Abstract
Aims
Thyroid dysfunction is considered the most frequent complication to amiodarone treatment, but data on its occurrence outside clinical trials are sparse. The present study aimed to examine the incidence of thyroid dysfunction following initiation of amiodarone treatment in a nationwide cohort of patients with and without heart failure (HF).
Methods and results
In Danish registries, we identified all patients with first-time amiodarone treatment during the period 2000–18, without prior thyroid disease or medication. The primary outcome was a composite of thyroid diagnoses and initiation of thyroid drugs. Outcomes were assessed at 1-year follow-up, and for patients free of events in the first year, in a landmark analysis for the subsequent 5 years. We included 43 724 patients with first-time amiodarone treatment, of whom 16 939 (38%) had HF. At 1-year follow-up, the cumulative incidence and adjusted hazard ratio (HR) of the primary outcome were 5.3% and 1.37 (95% confidence interval 1.25–1.50) in patients with a history of HF and 4.2% in those without HF (reference). In the 1-year landmark analysis, the subsequent 5-year cumulative incidences and adjusted HRs of the primary outcome were 5.3% (reference) in patients with 1-year accumulated dose <27.38 g [corresponding to average daily dose (ADD <75 mg)], 14.0% and HR 2.74 (2.46–3.05) for 27.38–45.63 g (ADD 75–125 mg), 20.0% and HR 4.16 (3.77–4.59) for 45.64–63.88 g (ADD 126–175 mg), and 24.5% and HR 5.30 (4.82–5.90) for >63.88 g (ADD >175 mg).
Conclusion
Among patients who initiated amiodarone treatment, around 5% had thyroid dysfunction at 1-year follow-up, with a slightly higher incidence in those with HF. A dose–response relationship was observed between the 1-year accumulated amiodarone dose and the subsequent 5-year cumulative incidence of thyroid dysfunction.
Keywords: Amiodarone, Hyperthyroidism, Hypothyroidism, Heart failure
What’s new?
Around 5% of patients with no prior history of thyroid disease, who initiated amiodarone treatment, were diagnosed and/or treated with thyroid drugs after 1 year.
The presence of heart failure (HF) was associated with a slightly higher risk of thyroid dysfunction, but patients with HF were also more sick and more likely to receiver higher accumulated doses of amiodarone.
Long-term risk of thyroid dysfunction had a dose–response relationship to the accumulated amiodarone dose within first year of treatment.
Short-term amiodarone treatment, as reflected by a low accumulated dose, was associated with a modest risk of thyroid dysfunction.
Introduction
Cardiac arrhythmias are a major cause of morbidity and mortality, particularly in patients with heart failure (HF). Amiodarone is effective for short- and long-term rhythm control of supraventricular tachyarrhythmias and may also be used following an event of ventricular tachycardia or after an event of worsening HF when tachycardia-induced cardiomyopathy is suspected.1–3
Amiodarone is a benzofuran, iodine-rich drug, which aside from its beneficial effects on cardiac arrhythmias, is known for its various adverse effects.1,4,5 Hyperthyroidism and hypothyroidism are well-recognized complications to amiodarone treatment, with reported overall risk ranging from 2 to 24%.2,6–8 Other complications include photosensitivity, and less frequently pulmonary fibrosis, and liver affection with elevated transaminase levels. Due to these adverse effects, amiodarone treatment requires close monitoring, and long-term treatment is often avoided in younger patients.1,2,6,9,10
While the relation between amiodarone and risk of thyroid dysfunction has been well described in clinical trials, population-based data on the short- and long-term adverse effects among all comers initiating oral amiodarone treatment are lacking.8,11–14 In addition, little is known about how the risk of thyroid dysfunction correlates to the dose of amiodarone accumulate over time.
Therefore, we set out to utilize the Danish nationwide registries to investigate the magnitude of the thyroid dysfunction risk associated with amiodarone treatment in patients with and without HF in order to guide clinicians and improve patient information.
Methods
Data sources
The Danish national administrative registries contain data on all Danish residents, and accurate linkage between multiple nationwide registries is possible using a unique and personal identification number which all Danish residents are given at date of birth or immigration. In the present study, we obtained data through linkage of the following registries: (i) the Danish Civil Registration System, which contains data on birth date, sex, vital status, and date of death,15 (ii) the Danish National Patient Registry, which holds information on all hospital admissions since 1977 and outpatient contacts since 1995, with diagnosis codes based on the International Classification of Diseases (ICD-8 and ICD-10 codes) and surgical procedures classified according to the Nordic Medico-Statistical Committee since 1996,16 and (iii) the Danish National Prescription Registry, which contains detailed information on dispensing date, strength, and quantity on all claimed drug prescriptions in Denmark.17
Study population and exposure
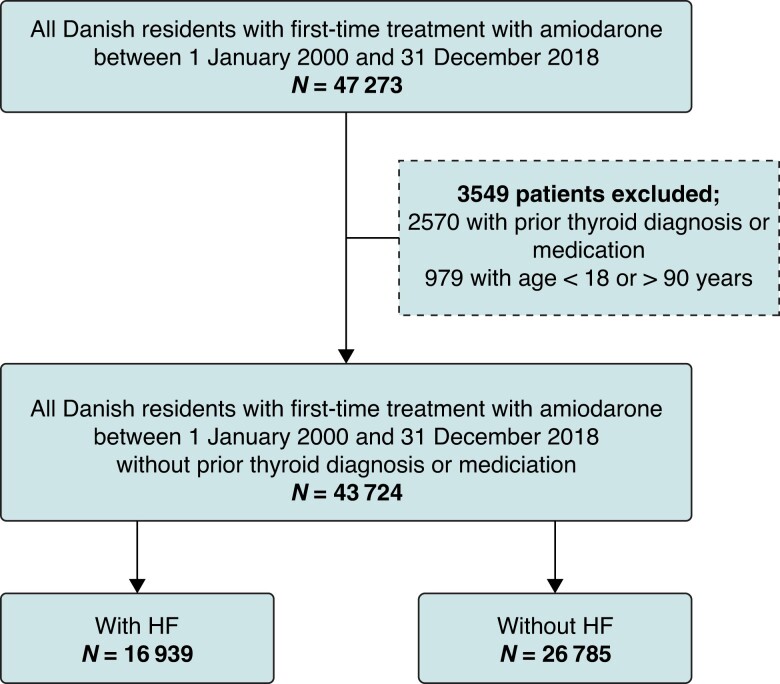
The study population comprised all Danish residents aged 18–90 years, with first-time treatment with amiodarone between 2000 and 2018, and no prior history of thyroid dysfunction or thyroid drug usage. The study population was categorized according to the presence of HF at any time prior to the index date (Figure 1). In patients alive and without thyroid dysfunction at 1-year follow-up, we performed a landmark analysis, examining the subsequent 5-year outcomes.
