Abstract
Aims
This retrospective study sought to compare complication rates and efficacy of power-controlled very high-power short-duration (vHPSD) and conventional catheter ablation in a large cohort of patients with atrial fibrillation (AF).
Methods and results
We analyzed 1115 consecutive patients with AF (38.7% paroxysmal, 61.3% persistent) who received first-time catheter ablation at our centre from 2015 to 2021. Circumferential pulmonary vein isolation ± additional substrate ablation using an irrigated-tip catheter was performed with vHPSD (70 W/5–7 s or 60 W/7–10 s) in 574 patients and with conventional power (30–35 W/15–30 s) in 541 patients. Baseline characteristics were well-balanced between groups (mean age 65.1 ± 11.2 years, 63.4% male). The 30-day incidence of cardiac tamponade [2/574 (0.35%) vs. 1/541 (0.18%), P = 0.598], pericardial effusion ≥ 10 mm [2/574 (0.35%) vs. 1/541 (0.18%), P = 0.598] and transient ischaemic attack [1/574 (0.17%) vs. 2/541 (0.37%), P = 0.529] was not significantly different between vHPSD and conventional ablation. No stroke, atrio-esophageal fistula, cardiac arrest or death occurred. Procedure (122.2 ± 46.8 min vs. 155.0 ± 50.5 min, P < 0.001), radiofrequency (22.4 ± 19.3 min vs. 52.9 ± 22.0 min, P < 0.001), and fluoroscopy (8.1 ± 7.2 vs. 9.2 ± 7.4, P = 0.016) duration were significantly shorter in the vHPSD group. At 12 months follow-up, freedom of any atrial arrhythmia was 44.1% vs. 34.2% (P = 0.010) in persistent AF and 78.1% vs. 70.2% in paroxysmal AF (P = 0.068).
Conclusion
vHPSD ablation is as safe as conventional ablation and is associated with an improved long-term efficacy in persistent AF.
Keywords: Atrial fibrillation, Catheter ablation, High-power short-duration, Ablation technologies, Procedural safety
Graphical Abstract
Graphical Abstract.
What’s new?
Catheter ablation using very high-power short-duration (vHPSD) (70 W/5–7 s and 60 W/7–10 s) shows a similar safety profile as conventional ablation (30–35 W/15–30 s) in a large cohort of 1115 patients.
vHPSD ablation is associated with a low 30-day incidence of cardiac tamponade (0.35%), transient ischaemic attack (0.17%), stroke (0%) and atrio-esophageal fistula (0%).
Long-term efficacy of vHPSD ablation is higher in persistent AF, whereas it is comparable with conventional ablation in paroxysmal AF.
Introduction
Pulmonary vein isolation (PVI) is an established treatment strategy for symptomatic atrial fibrillation (AF)(1). Radiofrequency catheter ablation (RFCA) is widely employed for PVI and aims at creating transmural, contiguous and irreversible lesions while avoiding collateral tissue damage. Despite recent advances in ablation technologies, PVI durability using conventional RFCA remains challenging and is limited by chronic pulmonary vein (PV) reconnection.1
Conventional power settings for PVI generally employ 20–40 W for a duration of 20–40 s, which is also known as low-power long-duration (LPLD) ablation. In recent years, the interest in using high-power short-duration (HPSD) ablation has grown after several ex vivo and in vivo studies suggested an improved efficacy and safety profile of this ablation strategy. HPSD ablation uses ≥45 W for a shorter duration (≤20 s) and has the particular biophysical characteristic of increasing the early resistive heating phase responsible for irreversible tissue necrosis, while reducing the late conductive heating which causes reversible damage in deep tissue.2,3 This ablation strategy generates a distinctively modified lesion geometry: HPSD lesions are half-oval-shaped and shallower,4 show a larger diameter4,5 and less endocardial sparing, which translates into an improved lesion to lesion contiguity.2 Furthermore, the shift towards resistive heating with consecutive reduction in conductive heating could potentially reduce collateral tissue damage.
Efficacy of HPSD has also been investigated in clinical studies, which reported significantly higher first-pass PVI rates,5,6 lower acute PV reconnections,5–8 improved long-term PVI durability,7 significantly shorter procedure and RF duration,5,8–11 and less arrhythmia recurrences with HPSD compared with LPLD.8,12 However, HPSD has a narrow efficacy to safety window,2 and there is very limited data on the safety profile of HPSD ablation above 50 W, since complication rates have been predominantly studied in relatively small patient groups for power settings ≤ 50 W. The incidence of rare complications in particular, such as stroke and atrio-esophageal fistula, requires further evaluation in large patient cohorts. This study investigated 30-day complication rates and long-term efficacy following AF catheter ablation in a large patient cohort treated with either very HPSD (vHPSD: 70 W/5–7 s and 60 W/7–10 s) or conventional ablation (LPLD: 30–35 W/15–30 s).
Methods
Study design
In this retrospective single-centre study, we analyzed 1115 consecutive patients with symptomatic paroxysmal or persistent AF who underwent first-time RFCA at the German Heart Centre in Munich. The vHPSD group comprising 574 patients ablated between April 2018 and March 2021 was compared with a historical LPLD cohort of 541 patients ablated between January 2015 and April 2018 using the same technologies and catheter set-ups.
Procedure-related complications occurring within 30 days of ablation and freedom of any atrial arrhythmia were assessed in both groups. Paroxysmal and persistent AF was defined according to current guidelines.1 All patients provided written informed consent for the procedure and the study was approved by the local ethics committee (approval #348/20 S-SR and #216/21 S-SR).
Ablation protocol
All patients received periprocedural uninterrupted oral anticoagulation. Left atrial thrombus was excluded ≤ 48 h before ablation using contrast-enhanced cardiac computed tomography (CT). Transesophageal echocardiography was performed in case of contraindications for contrast agent administration or CT unavailability. CT segmentation showing LA anatomy and oesophagus position relative to the LA posterior wall was reviewed during the procedure (Figure 1). Antiarrhythmic drugs were discontinued >5 half-lives prior to the procedure and were not re-started afterwards.
