Abstract
Aims
Atrial fibrillation (AF) and heart failure (HF) often coexist. However, whether AF onset before HF or vice versa is associated with the worst outcome remains unclear. A consensus of large studies can guide future research and preventive strategies to better target high-risk patients.
Methods and results
We included all Danish cases with the coexistence of AF and HF (2005–17) using nationwide registries. Patients were divided into three separate groups (i) AF before HF, (ii) HF before AF, or (iii) AF and HF diagnosed concurrently (±30 days). Adjusting landmark Cox analyses (index date was the time of the latter diagnosis of AF or HF) were used for evaluating the association of the three groups with a composite outcome of ischaemic stroke or death. Among a total of 49 042 patients included, 40% had AF before HF, 27% had HF before AF, and 33% had AF and HF diagnosed concurrently. The composite endpoint accrued more often in patients with HF before AF compared to the two other groups (<0.001), and this remained significant in the adjusted analyses with hazard ratios (95% confidence intervals) of 1.26 (1.22–1.30) compared to AF before HF. Finally, antihypertensive treatment, oral anticoagulants, amiodarone, statins, and AF ablation were associated with a lower hazard ratio of the composite endpoint (all < 0.001).
Conclusions
In this large Danish national cohort, diagnosis of HF before AF was associated with an increased absolute risk of death compared to AF before HF and AF and HF diagnosed concurrently. Antihypertensive treatment, oral anticoagulants, amiodarone, statins, and AF ablation may improve prognosis.
Keywords: Atrial fibrillation, Heart failure
What’s new?
Diagnosis of heart failure (HF) before atrial fibrillation (AF) seems to have a worse prognosis than AF before HF, and AF and HF diagnosed concurrently in a national patient cohort.
Antihypertensive treatment, oral anticoagulants, amiodarone, statins, and AF ablation may improve prognosis.
Introduction
Atrial fibrillation (AF) and heart failure (HF) are responsible for substantial economic costs, morbidity, and mortality. These two conditions often coexist, in part because they share antecedent risk factors, but also because one may directly predispose to the other.1 Individually, AF and HF increase the risk of stroke and death, and the synergistic combination of AF and HF creates a prothrombotic state that results in worse stroke morbidity and mortality than the mere presence of either condition separately.2,3
Various studies have demonstrated that the underlying pathophysiologic mechanism depends on which of the two conditions comes first.4 Accordingly, cardiomyopathies have been classified into atrial cardiomyopathies, ventricular cardiomyopathies, and mixed cardiomyopathies, maybe corresponding to AF occurring before HF, HF occurring before AF, and concurrent development of both conditions, respectively.5 This is important, because the absence of an overt ventricular pathology may enable the identification of patients most likely to benefit from a more aggressive rhythm control strategy for HF treatment, vs. therapies aimed at myocardial processes.6 Furthermore, the prognosis may vary depending on which of the two conditions comes first, however, results regarding the joint prognosis of AF and HF are conflicting. Some studies report that AF increases mortality,7,8 whereas others, that AF has no effect.9,10
However, the prognosis of the joint time course of AF and HF and the sequence in which they occur; has not been studied in a national population study. If more large studies agree on which sequence is associated with the worst prognosis, future research and preventive strategies can be targeted to these high-risk patients. In the present study, we, therefore, estimated prognosis and absolute rates of mortality and stroke in patients with coexisting AF and HF according to the sequence of which HF and AF occurred in a large national Danish cohort. Second, the outcome was compared between patients with AF alone and HF alone to patients with both conditions to generate hypotheses for the effects of the two conditions.
Methods
Study design
In this registry-based cohort study, information on demographics, comorbidities, procedures, concomitant medication, and outcome variables was identified using three different nationwide Danish registers. These registers were cross-linked using the unique personal identification number given to all Danish citizens at the date of birth or date of migration to Denmark. The Civil Registration System holds data on the age, sex, and vital status of patients, where all deaths are registered within 14 days of occurrence. The Danish National Patient Register contains information on every hospital admission in Denmark since 1978, in which each hospitalization is registered at discharge with one primary diagnosis and, if applicable, one or more secondary diagnoses according to the International Classification of Diseases; the 10th revision (ICD-10), since 1994. The Danish National Patient Register also holds information on operations and procedures that have been registered since 1996 and coded according to the Nordic Classification of Surgical Procedures (NCSP) by The Nordic Medico-Statistical Committee. Data on the date, quantity, strength, formulation, and affiliation of the prescribing physician for all prescriptions dispensed from Danish pharmacies have been accurately registered in The Danish Registry of Medicinal Product Statistics since 1995 and coded according to the Anatomical Therapeutic Chemical classification system.
The presence of AF was defined by a primary diagnosis of AF for a given hospital contact, both inpatient and outpatient, as defined by the ICD, Tenth Revision classification (DI-48). We did not differentiate between AF and atrial flutter. This definition has been validated with a positive predictive value of 92.6% [95% confidence interval (CI), 88.8–95.2] using the Danish National Patient Register.11 The diagnosis HF was identified using ICD-10 codes ‘I50’, ‘I11.0’, ‘I13.0’, or ‘I13.2’ in both inpatients and outpatients. Both inpatient and outpatient diagnoses were included. The positive predictive value of HF using these criteria has been estimated to be between 81 and 100%.12 Any cardiovascular admissions were identified using ICD-10 codes ‘I1’, ‘I12’, ‘I3’, ‘I4’, ‘I50’, ‘I63’, ‘I64.0’, ‘I9’, ‘G458’, and ‘G459’.