Figure 1.
Flow chart of the study population. HF, heart failure.
Eligible patients were grouped according to the accumulated amiodarone dose within the first year as follows: (i) <27.38 g [corresponding to average daily dose (ADD <75 mg)], (ii) 27.38–45.63 g (ADD 75–125 mg), 45.64–63.88 g (ADD 126–175 mg), and >63.88 g (ADD >175 mg). To put these groups into perspective, they may reflect a ‘standard’ daily dose of 200 mg for <4.5, 4.5–7.5, 7.6–10.5, and >10.5 months, within the first year of treatment, respectively, but may also reflect lower doses at longer duration or conversely shorter duration of higher dose treatment.
Concomitant pharmacotherapy and comorbidity
Patient medical history was obtained from the Danish National Patient Registry based on in-hospital and outpatient diagnosis codes at any time prior to the index date (see Supplementary material online, Tables S1 and S2). Concomitant pharmacotherapy was defined from the Danish National Prescription Registry as claimed prescriptions within 180 days prior to the index date (see Supplementary material online, Table S3). Heart failure was defined using in-hospital and outpatient diagnosis codes at any time prior to the index date. Aborted cardiac arrest was defined as a diagnosis with either cardiac arrest or ventricular fibrillation. Hypothyroidism was defined as a redeemed prescription of levothyroxine or hypothyroidism diagnosis, and hyperthyroidism was defined as a redeemed prescription of antithyroid medication or either a diagnosis of hyperthyroidism or thyroiditis, as done previously.18 Diabetes was defined as a redeemed prescription of anti-diabetic medication or a diabetes diagnosis.
Outcomes
The primary outcome was a composite of a redeemed prescription of either levothyroxine or antithyroid medication and in-hospital or outpatient contact with either of the following diagnoses: hypothyroidism, hyperthyroidism, or thyroiditis. The secondary outcomes were (i) hypothyroidism, (ii) hyperthyroidism, and (iii) all-cause death. Finally, two exploratory outcomes were observed: (i) an in-hospital and outpatient contact with a diagnosis of pulmonary fibrosis and (ii) an in-hospital and outpatient contact with a diagnosis of liver disease. For these analyses, we excluded patients who had a history of these outcomes prior to study start. In Denmark, initiation and monitoring of amiodarone treatment is restricted to outpatient cardiology departments, and measurement of thyroid-stimulating hormone (TSH) as well as liver parameters are recommended prior to initiation of amiodarone treatment and every 6 months during ongoing treatment.
The primary cohort was followed from the date of treatment initiation with amiodarone (i.e. index date) for a maximum of 1 year or until the occurrence of a primary outcome, death, or end of the study period (31 December 2018). Those without events in the first year were eligible for the landmark analysis which started 1 year from the index date and followed patients for up to 5 years or until the occurrence of the primary outcome, death, or end of the study period (31 December 2018), whichever came first.
Statistics
Baseline characteristics were reported using numbers and percentages for categorical variables and medians with 25–75th percentiles for continuous variables. Differences in baseline characteristics in those with and without HF were tested using the χ2 test for categorical variables and the Mann–Whitney test for continuous variables. Cumulative incidence curves were drawn for the composite primary and secondary outcomes using the Aalen–Johansen estimator assessing death as a competing risk. Differences between groups were assessed with Gray’s test. The cumulative incidence of all-cause death was examined using Kaplan–Meier estimates, and differences between groups were assessed using the log-rank test.19 A multivariable-adjusted Cox proportional hazard analysis was applied to compare risk across groups. Analysis was adjusted for the following covariates: sex, age, calendar period, prior hospital admission for any reason <2 weeks, treatment with statins, and known comorbidities prior to the index date (including ischaemic heart disease, peripheral artery disease, diabetes, malignancy, chronic renal disease, chronic obstructive pulmonary disease, and stroke). In the primary analysis, patients without HF were used as the reference group, while in the landmark analysis, the group of patients with an accumulated 1-year dose <27.38 g (ADD <75 mg) served as the reference group. The proportional hazards assumption was examined graphically using log [−log g (survival function)] vs. time plots for the exposure variable and found valid in the adjusted Cox analysis. Relevant interactions including sex and calendar period were tested and found insignificant. All statistical analyses are performed using the SAS statistical software (version 9.4, Cary, NC, USA) and R (version 3.6.1 The R Foundation, Vienna, Austria). For all analysis, a two-tailed P-value below 0.05 was considered statistically significant.
Sensitivity analyses
Four supplementary analyses were conducted. First, two consecutive matches on sex, age, and HF were conducted in a 1:1 ratio: (i) patients with first-time treatment with amiodarone (i.e. the primary cohort) matched with controls without prior thyroid diagnosis or medication and (ii) the primary cohort was matched with controls with a history of cardiac arrhythmias and without prior thyroid diagnosis or medication. Second, we repeated the 1-year outcome analyses restricting to patients with HF, stratified by HF severity as defined by daily dose of furosemide (i) furosemide (daily dose <40 mg), (ii) furosemide (daily dose 40–80 mg), (iii) furosemide (daily dose 81–160 mg), and (iv) furosemide (daily dose >160 mg). Third, we repeated the landmark analysis cohort restricted to patients who did not receive any further amiodarone treatment after the first year of follow-up. Fourth, in order to test whether short-term high-dose amiodarone was associated with higher risk of thyroid disorders, we repeated the landmark analysis with start at 3, 6, and 9 months, respectively, rather than 1 year after start of amiodarone treatment.