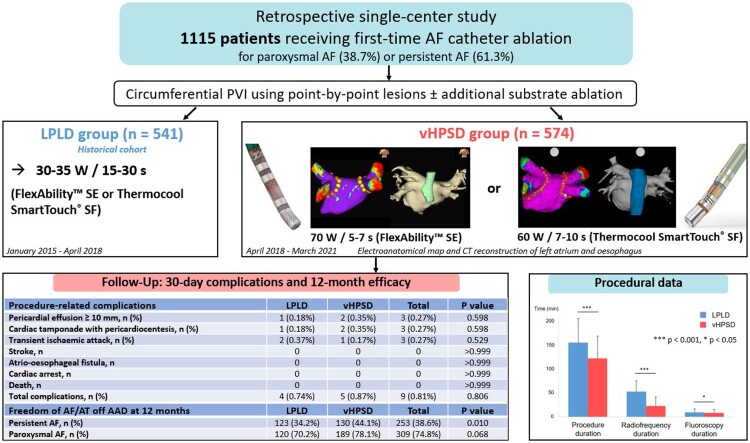
Figure 1.
Representative images showing electroanatomic maps (A–B) and vHPSD ablation settings (C–D) for the FlexAbility™ SE and the Thermocool SmartTouch® SF catheter. Voltage maps (0.05–0.5 mV) of the left atrium (LA) with ablation tags and corresponding CT reconstruction of the LA and oesophagus are shown in posterior-anterior view. Yellow ablation tags in A demonstrate the location of reduced RF application duration on the posterior wall.
The ablation procedure was performed under conscious sedation using midazolam, propofol and fentanyl. Femoral puncture was performed using an anatomical approach in the first 960 patients and under ultrasound guidance in all subsequent patients. After gaining venous femoral access, fluoroscopy-guided single transseptal puncture was performed using the Agilis™ steerable sheath (Abbott, Plymouth, MN) and double access to the LA was obtained. Unfractionated heparin was administered after transseptal puncture by continuous infusion to achieve an activated clotting time (ACT) ≥ 300 s throughout the entire procedure. High-density electroanatomical mapping was performed either using EnSite™ Velocity, EnSite™ Precision (Abbott) or CARTO® 3 system (Biosense Webster, Irvine, CA). Wide antral circumferential PVI was performed using point-by-point-lesions either with the 4 mm irrigated-tip catheter FlexAbility™ SE and the Ampere® RF generator (Abbott) or the 3.5 mm irrigated-tip catheter Thermocool Smarttouch® SF and the SmartAblate RF generator (Biosense Webster). The ablation catheter was not placed in the steerable sheath. In the conventional LPLD ablation group, 30–35 W were applied for 30 s on the anterior wall and for 15–20 s on the posterior wall using either the FlexAbility™ SE or the Thermocool Smarttouch® SF ablation catheter. In the HPSD group, 70 W/7 s (anterior wall) or 70 W/5 s (posterior wall) were applied with the Flexibility™ SE catheter, whereas 60 W/10 s (anterior wall) or 60 W/7 s (posterior wall) were applied with the Thermocool Smarttouch® SF catheter. The specific power and duration settings were based on in silico and ex vivo analyses previously described by Bourier et al.4 and were adapted to the specific catheter tip design. The FlexAbility™ catheter has a laser-cut enhanced irrigation tip with a very distal thermocouple for temperature feedback, while the Thermocool Smarttouch® SF catheter has 56 irrigation ports and a more proximally located thermocouple.13 Since the Thermocool Smarttouch® SF catheter was associated with a higher risk of steam pops compared with the FlexAbility™ catheter in a previous in vivo study,13 lower power settings (60 W) were used with the Thermocool Smarttouch® SF catheter in the current study.
Automatic temperature cut-off was set at 42° C (FlexAbility™ SE) and 40° C (Thermocool Smarttouch® SF). Both LPLD and vHPSD ablation were performed using a power-controlled mode and RF delivery duration was limited using an automated control. Irrigation was set to 20 mL/min (vHPSD group) and 17 mL/min (LPLD group), while the targeted impedance was between 110 and 140 Ω in both groups. If impedance was >140 Ω, repositioning of the neutral electrode or placement of a second neutral electrode was performed.
RF duration was limited in both vHPSD and LPLD groups on the posterior wall especially at sites were CT reconstruction of left atrial anatomy showed close proximity to the oesophagus (Figure 1). Real-time automated display of RF applications was employed with the following settings: For EnSite™ (Abbott), the AutoMark module was used (tag size 4 mm/minimum time 2 s for vHPSD, tag size 3 mm/minimum time 10 s for LPLD) and for CARTO®3 (Biosense Webster) the Visitag® function was enabled (tag size 3 mm, location stability 3 mm for 3 s and minimum contact force 25% of time >3 g). An inter-lesion distance of 5–6 mm (vHPSD) and ≤6 mm (LPLD) was targeted. Oesophageal temperature probes were not employed in this study. PVI was confirmed by entrance block by placing the mapping catheter in each PV antrum. Acute reconnection was assessed in each patient either by re-checking entrance block after ≥20 min waiting time or by adenosine administration. In case of documented PV reconnection, ablation was performed at the site of earliest activation.
In patients with persistent AF, additional substrate modification (ablation of low voltage areas and fractionation) was performed with the endpoint of AF cycle length prolongation or AF termination, as described previously.14 Left atrial lines (anterior line/roof line) and ablation of the cavotricuspid isthmus (CTI) were applied where appropriate. In the vHPSD group, anterior lines and CTI lines were ablated using moderate power settings (40–45 W/30 s).
Follow-up and complication assessment
Patient baseline and procedural characteristics were collected prospectively in a dedicated computerized database. Complications occurring in the first 30 days following ablation were assessed based on in-hospital monitoring and on routine follow-up visits at 1 or 3 months or on any unscheduled visits. Pericardial effusion was routinely assessed by transthoracic echocardiography at the end of each procedure, on the following morning and in case of haemodynamic deterioration. Pericardial effusion was defined as relevant if a new effusion ≥10 mm was detected on echocardiography after the procedure.
