Abstract
Aims
Pulsed-field ablation (PFA) can offer a novel perspective for atrial fibrillation (AF) ablation. We aimed to characterize the incidence of pulmonary vein (PV) reconnection, types of recurrent atrial tachyarrhythmia (ATa) and lesion quality after PFA-guided PV isolation (PVI).
Methods and results
Patients undergoing second ablation for recurrent ATa following the initial PVI using the pentaspline PFA catheter were investigated. The rate of PV reconnection, the features of recurrent ATa, and the amount of isolated posterior wall (PW) surface area (ISAPW%) (ratio of the isolated- to total surface area on PW) were analyzed.
Results
Among 360 patients treated with PFA, 25 patients (paroxysmal AF, n = 19) with 99 PVs underwent a second procedure 6.1 ± 4.0 months after the initial procedure. The rate of PV reconnection was 9.1% (9 PVs). Patients presented with atrial tachycardia (AT) (n = 16), AF (n = 8) and typical atrial flutter (n = 1). The mechanism of all but one AT was macro-reentry. The critical isthmus was found to be linked to the initial lesion set at the left atrial (LA) PW in eight patients and linked to pre-existing substrate at the LA anterior wall in four patients. One AT had a focal origin at the septum. In three patients, AT were unmappable. Mean ISAPW% was 72.7 ± 19.0%.
Conclusion
We revealed a remarkable low reconnection rate with a large antral lesion at the PW after pentaspline PFA catheter-guided PVI. However, macro-reentrant AT with a critical isthmus at the LAPW linked to the PVI lesion set was commonly observed.
Keywords: Pulsed-field ablation, Atrial fibrillation, Index lesion durability
What’s new?
The clinical findings were investigated in 25 patients with recurrent atrial tachyarrhythmia undergoing repeat ablation following pulmonary vein isolation (PVI) using the pentaspline pulsed-field ablation catheter.
We confirmed a low overall PV reconnection rate of 9.1% at the time of repeat ablation. The use of 31 mm-sized catheter was supposed to be associated with a less reconnection rate compared with 35 mm-sized catheter. In trend, LSPV was the vein with most frequent reconnection.
A large amount of isolated posterior wall (PW) surface area (ISAPW%) with this technology was confirmed at the second procedure.
The macro-reentrant atrial tachycardia (AT) associated with the PVI lesion set on PW was commonly observed in the patients with AT, which implied that the extensive lesion set on PW might create an unexpected potential slow conduction zone leading to recurrent AT.
Introduction
Pulmonary vein (PV) isolation (PVI) represents the procedural cornerstone in catheter ablation of atrial fibrillation (AF). To simplify the workflow as well as to guarantee the safety, pulsed-field ablation (PFA) has been recently developed as a new myocardial-specific ablation method where direct current electric energy, called electric-field, is applied to cells; disrupts cell membranes by creating pores.1–4 This mechanism offers lesion creation independent of contact force between catheter and left atrium (LA) without compromising safety.5
Recently, the FARAPULSE PFA system (FARAPULSETM, Boston Scientific) was regulatory approved in Europe. This system offers a seamless deflective function with variable shape from basket-like to flower-like and optional catheter size selection (31 mm or 35 mm). These unique features enable wide antral lesion creation without regression over time.6,7 Contemporary preclinical and early clinical studies focusing on AF ablation demonstrated promising data.2–4 Furthermore, our group reported the streamlined single-shot fashioned PVI, i.e. ‘5S-study strategy: Safe and Simple Single Shot pulmonary vein isolation with pulsed-field ablation using Sedation’ with this novel technology.8 Although the acute procedural data and freedom rate from atrial tachyarrhythmia (ATa) recurrence have been published in recent years,8,9 the real-word all-comer human data are still limited, especially on repeat procedure after PVI. Moreover, although the extensive antral isolation in this technology was reported,6,7,10 the isolated lesion in ‘the true single shot fashion’, i.e. without guidance using additional 3D mapping system and/or other imaging modalities, is still unclear.
In this study, therefore, we assessed the incidence and distribution of reconnected PVs, features of recurrent ATa, and isolated surface area following initial FARAPULSE-PFA system-guided PVI in patients who underwent repeat procedures for symptomatic ATa recurrences.
Methods
All patients provided written informed consent before undergoing the ablation procedure. The study was approved by the Institutional Review Board and complied with the Declaration of Helsinki.
Study population
Between May 2021 and April 2022, 360 consecutive patients with symptomatic paroxysmal or persistent AF underwent index PVI using FARAPULSE PFA system at our centre. Of these, patients who underwent a second ablation for recurrent ATa were analyzed.
Patients were excluded at the initial procedure if they were >85 years or <18 years, or ineligible for treatment with oral anticoagulation.
Pre-ablation protocol
Transesophageal echocardiography was routinely performed in patients with a CHA2DS2-VaSc score >/=2 to exclude intracardiac thrombi and assess potential mitral valve disease. Anticoagulation was continued until the morning of the procedure and resumed on the evening after the intervention. Pre-procedural imaging (computed tomography or cardiac MRI) to assess PV anatomy was not required.