Ethics
In Denmark, register-based studies that are conducted for the sole purpose scientific research do not require ethical approval or informed consent by law. However, the study is approved by the data responsible institute (Capital Region of Denmark—approval number: P-2019--191) in accordance with the General Data Protection Regulation.
Results
Baseline characteristics
From 1 January 2000 to 31 December 2018, 43 724 patients with first-time treatment with amiodarone were identified, with a median age of 71 years (25–75th percentile: 63–77), 69% men, and 16 939 (38%) with a history of HF. Compared with patients without HF, patients with a history of HF were of similar age (71 vs. 70 years), more often men (74 vs. 65%), and more likely to have a history of ventricular tachycardia (19 vs. 6.0%), ischaemic heart disease (60 vs. 43%), aborted cardiac arrest (8.0 vs. 3.0%), all P-values <0.001. There was little difference in the history of atrial fibrillation which was very common in both groups (72 vs. 73%). Cardiovascular pharmacotherapy and non-cardiac comorbidities were also more frequent in patients with HF than in those without HF (Table 1). In the landmark analysis, 36 697 patients were included, with a median age of 70 years (25–75th percentile: 62, 76), 69% men, of whom 15 282 (42%) had HF. The baseline characteristics for patients included in the landmark analysis are listed in Supplementary material online, Table S4.
Table 1.
Baseline characteristics of the study population
| Total | With HF | Without HF | P-value | |
|---|---|---|---|---|
| (n = 43 724) | (n = 16 939, 38%) | (n = 26 785, 62%) | ||
| Sex (men) | 29 946 (69.0) | 12 443 (74.0) | 17 503 (65.0) | <0.0001 |
| Age, median 25–75th percentile, years | 71 (63–77) | 71 (63–78) | 70 (62–77) | NA |
| Hospital admission for any reason <2 weeks | 29 627 (67.8) | 11 932 (70.4) | 17 695 (66.1) | <0.0001 |
| Calendar period | ||||
| ȃ2000–05 | 11 616 (26.6) | 4575 (27.0) | 7041 (26.3) | NA |
| ȃ2006–11 | 14 000 (32.0) | 5141 (30.35) | 8859 (33.1) | NA |
| ȃ2012–18 | 18 108 (41.4) | 7223 (42.64) | 10 885 (40.6) | NA |
| Prior cardiac procedures | ||||
| ȃCoronary angiography | 20 935 (47.9) | 9694 (57.2) | 11 241 (42.0) | <0.0001 |
| ȃPCI | 5976 (13.7) | 3201 (18.9) | 2775 (10.4) | <0.0001 |
| ȃCABG | 7497 (17.1) | 2692 (15.9) | 4805 (17.9) | <0.0001 |
| ȃHeart valve surgery | 4185 (9.6) | 1405 (8.3) | 2780 (10.4) | <0.0001 |
| ȃRadiofrequency ablation | 3026 (6.9) | 825 (4.9) | 2201 (8.2) | <0.0001 |
| ȃICD | 3422 (7.8) | 2702 (16.0) | 720 (2.7) | <0.0001 |
| ȃCRT-P | 305 (0.7) | 288 (1.7) | 17 (0.1) | <0.0001 |
| ȃCRT-D | 1664 (3.8) | 687 (4.1) | 977 (3.6) | 0.032 |
| ȃICD or CRT-P/D | 5292 (12.1) | 1326 (7.8) | 1322 (4.9) | <0.0001 |
| ȃBrady pacemaker | 2648 (6.1) | 3587 (21.2) | 1705 (6.4) | <0.0001 |
| Medical history | ||||
| ȃAtrial fibrillation | 31 952 (73.1) | 12 273 (72.5) | 19 679 (73.5) | 0.024 |
| ȃUnspecified supraventricular tachycardia | 5458 (12.5) | 1741 (10.3) | 3717 (13.9) | <0.0001 |
| ȃVentricular tachycardia | 4806 (11.0) | 3198 (19.0) | 1608 (6.0) | <0.0001 |
| ȃAborted cardiac arrest | 2080 (4.5) | 1359 (8.0) | 721 (3.0) | <0.0001 |
| ȃUnspecified arrhythmias | 6518 (14.9) | 2965 (17.5) | 3553 (13.3) | <0.0001 |
| ȃIschaemic heart disease | 21 653 (49.5) | 10 102 (60.0) | 11 551 (43.0) | <0.0001 |
| ȃHypertension | 19 496 (42.1) | 7514 (42.0) | 11 982 (42.3) | 0.440 |
| ȃStroke | 5963 (13.6) | 2644 (16.0) | 3319 (12.4) | <0.0001 |
| ȃDiabetes | 7058 (16.1) | 3585 (22.0) | 3472 (13.0) | <0.0001 |
| ȃPeripheral artery disease | 3032 (6.9) | 1603 (9.5) | 1429 (5.3) | <0.0001 |
| ȃBleeding | 7317 (16.7) | 3186 (18.8) | 4131 (15.4) | <0.0001 |
| ȃCOPD | 6527 (14.9) | 3330 (19.7) | 3197 (11.9) | <0.0001 |
| ȃMalignancy | 6670 (15.3) | 2299 (13.6) | 4371 (16.3) | <0.0001 |
| ȃChronic renal disease | 3496 (8.0) | 2026 (12.0) | 1470 (5.5) | <0.0001 |
| ȃLiver disease | 954 (2.2) | 449 (2.7) | 505 (1.9) | <0.0001 |
| ȃPulmonary fibrosis | 154 (0.4) | 81 (0.5) | 73 (0.3) | 0.0005 |
| Concomitant pharmacotherapy | ||||
| ȃBeta-blockers | 26 859 (61.4) | 10 796 (63.7) | 16 063 (60.0) | <0.0001 |
| ȃRAS inhibitors | 20 949 (47.9) | 10 572 (62.4) | 10 377 (38.7) | <0.0001 |
| ȃMineralocorticoid receptor antagonists | 4805 (11.0) | 3925 (23.2) | 880 (3.3) | <0.0001 |
| ȃDigoxin | 8987 (20.6) | 4549 (26.9) | 4438 (16.6) | <0.0001 |
| ȃLoop diuretics | 14 804 (33.9) | 9353 (55.2) | 5451 (20.4) | <0.0001 |
| ȃStatins | 17 468 (40.0) | 7128 (42.1) | 10 340 (38.6) | <0.0001 |
| ȃThiazides | 7191 (16.4) | 2358 (13.9) | 4833 (18.0) | <0.0001 |
| ȃCalcium channel blockers | 11 174 (25.6) | 3551 (21.0) | 7623 (28.5) | <0.0001 |
| ȃOral anticoagulant therapy | 19 298 (44.1) | 7493 (44.2) | 11 805 (44.1) | 0.754 |
| ȃAspirin | 17 774 (40.7) | 7468 (44.1) | 10 306 (38.5) | <0.0001 |
CABG, coronary artery bypass graft surgery; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; HF, heart failure; ICD, implantable cardioverter defibrillator; PCI, percutaneous coronary intervention; RAS, renin–angiotensin system.
