Background:
Multisystem inflammatory syndrome in children (MIS-C) is a multiorgan hyperinflammatory condition following SARS-CoV-2 infection. Data on COVID-19 vaccine adverse events and vaccine attitudes in children with prior MIS-C are limited. We described characteristics associated with COVID-19 vaccination, vaccine adverse events and vaccine attitudes in children with a history of MIS-C or COVID-19 and their parents/guardians.
Methods:
We enrolled children previously hospitalized for MIS-C or COVID-19 from 3 academic institutions. We abstracted charts and interviewed children and parents/guardians regarding vaccine adverse events and acceptability.
Results:
Of 163 vaccine-eligible children enrolled with a history of MIS-C and 70 with history of COVID-19, 51 (31%) and 34 (49%), respectively, received mRNA COVID-19 vaccine a median of 10 (Interquartile Range 6–13) months after hospital discharge. Among 20 children with MIS-C and parents/guardians who provided interviews, local injection site reaction of brief duration (mean 1.8 days) was most commonly reported; no children required medical care within 2 weeks postvaccination. Vaccine survey results of interviewed, vaccinated children and their parents/guardians: of 20 children with MIS-C and 15 children with COVID-19, 17 (85%) and 13 (87%), respectively, listed doctors in the top 3 most trusted sources for vaccine information; 13 (65%) and 9 (60%) discussed vaccination with their doctor.
Conclusions:
COVID-19 vaccination was well tolerated in children with prior MIS-C or COVID-19 participating in our investigation. Parents/guardians regarded their children’s doctors as a trusted source of information for COVID-19 vaccines, and most vaccinated children’s parents/guardians had discussed COVID-19 vaccination for their child with their doctor.
Keywords: MIS-C, COVID-19, vaccine, adverse events
Multisystem inflammatory syndrome in children (MIS-C) is a rare but serious sequela of SARS-CoV-2 infection with >9000 cases and 74 deaths reported to US national surveillance as of October 3, 2022.1 MIS-C is a systemic hyperinflammatory response typically occurring 2–6 weeks after SARS-CoV-2 infection.2–4 The pathogenesis of MIS-C is not fully understood; etiologic hypotheses include a dysregulated immune response to SARS-CoV-2 infection,5 underlying host genetic susceptibility,6 and persistent SARS-CoV-2 antigenemia leading to superantigenic response.7
Before the initiation of our investigation in September 2021, the Advisory Committee on Immunization Practices (ACIP) recommended use of Pfizer-BioNTech mRNA COVID-19 vaccine in persons ≥16 years old on December 12, 2020,8 children 12–15 years on May 12, 2021,9 and use of Moderna mRNA COVID-19 vaccine in persons ages ≥18 years on December 19, 2020.10 During our investigation, in May 2022, ACIP recommended Pfizer COVID-19 vaccine in children ages 5–11 years on November 2, 2021.11
The US safety monitoring of mRNA COVID-19 vaccines in children ages 5–17 years and persons ages ≥18 years has found that the majority of reported vaccine adverse events are mild and self-limited.12–15 COVID-19 vaccines are effective at preventing COVID-19-related hospitalizations and death in children and adolescents.16–18 Despite this, COVID-19 pediatric vaccination coverage remains low; 32% of children 5–11 years and 61% of children 12–17 years of age in the United States received their primary COVID-19 vaccine series as of October 12, 2022.19
Data regarding mRNA COVID-19 vaccine safety in children who have had MIS-C are scarce. Because of the known association of MIS-C with SARS-CoV-2 infection, the Pfizer-BioNTech and Moderna COVID-19 vaccine emergency use authorizations (EUAs) named multisystem inflammatory syndrome (MIS) an adverse event of special interest and mandated that vaccine providers report MIS occurring after receipt of vaccine.20 Combined surveillance data from national MIS-C surveillance and the Vaccine Adverse Event Reporting System (VAERS) found that MIS-C after COVID-19 vaccination in persons 12−20 years was rare, with 1 reported case per million vaccinated persons ages 12–20 years; most cases had evidence of SARS-CoV-2 infection.21 One case series of 15 patients with a history of MIS-C reported that mRNA COVID-19 vaccines were well tolerated.22 Interview of 29 children who received COVID-19 vaccine after MIS-C also found the vaccines were well tolerated with no severe reactions.23 At the time of this investigation Centers for Disease Control and Prevention (CDC) COVID-19 vaccine interim clinical considerations for initiating COVID-19 vaccination in children with a history of MIS-C noted that experts “consider the benefits of COVID-19 vaccination for children and adolescents with a history of MIS-C (ie, a reduced risk of severe disease including potential recurrence of MIS-C after reinfection) to outweigh a theoretical risk of an MIS-like illness or the risk of myocarditis following COVID-19 vaccination” in children who have achieved clinical recovery (including return to normal cardiac function), are at least 90 days from their MIS-C diagnosis, and reside in an area where the COVID-19 community transmission is high.24
Our objectives were to describe (1) demographic and clinical characteristics of a cohort of children ages <21 years who had MIS-C and subsequently initiated mRNA COVID-19 vaccination; (2) COVID-19 vaccine adverse events in children previously hospitalized for MIS-C or acute COVID-19; and (3) vaccine attitudes of children and parents/guardians of children with a history of MIS-C or acute COVID-19 who received vaccination compared with those who did not.
