Background:
With the progression of the Coronavirus disease pandemic, the number of mutations in the viral genome has increased, showing the adaptive evolution of severe acute respiratory syndrome coronavirus 2 in humans and intensification in transmissibility. Long-term infections also allow the development of viral diversity. In this study, we report the case of a child with severe combined immu presenting a prolonged severe acute respiratory syndrome coronavirus 2 infection. We aimed to analyze 3 naso-oropharyngeal swab samples collected between August and December 2021 to describe the amino acid changes present in the sequence reads that may have a role in the emergence of new viral variants.
Methods:
The whole genome from clinical samples was sequenced through high throughput sequencing and analyzed using a workflow to map reads and then find variations/single-nucleotide polymorphisms. In addition, the samples were isolated in cell culture, and a plaque forming units assay was performed, which indicates the presence of viable viral particles.
Results:
The results obtained showed that the virus present in all samples is infectious. Also, there were 20 common mutations among the 3 sequence reads, found in the ORF1ab and ORF10 proteins. As well, a considerable number of uncommon mutations were found.
Conclusions:
In conclusion, we emphasize that genomic surveillance can be a useful tool to assess possible evolution signals in long-term patients.
Keywords: amino acid substitution, genomic surveillance, persistent infection, SARS-CoV-2
Since the discovery of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants, such as Alpha, Gamma, Delta and Omicron, it has been possible to note changes in the Coronavirus disease (COVID-19) range of symptoms and transmission rates, which are more heterogeneous and more frequent in younger adults, adding the fact of intense vaccination programs, cases of previous natural infection and re-infections.1–3
The SARS-CoV-2 incubation period has a wide range, ranging from 2.87 to 17.6 days, and it can be different among age groups.4,5 It is estimated, based on several published reports, that higher levels of IgM and IgG antibodies occur in the second and third week of infection.6 However, it is indicated that immunosuppression can be related to prolonged periods of COVID-19 and the persistence of SARS-CoV-2.7 Also, the infection period can be different considering some factors such as the immunological status of the patient, viral load and disease severity.8
Prolonged infections in pediatric immunosuppressed patients have already been reported and are a source of concern for these patients. Although persistent infections are not fully understood, these cases can increase the risk of contamination for other individuals as the patient remains infected much longer and may also need hospital assistance for a longer time.9 Besides, mutations in the virus genome can occur during the long time the patient is infected and under antivirals, convalescent or monoclonal antibodies treatment. It also could select the virus and make it more resistant to antivirals or able to escape from the immune system.10
Therefore, the objective of the present work was to evaluate the viability of viral particles from the samples, as well as to describe and analyze the presence of amino acid changes present in the reads of SARS-CoV-2 sequences indicating intra-host evolution from a prolonged infection in a child with innate severe combined immunodeficiency after a procedure of hematopoietic stem-cell transplantation.
MATERIAL AND METHODS
Case Description
A 1-year-old child with a history of severe combined immunodeficiency treated with haploidentical hematopoietic stem cell transplant was admitted to the Hospital de Clínicas de Porto Alegre, Rio Grande do Sul, Brazil on July 27, 2021, presenting with nausea, vomit, abdominal pain, dysuria and right leg edema as well as hyperemia. The initial investigation showed no signs of thrombosis, but urine culture showed infection by a cefepime-sensitive Enterobacter. Treatment was initiated with amoxicillin-clavulanate, but, with a new rise in reactive protein C levels and fever, treatment was changed to cefepime. On August 3rd he was submitted to an elective computer tomography as a follow-up to rifampicin and isoniazid treatment, under regular schemes and dosages. This examination showed ground glass areas and the progression of centrilobular opacities and nodules. On the same day, a nasopharyngeal sample was positive for COVID-19 testing. The patient was discharged on August 12 but was readmitted multiple times for urinary infections. A second sample for COVID-19 was collected on November 5 and the third sample on December 3. Samples 1, 2 and 3 were evaluated using Quantitative reverse transcription Polymerase Chain Reaction for SARS-CoV-2 cDNA detection targeting the viral E gene, according to the Charité Institute, Berlin, Germany, protocols11 and were conducted using AgPath-ID One-Step RT-PCR Reagents (Thermo Fisher Scientific, Waltham, MA).
