Abstract
Objective
This study sought to assess the effect of ethanol infusion into the vein of Marshall (EIVOM) on the acute success of left pulmonary vein (LPV) isolation in persistent atrial fibrillation (PeAF).
Methods and results
A total of 313 patients with drug-resistant PeAF were enrolled (135 in Group 1 and 178 in Group 2). In Group 1, EIVOM was firstly performed, followed by radiofrequency ablation (RFA) including bilateral pulmonary vein isolation (PVI) and linear ablation at roofline, cavotricuspid isthmus, and mitral isthmus (MI). In Group 2, PVI and linear ablations were completed with RFA. First-pass isolation of the LPV was achieved in 119 (88.1%) and 132 (74.2%) patients in Groups 1 and 2, respectively (P = 0.002). The rate of acute pulmonary vein reconnection (PVR) was significantly lower in Group 1 (9.6% vs. 22.5%, P = 0.003). About half of acute PVR occurred in the carina with or without EIVOM.
Conclusion
EIVOM is effective in achieving a higher first-pass isolation and a lower acute PVR of LPV in PeAF.
Keywords: Atrial fibrillation, Catheter ablation, Ethanol, Vein of Marshall, Pulmonary vein
What’s new?
The adjunctive effect of EIVOM on the acute success of LPV isolation has never been discussed. EIVOM is effective in achieving a higher rate of first-pass isolation and a lower rate of acute PVR of LPV in PeAF compared with the conventional RFA.
The lesions intervened by ethanol are not limited to the anterior LPV antrum, which are dependent on the anatomical distribution of the branches of the VOM. Part of regions of the LPV antrum do not require RFA.
PV carina is the most common location of acute PVR. EIVOM is helpful to eliminate the epicardial connections between LPV and LA.
Introduction
Pulmonary vein isolation (PVI) is considered the cornerstone for successful ablation in persistent atrial fibrillation (PeAF).1 Pulmonary vein reconnection (PVR) is a major contributor to AF recurrence after previous ablation,2,3 with an occurrence rate ranging from 19 to 64%.4 Achieving durable PVI is still challenging.5,6
Recently, the value of ethanol infusion into the vein of Marshall (EIVOM) in the treatment of PeAF has been widely reported.7,8 We previously reported that employing EIVOM as the first step of the ablation procedure before radiofrequency ablation (RFA) was associated with a higher rate of mitral isthmus (MI) block and less AF/atrial tachycardia (AT) recurrence.9 Besides, we found that EIVOM not only improve the success rate of MI block, but also contribute to the isolation of LPV as firstly reported by Valderrábano et al.10 However, the effect of EIVOM on the acute success of LPV isolation has not been well illustrated. In this present prospective study, we evaluated the value of EIVOM as the first step for isolation of LPV in PeAF, aiming to achieve a higher first-pass isolation rate of LPV and a lower acute PVR rate.
Methods
Population and study design
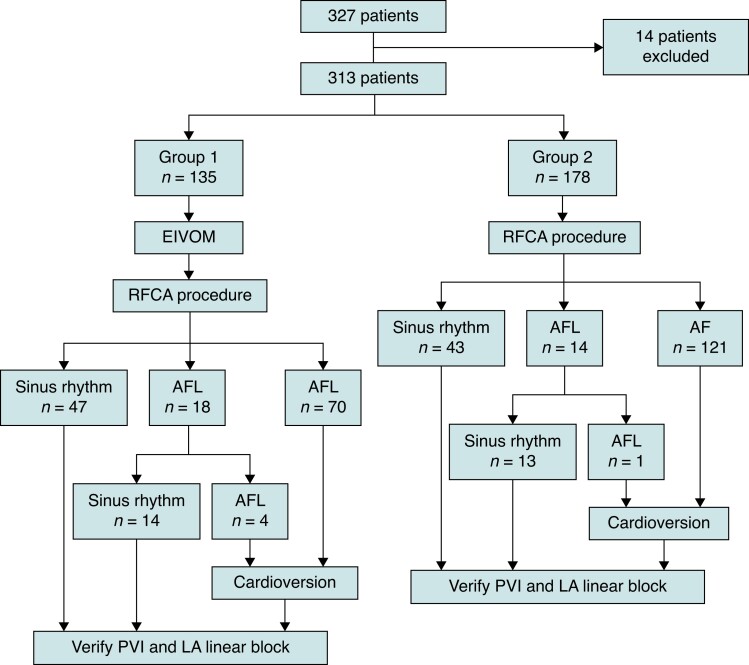
From November 2019 to March 2022, patients with drug-refractory PeAF undergoing the first ablation at Beijing Anzhen Hospital were prospectively enrolled. PeAF was defined as AF lasting for more than 7 d and long-standing PeAF as AF lasting for >12 months. The inclusion criteria were: (1) age over 18 years; (2) AF persisting over 3 months; (3) refractory to at least one Class I or III antiarrhythmic drug; and (4) no previous history of ablation for AF. All patients signed informed consent before the procedure. The study protocol was approved by the institutional review board of Anzhen hospital. All the antiarrhythmic drugs were discontinued for at least 5 half-lives before the procedure. Patients who underwent EIVOM followed by RFA including bilateral PVI as well as linear ablation at MI, left atrial roofline, and cavotricuspid isthmus (CTI) were included in Group 1, while patients who underwent only the RFCA were included in Group 2. Patients who experienced failed EIVOM due to the inability to visualize or cannulate the VOM were excluded. The flowchart of the study is shown in Figure 1.
Figure 1.