MATERIALS AND METHODS
CDC, in collaboration with 3 institutions, performed an investigation of COVID-19 vaccine side effects and vaccination attitudes in children who had been hospitalized for MIS-C or COVID-19 and their parents/guardians. This collaboration augmented a study that previously identified a cohort of children ages <21 years hospitalized for MIS-C or COVID-19 and performed in-depth phenotypic illness description.25 MIS-C was defined according to the CDC case definition26 and acute COVID-19 was defined as an illness requiring hospitalization and meeting the Council for State and Territorial Epidemiologists (CSTE) interim COVID-19 case definition.27 Starting in September 2021, children previously hospitalized for MIS-C or acute COVID-19 and previously enrolled in the initial phenotype investigation and their parents/guardians were approached regarding participation in this follow-up investigation. All who chose to participate in interviews were interviewed via phone using a standardized questionnaire regarding receipt of COVID-19 vaccination, adverse events following dose 1 and dose 2 and attitudes about COVID-19 vaccination. Interviews were conducted with parents/guardians of children ages <18 years and with the child and/or parent/guardian for ages 18–20 years. Interviewees who participated in the initial interview were approached for a follow-up interview to collect further clinical information, and capture persons not yet vaccinated at time of first interview. Follow-up interviews were completed a median of 5 (Interquartile Range [IQR]: 4–5) months after initial interviews and did not include vaccine attitude questions. Chart abstraction to collect COVID-19 vaccine information was performed for all children (interviewed and not interviewed) enrolled in the initial study who survived their MIS-C or acute COVID-19 hospitalization and who remained in the participating institution medical care system. Vaccination status and vaccine information was verified for all vaccine-eligible children using the state immunization information systems.
We determined if a child was COVID-19 vaccine-eligible at the time of interview and/or chart abstraction. Vaccine eligibility was defined according to ACIP’s age-based COVID-19 vaccine recommendations at the time of the interview for those interviewed or chart abstraction for those not interviewed. In accordance with timing of the ACIP recommendation for COVID-19 vaccination in children ages 5–11 years, vaccination eligibility at time of interview or chart abstraction was defined as: age ≥12 years if interviewed or abstracted before November 2, 2021, and age ≥5 years if interviewed or abstracted on or after November 2, 2021. For those with 2 interviews, date of the most recent interview was used to calculate eligibility. Interviews were completed by May 31, 2022.
We described demographic and clinical characteristics of vaccinated and unvaccinated vaccine-eligible children. We used Chi square, Fisher’s Exact and Kruskal–Wallis tests to evaluate characteristics associated with receipt of COVID-19 vaccination. We reviewed reported COVID-19 vaccine local and systemic reactions and health care utilization after dose 1 and 2 for all vaccinated children enrolled. We did not collect this information after dose 3 as at the time of our investigation Pfizer and Moderna boosters were recommended only for those with moderate to severe immunocompromise ages ≥12 and ≥18 years, respectively, and few children were eligible (Table 2, Supplemental Digital Content 1, http://links.lww.com/INF/E907). Vaccine attitudes were described for all interviewees, including vaccinated and unvaccinated vaccine-eligible and vaccine-ineligible. Responses to vaccine attitude questions were compared between vaccinated and unvaccinated vaccine-eligible interviewees by assigning numeric values to the Likert scale and performing a Wilcoxon signed-rank test. Exact P values were used. Reponses of “not sure” were not included in the Wilcoxon signed-rank test.
This study was approved by the sites’ institutional review boards and deemed by CDC to meet criteria for public health surveillance as defined in 45 CFR §46.102(I)(2). Informed consent for interviews was obtained from children ages ≥18 years or the parents/guardians of children ages <18 years. Assent was also obtained, as appropriate for each institution, from children ages 6–17 years.
RESULTS
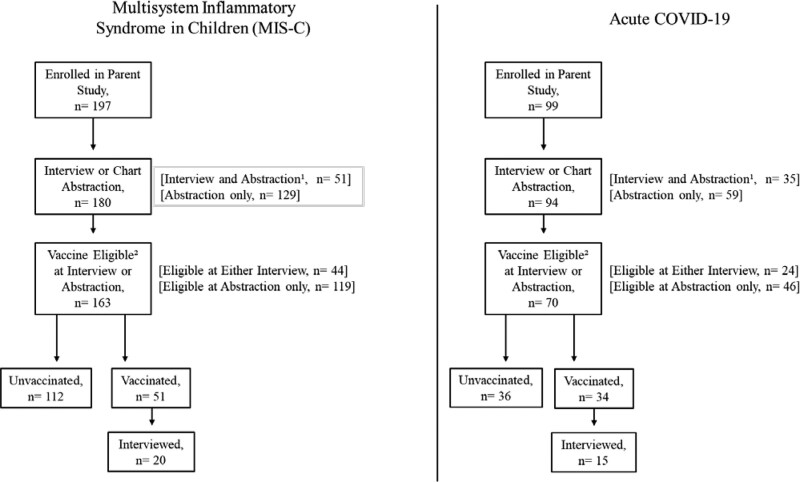
In total, 180 (91%) of the 197 children with MIS-C and 94 (95%) of the 99 children with acute COVID-19 enrolled in the initial phenotype study participated in this follow-up study (Fig. 1). Of these, 163 with prior MIS-C and 70 with prior acute COVID-19 were vaccine-eligible at the time of their most recent interview or chart abstraction; 51 (31%) of 163 and 34 (49%) of 70 had received COVID-19 vaccine.
FIGURE 1.
Investigation participants.
Characteristics of Vaccine-eligible Vaccinated and Unvaccinated Children With History of MIS-C
Among children with a history of MIS-C, 38 of 51 (75%) vaccinated children were ages ≥12 years compared with 41 of 112 (37%) unvaccinated children (P < 0.001) (Table 1). Thirty-eight (75%) vaccinated and 61 (54%) unvaccinated children were male (P = 0.02). Seventeen (33%) of vaccinated children were of Hispanic ethnicity compared with 18 (16%) of unvaccinated children (P = 0.03). A greater proportion of vaccinated children had an underlying medical condition compared with unvaccinated children (28 [55%] vs 40 [36%] P = 0.02). There were no statistically significant differences in severity of prior MIS-C illness (cardiac involvement, intensive care unit admission, receipt of life support, or length of stay) among those who did and did not receive COVID-19 vaccination. No significant differences were found comparing vaccinated and unvaccinated children with a history of COVID-19 except for age distribution (Table 1, Supplemental Digital Content 1, http://links.lww.com/INF/E907).