All patients presenting any in-hospital neurological deficit post-ablation were assessed by a neurologist. Depending on the clinical presentation and neurologist’s recommendation, brain imaging with either CT or magnetic resonance imaging (MRI) was performed. TIA was defined according to current guidelines as an acute loss of focal cerebral or ocular function without evidence of ischaemia on cerebral imaging and with complete resolution of symptoms within 24 h.15 Stroke was defined as new-onset focal neurological deficit with evidence of acute ischaemia or haemorrhage on brain imaging.16 TIA and stroke were confirmed by a neurologist. In case of symptoms suggestive of oesophageal injury, CT scan and/or endoscopy were performed, depending on clinical presentation. All patients received medication with a proton pump inhibitor (pantoprazole 40 mg twice daily) after PVI which was continued for 4 weeks. Vascular complications were detected using routine ultrasound the day after the procedure and were defined as any pseudoaneurysm, arteriovenous fistula or active bleeding at the site of vascular access.
Arrhythmia recurrence was assessed on 3-, 6-, and 12-month follow-up visits using 7-day Holter ECGs. A blanking period of 6 weeks was implemented. Follow-up data beyond the blanking period were available in 1068/1115 (95.8%) patients.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation and compared by t tests. Categorical variables are presented as frequencies or percentages and compared by χ2 tests. Time to first AF/AT recurrence was plotted using the Kaplan–Meier product limit method and compared by the log-rank test. Univariate und multivariate backward logistic regression analyses were performed to identify factors associated with atrial arrhythmia recurrence. Factors with a P-value <0.1 on univariate analysis were considered in the multivariate model. Statistical tests and confidence intervals with two-sided P < 0.05 were considered statistically significant. Statistical analysis was performed using the SPSS version 28.0 (IBM Inc., Armonk, NY).
Results
Patient population
A total of 1115 patients with paroxysmal AF (38.7%) and persistent AF (61.3%) were analyzed in this study. The vHPSD group comprised 574 patients and the LPLD group 541 patients. Patient baseline characteristics were well-balanced between groups and are presented in Table 1. Mean age (65.2 ± 11.7 vs. 65.0 ± 10.7, P = 0.723), male gender [355/574 (61.8%) vs. 352/541 (65.1%), P = 0.265], mean BMI (28.3 ± 8.4 vs. 28.6 ± 13.0, P = 0.670) and mean CHA2DS2VASC Score (2.5 ± 1.8 vs. 2.3 ± 1.6, P = 0.074) were similar between groups. Mean left ventricular ejection fraction was significantly lower in the vHPSD group (54.6 ± 9.4 vs. 56.1 ± 7.2, P = 0.003). The proportion of patients suffering from hypertension, diabetes, vascular disease, and previous TIA/stroke was comparable. Paroxysmal/persistent AF was present in 44.1%/55.9% of patients in the vHPSD group and in 33.1%/66.9% of patients in the LPLD group.
Table 1.
Baseline characteristics and procedural data
| vHPSD | LPLD | Total | P-value | |
|---|---|---|---|---|
| (n = 574) | (n = 541) | (n = 1115) | ||
| Baseline characteristics | ||||
| Age, years | 65.2 ± 11.7 | 65.0 ± 10.7 | 65.1 ± 11.2 | 0.723 |
| Male, n (%) | 355 (61.8%) | 352 (65.1%) | 707 (63.4%) | 0.265 |
| Body mass index, kg/m2 | 28.3 ± 8.4 | 28.6 ± 13.0 | 28.4 ± 10.9 | 0.670 |
| Hypertension, n (%) | 372 (64.8%) | 348 (64.3%) | 720 (64.6%) | 0.866 |
| Diabetes, n (%) | 61 (10.6%) | 53 (9.8%) | 114 (10.2%) | 0.647 |
| Vascular disease, n (%) | 145 (25.3%) | 148 (27.4%) | 293 (26.3%) | 0.427 |
| Previous stroke or transient ischaemic attack, n (%) | 49 (8.5%) | 42 (7.8%) | 91 (8.2%) | 0.625 |
| Left ventricular ejection fraction, % | 54.6 ± 9.4 | 56.1 ± 7.2 | 55.8 ± 8.1 | 0.003 |
| CHA2DS2VASC Score | 2.5 ± 1.8 | 2.3 ± 1.6 | 2.4 ± 1.7 | 0.074 |
| Procedural data | ||||
| Pulmonary vein isolation, n (%) | 574 (100%) | 541 (100%) | 1115 (100%) | >0.999 |
| Additional substrate ablation, n (%) | 286 (49.8%) | 265 (49.0%) | 551 (49.4%) | 0.779 |
| Contact force catheter, n (%) | 74 (12.9%) | 70 (12.9%) | 144 (12.9%) | 0.981 |
| Mean temperature, °C | 31.2 ± 4.0 | 31.3 ± 2.2 | 31.3 ± 3.2 | 0.425 |
| Mean power, W | 56.0 ± 7.9 | 31.5 ± 3.9 | 44.1 ± 13.8 | <0.001 |
| Procedure duration, min | 122.2 ± 46.8 | 155.0 ± 50.5 | 138.1 ± 51.3 | <0.001 |
| Radiofrequency duration, min | 22.4 ± 19.3 | 52.9 ± 22.0 | 37.3 ± 25.7 | <0.001 |
| Fluoroscopy duration, min | 8.1 ± 7.2 | 9.2 ± 7.4 | 8.6 ± 7.3 | 0.016 |
LPLD = low-power long-duration ablation; vHPSD = very high-power short-duration ablation
Procedural characteristics
Procedural data are summarized in Table 1. Mean power was 56.0 ± 7.9 W in the vHPSD group and 31.5 ± 3.9 W in the LPLD group (P < 0.001), while mean temperature was similar (31.2 ± 4.0 vs. 31.3 ± 2.2, P = 0.425). There was no difference in contact force catheter use: The Thermocool Smarttouch® SF catheter was employed in 12.9% of patients in both groups, while the FlexAbility™ SE catheter was used in 87.1% of patients (P = 0.981).
