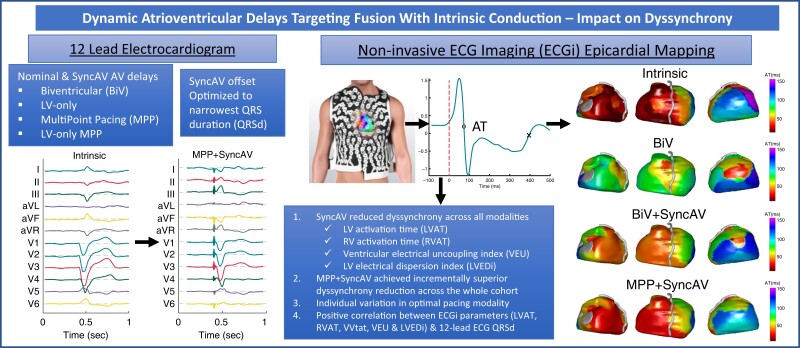
Abstract
Aims
Cardiac resynchronization therapy programmed to dynamically fuse pacing with intrinsic conduction using atrioventricular (AV) timing algorithms (e.g. SyncAV) has shown promise; however, mechanistic data are lacking. This study assessed the impact of SyncAV on electrical dyssynchrony across various pacing modalities using non-invasive epicardial electrocardiographic imaging (ECGi).
Methods and results
Twenty-five patients with left bundle-branch block (median QRS duration (QRSd) 162.7 ms) and intact AV conduction (PR interval 174.0 ms) were prospectively enrolled. ECGi was performed acutely during biventricular pacing with fixed nominal AV delays (BiV) and using SyncAV (optimized for the narrowest QRSd) during: BiV + SyncAV, LV-only single-site (LVSS + SyncAV), MultiPoint pacing (MPP + SyncAV), and LV-only MPP (LVMPP + SyncAV). Dyssynchrony was quantified via ECGi (LV activation time, LVAT; RV activation time, RVAT; LV electrical dispersion index, LVEDi; ventricular electrical uncoupling index, VEU; and biventricular total activation time, VVtat). Intrinsic conduction LVAT (124 ms) was significantly reduced by BiV pacing (109 ms) (P = 0.001) and further reduced by LVSS + SyncAV (103 ms), BiV + SyncAV (103 ms), LVMPP + SyncAV (95 ms), and MPP + SyncAV (90 ms). Intrinsic RVAT (93 ms), VVtat (130 ms), LVEDi (36 ms), VEU (50 ms), and QRSd (163 ms) were reduced by SyncAV across all pacing modes. More patients exhibited minimal LVAT, VVtat, LVEDi, and QRSd with MPP + SyncAV than any other modality.
Conclusion
Dynamic AV delay programming targeting fusion with intrinsic conduction significantly reduced dyssynchrony, as quantified by ECGi and QRSd for all evaluated pacing modes. MPP + SyncAV achieved the greatest synchrony overall but not for all patients, highlighting the value of pacing mode individualization during fusion optimization.
Keywords: CRT optimization, SyncAV, Electrocardiographic imaging, Atrioventricular delay, MultiPoint pacing, Fusion pacing
Graphical Abstract
Graphical Abstract.
Dynamic atrioventricular delays targeting fusion with intrinsic conduction reduce electrical dyssynchrony.
What’s new?
High resolution, multi-parametric, quantitative assessment of dyssynchrony using electrocardiographic imaging (ECGi) was used to evaluate the implementation of dynamic fusion optimization across the full range of available cardiac resynchronization therapy (CRT) pacing modes.
Superior resynchronization was demonstrated with dynamic atrioventricular delay (AVD) programming (SyncAV) individualized to patient-specific offset vs. fixed, nominal AVD programming across all evaluated pacing modalities [biventricular, left ventricular (LV)-only single site, MultiPoint pacing [MPP], and LV-only MPP].
Right ventricle activation time (RVAT) was significantly reduced using dynamic fusion optimization across all pacing modalities, highlighting this as a mechanism of efficacy.
MPP + SyncAV achieved the greatest resynchronization across the whole cohort but not in all patients, highlighting the benefit of individualizing CRT programming mode even in a highly selected cohort.
Introduction
The benefits of cardiac resynchronization therapy (CRT) for symptomatic heart failure with reduced ejection fraction (HFrEF) and conduction delay are well established, with reduced morbidity and mortality.1 Clinical response to CRT remains variable, with a substantial minority lacking therapeutic benefit. In well-selected patients, response is influenced by device-related factors, including left ventricular (LV) lead location and programming of atrioventricular (AV), inter-ventricular, and intra-ventricular delays. Advances in quadripolar leads and device algorithms have broadened programming options, improving patient response; however, selection of optimal settings remains challenging.
The dynamic algorithm, SyncAV2 (Abbott, Sylmar, CA), continually programmes the AV delay (AVD) shorter than the intrinsic PR interval by a customizable offset (either fixed or percent of PR interval) to synchronize the paced ventricular activation wavefronts to achieve fusion with intrinsic conduction. This has demonstrated improved electrical resynchronization, acutely post-implant,2,3 and at CRT optimization during follow-up.4 Combining SyncAV with MultiPoint pacing (MPP) has shown potential for further narrowing of the paced QRS duration (QRSd) when used in patients with intact AV conduction and left bundle-branch block (LBBB).2,5 Greater magnitude of QRSd narrowing likely represent greater reduction in electrical dyssynchrony and may be associated with improved outcomes for patients with LBBB receiving CRT.6
The manual fusion optimization interval (FOI) technique has similar aims, and its efficacy has been demonstrated with improved QRSd narrowing and LV reverse remodelling.7 The AdaptivCRT (Medtronic, Minneapolis, MN) algorithm was associated with reduced incidence of atrial fibrillation and improved survival8 from non-randomized registry data; it dynamically targets fusion optimization by predominantly pacing LV-only, however lacks customizability of the AVD.
Studies demonstrating improved electrical synchrony with dynamic fusion optimization predominantly utilize 12-lead electrocardiography (ECG) assessment of the QRSd.2,4,9 However, the 12-lead ECG lacks details of activation propagation and cardiac geometry obtained by high-resolution mapping required to further mechanistic understanding. This study aims to use non-invasive epicardial mapping (ECG imaging, ECGi) to systematically evaluate the impact of SyncAV on electrical dyssynchrony in patients with intact AV conduction and LBBB across the full range of available pacing modes, acutely post CRT implantation.