Workflow of the patient enrollment and procedure. The outcome of two groups after subsequent ablation steps. EIVOM, ethanol infusion into the vein of Marshall; RFCA, radiofrequency catheter ablation; AF, atrial fibrillation; AFL, atrial flutter; PVI, pulmonary vein isolation; LA, left atrial.
Ablation procedural
Electrophysiological study and ablation procedure were performed under conscious sedation with fentanyl and midazolam and uninterrupted anticoagulation. Heparin was administered to maintain an activated clotting time target of > 300 s. A 6F steerable decapolar catheter (Access Point Technologies Medical Inc.) was positioned in the coronary sinus (CS). Double transseptal access to the left atrium (LA) was performed under fluoroscopic guidance and intracardiac echocardiography (Cartosound, Biosense-Webster Inc.). The LA was reconstructed under the guidance of a three-dimensional electroanatomic mapping system (CARTO, Biosense-Webster Inc.) using a high-density mapping catheter (Pentaray® Catheter, Biosense-Webster Inc.).
EIVOM was performed immediately after LA mapping in Group 1. An 8.5F-long sheath (SL1; St. Jude Medical) was inserted into the right atrial just below the CS ostium, and a steerable long sheath (Agilis NxT; Abbott) was used if necessary. Under the right anterior oblique view, a 6F guiding catheter (Judkins R4.0) was inserted into the CS pointing posteriorly and superiorly in the vicinity of the Vieussens Valve in search of the ostium of VOM, and then a selective VOM venogram was acquired. A BMW guiding wire (0.014 in.×190 cm; Abbott) supported by an over-the-wire (OTW) balloon (1.5–2.5 mm diameter and 8–12 mm length, Boston Scientific) catheter was advanced into the visualized VOM. An appropriately sized balloon was inflated with a pressure of 6–8 atm. A selective venography of the VOM was obtained by injecting 1 mL of contrast medium to confirm complete occlusion with the OTW balloon and to attain a detailed distribution of VOM beyond the balloon occlusion. Subsequently, 6–10 mL of 95% ethanol would be slowly injected into the VOM and repeated venography of the VOM would reveal contrast staining of the affected myocardium colocalizing with the course of VOM.
RFA was performed in both Groups with a 3.5-mm cool saline-irrigated ablation catheter (ThermoCool Smart Touch SF® Catheter, Biosense-Webster Inc.). Ablation was performed with a point-by-point application fashion under power-control mode, with a temperature limited to 43°C and a saline flow rate of 8–15 mL/min. RF current was delivered for 8–30 s (power 45–50 W PVI; 35–45 W all other atrial sites; 25 W CS). Ablation within CS would be performed if necessary. RF applications were depicted using automated tagging technology (Carto VisiTagTM, Biosense-Webster Inc.), with a filter threshold of catheter motion <2.5 mm within 4 s and contact force ≥8 g for 70% of the time. Inter lesion distance was <6 mm. Targets for ablation index were (1) 500–550 for anterior wall; (2) 350–400 for posterior wall; (3) 450–500 for the LA roof and CTI; (4) 550–600 for MI; and (5) 300–350 for CS. If an organized AT occurred during the procedure, mapping-guided ablation would be performed. At the end of these steps, if AF persisted, electrical cardioversion would be performed to restore sinus rhythm. The details of the two approaches were demonstrated in our previous study.9
Assessment of PVR
The Pentaray catheter was positioned at the ostium of each PV. Complete isolation was confirmed by the Pentaray catheter with the absence of PV potential or the presence of dissociated PV potential. First-pass isolation was defined as achieving PVI after completing an ipsilateral encirclement with a Pentaray catheter recording PV electrical activity simultaneously. Acute PVR was defined as spontaneous PVR after a waiting time of 20 min since the initial verification of PVI. Conduction gaps were recognized as the site demonstrating the shortest atrium to PV conduction interval mapped with the Pentaray catheter in PV. The whole circle of PV antrum was divided into eight anatomic segments, including the anterior and posterior antrum of the superior PV, anterior and posterior antrum of the inferior PV, anterior and posterior carina, roof, and bottom. Sites of PVR were recorded and reisolated with touch-up ablation. Repeated assessment of PVI would be performed after the completion of touch-up ablation. The final PVI was confirmed by the isolation of all PVs after more than a 20 min waiting period. The endpoint of the procedure is bilateral PVI and bidirectional linear block.
Statistical analysis
Continuous variables were presented as mean ± standard deviation. Continuous data were compared using Student's t-test. Categorical variables were expressed as absolute numbers (percentages) and were compared by Chi-square or McNemar’s test as appropriate. All analyses were performed using the SPSS 28.0 software package. P-values <0.05 were considered significant.
Results
Baseline characteristics
Fourteen patients with failure of EIVOM due to an inability to find the VOM (n = 10) or unsuccessful cannulation (n = 4) were excluded. A total of 313 patients (243 males, mean age of 61.0 ± 10.2 years) were included. Of them, 135 (43.1%) patients were included in Group 1. There was a statistically significant difference in LA dimension (44.8 ± 5.6 vs. 43.3 ± 5.1 mm, P = 0.009), left ventricular ejection fraction (57.7 ± 11.8 vs. 60.3 ± 8.4, P = 0.011), and left ventricular end-diastolic diameter (50.9 ± 8.8 vs. 48.2 ± 4.4 mm, P < 0.001) between Group 1 and Group 2. The detailed baseline characteristics between groups are illustrated in Table 1.
Table 1.