Index ablation procedure
Index procedure was conducted according to the previously described ‘5S study’ for PVI8 without additional 3D mapping system. In brief, after femoral venous access, selective PV angiographies following single transseptal puncture were conducted with a 7Fr-multipurpose catheter in standard angulation (RAO 30°/LAO 40°). Thereafter the transseptal sheath was then exchanged for the catheter (13.8 F inner diameter; FARADRIVETM, Boston Scientific) sheath using a guidewire. Device size (31 or 35 mm FARAWAVETM, Boston Scientific) was left at the discretion of the operator according to PV angiographies and the position of transseptal puncture. After the activated clotting time ≥300 s was confirmed at least one time, the PFA catheter was introduced to the LA over a guidewire. PFA ablation started from the left superior PV (LSPV) and was conducted in the following order (LSPV, left inferior PV (LIPV), right inferior PV (RIPV), and right superior (RSPV)). Left common PVs (LCPVs) with a long common trunk were treated as a single PV. After positioning the catheter at the PV ostium, eight applications were delivered as the initial lesion set for each PV, i.g. two pairs of two applications with basket- and flower-like configuration with a different 36°-rotated position of the catheter spline. All applications were conducted as a biphasic waveform in a microsecond scale unsynchronized to cardiac rhythm. Energy output with 1.8 and 1.9 Kv were utilized for 31 and 35 mm catheter at the beginning of the learning curve according to the manufacturer’s recommendation. A full output of 2.0 Kv was standardized after confirming oesophageal safety.11 After the first and last energy application at each PV, the catheter was deployed at the PV ostium in a basket form to assess PVI.
All ablation were conducted under intravenous sedation using propofol, midazolam, and fentanyl following the recommendations of a German positional paper.12
Mapping protocol at repeat ablation
All repeat procedures were performed using 3D mapping system (CARTO3TM, Biosense Webstar) with a double transseptal puncture. Thereafter, selective PV angiographies were performed to exclude PV stenosis. In case of atrial tachycardia (AT) or atrial flutter (AFL), a high-density local activation map using 3D mapping system was constructed to identify the re-entry circuit or the earliest local activation site. Additionally, entrainment manoeuvers were carried out to confirm the diagnosis. In case of AF, normal sinus rhythm was restored by external electrical cardioversion. A spiral or multispline shaped mapping catheter (Lasso NAVTM/Pentaray NAVTM, Biosense Webstar) was used to evaluate persistency of electrical conduction block of PVs and location of conduction gap. In case of resumed LA to PV conduction, gap localization was identified with combination to intracardiac electrogram on the mapping catheter and detailed activation mapping using a 3.5 mm-irrigated tip catheter (THERMOCOOL SMARTTOUTCH SF TM, Biosense Webster). Radiofrequency current ablation was carried out to reisolate PVs using standard energy settings (30–40 W, flush rate 8–25 mL/min). Gap location was defined as the site of successful re-isolation of the PV or the site of clear change in the PV electric activation pattern, as previously published.13 All PVs were divided into four quadrants [anterior–superior (AS); anterior–inferior (AI); posterior–inferior (PI); posterior–superior (PS)] to categorize the gap location.
Measurement of the isolated surface area on posterior wall
Low-voltage area (LVA) was defined as an area with ≥3 adjacent points with a peak-to-peak bipolar electrogram amplitude of ≤0.5 mV or ≤0.3 mV during sinus rhythm or atrial flutter/AT, respectively.14 The total LA posterior wall (PW) area was defined as the quadrangle area connecting the ostia of LSPV/RSPV roofs and LIPV/RIPV bottom. The ratio of the isolated surface area on posterior wall (ISAPW)/total LAPW surface area (ISAPW (%)) was measured by two electrophysiologists based on calculating software in 3D mapping area allowing for exact determination of the map’s surface area within an area of manually selected points: ISAPW(%) = (isolated PW surface area)/(total LAPW surface area) × 100. (see Supplementary material online, Figure S1).
Endpoints
The endpoints of this study were the rate and distribution of PV reconnection, the features of recurrent ATa, and ISAPW(%) after the current pentaspline PFA catheter-guided PVI.
Statistical analysis
Data were expressed as mean ± SD. Categorical variables are expressed as number and percentage. For comparison between two groups, unpaired Student’s t-test, Mann–Whitney U test, or χ 2 test/Fisher’s exact test were used. A P-value of <0.05 was considered statistically significant. Analyses were conducted using JMP, version 11.0 software (SAS Institute, Cary, NC, USA).