Methods
Study design
This prospective, single-centre study was undertaken at St. Bartholomew’s Hospital, London, United Kingdom. This was a sub-study of a multicentre, international study (NCT03567096). All patients provided written informed consent, and the study protocol was approved by the local research ethics committee (18/LO/0996). The study adhered to the Helsinki Declaration.
Study population
Enrolment required patients with guideline-directed indications for CRT implantation (de novo or upgrade of existing non-CRT device), New York Heart Association (NYHA) functional class II–IV symptoms, intrinsic QRSd ≥150 ms, sinus or atrial paced rhythm with intact AV conduction (resting PR interval ≤ 250 ms), permanent LBBB as per Strauss’ criteria,10 absence of atrial tachyarrhythmia at the time of enrolment, and stability on optimal medical therapy for at least 3 months. Patients were excluded if MPP was not programmable using non-adjacent cathodes with at least 30 mm anatomical separation.
Enrolled patients were implanted with a CRT-D (Abbott Quadra Assura or Gallant HF) or CRT-P (Abbott Quadra Allure) device and quadripolar LV lead (Abbott Quartet) positioned at the discretion of the implanting physician.
CRT pacing protocol and QRSd measurement
The pacing protocol included intrinsic conduction and the following pacing modes: biventricular pacing with the nominal AVD (BiV, static paced/sensed AVD of 140/110 ms), LV-only single-site pacing with SyncAV (LVSS + SyncAV), biventricular pacing with SyncAV (BiV + SyncAV), LV-only MPP with SyncAV (LVMPP + SyncAV), and biventricular MPP with SyncAV (MPP + SyncAV). The SyncAV offset was individualized for each patient (10–60% of the right atrium (RA)-right ventricle (RV) sensed interval, i.e. PR interval) to yield the shortest QRSd.
Continuous ECG recordings of at least 30 s per setting were performed using Bard Labsystem Pro EP recording system (Boston Scientific, Marlborough, MA), and QRSd measurements were performed manually by two blinded expert observers at a sweep speed of 200 mm/s, measuring from earliest onset of activation (deflection from isoelectric baseline) to the latest offset (return to isoelectric baseline) across all 12-ECG leads, excluding the pacing artefact. The mean QRSd was then derived from three consecutive QRS complexes.
LV pacing cathodes were selected for each pacing mode from the subset associated with pacing capture thresholds below 2.5 V (0.5 ms pulse width) without phrenic nerve stimulation. For BiV and LVSS, the latest activating LV cathode was selected, as measured by device-based intracardiac RVsensed-LVsensed conduction time using the programmer CRT toolkit. For MPP and LVMPP, LV1 and LV2 were selected as the cathodes with the widest anatomical spacing of at least 30 mm (e.g. D1 and P4, or D1 and M3). Following completion of the pacing protocol, the SyncAV offset yielding narrowest QRSd for each pacing mode was used for the non-invasive epicardial mapping procedure.
Non-invasive ECGi mapping
The ECGi mapping procedure was performed acutely, post CRT implantation. A 252-electrode vest (CardioInsight, Medtronic, MN) was used to record body surface potentials (1000 Hz sampling rate), as previously described.11 The vest remained in situ for the duration of the mapping procedure following a low dose non-contrast axial computed tomography (CT) scan with 3-mm slice thickness. Continuous recording was completed for the duration of the pacing protocol using the EcVue CardioInsight workstation.
Each programming mode was mapped for a minimum of three consecutive QRS complexes, selected by absence of ectopic beats and minimizing noise artefact. Epicardial unipolar electrograms were then computed over approximately 1500 epicardial points covering both ventricles, with those over the atrioventricular valves manually excluded.
ECGi data analysis
Raw data were imported into Matlab (The Mathworks, Inc, MA) files using a custom script and analysed as previously described.12 Following pacing artefact removal, signals were band-pass filtered between 0.5 and 80 Hz for activation time (AT) measurements. AT was then measured as the time of steepest signal downslope (dV/dtmin) during the QRS complex of the unipolar electrogram. All signals were carefully reviewed for outliers and semi-automatically corrected to avoid miss-annotation.
ECGi-derived parameters of electrical dyssynchrony were quantified by calculation of the total LV, RV, and biventricular activation times (LVAT, RVAT, VVtat, respectively). Inter-ventricular dyssynchrony was quantified by the ventricular uncoupling index (VEU), calculated as the difference between the mean LV and mean RV activation times. Intra-ventricular dyssynchrony was quantified by the LV electrical dispersion index (LVEDi), calculated as the standard deviation of LV activation times. High LVEDi and VEU values >50 ms during intrinsic conduction have been associated with response to CRT [LV ejection fraction, (LV EF) and LV end-systolic volume, increase] with high sensitivity and specificity.13–15
Biventricular activation maps and cines were reviewed by two expert reviewers blinded to programming. Intra-patient comparisons were performed between intrinsic conduction and each pacing modality of interest to identify:
Latest activating LV segment(s), according to the American Heart Association (AHA) segmentation model.16
The anatomical relation of the LV segment of latest activation to the LV lead location, defined as concordant, adjacent, or remote (i.e. 2 or more segments from latest site).
Presence and location of lines of conduction discontinuity, high spatial gradient of activation (≥50 ms per 10 mm).
Statistical analysis
Categorical variables were expressed as number and percentage. QRSd and ECGi parameters, which were not normally distributed, were expressed as median (interquartile range, IQR). QRSd and ECGi metrics were reported as absolute (ms) and reduction relative to intrinsic conduction (% reduction). Differences in metrics across pacing modes were tested using Wilcoxon signed rank test. Correlations between QRSd and ECGi parameters were assessed by the Spearman rank correlation coefficient (ρ). Bonferroni correction for multiple comparisons was used for all tests, with P < 0.05/15 (0.0033) deemed statistically significant across 15 pair-wise comparisons (i.e. 6 pacing modes compared for QRSd and ECGi) and P < 0.05/10 (0.005) deemed statistically significant across 10 pair-wise comparisons.