Baseline characteristics
| Group 1 (n = 135) | Group 2 (n = 178) | P-value | |
|---|---|---|---|
| Age (years) | 60.1 ± 11.0 | 61.7 ± 9.6 | 0.096 |
| Male, n (%) | 109 (80.7) | 134 (75.3) | 0.251 |
| BMI (kg/m2) | 26.4 ± 3.7 | 26.4 ± 3.6 | 0.466 |
| Long-standing PeAF, n (%) | 73 (54.1) | 84 (47.2) | 0.280 |
| Hypertension | 76 (56.3) | 101 (56.7) | 0.937 |
| Diabetes mellitus | 27 (20) | 42 (23.6) | 0.447 |
| Heart failure | 36 (26.7) | 38 (21.3) | 0.273 |
| Stroke/TIA | 11 (8.1) | 25 (14) | 0.105 |
| CHADS2-Vasc score, n (%) | |||
| ȃ0,1 | 56 (41.5) | 72 (40.4) | 0.854 |
| ȃ2 | 30 (22.2) | 36 (20.2) | 0.668 |
| ȃ ≥ 3 | 49 (36.3) | 70 (39.3) | 0.584 |
| LA dimension (mm) Left ventricular ejection fraction (%) |
44.8 ± 5.6 57.7 ± 11.8 |
43.3 ± 5.1 60.3 ± 8.4 |
0.009 0.011 |
| Left ventricular end-diastolic diameter (mm) | 50.9 ± 8.8 | 48.2 ± 4.4 | <0.001 |
BMI, body mass index; PeAF, persistent atrial fibrillation; TIA, transient ischaemic attack; LA, left atrium.
Procedural results
All procedures were performed successfully, with a mean procedure duration of 146.5 ± 36.4 min and a mean fluoroscopic time of 8.2 ± 9.6 min. The total procedure time and fluoroscopy time in the Group 1 were longer compared with Group 2 (152.9 ± 39.1 vs. 141.9 ± 33.6 min, P = 0.005; 12.5 ± 9.8 vs. 4.9 ± 8.0 min, P < 0.001; respectively). Finally, all PVs were successfully isolated. In Group 1, the RFA time for initial isolation of LPV and right PV (RPV) were 12.2 ± 7.8 and 17.2 ± 7.3 min, respectively (P < 0.001). In Group 2, those were 15.5 ± 7.1 and 14.5 ± 6.0 min, respectively (P = 0.152). RFA time for RPV isolation in Group 1 was longer than that of Group 2 (P < 0.001), whereas RFA time for LPV isolation in Group 1 was significantly shorter than that of Group 2 (P < 0.001). A representative procedure is illustrated in Figure 2. The rate of first-pass isolation of LPV was significantly higher in Group 1 compared with Group 2 (88.1% vs. 74.2%, P = 0.002). In two patients, LPV isolation was achieved immediately after EIVOM. No significant difference was not found between the two groups in the rate of first-pass isolation rate of RPV (69.6% vs. 70.2%, P = 0.909). The rate of MI bidirectional block was significantly higher in Group 1 (95.6% vs. 87.6%, P = 0.015), while MI block was achieved directly after EIVOM in 6 (4.4%) patients. RFA time for MI in Group 1 was shorter than that in Group 2 (6.6 ± 4.4 vs. 7.8 ± 4.3 min, P = 0.013). The rate of acute MI reconnection was significantly lower in Group 1 (12.6% vs. 30.9%, P < 0.001). The detailed procedural characteristics between groups are illustrated in Table 2.
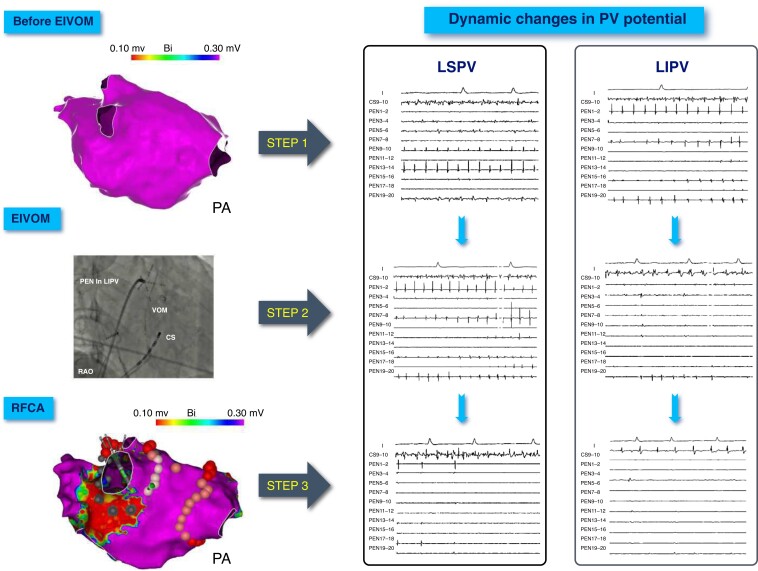
Figure 2.
Dynamic changes in LPV potential during EIVOM and RFCA. Step 1: LA voltage mapping and PV potential recording during AF rhythm before EIVOM. No low voltage area was found in LA. Step 2: after EIVOM, the change of the LSPV potential is not obvious, but LIPV is nearly isolated. Step 3: RFCA was implemented from the anterior wall to the posterior wall of the LSPV. The LSPV potential became slower gradually. PVI was achieved after ablation at the site showed by the catheter. Need for RF ablation was obviated in the majority parts of the LPV antrum. LPV, pulmonary vein; EIVOM, ethanol infusion into the vein of Marshall; RFCA, radiofrequency catheter ablation; LA, left atrial; AF, atrial fibrillation; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; PVI, pulmonary vein isolation.
