Background:
The clinical features of coronavirus disease 2019 (COVID-19) in children have been changing because of the emergence and rapid spread of variants of concern (VOC). The increase in cases infected with VOC has brought concern with persistent symptoms after COVID-19 in children. This survey aimed to analyze the clinical manifestations and persistent symptoms of pediatric COVID-19 cases in Japan.
Methods:
We analyzed the clinical manifestations of pediatric COVID-19 cases reported between February 2020 and April 2022 in Japan, using a dedicated database updated voluntarily by the members of the Japan Pediatric Society. Using the same database, we also analyzed persistent symptoms after COVID-19 in children who were diagnosed between February 2020 and November 2021.
Results:
A total of 5411 and 1697 pediatric COVID-19 cases were included for analyzing clinical manifestations and persistent symptoms, respectively. During the Omicron variant predominant period, the percentage of patients with seizures increased to 13.4% and 7.4% in patient groups 1–4 and 5–11 years of age, respectively, compared with the pre-Delta (1.3%, 0.4%) or Delta period (3.1%, 0.0%). Persistent and present symptoms after 28 days of COVID-19 onset were reported in 55 (3.2%).
Conclusions:
Our survey showed that the rate of symptomatic pediatric COVID-19 cases increased gradually, especially during the Omicron variant predominant period, and a certain percentage of pediatric cases had persistent symptoms. Certain percentages of pediatric COVID-19 patients had severe complications or prolonged symptoms. Further studies are needed to follow such patients.
Keywords: COVID-19, pediatrics, clinical characteristics, severity, postacute
Most of the reports published during the early period of the coronavirus disease 2019 (COVID-19) pandemic suggested that the clinical manifestations of COVID-19 were generally milder and sometimes asymptomatic in the pediatric population compared with the adults.1–3 However, information regarding the impact of the predominant variants of concern (VOC) on clinical manifestations and severity in pediatric COVID-19 patients is limited. In addition, information about the frequency of and risk factors for persistent symptoms after COVID-19 in children is also not well characterized.4 The objective of this study is to analyze the acute and postacute clinical manifestations of pediatric COVID-19 using a nationwide survey, which used a dedicated database of outpatient and inpatient pediatric COVID-19 cases in Japan.
MATERIALS AND METHODS
Survey Population
We included pediatric patients <20 years of age who were diagnosed with COVID-19 by real-time reverse transcriptase polymerase chain reaction (PCR), loop-mediated isothermal amplification (LAMP) test, or antigen test. The survey period was set from February 2020 to April 2022. Clinical information of pediatric COVID-19 patients, including the epidemiologic characteristics, clinical manifestations, treatment, hospitalization status, complications, and prognosis at the time of discharge from the hospital was collected into the dedicated database that was updated and maintained voluntarily by physicians who were the members of the Japan Pediatric Society (JPS).
In Japan, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Delta and Omicron variants became predominant in August 2021 and January 2022, respectively.5 Hence, we divided the survey periods by VOC into the pre-Delta period (from February 2020 to July 2021), the Delta period (from August 2021 to December 2021), and the Omicron period (from January 2022 to April 2022). Then, we analyzed the influence of age and the predominant SARS-CoV-2 variant on the clinical manifestations and severity of pediatric COVID-19 patients. We set the analysis period for persistent symptoms after COVID-19 in children between February 1, 2020, and November 4, 2021. Six months after enrollment, an e-mail was sent automatically to the patients’ physicians inquiring about symptoms that were present 28 days after the onset of COVID-19.
Statistical Analysis
Statistical analyses were performed using the statistical analysis software R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria) or SPSS Statistics 27 (IBM Corporation, Armonk, NY). The chi-square test and Fisher exact test were used to assess the categorical variables as appropriate, and the Mann-Whitney U test was used to assess the continuous variables. To identify the risk factors for having symptoms 28 days after the onset of COVID-19 in children and adolescents, we performed a multivariate logistic regression analysis of variables for which P < 0.10 in univariate analysis. A two-tailed P value of <0.05 was considered statistically significant.
Ethical Considerations
This survey was following the Helsinki Declaration as revised in 2013 and was approved by the Ethics Review Committee of the JPS (approval number: 32).