Successful PVI was achieved in 100% of patients in both the vHPSD and the LPLD group. The fraction of patients receiving additional substrate ablation (including substrate modification and atrial lines) was similar between groups [286/574 (49.8%) vs. 265/541 (49.0%), P = 0.946].
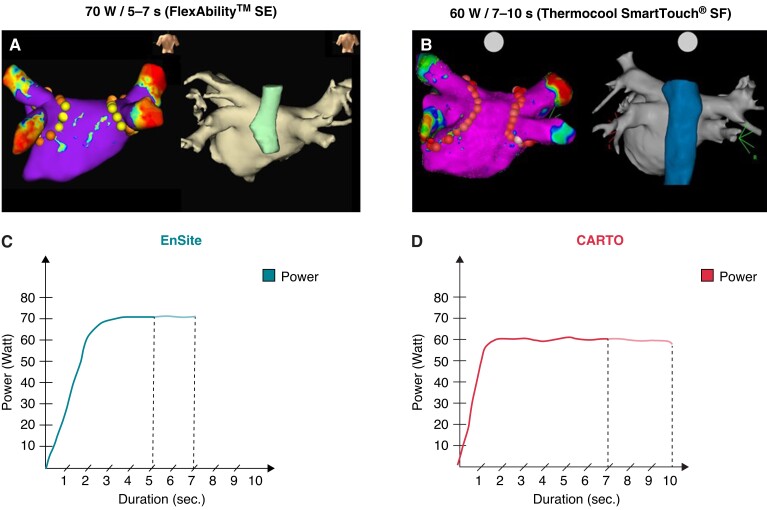
In the vHPSD group, skin-to-skin procedure duration (122.2 ± 46.8 min vs. 155.0 ± 50.5 min, P < 0.001), radiofrequency duration (22.4 ± 19.3 min vs. 52.9 ± 22.0 min, P < 0.001), and fluoroscopy time (8.1 ± 7.2 vs. 9.2 ± 7.4 min, P = 0.016) were significantly lower compared with the LPLD group (Figure 2).
Figure 2.
Procedural characteristics of LPLD and vHPSD ablation. * P < 0.05, *** P < 0.001.
Complications
Complications occurring in the first 30 days of ablation are summarized in Table 2. The rate of pericardial effusion ≥ 10 mm was similar in the vHPSD and the LPLD group [2/574 (0.35%) vs. 1/541 (0.18%), P = 0.598]. All cases of pericardial effusion presented spontaneous remission in control echocardiography 48 h after the procedure and had completely resolved on follow-up visit.
Table 2.
Procedure-related and vascular access-related complications
| Complications | vHPSD | LPLD | Total | P-value |
|---|---|---|---|---|
| (n = 574) | (n = 541) | (n = 1115) | ||
| Procedure-related | ||||
| Pericardial effusion ≥ 10 mm, n (%) | 2 (0.35%) | 1 (0.18%) | 3 (0.27%) | 0.598 |
| Cardiac tamponade with pericardiocentesis, n (%) | 2 (0.35%) | 1 (0.18%) | 3 (0.27%) | 0.598 |
| Transient ischaemic attack, n (%) | 1 (0.17%) | 2 (0.37%) | 3 (0.27%) | 0.529 |
| Stroke, n | 0 | 0 | 0 | >0.999 |
| Atrio-oesophageal fistula, n | 0 | 0 | 0 | >0.999 |
| Cardiac arrest, n | 0 | 0 | 0 | >0.999 |
| Death, n | 0 | 0 | 0 | >0.999 |
| Total complications, n (%) | 5 (0.87%) | 4 (0.74%) | 9 (0.81%) | 0.806 |
| Vascular access-related | ||||
| Vascular complications, n (%) | 33 (5.7%) | 54 (10.0%) | 87 (7.8%) | 0.008 |
LPLD = low-power long-duration ablation; vHPSD = very high-power short-duration ablation
Cardiac tamponade occurred in 2/574 (0.35%) patients ablated with vHPSD and in 1/541 (0.18%) patients ablated with LPLD (P = 0.598). Details of cardiac tamponade presentation are summarized in Table 3. Cardiac tamponade occurring in the vHPSD group was related to the transseptal puncture in one case and to a steam pop occurring during CTI ablation using moderate power (40 W) in the second case. In the LPLD group, one tamponade occurred as a result of a post-cardiac injury syndrome. All cases were successfully treated by pericardiocentesis and showed full recovery after 4 weeks.
Table 3.
Characteristics of severe complications
| Cardiac tamponade with pericardiocentesis (n = 3) | |||||
|---|---|---|---|---|---|
| Timing (post-ablation) | Aspirated volume | Aspirate characteristics | Presumed mechanism | Consequence | |
| Patient 1 (vHPSD) | intraprocedural | 1000 mL | Arterial blood | Transseptal puncture | Dressler syndrome, prolonged hospitalization |
| Patient 2 (vHPSD) | 2 h | 500 mL | Venous blood | Steam pop during ablation of CTI with 40 W | Prolonged hospitalization |
| Patient 3 (LPLD) | 5 h | 300 mL | Serous fluid | Post-cardiac injury syndrome | Prolonged hospitalization, repeat pericardiocentesis after two weeks |
| Transient ischaemic attack (n = 3) | |||||
| Timing (post-ablation) | Symptom duration | Symptoms | Presumed mechanism | Imaging | |
| Patient 4 (vHPSD) | 24 h | 15 min | Paresthesia (left face side and left arm), dysphasia | Unknown | CT |
| Patient 5 (LPLD) | 4 days | 20 min | Hemianopsia (left-sided) and hypoesthesia (left arm) | mean ACT ≤280 s | CT |
| Patient 6 (LPLD) | 48 h | 6 h | hypoesthesia (left fingers, left corner of the mouth) | mean ACT ≤280 s | CT, MRI |
ACT = activated clotting time; CT = computed tomography; CTI = cavotricuspid isthmus; LPLD = low-power long-duration ablation; MRI = magnetic resonance imaging; vHPSD = very high-power short-duration ablation.