Isolation in Cell Culture
Viral isolation protocol in cell culture was performed at Laboratório de Estudos de Vírus Emergentes at Universidade Estadual de Campinas (UNICAMP), under biosafety level 3 (BSL-3) facilities where it was possible to verify the presence of viable viral particles of SARS-CoV-2. For this, the samples were processed according to a protocol previously described12 and inoculated into 6-well plates containing Vero CCL81 cells (CCL-81, ATCC, Manassas, VA). The culture was monitored daily to verify the appearance of cytopathic effects (CPE) on the cells, which is indicative of cell damage caused by viral replication. The culture was followed until the 4th day postinfection. After the first passage in culture, the supernatants were successively passaged 2 more times in cell culture to verify if CPE formation was maintained (passages 1, 2 and 3).
RNA Extraction and Quantitative reverse transcription Polymerase Chain Reaction of Cell Culture
Cell supernatant was collected for RNA extraction using the Quick-DNA/RNA viral kit (Catalog number: D7021, Zymo Research, Irvine, CA) according to the manufacturer’s instructions, and Quantitative reverse transcription Polymerase Chain Reaction for SARS-CoV-2 detection targeting the viral E gene was performed according to the Charité Institute, Berlin, Germany, protocols using the qPCR BIO Probe 1-Step Go Lo-Rox (Catalog number: PB25.41-05, PCR Biosystems, London, UK) according to manufacturer’s instructions. This step was performed to verify and prove the presence of SARS-CoV-2-specific genetic material in the cultures.
Plaque Forming Units Assay
Supernatants were collected for the Plaque Forming Units (PFU), which identifies the presence of viable viral particles and allows viral titration in plaque-forming units per milliliter (PFU/mL) as described elsewhere,13 with modifications. Briefly, Vero CCL81 cells (CCL-81; ATCC, Manassas, VA) were seeded in 24-well plates (2 × 105 cells/mL). An aliquot of each supernatant was 10-fold serially diluted (10-1 to 10-6) and inoculated in an 80%–90% confluent Vero cells monolayer. The plates were incubated for 1 hour at room temperature at constant and gentle homogenization. Then, the inoculum was removed, and it was added 1 mL of a semisolid medium composed of 1% carboxymethylcellulose (Sigma-Aldrich, St Louis, MO), 1% Dulbecco’s modified Eagle’s medium (Vitrocell Embriolife, Campinas, Brazil), 1% penicillin-streptomycin (10,000 U/mL penicillin and 10 mg/mL streptomycin; Sigma-Aldrich) and 5% fetal bovine serum (Gibco by Life Technologies, Carlsbad, CA). The plates were incubated at 37ºC, 5% CO2, for 4 days. The semisolid medium was removed, and the cells were fixed with 2 mL of 8% formaldehyde solution overnight and stained with 1% methylene blue (Sigma-Aldrich) for 30 minutes. The results are expressed in PFU/mL.