Study cohort
Patients 18–90 years old without prior ischaemic stroke were included between 2005 and May 2017. Patients were divided into the following three groups: (i) AF before HF, (ii) HF before AF, or (iii) AF and HF diagnosed concurrently (±30 days). ± 30 days window was chosen to assure that the onset of AF and HF was related. Patients were followed from the second diagnosis of HF or AF. This date is referred to as the index date.
Study endpoint
The primary endpoint was a composite of ischaemic stroke and all-cause mortality, and secondary endpoints were the two endpoints separately.
Statistics
Categorical data were presented as counts with percentages, and statistical differences were tested using χ2 tests. Continuous variables were presented as medians with interquartile ranges, and the statistical difference was tested using Wilcoxon rank-sum tests. Baseline characteristics were compared at the index date, which was defined as the time of the second diagnosis of either AF or HF occurred. Second, baseline characteristics were compared at the time of the first diagnosis of the AF alone or HF alone, or the time of the second diagnosis of either AF or HF occurred in the AF and HF group. The association between HF onset before AF, concurrently with AF or after AF, and a composite endpoint of stroke and death, and both endpoints separately, was analyzed using multivariable Cox regression, adjusted for sex, age, chronic obstructive pulmonary disease, chronic kidney disease (CKD), diabetes mellitus, hypertension, ischaemic heart disease, and disease duration. Schoenfeld residuals were used to check the proportional hazards assumption. The models were tested for interaction with sex, calendar year, and time between HF and AF. Furthermore, the incident composite endpoint and death were shown using Kaplan Meier plots and incident stroke by plots of cumulative incidence using Fine and Gray competing risk regression, using all-cause mortality as a competing event. Finally, we compared the odds ratio for 1-year outcome of the composite endpoints or stroke between the three groups, whichever came first, patients with AF, HF, or both AF and HF, with AF as the reference.
Data management and statistical analyses were conducted using R statistics.13 Two-tailed P < 0.05 was considered significant.
Ethics
In Denmark, registry-based studies that are conducted for the sole purpose of statistics and scientific research do not require ethical approval or informed consent by law. However, the study is approved by the data responsible institute [the Capital Region of Denmark (approval number: P-2019–191)] in accordance with the General Data Protection Regulation.
Results
The total number of patients included in the study was 49 042. Of these, 19 493 (40%) patients had AF diagnosed before HF, 13 356 (27%) patients had HF diagnosed before AF, and 16 193 (33%) patients had AF and HF diagnosed concurrently (see Supplementary material online, Figure S1). The median time between AF in HF patients was 1 309 days (Q1: 382; Q3: 2 851) and 1 372 days (Q1: 416; Q3: 2 770) between HF in AF patients.
The patients with AF diagnosed before HF were older, more were women, and more used calcium antagonists, amiodarone, and digoxin (Table 1) compared with the other study groups. Patients with HF before AF had more ischaemic heart disease, chronic obstructive pulmonary disease, CKD, hypertension, and diabetes; used more diuretics, angiotensin-2-antagonists, and ace-inhibitors; and more had received the percutaneous coronary intervention, by-pass surgery, and implantable cardioverter-defibrillator.
Table 1.
Baseline characteristics by initial diagnosis