The rate of TIA [1/574 (0.17%) vs. 2/541 (0.37%), P = 0.529] was similar in the vHPSD and LPLD group. Symptom characteristics are summarized in Table 3. Inguinal vascular complications occurred in 33/574 (5.7%) vs. 54/541 (10.0%) patients in the vHPSD and LPLD group, respectively (P = 0.008). Endoscopy was performed in 3/1115 patients (1/574 vs. 2/541 in the vHPSD vs. LPLD group) in the first 30 days following ablation due to symptoms suggestive of oesophageal injury. One thermal oesophageal injury measuring 5 mm was detected in one patient in the LPLD group only and showed complete remission on follow-up endoscopy. No atrio-esophageal fistula, stroke, cardiac arrest or death occurred in any group.
Ablation outcomes
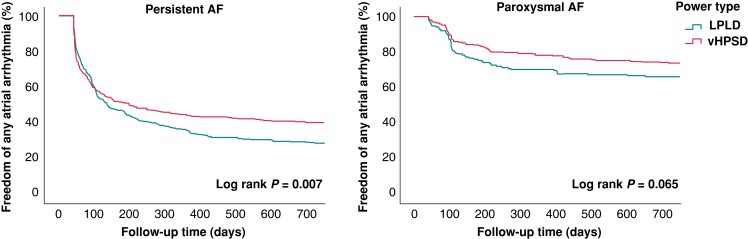
Freedom of any atrial arrhythmia off AAD after a single procedure was assessed at 12-month follow-up based on 7-day Holter monitoring at 3-, 6-, and 12-month visits. The median follow-up was 366 days (IQR: 168; 554). Kaplan–Meier analyses are shown in Figure 3. In patients with persistent AF, freedom of any arrhythmia was achieved in 130/295 (44.1%) patients in the vHPSD group vs. 123/360 (34.2%) patients in the LPLD group (P = 0.010). vHPSD ablation was an independent negative predictor of arrhythmia recurrence (OR: 0.608, CI: 0.434–0.851, P = 0.004) on multivariate analysis (Table 4). In patients with paroxysmal AF, freedom of any arrhythmia was observed in 189/242 (78.1%) vs. 120/171 (70.2%) patients in the vHPSD and LPLD group, respectively (P = 0.068). Female gender (OR: 2.060, 95% CI: 1.243–3.413, P = 0.005) and vascular disease (OR: 1.945, 95% CI: 1.047–3.610, P = 0.035) independently predicted arrhythmia recurrence (Table 4).
Figure 3.
Freedom from any atrial arrhythmia off AAD after a single ablation procedure in patients with persistent AF (n = 655) and paroxysmal AF (n = 413) after a blanking period of 6 weeks.
Table 4.
Recurrence of any atrial arrhythmia at 12 months of AAD after a single procedure
| Persistent AF—Univariate analysis | |||
|---|---|---|---|
| Variable | Recurrence | No recurrence | P-value |
| n = 402 | n = 253 | ||
| Age, years | 67.9 ± 9.8 | 65.5 ± 11.1 | 0.005 |
| Female, n (%) | 146 (36.3%) | 72 (28.5%) | 0.038 |
| Body mass index, kg/m2 | 28.7 ± 5.4 | 28.9 ± 10.9 | 0.777 |
| Hypertension, n (%) | 288 (71.6%) | 168 (66.4%) | 0.156 |
| Diabetes, n (%) | 47 (11.7%) | 31 (12.3%) | 0.829 |
| Vascular disease, n (%) | 121 (30.1%) | 71 (28.1%) | 0.577 |
| Previous stroke or transient ischaemic attack, n (%) | 38 (9.5%) | 17 (6.7%) | 0.219 |
| Left ventricular ejection fraction, % | 52.2 ± 10.6 | 55.6 ± 7.5 | <0.001 |
| CHA2DS2VASC Score | 2.8 ± 1.6 | 2.5 ± 1.7 | 0.05 |
| Additional substrate ablation, n (%) | 325 (80.8%) | 200 (79.1%) | 0.575 |
| vHPSD ablation | 165 (41.0%) | 130 (51.4%) | 0.010 |
| Persistent AF—Multivariate analysis for atrial arrhythmia recurrence | |||
| Variable | Odds ratio | 95% confidence interval | P-value |
| Age | 1.018 | 0.996–1.040 | 0.119 |
| Female | 1.442 | 0.980–2.123 | 0.064 |
| Left ventricular ejection fraction | 1.001 | 0.982–1.020 | 0.936 |
| CHA2DS2VASC Score | 1.019 | 0.880–1.179 | 0.806 |
| vHPSD ablation | 0.608 | 0.434–0.851 | 0.004 |
| Paroxysmal AF—Univariate analysis | |||
| Variable | Recurrence | No recurrence | P-value |
| n = 104 | n = 309 | ||
| Age, years | 64.7 ± 9.2 | 60.9 ± 12.2 | 0.002 |
| Female, n (%) | 56 (53.8%) | 118 (38.2%) | 0.005 |
| Body mass index, kg/m2 | 27.8 ± 4.9 | 28.2 ± 17.4 | 0.795 |
| Hypertension, n (%) | 66 (63.5%) | 166 (53.7%) | 0.083 |
| Diabetes, n (%) | 7 (6.7%) | 24 (7.8%) | 0.729 |
| Vascular disease, n (%) | 29 (27.9%) | 54 (17.5%) | 0.022 |
| Previous stroke or transient ischaemic attack, n (%) | 9 (8.7%) | 23 (7.4%) | 0.677 |
| Left ventricular ejection fraction, % | 58.3 ± 5.9 | 57.8 ± 6.8 | 0.472 |
| CHA2DS2VASC Score | 2.3 ± 1.5 | 1.8 ± 1.7 | 0.015 |
| vHPSD ablation | 53 (51.0%) | 189 (61.2%) | 0.068 |
| Paroxysmal AF—Multivariate analysis for atrial arrhythmia recurrence | |||
| Variable | Odds ratio | 95% confidence interval | P-value |
| Age | 1.024 | 0.995–1.054 | 0.109 |
| Female | 2.060 | 1.243–3.413 | 0.005 |
| Hypertension | 1.337 | 0.744–2.404 | 0.331 |
| Vascular disease | 1.945 | 1.047–3.610 | 0.035 |
| CHA2DS2VASC Score | 0.885 | 0.699–1.122 | 0.314 |
| vHPSD ablation | 0.640 | 0.403–1.017 | 0.059 |
AAD = antiarrhythmic drugs; AF = atrial fibrillation; vHPSD = very high-power short-duration.