Primary outcome
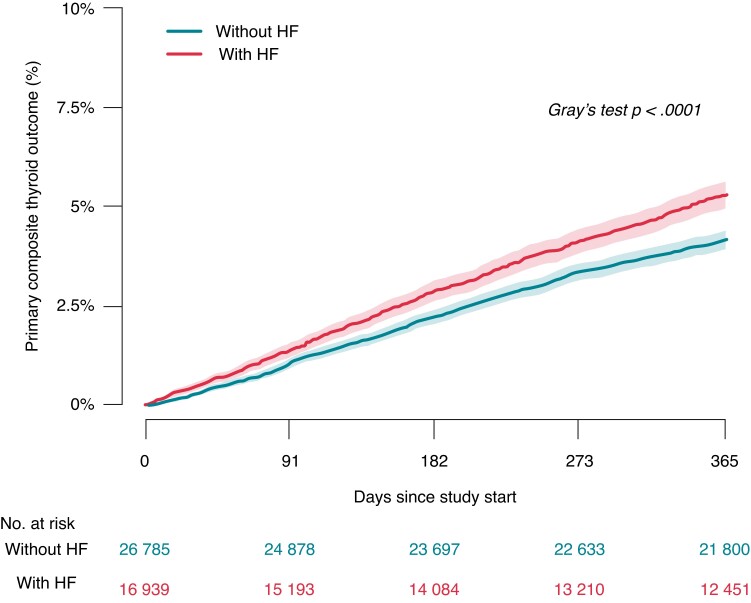
The crude cumulative 1-year incidence of the primary composite thyroid outcome was 5.3% in patients with a history of HF, and 4.2% in those without HF (Figure 2). Compared with patients without HF, adjusted analysis yielded an increased associated rate of the primary composite thyroid outcome in patients with a history of HF hazard ratio (HR) 1.37 [95% confidence interval (CI) 1.25–1.50]. Details on occurrence of hypo- and hyperthyroidism are listed in Table 2. The corresponding 1-year incidence of the primary composite thyroid outcome was 0.5% in controls from the general population, matched on age, sex, and history of HF (see Supplementary material online, Tables S5 and S6).
Figure 2.
Cumulative 1-year incidence of the composite primary thyroid outcome according to the presence of heart failure. HF, heart failure.
Table 2.
One-year clinical outcomes
| No. (crude cumulative 1-year incidence, %) | Adjusted modela | |||
|---|---|---|---|---|
| Total | With HF | Without HF | HR (95% CI), P-value | |
| (n = 43 724) | (n = 16 939) | (n = 26 785) | ||
| Primary outcome | ||||
| ȃComposite thyroid outcome | 1945 (4.5) | 868 (5.3) | 1077 (4.2) | 1.37 (1.25–1.50), <0.0001 |
| Secondary outcomes | ||||
| ȃComposite hypothyroidism | 969 (2.2) | 494 (3.0) | 475 (2.0) | 1.73 (1.52–2.00), <0.0001 |
| ȃComposite hyperthyroidism | 993 (2.3) | 384 (2.3) | 609 (2.3) | 1.10 (0.95–1.24), 0.21 |
| Any thyroid diagnosis | 1433 (3.3) | 623 (3.8) | 810 (3.1) | 1.33 (1.20–1.48), <0.0001 |
| ȃHypothyroidism | 689 (1.6) | 335 (2.0) | 354 (1.4) | 1.61 (1.38–1.87), <0.0001 |
| ȃHyperthyroidism | 696 (1.6) | 270 (1.6) | 426 (1.6) | 1.12 (0.95–1.31), 0.17 |
| ȃThyroiditis | 48 (0.1) | 18 (0.1) | 30 (0.1) | NA |
| Any thyroid treatment | 879 (2.0) | 408 (2.5) | 471 (1.8) | 1.42 (1.24–1.63), <0.0001 |
| ȃLevothyroxine | 404 (0.9) | 227 (1.3) | 177 (0.7) | 2.03 (1.66–2.49), <0.0001 |
| ȃPropylthiouracil | ≤3b | ≤3b | ≤3b | NA |
| ȃMethimazole | 470 (1.1) | 178 (1.1) | 292 (1.1) | 1.03 (0.85–1.25), 0.74 |
| ȃAll-cause death | 5432 (12.4) | 2931 (18.0) | 2501 (9.6) | 1.66 (1.57–1.76), <0.0001 |
| Exploratory outcomes | ||||
| ȃLiver disease | 166 (0.4) | 88 (0.5) | 78 (0.3) | NA |
| ȃPulmonary fibrosis | 83 (0.2) | 42 (0.2) | 41 (0.1) | NA |
This table shows the absolute numbers (No.), crude cumulative 1-year incidence, and adjusted HRs for the primary, secondary, and exploratory outcomes, individually and as a composite, according to HF.