Viral Whole Genome Sequencing and Bioinformatics Analysis
RNA was extracted from naso-oropharyngeal swab samples and a reverse transcription reaction was performed using the SuperScript IV reverse transcriptase kit (Thermo Fisher Scientific). Preparation of the whole viral genome library was performed using the QIAseq SARS-CoV-2 primer panel paired for library enrichment and QIAseq FX DNA Library UDI Kit, according to the manufacturer’s instructions (QIAGEN, Hilden, Germany). Sequencing was implemented in an Illumina MiSeq platform using MiSeq Reagent Kit v3 (600-cycle) from Illumina Inc. (Foster City, CA). The FASTq reads were imported to Geneious Prime, trimmed (BBDuk 37.25), and mapped against the reference sequence hCoV-19/Wuhan/WIV04/2019 (EPI_ISL_402124) available in the EpiCoV database from Global Initiative on Sharing Avian Influenza Data. The sequences were characterized using the Pangolin COVID-19 Lineage Assigner tool (https://github.com/hCoV-2019/pangolin).14 The sequences generated in this study were aligned with 20 SARS-CoV-2 complete genomes and the reference sequence that were retrieved from the Global Initiative on Sharing Avian Influenza Data database using muscle alignment on geneious prime and a phylogenetic tree was built using the maximum likelihood method and Tamura-Nei model using MEGA software applying 1000 bootstrap replications. Bioinformatics analysis was performed based on the FASTq reads obtained from the sequencing and was analyzed in the software Geneious Prime using a workflow to map reads and then find variations/single-nucleotide polymorphism that consider the type of polymorphism, amino acid change, CoDing Sequence position, codon change, protein effect and variant frequency. All changes that appear in the reads at a frequency equal to or greater than 80% were not considered because this is the cutoff point to be part of the consensus sequence, and the purpose of the work was to evidence those that appear only in minority reads.
RESULTS
Case Description
The child tested positive for SARS-CoV-2 by molecular methods, with Ct values targeting the E gene (Ct = 20.72) (sample 1). The second sample kept positive with a Ct value of 23.71 (sample 2) and the last sample obtained in December remained positive with a Ct value of 28.22 (sample 3). The patient did not receive any dose of the COVID-19 vaccine before becoming infected with the virus. In August 2022, the patient was retested for SARS-CoV-2 and remains positive, but he is no longer hospitalized.
Detection of Viable Viral Particles in the Course of Persistent Infection
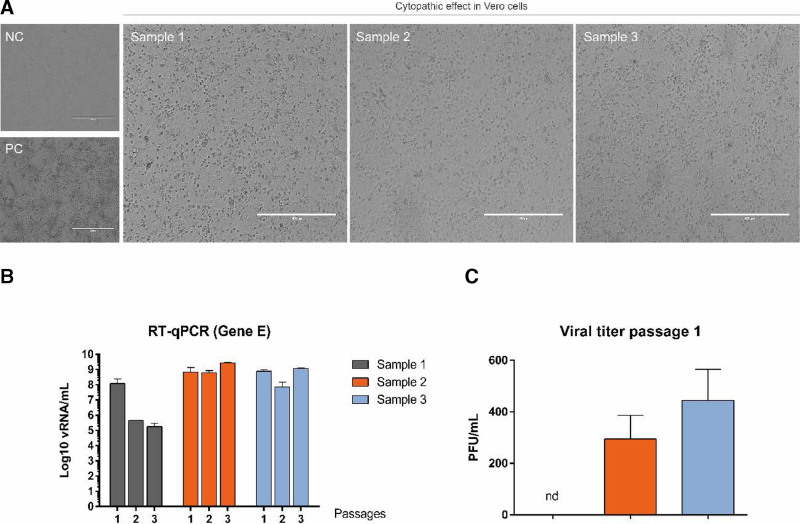
The viral isolation protocol allowed the observation of CPE in cell culture, indicating the presence of viable viral particles in the 3 samples (Fig. 1A) after 4 days of infection. With successive passages, it was observed that the amount of viral RNA remained high, except for sample 1, where there was a decrease in the amount of viral RNA (Fig. 1B). Also, it was not possible to observe the formation of lysis plaques in the culture performed with sample 1, despite the presence of CPE in the culture (Fig. 1C).
FIGURE 1.