Discussion
The current study compared 30-day complication rates and ablation outcomes following first-time AF catheter ablation in a large cohort of patients treated with either vHPSD or LPLD. The main finding of this study is that vHPSD ablation (70 W/5–7 s and 60 W/7–10 s) shows a similarly low complication rate as conventional LPLD (30–35 W for 15–30 s). A significant reduction in procedure duration, RF energy delivery, and fluoroscopy duration was observed in the vHPSD group. Long-term efficacy of vHPSD vs. conventional ablation at 12 months was significantly higher in persistent AF and comparable in paroxysmal AF.
While an improved procedural efficacy of HPSD ablation has been suggested in previous animal and clinical studies,5–12 definite assessment of HPSD safety profile remains to be established. HPSD has not been widely adopted in the clinical setting due to concerns regarding its narrow efficacy to safety window: slight variations in power and/or duration may compromise safety by resulting in steam pops, charring and collateral tissue damage, while inadequate combination of power and duration may reduce efficacy by generating superficial and non-transmural lesions.2,3 Several ex vivo and in vivo experiments showed that RFCA using HPSD is associated with a more favourable lesion geometry than LPLD while presenting a similar safety profile.4,5 HPSD generates a considerable shift towards resistive heating during lesion creation, while reducing the conductive heating phase. This may decrease thermal effects on deep tissue and thus reduce the risk of collateral damage to adjacent structures2 such as the pericardium, phrenic nerve and oesophagus. However, it remains unknown whether this translates into an improved safety profile in the clinical setting.
The present study is to the best of our knowledge the largest study comparing the safety profile and long-term efficacy of vHPSD and LPLD ablation. As shown by a recent meta-analysis, complication rates following HPSD and LPLD catheter ablation have been investigated in a limited number of small studies.17 Considering the low incidence of PVI-related complications such as cardiac tamponade, stroke and atrio-esophageal fistula, assessment of outcomes in large patient cohorts is warranted for accurate characterization of HPSD safety. Definite evaluation of HPSD safety profile is also hampered by heterogeneous power/duration settings and RF catheters employed in previous reports. The vast majority of studies investigating outcomes of HPSD ablation used power settings of 40–50 W for a duration of 2–15 s, while conventional power was applied on the posterior wall in some studies.3,17 Therefore, data on the safety profile and efficacy of HPSD above 50 W are very limited.
HPSD ablation is generally defined as the application of ≥45 W for a duration <20 s. Considering its narrow safety and efficacy window, careful selection of power and duration settings is warranted. The settings used in this study are based on in silico and ex vivo analyses which demonstrated that ablation with 60 W/10 s and 70 W/7 s generates similar lesion volumes and ablation index values as ablation with 30 W/30 s when applying the same contact force.4 However, the ideal combination of power and duration settings that generates transmural lesions at the lowest risk of collateral damage remains to be established.
This study demonstrates that AF catheter ablation using power-controlled vHPSD is associated with low procedure-related complication rates that did not significantly differ from conventional LPLD ablation. The rate of cardiac tamponade (0.35% vs. 0.18%) and TIA (0.17% vs. 0.37%) was comparable. The significantly lower vascular access complication rate in our vHPSD patient cohort is likely related to the use of ultrasound-guided femoral puncture in 155/574 (27%) patients included in this group, as previously reported.18 No further procedure-related complications occurred after 30 days, although a potential bias due to underreporting cannot be excluded beyond the first month of follow-up.
The findings of this study are in agreement with previous reports, which showed a favourable safety profile of AF catheter ablation using HPSD. In a multi-centre study of 13 974 procedures, HPSD ablation with 45–50 W/5–15 s (anterior wall) and 45–50 W/2–10 s or 35 W/20 s (posterior wall) was associated with very low complication rates: 0.24% cardiac tamponade, 0.086% stroke, 0.014% PV stenosis requiring intervention, 0.028% atrio-esophageal fistula and 0.014% death.19 Smaller comparator studies also reported similarly low rates of cardiac tamponade and oesophageal injury for HPSD.8,9,20 In a recent review, overall complication rates for HPSD and LPLD were 2.1% and 4.5%,3 which suggests a favourable safety profile for HPSD. However, only a limited number of studies have so far presented comprehensive complication rates, and incidences of TIA/stroke were only infrequently reported.17 The current finding of very low TIA rates and the lack of any stroke in both ablation groups is reassuring.
In our study, no atrio-esophageal fistula occurred and thermal oesophageal injury was confirmed in only one patient in the LPLD group. When interpreting the current results, our centre-specific approach aimed at minimizing oesophageal injury should be considered: (i) routine review of LA anatomy and oesophagus location on CT reconstruction before PVI, (ii) reduction of RF delivery on the posterior wall (7–5 s for 70 W and 10–7 s for 60 W) and (iii) medication with a proton pump inhibitor for 4 weeks following PVI. While the present data does not allow a systematic assessment of silent oesophageal injuries, prior studies found a comparable incidence in HPSD and conventional ablation. Baher et al.9 investigated thermal oesophageal injuries using same-day MRI and reported no significant difference in patients treated with HPSD (50 W/5 s) and LPLD (≤35 W/10–30 s). Another study using routine endoscopy reported fewer oesophageal lesions with HPSD (50–60 W) compared with LPLD (30 W).20
Long-term efficacy of vHPSD at 12 months was significantly higher in persistent AF, whereas it was comparable to the conventional approach in paroxysmal AF. Previous studies reported similar rates of AF freedom for HPSD and conventional ablation while a recent review3 and meta-analysis17 suggest a slight trend favouring HPSD. Lower acute and chronic PV reconnection rates have been previously reported for HPSD ablation.5–8 However, further prospective data are needed in order to establish whether vHPSD is associated with an improved outcome.