Table 2.
Procedural characteristics and complications
| Group 1 (n = 135) | Group 2 (n = 178) | P-value | |
|---|---|---|---|
| Total procedure time (min) | 152.9 ± 39.1 | 141.9 ± 33.6 | 0.005 |
| Total fluoroscopy time (min) | 12.5 ± 9.8 | 4.9 ± 8.0 | <0.001 |
| LPV ablation time (min) | 12.2 ± 7.8 | 15.5 ± 7.1 | <0.001 |
| RPV ablation time (min) | 17.2 ± 7.3 | 14.5 ± 6.0 | <0.001 |
| First-pass isolation, n (%) | |||
| ȃLPV | 119 (88.1) | 132 (74.2) | 0.002 |
| ȃRPV | 94 (69.6) | 125 (70.2) | 0.909 |
| Acute PVR, n (%) | |||
| ȃLPV | 13 (9.6) | 40 (22.5) | 0.003 |
| ȃRPV | 37 (27.4) | 51 (28.7) | 0.808 |
| Duration from initial isolation to PVR (min) | |||
| ȃLPV | 37.4 ± 16.5 | 35.6 ± 11.5 | 0.336 |
| ȃRPV | 42.7 ± 21.5 | 46.7 ± 16.3 | 0.167 |
| Duration from initial success to final check in PV (min) | |||
| ȃLPV | 50.5 ± 25.2 | 47.8 ± 23.6 | 0.426 |
| ȃRPV | 55.9 ± 29.4 | 60.3 ± 26.1 | 0.238 |
| MI success rate, n (%) | 129 (95.6) | 156 (87.6) | 0.015 |
| MI ablation time (min) | 6.6 ± 4.4 | 7.8 ± 4.3 | 0.013 |
| Acute MI reconnection, n (%) | 17 (12.6) | 55 (30.9) | <0.001 |
LPV, left pulmonary vein; RPV, right pulmonary vein; MI, mitral isthmus; PVR, pulmonary vein reconnection.
Incidence and distribution of acute PVR
Acute PVR after a 20-min observational period occurred in 119/313 (38.0%) patients. Of these, the rate of acute PVR of LPV was significantly lower in Group 1 compared with Group 2 (9.6% vs. 22.5%, P = 0.003). However, no statistically significant difference was observed in the rate of acute PVR in RPV between the two groups (27.4% vs. 28.7%, P = 0.808). Of the total 313 patients, no patient was found to have reconnections in more than 2 PVs, while 12 patients (3.8%) had reconnections in 2 PVs. Sixty-one patients (19.5%) had single reconnection in 1 PV, with 87 (27.8%) reconnections located at the carinas. Twenty-two patients (7.0%) had bilateral PVR.
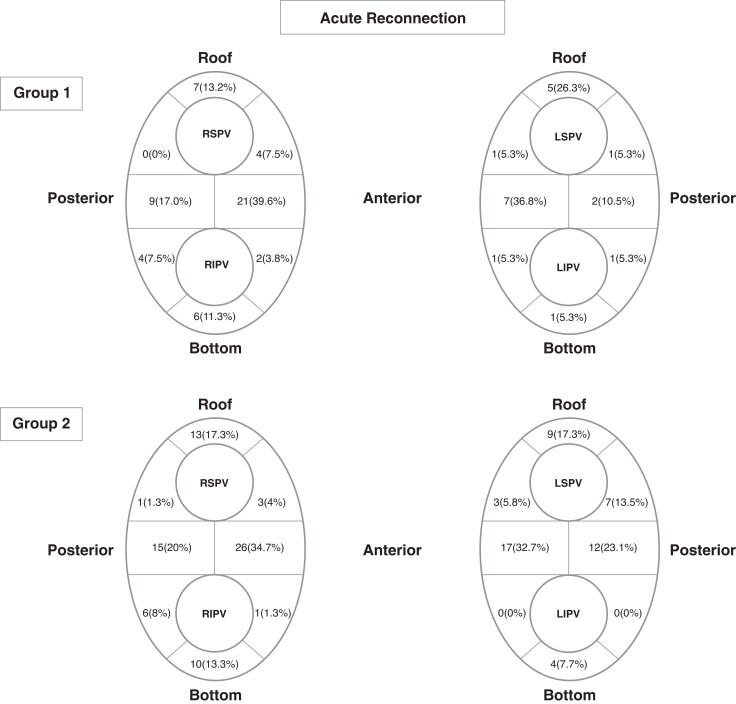
In Group 1, LPVs demonstrated significantly much lesser acute PVR than RPVs (9.6% vs. 27.4%, P < 0.001). Fifty-three reconnections were recorded in RPV while only 19 in LPVs. Eleven (20.8%) reconnections located at right superior PV (RSPV), while 12 (22.6%) at right inferior PV (RIPV), and 30 (56.6%) at right carina. For LPV, 7 (36.8%) located at left superior PV (LSPV), and 3 (15.8%) at LIPV, with 9 (47.4%) at left carina. In Group 2, acute PVR was observed in 40 (22.5%) LPVs and 51 (28.7%) RPVs, respectively (P = 0.178). Seventy-five reconnections were documented in RPVs and 52 in LPVs. Of these, 17 (22.7%) were at RSPV, 17 (22.7%) at RIPV, and 41 (36.5%) at right carina; 19 (36.5%) at LSPV, 4 (7.7%) at LIPV, and 29 (55.8%) at left carina. The detailed distributions of PVR in two groups are illustrated in Figure 3. The acute PVR rates of the typical distribution region of VOM (i.e. anterior carina, anterior LIPV, and bottom) in Group 1 and 2 were 9/135 (6.7%) and 21/178 (11.8%), respectively (P = 0.127). No significant difference was found in the distribution of the connection gap between the two groups.