Samples inoculation in cell culture. A: Cytopathic effect on cultures of samples 1, 2 and 3, observed 4 days after infection. PC= positive control (Gamma variant stock inoculated at MOI* of 0.01); CN= negative control. Photos obtained from the 20× objective, 400 µm scale. B: Figure showing viral titer in the supernatant of samples inoculated into Vero cell culture (PFU/mL), and results of viral RNA quantification in successive passages in culture, obtained by Quantitative reverse transcription Polymerase Chain Reaction. *MOI = Multiplicity of infection; multiplicity of infection or ratio of viral particles to each cell.
Genome Sequencing and Bioinformatics Analysis
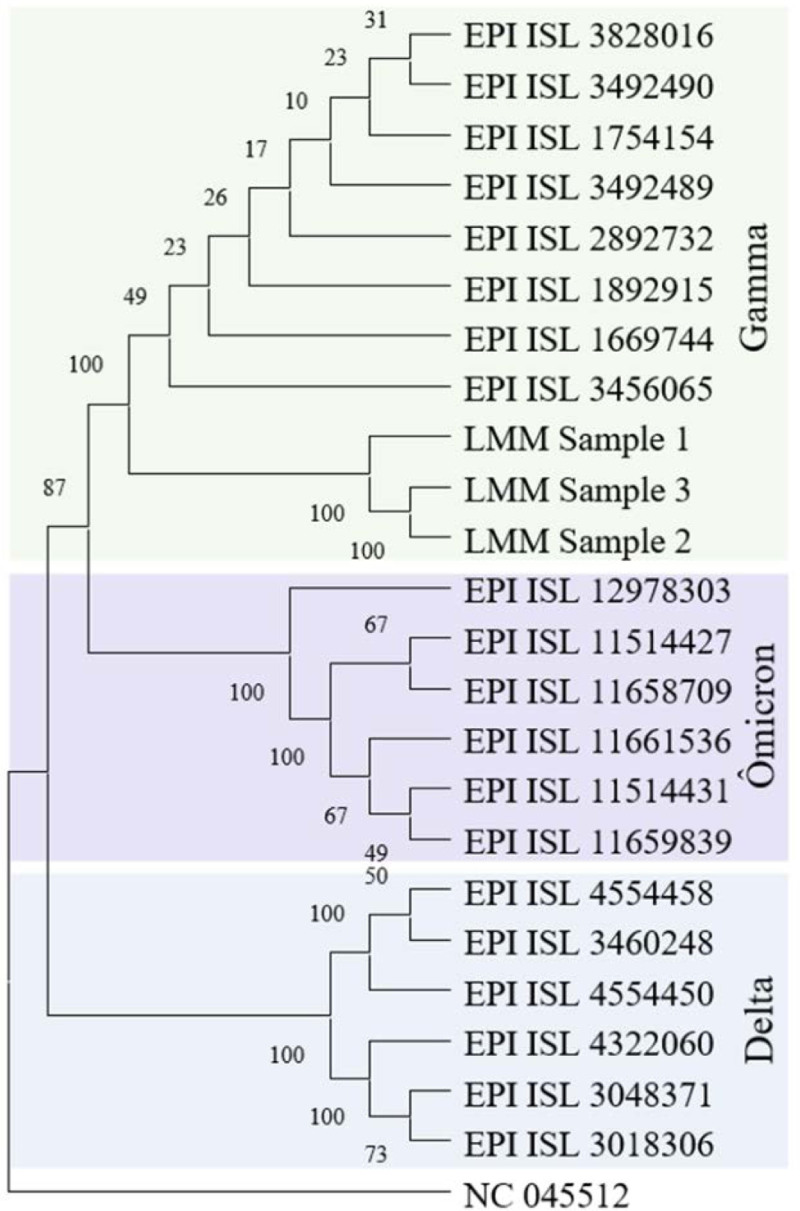
After sequencing, the samples were identified as Gamma lineage which can be seen in the phylogeny (Fig. 2). The consensus sequences were submitted to the EpiCoV database from Global Initiative on Sharing Avian Influenza Data and are available with the access numbers EPI_ISL_11514417 (sample 1), EPI_ISL_11514383 (sample 2) and EPI_ISL_11514402 (sample 3).
