Chun (Chair), Sergio Castrejon (Co-Chair), Ante Anic, G.C., Piotr Futyma, Andreas Metzner, Federico Migliore, Giacomo Mugnai, Laura Perrotta, Rui Providencia, Sergio Richter, Laurent Roten, and Arian Sultan. The authors acknowledge the EHRA Scientific Research Network centres participating in this survey. A list of these centres can be found on the EHRA website.
Contributor Information
Konstantinos Iliodromitis, Klinik für Kardiologie und Rhythmologie, Evangelisches Krankenhaus Hagen-Haspe, Brusebrinkstraße 20, 58135 Hagen, Germany; School of Medicine, Witten/Herdecke University, Alfred-Herrhausen-Straße 50, 58455 Witten, Germany.
Radoslaw Lenarczyk, Division of Medical Sciences in Zabrze, Department of Cardiology, Congenital Heart Diseases and Electrotherapy, Silesian Center for Heart Diseases Zabrze, The Medical University of Silesia, Zabrze, Poland.
Daniel Scherr, Division of Cardiology, Department of Medicine, Medical University of Graz, Auenbruggerplatz 15, 8036 Graz, Austria; Department of Cardiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Center+, Maastricht, The Netherlands.
Giulio Conte, Division of Cardiology, Cardiocentro Ticino, Lugano, Switzerland.
Michal M Farkowski, II Department of Heart Arrhythmia, National Institute of Cardiology, Warsaw, Poland.
Francisco Marin, Department of Cardiology, Hospital Clínico Universitario Virgen de la Arrixaca, IMIB-Arrixaca, CIBERCV, University of Murcia, Murcia, Spain.
Javier Garcia-Seara, Arrhythmia Unit, Cardiology Department, CIBER-CV and IDIS Foundation, University Hospital of Santiago de Compostela, Compostela, Spain.
Stefan Simovic, Department of Internal Medicine, Faculty of Medical Sciences, University of Kragujevac, Kragujevac, Serbia; Clinic for Cardiology, University Clinical Center Kragujevac, Kragujevac, Serbia.
Tatjana Potpara, School of Medicine, University of Belgrade, Beograd, Serbia; Cardiology Clinic, Clinical Centre of Serbia, Belgrade, Serbia.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei Bet al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–78. [DOI] [PubMed] [Google Scholar]
- 2. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJet al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 3. Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KGet al. 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace 2021;23:1612–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga Let al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace 2018;20:157–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dagres N, Bongiorni MG, Larsen TB, Hernandez-Madrid A, Pison L, Blomsträm-Lundqvist C. Current ablation techniques for persistent atrial fibrillation: results of the European Heart Rhythm Association survey. Europace 2015;17:1596–600. [DOI] [PubMed] [Google Scholar]
- 6. Potpara TS, Larsen TB, Deharo JC, Rossvoll O, Dagres N, Todd Det al. Oral anticoagulant therapy for stroke prevention in patients with atrial fibrillation undergoing ablation: results from the first European Snapshot Survey on Procedural Routines for Atrial Fibrillation Ablation (ESS-PRAFA). Europace 2015;17:986–93. [DOI] [PubMed] [Google Scholar]
- 7. Chen J, Dagres N, Hocini M, Fauchier L, Bongiorni MG, Defaye Pet al. Catheter ablation for atrial fibrillation: results from the first European Snapshot Survey on Procedural Routines for Atrial Fibrillation Ablation (ESS-PRAFA) part II. Europace 2015;17:1727–32. [DOI] [PubMed] [Google Scholar]
- 8. Lip GY, Laroche C, Dan GA, Santini M, Kalarus Z, Rasmussen Let al. A prospective survey in European Society of Cardiology member countries of atrial fibrillation management: baseline results of EURObservational Research Programme Atrial Fibrillation (EORP-AF) pilot general registry. Europace 2014;16:308–19. [DOI] [PubMed] [Google Scholar]
- 9. Lip GY, Laroche C, Ioachim PM, Rasmussen L, Vitali-Serdoz Let al. Prognosis and treatment of atrial fibrillation patients by European cardiologists: one year follow-up of the EURObservational Research Programme-Atrial Fibrillation general registry pilot phase (EORP-AF pilot registry). Eur Heart J 2014;35:3365–76. [DOI] [PubMed] [Google Scholar]
- 10. Akoum N, Daccarett M, McGann C, Segerson N, Vergara G, Kuppahally S et al. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a DE-MRI guided approach. J Cardiovasc Electrophysiol 2011;22:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gallagher C, Elliott AD, Wong CX, Rangnekar G, Middeldorp ME, Mahajan Ret al. Integrated care in atrial fibrillation: a systematic review and meta-analysis. Heart 2017;103:1947–53. [DOI] [PubMed] [Google Scholar]
- 12. Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba Wet al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005;293:2634–40. [DOI] [PubMed] [Google Scholar]
- 13. Morillo CA, Verma A, Connolly SJ, Kuck KH, Nair GM, Champagne Jet al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA 2014;311:692–700. [DOI] [PubMed] [Google Scholar]