RESULTS
Clinical Characteristics of the Patients During the Acute Phase of COVID-19
Patient Demographics
As of April 30, 2022, 5621 laboratory-confirmed pediatric COVID-19 cases, less than 20 years of age, were reported to the JPS database. We excluded the patients who were 16–19 years old as most of them preferred to visit clinics in internal medicine in Japan, which resulted in lower registration rates, and it was not possible to generalize the survey results of the small group. Finally, 5411 pediatric COVID-19 patients who were <16 years of age were enrolled in the survey (Table 1). The median age of the study population was 6.1 years (interquartile range [IQR]: 2.1–10.3), and 2889 (53.4%) were female patients. There were 2693 (49.8%), 1193 (22.0%), and 1525 (28.2%) patients registered in the pre-Delta, Delta, and Omicron periods, respectively. Among the 5–15 years age group patients, only 34 patients (0.6%) had received at least 1 dose of COVID-19 vaccination.
TABLE 1.
Demographic and Clinical Characteristics of Pediatric COVID-19 Cases in Each Age Group
| Characteristic | Total (n = 5411) | <1 years (n = 768) | 1–4 years (n = 1566) | 5–11 years (n = 2170) | 12–15 years (n = 907) |
|---|---|---|---|---|---|
| Median age (IQR), years | 6.1 (2.1–10.3) | 0.4 (0.3–0.7) | 2.6 (1.8–3.8) | 8.4 (6.6–10.2) | 13.8 (12.8–14.7) |
| Male sex, n (%) | 2889 (53.4) | 388 (50.5) | 819 (52.3) | 1184 (54.6) | 498 (54.9) |
| Hospitalization status, n (%) | |||||
| Outpatient | 1976 (36.5) | 161 (21.0) | 536 (34.2) | 943 (43.5) | 336 (37.0) |
| Inpatient | 3435 (63.5) | 607 (79.0) | 1030 (65.8) | 1227 (56.5) | 571 (63.0) |
| Period separated by predominant variant, n (%) | |||||
| Pre Delta | 2693 (49.8) | 376 (48.9) | 835 (53.3) | 986 (45.4) | 496 (54.7) |
| Delta | 1193 (22.0) | 168 (21.9) | 322 (20.6) | 484 (22.3) | 219 (24.1) |
| Omicron | 1525 (28.2) | 224 (29.2) | 409 (26.1) | 700 (32.3) | 192 (21.2) |
| Household contact infection, n (%) | |||||
| Pre Delta | 1962 (72.9) | 328 (87.2) | 620 (74.3) | 705 (71.5) | 309 (62.3) |
| Delta | 833 (69.8) | 155 (92.3) | 230 (71.4) | 310 (64.1) | 138 (63.0) |
| Omicron | 777 (51.0) | 195 (87.1) | 226 (55.3) | 282 (40.3) | 74 (38.5) |
| Underlying medical conditions, n (%) | |||||