FIGURE 2.
Phylogenetic tree based on the SARS-CoV-2 complete genomes of the samples, pointing to the microevolution occurred in the course of infection.
In the consensus sequences, it was identified 9 nonsynonymous mutations that are considered as Gamma lineage definition (P80R, S3675-, G3676-, F3677-, L18F, K417T, E484K, N501Y and H655Y). While analyzing the reads mapped against the reference, it was possible to identify the presence of common and uncommon amino acid changes between the 3 samples.
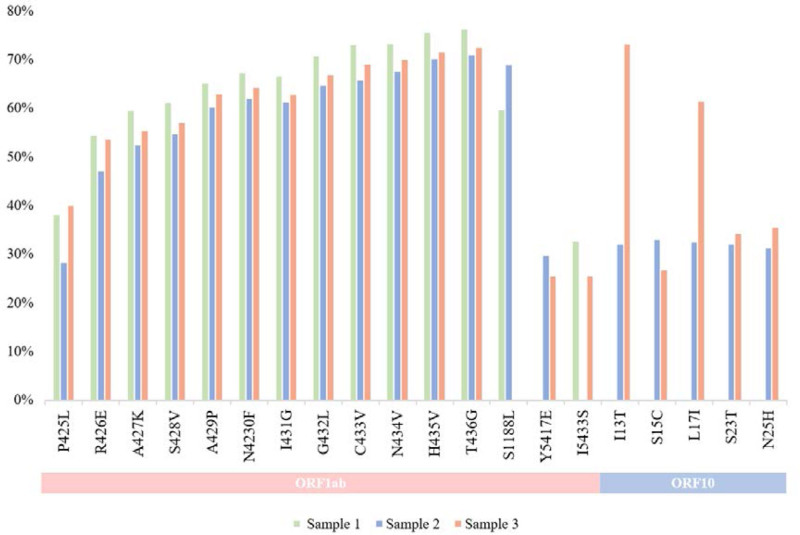
From the reads of the 3 sequences analyzed in this study, a total of 20 common mutations between the samples were identified, 15 of them were found in the ORF1ab polyprotein and 5 in the ORF10, as well as mutations that appeared in only 2 of the 3 samples where 3 of them were found in ORF1ab and 5 in ORF10, which can be seen in Fig. 3 along with their respective frequencies.
FIGURE 3.
Profile of common mutations among the sequences reads and their frequency. Below, it is possible to see the distribution of the amino acid changes in the specific genes of these SARS-CoV-2 samples.
A considerable number of uncommon mutations were found, and these changes were unique to a given sample. We identified in sample 1, the presence of 24 unique mutations. Meanwhile, 10 mutations were unique to sample 2. Sample 3 presented a total of 22 unique mutations. The data related to unique mutations can be seen in Table 1.
TABLE 1.