Results
Demographic data
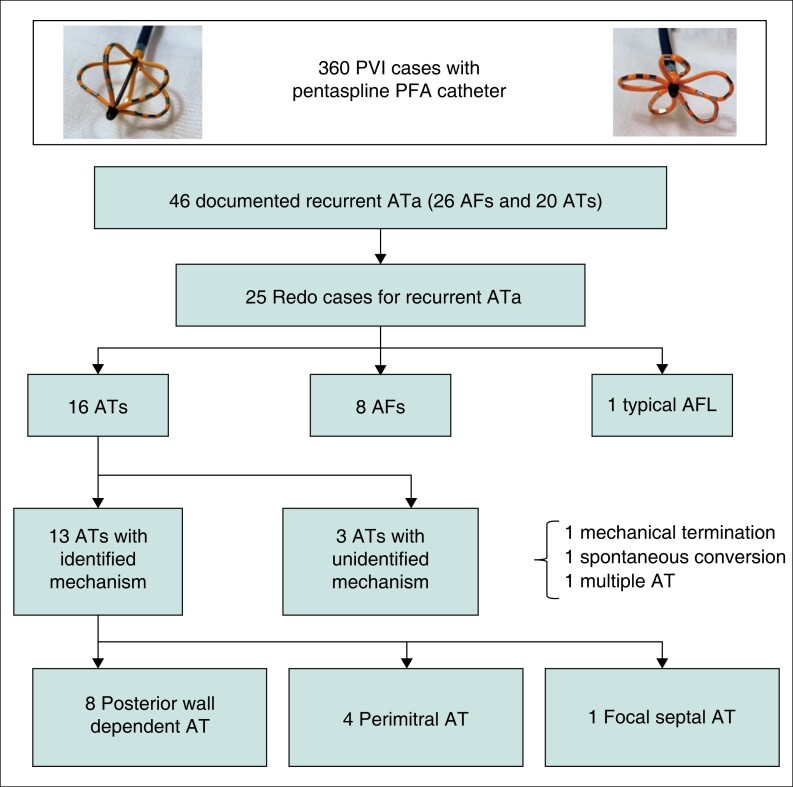
A total of 360 patients underwent initial PVI with the pentaspline PFA catheter in our centre (31 mm catheter, n = 215, 35 mm catheter, n = 145). Recurrent ATa was documented in 46 patients (26 AF and 20 AT) until the study enrolment. Of these, 25 patients (99 PVs) underwent a second ablation for symptomatic ATa recurrences 6.1 ± 4.0 months after the PFA index procedure. PVI was achieved solely with the PFA catheter (31 mm catheter, n = 15, 35 mm catheter, n = 10) and no radiofrequency touch-up ablation was utilized in the initial procedures.
Demographic and procedural data on initial procedure are depicted in Table 1. The maximum PV diameter in all four PVs was larger in patients treated with 35 mm catheter (20.8 ± 2.0 vs. 31 mm catheter: 24.5 ± 4.5 mm, P = 0.0245). A single stroke event occurred in one patient at initial procedure. The transient paralysis of the right hand was observed in this patient on the day of procedure and the symptom resolved completely at the time of discharge.
Table 1.
Demographic and procedural data at initial procedure
| Data at index procedure | Overall 25 Pt |
|---|---|
| Age, years | 72 ± 9 |
| Gender, female, n, % | 16 (64%) |
| BMI, n, % | 28 ± 5 |
| ȃParoxysmal, n, % | 19 (76%) |
| ȃPersistent, n, % | 5 (20%) |
| ȃLong-standing persistent, n, % | 1 (4%) |
| History of atrial flutter, n, % | 1 (4%) |
| Hypertension, n, % | 20 (80%) |
| Coronary artery disease, n, % | 1 (8%) |
| Heart failure, n, % | 4 (16%) |
| Diabetes mellitus, n, % | 2 (8%) |
| History of stroke, n, % | 1 (4%) |
| Left atrial diameter, mm | 45 ± 14 |
| Ejection fraction, % | 53 ± 15 |
| Class I, n, % | 3 (12%) |
| Class II, n, % | 17 (68%) |
| Class III, n, % | 5 (20%) |
| RSPV diameter, mm | 19.7 ± 2.8 |
| RIPV diameter, mm | 18.4 ± 2.9 |
| LSPV diameter, mm | 20.4 ± 3.5 |
| LIPV diameter, mm | 19.1 ± 3.9 |
| LCPV diameter, mm | 22 |
| Maximum diameter of all four PVs, mm | 22 ± 3.7 |
| Procedural data | |
| Procedure time | 40 ± 14 |
| Fluoroscopic time | 9.4 ± 3.7 |
| Catheter size selection (31 mm/35 mm) | 15/10 (60%/40%) |
| Energy output (1.8/1.9/2.0 Kv), n, % | 1/13/11 (4%/52%/44%) |
| Use of touch-up catheter, n, % | 0 (0%) |
| Complication | |
| Stroke | 1 (4%) |
Pulmonary vein reconnection at the repeat procedure
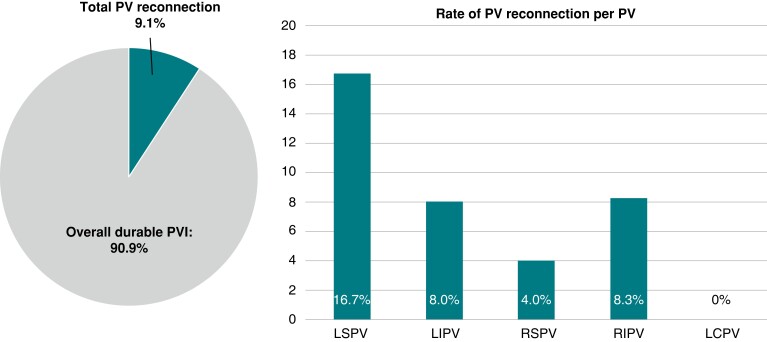
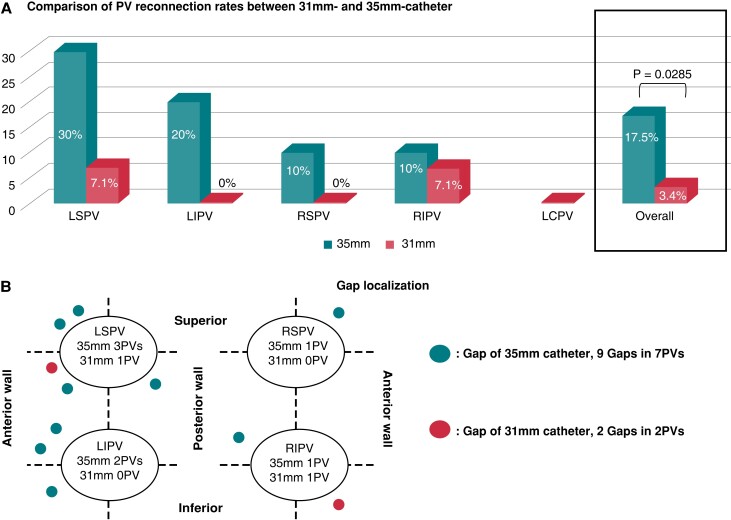
A total of 99 PVs were identified, including one LCPV with a long common trunk. Selective angiogram at the second procedure revealed no PV narrowing (<25%). In total, PV reconnection was documented in nine PVs (9.1%). On a per vein basis, the PV reconnection rates were 4% (1/25), 8% (2/25), 16.7% (4/24), 8.3% (2/24) and 0% (0/1), for RSPV, RIPV, LSPV, LIPV, and LCPV, respectively (Figure 1). In trend, LSPV was the vein with most frequent reconnection. On a per catheter size basis, reconnection rate was in trend lower in PVs treated with the 31 mm catheter than in PVs treated with the 35 mm catheter (31 mm: 3.4% (2/59) PVs vs. 35 mm: 17.5% (7/40) PVs, P = 0.0285, Figure 2A). Of note, the diameter of isolated PVs was significantly smaller than that of reconnected PVs (isolated PVs: 19.0 ± 2.9 mm vs. reconnected PVs: 23.1 ± 5.2, P = 0.0088).