Figure 3.
Acute reconnection distribution. Location at areas of acute pulmonary vein reconnection determined by the mapping of gaps in ablation lesions set. RSPV, right superior pulmonary vein; RIPV, right inferior pulmonary vein; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein.
Distribution of lesions created by EIVOM at LPVs antrum
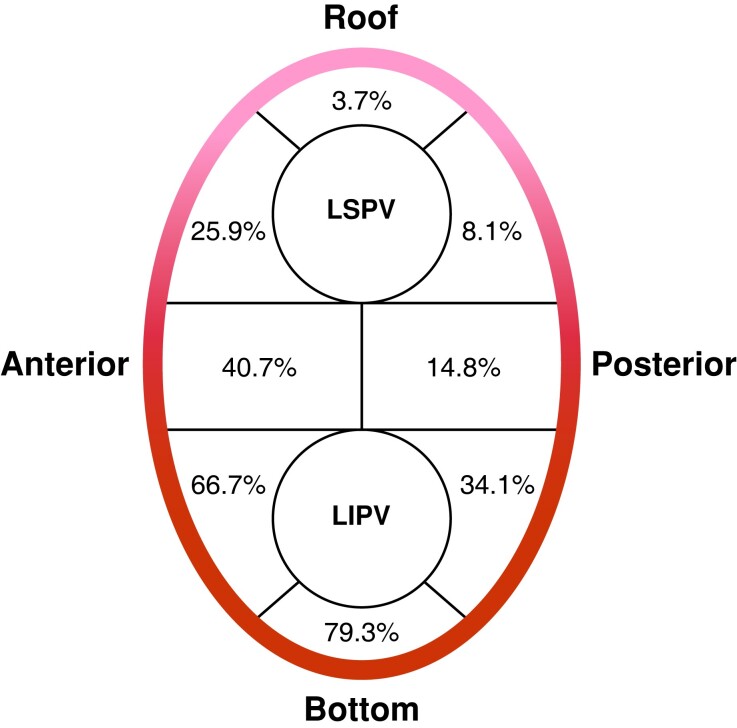
The distribution pattern of lesions created by EIVOM at LPV antrum is illustrated in Figure 4. Two most frequently impacted segments were the bottom [in 107 patients (79.3%)] and the anterior wall [in 90 patients (66.7%)] of LPVs. In 108 patients, at least one lesion created by EIVOM were observed at these two segments. Lesions created by EIVOM could also be seen at the anterior carina (55, 40.7%), posterior antrum of the LIPV (46, 34.1%), and anterior antrum of the LSPV (35, 25.9%). In rarer cases, lesions could extend to the following segments: posterior carina in 20 patients (14.8%), posterior antrum of the LSPV in 11 patients (8.1%), and roof in 5 patients (3.7%).
Figure 4.
Distribution of LPVs antrum ablated by EIVOM. The lesions ablated by ethanol were not limited to the anterior LPV, but extended to posterior LPV and even the entire LPV antrum. LPV, left pulmonary vein; EIVOM, ethanol infusion into the vein of Marshall; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein.
Repeat ablation in patients
Seventeen patients received repeated ablation for recurrence of AF/AT/atrial flutter (AFL), with 5 (29.4%) patients in Group 1 and 12 (70.6%) in Group 2. The mean procedure to recurrence time was 4.9 ± 4.3 months after the first ablation, while mean time between the index and the repeated procedure was 10.5 ± 7.1 months. Of these, 4 (23.5%) patients had recurrence as paroxysmal AF, 2 (11.8%) as PeAF, 2 (11.8%) as AT and 10 (58.8%) as AFL. One patient had two types of tachycardia with paroxysmal AF and AFL. The mechanisms of the two ATs were focal origin from superior vena cava and LIPV, respectively. Six of the 10 cases of AFLs were perimitral, with two cases being VOM-dependent (one patient in Group 1). Roofline-dependent and CTI-dependent AFL accounted for 1 case, respectively. In the remaining two patients, multiple AFLs with different mechanisms presented during repeated procedure.
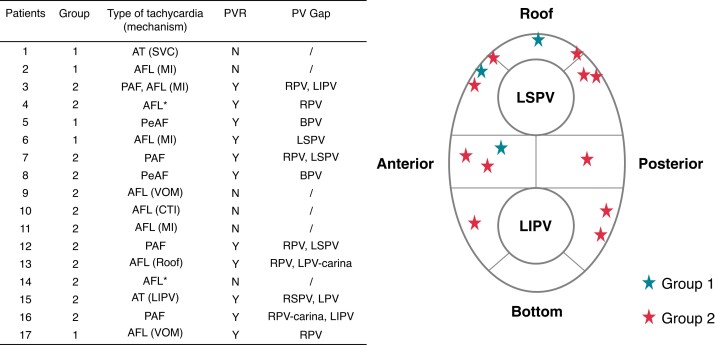
In patients underwent repeated ablation, 6 (35.3%) patients had all veins being isolated, whereas 11 (64.7%) presented with PVR. Two of the 5 patients in Group 1 presented with reconnections in LPV, while 8 of the 12 patients in Group 2 presented with reconnections in LPV (40% vs. 66.7%, P = 0.633). The detailed characteristics of repeated ablation and the location of the LPV gaps are shown in Figure 5.