Profile of unique mutations among the sequences reads, their frequency and the respective gene
| Sample | Gene | Amino acid change | Variant frequency |
|---|---|---|---|
| 1 | ORF1ab | W423L | 26.3% |
| V424L | 22.8% | ||
| A2710V | 54.5% | ||
| I2712T | 41.9% | ||
| L3201P | 40.6% | ||
| V5427D | 30.0% | ||
| N5431H | 25.0% | ||
| A5432V | 26.3% | ||
| AT5434AP | 28.0% | ||
| D6249Y | 37.1% | ||
| ORF3a | S74F | 31.6% | |
| E | V70I | 37.4% | |
| ORF7b | H37R | 51.9% | |
| N38R | 50.0% | ||
| NE38TS | 44.2% | ||
| E39V | 50.0% | ||
| T40R | 50.0% | ||
| T40K | 44.2% | ||
| C41L | 46.2% | ||
| C41K | 50.0% | ||
| H42D | 50.0% | ||
| A423G | 50.0% | ||
| N | DEL204 | 31.7% | |
| 2 | ORF1ab | G5423D | 25.7% |
| N5426T | 27.3% | ||
| V5427I | 26.9% | ||
| D5429G | 26.6% | ||
| N5431S | 25.3% | ||
| I5433M | 37.8% | ||
| VDG6634TLF | 43.2% | ||
| VDG6634VLF | 31.9% | ||
| ORF10 | L18C | 29.5% | |
| I27K | 26.3% | ||
| 3 | ORF1ab | V5422R | 25.3% |
| G5423E | 26.3% | ||
| N5426S | 25.6% | ||
| T5428N | 26.6% | ||
| A5432L | 26.3% | ||
| A5434G | 28.9% | ||
| T5435P | 29.6% | ||
| N | M210I | 27.7% | |
| ORF10 | Y14F | 39.0% | |
| L16R | 25.6% | ||
| L18Q | 25.6% | ||
| L18S | 35.9% | ||
| CR19IL | 36.8% | ||
| C19N | 25.6% | ||
| R20I | 26.3% | ||
| M21P | 25.3% | ||
| M21T | 43.6% | ||
| N22H | 35.0% | ||
| R24K | 34.8% | ||
| Y26S | 44.8% | ||
| IA27HQ | 36.4% |
DISCUSSION
During 2021, the highest wave of SARS-CoV-2 cases in Brazil was caused by the predominance of the Gamma lineage (P.1). This lineage was responsible for many positive cases and hospitalizations, as well as a large number of deaths.15 Besides, cases of more extended infection periods than usual were reported. These persistent infections may play a significant role in transmission rates and in the possibility of new mutations in the SARS-CoV-2 genome, which may cause new variants to appear. In addition, immune system disorders leave the patient weakened for a prolonged period caused by the immune system’s difficulty in eliminating the virus, requiring medical assistance for a longer time.16
PFU assays are known to be the “gold standard” for quantifying viruses in their infectious form. Through it, in regions, where there is cell death caused by the virus, the formation of plaques, is visualized.17 In our samples, the presence of infectious viral particles was observed in the nasopharyngeal swab samples, with an increase in the viral load of samples 2 and 3. While sample 1 presented only CPE, it was not possible to observe the formation of lysis plaques in the culture. It was possible to see that the virus remained infectious in the patient, not just genomic fragments. Although in sample 1 it was not possible to calculate the titer, the virus had a cytopathic effect when isolated in cell culture, and the PCR results of the supernatant were positive, showing that the virus was viable.
The patient whose case was presented in the present study remained hospitalized for 3 months. The samples collected during this period were sequenced and showed 9 nonsynonymous defining mutations in its consensus sequences (P80R, S3675-, G3676-, F3677-, L18F, K417T, E484K, N501Y and H655Y), these alterations are known as signature mutations of Gamma lineage and are already known to lead to immune escape or affect viral infectivity.18
ORF1ab polyprotein region concentrates most of the amino acid changes, and this can be related to the size of this gene that covers two-thirds of the entire genome of SARS-CoV-2. This region is known for its variability due to flexible binding regions.19 All the amino acid changes present in the sequence reads in this study are classified as nondefining mutations but can lead to impacts on the virus pathogenicity.
The S1188L mutation found in the ORF1ab polyprotein is in the Mac1 macrodomain within the nsp3 region. This domain is conserved in all coronaviruses and is essential in viral pathogenesis.20 As it is a conserved region, alterations in the sequence in Mac1 may not be as significant for viral replication but may be relevant in the pathogenesis of the disease.21
The ORF10 protein can interact with several host proteins, which can lead to a change in functionality caused by the presence of point mutations in this region. Based on our results, 20 amino acid changes were found in this protein, among them, we draw attention to the I13T and N22H because these two mutations were previously reported by another study.22
The N protein is a relevant structural protein in the capsid formation process and has a high immunogenic potential as it is strongly linked to the viral replication process. In addition, it is used as a target for disease diagnosis and can also be a target for therapeutic approaches.23 Therefore, changes in this region can be significant. In this study, just 1 amino acid change was found in the N gene.