| AF before HF (A) | Concurrently AF and HF (B) | HF before AF (C) | A vs. C | B vs. C | A vs. B | |
|---|---|---|---|---|---|---|
| N | 19 493 | 16 193 | 13 356 | |||
| Age [median (Q1, Q3)] | 77 (70, 83) | 75 (66, 82) | 76 (69, 83) | <0.001 | <0.001 | <0.001 |
| Male sex, n (%) | 11651 (59.8) | 9877 (61.0) | 8211 (61.5) | 0.002 | 0.404 | 0.019 |
| Disease duration [median days (Q1, Q3)] | 1309 (382, 2851) | 0 (0, 0) | 1372 (416, 2770) | 0.506 | <0.001 | <0.001 |
| Comorbidity | ||||||
| Ischaemic heart disease, n (%) | 7080 (36.3) | 4177 (25.8) | 6646 (49.8) | <0.001 | <0.001 | <0.001 |
| Chronic obstructive pulmonary disease, n (%) | 4043 (20.7) | 2319 (14.3) | 3508 (26.3) | <0.001 | <0.001 | <0.001 |
| Chronic kidney disease, n (%) | 1708 (8.8) | 830 (5.1) | 1677 (12.6) | <0.001 | <0.001 | <0.001 |
| Hypertension, n (%) | 17 791 (91.3) | 14 308 (88.4) | 12 208 (91.4) | 0.682 | <0.001 | <0.001 |
| Diabetes, n (%) | 3835 (19.7) | 2248 (13.9) | 3135 (23.5) | <0.001 | <0.001 | <0.001 |
| CHA2DS2-VASc, category (%) | <0.001 | <0.001 | <0.001 | |||
| 1 | 212 (1.1) | 351 (2.2) | 96 (0.7) | |||
| 2 | 1935 (9.9) | 2606 (16.1) | 1344 (10.1) | |||
| 3 | 3817 (19.6) | 3692 (22.8) | 2771 (20.7) | |||
| ≥4 | 13 529 (69.4) | 9544 (58.9) | 9145 (68.5) | |||
| Medication | ||||||
| Thiazide, n (%) | 5137 (26.4) | 3999 (24.7) | 2585 (19.4) | <0.001 | <0.001 | <0.001 |
| Spironolactone, n (%) | 5001 (25.7) | 3842 (23.7) | 4657 (34.9) | <0.001 | <0.001 | <0.001 |
| Loop diuretics, n (%) | 14 800 (75.9) | 11 887 (73.4) | 10 427 (78.1) | <0.001 | <0.001 | <0.001 |
| Beta-blocker, n (%) | 14 501 (74.4) | 11 995 (74.1) | 9793 (73.3) | 0.031 | 0.147 | 0.505 |
| Calcium channel blocker, n (%) | 7394 (37.9) | 4750 (29.3) | 3907 (29.3) | <0.001 | 0.889 | <0.001 |
| Renin–angiotensin blocker, n (%) | 13 208 (67.8) | 11 382 (70.3) | 10 005 (74.9) | <0.001 | <0.001 | <0.001 |
| Verapamil, n (%) | 2252 (11.6) | 738 (4.6) | 603 (4.5) | <0.001 | 0.883 | <0.001 |
| Amiodarone, (%) | 2373 (12.2) | 1751 (10.8) | 1458 (10.9) | 0.001 | 0.791 | <0.001 |
| Digoxin, n (%) | 9116 (46.8) | 7343 (45.3) | 4668 (35.0) | <0.001 | <0.001 | 0.008 |
| Oral anticoagulants, n (%) | 14 552 (74.7) | 10 351 (63.9) | 7403 (55.4) | <0.001 | <0.001 | <0.001 |
| Dabigatran, n (%) | 1397 (7.2) | 1165 (7.2) | 664 (5.0) | <0.001 | <0.001 | 0.936 |
| Rivaroxaban, n (%) | 885 (4.5) | 950 (5.9) | 614 (4.6) | 0.828 | <0.001 | <0.001 |
| Apixaban, n (%) | 920 (4.7) | 1024 (6.3) | 710 (5.3) | 0.016 | <0.001 | <0.001 |
| Warfarin, n (%) | 11 517 (59.1) | 7426 (45.9) | 5501 (41.2) | <0.001 | <0.001 | <0.001 |
| Procedure | ||||||
| ICD, n (%) | 434 (2.2) | 121 (0.7) | 1074 (8.0) | <0.001 | <0.001 | <0.001 |
| Percutaneous coronary intervention, n (%) | 2176 (11.2) | 1315 (8.1) | 2642 (19.8) | <0.001 | <0.001 | <0.001 |
| By-pass surgery, n (%) | 1750 (9.0) | 820 (5.1) | 1839 (13.8) | <0.001 | <0.001 | <0.001 |
AF, atrial fibrillation; HF, heart failure; ICD, implantable cardioverter-defibrillator.
Temporal trends in HF and AF
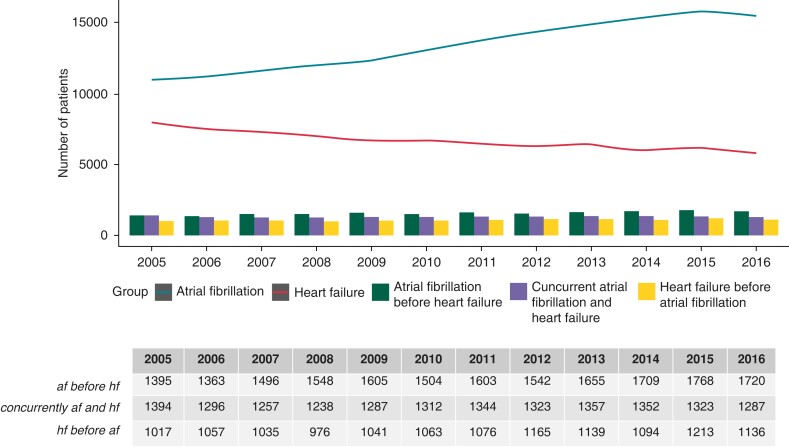
The incidence of HF per se steadily decreased during the period, whereas AF per se increased during the study period (Figure 1). Incidence of AF before HF, HF before AF, and AF and HF diagnosed concurrently all increased slightly through the study period.
Figure 1.
Incidence of atrial fibrillation, heart failure, and temporal relations. Lines represent incidence of atrial fibrillation alone and heart failure alone per year. The bar chart represents number of patients with both atrial fibrillation and heart failure; divided by which condition came first or if they occurred concurrently.