TABLE 1.
Characteristics and Vaccination Status of Children With a History of MIS-C Who Were COVID-19 Vaccine-eligible* at Time of Interview and/or Chart Abstraction, N = 163
| Vaccinated, n, (%) (n = 51) | Unvaccinated, n, (%) (n = 112) | P † | |
|---|---|---|---|
| Age group (y)‡ | |||
| 5–11 | 13 (25) | 71 (63) | <0.001 |
| 12–15 | 16 (31) | 24 (21) | 0.242 |
| 16–20 | 22 (43) | 17 (15) | <0.001 |
| Sex | |||
| Male | 38 (75) | 61 (54) | 0.015 |
| Race/ethnicity§ | |||
| Hispanic | 17 (33) | 18 (16) | 0.027 |
| Non-Hispanic Black | 17 (33) | 61 (54) | 0.079 |
| Non-Hispanic White | 13 (25) | 26 (23) | 0.432 |
| Non-Hispanic other race¶ | 2 (4) | 5 (4) | 0.488 |
| Location | |||
| Institution 1 | 46 (90) | 94 (84) | 0.287 |
| Institution 2 | 5 (10) | 13 (12) | 0.733 |
| Institution 3 | 0 (0) | 5 (4) | 0.326 |
| Underlying medical conditions | |||
| None | 23 (45) | 72 (64) | 0.021 |
| Obesity | 22 (43) | 31 (28) | 0.051 |
| Other‖ | 12 (24) | 16 (14) | 0.814 |
| Severity of previous MIS-C illness | |||
| Cardiac MIS-C involvement** | 24 (47) | 63 (56) | 0.275 |
| Intensive care unit admission | 29 (57) | 62 (55) | 0.858 |
| Life support†† | 23 (45) | 50 (45) | 0.957 |
| Median hospital length of stay, d (IQR) | 6 (4–8) | 6 (4–8) | 0.550 |
| Median time from MIS-C hospital discharge to initial interview for those interviewed, mo (IQR) | 11 (9–14) | 12 (10–14) | 0.858 |
Vaccine eligible: age ≥12 years if interview or chart abstraction completed before November 2, 2021, and age ≥5 years if interview or chart abstraction completed on or after November 2, 2021. For those interviewed, date of most recent interview was used to calculate eligibility for vaccination.
Chi square or Fisher’s Exact two-sided P value if cell <5 for categorical variables; Kruskal–Wallis for continuous variables.
Age calculated at time of most recent interview or chart abstraction.
Race was unknown for 2 individuals in the vaccinated group and 2 individuals in the unvaccinated group.
Other race = Asian, Native Hawaiian, multiple and other.
Conditions in the vaccinated group: chronic lung disease (n = 8; one also with sickle cell disease); Klippel-Feil syndrome (n = 1); congenital heart disease (n = 1), immunocompromising condition (n = 1), Trisomy 21 and obstructive sleep apnea (n = 1). Conditions in the unvaccinated group: chronic lung disease (n = 13; one also with vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies and limb abnormalities (VACTERL) syndrome and one also with Trisomy 21, congenital heart disease and obstructive sleep apnea), congenital heart disease (n = 1), Trisomy 21 (n = 1) and type 2 diabetes (n = 1).
Cardiac involvement: congestive heart failure, myocarditis, pericarditis noted in the chart or an echocardiogram finding (coronary aneurysm, coronary artery dilation or cardiac dysfunction).
Life support: receipt of vasopressors, intubation/mechanical ventilation or extracorporeal membranous oxygenation.
COVID-19 Vaccine Reactogenicity and Adverse Events Among Children With History of MIS-C or COVID-19 Hospitalization
Of the 51 children who received COVID-19 vaccine after MIS-C hospitalization, 6 (12%) received 1 dose, 39 (76%) received 2 doses and 6 (12%) received >2 doses (Table 2, Supplemental Digital Content 1, http://links.lww.com/INF/E907). Vaccine dose 1 was given a median of 10 (IQR 6–13) months after MIS-C hospital discharge; the earliest first doses were given to 3 children within 90 days of discharge (on days 9, 50 and 82) and the latest at 21 months.
Interview data were collected for 20 (39%) of the 51 vaccinated children with MIS-C, while the remaining 31 (61%) had vaccine data collected through chart abstraction only. The most reported reaction was injection site pain: 10 (50%) after dose 1 and 4 (21%) after dose 2 (Table 2). Five (25%) and 3 (16%) of children took fever-reducing or pain-relieving medicine for side effects after dose 1 and dose 2, respectively. Two (10%) children reported symptoms interfered with daily activities after dose 1 (for a mean of 1 day), and none after dose 2.
TABLE 2.