Results
Baseline characteristics
Twenty-five subjects were prospectively enrolled and completed study investigations from January 2019 to September 2021, and baseline characteristics are displayed in Table 1. Subjects were 28% female, age 66 (57–74) years, 48% ischaemic cardiomyopathy, LV EF 29 (23–32) %, QRSd 163 (154–177) ms, and PR interval 174 (160–189) ms. Twenty-three patients (92%) received CRT-defibrillators (14 Abbott Quadra Assura, 9 Abbott Gallant), and two patients (8%) received CRT-pacemakers (Abbott Quadra Allure). The LV lead location was in a lateral vein in 15 (60%), posterolateral vein in 7 (28%), and anterolateral vein in 3 (12%) patients. Quartet quadripolar LV leads were implanted in all subjects (20 × 1458QL, 4 × 1456Q, 1 × 1457Q). The RV lead location was apical in 11 (44%) and septal in 14 (56%) patients, giving the following combinations of RV lead + LV lead locations: septal + lateral in 11/25 (44%), septal + posterolateral in 1/25 (4%), septal + anterolateral in 2/25 (8%), apical + lateral in 4/25 (16%), apical + posterolateral in 6/25 (24%), and apical + anterolateral in 1/25 (4%). Four additional subjects were excluded due to inability to programme MPP with adequate separation (30 mm or more) of electrodes.
Table 1.
Patient characteristics at enrolment
| Characteristic | All patients (n = 25) |
|---|---|
| Age, years | 66 (57–74) |
| Male, n (%) | 18 (72) |
| Body surface area, m2 | 1.88 (1.67–2.11) |
| Ischaemic cardiomyopathy, n (%) | 11 (44) |
| Chronic kidney disease | 0 (0) |
| Diabetes mellitus | 9 (36) |
| NYHA functional class at enrolment, n (%) | |
| ȃClass II | 18 (72) |
| ȃClass III | 7 (28) |
| QRS duration, ms | 163 (154–177) |
| QRS morphology, LBBB, n (%) | 25 (100) |
| PR interval, ms | 174 (161–189) |
| Heart Failure Questionnaire score | 31 (17–55) |
| Echocardiographic indices | |
| ȃLV EF, % | 29 (23–32) |
| ȃLVESV, mL | 120 (101–154) |
| ȃLVEDV, mL | 175 (144–207) |
| Pharmacotherapy, n (%) | |
| ȃBeta-blocker | 25 (100) |
| ȃACEi or ARB | 18 (72) |
| ȃAldosterone antagonist | 19 (76) |
| ȃSacubitril/valsartan | 7 (28) |
Continuous metrics are displayed as median (interquartile range), categorical variables as number (%).
NYHA, New York Heart Association; LV EF, left ventricle ejection fraction; LVESV, left ventricle end systolic volume; LVEDV, left ventricle end diastolic volume.
Intracardiac conduction intervals and syncAV offsets
Device measured intracardiac conduction times were recorded in all subjects, with RVsensed-LVsensed, RVpaced-LVsensed, LVpaced-RVsensed and Q-LV times of 131 (118–151) ms, 170 (147–182) ms, 143 (124–175) ms, and 137 (119–151) ms, respectively. Following individualization of SyncAV offset for each subject across programming modes, the following offsets were used: LVSS + SyncAV 30 (20–60) ms, BiV + SyncAV 20 (10–30) ms, LVMPP + SyncAV 40 (20–60) ms, MPP + SyncAV 20 (20–40) ms.
Intrinsic conduction mapping
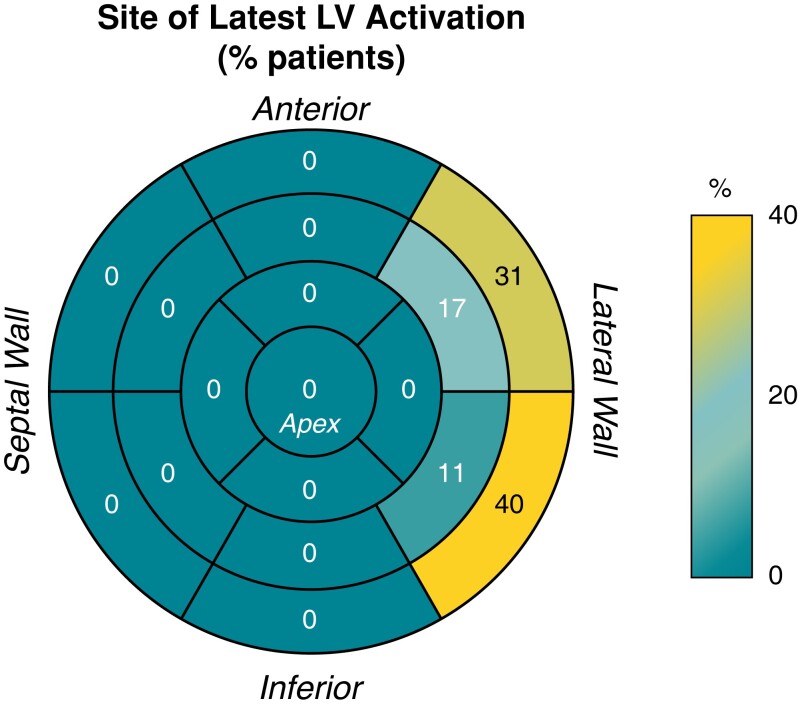
During intrinsic conduction, the latest activating LV segment was basally located in all patients, extending to mid segments in 8 patients (32%) and was in the lateral LV in all: anterolateral in 15 (60%), infero-lateral in 17 (68%) and including both antero/inferolateral in 7 (28%). These data are displayed in Figure 1. The LV lead location was concordant with the latest activating segment in 15 (60%), adjacent in 9 (36%), and remote in 1 (4%).
Figure 1.
Distribution of latest segment of left ventricular (LV) activation during intrinsic conduction. Values represent % of patients with latest activation in each segment.
Abnormal activation propagation during intrinsic conduction was present in all patients, with at least one line of activation discontinuity, present from right-to-left within the basal-mid anterior LV segments in 24 (96%) and limited to the basal-mid inferior segments in 1 (4%). Figure 2 displays a typical example with intrinsic conduction in 3 views. The extent and distribution of lines of activation discontinuity was a full U-shape from basal to apical segments, extending to the inferior wall in 12 patients (48%) with the line approximately parallel to the cardiac long axis. The distribution was a partial U-shape extending to segments in the inferior wall in 11 patients (44%), and a single line limited to 1–2 segments in 2 patients (8%). The pattern of LV wavefront propagation was U-shaped, with a turning point in apical segments in 24 (96%) and in the mid LV segments in 1 (4%).