CI, confidence interval; HF, heart failure; HR, hazard ratio.
Fully adjusted for sex, age, calendar period, hospital admission for any reason <2 weeks, ischaemic heart disease, peripheral artery disease, diabetes, malignancy, chronic obstructive pulmonary disease, chronic renal disease, stroke, and treatment with statins.
The exact number of patients is withheld to maintain confidentiality.
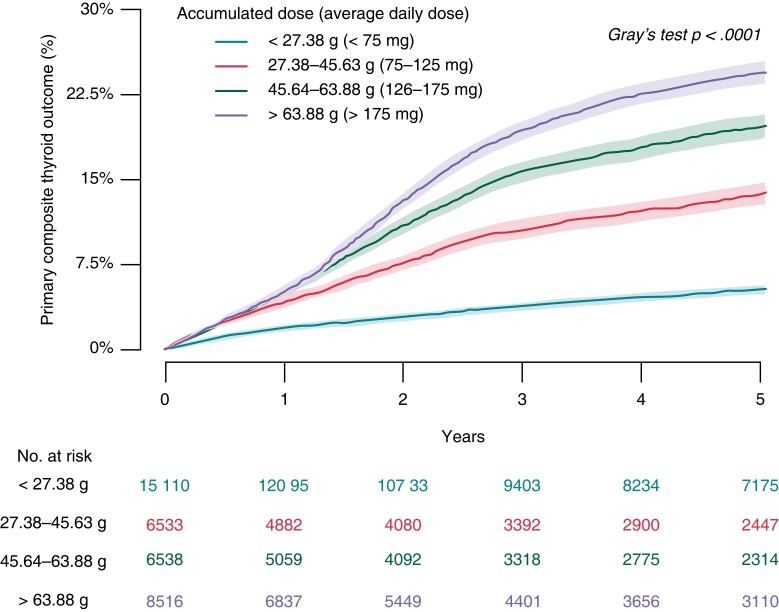
In the landmark analysis, the crude cumulative 5-year incidence of the primary composite thyroid outcome was 5.3, 14.0, 20.0, and 24.5% for patients with 1-year accumulated dose <27.38 g (ADD <75 mg), 27.38–45.63 g (ADD 75–125 mg), 45.64–63.88 g (ADD 126–175 mg), and >63.88 g (ADD >175 mg), respectively (Figure 3). Compared with 1-year accumulated dose <27.38 g (ADD <75 mg), the corresponding associated rates of the primary composite thyroid outcome were HR 2.74 (95% CI 2.46–3.05), HR 4.16 (95% CI 3.77–4.59), and HR 5.28 (95% CI 4.82–5.90), respectively (Table 3). The median accumulated dose of amiodarone among the 7367 patients with the primary composite thyroid outcome, at time of event, was 38.2 g (25–75th percentile: 5.8–123.6 g).
Figure 3.
Cumulative 5-year incidence of the primary composite thyroid outcome from the landmark analysis starting at 1-year follow-up.
Table 3.
Long-term outcomes from the landmark analysis starting at 1-year follow-up
| No. (crude cumulative 5-year incidence, %) adjusted HR (95% CI)a | ||||
|---|---|---|---|---|
| Accumulated 1-year dose (n = 36 697) | <27.38 g (ADD < 75 mg), n = 15 110 (42%) | 27.38–45.63 g (ADD 75–125 mg), n = 6533 (18%) | 45.64–63.88 g (ADD 126–175 mg), n = 6538 (18%) | >63.88 g ADD >175 mg), n = 8516 (23%) |
| Primary outcome | ||||
| ȃComposite thyroid outcome | 636 (5.3) 1.00 (Ref.) | 698 (14.0) 2.74 (2.46–3.05) P < 0.0001 | 1078 (20.0) 4.16 (3.77–4.59) P < 0.0001 | 1830 (24.5) 5.28 (4.82–5.90) P < 0.0001 |
| Secondary outcomes | ||||
| ȃComposite hypothyroidism | 309 (3.0) 1.00 (Ref.) | 300 (6.1) 2.30 (1.93–2.66) P < 0.0001 | 424 (8.0) 2.96 (2.55–3.43) P < 0.0001 | 664 (9.0) 3.40 (2.96–3.90) P < 0.0001 |
| ȃComposite hyperthyroidism | 338 (3.0) 1.00 (Ref.) | 416 (8.1) 3.10 (2.68–3.60) P < 0.0001 | 675 (12.3) 5.10 (4.44–5.80) P < 0.0001 | 1231 (16.4) 6.95 (6.15–7.87) P < 0.0001 |
| Any thyroid diagnosis | 398 (3.3) 1.00 (Ref.) | 470 (9.3) 2.90 (2.52–3.30) P < 0.0001 | 721 (13.2) 4.50 (4.00–5.04) P < 0.0001 | 1263 (17.0) 6.00 (5.23–6.58) P < 0.0001 |
| ȃHypothyroidism | 186 (1.6) 1.00 (Ref.) | 169 (3.5) 2.13 (1.73–2.63) P < 0.0001 | 265 (5.0) 3.11 (2.57–3.76) P < 0.0001 | 425 (5.8) 3.64 (3.10–4.34) P < 0.0001 |
| ȃHyperthyroidism | 205 (1.7) 1.00 (Ref.) | 275 (5.4) 3.30 (2.72–4.00) P < 0.0001 | 413 (7.5) 5.10 (4.31–6.10) P < 0.0001 | 788 (11.0) 7.40 (6.32–8.63) P < 0.0001 |
| ȃThyroiditis | 7 NA | 26 NA | 43 NA | 50 NA |
| Any thyroid treatment | 442 (3.7) 1.00 (Ref.) | 486 (9.9) 2.73 (2.40–3.11) P < 0.0001 | 775 (14.4) 4.17 (3.71–4.70) P < 0.0001 | 1332 (18.1) 5.30 (4.74–6.00) P < 0.0001 |
| ȃLevothyroxine | 189 (1.6) 1.00 (Ref.) | 193 (4.0) 2.31 (1.90–2.83) P < 0.0001 | 258 (4.9) 2.80 (2.31–3.40) P < 0.0001 | 374 (5.1) 3.00 (2.50–3.55) P < 0.0001 |
| ȃPropylthiouracil | ≤3b NA | 9 NA | 10 NA | 20 NA |
| ȃMethimazole | 250 (2.1) 1.00 (Ref.) | 284 (5.6) 3.00 (2.42–3.40) P < 0.0001 | 507 (9.4) 5.01 (4.30–5.84) P < 0.0001 | 938 (13.0) 6.86 (6.00–7.91) P < 0.0001 |
| All-cause death | 2487 (21.4) 1.00 (Ref.) | 1332 (27.2) 1.22 (1.14–1.31) P < 0.0001 | 1798 (34.0) 1.42 (1.33–1.51) P < 0.0001 | 2447 (33.1) 1.42 (1.34–1.50) P < 0.0001 |
| Exploratory outcomes | ||||
| ȃLiver disease | 130 (1.1) | 63 (1.3) | 68 (1.3) | 92 (1.2) |
| ȃPulmonary fibrosis | 45 (0.3) | 24 (0.5) | 35 (0.6) | 48 (0.6) |
ADD, average daily dose; CI, confidence interval; HR, hazard ratio; Ref., reference.
Fully adjusted for sex, age, ischaemic heart disease, peripheral artery disease, diabetes, malignancy, chronic obstructive pulmonary disease, chronic renal disease, stroke, and treatment with statins.