COVID-19 Vaccine Reactogenicity After Dose 1 and Dose 2 Reported During Parent/Guardian Interview for Children With History of Hospitalization for MIS-C or Acute COVID-19
| Multisystem Inflammatory Syndrome in Children | Acute COVID-19 | |||
|---|---|---|---|---|
| Dose 1, n (%) (n = 20)* | Dose 2, n (%) (n = 19)† | Dose 1, n (%) (n = 15)‡ | Dose 2, n (%) (n = 14)§ | |
| Pain at injection site | 10 (50) | 4 (21) | 8 (53) | 7 (50) |
| Redness at injection site | 1 (5) | 2 (11) | 1 (7) | 2 (14) |
| Swelling at injection site | 1 (5) | 1 (5) | 3 (20) | 2 (14) |
| Subjective or objective (≥38 °C) fever | 0 (0) | 1 (5) | 3 (20) | 2 (14) |
| Chills | 2 (10) | 1 (5) | 2 (13) | 1 (7) |
| Fatigue | 2 (10) | 2 (11) | 2 (13) | 4 (29) |
| Headache | 3 (15) | 2 (11) | 2 (13) | 2 (14) |
| Vomiting | 0 (0) | 0 (0) | 1 (7) | 0 (0) |
| Diarrhea | 0 (0) | 0 (0) | 1 (7) | 0 (0) |
| New or worsened muscle pain | 4 (20) | 2 (11) | 1 (7) | 1 (7) |
| New or worsened joint pain | 2 (10) | 2 (11) | 1 (7) | 1 (7) |
| Rash | 2 (10) | 1 (5) | 0 (0) | 0 (0) |
| Other symptom¶ | 0 (0) | 1 (5) | 1 (7) | 0 (0) |
| Used fever-reducing or pain medicine for symptoms/side effects | 5 (25) | 3 (16) | 7 (47) | 6 (43) |
| Symptoms/side effects interfered with normal daily activities‖ | 2 (10) | 0 (0) | 2 (13) | 1 (7) |
Symptoms after dose 1 had a mean duration of 1.8 (range 1–3) days.
Symptoms after dose 2 had a mean duration of 1.8 (range 1–3) days.
Symptoms after dose 1 had a mean duration of 2 (range 1–4) days.
Symptoms after dose 2 had a mean duration of 1.9 (range 1–5) days.
One individual with runny nose/nasal congestion and one with epistaxis; interviewees were also asked about chest pain or pressure, and none reported these symptoms.
Among children with MIS-C, symptoms interfered with activities of daily living for 1 day; among children with acute COVID-19, interference duration was 2–3 days.
Of the 34 children who received COVID-19 vaccine after hospitalization for acute COVID-19, 6 (18%) received 1 dose, 25 (74%) received 2 doses and 3 (9%) received >2 doses at the time of interview (Table 2, Supplemental Digital Content 1, http://links.lww.com/INF/E907). Vaccine dose 1 was given a median of 9 (IQR 8–12) months after acute COVID-19 hospital discharge. Interview data were collected for 15 (44%) of the 34 vaccinated children. The most reported reaction was injection site pain: 8 (53%) after dose 1 and 7 (50%) after dose 2 (Table 2). Symptoms lasted a mean duration of 2 (range 1–4) days after dose 1 and 1.9 (range 1–5) days after dose 2.
No vaccinated children with a history of MIS-C (n = 20 with interview) or acute COVID-19 (n = 15 with interview) reported seeking medical care during the 2 weeks after receiving either vaccine dose. No vaccine-ineligible children in the investigation received COVID-19 vaccine. Interviewees reported receipt of Pfizer or Moderna vaccines; none reported Janssen (Table 2, Supplemental Digital Content 1, http://links.lww.com/INF/E907).
COVID-19 Vaccine Attitudes Among Children/Guardians After MIS-C Hospitalization
Of the 51 children/guardians interviewed after MIS-C hospitalization, 36 were vaccine-eligible at the time of initial interview (20 vaccinated, 16 unvaccinated) and 15 were not vaccine-eligible (all unvaccinated) (Table 3).
TABLE 3.
COVID-19 Vaccine Interest and Vaccine Attitudes After MIS-C Hospitalization From Children and Their Parents/Guardians Who Completed Initial Interview, N = 51
| Vaccine-eligible at Time of Initial Interview | Not Vaccine-eligible at Time of Initial Interview* | |||
|---|---|---|---|---|
| Vaccinated, n (%) (n = 20) | Unvaccinated, n (%) (n = 16) | P† | Unvaccinated, n (%) (n = 15) | |
| Have you discussed with a doctor if they would recommend a COVID-19 vaccine for [you/your child]? | ||||
| Yes | 13 (65) | 6 (38) | 0.095 | 1 (7) |
| No | 6 (30) | 10 (63) | 14 (93) | |
| Has [your/your child’s] doctor(s) recommended that [you/your child] get a COVID-19 vaccine, if or when available for [your/their] age group? | ||||
| Yes, recommended to get it as soon as possible | 10 (50) | 4 (25) | 0.176 | 0 (0) |
| Yes, but recommended to wait to get it | 3 (15) | 2 (13) | 1.000 | 1 (7) |
| No | 5 (25) | 10 (63) | 0.041 | 12 (80) |
| Not sure | 2 (10) | 0 (0) | 0.492 | 2 (13) |
| If a COVID-19 vaccine were available to [you/your child], would [you/your child] get it? | ||||
| Yes, would get it as soon as possible | 1 (5)‡ | 2 (13) | 0.574 | 2 (13) |
| Yes, but would wait to get it | 0 (0) | 0 (0) | – | 2 (13) |
| No | 0 (0) | 5 (31) | 0.012 | 4 (27) |
| Not sure | 1 (5)‡ | 9 (56) | 0.002 | 7 (47) |
| If [you/your child] will NOT get the vaccine, please explain why not | ||||
| No trusted sources for vaccine information | NA | 1 (6) | – | 0 (0) |
| Not enough information on the vaccine side effects in children with underlying conditions | NA | 7 (44) | – | 1 (7) |
| Not answered/ does not wish to answer | NA | 5 (31) | – | 12 (80) |
| Worried about heart inflammation from vaccine | NA | 2 (13) | – | 1 (7) |
| Wants to see if MIS-C recurs first | NA | 1 (6) | – | 0 (0) |
| Do not believe in the vaccine | NA | 0 (0) | – | 1 (7) |
| How concerned are you about [you/your child] getting reinfected with COVID-19? | ||||
| Not at all | 3 (15) | 1 (6) | 0.894 | 2 (13) |
| A little | 4 (20) | 4 (25) | 2 (13) | |
| Moderately | 6 (30) | 6 (38) | 2 (13) | |
| Very | 7 (35) | 5 (31) | 9 (60) | |