Figure 1.
The rate of PV reconnection at the repeat procedure after pentaspiline PFA catheter-guided ablation.
Figure 2.
The comparison between 31 and 35 mm catheter. The rates of PV reconnection (A) and gap localization (B) at the repeat procedure after PVI using the pentaspline PFA catheter.
On a per patient basis, persistent durable isolation of all four PVs was recorded in 19 (76%) patients. Regarding to energy output, persistent durable isolation of all four PVs was confirmed in 10 of 11 patients (90.9%) treated with a full output of 2.0 Kv while it was conformed in 9 of 14 patients (64.2%) treated with an output of 1.8 or 1.9 Kv. The numbers of reconnected PVs were one in three patients and two in three patients.
In total, 11 conduction gaps were detected in 9 PVs. In one patient, broad conduction gap was found at the anterior part of the left ipsilateral PVs so that the two conduction gaps were identified both on AS and AI in each PV. The distribution of gap localization was summarized in Figure 2B.
Findings during repeat ablation
The type of recurrent ATa was summarized in Figure 3. For the repeat procedure 16, 8 and 1 patient presented with AT, AF and typical AFL, respectively. Notably, durable isolation of all four PVs was observed in 82.4% (14/17) of non-AF patients (16 ATs and 1 AFL) while it was present in 62.5% (5/8) of patients with recurrent AF (P = 0.34). At the initial procedure, the 31 mm catheter was utilized in nine patients with recurrent AT, in five patients with recurrent AF, and in one patient with recurrent typical AFL. The 35 mm catheter was utilized in six patients with recurrent AT and in four patients with recurrent AF.
Figure 3.
Patients flow chart and the type of recurrent ATa.
In patients with AT recurrence, the mechanism was identified in 13 patients. The remaining 3 Ats were unmappable for non-inducibility after electrical cardioversion (n = 2) or instability with multiple different ATs (n = 1).
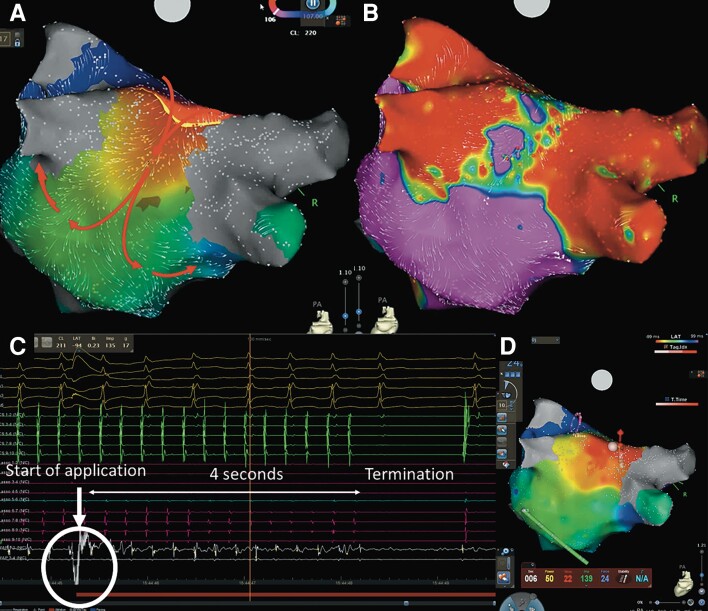
In 8 of 13 (61.5%) patients (four patients with 31 mm and four patients with 35 mm catheter), the critical isthmus of the macro-re-entrant AT was localized at the PW in a narrow zone of viable myocardium in between the previous PVI lesions (Figure 4, Supplementary material online, Video S1). In 4 of 13 (30.8%) patients, the critical isthmus was located in LVA at the anterior LA wall. In one patient, a focal AT from the non-coronary cusp was found.