In the present study, HPSD ablation was associated with a significant decrease in procedure (−21%), RF (−58%), and fluoroscopy (−12%) duration. Reduction of procedural duration and RF energy delivery is the most consistent finding for HPSD ablation.5–9,20 A recent review found a mean reduction of 26.5% in procedure duration and 48.5% in RF duration for HPSD.3 Shorter procedure duration is a potential advantage of HPSD ablation as it may reduce left atrial dwell time, sedation and intravenous fluid volumes, while reduction of RF duration is associated with improved catheter stability during energy delivery.7
Limitations
This is a retrospective, non-randomized single-centre study with the inherent limitations of this study design. An important limitation is the comparison of the vHPSD group with a historical cohort. Since the LPLD group was ablated up to 3 years before the vHPSD cohort, workflow optimization might have influenced procedural characteristics and results. However, ablation procedures were performed by the same team of experienced operators using the same ablation strategy, catheters and technologies, while baseline characteristics were well-balanced between groups. Since the majority of ablations (87%) were conducted with the non-contact force FlexAbility™ SE catheter, current results and power settings may not be extended to the use of contact force catheters or to other technologies with different catheter tip designs. The study was not powered to detect differences in rare complications such as cardiac tamponade, stroke or oesophageal fistula. As systematic imaging to assess silent cerebral and oesophageal lesions was not performed, further studies are warranted until vHPSD ablation can be routinely recommended for clinical practice. Finally, safety profile of HPSD ablation is also determined by additional parameters, such as baseline impedance values, contact force, catheter stability and tissue properties,7 which were not systematically assessed in this study.
Conclusion
AF catheter ablation using vHPSD (70 W/5–7 s and 60 W/7–10 s) has a similar safety profile as conventional LPLD ablation and is associated with an improved long-term efficacy in persistent AF. Future randomized trials are warranted for definite assessment of safety and efficacy of vHPSD ablation.
Acknowledgements
We would like to thank our study nurse Sabine Brandhorst for excellent study management.
Contributor Information
Miruna A Popa, German Heart Center Munich, Department of Electrophysiology, Lazarettstraße 36, 80636 Munich, Germany; Munich Arrhythmia Research and Study Center (MARS), Lazarettstraße 36, 80636 Munich, Germany.
Felix Bourier, German Heart Center Munich, Department of Electrophysiology, Lazarettstraße 36, 80636 Munich, Germany; Munich Arrhythmia Research and Study Center (MARS), Lazarettstraße 36, 80636 Munich, Germany.
Sarah Lengauer, German Heart Center Munich, Department of Electrophysiology, Lazarettstraße 36, 80636 Munich, Germany; Munich Arrhythmia Research and Study Center (MARS), Lazarettstraße 36, 80636 Munich, Germany.
Hannah Krafft, German Heart Center Munich, Department of Electrophysiology, Lazarettstraße 36, 80636 Munich, Germany; Munich Arrhythmia Research and Study Center (MARS), Lazarettstraße 36, 80636 Munich, Germany.
Fabian Bahlke, German Heart Center Munich, Department of Electrophysiology, Lazarettstraße 36, 80636 Munich, Germany; Munich Arrhythmia Research and Study Center (MARS), Lazarettstraße 36, 80636 Munich, Germany.
Leonie V Förschner, German Heart Center Munich, Department of Electrophysiology, Lazarettstraße 36, 80636 Munich, Germany; Munich Arrhythmia Research and Study Center (MARS), Lazarettstraße 36, 80636 Munich, Germany.
Stephan Dorfmeister, German Heart Center Munich, Department of Electrophysiology, Lazarettstraße 36, 80636 Munich, Germany; Munich Arrhythmia Research and Study Center (MARS), Lazarettstraße 36, 80636 Munich, Germany.
Susanne Kathan, German Heart Center Munich, Department of Electrophysiology, Lazarettstraße 36, 80636 Munich, Germany; Munich Arrhythmia Research and Study Center (MARS), Lazarettstraße 36, 80636 Munich, Germany.
Marta Telishevska, German Heart Center Munich, Department of Electrophysiology, Lazarettstraße 36, 80636 Munich, Germany; Munich Arrhythmia Research and Study Center (MARS), Lazarettstraße 36, 80636 Munich, Germany.
Florian Englert, German Heart Center Munich, Department of Electrophysiology, Lazarettstraße 36, 80636 Munich, Germany; Munich Arrhythmia Research and Study Center (MARS), Lazarettstraße 36, 80636 Munich, Germany.
Carsten Lennerz, German Heart Center Munich, Department of Electrophysiology, Lazarettstraße 36, 80636 Munich, Germany; Munich Arrhythmia Research and Study Center (MARS), Lazarettstraße 36, 80636 Munich, Germany.
Tilko Reents, German Heart Center Munich, Department of Electrophysiology, Lazarettstraße 36, 80636 Munich, Germany; Munich Arrhythmia Research and Study Center (MARS), Lazarettstraße 36, 80636 Munich, Germany.
Gabriele Hessling, German Heart Center Munich, Department of Electrophysiology, Lazarettstraße 36, 80636 Munich, Germany; Munich Arrhythmia Research and Study Center (MARS), Lazarettstraße 36, 80636 Munich, Germany.
Isabel Deisenhofer, German Heart Center Munich, Department of Electrophysiology, Lazarettstraße 36, 80636 Munich, Germany; Munich Arrhythmia Research and Study Center (MARS), Lazarettstraße 36, 80636 Munich, Germany.
Marc Kottmaier, German Heart Center Munich, Department of Electrophysiology, Lazarettstraße 36, 80636 Munich, Germany; Munich Arrhythmia Research and Study Center (MARS), Lazarettstraße 36, 80636 Munich, Germany.