| How safe do you think a COVID-19 vaccine will be for [you/your child]? | ||||
| Not at all | 0 (0) | 2 (13) | <0.001 | 2 (13) |
| A little | 0 (0) | 3 (19) | 4 (27) | |
| Moderately | 3 (15) | 0 (0) | 5 (33) | |
| Very | 15 (75) | 1 (6) | 3 (20) | |
| Not sure | 2 (10) | 10 (63) | 0.002 | 1 (7) |
| How safe do you think a COVID-19 vaccine will be for children who have NOT had MIS-C or severe COVID-19? | ||||
| Not at all | 0 (0) | 2 (13) | <0.001 | 2 (13) |
| A little | 0 (0) | 3 (19) | 4 (27) | |
| Moderately | 3 (15) | 3 (19) | 3 (20) | |
| Very | 15 (75) | 2 (13) | 4 (27) | |
| Not sure | 2 (10) | 6 (38) | 0.103 | 2 (13) |
| How much do you trust public health agencies’ recommendations about getting COVID-19 vaccines? | ||||
| Not at all | 0 (0) | 3 (19) | 0.002 | 3 (20) |
| A little | 1 (5) | 3 (19) | 2 (13) | |
| Moderately | 7 (35) | 6 (38) | 5 (33) | |
| Very much | 12 (60) | 2 (13) | 5 (33) | |
| Top 3 most trusted sources of information about COVID-19 vaccines | ||||
| Centers for Disease Control and Prevention | 13 (65) | 8 (50) | 0.500 | 9 (60) |
| Doctors | 17 (85) | 12 (75) | 0.678 | 9 (60) |
| Public health or clinical organizations§ | 7 (35) | 8 (50) | 0.493 | 5 (33) |
| Family and friends | 9 (45) | 4 (25) | 0.301 | 8 (53) |
| News sources (eg, television, internet and radio) | 7 (35) | 0 (0) | 0.011 | 5 (33) |
| Other health care providers¶ | 2 (10) | 3 (19) | 0.637 | 3 (20) |
| Social media‖ | 1 (5) | 0 (0) | 1.000 | 0 (0) |
| No trusted sources | 0 (0) | 2 (13) | 0.191 | 1 (7) |
Eleven children were interviewed before November 2, 2021, and were <12 years old, 4 children were interviewed after November 2, 2021, and were <5 years old.
We compared responses from vaccinated to unvaccinated children by assigning numeric values to the Likert response scale (excluding “not sure” responses) and performing a Wilcoxon signed-rank test; exact P values used.
Eighteen (90%) of vaccinated children were already vaccinated at time of initial interview so for them this question was not applicable. Two (10%) of vaccinated children were not yet vaccinated at the time of initial interview so an answer was provided for this question.
Food and Drug Administration, leaders at the National Institute of Health, World Health Organization, state or local health departments, hospital systems, professional organization, health insurers.
Nurses, pharmacists.
Facebook, Twitter, Instagram, LinkedIn, WhatsApp and TikTok.
Among vaccine-eligible children, a greater proportion of parents/guardians of vaccinated children reported having discussed COVID-19 vaccine recommendations with their child’s doctor compared with those of unvaccinated (65% vs. 38%; P = 0.10). Ten (50%) parents/guardians reported they had received a doctor’s recommendation to vaccinate as soon as possible after MIS-C, compared with 4 (25%) of unvaccinated children (P = 0.18). Both vaccine-eligible parent/guardian groups were moderately to very concerned about SARS-CoV-2 reinfection: 13 (65%) of vaccinated and 11 (69%) of unvaccinated (P = 0.92). Eighteen (90%) parents/guardians of vaccinated children were moderately to very sure the COVID-19 vaccine was safe for their child compared with 1 (6%) of unvaccinated children (P = 0.001). Ten (63%) parents/guardians of unvaccinated were unsure if the vaccine was safe for their child. Nineteen (95%) of parents/guardians of vaccinated children versus 8 (51%) of unvaccinated reported trusting public health agency vaccine recommendations moderately to very much (P = 0.007). Doctors were the group most frequently placed in the top 3 trusted sources of information [17 (85%) among vaccinated and 12 (75%) among unvaccinated parents/guardians; P = 0.68].
COVID-19 Vaccine Attitudes Among Children/Guardians After COVID-19 Hospitalization
Of the 35 interviewed children/guardians with a history of COVID-19, 22 were vaccine-eligible at time of initial interview (15 vaccinated, 7 unvaccinated) and 13 were not vaccine-eligible (all unvaccinated) (Table 4). Parents/guardians of 9 (60%) of 15 vaccinated children, and 4 (57%) of 7 unvaccinated children had discussed COVID-19 vaccine recommendations with their doctor after COVID-19 hospitalization (P = 1.00). Parents/guardians of seven (47%) vaccinated children and 1 (14%) unvaccinated child reported they had received a recommendation to vaccinate as soon as possible after COVID-19 (P = 0.19). Parents/guardians in both vaccine-eligible groups were moderately to very concerned about SARS-CoV-2 reinfection: 11 (73%) of vaccinated children and 5 (72%) of unvaccinated (P = 0.67). However, 5 (71%) of unvaccinated children’s parents/guardians said that their child would not receive COVID-19 vaccine if it were available to them. One (14%) parent/guardian of an unvaccinated child was moderately to very sure the vaccine was safe for their child compared with 14 (93%) of those with vaccinated children (P = 0.001). Only one (14%) parent/guardian of an unvaccinated child was unsure about vaccine safety for their child. All parents/guardians of vaccinated children reported trusting public health agencies’ vaccine recommendations moderately to very much compared with 2 (29%) of the unvaccinated (P = 0.002). Doctors were the group most frequently placed in the top 3 trusted sources of information: 13 (87%) for vaccinated and 5 (71%) for unvaccinated (P = 0.57).