Other genes such as M (packaging protein of the viral genome) and E (membrane protein that forms the viral envelope) are highly conserved, in addition to being small regions of the SARS-CoV-2 genome,24 this may explain the absence of mutations in these regions in our study.
Figure 3 shows that in ORF10 the last 4 mutations are in samples 2 and 3, demonstrating that at the beginning of the infection, these mutations were not present and appeared over time. Besides, as can be seen in our phylogenetic tree, samples 2 and 3 are more evolved compared to sample 1 showing that this case of persistent infection may have contributed to the viral evolution. In addition, RNA viruses have high mutation rates provenience from the accumulation of alterations caused by the low fidelity of the RNA-dependent RNA polymerase.25 The presence of repair mechanisms and their influence on the repair of errors during replication is still studied in coronaviruses.26
Most of the amino acid changes found in this study have never been previously described by other studies, showing that they are unique to our samples. This shows that the frequency of this intrahost heterogeneity can increase during the long time the patient is infected with SARS-CoV-2 and competition can occur between viral populations carrying different mutations. Furthermore, it is not possible to identify differences in the presence of specific mutations related to the age of the patient.
In conclusion, we emphasize that even though the infection persisted for months, several unique mutations present in the FASTq reads did not become frequent enough to be found in the consensus sequences. With this, it is important to evaluate the genomic profile of SARS-CoV-2 lineages because some mutations may become more frequent as the virus spread. Besides, it could affect viral fitness and bring benefits to the pathogen.
Footnotes
This work is an initiative of Rede Corona-ômica BR MCTI/FINEP affiliated to RedeVírus/MCTI (FINEP = 01.20.0029.000462/20, CNPq = 404096/2020-4). The study was also supported by FAPERGS/MS/PPSUS (21/2551-0000081-3).
The authors have no conflict of interests to disclose.
Contributor Information
Mariene Ribeiro Amorim, Email: mariene.ramorim@gmail.com.
Mariana Soares da Silva, Email: marisoares.vet@gmail.com.
Juliana Schons Gularte, Email: julianaschons@hotmail.com.
Meriane Demoliner, Email: merianedemoliner@gmail.com.
Viviane Girardi, Email: vivi.girardi@hotmail.com.
Vyctoria Malayhka de Abreu Goes Pereira, Email: vyctoriamalayhkaa@gmail.com.
Alana Witt Hansen, Email: alanahansen@feevale.br.
Juliane Deise. Fleck, Email: julianefleck@feevale.br.
Fernanda de-Paris, Email: fparis@hcpa.edu.br.
Grazielle Motta Rodrigues, Email: gmorodrigues@hcpa.edu.br.
Janaina Aparecida Risczik Arruda Correa, Email: jarcorrea@hcpa.edu.br.
Elissandra Machado Arlindo De Mattos, Email: earlindo@hcpa.edu.br.
Rodrigo Minuto Paiva, Email: rpaiva@hcpa.edu.br.
Caroline Deutschendorf, Email: cdeutschendorf@hcpa.edu.br.
Frederico Soares Falcetta, Email: ffalcetta@hcpa.edu.br.
José Luiz Proença Modena, Email: jlmodena@unicamp.br.
Fernando Rosado Spilki, Email: fernandors@feevale.br.