Funding
None.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Leshem E, Zilberman I, Tschabrunn CM, Barkagan M, Contreras-Valdes FM, Govari Aet al. . High-Power and short-duration ablation for pulmonary vein isolation: biophysical characterization. JACC Clin Electrophysiol 2018;4:467–79. [DOI] [PubMed] [Google Scholar]
- 3. Winkle RA. HPSD Ablation for AF high-power short-duration RF ablation for atrial fibrillation: a review. J Cardiovasc Electrophysiol 2021;32:2813–23. [DOI] [PubMed] [Google Scholar]
- 4. Bourier F, Duchateau J, Vlachos K, Lam A, Martin CA, Takigawa Met al. . High-power short-duration versus standard radiofrequency ablation: insights on lesion metrics. J Cardiovasc Electrophysiol 2018;29:1570–5. [DOI] [PubMed] [Google Scholar]
- 5. Pambrun T, Durand C, Constantin M, Masse A, Marra C, Meillet Vet al. . High-Power (40–50 W) radiofrequency ablation guided by unipolar signal modification for pulmonary vein isolation: experimental findings and clinical results. Circ Arrhythm Electrophysiol 2019;12:e007304. [DOI] [PubMed] [Google Scholar]
- 6. Dhillon G, Ahsan S, Honarbakhsh S, Lim W, Baca M, Graham Aet al. . A multicentered evaluation of ablation at higher power guided by ablation index: establishing ablation targets for pulmonary vein isolation. J Cardiovasc Electrophysiol 2019;30:357–65. [DOI] [PubMed] [Google Scholar]
- 7. Yavin HD, Leshem E, Shapira-Daniels A, Sroubek J, Barkagan M, Haffajee CIet al. . Impact of high-power short-duration radiofrequency ablation on long-term lesion durability for atrial fibrillation ablation. JACC Clin Electrophysiol 2020;6:973–85. [DOI] [PubMed] [Google Scholar]
- 8. Kottmaier M, Popa M, Bourier F, Reents T, Cifuentes J, Semmler Vet al. . Safety and outcome of very high-power short-duration ablation using 70 W for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Europace 2020;22:388–93. [DOI] [PubMed] [Google Scholar]
- 9. Baher A, Kheirkhahan M, Rechenmacher SJ, Marashly Q, Kholmovski EG, Siebermair Jet al. . High-Power radiofrequency catheter ablation of atrial fibrillation: using late gadolinium enhancement magnetic resonance imaging as a novel Index of esophageal injury. JACC Clin Electrophysiol 2018;4:1583–94. [DOI] [PubMed] [Google Scholar]
- 10. Yazaki K, Ejima K, Kanai M, Kataoka S, Higuchi S, Yagishita Det al. . Impedance drop predicts acute electrical reconnection of the pulmonary vein-left atrium after pulmonary vein isolation using short-duration high-power exposure. J Interv Card Electrophysiol 2020;59:575–84. [DOI] [PubMed] [Google Scholar]
- 11. Hansom SP, Alqarawi W, Birnie DH, Golian M, Nery PB, Redpath CJet al. . High-power, short-duration atrial fibrillation ablation compared with a conventional approach: outcomes and reconnection patterns. J Cardiovasc Electrophysiol 2021;32:1219–28. [DOI] [PubMed] [Google Scholar]
- 12. Winkle RA, Mead RH, Engel G, Patrawala RA. Atrial fibrillation ablation: “perpetual motion” of open irrigated tip catheters at 50 W is safe and improves outcomes. Pacing Clin Electrophysiol 2011;34:531–9. [DOI] [PubMed] [Google Scholar]
- 13. Winterfield JR, Jensen J, Gilbert T, Marchlinski F, Natale A, Packer Det al. . Lesion size and safety comparison between the novel flex tip on the FlexAbility ablation catheter and the solid tips on the ThermoCool and ThermoCool SF ablation catheters. J Cardiovasc Electrophysiol 2016;27:102–9. [DOI] [PubMed] [Google Scholar]
- 14. Ammar S, Hessling G, Reents T, Paulik M, Fichtner S, Schön Pet al. . Importance of sinus rhythm as endpoint of persistent atrial fibrillation ablation. J Cardiovasc Electrophysiol 2013;24:388–95. [DOI] [PubMed] [Google Scholar]
- 15. Fonseca AC, Merwick Á, Dennis M, Ferrari J, Ferro JM, Kelly Pet al. . European Stroke organisation (ESO) guidelines on management of transient ischaemic attack. Eur Stroke J 2021;6:CLXIII-CLXXXVI. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker Ket al. . Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 2019;50:e344–418. [DOI] [PubMed] [Google Scholar]
- 17. Kewcharoen J, Techorueangwiwat C, Kanitsoraphan C, Leesutipornchai T, Akoum N, Bunch TJet al. . High-power short duration and low-power long duration in atrial fibrillation ablation: a meta-analysis. J Cardiovasc Electrophysiol 2021;32:71–82. [DOI] [PubMed] [Google Scholar]
- 18. Ströker E, de Asmundis C, Kupics K, Takarada K, Mugnai G, De Cocker Jet al. . Value of ultrasound for access guidance and detection of subclinical vascular complications in the setting of atrial fibrillation cryoballoon ablation. Europace 2018;21:434–9. [DOI] [PubMed] [Google Scholar]
- 19. Winkle RA, Mohanty S, Patrawala RA, Mead RH, Kong MH, Engel Get al. . Low complication rates using high power (45–50 W) for short duration for atrial fibrillation ablations. Heart Rhythm 2019;16:165–9. [DOI] [PubMed] [Google Scholar]
- 20. Castrejón-Castrejón S, Martínez Cossiani M, Ortega Molina M, Escobar C, Froilán Torres C, Gonzalo Bada Net al. . Feasibility and safety of pulmonary vein isolation by high-power short-duration radiofrequency application: short-term results of the POWER-FAST PILOT study. J Interv Card Electrophysiol 2020;57:57–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.