TABLE 4.
COVID-19 Vaccine Interest and Vaccine Attitudes After COVID-19 Hospitalization From Children and Their Parents/Guardians Who Completed Initial Interview, N = 35
| Vaccine-eligible at Time of Initial Interview | Not Vaccine-eligible at Time of Initial Interview* | |||
|---|---|---|---|---|
| Vaccinated, n (%) (n = 15) | Unvaccinated, n (%) (n = 7) | P † | Unvaccinated, n (%) (n = 13) | |
| Have you discussed with a doctor if they would recommend a COVID-19 vaccine for [you/your child]? | ||||
| Yes | 9 (60) | 4 (57) | 1.000 | 0 (0) |
| No | 6 (40) | 3 (43) | 13 (100) | |
| Has [your/your child’s] doctor(s) recommended that [you/your child] get a COVID-19 vaccine, if or when available for [your/their] age group? | ||||
| Yes, recommended to get it as soon as possible | 7 (47) | 1 (14) | 0.193 | 0 (0) |
| Yes, but recommended to wait to get it | 0 (0) | 1 (14) | 0.318 | 0 (0) |
| No | 7 (47) | 5 (71) | 0.381 | 13 (100) |
| Not sure | 1 (7) | 0 (0) | 1.000 | 0 (0) |
| If a COVID-19 vaccine were available to [you/ your child], would [you/ your child] get it? | ||||
| Yes, would get it as soon as possible | 0 (0) | 0 (0) | – | 1 (8) |
| Yes, but would wait to get it | 0 (0) | 0 (0) | – | 3 (23) |
| No | 1‡ (7) | 5 (71) | 0.004 | 6 (46) |
| Not sure | 0 (0) | 2 (29) | 0.091 | 3 (23) |
| If [you/your child] will NOT get the vaccine, please explain why not | ||||
| Not enough information on the vaccine side effects in children with underlying conditions | NA | 2 (29) | – | 2 (15) |
| Not answered/ does not wish to answer | NA | 0 (0) | – | 2 (15) |
| Worried about heart inflammation from vaccine | NA | 0 (0) | – | 0 (0) |
| Worried about COVID-19 vaccine side effects | NA | 2 (29) | – | 1 (8) |
| Child does not want the COVID-19 vaccine | NA | 1 (14) | – | 0 (0) |
| How concerned are you about [you/your child] getting reinfected with COVID-19? | ||||
| Not at all | 1 (7) | 2 (29) | 0.329 | 2 (15) |
| A little | 3 (20) | 0 (0) | 3 (23) | |
| Moderately | 3 (20) | 3 (43) | 1 (8) | |
| Very | 8 (53) | 2 (29) | 7 (47) | |
| How safe do you think a COVID-19 vaccine will be for [you/your child]? | ||||
| Not at all | 0 (0) | 4 (57) | <0.001 | 6 (46) |
| A little | 0 (0) | 1 (14) | 0 (0) | |
| Moderately | 1 (7) | 0 (0) | 1 (8) | |
| Very | 13 (87) | 1 (14) | 3 (23) | |
| Not sure | 1 (7) | 1 (14) | 1.000 | 3 (23) |
| How safe do you think a COVID-19 vaccine will be for children who have NOT had MIS-C or severe COVID-19? | ||||
| Not at all | 0 (0) | 1 (14) | 0.080 | 3 (23) |
| A little | 0 (0) | 1 (14) | 1 (8) | |
| Moderately | 2 (13) | 0 (0) | 2 (15) | |
| Very | 12 (80) | 2 (29) | 4 (31) | |
| Not sure | 1 (7) | 3 (43) | 0.077 | 4 (31) |
| How much do you trust public health agencies’ recommendations about getting COVID-19 vaccines? | ||||
| Not at all | 0 (0) | 2 (29) | <0.001 | 5 (38) |
| A little | 0 (0) | 2 (29) | 3 (23) | |
| Moderately | 5 (33) | 2 (29) | 1 (8) | |
| Very much | 10 (67) | 0 (0) | 4 (31) | |
| Top 3 most trusted sources of information about COVID-19 vaccines | ||||
| Centers for Disease Control and Prevention | 10 (67) | 2 (29) | 0.172 | 9 (69) |
| Doctors | 13 (87) | 5 (71) | 0.565 | 11 (85) |
| Public health or clinical organizations§ | 4 (27) | 1 (14) | 1.000 | 9 (69) |
| Family and friends | 5 (33) | 4 (57) | 0.376 | 5 (38) |
| News sources (eg, television, internet and radio) | 3 (20) | 1 (14) | 1.000 | 1 (8) |
| Other health care providers¶ | 2 (13) | 1 (14) | 1.000 | 1 (8) |
| Social media‖ | 2 (13) | 0 (0) | 1.000 | 2 (15) |
| Other** | 2 (13) | 1 (14) | 1.000 | 0 (0) |
Average age at time of interview was 2.3 (range 1–6) years. Eleven children were interviewed before November 2, 2021, and were <12 years old, 2 children were interviewed after November 2, 2021, and were <5 years old.
Comparing responses from vaccinated to unvaccinated children by assigning numeric values to the Likert response scale (excluding “not sure” responses) and performing a Wilcoxon signed-rank test; exact P values used.
Fourteen (93%) of vaccinated children were already vaccinated at time of initial interview so for them this question was not applicable. One (7%) vaccinated child was not yet vaccinated at the time of initial interview so an answer was provided for this question.
Food and Drug Administration, leaders at the National Institute of Health, World Health Organization, state or local health departments, hospital systems, professional organization, health insurers.
Nurses, pharmacists.
Facebook, Twitter, Instagram, LinkedIn, WhatsApp and TikTok.
Union leaders, community-based resources, personal research.