Figure 5.
Procedural characteristics and the locations of the left PV gaps received repeat ablation. *Multiple mechanisms participated in the development of AFL in these two patients. AT, atrial tachycardia; AFL, atrial flutter; PAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; SVC, superior vena cava; MI, mitral isthmus; VOM, vein of Marshall; CTI, cavotricuspid isthmus; RPV, right pulmonary vein; LPV, left pulmonary vein; BPV, bilateral pulmonary vein; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; RSPV, right superior pulmonary vein; PVR, pulmonary vein reconnection.
Procedure-related complications
Dissection at the proximal CS occurred in two patients in an attempt to cannulate the VOM, which no subsequent complications or significant pericardial effusion. After observing for 30 min, EIVOM were performed as usual. No serious complications such as cardiac tamponade, thromboembolic complications, phrenic palsy, PV stenosis, or atrio-esophageal fistula occurred in our cohort.
Discussion
Our study systematically evaluated the effect of EIVOM on acute success of LPV isolation when performed as the first step of ablation for PeAF. The main findings of the current study are as follows: (1) Compared with conventional RFCA, EIVOM can significantly improve the first-pass isolation rate of LPV; (2) EIVOM can significantly reduce the acute PVR rate of LPV; and (3) PV carina is the most common location of acute PVR after RF with or without EIVOM.
Importance and challenge in PVI
Durable PVI is the cornerstone for AF ablation. First-pass PVI is associated with durable PVI and good AF ablation outcomes.11,12 Much effort has been made to enhance the acute as well as the long-term success rate of PVI. However, conduction recovery remains to be a challenging issue in some cases. A recent meta-analysis that reported outcomes of ablation index guided ablation, 42.0% of which was PeAF, demonstrated that the success rate of first-pass PVI ranged from 61.2% to 98%, while acute PVR observed in 6% to 32% of cases.13 Yamaguchi et al.14 aimed to assess the efficacy of PVI using VISITAG SURPOINT in PeAF patients and showed that the first-pass PVI rate was 91% for LPVs and the rate of acute PVR of the LPVs after 30 min waiting time was 31%. In our study, although the patients in the EIVOM group with larger LA dimension, left ventricular end-diastolic diameter, and lower left ventricular ejection fraction, first-pass isolation of LPVs was achieved in 119 (88.1%), which was significantly higher than that of the conventional ablation group (132, 74.2%). In addition, the acute PVR rate of LPVs was also significantly lower in the EIVOM group compared with the conventional ablation group (9.6% vs. 22.5%, P < 0.001). These outcomes were consistent with previous studies.
Contributions of EIVOM in achieving PVI
Early in 2009, Valderrábano et al.10 discussed the feasibility of EIVOM in PVI and showed that EIVOM significantly decreased RFA time for LIPV. Subsequently, their team demonstrated that EIVOM could be useful in patients with recurrent AF after PVI, with EIVOM facilitating the isolation of reconnected LPVs.15 Our study demonstrated that EIVOM is critical to enhancing the acute success of durable PVI, the reasons can be explained by: (1) The course of VOM: anatomically, the prominently thick pectinate muscle at the left lateral ridge poses great challenges to catheter stability and the formation of transmural injury. However, the VOM exactly lies at the corresponding epicardial aspect of this tough region. In previous reports, Żabówka et al.16 categorized the VOM into 4 types from observations of 200 autopsied adult human hearts: extended below the level of the LIPV (21.9%); to the level of the LIPV (47.7%); to the level of the interpulmonary area (17.2%); and to the level of the LSPV (13.3%). Valderrábano et al.17 also demonstrated that VOM was visible up to the level of LIPV in 72.8% patients, and went past the LIPV, reaching the LSPV in 9.6% of patients. Thus, ethanol infusion into VOM can facilitate the creation of durable tissue lesions along its course at PV antrum. Our study verified the injury distribution at PV-LA antrum by EIVOM through electroanatomic mapping. In the majority of patients, the bottom of the LPV did not require RFA (79.3%). The need for additional RFA was also obviated at the anterior wall of LIPV (66.7%), anterior carina (40.7%), and posterior wall of LIPV (34.1%). However, the injury lesions were not limited in the typical distribution regions of VOM. The regions intervened by ethanol was dependent on the anatomical distribution of the branches of the VOM.18 (2) The connections between VOM and left PV: There are abundant junctions between VOM and left PV. Some of the branching courses of the VOM insert into the PV directly from epicardial access. The connections between VOM and LPV as well as VOM and LA could provide an electrical shortcut between LPV and LA.19 Therefore, even if circumferential isolation between LPV and LA antrum has been achieved, rapid electrical activations within LPV could persistently conduct to LA through VOM, causing difficulty in achieving thorough disconnection between LPV and LA. In addition, eliminating these epicardial connections could be futile by ablation only at the endocardial aspect.20 In these cases, EIVOM can provide an easy solution as illustrated in Figure 6. Common LPV insertions of the epicardial connections locate at the carina, which could be demonstrated by the presence of contrast drainage into the PVs during VOM venogram, with 37.7% appeared at the LPV carina.17 In our study, PV carina is the most common location of acute PVR (55.8%). EIVOM is helpful to decrease the acute PVR rate of LPV carina compared with the RF group. However, a small portion (9.6%) of patients in the EIVOM group still presented with acute PVR in our study, which implied that transmural injury may not be achieved solely by EIVOM, and touch-up ablation at the endocardial aspect may be necessary even if apparent PVI is attained.