Impact of developing AF after HF and vice versa
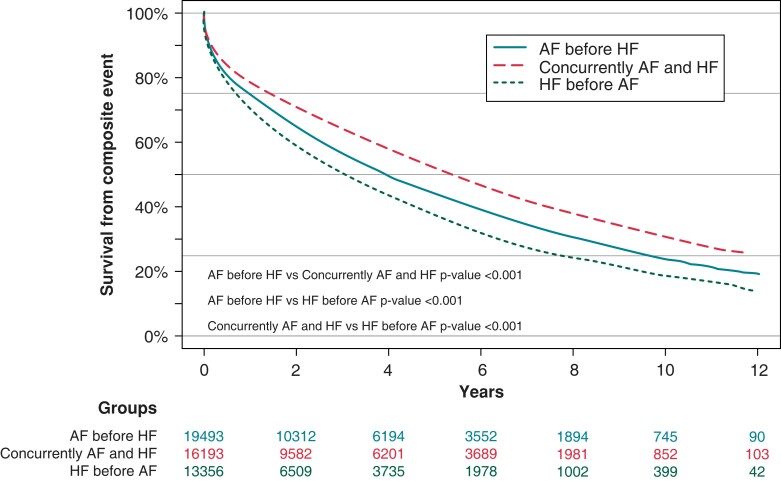
The cumulative incidence for the composite endpoint was highest in patients with HF before AF (Figure 2). The 10-year incidence was 81.5% compared to 76.1% (P < 0.001) and 69.0% (P < 0.001) in HF before AF, AF before HF, and AF concurrently with HF, respectively (Figure 2).
Figure 2.
Atrial fibrillation and heart failure onset and freedom from death and stroke. AF, atrial fibrillation; HF, heart failure.
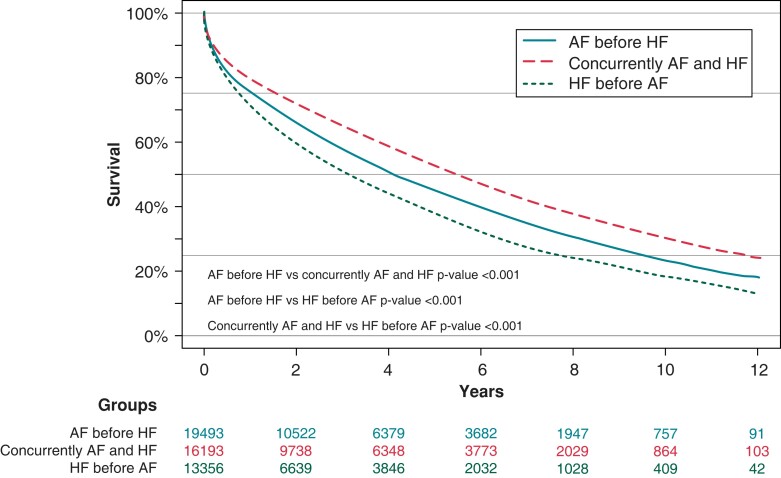
For death, the mortality rate was also higher in patients with HF before AF. The 10-year mortality rate was 81.8% compared to 76.8% (P < 0.001) and 69.6% (P < 0.001) in patients with HF before AF, AF before HF, and for AF concurrently with HF, respectively (Figure 3).
Figure 3.
Atrial fibrillation and heart failure onset and survival. AF, atrial fibrillation; HF, heart failure.
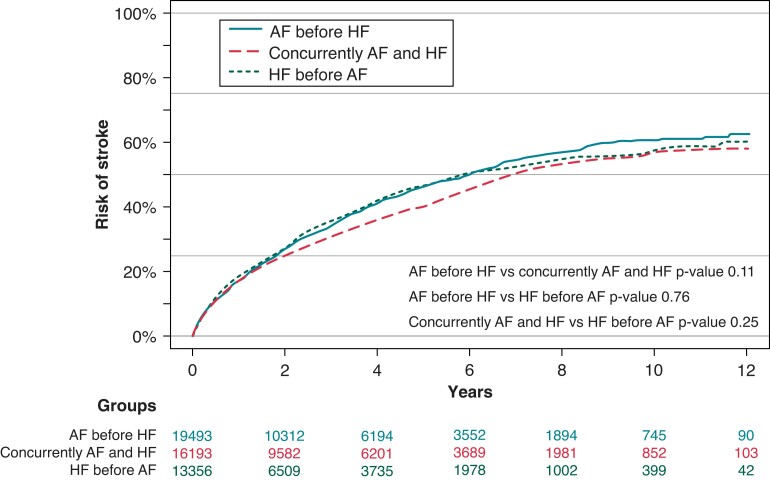
The cumulative incidence of stroke was 5.8% for HF before AF, 6.0% in AF before HF, and 5.7% in patients with AF concurrently with HF, with no significant difference found among the three groups (Figure 4).
Figure 4.
Atrial fibrillation and heart failure onset and risk of stroke using Aalen-Johansen with death as competing risk. AF, atrial fibrillation; HF, heart failure.