DISCUSSION
We investigated characteristics associated with COVID-19 vaccination, vaccine adverse events and vaccine attitudes of children and their parents/guardians after hospitalization for MIS-C or COVID-19. Our cohort of children with history of MIS-C who subsequently initiated COVID-19 mRNA vaccination reported mild and brief vaccine reactogenicity events that did not require medical evaluation. Children who received vaccine after MIS-C tended to be older (≥12 years) and have an underlying medical condition (predominantly obesity). Regardless of vaccination status, children with history of MIS-C and their parents/guardians were concerned with SARS-CoV-2 reinfection and considered doctors a trusted source of information. However, the parents/guardians of two-thirds of vaccinated children with history of MIS-C had discussed vaccination with their doctors compared with only one-third of the unvaccinated. Attitudes toward COVID-19 vaccines were similar between parents/guardians of vaccinated children with a history of MIS-C and those with a history of COVID-19.
COVID-19 vaccination after MIS-C or COVID-19 hospitalization was well tolerated by our cohort of children. Local and systemic reactions in children who initiated mRNA COVID-19 vaccine after MIS-C were similar to those reported nationally among children without history of MIS-C. The US vaccine safety monitoring in children ages 5–11 and 12–17 years found that pain at the injection site was the most commonly reported reaction.12,13 During national safety monitoring from December 2020 to July 2021, 5%–7% of children ages 5–11 years and 10%–28% of adolescents ages 12–17 years providing v-safe28 responses reported reactions that interfered with daily activities, while 1% of children ages 5–11 years and 0.5%–0.8% of adolescents ages 12–17 years required medical attention for their symptoms.12,13 We did not find any significant differences in the frequency of reactions between persons in our cohort with a history of MIS-C versus those with COVID-19.
When evaluating characteristics associated with COVID-19 vaccination in our cohort, Vaccination coverage increased with age, likely a reflection of age-based vaccine availability and recommendations. Although MIS-C predominantly occurs in children without underlying conditions, we found that children with underlying conditions, primarily obesity, were overrepresented among the vaccinated group. This may reflect education efforts on the risk factors for developing severe COVID-19. However, it also highlights the need for better education regarding vaccination for MIS-C prevention. Most children with MIS-C do not have underlying medical conditions, so it is important to emphasize that previously healthy children should also receive COVID-19 vaccination to prevent both COVID-19 and MIS-C.16,29,30
Results of the vaccine attitude survey were similar for vaccinated children and their parents/guardians with a history of MIS-C and those with a history of COVID-19: both groups were concerned about SARS-CoV-2 reinfection, were more likely to have discussed COVID-19 vaccination with their doctor after their illness, and more likely to have received a vaccine recommendation from their doctor compared with their unvaccinated counterparts. Parents/guardians of both vaccinated and unvaccinated MIS-C and COVID-19 children listed doctors as one of their top 3 most trusted sources of information. However, parents/guardians of vaccine-eligible but unvaccinated children with a history of MIS-C or COVID-19 were less likely to have discussed the vaccine with their doctor or view the vaccine as safe. Interestingly, among parents/guardians of unvaccinated children with a history of MIS-C, 63% were unsure if the vaccine was safe and 13% felt it was not at all safe, whereas for the COVID-19 group, 14% were unsure and 57% responded that it was not at all safe. These findings highlight the pivotal role pediatricians and healthcare providers may play in COVID-19 vaccine acceptance and uptake, and the benefits of caregiver education regarding COVID-19 vaccination in children who have a history of MIS-C or COVID-19. Clinicians can use opportunities before hospital discharge and at follow-up visits to educate parents/guardians regarding COVID-19 vaccination.
Our investigation has several limitations. Our cohort is small, enrolled from 3 institutions, and may not be a representative of the general population. Parents/guardians who could not be contacted for interview or declined participation could be systematically different from those who did participate. Interviews were conducted months after discharge and are subject to recall bias. Because the study concluded data collection before EUA of COVID-19 vaccination in children ages <5 years, data about adverse effects were not available for this age group. Adverse event data were collected for only 2 weeks postvaccination, which does not exclude long-term outcomes. We did not collect data after vaccine dose 3.
COVID-19 vaccines were well tolerated with only mild and brief local and systemic reactions in our cohort of children vaccinated after MIS-C. Our findings, although limited by small sample size, support the current CDC interim clinical considerations expert opinion that the benefits of initiating COVID-19 vaccination in children with a history of MIS-C outweigh the potential risks as long as certain criteria are met.31 COVID-19 vaccines remain the most effective tool for preventing severe COVID-19 and MIS-C.30,32,33 Parents/guardians regard their doctors as a trusted source of COVID-19 vaccine information, highlighting the important opportunity providers have to give COVID-19 vaccine recommendations.
Supplementary Material
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
This investigation was supported by the Centers for Disease Control and Prevention (CDC). The CDC provided funds for the principal investigators and abstractors and laboratory sample collection and testing (contract #75D30120C09256).
F.R.L. received a grant from Lilly for work unrelated to this investigation. D.A.H. serves on the Board of Directors of BioVersys AG, Basel, Switzerland (work unrelated to this manuscript), and has received funding from BioAge Laboratories for research work unrelated to this manuscript. His institution has received NIH research funding to conduct Moderna COVID-19 vaccine clinical trials. C.A.R.’s institution has received funding to conduct clinical research unrelated to this manuscript from BioFire Inc., GSK, MedImmune, Micron, Merck, Novavax, PaxVax, Regeneron, Pfizer and Sanofi-Pasteur. She is a coinventor of patented respiratory syncytial virus (RSV) vaccine technology unrelated to this manuscript, which has been licensed to Meissa Vaccines, Inc. Her institution has received funding from NIH to conduct clinical trials of Moderna and Janssen COVID-19 vaccines. All other authors have no conflicts of interest or funding to disclose.
A.P.C. and E.D.B. contributed equally.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Amber Kunkel, Email: agkunkel@gmail.com.