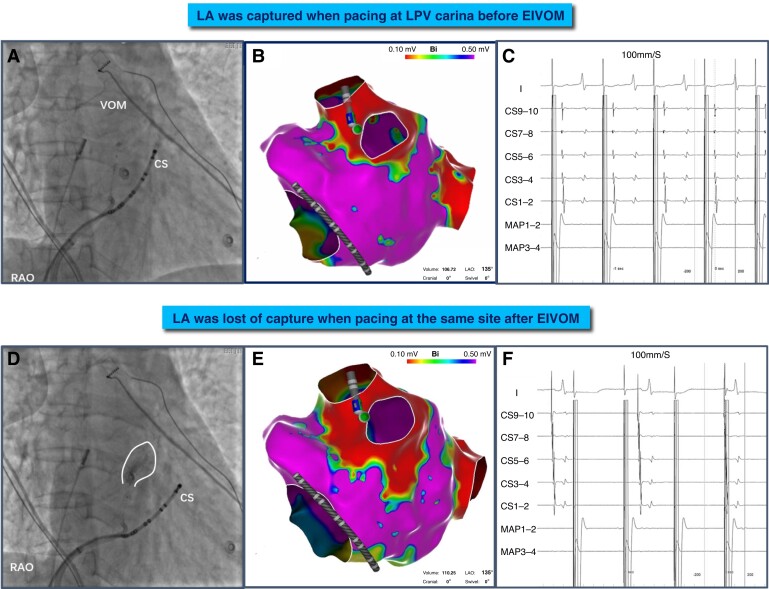
Figure 6.
A representative procedural example of VOM-mediated PV connection ablated by ethanol infusion. (A, D) VOM venogram before and after EIVOM. VOM ostia was adjacent to CS7–8 through venography. (B, C) Pacing at the conductive gap (site of the catheter), the atrium was captured and the earliest atrial activation was recorded at the CS7–8. (E, F) After EIVOM, the low voltage area extended to the ridge and posterior wall. Pacing at this site again, the atrium was lost of capture and the atrial activation sequence was changed. CS, coronary sinus; EIVOM, ethanol infusion into the vein of Marshall; RAO, right anterior oblique view.
The rationale of EIVOM as the first step during ablation for PeAF
Recent studies have recommended EIVOM as the first step for MI ablation before RF ablation.21,22 In the current study, we confirmed the significant improvement of the acute success in MI block by EIVOM. With the proven benefit to MI block and PVI by EIVOM, we recommended EIVOM to be the first step in the whole ablation procedure before LA access to reduce the manipulation time in LA, which could lower the risk of thrombosis complications. In addition, the total procedure time in EIVOM group was not significantly prolonged as the technique of EIVOM was straightforward. Each operator in our centre with about 50 cases’ experience could complete the procedure skillfully. Another important reason was due to significantly shortened RF ablation time, as RF ablation at LPV antrum and MI region could be tailored with electroanatomic mapping revealing the lesion created by EIVOM. Theoretically, RF ablation after EIVOM is safer than performing RF ablation firstly, as the ablation index could be adjusted according to the local injury situation. Further randomized controlled study is still needed to validate the safety of performing EIVOM as the initial step in the future.
Limitations
Our study has some limitations: (1) The study was an observational study instead of a randomized design. (2) Although EIVOM contributed to left PVI, there was still a lack of electrophysiological mapping approach for the identification and ablation of VOM-mediated PVR and VOM-mediated AF. (3) This was an acute study without long-term follow-up. The number of patients who accepted repeat ablation was too small, although we found the location of the PVR in the EIVOM group may be out of the distribution of VOM (roof, anterior wall of LSPV, and carina). It was unable to give a conclusion on the effect of EIVOM on late PVR and AF/AT recurrence. The long-term effect of EIVOM on PVI durability and MI block needs to be further investigated.
Conclusion
EIVOM is effective in achieving higher first-pass isolation and lower acute PVR of LPV in PeAF.
Contributor Information
Lihong Huang, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Mingyang Gao, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Yiwei Lai, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Qi Guo, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Songnan Li, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Changyi Li, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Nian Liu, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Wei Wang, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Xiaoxia Liu, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Song Zuo, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Xueyuan Guo, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Xin Zhao, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Chenxi Jiang, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Caihua Sang, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Ribo Tang, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Deyong Long, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Xin Du, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Jianzeng Dong, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Chang-sheng Ma, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University, No 2, Anzhen Rd, Chaoyang District, 100029 Beijing, China.