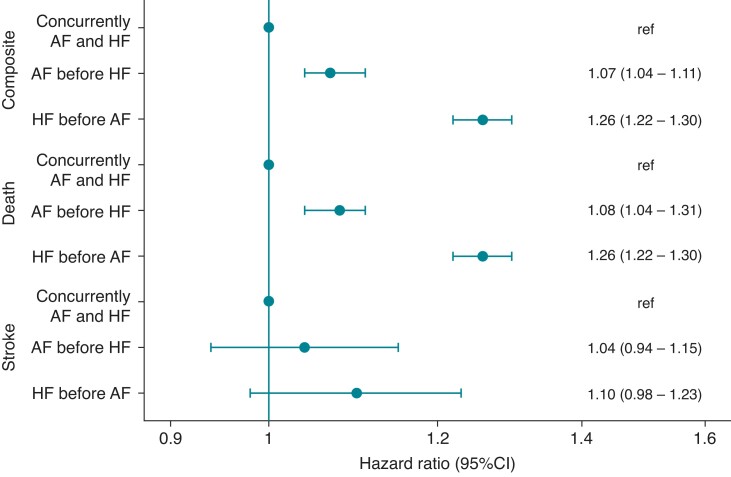
In multivariable Cox analyses, HF before AF was associated with an increased hazard of both the composite endpoint and death compared to both AF before HF and AF concurrently with HF (Figure 5). For stroke alone, there was no multivariable-adjusted difference among the three groups (Figure 5). There was no interaction with the calendar year or time between HF and AF (both P > 0.05) on hazard ratios (HRs) of the primary endpoint, but we found interaction with gender (P < 0.001). Although both AF before HF and HF before AF seemed to be worse in men, the HR was smaller and not significant in women with AF before HF, and no clinically relevant difference was found in the gender-stratified analyses (see Supplementary material online, Figures S2–S5). To show which factors are associated with stroke and death, several important additional factors were included in the multivariable Cox model (see Supplementary material online, Figure S6). This revealed that antihypertensive treatment, oral anticoagulants, amiodarone, statins, and AF ablation were associated with a lower hazard of stroke and death, whereas ischaemic heart disease, chronic obstructive pulmonary disease, diabetes, male gender, older age, and digitalis were associated with higher hazards of stroke and death.
Figure 5.
Hazard ratios for the composite, death, and stroke with AF before HF as reference. Adjusted to age, chronic obstructive pulmonary disease, chronic kidney disease, diabetes mellitus, hypertension, ischaemic heart disease, and disease duration of atrial fibrillation or heart failure. AF, atrial fibrillation; HF, heart failure.
To generate hypotheses for effects, the crude odds ratio for 1-year outcome of the composite endpoint or stroke was compared between the three groups, whichever came first, AF alone, HF alone, or both AF and HF, with AF as the reference. This showed that HF had a higher odds ratio of both stroke alone and the composite endpoint of stroke and death compared to AF alone, and furthermore, that patients with concurrent AF and HF had an even higher hazard of both outcomes (see Supplementary material online, Figures S7 and S8). The baseline characteristics between the three groups are shown in Supplementary material online, Table S1). Importantly, this showed that patients with concurrent AF and HF had more hypertension than the two other groups and that HF patients had more hypertension than AF patients. Furthermore, patients had more ischaemic heart disease in the HF group and in group with concurrent AF and HF compared to the AF group.
Disease burden of developing AF after HF and vice versa
Cardiovascular admissions in the three groups AF before HF, HF before AF, and AF concurrent with HF are shown in Supplementary material online, Figure S9. This showed significantly more admissions for cardiovascular disease in the group HF before AF compared to the two other groups, and significantly more admissions in the group AF before HF compared to AF concurrent with HF.
Discussion
This study reports 4 important findings, (i) number of patients diagnosed with both AF before HF, vice versa, and AF and HF diagnosed concurrently increased in the period from 2005 to 2017, despite decreasing incidence of HF, (ii) coexisting AF and HF is associated with a higher risk of stroke and death compared to AF and HF alone, (iii) HF before AF is strongly associated with death, but not stroke, compared to AF diagnosed before or within 30 days of HF in a national Danish cohort, and (iv) antihypertensive treatment, oral anticoagulants, amiodarone, statins, and AF ablation were associated with a lower hazard of stroke and death in patients with coexisting AF and HF.
The continuing decrease in HF could result from better treatment preventive strategies and acute treatment of myocardial infarction. The increase in AF could reflect the aging population and, therefore, age-related comorbidity. Another, and maybe more important aspect, is the increasing focus and detection of AF. With catheter ablation of AF being introduced in Denmark in 2008, the attention on AF likely increased in the following years. Furthermore, the introduction of the first direct oral anticoagulant in 2011 started a new paradigm in AF patients’ anticoagulant treatment, putting even more focus on AF. A study of temporal trends of AF and HF admissions and death in the United States between 1991 and 2015 demonstrated similar decreasing hospitalization and mortality rates for HF vs. increasing rates for AF.14 The first could result from better and faster treatment of myocardial infarction reducing infarct size, but also improved treatment of HF during the last decades. The increasing rate of AF admissions corresponds to our observations and could have similar reasons as suspected to drive our study trends.