| Obesity | 74 (1.4) | 4 (0.5) | 3 (0.2) | 31 (1.4) | 36 (4.0) |
| Chronic cardiac disease | 80 (1.5) | 16 (2.1) | 26 (1.7) | 28 (1.3) | 10 (1.1) |
| Chronic lung disease | 17 (0.3) | 5 (0.7) | 10 (0.6) | 1 (0.05) | 1 (0.1) |
| Athma | 254 (4.7) | 3 (0.4) | 63 (4.0) | 133 (6.1) | 55 (6.1) |
| Chronic Kidney disease | 35 (0.6) | 1 (0.1) | 6 (0.4) | 13 (0.6) | 15 (1.7) |
| Chronic liver disease | 10 (0.2) | 1 (0.1) | 3 (0.2) | 4 (0.2) | 2 (0.2) |
| Chronic neurologic disorder | 38 (0.7) | 0 (0.0) | 7 (0.4) | 17 (0.8) | 14 (1.5) |
| Diabets | 8 (0.1) | 0 (0.0) | 0 (0.0) | 3 (0.1) | 5 (0.6) |
| Malignant neoplasm | 6 (0.1) | 0 (0.0) | 3 (0.2) | 2 (0.1) | 1 (0.1) |
| Primary Immunodeficiency | 5 (0.1) | 0 (0.0) | 0 (0.0) | 4 (0.2) | 1 (0.1) |
| COVID-19 vaccination status, n (%) | |||||
| BNT162b2 | |||||
| 1 dose | 5 (0.1) | N/A | N/A | 3 (0.2) | 2 (0.2) |
| 2 doses | 20 (0.4) | 2 (0.0) | 18 (2.0) | ||
| mRNA-1273 | |||||
| 1 dose | 0 (0.0) | N/A | N/A | N/A | 0 (0.0) |
| 2 doses | 1 (0.02) | 1 (0.1) | |||
| vaccine type unknown | |||||
| 1 dose | 1 (0.02) | N/A | N/A | N/A | 1 (0.1) |
| 2 doses | 7 (0.1) | 7 (0.8) | |||
Abbreviations: N/A, not applicable
Clinical Manifestations
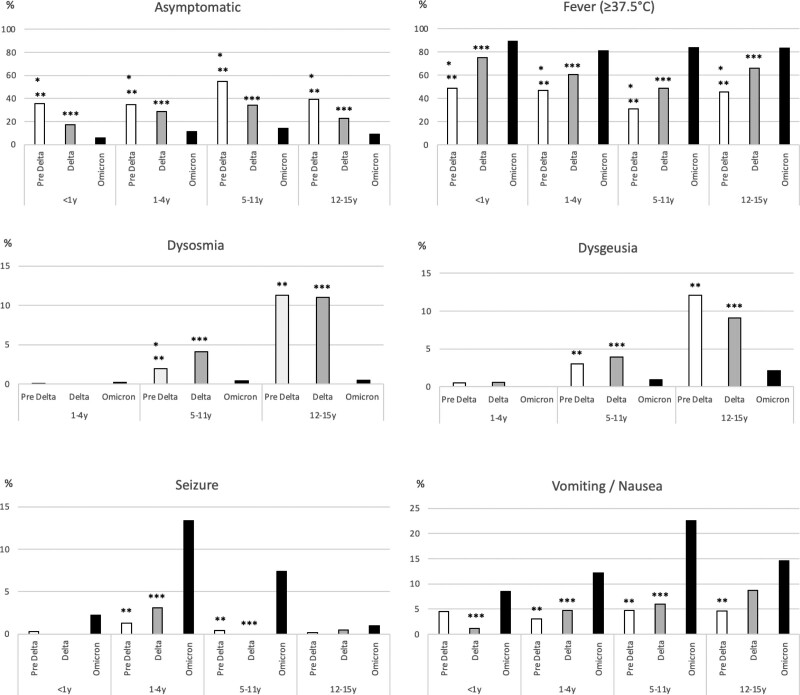
The percentage of asymptomatic patients decreased gradually in each age group during the Delta and Omicron periods (Fig. 1). In contrast, the percentage of patients with fever (≥37.5°C) increased gradually in each age group. During the Omicron period, the percentage of patients with seizures increased to 13.4% and 7.4% among patient groups 1–4 and 5–11 years old, respectively, compared with the pre-Delta (1.3%, 0.4%) or Delta period (3.1%, 0.0%). Similarly, the percentage of patients with vomiting and nausea increased significantly, by 12.2%, 22.6%, and 14.6% in the patient groups 1–4, 5–11, and 12–15 years of age, respectively, during the Omicron period compared with the pre-Delta (3.0%, 4.7%, 4.6%) or Delta period (4.7%, 6.0%, 8.7%).
FIGURE 1.
Clinical manifestations of pediatric COVID patients by variant predominance period and age group between February 2020 and April 2022. Pre Delta versus Delta P < 0.05, **Pre Delta versus Omicron P < 0.05, ***Delta versus Omicron P < 0.05.