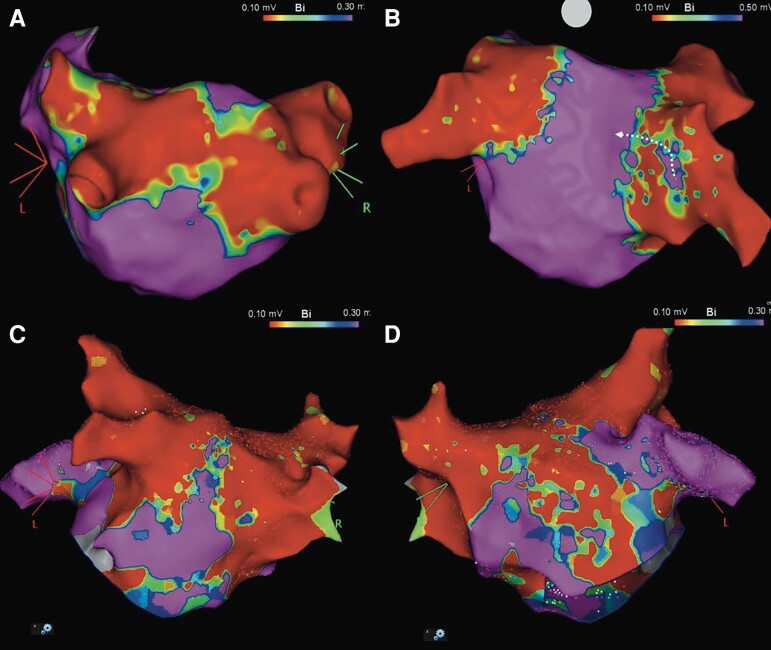
Figure 4.
A case of PW lesion set related AT. A local activation map (A) and a voltage map (B) of LA with posterior–anterior oblique using 3D mapping system constructed during a recurrent AT, which indicated a roof-dependent AT with a critical isthmus linked to the lesion set of the index procedure at the LAPW. The tachycardia was terminated 4 s with the first application on the LA roof after starting the application (C, D).
A roof line was deployed successfully for the PW associated ATs in all patients. Anterior linear ablation from the mitral valve to the RSPV was carried out in four cases with perimitral AT.
In three patients with unidentified AT mechanism, left atrial (LA) substrate modification with a combination of anterior and roof linear ablations was performed. In a single case of typical AFL, a cavotricuspid isthmus line was deployed.
Excluding antral and PW lesions, LVA was observed on LA septal area in 11 patients (perimitral AT: 3, roof-dependent AT: 2, unmappable AT: 1, AF: 5) and LA anterior area in nine patients (peri-mitral AT: 3, AF: 5, unidentified AT: 1).
In eight patients with recurrent AF, LA–PV reconnection was observed in three patients. PV re-isolation at the conduction gap sites was performed with focal ablation.
A single case of post postprocedural Dressler’s syndrome was observed 4 weeks after the second ablation. This patient was treated conservatively.
Procedural data on the repeat procedure was summarized in Table 2.
Table 2.
Procedural data on repeat procedure
| Overall 25 patients | Patients with AT (n = 16) | Patients with AF (n = 8) | Patient with AFL (n = 1) | |
|---|---|---|---|---|
| Procedure time, min | 66 ± 14 | 63 ± 11 | 69 ± 16 | 65 |
| Fluoroscopic time, min | 6.0 ± 2.3 | 5.6 ± 2.1 | 6.5 ± 2.7 | 7.9 |
| Cycle length of tachycardia | 297 ± 103 | 299 ± 106 | — | 260 |
| Re-PVI, n, % | 6 (24%) | 3 (19%) | 3 (38%) | 0 (0%) |
| Exclusively re-PVI, n, % | 1 (4%) | 0 (0%) | 1 (13%) | 0 (0%) |
| Anterior linear ablation, n, % | 13 (52%) | 8 (50%) | 5 (62.5%) | 0 (0%) |
| Roof linear ablation, n, % | 21 (84%) | 13 (81%) | 7 (88%) | 1 (100%) |
| CTI block, n, % | 3 (12%) | 2 (13%) | 0 (0%) | 1 (100%) |
| Complication | ||||
| Dressler’s Syndrome | 1 (4%) | 1 (4%) | 0 (0%) | 0 (0%) |
Isolated surface area on posterior wall(%)
The overall ISAPW(%) was calculated at 72.7 ± 19.0%. Excluding the patient with AFL, ISAPW(%) was higher in patients with AT than patients with AF (AT: 76.1 ± 17.3 vs. AF: 54.3 ± 16.5, P = 0.0357). If analyzed by catheter size, ISAPW(%) was 67.3 ± 20.6% in 35 mm catheter and 75.8 ± 18.0% in 31 mm catheter (P = 0.245).
Follow-up
Mean follow-up period after the second procedure was 4.8 ± 3.6 months. The number of patients who came to outpatient clinic after a blanking period (3 months) was 17. Of those, four patients (23.5%) presented with a recurrent AF.