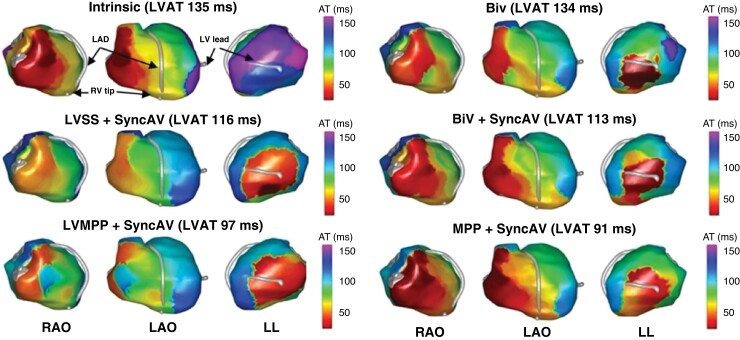
Figure 2.
Electrocardiographic imaging (ECGi) epicardial maps of activation time in a representative case for each modality of interest. From left to right, images correspond to right anterior oblique (RAO), left anterior oblique (LAO), and left lateral (LL) views. Grey geometries correspond to left anterior descending artery (LAD), right ventricular lead tip (RV tip), and left ventricular lead (LV lead); LVAT, left ventricular activation time.
Evaluation of ECG and ECGi metrics
QRS duration
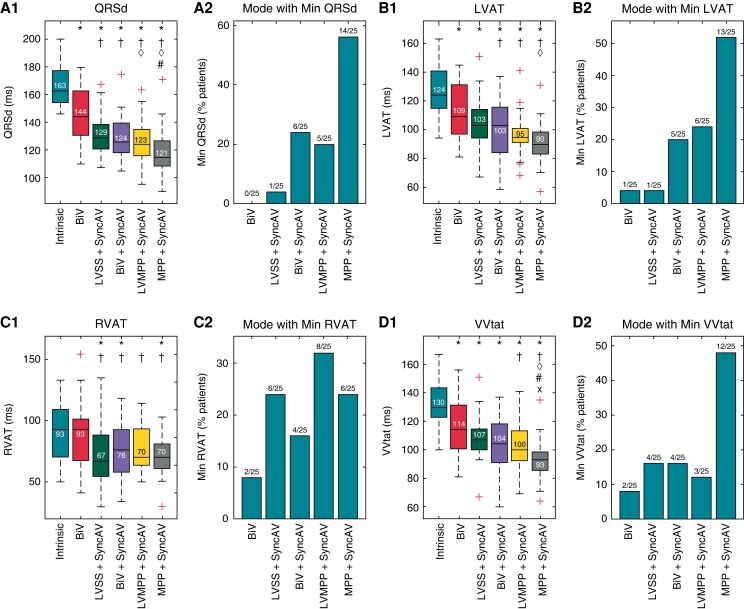
12-lead ECG QRSd measurements were completed in all subjects and across all pacing modalities. As shown in Figure 3A, the QRSd during intrinsic conduction of 163 (154–177) ms was reduced by all CRT pacing modalities (P < 0.001 for all). Relative to intrinsic conduction, QRSd was reduced with BiV by 10.4% to 144 (130–163) ms, with LVSS + SyncAV by 19.4% to 129 (121–139) ms, with BiV + SyncAV by 22.6% to 124 (118–139) ms, with LVMPP + SyncAV by 22.3% to 123 (116–135) ms, and with MPP + SyncAV by 27.2% to 121 (108–127) ms. MPP + SyncAV demonstrated significantly narrower QRSd than BiV, BiV + SyncAV, and LVSS + SyncAV (P < 0.002).
Figure 3.
Electrocardiographic (ECG) QRS duration and ECG imaging (ECGi) activation times for intrinsic conduction and each pacing mode. (A) QRS duration, (B) LVAT, (C) RVAT, and (D) VVtat panels show box plots of absolute values and bar graphs of proportion of patients in which each mode delivered the minimum value. Standard box plots show median (horizontal line), interquartile range (box), non-outlier range (dashed whiskers), and individual outlier values beyond 1.5 × (interquartile range) from each of the two quartiles (‘+’ symbol). BiV, biventricular pacing with nominal fixed atrioventricular delays; LVSS, left ventricular single site pacing, LVMPP, left ventricular MultiPoint Pacing (dual site), MPP, biventricular MultiPoint Pacing; QRSd, QRS duration; LVAT, left ventricular activation time; RVAT, right ventricular activation time; VVtat, total ventricular activation time. Statistical significance (P < 0.05/15 using Bonferroni correction) indicated relative to intrinsic conduction (*), BiV (†), LVSS + SyncAV (◊), BiV + SyncAV (#), and LVMPP + SyncAV (x).
The pacing modality resulting in the narrowest QRSd for the most patients was MPP + SyncAV in 14 patients (56%), followed by BiV + SyncAV in 6 patients (24%), LVMPP + SyncAV in 5 patients (20%), and LVSS + SyncAV in 1 patient (4%).
Ventricular activation time
ECGi mapping was completed in all subjects, for all pacing modalities of interest. Figure 3B displays the LVAT during intrinsic conduction of 124 (115–141) ms which was significantly reduced by all CRT pacing modalities (P < 0.002 for all). Relative to intrinsic conduction, LVAT was reduced with BiV by 12.3% to 109 (97–132) ms, with LVSS + SyncAV by 14.5% to 103 (94–114) ms, with BiV + SyncAV by 19.4% to 103 (84–116) ms, with LVMPP + SyncAV by 24.6% to 95 (91–101) ms, and with MPP + SyncAV by 27.4% to 90 (83–98) ms. The magnitude of LVAT reduction with MPP + SyncAV was significantly greater than BiV (P < 0.001) and LVSS + SyncAV (P < 0.001). More patients exhibited the shortest LVAT with MPP + SyncAV than any other pacing mode (13/25, 52%). In the 12 patients for whom MPP + SyncAV did not yield the shortest LVAT, 9/12 (75%) achieved within 10 ms of shortest LVAT and 3/12 (25%) within 20 ms of the shortest LVAT by MPP + SyncAV.
Therefore MPP + SyncAV achieved either shortest LVAT or within 10 ms (non-inferior) for 22/25 (80%). Comparing LVMPP + SyncAV vs. MPP + SyncAV, the magnitude of LVAT reduction was superior during LVMPP + SyncAV for 6 patients (24%) and within 10 ms in 14 patients (56%), i.e. non-inferior in 20 (80%).