REFERENCES
- 1.Zhang J, Chen N, Zhao D, et al. Clinical characteristics of COVID-19 patients infected by the omicron variant of SARS-CoV-2. Front Med. 2022;9:912367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maslo C, Friedland R, Toubkin M, et al. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 omicron wave compared with previous waves. JAMA. 2022;327:583–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399:437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chun JY, Baek G, Kim Y. Transmission onset distribution of COVID-19. Int J Infect Dis. 2020;99:403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie S, Zhang G, Yu H, et al. The epidemiologic and clinical features of suspected and confirmed cases of imported 2019 novel coronavirus pneumonia in north Shanghai, China. Ann Transl Med. 2020;8:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323:2249–2251. [DOI] [PubMed] [Google Scholar]
- 7.Avanzato VA, Matson MJ, Seifert SN, et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183:1901–1912.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh KA, Jordan K, Clyne B, et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect. 2020;81:357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang B, Fan J, Huang J, et al. Clinical and molecular characteristics of COVID-19 patients with persistent SARS-CoV-2 infection. Nat Commun. 2021;12:3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Vidal C, Iglesias-Caballero M, Puerta-Alcalde P, et al. ; HEMATOCOVID19-Researchers Group. Emergence of progressive mutations in SARS-CoV-2 from a hematologic patient with prolonged viral replication. Front Microbiol. 2022;13:826883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Souza WM, Amorim MR, Sesti-Costa R, et al. Neutralization of SARS-CoV-2 lineage P.1 by antibodies elicited through natural SARS-CoV-2 infection or vaccination with an inactivated SARS-CoV-2 vaccine: an immunological study. Lancet Microbe. 2021;2:e527–e535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araújo DB, Machado RRG, Amgarten DE, et al. SARS-CoV-2 isolation from the first reported patients in Brazil and establishment of a coordinated task network. Mem Inst Oswaldo Cruz. 2020;115:e200342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Toole A, Scher E, Underwood A, et al. Assignment of epidemiological lineages in an emerging pandemic using the pangolin tool. Virus Evol. 2021;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demoliner M, Silva MS, Gularte JS, et al. Predominance of SARS-CoV-2 P.1 (Gamma) lineage inducing the recent COVID-19 wave in southern Brazil and the finding of an additional S: D614A mutation. Infect Genet Evol. 2021;96:105134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fung M, Babik JM. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis. 2021;72:340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Case JB, Bailey AL, Kim AS, et al. Growth, detection, quantification, and inactivation of SARS-CoV-2. Virology. 2020;548:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naveca FG, Nascimento V, Souza V, et al. ; Fiocruz COVID-19 Genomic Surveillance Network. Spread of Gamma (P.1) sub-lineages carrying Spike mutations close to the furin cleavage site and deletions in the N-terminal domain drives ongoing transmission of SARS-CoV-2 in Amazonas, Brazil. Microbiol Spectr. 2022;10:e0236621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson SAJ, Richter A, Casey A, et al. Recurrent SARS-CoV-2 mutations in immunodeficient patients. Virus Evol. 2022;8:veac050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alhammad YMO, Kashipathy MM, Roy A, et al. The SARS-CoV-2 conserved macrodomain is a mono-ADP-ribosylhydrolase. J Virol. 2020;95:e01969–e01920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caccuri F, Zani A, Messali S, et al. A persistently replicating SARS-CoV-2 variant derived from an asymptomatic individual. J Transl Med. 2020;18:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassan SS, Lundstrom K, Serrano-Aroca A, et al. Emergence of unique SARS-CoV-2 ORF10 variants and their impact on protein structure and function. Int J Biol Macromol. 2022;194:128–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arya R, Kumari S, Pandey B, et al. Structural insights into SARS-CoV-2 proteins. J Mol Biol. 2021;433:166725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yavarian J, Nejati A, Salimi V, et al. Whole genome sequencing of SARS-CoV2 strains circulating in Iran during five waves of pandemic. PLoS One. 2022;17:e0267847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hui EK. Reasons for the increase in emerging and re-emerging viral infectious diseases. Microbes Infect. 2006;905:e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domingo E, García-Crespo C, Lobo-Vega R, et al. Mutation rates, mutation frequencies, and proofreading-repair activities in RNA virus genetics. Viruses. 2021;13:1882. [DOI] [PMC free article] [PubMed] [Google Scholar]