Discussion
Main findings
To the best of our knowledge, this is the first report on the electrophysiological findings in patients with recurrent ATa following PVI using the novel pentaspline PFA catheter. The main findings are as follows: (i) an overall low rate of PV reconnection (9.1%); (ii) a less reconnection rate in PVs treated with 31 mm catheter (3.4% vs. 35 mm: 17.5%, P = 0.0285); (iii) a complete durable isolation of all four PVs in 76% of patients; (iv) PVI lesion set associated PW-dependent AT as the most common ATa mechanism (8/13 Ats, 61.5%), at the repeat procedure.
Index pulmonary vein isolation durability
Electrical PV reconnection is generally associated to AF recurrence.15 Therefore, creating durable PV lesion sets has been a crucial task to improve the long-term freedom from recurrent arrhythmia. A preclinical study demonstrated the marvellous rate (96%) of durable PVI with invasive remapping study 3 months after procedure.2 The durability was assessed irrespective of ATa recurrence in that study. In contrast, our study investigated the index lesion durability only in patients with recurrent ATa. Nevertheless, the low incidence of PV reconnection was remarkable. Of note, the PVs treated with 31 mm catheter resulted in an excellent low reconnection rate (3.4%) compared with previous data regarding to repeat ablation using other technologies at initial procedure.13,16,17
Interestingly, the predominantly reconnected PV was LSPV in our data. Indeed, Gunawardene et al.10 reported a 6.25% rate of acute PV reconnection only in superior PVs (3.75% in RSPV and 2.5% in LSPV) using the pentaspline PFA catheter with an ultra-high-density 3D mapping system. Superior PVs are generally larger than the ipsilateral inferior PV. Additionally, the LSPV ostium is a complex anatomy consisting of LIPV and left atrial appendage. Thus, the 35 mm catheter was selected in larger superior PVs to cover anatomical gap between catheter and PV ostium. However, the effective electric-field’s strength may decrease as the distance between its source and the target tissue increases.5 A combination of anatomical feature and size selection could lead to a trend towards reconnection in LSPV.
The pattern of recurrent atrial tachyarrhythmia
AF has been demonstrated as the most dominant recurrent ATa irrespective of energy sources after PVI.13,15–19 Recurrent ATa as macro-reentrant AT following initial PVI is uncommon in general. Our group also reported the low incidence of lesion set related AT after contemporary balloon-based ablations.17,18 Unexpectedly, lesion set associated AT was frequently observed in our study. The presence of proarrhythmic substrate was reported to be associated with post-ablation macro-reentry circuits.18–20 Such substrate is theoretically based on either lesion set related- or pre-existing scar lesion represented by LVA. Considering the distribution of LVA, the critical isthmus of the AT was linked to the PVI lesion set of the index procedure at the LAPW in the majority of cases (Figure 5A), while two types of isolated surface lesion on PW were observed in patients with other types of recurrent ATa; (i) the distance on PW between isolated border zone was wide enough to avoid a potential slow conduction corridor on PW (Figure 5B); (ii) septal and lateral ablated antral lesions were fused at the LA roof and connected to LVA at the anterior lesion (Figure 5C and D), which blocked unintentionally a roof line.
Figure 5.
Comparison of voltage maps according to types of recurrent ATa coloured according to the defined setting of the reference color bar; red indicates low voltage area with a bipolar voltage amplitude of ≤0.1 mV, and purple indicates preserved voltage area; ≤0.5 mV or ≤0.3 mV during sinus rhythm or atrial tachycardia, respectively. Representative voltage maps with posterior–anterior oblique in patients with recurrent PW related AT (A), AF (B) and perimitral AT (C). The white arrow on Figure 4B indicates a reconnected RIPV with a conduction breakthrough. Three perimitral AT cases presented the LVA on septal or anterior LA fused with isolated area, that was speculated to block a roof line unintentionally (D).
Perimitral AT is not uncommon after PVI and also non-negligible in PFA-guided PVI. Wasmer et al. reported that differences in the LA substrate in addition to ablation lesions and the protocol of PVI most likely contribute to the development of different AT mechanisms under comparable conditions.20 From the combination of LVA distant from the index ablation set and a history of persistent AF, perimitral AT was diagnosed as lesion set independent AT.
Extensive lesion set on posterior wall
Kawamura et al.7 described that there was no significant difference between the PFA and thermal fashioned ablation, i.e. radiofrequency circumferential PVI, laser balloon- and cryoballoon-guided PVI, in either the left- and right-sided PV isolation areas, or the non-ablated PW area. In line with this report, Gunewardene et al.10 demonstrated the compatible result about lesion set.
On the other hand, our data showed an extensive isolation area on the PW beyond the PV antrum. Different from previous studies, our study enrolled patients with persistent AF including a case of long-standing persistent AF. Also, the intracardiac echocardiography and/or 3D mapping system was not integrated based on the recently published single-shot fashioned 5S ablation strategy.8 The current pentaspline PFA catheter has two catheter size options (31 or 35 mm), and its selection could be decided according to the LA/PV anatomy. Therefore, a narrow zone of viable myocardium in between the previous PVI lesions could be formed irrespective of catheter size, which potentially creates an unexpected critical isthmus for macro-reentry circuit. Thus, there is potential usefulness to evaluate created lesion and pre-exiting scar lesion using 3D mapping system at the initial procedure to predict the future clinical course. Furthermore, anatomical predictor by pre-procedural or fluoroscopic imaging modalities can offer the answer.