The exact number of patients is withheld to maintain confidentiality.
Secondary outcomes
The crude cumulative 1-year incidence of all-cause death was 18.0% among patients with a history of HF, and 10.0% among patients without HF (reference), adjusted HR 1.66 (95% CI 1.57–1.76; see Supplementary material online, Figure S1). The crude cumulative 1-year incidence of hypothyroidism was 3.0% in patients with a history of HF, and 2.0% in those without HF, and corresponding numbers for hyperthyroidism were 2.3 and 2.3%, respectively (Table 2).
In the landmark analysis, the crude cumulative 5-year incidence of all-cause death was 21.4, 27.2, 34.0, and 33.1% for patients with 1-year accumulated dose <27.38 g (ADD <75 mg), 27.38–45.63 g (ADD 75–125 mg), 45.64–63.88 g (ADD 126–175 mg), and >63.88 g (ADD >175 mg), respectively (see Supplementary material online, Figure S2). The crude cumulative 5-year incidences of hypothyroidism and hyperthyroidism according to accumulated dose of amiodarone are listed in Table 3. Each composite of the primary outcome is listed in Table 3.
Exploratory outcomes
The crude cumulative 1-year incidences of liver disease (0.5 vs. 0.3% in those with and without HF), and pulmonary fibrosis (0.2 vs. 0.1%) did not differ substantively according to HF (Table 2). In the landmark analysis, we found no relation between the accumulated amiodarone dose within the first year and risk of liver disease or pulmonary fibrosis (Table 3).
Sensitivity analyses
First, the crude cumulative 1-year incidence of the primary composite thyroid outcome was much lower in an age-, sex-, and HF-matched sample of the general population (0.5%), and among matched control with cardiac arrhythmias not treated with amiodarone (0.6%) (see Supplementary material online, Tables S5 and S6). Second, we restricted the analysis to only include HF patients and further stratified them by daily dose of furosemide (i) <40 mg, (ii) 40–80 mg, (iii) 81–160 mg, and (iv) >160 mg. The corresponding crude cumulative 1-year incidences of the composite thyroid outcome were 5.2, 5.1, 5.7, and 5.5%, and all-cause deaths were 13, 23, 25, and 33%, respectively (see Supplementary material online, Figures S3 and S4). Third, we performed a modified landmark analysis where patients who were treated with amiodarone beyond the first year were censored, and the crude cumulative 5-year incidence of the primary composite thyroid outcome was 4.6, 10.2, 13.4, and 16.3% (see Supplementary material online, Figure S5 and Table S7). Fourth, we performed landmark analyses starting at 3, 6, and 9 months according to accumulated doses and found that patients in the groups with the highest accumulated doses (ADD >175 mg) had 5-year incidence of thyroid dysfunction of 18, 21, and 23%, when compared with 24.5% in the primary landmark analysis (see Supplementary material online, Figures S6–S8).
Discussion
In this nationwide study, we found that ∼5% of patients who initiated treatment with amiodarone were diagnosed with or treated for thyroid dysfunction within 1 year of follow-up. A history of HF at time of amiodarone treatment was associated with a higher likelihood of prior ventricular arrhythmia, higher accumulated doses of amiodarone within the first year, and a significantly higher risk of thyroid dysfunction, as well as all-cause death. The long-term risk of thyroid dysfunction had a dose–response relationship to the accumulated dose of amiodarone within the first year of treatment.
Compared with matched controls from the general population, patients treated with amiodarone had ∼10× higher incidence of thyroid dysfunction and more comorbidity and a much higher risk of all-cause death.
When we further stratified patients with HF according to daily dose of furosemide at study start (as a proxy for HF severity), we found a clear relation to 1-year risk of death but not incidence of thyroid dysfunction.