Treatment and Outcomes
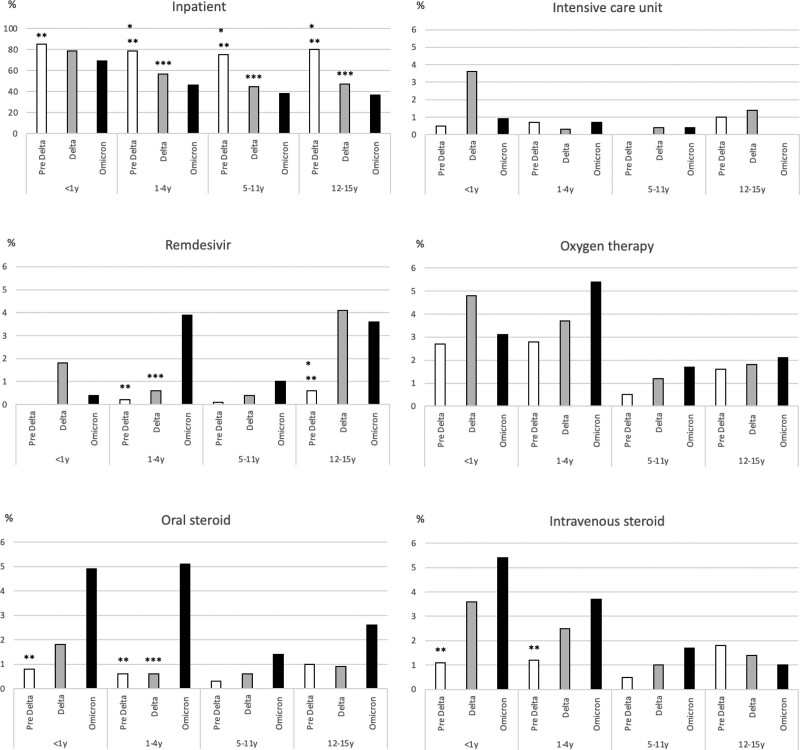
The percentage of hospitalization decreased gradually in each age group (Fig. 2), and during the Omicron period, only 682 cases (44.7%) were hospitalized in all age groups. In contrast, the pediatric intensive care unit (PICU) admission rate did not change during the period. Among patient groups 1–4 years of age, remdesivir was used more frequently during the Omicron period (3.9%), compared with the pre-Delta (0.2%) or Delta period (0.6%) (Fig. 2).
FIGURE 2.
Clinical management and treatment of pediatric COVID patients by variant predominance period and age group between February 2020 and April 2022. *Pre Delta versus Delta P < 0.05, **Pre Delta versus Omicron P < 0.05, ***Delta versus Omicron P < 0.05.
The frequent complications observed were convulsive disorder (n = 109, 2.0%), followed by pneumonia (n = 65, 1.2%). Five cases of multisystem inflammatory syndrome in children (MIS-C) (0.1%) were reported. Twelve cases (0.2%) of croup syndrome were reported during the Omicron variant predominant period. Although the frequency was not high, severe complications, such as 7 myocarditis/pericarditis cases (0.13%) and 3 encephalopathy cases (0.06%) were reported. None of the 34 patients who had at least 1 dose of COVID-19 immunization history had serious complications including convulsive disorder, pneumonia, MIS-C, myocarditis, or encephalopathy.
Postacute Clinical Manifestations of Children With COVID-19
In total, 3987 patients with SARS-CoV-2–positive results were registered during the study period for a survey of postacute clinical manifestations, and 1697 patients (42.6%) who responded completely were subjected to the analyses. Present symptoms after 28 days of COVID-19 onset were reported in 55 (3.2%) (Table 2). Persistent symptoms were most common in older children. The most frequent symptoms were dysosmia (1.1%), followed by dysgeusia (1.0%), fever (0.8%), fatigue (0.7%), and cough (0.5%). Daily lives were affected by regular outpatient visits or interruption of day-care or school in 1.1% of the cases. The presence of symptoms 28 days after the onset of COVID-19 was independently associated with older age (odds ratio [OR], 1.10; 95% confidence interval [CI]: 1.04–1.17) and the presence of fever (OR, 2.38; 95% CI: 1.29–4.38) or dysosmia (OR, 2.73; 95% CI: 1.15–6.49) during the initial stage of COVID-19. When the patients in whom symptoms were present at 28 days after onset were compared with those who did not have symptoms at that time, the gender ratios of the 2 groups were similar (P = 0.76), and the rates of complications of pneumonia (P = 0.43), myocarditis/pericarditis (P = 0.15), and encephalitis/encephalopathy (P = 1.00) did not differ significantly between the 2 groups.