Results for RVAT are displayed in Figure 3C. RVAT during intrinsic conduction was 93 (70–109) ms. This was significantly reduced with LVSS + SyncAV by 17.2% to 67 (55–88) ms (P < 0.001), with BiV + SyncAV by 19.0% to 76 (58–93) ms (P < 0.001), and with MPP + SyncAV by 19.5% to 70 (61–81) ms (P < 0.002). RVAT reduction vs. intrinsic conduction was non-significant with BiV by 6.2% to 93 (68–101) ms (P = 0.628) or with LVMPP + SyncAV by 16.5% to 70 (64–94) ms (P = 0.009).
Results for biventricular activation time (VVtat) are displayed in Figure 3D. The VVtat during intrinsic conduction of 130 (123–144) ms was significantly reduced by all pacing modes (P < 0.001 for all). VVtat was reduced with BiV by 15.3% to 114 (101–132) ms, with LVSS + SyncAV by 16.4% to 107 (100–115) ms, with BiV + SyncAV by 19.5% to 104 (91–118) ms, with LVMPP + SyncAV by 24.4% to 100 (92–113) ms, and with MPP + SyncAV by 30.6% to 92 (86–98) ms. Only MPP + SyncAV significantly reduced VVtat with respect to all other modalities (P < 0.003 for all) and resulted in the minimum VVtat in the greatest proportion of patients (48%, 12/25).
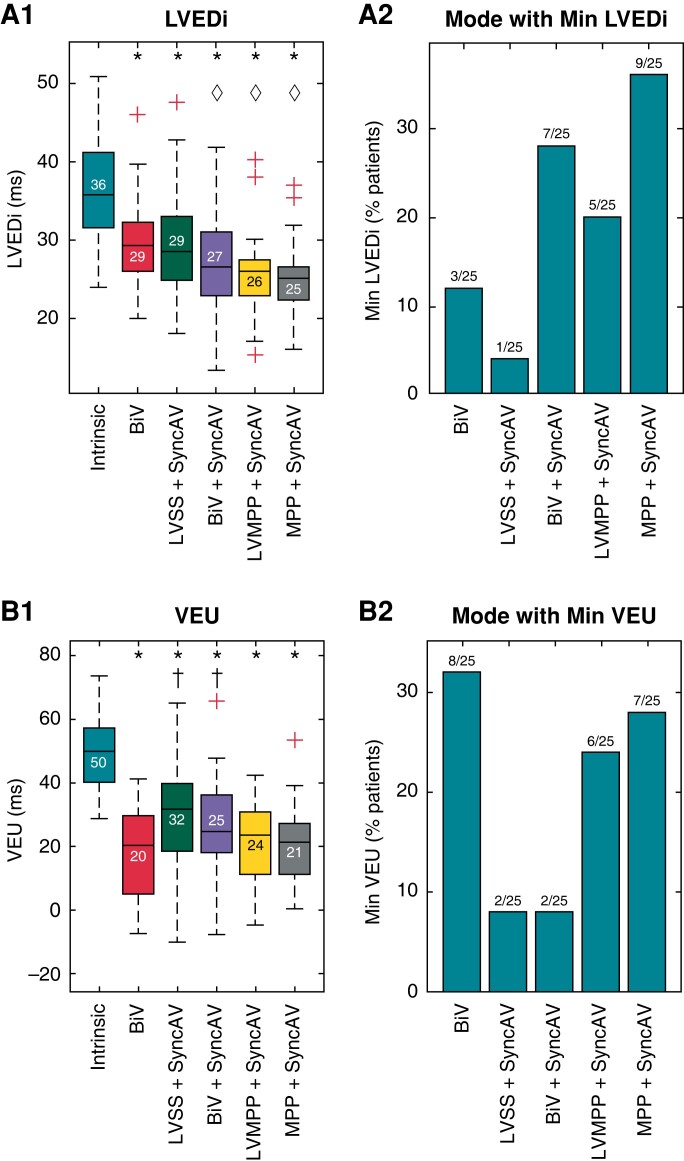
LV electrical dispersion index
Changes in LV electrical dispersion index (LVEDi) are displayed in Figure 4A. During intrinsic conduction, LVEDi was prolonged at 36 (32–41) ms and was significantly reduced by all pacing modes (P < 0.001 for all). LVEDi was reduced with BiV by 19.0% to 29 (26–32) ms, with LVSS + SyncAV by 16.9% to 29 (25–33) ms, with BiV + SyncAV by 20.7% to 27 (23–31) ms, with LVMPP + SyncAV by 31.3% to 26 (23–28) ms, and with MPP + SyncAV by 31.3% to 25 (22–27) ms.
Figure 4.
Electrocardiographic imaging (ECGi) metrics of dyssynchrony for intrinsic conduction and each pacing mode. (A) LVEDi and (B) VEU panels show box plots of absolute values and bar graphs of proportion of patients in which each mode delivered the minimum value. LVEDi, left ventricular dispersion index; VEU, ventricular electrical uncoupling index; all other plot characteristics, abbreviations, and symbols follow Figure 3.
Ventricular electrical uncoupling index
Ventricular electrical uncoupling index (VEU) during intrinsic conduction of 50 (40–57) ms was ≥ 50 ms in 13 patients (52%). Figure 4B demonstrates that VEU was significantly reduced by all pacing modalities (P < 0.001 for all). MPP + SyncAV (21 [(11–27) ms] produced the greatest reduction in VEU of 55.9%, followed by BiV [20 (5–30) ms] with 55.5%, LVMPP + SyncAV [24 (11–31) ms] with 50.6%, BiV + SyncAV [25 (18–36) ms] with 39.3%, and LVSS + SyncAV [32 (18–40) ms] with 34.8%.