The relation between amiodarone treatment and the 1-year incidence of thyroid events has previously been evaluated in the Randomized, Double-Blind Trial to Evaluate the Efficacy and Safety of Dronedarone versus Amiodarone (DIONYSOS), where 5.9% in the amiodarone group compared with 0.8% in the dronedarone group developed thyroid events.7 A meta-analysis of 13 randomized trials, involving 6553 patients with a mean follow-up of 1 year, showed a higher net absolute difference in the amiodarone group compared with a placebo group for hypothyroidism of 5.9%, 0.9% for hyperthyroidism, 1.1% for lung infiltrates, and 0.6% for liver toxicity.20 In a post hoc analysis of the Double-blind Sotalol Amiodarone Atrial Fibrillation Efficacy Trial (SAFE-Trial), 5% of the amiodarone-treated patients developed hypothyroidism compared with 0.3% of controls (treated with either sotalol or placebo), whereas 5.3% in the amiodarone group developed hyperthyroidism compared with 2.4% in the control group.21 In line with these studies, we found that ∼2.2% experienced hypothyroidism, 2.3% hyperthyroidism, 0.2% pulmonary fibrosis, and 0.4% liver toxicity, within the first year of amiodarone treatment. Notably, the risk of pulmonary fibrosis and liver disease did not seem to increase with higher accumulated doses of amiodarone, albeit with the caveat that these complications were rare.
Earlier studies report inconsistent results regarding the accumulated amiodarone dose needed to cause thyroid dysfunction in a substantial fraction of patients. Notably, a meta-analysis of four randomized trials involving 1465 patients on ‘low-dose’ amiodarone (ADD <400 mg) reported thyroid dysfunction in 3.7% in the amiodarone group vs. 0.4% in the placebo group during a mean follow-up of 1 year.22 A small, nested case–control study found that accumulated amiodarone dose >144 g was related to a higher risk of thyroid dysfunction, particularly hyperthyroidism.14 The median accumulated dose of amiodarone at the time of thyroid dysfunction in our study was 38 g, and the above-mentioned study is fully consistent with our finding of a clear dose–response relationship between accumulated dose of amiodarone within the first year and subsequent risk of thyroid dysfunction. Further, we evaluated whether shorter-duration high-dose amiodarone treatment was associated with even higher risk, but this was not evident.
In the landmark analysis that censored patients treated with amiodarone after the first year, the 5-year incidence of thyroid dysfunction was somewhat lower. For the group with the lowest accumulated dose <27.38 g (ADD <75 mg), 5-year incidence remained unchanged around 5%, thus indicating that short-term amiodarone treatment may be relatively low risk.
In the present study, there was a slightly higher 1-year incidence of the composite primary thyroid outcome in patients with HF compared with those without HF (5.3 vs. 4.2%). Patients with more severe HF (as defined by higher doses of loop diuretics) did not seem to have a higher incidence of thyroid dysfunction.
Limitations
The Danish administrative registries are validated and of high quality with high positive predictive values (>80%) for HF, cardiac arrhythmia, and other cardiovascular diseases (see Supplementary material online, Table S8).23–25 The accuracy of thyroid disorder diagnoses has not been validated in the Danish registries, although in a Canadian study, a diagnosis of hypothyroidism was shown to have a positive predictive value of 93%.26 Similarly, the diagnoses used for the exploratory outcomes of liver disease and pulmonary fibrosis have not been validated. We lacked information on important clinical parameters, both for HF severity such as ejection fraction, blood pressure, kidney function, and natriuretic peptides and for better characterization of the thyroid outcomes such as thyroid ultrasound and scintigraph reports, and laboratory measurements such as TSH, triiodothyronine (T3), free T3, thyroxine (T4), free T4, and thyroid antibodies were not available. Thus, a differentiation between amiodarone-induced hypothyroidism as well as amiodarone-induced thyrotoxicosis Types 1 and 2 was not possible. Detection of previously overlooked thyroid diseases due to frequent testing after initiation of amiodarone treatment cannot be fully ruled out, although thyroid testing prior to initiation of amiodarone treatment is recommended in Denmark.
Conclusions
In this nationwide study, we found that ∼5% of patients who initiated treatment with amiodarone had thyroid dysfunction at 1-year follow-up. In addition, a dose–response relationship was observed between the accumulated dose of amiodarone within the first year and the subsequent 5-year incidence of thyroid dysfunction. Patients with HF who initiated amiodarone received higher accumulated doses of amiodarone and displayed significantly higher associated rates of thyroid dysfunction, as well as all-cause death.
Supplementary Material
Contributor Information
Sam Aiyad Ali, Department of Cardiology, Rigshospitalet, University of Copenhagen, Blegdamsvej 9, Copenhagen 2100, Denmark.
Mads Ersbøll, Department of Cardiology, Rigshospitalet, University of Copenhagen, Blegdamsvej 9, Copenhagen 2100, Denmark.
Naja Emborg Vinding, Department of Cardiology, Rigshospitalet, University of Copenhagen, Blegdamsvej 9, Copenhagen 2100, Denmark.
Jawad Haider Butt, Department of Cardiology, Rigshospitalet, University of Copenhagen, Blegdamsvej 9, Copenhagen 2100, Denmark.
Rasmus Rørth, Department of Cardiology, Rigshospitalet, University of Copenhagen, Blegdamsvej 9, Copenhagen 2100, Denmark.
Christian Selmer, Department of Endocrinology, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark.
Lucas Malta Westergaard, Department of Cardiology, Rigshospitalet, University of Copenhagen, Blegdamsvej 9, Copenhagen 2100, Denmark.
Ulrik Madvig Mogensen, Department of Cardiology, Zealand University Hospital, Roskilde, Denmark.
Peter E Weeke, Department of Cardiology, Rigshospitalet, University of Copenhagen, Blegdamsvej 9, Copenhagen 2100, Denmark.
Christian Jøns, Department of Cardiology, Rigshospitalet, University of Copenhagen, Blegdamsvej 9, Copenhagen 2100, Denmark.
Finn Gustafsson, Department of Cardiology, Rigshospitalet, University of Copenhagen, Blegdamsvej 9, Copenhagen 2100, Denmark.