TABLE 2.
Characteristics of Children and Adolescents With Persistent Symptoms of COVID-19 Atleast 28 Days After Onset
| Characteristic | Total (n = 1697) | 0–4 years (n = 699) | 5–11 years (n = 627) | 12–15 years (n = 315) | 16–19 years (n = 56) |
|---|---|---|---|---|---|
| Median age (IQR), years | 6.3 (2.2–11.3) | 1.7 (0.7–3.0) | 8.4 (6.5–10.2) | 13.7 (12.8–14.8) | 17.8 (16.8–19.0) |
| Male sex, n (%) | 899 (53.0) | 352 (50.4) | 340 (54.2) | 173 (54.9) | 34 (60.7) |
| No. of symptoms, n (%) | |||||
| Any | 55 (3.2) | 12 (1.7) | 14 (2.2) | 22 (7.0) | 7 (12.5) |
| 1 | 28 (1.6) | 10 (1.4) | 7 (1.1) | 8 (2.5) | 3 (5.4) |
| 2 | 17 (1.0) | 1 (0.1) | 4 (0.6) | 9 (2.9) | 3 (5.4) |
| 3 | 4 (0.2) | 0 (0) | 1 (0.2) | 2 (0.6) | 1 (1.8) |
| 4 | 3 (0.2) | 1 (0.1) | 1 (0.2) | 1 (0.3) | 0 (0) |
| 5 | 3 (0.2) | 0 (0) | 1 (0.2) | 2 (0.6) | 0 (0) |
| Symptoms, n (%) | |||||
| Fever (≥38°C) | 13 (0.8) | 6 (0.9) | 3 (0.5) | 3 (1.0) | 1 (1.8) |
| Cough | 9 (0.5) | 5 (0.7) | 2 (0.3) | 1 (0.3) | 1 (1.8) |
| Shortness of breath | 1 (0.1) | 0 (0) | 0 (0) | 1 (0.3) | 0 (0) |
| Retractive breathing | 1 (0.1) | 1 (0.1) | 0 (0) | 0 (0) | 0 (0) |
| Wheezing | 1 (0.1) | 1 (0.1) | 0 (0) | 0 (0) | 0 (0) |
| Sore throat | 2 (0.1) | 0 (0) | 1 (0.2) | 1 (0.3) | 0 (0) |
| Dysgeusia | 17 (1.0) | 0 (0) | 4 (0.6) | 11 (3.5) | 2 (3.6) |
| Dysosmia | 19 (1.1) | 0 (0) | 3 (0.5) | 12 (3.8) | 4 (7.1) |
| Chest pain | 2 (0.1) | 0 (0) | 1 (0.2) | 1 (0.3) | 0 (0) |
| Myalgia | 1 (0.1) | 0 (0) | 0 (0) | 1 (0.3) | 0 (0) |
| Arthralgia | 2 (0.1) | 0 (0) | 1 (0.2) | 1 (0.3) | 0 (0) |
| Fatigue | 12 (0.7) | 1 (0.1) | 3 (0.5) | 6 (1.9) | 2 (3.6) |
| Headache | 6 (0.4) | 1 (0.1) | 2 (0.3) | 1 (0.3) | 2 (3.6) |
| Altered consciousness | 2 (0.1) | 0 (0) | 1 (0.2) | 1 (0.3) | 0 (0) |
| Depression | 1 (0.1) | 0 (0) | 0 (0) | 1 (0.3) | 0 (0) |
| Abdominal pain | 6 (0.4) | 0 (0) | 3 (0.5) | 3 (1.0) | 0 (0) |
| Nausea/vomiting | 2 (0.1) | 1 (0.1) | 1 (0.2) | 0 (0) | 0 (0) |
| Diarrhea | 4 (0.2) | 0 (0) | 2 (0.3) | 2 (0.6) | 0 (0) |
| Outcome | |||||
| Regular outpatient visit | 18 (1.1) | 4 (0.6) | 1 (0.2) | 8 (2.5) | 5 (8.9) |
| Hospitalization | 2 (0.1) | 1 (0.1) | 1 (0.2) | 0 (0) | 0 (0) |
| Interruption of nursery/school | 18 (1.1) | 8 (1.1) | 4 (0.6) | 6 (1.9) | 0 (0) |
COVID-19, coronavirus disease 2019; IQR, interquartile range.