HF has been shown to account for twice as many hospitalizations and >3 times as many deaths compared with AF.14 Several studies have demonstrated a close relationship between the development of AF in HF and vice versa.1,15–17 The association of AF with increased risk of subsequent HF may be explained by the higher ventricular rate and stress in AF, often leading to HF, also known as tachyarrhythmia-induced cardiomyopathy.18 AF onset in HF patients is probably due to the inevitable left ventricular overload in HF being transferred to the atria thru the mitral valve causing atrial stretch and fibrosis that leads to AF at an advanced stage of HF.4 This is supported by recent expert consensus on atrial cardiomyopathies arguing that HF triggers the occurrence of atrial cardiomyopathy before the development of AF.19 Analyzing the temporal relationship between HF and AF, the Framingham Heart Study showed a higher hazard of developing HF in AF patients and AF in HF patients compared to patients without these conditions.15 However, in multivariable analysis adjusted for several underlying conditions and chronic diseases, prevalent HF remained associated with AF, but prevalent AF was no longer associated with HF with reduced ejection fraction. This suggests aging and chronic underlying diseases including hypertension, myocardial infarction, diabetes, and left ventricular hypertrophy are important factors in the pathophysiology of HF, and maybe more important than AF per se. It also supports the belief that AF onset in HF patients is a sign of cardiac stress and incipient failure of the cardiovascular system to compensate for physiologic demands.
Confirming our findings, a recently published study from the Global Anticoagulant Registry in the FIELD-Atrial Fibrillation (GARFIELD-AF) showed that patients with HF and AF had an increased risk of death, stroke and myocardial infarction compared to AF patients without HF.3 The Framingham Heart group has previously shown that AF was associated with death independent of preexisting cardiovascular conditions related to AF and independent of gender.17 In contrast, in a study with 409 patients with moderate to severe HF, including 84 patients with AF, AF was not associated with increased mortality after adjusting for important prognostic variables, such as age, left ventricular ejection fraction, NYHA class, renal function, and blood pressure.20 The V-HeFT trial also failed to show that AF in patients with mild to moderate HF was associated with a higher risk of all-cause mortality or sudden death.10 However, the latter two studies were limited by their size and only included a limited number of patients with myocardial infarction. Results from the Danish Investigations of Arrhythmia and Mortality On Dofetilide study (DIAMOND) showed that AF was related to poor prognosis, but only in patients with HF and ischaemic heart disease, and not in patients without the ischaemic component.21 These results are supported by a Danish national study including 89 702 patients with myocardial infarction,22 showing that new-onset AF was associated with a two-fold increase in all-cause mortality, cardiovascular death, stroke, and re-infarction. In accordance with these results, another large registry study showed 23 that patients with ischaemic HF, but not those with nonischaemic HF, had an increased risk of death if chronic AF was present. In contrast, a recent substudy from the DANISH trial using a cardiac electronic implantable device to detect AF, AF onset in nonischaemic HF was associated with mortality, questioning myocardial infarction as a key factor for the association with mortality.16
Comparing the temporal relations of AF and HF with risk of mortality, the Framingham Heart Study demonstrated that coexisting AF and HF were associated with a higher hazard ratio of mortality, but only in patients who developed HF first.1 In concordance, the present study showed a stronger association with mortality in patients who developed HF before AF than vice versa compared to developing AF and HF concurrently. Accordingly, cardiomyopathies have been classified into atrial cardiomyopathies, ventricular cardiomyopathies, and mixed cardiomyopathies, maybe corresponding to AF occurring before HF, AF occurring before HF, and concurrent development of both conditions, respectively.5 If HF is diagnosed before AF, the HF is most likely a result of chronic underlying conditions that contribute significantly to the chronic poor state. Therefore, it is likely to drive increased mortality, and the development of AF is a parallel sign of underlying cardiovascular failure. When AF is diagnosed before HF, HF may reflect a tachycardia-triggered failure of the cardiovascular system, which is often a reversible condition, which potentially could explain its less strong association with death. However, this interpretation is not investigated in the present study, and therefore, can only be speculative. We found that the association between the composite and death was less strong for women with AF before HF compared to men, but the difference was not clinically relevant and was therefore not investigated further.
Our findings, confirming that patients with HF before AF seem to have a worse prognosis than patients with AF before HF, or AF and HF diagnosed concurrently, suggest that the clinician should pay attention to which condition appeared first, as patients with HF before AF are a high-risk group probably needing closer and more careful follow-up. Recently, the RATE-AF study compared digoxin and bisoprolol for rate control in AF patients with NYHA II or more. They found no difference in the rate control, but interestingly, NYHA class improved, and natriuretic peptides significantly reduced in digoxin-treated patients compared to beta-blocker-treatedpatients.24 Our study showed, that patients with HF before AF, received significantly less digoxin than the two other groups, which could have contributed to the worse prognosis of these patients. However, in the multivariable analysis digoxin seemed to be associated with slightly worse outcomes. Currently, there are two prospective, placebo-controlled randomized trials underway which are studying the effects of low-dose digoxin on outcome in patients with HF, the DIGIT-HF trial (DIGitoxinto Improve ouTcomes in patients with advanced chronic Heart Failure) trial and DECISION trial (Digoxin Evaluation in Chronic heart failure: Investigational Study In Outpatients in the Netherlands), that will both add valuable knowledge about digoxin use in HF patients.25
In the present study, patients with HF before AF had more chronic comorbidity, which was also reflected in the higher use of HF treatment and diuretics; and more percutaneous coronary intervention, by-pass surgery, and implantable cardioverter-defibrillators. This suggests that treatment strategies maybe should target chronic underlying diseases especially in patients with HF before AF including hypertension, myocardial infarction, diabetes, and left ventricular hypertrophy, which is also supported in our multivariable analyses showing that antihypertensive treatment, oral anticoagulants, amiodarone, statins, and AF ablation were associated with improved prognosis. Whether AF should be a target per se is controversial. The Atrial Fibrillation and Congestive Heart Failure (AF-CHF) trial, including 1376 patients with AF and HF, most developing HF before AF, demonstrated that rhythm control with amiodarone did not prevent poor outcomes compared to rate control.26 However, randomized controlled trials have reported that catheter ablation of AF might reduce the risk of mortality in patients with coexisting AF and HF regardless of which came first.27 Therefore, it may be reasonable, to treat AF in addition to underlying chronic diseases, and maybe regardless of whatever came first. This is recently confirmed in the EAST trial, showing that early rhythm control including a substantial number of catheter ablations was associated with a lower risk of adverse cardiovascular outcomes than usual care among patients with early AF, regardless of HF and obesity.28 Surprisingly, there was no change in left ventricular function after 2 years, which demonstrates that prognosis in AF patients is dependent on several other factors than HF.