Joseph Y. Abrams, Email: hus4@cdc.gov.
Ami B. Shah, Email: hiz4@cdc.gov.
Teresa A. Hammett, Email: tah5@cdc.gov.
Yajira L. Beltran, Email: yajira.beltran@orlandohealth.com.
Federico R. Laham, Email: flaham@gmail.com.
Carol M. Kao, Email: kaoc@wustl.edu.
David A. Hunstad, Email: dhunstad@wustl.edu.
Laila Hussaini, Email: lhussai@emory.edu.
Christina A. Rostad, Email: christina.rostad@emory.edu.
Shana Godfred-Cato, Email: nzt6@cdc.gov.
Angela P. Campbell, Email: app4@cdc.gov.
Ermias D. Belay, Email: ebb8@cdc.gov.
REFERENCES
- 1.CDC. Multisystem inflammatory syndrome in children cases in the U.S. Available at: https://www.cdc.gov/mis-c/cases/index.html. Accessed October 14, 2022.
- 2.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldstein LR, Tenforde MW, Friedman KG, et al. ; Overcoming COVID-19 Investigators. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payne AB, Gilani Z, Godfred-Cato S, et al. ; MIS-C Incidence Authorship Group. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw Open. 2021;4:e2116420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Consiglio CR, Cotugno N, Sardh F, et al. ; CACTUS Study Team. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:968–981.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou J, Platt CD, Habiballah S, et al. ; Taking on COVID-19 Together Study Investigators. Mechanisms underlying genetic susceptibility to multisystem inflammatory syndrome in children (MIS-C). J Allergy Clin Immunol. 2021;148:732–738.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yonker LM, Gilboa T, Ogata AF, et al. Multisystem inflammatory syndrome in children is driven by Zonulin-dependent loss of gut mucosal barrier. J Clin Invest. 2021;131:e149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliver S, Gargano J, Marin M, et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. Morb Mortal Wkly Rep. 2020;69:1922–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace MW, Woodworth K, Gargano JW, et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12–15 years—United States, May 2021. MMWR Morb Mortal Wkly Rep. 2021;70:749–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliver SG, Gargano J, Marin M, et al. The advisory committee on immunization practices’ interim recommendation for use of moderna COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1653–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodworth KM, Moulia D, Collins JP, et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in children aged 5–11 Years—United States, November 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1579–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hause AB, Baggs J, Marquez P, et al. COVID-19 vaccine safety in children aged 5–11 years—United States, November 3–December 19, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1755–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hause AM, Gee J, Baggs J, et al. COVID-19 vaccine safety in adolescents aged 12-17 years—United States, December 14, 2020-July 16, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenblum HG, Gee J, Liu R, et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the vaccine adverse event reporting system and v-safe. Lancet Infect Dis. 2022;22:802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein NP, Stockwell MS, Demarco M, et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA vaccination in preventing COVID-19–associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5–17 years—VISION Network, 10 States, April 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price AM, Olson SM, Newhams MM, et al. ; Overcoming Covid-19 Investigators. BNT162b2 protection against the omicron variant in children and adolescents. N Engl J Med. 2022;386:1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenforde MS, Self WH, Naioti EA, et al. Sustained effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 associated hospitalizations among adults—United States, March–July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CDC. COVID-19 vaccinations in the United States. Available at: https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends. Accessed October 18.
- 20.FDA. COVID-19 Vaccines. U.S. Food and Drug Administration; 2022. [Google Scholar]
- 21.Yousaf AR, Cortese MM, Taylor AW, et al. ; MIS-C Investigation Authorship Group. Reported cases of multisystem inflammatory syndrome in children aged 12-20 years in the USA who received a COVID-19 vaccine, December, 2020, through August, 2021: a surveillance investigation. Lancet Child Adolesc Health. 2022;6:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wisniewski M, Chun A, Volpi S, et al. Outcomes after SARS-CoV-2 vaccination among children with a history of multisystem inflammatory syndrome. JAMA Netw Open. 2022;5:e224750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aykac K, Ozturk K, Demir OO, et al. Frequency and safety of COVID-19 vaccination in children with multisystem inflammatory syndrome: a telephonic interview-based analysis. World J Pediatr. 2022;18:700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Available at: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#covid19-vaccination-misc-misa. Accessed July 8, 2022.
- 25.Godfred-Cato S, Abrams JY, Balachandran N, et al. Distinguishing multisystem inflammatory syndrome in children from COVID-19, Kawasaki disease and toxic shock syndrome. Pediatr Infect Dis J. 2022;41:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CDC. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). Health Advisory. CDC Health Alert Network; 2020. [Google Scholar]
- 27.NNDSS. CSTE Position Statement: Coronavirus Disease 2019 (COVID-19) 2020 Interim Case Definition, Approved April 5, 2020. CDC; 2021. [Google Scholar]
- 28.CDC. V-safe after vaccination health checker. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafe.html. Accessed July 18, 2022.
- 29.Miller AD, Zambrano LD, Yousaf AR, et al. Multisystem inflammatory syndrome in children-United States, February 2020-July 2021. Clin Infect Dis. 2021;75:e1165–e1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zambrano LN, Olson SM, Halasa NB, et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12–18 years—United States, July–December 2021. Morb Mortal Wkly Rep. 2022;71:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodworth K. Clinical Considerations for Pfizer-BioNTech COVID-19 Vaccination in Adolescents. ACIP; 2021. [Google Scholar]
- 32.Levy M, Recher M, Hubert H, et al. Multisystem inflammatory syndrome in children by COVID-19 vaccination status of adolescents in France. JAMA. 2022;327:281–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holm M, Espenhain L, Glenthoj J, et al. Risk and phenotype of multisystem inflammatory syndrome in vaccinated and unvaccinated Danish children before and during the omicron wave. JAMA Pediatr. 2022;176:821–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]