Funding
This work was funded by the National Key Research and Development Program of China, 2018YFC1312501; Beijing Municipal Commission of Science and Technology Grant/Award Numbers: D171100006817001; Zhongnanshan Medical Foundation of Guangdong Province: ZNSA-202001.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga Let al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nery PB, Belliveau D, Nair GM, Bernick J, Redpath CJ, Szczotka Aet al. Relationship between pulmonary vein reconnection and atrial fibrillation recurrence: a systematic review and meta-analysis. JACC Clin Electrophysiol 2016;2:474–83. [DOI] [PubMed] [Google Scholar]
- 3. Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FTet al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation 2005;111:127–35. [DOI] [PubMed] [Google Scholar]
- 4. Aryana A, Singh SM, Mugnai G, de Asmundis C, Kowalski M, Pujara DK et al. Pulmonary vein reconnection following catheter ablation of atrial fibrillation using the second-generation cryoballoon versus open-irrigated radiofrequency: results of a multicenter analysis. J Intervent Cardiac Electrophysiol 2016;47:341–8. [DOI] [PubMed] [Google Scholar]
- 5. Callans DJ, Gerstenfeld EP, Dixit S, Zado E, Vanderhoff M, Ren JF et al. Efficacy of repeat pulmonary vein isolation procedures in patients with recurrent atrial fibrillation. J Cardiovasc Electrophysiol 2004;15:1050–5. [DOI] [PubMed] [Google Scholar]
- 6. Mulder MJ, Kemme MJB, Allaart CP. Radiofrequency ablation to achieve durable pulmonary vein isolation. Europace 2022;24:874–86. [DOI] [PubMed] [Google Scholar]
- 7. Valderrábano M, Peterson LE, Swarup V, Schurmann PA, Makkar A, Doshi RN et al. Effect of catheter ablation with vein of Marshall ethanol infusion vs catheter ablation alone on persistent atrial fibrillation: the VENUS randomized clinical trial. JAMA 2020;324:1620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pambrun T, Denis A, Duchateau J, Sacher F, Hocini M, Jaïs P, et al. MARSHALL bundles elimination, pulmonary veins isolation and lines completion for anatomical ablation of persistent atrial fibrillation: MARSHALL-PLAN case series. J Cardiovasc Electrophysiol 2019;30:7–15. [DOI] [PubMed] [Google Scholar]
- 9. Lai Y, Liu X, Sang C, Long D, Li M, Ge W et al. Effectiveness of ethanol infusion into the vein of Marshall combined with a fixed anatomical ablation strategy (the “upgraded 2C3L” approach) for catheter ablation of persistent atrial fibrillation. J Cardiovasc Electrophysiol 2021;32:1849–56. [DOI] [PubMed] [Google Scholar]
- 10. Valderrábano M, Liu X, Sasaridis C, Sidhu J, Little S, Khoury DS. Ethanol infusion in the vein of Marshall: adjunctive effects during ablation of atrial fibrillation. Heart Rhythm 2009;6:1552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Osorio J, Hunter TD, Rajendra A, Zei P, Silverstein J, Morales G. Predictors of clinical success after paroxysmal atrial fibrillation catheter ablation. J Cardiovasc Electrophysiol 2021;32:1814–21. [DOI] [PubMed] [Google Scholar]
- 12. Pranata R, Vania R, Huang I. Ablation-index guided versus conventional contact-force guided ablation in pulmonary vein isolation – systematic review and meta-analysis. Indian Pacing Electrophysiol J 2019;19:155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ioannou A, Papageorgiou N, Lim WY, Wongwarawipat T, Hunter RJ, Dhillon G et al. Efficacy and safety of ablation index-guided catheter ablation for atrial fibrillation: an updated meta-analysis. Europace 2020;22:1659–71. [DOI] [PubMed] [Google Scholar]
- 14. Yamaguchi J, Takahashi Y, Yamamoto T, Amemiya M, Sekigawa M, Shirai Y et al. Clinical outcome of pulmonary vein isolation alone ablation strategy using VISITAG SURPOINT in nonparoxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2020;31:2592–9. [DOI] [PubMed] [Google Scholar]
- 15. Dave AS, Báez-Escudero JL, Sasaridis C, Hong TE, Rami T, Valderrábano M.. Role of the vein of Marshall in atrial fibrillation recurrences after catheter ablation: therapeutic effect of ethanol infusion. J Cardiovasc Electrophysiol 2012;23:583–91. [DOI] [PubMed] [Google Scholar]
- 16. Żabówka A, Jakiel M, Bolechała F, Jakiel R, Jasińska KA, Hołda MK. Topography of the oblique vein of the left atrium (vein of Marshall). Kardiol Pol 2020;78:688–93. [DOI] [PubMed] [Google Scholar]
- 17. Valderrábano M, Morales PF, Rodríguez-Mañero M, Lloves C, Schurmann PA, Dave AS. The human left atrial venous circulation as a vascular route for atrial pharmacological therapies: effects of ethanol infusion. JACC Clin Electrophysiol 2017;3:1020–32. [DOI] [PubMed] [Google Scholar]
- 18. Kamakura T, André C, Duchateau J, Nakashima T, Nakatani Y, Takagi T et al. Distribution of atrial low voltage induced by vein of Marshall ethanol infusion. J Cardiovasc Electrophysiol 2022;33:1687–93. [DOI] [PubMed] [Google Scholar]
- 19. Tan AY, Chou CC, Zhou S, Nihei M, Hwang C, Peter CT et al. Electrical connections between left superior pulmonary vein, left atrium, and ligament of Marshall: implications for mechanisms of atrial fibrillation. Am J Physiol Heart Circ Physiol 2006;290:H312–22. [DOI] [PubMed] [Google Scholar]
- 20. Barrio-Lopez MT, Sanchez-Quintana D, Garcia-Martinez J, Betancur A, Castellanos E, Arceluz M et al. Epicardial connections involving pulmonary veins: the prevalence, predictors, and implications for ablation outcome. Circ Arrhythm Electrophysiol 2020;13:e007544. [DOI] [PubMed] [Google Scholar]
- 21. Gillis K, O'Neill L, Wielandts JY, Hilfiker G, Almorad A, Lycke M et al. Vein of Marshall ethanol infusion as first step for mitral isthmus linear ablation. JACC Clin Electrophysiol 2022;8:367–76. [DOI] [PubMed] [Google Scholar]
- 22. Takigawa M, Vlachos K, Martin CA, Bourier F, Denis A, Kitamura T et al. Acute and mid-term outcome of ethanol infusion of vein of Marshall for the treatment of perimitral flutter. Europace 2020;22:1252–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.