- 14. Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad Oet al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med 2012;367:1587–95. [DOI] [PubMed] [Google Scholar]
- 15. Kuniss M, Pavlovic N, Velagic V, Hermida JS, Healey S, Arena Get al. Cryoballoon ablation vs. antiarrhythmic drugs: first-line therapy for patients with paroxysmal atrial fibrillation. Europace 2021;23:1033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne Jet al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 2021;384:305–15. [DOI] [PubMed] [Google Scholar]
- 17. Wazni OM, Dandamudi G, Sood N, Hoyt R, Tyler J, Durrani Set al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med 2021;384:316–24. [DOI] [PubMed] [Google Scholar]
- 18. Kuck KH, Lebedev DS, Mikhaylov EN, Romanov A, Gellér L, Kalējs Oet al. Catheter ablation or medical therapy to delay progression of atrial fibrillation: the randomized controlled atrial fibrillation progression trial (ATTEST). Europace 2021;23:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mont L, Bisbal F, Hernández-Madrid A, Pérez-Castellano N, Viñolas X, Arenal Aet al. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study). Eur Heart J 2014;35:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parkash R, Wells GA, Rouleau J, Talajic M, Essebag V, Skanes Aet al. Randomized ablation-based rhythm-control versus rate-control trial in patients with heart failure and atrial fibrillation: results from the RAFT-AF trial. Circulation 2022;145:1693–704. [DOI] [PubMed] [Google Scholar]
- 21. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy Det al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation 2016;133:1637–44. [DOI] [PubMed] [Google Scholar]
- 22. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. [DOI] [PubMed] [Google Scholar]
- 23. Kuck KH, Merkely B, Zahn R, Arentz T, Seidl K, Schlüter Met al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA trial. Circ Arrhythm Electrophysiol 2019;12:e007731. [DOI] [PubMed] [Google Scholar]
- 24. Noseworthy PA, Van Houten HK, Gersh BJ, Packer DL, Friedman PA, Shah NDet al. Generalizability of the CASTLE-AF trial: catheter ablation for patients with atrial fibrillation and heart failure in routine practice. Heart Rhythm 2020;17:1057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kheiri B, Osman M, Abdalla A, Haykal T, Ahmed S, Bachuwa Get al. Catheter ablation of atrial fibrillation with heart failure: an updated meta-analysis of randomized trials. Int J Cardiol 2018;269:170–3. [DOI] [PubMed] [Google Scholar]
- 26. van Vugt SPG, Westra SW, Volleberg RHJA, Hannink G, Nakamura R, de Asmundis Cet al. Meta-analysis of controlled studies on minimally interrupted vs. continuous use of non-vitamin K antagonist oral anticoagulants in catheter ablation for atrial fibrillation. Europace 2021;23:1961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KRet al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–45. [DOI] [PubMed] [Google Scholar]
- 28. Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle Let al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring. Circulation 2019;140:1779–88. [DOI] [PubMed] [Google Scholar]
- 29. le Polain de Waroux JB, Weerasooriya R, Anvardeen K, Barbraud C, Marchandise S, De Meester Cet al. Low contact force and force-time integral predict early recovery and dormant conduction revealed by adenosine after pulmonary vein isolation. Europace 2015;17:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phlips T, Taghji P, El Haddad M, Wolf M, Knecht S, Vandekerckhove Y et al. Improving procedural and one-year outcome after contact force-guided pulmonary vein isolation: the role of interlesion distance, ablation index, and contact force variability in the ‘CLOSE’-protocol. Europace 2018;20:f419–27. [DOI] [PubMed] [Google Scholar]
- 31. Scherr D, Khairy P, Miyazaki S, Aurillac-Lavignolle V, Pascale P, Wilton SB et al. Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol 2015;8:18–24. [DOI] [PubMed] [Google Scholar]
- 32. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 33. Rolf S, Kircher S, Arya A, Eitel C, Sommer P, Richter S et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7:825–33. [DOI] [PubMed] [Google Scholar]
- 34. Clarnette JA, Brooks AG, Mahajan R, Elliott AD, Twomey D, Pathak RKet al. Outcomes of persistent and long-standing persistent atrial fibrillation ablation: a systematic review and meta-analysis. Europace 2018;20:f366–76. [DOI] [PubMed] [Google Scholar]
- 35. Mohanty S, Mohanty P, Trivedi C, Gianni C, Della Rocca DG, Di Biase Let al. Long-term outcome of pulmonary vein isolation with and without focal impulse and rotor modulation mapping. Circ Arrhythm Electrophysiol 2018;11:e005789. [DOI] [PubMed] [Google Scholar]
- 36. Marrouche NF, Wazni O, McGann C, Greene T, Dean JM, Dagher Let al. Effect of MRI-guided fibrosis ablation vs conventional catheter ablation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the DECAAF II randomized clinical trial. JAMA 2022;327:2296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.