Clinical implications
Our data demonstrated a high durable PVI rate in patients with recurrent ATa, which highlighted the persistent effect of PFA without regression in all-comer patients. One of the possible points of improvements is energy output. Based on the recommendation from the manufacturer, energy output with 1.8 and 1.9 Kv were used for 31 and 35 mm catheter at the beginning of the learning curve, respectively. The energy output of 2.0 Kv was standardized after confirming oesophageal safety.11 Thus, if clinical safety is completely ensured, irreversible lesion formation with the output of 2.0 Kv should be preferred.
Regarding to the recurrent ATa, our data implied a need to reconsider the way of lesion creation in anticipation of the future clinical course. Especially, extra attention should be paid for the PW area at the initial procedure. More small lesion formation by adjusting catheter configuration at energy delivery can be a possible option to prevent lesion set associated critical isthmus. Oppositely, more extensive lesion formation is also one of possible options. Although the prophylactic PW ablation on top of PVI has not reached to the consensus, Reddy et al reported the high durable PW isolation rate with the pentaspline PFA catheter.3 Hence, the indication for preventive elimination of the potential isthmus on PW could be debated for the future tailored ablation strategy. Alternatively, investing predictive marker is one of the future tasks to identify the specific patients that may require the prophylactic PW ablation.
Limitations
This study has several limitations. First, this is a single-centre small-sized study due to peculiarity of patient backgrounds as repeat procedure. Only selected patients with symptomatic recurrent tachyarrhythmia after PFA-guided PVI have been investigated. Therefore, the PVI durability in the overall patient population remains unclear. Second, a repeat procedure was not conducted in all patients with documented recurrent ATa. This was mainly because the symptom tended to be stronger in patients with recurrent AT than patients with recurrent AF. Hence, the selection bias for the repeat procedure could not be excluded in this study. Third, no control group was investigated. Fourth, no pre- and postprocedural imaging modality was conducted in this study. Fifth, LVA was determined according to a report of variable cut-off points in different rhythms.18 The cut-off point of LVA was based on the report demonstrating its correlation among different rhythms, which has not been yet widely established. Finally, the gap location was based on combination of information from 3D mapping system and electrophysiological findings. However, surrounding ablation points which did not critically affect sequential morphology on intra cardiogram could be underestimated.
Conclusion
We confirmed a low incidence of PV reconnection (9.1%) at the time of repeat ablation following the current pentaspline PFA catheter-guided PVI in patients with recurrent ATa. The use of 31 mm-sized catheter was supposed to be associated with a lower reconnection rate. In trend, LSPV was the vein with most frequent reconnection. Unexpectedly, lesion linked electrical isthmus formation was frequently observed predisposing to LAPW-dependent AT, which implied a need for lesion set adjustment.
IRB information
The study was reviewed by the institutional review board and complies with the Declaration of Helsinki.
Supplementary Material
Contributor Information
Shota Tohoku, Cardioangiologisches Centrum Bethanien, Wilhelm-Epstein Str. 4, 60431 Frankfurt, Germany.
K R Julian Chun, Cardioangiologisches Centrum Bethanien, Wilhelm-Epstein Str. 4, 60431 Frankfurt, Germany; Die Sektion Medizin, Universität zu Lübeck, Ratzeburger Allee 160, 23562 Lübeck, Germany.
Stefano Bordignon, Cardioangiologisches Centrum Bethanien, Wilhelm-Epstein Str. 4, 60431 Frankfurt, Germany.
Shaojie Chen, Cardioangiologisches Centrum Bethanien, Wilhelm-Epstein Str. 4, 60431 Frankfurt, Germany; Die Sektion Medizin, Universität zu Lübeck, Ratzeburger Allee 160, 23562 Lübeck, Germany.
David Schaack, Cardioangiologisches Centrum Bethanien, Wilhelm-Epstein Str. 4, 60431 Frankfurt, Germany.
Lukas Urbanek, Cardioangiologisches Centrum Bethanien, Wilhelm-Epstein Str. 4, 60431 Frankfurt, Germany.
Ramin Ebrahimi, Cardioangiologisches Centrum Bethanien, Wilhelm-Epstein Str. 4, 60431 Frankfurt, Germany.
Jun Hirokami, Cardioangiologisches Centrum Bethanien, Wilhelm-Epstein Str. 4, 60431 Frankfurt, Germany.
Fabrizio Bologna, Cardioangiologisches Centrum Bethanien, Wilhelm-Epstein Str. 4, 60431 Frankfurt, Germany.
Boris Schmidt, Cardioangiologisches Centrum Bethanien, Wilhelm-Epstein Str. 4, 60431 Frankfurt, Germany; Universitätsklinikum Frankfurt, Medizinische Klinik 3- Klinik für Kardiologie, Theodor-Stern-Kai 7, 60590 Frankfurt am Main, Germany.
Supplementary material
Supplementary material is available at Europace online.