Limitations
Our study suffers the limitations with any analysis of longitudinal registry data. Notably, we could not differentiate between patients with HF and reduced vs. preserved ejection fraction because we did not have measurements of left ventricular ejection fraction and other important echocardiographic parameters. Unmeasured confounding due to lack of information on important clinical variables such as functional class, NT-proBNP, left ventricular ejection fraction, and residual confounding; for example, renal function is estimated based on a diagnostic code for CKD, and cannot be excluded in our study. Furthermore, HF and AF numbers may be underestimated, as both diseases do not necessarily require hospital care. We did not differentiate between patterns of AF (paroxysmal, persistent, chronic, or post-operative). In the present study, the patients were included in the analyses at the first time they were diagnosed with AF. As AF usually is paroxysmal in the beginning, it is likely that most of the cases were paroxysmal. However, the group ‘AF before HF’ could include cases with more persistent AF, as the median time from AF to HF was 1309 days (Table 1). Finally, our study is observational, and our findings are associations and do not reflect causality. However, the completeness of data, which enabled comprehensive follow-up of large numbers of patients for 10 years, adds strength to this study, and the study may give a real-world estimate of the association of the temporal relationship of AF and HF with mortality and stroke.
Conclusion
In this large Danish national cohort, the coexistence of AF and HF was associated with higher hazards of stroke and death compared to AF and HF alone. In patients with coexisting AF and HF, the development of HF before AF was associated with a higher rate of death compared to AF before HF and AF and HF occurring within 30 days. Antihypertensive treatment, oral anticoagulants, amiodarone, statins, and AF ablation, but not digoxin, were associated with lower hazards of stroke and death.
Supplementary Material
Contributor Information
Jannik Pallisgaard, Department of Cardiology, Herlev and Gentofte Hospital, Copenhagen University, Copenhagen 2900, Denmark.
Anders M Greve, Department of Clinical Biochemistry, Rigshospitalet, Copenhagen University, Copenhagen 2100, Denmark.
Morten Lock-Hansen, Department of Cardiology, Herlev and Gentofte Hospital, Copenhagen University, Copenhagen 2900, Denmark.
Jens Jakob Thune, Department of Cardiology, Bispebjerg and Frederiksberg Hospital, Copenhagen University, Copenhagen 2400, Denmark.
Emil Loldrup Fosboel, Department of Cardiology, The Heart Center, Rigshospitalet, Copenhagen University, Copenhagen 2100, Denmark.
Richard B Devereux, Department of Medicine, Division of Cardiology, Weill Cornell Medical College, New York, NY 10065, USA.
Peter M Okin, Department of Medicine, Division of Cardiology, Weill Cornell Medical College, New York, NY 10065, USA.
Gunnar H Gislason, Department of Cardiology, Herlev and Gentofte Hospital, Copenhagen University, Copenhagen 2900, Denmark; Department of Research, Danish Heart Foundation, Copenhagen 1120, Denmark.
Christian Torp-Pedersen, Department of Cardiology, North Zealand Hospital, Copenhagen University, Copenhagen 3400, Denmark.
Casper N Bang, Department of Cardiology, Bispebjerg and Frederiksberg Hospital, Copenhagen University, Copenhagen 2400, Denmark.
Supplementary material
Supplementary material is available at Europace online.
Funding
No funding declared.
Data availability
The data and study materials are not available to other researchers for purposes of reproducing the results or replicating the procedure.