Emil Fosbøl, Department of Cardiology, Rigshospitalet, University of Copenhagen, Blegdamsvej 9, Copenhagen 2100, Denmark.
Lars Køber, Department of Cardiology, Rigshospitalet, University of Copenhagen, Blegdamsvej 9, Copenhagen 2100, Denmark.
Søren Lund Kristensen, Department of Cardiology, Rigshospitalet, University of Copenhagen, Blegdamsvej 9, Copenhagen 2100, Denmark.
Supplementary material
Supplementary material is available at Europace online.
Funding
The work was supported by an internal grant from the Department of Cardiology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark. The funding source had no role in the design, conduct, analysis, or reporting of the submitted work.
Data availability
The study was conducted using Statistics Denmark’s registers. We were authorized online access to the data sets, but we do not have authorization to share data sets.
References
- 1. Hindricks G, Potpara T, Dagres N, Bax JJ, Boriani G, Dan GAet al. . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Vassallo P, Trohman RG. Prescribing amiodarone. JAMA 2007;298:1312. [DOI] [PubMed] [Google Scholar]
- 3. Um KJ, McIntyre WF, Healey JS, Mendoza PA, Koziarz A, Amit Get al. . Pre- and post-treatment with amiodarone for elective electrical cardioversion of atrial fibrillation: a systematic review and meta-analysis. Europace 2019;21:856–63. [DOI] [PubMed] [Google Scholar]
- 4. Bartalena L, Bogazzi F, Chiovato L, Hubalewska-Dydejczyk A, Links TP, Vanderpump M. 2018 European Thyroid Association (ETA) Guidelines for the management of amiodarone-associated thyroid dysfunction. Eur Thyroid J 2018;7:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basaria S, Cooper DS. Amiodarone and the thyroid. Am J Med 2005;118:706–14. [DOI] [PubMed] [Google Scholar]
- 6. Trohman RG, Sharma PS, McAninch EA, Bianco AC. Amiodarone and thyroid physiology, pathophysiology, diagnosis and management. Trends Cardiovasc Med 2019;29:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. le Heuzey JY, de Ferari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol 2010;21:597–605. [DOI] [PubMed] [Google Scholar]
- 8. Yagishita A, Hachiya H, Kawabata M, Nakamura T, Sugiyama K, Tanaka Yet al. . Amiodarone-induced thyrotoxicosis late after amiodarone withdrawal. Circ J 2013;77:2898–903. [DOI] [PubMed] [Google Scholar]
- 9. Burgess C, Blaikie A, Ingham T, Robinson G, Narasimhan S. Monitoring the use of amiodarone: compliance with guidelines. Intern Med J 2006;36:289–93. [DOI] [PubMed] [Google Scholar]
- 10. Mihajlovic M, Mihajlovic A, Marinkovic M, Kovacevic V, Simic J, Mujovic Net al. . Main determinants of physician-driven amiodarone discontinuation in clinical practice. Europace 2021;23:euab116.051. [Google Scholar]
- 11. Trip MD, Wiersinga W, Plomp TA. Incidence, predictability, and pathogenesis of amiodarone-induced thyrotoxicosis and hypothyroidism. Am J Med 1991;91:507–11. [DOI] [PubMed] [Google Scholar]
- 12. Rose EP. Thyroid dysfunction during chronic amiodarone therapy. J Am Coll Cardiol 1987;9:175–83. [DOI] [PubMed] [Google Scholar]
- 13. Kinoshita S, Hayashi T, Wada K, Yamato M, Kuwahara T, Anzai Tet al. . Risk factors for amiodarone-induced thyroid dysfunction in Japan. J Arrhythm 2016;32:474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bouvy ML, Heerdink ER, Hoes AW, Leufkens HGM. Amiodarone-induced thyroid dysfunction associated with cumulative dose. Pharmacoepidemiol Drug Saf 2002;11:601–6. [DOI] [PubMed] [Google Scholar]
- 15. Pedersen CB. The Danish civil registration system. Scand J Public Health 2011;39:22–5. [DOI] [PubMed] [Google Scholar]
- 16. Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health 2011;39:30–3. [DOI] [PubMed] [Google Scholar]
- 17. Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health 2011;39:38–41. [DOI] [PubMed] [Google Scholar]
- 18. Brandt F, Thvilum M, Almind D, Christensen K, Green A, Hegedüs Let al. . Morbidity before and after the diagnosis of hyperthyroidism: a nationwide register-based study. PLoS One 2013;8:e66711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Statist. 1988;16:1141–54. [Google Scholar]
- 20. Connolly S, Cairns J, Gent M, Roberts R, Yusuf S. Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure. Lancet 1997;350:1417–24. [PubMed] [Google Scholar]
- 21. Batcher EL, Tang XC, Singh BN, Singh SN, Reda DJ, Hershman JM. Thyroid function abnormalities during amiodarone therapy for persistent atrial fibrillation. Am J Med 2007;120:880–5. [DOI] [PubMed] [Google Scholar]
- 22. Vorperian VR, Havighurst TC, Miller S, January CT. Adverse effects of low dose amiodarone: a meta-analysis. J Am Coll Cardiol 1997;30:791–8. [DOI] [PubMed] [Google Scholar]
- 23. Sundbøll J, Adelborg K, Munch T, Frøslev T, Sørensen HT, Bøtker HEet al. . Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open 2016;6:e012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kümler T, Gislason GH, Kirk V, Bay M, Nielsen OW, Køber Let al. . Accuracy of a heart failure diagnosis in administrative registers. Eur J Heart Fail 2008;10:658–60. [DOI] [PubMed] [Google Scholar]
- 25. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quan H, Li B, Duncan Saunders L, Parsons GA, Nilsson CI, Alibhai Aet al. . Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res 2008;43:1424–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study was conducted using Statistics Denmark’s registers. We were authorized online access to the data sets, but we do not have authorization to share data sets.