Funding
None declared.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Wittkampf FHM, van Es R, Neven K. Electroporation and its relevance for cardiac catheter ablation. JACC Clin Electrophysiol 2018;4:977–86. [DOI] [PubMed] [Google Scholar]
- 2. Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet Het al. . Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol 2019;74:315–26. [DOI] [PubMed] [Google Scholar]
- 3. Reddy VY, Anic A, Koruth J, Petru J, Funasako M, Minami Ket al. . Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardiol 2020;76:1068–80. [DOI] [PubMed] [Google Scholar]
- 4. Reddy VY, Dukkipati SR, Neuzil P, Anic A, Petru J, Funasako Met al. . Pulsed field ablation of paroxysmal atrial fibrillation. JACC Clin Electrophysiol 2021;7:614–27. [DOI] [PubMed] [Google Scholar]
- 5. Ramirez FD, Reddy VY, Viswanathan R, Hocini M, Jaïs P. Emerging technologies for pulmonary vein isolation. Circ Res 2020;127:170–83. [DOI] [PubMed] [Google Scholar]
- 6. Kawamura I, Neuzil P, Shivamurthy P, Petru J, Funasako M, Minami Ket al. . Does pulsed field ablation regress over time? A quantitative temporal analysis of pulmonary vein isolation. Heart Rhythm 2021;18:878–84. [DOI] [PubMed] [Google Scholar]
- 7. Kawamura I, Neuzil P, Shivamurthy P, Kuroki K, Lam J, Musikantow Det al. . How does the level of pulmonary venous isolation compare between pulsed field ablation and thermal energy ablation (radiofrequency, cryo, or laser)? EP Europace 2021;23:1757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmidt B, Bordignon S, Tohoku S, Chen S, Bologna F, Urbanek Let al. . 5 S study: safe and simple single shot pulmonary vein isolation with pulsed field ablation using sedation. Circ Arrhythm Electrophysiol 2022;15:e010817. [DOI] [PubMed] [Google Scholar]
- 9. Ekanem E, Reddy VY, Schmidt B, Reichlin T, Neven K, Metzner Aet al. . Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace 2022;24:1256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gunawardene MA, Schaeffer BN, Jularic M, Eickholt C, Maurer T, Akbulak RÖet al. . Pulsed-field ablation combined with ultrahigh-density mapping in patients undergoing catheter ablation for atrial fibrillation: practical and electrophysiological considerations. Cardiovasc Electrophysiol 2022;33:345–56. [DOI] [PubMed] [Google Scholar]
- 11. Koruth JS, Kuroki K, Kawamura I, Brose R, Viswanathan R, Buck EDet al. . Pulsed field ablation versus radiofrequency ablation: esophageal injury in a novel porcine model. Circ Arrhythm Electrophysiol 2020;13:e008303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tilz RR, Chun KRJ, Deneke T, Kelm M, Piorkowski C, Sommer Pet al. . Position paper of the German society of cardiology on cardioanalgosedation: focus on interventions in rhythmology. Kardiologe 2017;11:369–82. [Google Scholar]
- 13. Tohoku S, Bordignon S, Chen S, Bologna F, Urbanek L, Operhalski Fet al. . Validation of lesion durability following pulmonary vein isolation using the new third-generation laser balloon catheter in patients with recurrent atrial fibrillation. J Cardiol 2021;78:388–96. [DOI] [PubMed] [Google Scholar]
- 14. Rodríguez-Mañero M, Valderrábano M, Baluja A, Kreidieh O, Martínez-Sande JL, García-Seara Jet al. . Validating left atrial low voltage areas during atrial fibrillation and atrial flutter using multielectrode automated electroanatomic mapping. JACC Clin Electrophysiol 2018;4:1541–52. [DOI] [PubMed] [Google Scholar]
- 15. Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FTet al. . Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double lasso technique. Circulation 2005;111:127–35. [DOI] [PubMed] [Google Scholar]
- 16. Kuck KH, Albenque JP, Chun KRJ, Fürnkranz A, Busch M, Elvan Aet al. . Repeat ablation for atrial fibrillation recurrence post cryoballoon or radiofrequency ablation in the FIRE AND ICE trial. Circ Arrhythm Electrophysiol 2019;12:e007247. [DOI] [PubMed] [Google Scholar]
- 17. Chun JKR, Bordignon S, Last J, Mayer L, Tohoku S, Zanchi Set al. . Cryoballoon versus laserballoon: insights from the first prospective randomized balloon trial in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2021;14:e009294. [DOI] [PubMed] [Google Scholar]
- 18. Nagase T, Bordignon S, Perrotta L, Bologna F, Tsianakas N, Chen Set al. . HEartlight guided—PUre pulmonary vein isolation regardless of concomitant atrial substrate: HEURECA study. Pacing Clin Electrophysiol 2019;42:22–30. [DOI] [PubMed] [Google Scholar]
- 19. Masuda M, Asai M, Iida O, Okamoto S, Ishihara T, Nanto Ket al. . Low-Voltage-Area ablation in paroxysmal atrial fibrillation-extended follow-up results of the VOLCANO trial. Circ J 2022;86:245–52. [DOI] [PubMed] [Google Scholar]
- 20. Wasmer K, Mönnig G, Bittner A, Dechering D, Zellerhoff S, Milberg Pet al. . Incidence, characteristics, and outcome of left atrial tachycardias after circumferential antral ablation of atrial fibrillation. Heart Rhythm 2012;9:1660–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.