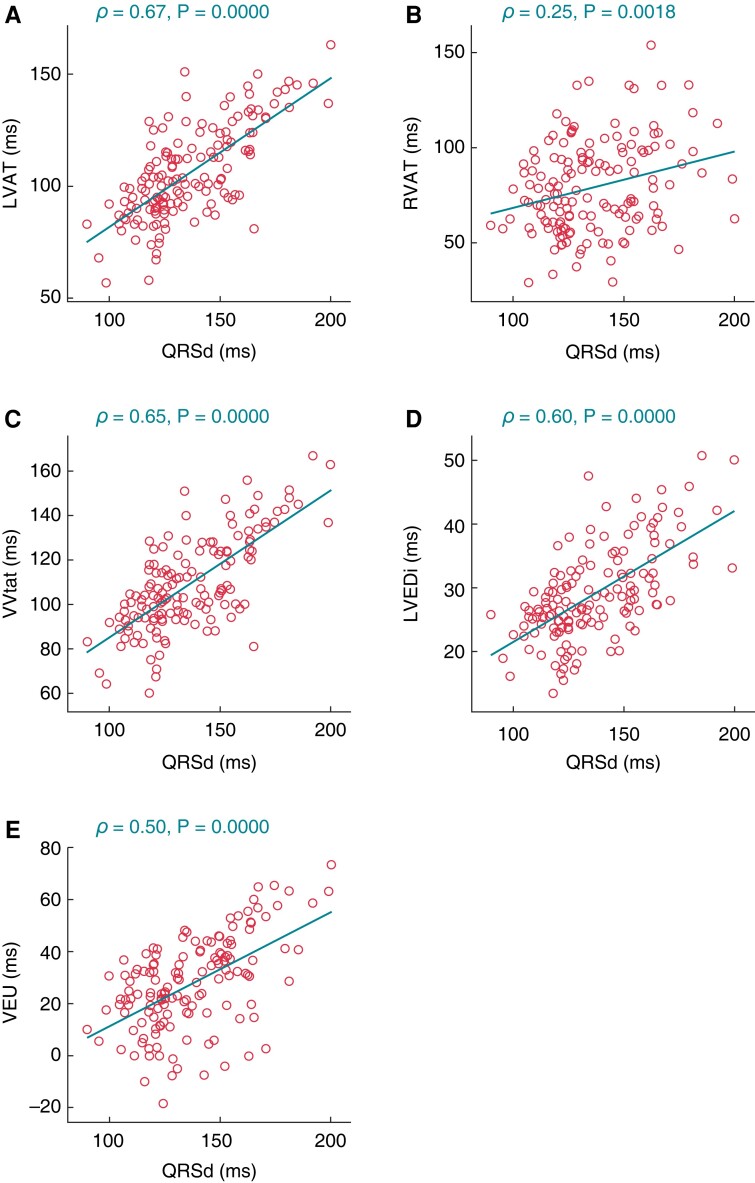
Correlations between ECGi dyssynchrony and QRSd
Assessment of the correlation between QRSd and indices of dyssynchrony derived from ECGi revealed a positive correlation for all metrics (LVAT ρ = 0.35; RVAT ρ = 0.25; VVtat ρ = 0.65; LVEDi ρ = 0.60; VEU ρ = 0.50; P < 0.002 for all), as shown in Figure 5. Assessment of the correlation between the delta percent change, relative to intrinsic conduction, of QRSd vs. for ECGi metrics revealed a significant positive correlation for LVAT ρ = 0.40, VVtat ρ = 0.38 and LVEDi ρ = 0.40 (P < 0.001 for all) but not RVAT (ρ = 0.02, P = 0.862) or VEU (ρ = 0.16, P = 0.080).
Figure 5.
Correlation between QRS duration (QRSd) and each electrocardiographic imaging (ECGi) dyssynchrony metric across all pacing modes: (A) LVAT, (B) RVAT, (C) VVtat, (D) LVEDi and (E) VEU. Acronyms follow those of Figures 3 and 4.
Analysis of activation propagation
Assessment of intra-patient change in activation propagation was completed in all patients by two expert reviewers. Figure 2 displays an example of intra-patient changes for each pacing mode. Compared to intrinsic conduction maps, the extent of lines of activation discontinuity (i.e. slow conduction) reduced in 10/25 patients (40%) with BiV, 18/25 patients (72%) with BiV + SyncAV (P = 0.045 vs. BiV), 15/25 patients (60%) with LVSS + SyncAV (P = 0.258 vs. BiV), 17/25 with LVMPP + SyncAV (P = 0.088 vs. BiV) and 17/25 with MPP + SyncAV (P = 0.088 vs. BiV). Compared to BiV, the extent of lines of slow conduction was further reduced in 18/25 patients (72%, P = 0.045) with BiV + SyncAV, 15/25 patients (60%, P = 0.278) with LVSS + SyncAV, 17/25 patients (68%, P = 0.088) with LVMPP + SyncAV, and 21/25 patients (84%, P = 0.003) by MPP + SyncAV.
Discussion
The objective of this study was to investigate the impact of dynamic fusion optimization during CRT across a range of pacing modalities, using ECG QRSd measurements and high precision non-invasive ECGi epicardial mapping. These aims were completed successfully, demonstrating the following key findings:
CRT programming using SyncAV to achieve dynamic fusion optimization with individualized patient-specific offset provides superior electrical resynchronization when compared to fixed, nominal AVD programming, effective across all evaluated pacing modalities.
While MPP + SyncAV provided the greatest resynchronization across the whole cohort, the greatest electrical response magnitude was not observed in all patients. This highlights the value of individualizing programming strategies even in this highly selected cohort with preserved AV conduction and LBBB. When targeting fusion with intrinsic conduction, LV-only pacing and LVMPP may provide additional benefit.
Dynamic fusion optimization with SyncAV demonstrated reduced RVAT vs. fixed, nominal AV delay programming. This may represent reduced RV pacing induced dyssynchrony achieved by fusion optimization with SyncAV.
A significant positive correlation was present between QRSd and ECGi-derived LVAT, VVtat and LVEDi. Thus, supporting that programming optimization targeting narrowest QRSd by 12-lead ECG is effective and pragmatic.
This study confirms that acute fusion optimization can be successfully and reliably achieved with the use of a device algorithm, the dynamic component of which may be more likely to maintain programming efficacy and durability. Investigating all available pacing modalities confirmed the benefits of individualization, and showed that LVMPP + SyncAV is effective, achieving greatest resynchronization by LVAT reduction in 20% of patients, and was non-inferior vs. MPP + SyncAV (LVAT within 10 ms) in 56% of patients.