References
- 1. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PAet al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the framingham heart study. Circulation 2003;107:2920–5. [DOI] [PubMed] [Google Scholar]
- 2. Haeusler KG, Laufs U, Endres M. Chronic heart failure and ischemic stroke. Stroke 2011;42:2977–82. [DOI] [PubMed] [Google Scholar]
- 3. Ambrosio G, Bassand JP, Corbalan R, Kayani G, Carluccio E, Mantovani LGet al. Characteristics, treatment, and outcomes of newly diagnosed atrial fibrillation patients with heart failure: GARFIELD-AF. ESC Hear Fail 2021;8:1139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crijns HJGM, Van den Berg MP, Van Gelder IC, Van Veldhuisen DJ. Management of atrial fibrillation in the setting of heart failure. Eur Heart J 1997;18:C45–9. [DOI] [PubMed] [Google Scholar]
- 5. Hirsh BJ, Copeland-Halperin RS, Halperin JL. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism: mechanistic links and clinical inferences. J Am Coll Cardiol 2015;65:2239–51. [DOI] [PubMed] [Google Scholar]
- 6. Providencia R, Lambiase PD. Letter by Providencia and lambiase regarding article, ‘atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation 2016;133:e691. [DOI] [PubMed] [Google Scholar]
- 7. Middlekauff HR, Stevenson WG, Stevenson LW. Prognostic significance of atrial fibrillation in advanced heart failure. A study of 390 patients. Circulation 1991;84:40–8. [DOI] [PubMed] [Google Scholar]
- 8. Dries DL, Exner D V, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of left ventricular dysfunction. J Am Coll Cardiol 1998;32:695–703. [DOI] [PubMed] [Google Scholar]
- 9. Mahoney P, Kimmel S, Denofrio D, Wahl P, Loh E. Prognostic significance of atrial fibrillation in patients at a tertiary medical center referred for heart transplantation because of severe heart failure. Am J Cardiol Am J Cardiol 1999;83:1544–7. [DOI] [PubMed] [Google Scholar]
- 10. Carson PE, Johnson GR, Dunkman WB, Fletcher RD, Farrell L, Cohn JN. The influence of atrial fibrillation on prognosis in mild to moderate heart failure. The V-HeFT studies. The V-HeFT VA cooperative studies group. Circulation 1993;87:VI102–10. [PubMed] [Google Scholar]
- 11. Rix TA, Riahi S, Overvad K, Lundbye-Christensen S, Schmidt EB, Joensen AM. Validity of the diagnoses atrial fibrillation and atrial flutter in a danish patient registry. Scand Cardiovasc J 2012;46:149–53. [DOI] [PubMed] [Google Scholar]
- 12. Mard S, Nielsen FE. Positive predictive value and impact of misdiagnosis of a heart failure diagnosis in administrative registers among patients admitted to a university hospital cardiac care unit. Clin Epidemiol Dove Press2010;2:235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. R Foundation for Statistical Computing RCT. R: a language and environment for statistical computing. R core team 2019. Available: https://www.R-project.org/ .
- 14. Vasan RS, Zuo Y, Kalesan B. Divergent temporal trends in morbidity and mortality related to heart failure and atrial fibrillation: age, sex, race, and geographic differences in the United States, 1991–2015. J Am Heart Assoc 2019;8:e010756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SAet al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation 2016;133:484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boas R, Jakob Thune J, Pehrson S, Køber L, Nielsen JC, Videbaek Let al. Atrial fibrillation is a marker of increased mortality risk in nonischemic heart failure-results from the DANISH trial. Am Heart J 2021;232:61–70. [DOI] [PubMed] [Google Scholar]
- 17. Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham heart study. JAMA 1994;271:840–4. [PubMed] [Google Scholar]
- 18. Shinbane JS, Wood MA, Jensen DN, Ellenbogen KA, Fitzpatrick AP, Scheinman MM. Tachycardia-induced cardiomyopathy: A review of animal models and clinical studies. J Am Coll Cardiol 1997;29:709–15. [DOI] [PubMed] [Google Scholar]
- 19. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SAet al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace 2016;18:1455–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crijns H. Prognostic value of the presence and development of atrial fibrillation in patients with advanced chronic heart failure. Eur Heart J 2000;21:1238–45. [DOI] [PubMed] [Google Scholar]
- 21. Pedersen OD, Søndergaard P, Nielsen T, Nielsen SJ, Nielsen ES, Falstie-Jensen Net al. Atrial fibrillation, ischaemic heart disease, and the risk of death in patients with heart failure. Eur Heart J 2006;27:2866–70. [DOI] [PubMed] [Google Scholar]
- 22. Bang CN, Gislason GH, Greve AM, Bang CA, Lilja A, Torp-Pedersen Cet al. New-onset atrial fibrillation is associated with cardiovascular events leading to death in a first time myocardial infarction population of 89,703 patients with long-term follow-up: a nationwide study. J Am Heart Assoc 2014;3:e000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raunsø J, Pedersen OD, Dominguez H, Hansen ML, Møller JE, Kjaergaard Jet al. Atrial fibrillation in heart failure is associated with an increased risk of death only in patients with ischaemic heart disease. Eur J Heart Fail 2010;12:692–7. [DOI] [PubMed] [Google Scholar]
- 24. Kotecha D, Bunting K V, Gill SK, Mehta S, Stanbury M, Jones JCet al. Effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life: the RATE-AF randomized clinical trial. JAMA 2020;324:2497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van der Meer P, Rienstra M, van Veldhuisen DJ. A deleterious interaction between omecamtiv mecarbil and atrial fibrillation in patients with heart failure: an influence of digoxin? Eur Heart J 2022;43:2221–3. [DOI] [PubMed] [Google Scholar]
- 26. Talajic M, Khairy P, Levesque S, Connolly SJ, Dorian P, Dubuc Met al. Maintenance of sinus rhythm and survival in patients with heart failure and atrial fibrillation. J Am Coll Cardiol 2010;55:1796–802. [DOI] [PubMed] [Google Scholar]
- 27. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens Let al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. [DOI] [PubMed] [Google Scholar]
- 28. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan Aet al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and study materials are not available to other researchers for purposes of reproducing the results or replicating the procedure.