ECGi mapping revealed that dynamic fusion optimization with SyncAV improved electrical resynchronization by a combination of shortened LV and RV activation time duration, reduced dispersion of LV activation (LVEDi) reflecting improved intraventricular conduction delay, and reduced VEU reflecting reduced inter-ventricular dyssynchrony. This illustrates that programming SyncAV at an individualized offset enables coordinated fusion of paced activation wavefronts with intrinsic conduction activation. It is noteworthy that the largest VEU reduction was most frequently seen with BiV and may be explained by the relatively short AV delay (paced 140/sensed 110 ms) during BiV, minimizing fusion and altering both LVAT and RVAT to a common level. However, LVAT, RVAT, and LVEDi reductions during BiV are modest, in keeping with small QRSd reductions, reflecting the lost potential for further synchrony with residual intraventricular dyssynchrony.
The improvements in electrical synchrony demonstrated by ECGi support previous studies evaluating SyncAV and MPP using 12-lead ECG QRSd.2,3,4 ECGi mapping enables differentiation between RV, LV, and biventricular activation times, as well as intra-LV electrical dispersion, facilitating assessment of intra and inter-ventricular dyssynchrony whilst displaying the relationship between activation and cardiac geometry.
Depending on the pacing modality, coordinated fusion may take the form of double (LVSS = intrinsic + LV), triple (BiV = intrinsic + RV + LV, or LVMPP = intrinsic + LV1 + LV2), or quadruple wavefront fusion (MPP = intrinsic + RV + LV1 + LV2). Quadruple ventricular wavefront fusion may most closely mimic ventricular activation seen during normal conduction which typically display 4–5 epicardial breakthrough sites occurring within 40–50 ms of QRS onset.17
Without wide separation of MPP cathodes, quadruple fusion may not occur due to a reduction in the relative area of stimulated epicardium resulting in LV paced wavefronts coalescing into a single wavefront. Lambiase et al.18 demonstrated with invasive non-contact mapping that the LV lead location in relationship to zones of distal slow conduction has a crucial effect on both acute haemodynamic response and shortening of LV activation time during LV pacing.
Programming MPP with wide electrode separation may be more likely to stimulate a region with intact myocardial depolarization improving local activation wavefront propagation, reducing latency from stimulation to wave-front propagation, improving LVEDi and shortening LVAT. Fusion optimization then maximizes the impact of MPP through optimal coordination of activation wavefronts including intrinsic conduction. Factors influencing this mechanism will include the LV lead position, local epicardial conduction velocity, as well as the presence and extent of anisotropic conduction within the epicardium.
Auricchio et al.19 described new lines of functional conduction block occurring during asynchronous pacing at the RV apex, anterior, and lateral LV. Fusion optimization may reduce the propensity for new lines of functional conduction block to emerge at sites with prolonged activation recovery intervals. The reduction in extent of lines of activation discontinuity with BiV + SyncAV vs. BiV in our data supports this mechanism.
It is noteworthy that during BiV, RVAT was not significantly reduced vs. intrinsic conduction and in several individuals RVAT increased with BiV. This is in keeping with previous studies demonstrating RV ‘desynchronization’ during BiV without fusion strategies.20,21 Additionally, SyncAV significantly reduced RVAT with respect to BiV across all pacing modes.
These findings may be explained by the mitigation of RV pacing induced dyssynchrony, a plausible mechanism of benefit for CRT utilizing fusion optimization to enhance electrical resynchronization. Iatrogenic electropathy is a fundamental factor in non-response to CRT and frequently overlooked in the RV.
Limitations
Due to the time-consuming nature of electrical mapping, the small sample size posed the main limitation of the study. This study was also single-centre, with the accompanying inherent limitations. A single manufacturer of CRT devices was used throughout the study, limiting the applicability of these findings to other CRT devices. However, these findings have relevance for fusion optimization programming generally, including overlap with the non-proprietary FOI methodology.7 The heterogeneity of the patient population, both in terms of cardiomyopathy and RV/LV lead placement, did not allow patient-specific conclusions to be made.
The pacing protocol was limited to simultaneous RV-LV pacing, potentially missing opportunities for further incremental resynchronization in some patients. However, RV-LV offset adjustment would have further increased the complexity and duration of an already comprehensive pacing protocol and was omitted for pragmatic reasons. This restriction standardized the intra-patient pacing mode comparison of AVD optimization targeting fusion, of relevance where altering RV-LV1 timing offset is unavailable (LVSS and LVMPP).
The MPP vectors were limited to the widest spacing of available cathodes with separation of 30 mm or greater. Although this could be considered a strength, patients lacking two LV cathodes with adequate anatomical separation were excluded. This acute study does not include long-term clinical response, which is part of an ongoing larger study (NCT03567096). Additionally, panoramic single beat epicardial ECGi lacks the interventricular septal geometry, omitting specific information regarding timing of septal activation. We are therefore unable to assess alteration of septal activation by programming strategies used in this study.
Furthermore, caution is needed when interpreting lines of activation discontinuity evident from ECGi, given the previously described discrepancies between invasive contact mapping and epicardial ECGi reported by Duchateau et al.22 However, the serial intra-patient activation map assessments performed in our study aimed to mitigate the impact of error during electrical dyssynchrony quantification. Finally, complementary hemodynamic studies are also warranted, as the optimal AV timing for electrical AV synchrony may not reflect the optimal timing for ventricular filling, as described by data from the BRAVO study.23
Conclusions
Programming CRT using SyncAV at an offset individualized to target narrowest QRSd was associated with reduced electrical dyssynchrony, quantified by ECGi epicardial mapping. Dynamic fusion optimization of the AVD was effective across all available pacing modalities including LV-only pacing. The addition of biventricular MPP to SyncAV incrementally improved resynchronization across the whole cohort; however, LVMPP + SyncAV was non-inferior in several cases. This study highlights the value of individualizing programming modality in addition to timing intervals targeting fusion optimization.
Contributor Information
Peter H Waddingham, Barts Heart Centre, St. Bartholomew’s Hospital, West Smithfield, London EC1A 7BE, United Kingdom; William Harvey Research Institute, Charterhouse Square, Queen Mary University of London, London EC1M 6BQ, UK.
Jan O Mangual, Abbott, Sylmar, CA, USA.
Michele Orini, Institute of Cardiovascular Science, University College London, London, UK.
Nima Badie, Abbott, Sylmar, CA, USA.
Amal Muthumala, Barts Heart Centre, St. Bartholomew’s Hospital, West Smithfield, London EC1A 7BE, United Kingdom.
Simon Sporton, Barts Heart Centre, St. Bartholomew’s Hospital, West Smithfield, London EC1A 7BE, United Kingdom.
Luke C McSpadden, Abbott, Sylmar, CA, USA.
Pier D Lambiase, Barts Heart Centre, St. Bartholomew’s Hospital, West Smithfield, London EC1A 7BE, United Kingdom; Institute of Cardiovascular Science, University College London, London, UK.
Anthony W C Chow, Barts Heart Centre, St. Bartholomew’s Hospital, West Smithfield, London EC1A 7BE, United Kingdom; William Harvey Research Institute, Charterhouse Square, Queen Mary University of London, London EC1M 6BQ, UK.
Funding
This study was funded by Abbott.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Cleland JG, Abraham WT, Linde C, Gold MR, Young JB, Claude Daubert J, et al. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J 2013;34:3547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Donnell D, Wisnoskey B, Badie N, Odgers L, Smart T, Ord M, et al. Electrical synchronization achieved by multipoint pacing combined with dynamic atrioventricular delay. J Interv Card Electrophysiol 2021;61:453–460. [DOI] [PubMed] [Google Scholar]
- 3. Varma N, O'Donnell D, Bassiouny M, Ritter P, Pappone C, Mangual J, et al. Programming cardiac resynchronization therapy for electrical synchrony: reaching beyond left bundle branch block and left ventricular activation delay. J Am Heart Assoc 2018;7:e007489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thibault B, Ritter P, Bode K, Calo L, Mondesert B, Mangual JO, et al. Dynamic programming of atrioventricular delay improves electrical synchrony in a multicenter cardiac resynchronization therapy study. Heart Rhythm 2019;16:1047–56. [DOI] [PubMed] [Google Scholar]
- 5. Waddingham PH, Mangual J, Orini M, Badie N, McSpadden L, Lambiase PD, et al. Noninvasive electrocardiographic imaging of dynamic atrioventricular delay programming in a patient with left bundle branch block. HeartRhythm Case Rep 2021;7:849–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jastrzebski M, Baranchuk A, Fijorek K, Kisiel R, Kukla P, Sondej T, et al. Cardiac resynchronization therapy-induced acute shortening of QRS duration predicts long-term mortality only in patients with left bundle branch block. Europace 2019;21:281–9. [DOI] [PubMed] [Google Scholar]
- 7. Trucco E, Tolosana JM, Arbelo E, Doltra A, Castel M, Benito E, et al. Improvement of reverse remodeling using electrocardiogram fusion-optimized intervals in cardiac resynchronization therapy: a randomized study. JACC Clin Electrophysiol 2018;4:181–9. [DOI] [PubMed] [Google Scholar]
- 8. Singh JP, Cha YM, Lunati M, Chung ES, Li S, Smeets P, et al. Real-world behavior of CRT pacing using the AdaptivCRT algorithm on patient outcomes: effect on mortality and atrial fibrillation incidence. J Cardiovasc Electrophysiol 2020;31:825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waddingham PH, Lambiase P, Muthumala A, Rowland E, Chow AW. Fusion pacing with biventricular, left ventricular-only and multipoint pacing in cardiac resynchronisation therapy: latest evidence and strategies for use. Arrhythm Electrophysiol Rev 2021;10:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol 2011;107:927–34. [DOI] [PubMed] [Google Scholar]
- 11. Ramanathan C, Ghanem RN, Jia P, Ryu K, Rudy Y. Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nat Med 2004;10:422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graham AJ, Orini M, Zacur E, Dhillon G, Daw H, Srinivasan NT, et al. Simultaneous comparison of electrocardiographic imaging and epicardial contact mapping in structural heart disease. Circ Arrhythm Electrophysiol 2019;12:e007120. [DOI] [PubMed] [Google Scholar]
- 13. Bear LR, Huntjens PR, Walton RD, Bernus O, Coronel R, Dubois R. Cardiac electrical dyssynchrony is accurately detected by noninvasive electrocardiographic imaging. Heart Rhythm 2018;15:1058–69. [DOI] [PubMed] [Google Scholar]
- 14. Ploux S, Lumens J, Whinnett Z, Montaudon M, Strom M, Ramanathan C, et al. Noninvasive electrocardiographic mapping to improve patient selection for cardiac resynchronization therapy: beyond QRS duration and left bundle branch block morphology. J Am Coll Cardiol 2013;61:2435–43. [DOI] [PubMed] [Google Scholar]
- 15. Ghosh S, Silva JN, Canham RM, Bowman TM, Zhang J, Rhee EK, et al. Electrophysiologic substrate and intraventricular left ventricular dyssynchrony in nonischemic heart failure patients undergoing cardiac resynchronization therapy. Heart Rhythm 2011;8:692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American heart association. Circulation 2002;105:539–42. [DOI] [PubMed] [Google Scholar]
- 17. Wyndham CR, Meeran MK, Smith T, Saxena A, Engelman RM, Levitsky S, et al. Epicardial activation of the intact human heart without conduction defect. Circulation 1979;59:161–8. [DOI] [PubMed] [Google Scholar]
- 18. Lambiase PD, Rinaldi A, Hauck J, Mobb M, Elliott D, Mohammad S, et al. Non-contact left ventricular endocardial mapping in cardiac resynchronisation therapy. Heart 2004;90:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Auricchio A, Fantoni C, Regoli F, Carbucicchio C, Goette A, Geller C, et al. Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation 2004;109:1133–9. [DOI] [PubMed] [Google Scholar]
- 20. Ploux S, Eschalier R, Whinnett ZI, Lumens J, Derval N, Sacher F, et al. Electrical dyssynchrony induced by biventricular pacing: implications for patient selection and therapy improvement. Heart Rhythm 2015;12:782–91. [DOI] [PubMed] [Google Scholar]
- 21. Eschalier R, Ploux S, Lumens J, Whinnett Z, Varma N, Meillet V, et al. Detailed analysis of ventricular activation sequences during right ventricular apical pacing and left bundle branch block and the potential implications for cardiac resynchronization therapy. Heart Rhythm 2015;12:137–43. [DOI] [PubMed] [Google Scholar]
- 22. Duchateau J, Sacher F, Pambrun T, Derval N, Chamorro-Servent J, Denis A, et al. Performance and limitations of noninvasive cardiac activation mapping. Heart Rhythm 2019;16:435–42. [DOI] [PubMed] [Google Scholar]
- 23. Jones S, Lumens J, Sohaib SMA, Finegold JA, Kanagaratnam P, Tanner M, et al. Cardiac resynchronization therapy: mechanisms of action and scope for further improvement in cardiac function. Europace 2017;19:1178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.