DISCUSSION
This is the nationwide survey focused on pediatric inpatient and outpatient COVID-19 cases and analyzed the acute and postacute clinical manifestations in Japan. We demonstrated the rate of symptomatic pediatric COVID-19 cases increased gradually, especially during the Omicron variant predominant period, and a few percent of pediatric cases had persistent symptoms.
The rate of asymptomatic cases decreased gradually in each age group during the survey period, especially during the Omicron predominant period. Thus, it can be postulated that the recent pediatric COVID-19 cases tend to be more symptomatic. However, at the beginning of the COVID-19 pandemic, most asymptomatic children with a history of close contact with COVID-19 patients were tested as part of a cluster survey in Japan and hospitalized if they showed positive for the SARS-CoV-2 test. After the Delta predominant period, asymptomatic children might not have been tested even if they had close contact history with COVID-19 in Japan. The percentage of patients with fever also increased gradually over time in each age group. Although it is difficult to discuss the relationship between fever elevation and seizure rate from this survey, the percentage of patients with seizures also increased significantly among patient groups 1–4 and 5–11 years of age during the Omicron predominant period compared with the pre-Delta or Delta predominant period (Fig. 1). Notably, febrile seizures were observed even in patients who were of 5 years of age or older, including those outside the predominant age range of febrile seizures. Similar results were reported from other countries.6–8 In Sweden, 9% of pediatric hospitalized COVID-19 cases had seizures between March and December 2020. This percentage increased up to 21% in January 2022 during the Omicron variant predominant period.6 These results suggest that febrile seizures may be one of the predominant symptoms of pediatric COVID-19, especially after the Omicron variant predominated.
The percentage of patients with vomiting and nausea also increased significantly among patient groups 1–4, 5–11, and 12–15 years old during the Omicron period compared with the pre-Delta or Delta predominant period (Fig. 1). In the context of these results, COVID-19 may need to be added to the differential diagnosis for children who visit the hospital with vomiting as an initial complaint.
On May 7, 2020, the Ministry of Health, Labour, and Welfare in Japan granted special emergency approval for remdesivir. In this survey, the use of remdesivir for the 1–4 years group increased significantly after the Omicron predominant period. This result suggests that the clinical severity of pediatric COVID-19 patients became significant during the Omicron predominant period. However, hospital admission rates in pediatric COVID-19 cases decreased, and the PICU admission rate did not change over time during this survey. Several reasons can be presumed for such inconsistency. First, the threshold of treatment by remdesivir for pediatric COVID-19 cases may have been kept high during the early stage of the COVID-19 pandemic because clinical information and evidence about the effectiveness and safety of remdesivir in children was limited. Currently, additional information on the use of remdesivir in children has been published.9–13 As a result, it is assumed that the current threshold for the use of remdesivir in children has been lowered compared with the pre-Delta predominant period. Second, the recent increase in the frequency of using remdesivir may be the result of a sharp increase in the absolute number of pediatric patients during the Omicron pandemic.
Based on this survey, the most frequent complication was convulsive disorder. However, it seems that most cases might be composed of mild diseases such as febrile seizures. Although pneumonia was the second most frequent complication, the severity was also generally mild. Recently, especially during the Omicron predominant period, infant COVID-19 cases complicated with croup have been reported.14–16 In this survey, all croup cases were reported during the Omicron period, and most cases were observed in individuals under 5 years of age.
However, in this survey, none of the patients with at least 1 dose of COVID-19 immunization history had severe complications.
According to the current study, most cases improved without admission, and 2 pediatric fatalities were reported. However, this result does not mean that the clinical manifestations of pediatric COVID-19 cases were mild. The National Institute of Infectious Diseases in Japan reported 41 pediatric COVID-19 fatality cases during the Omicron variant predominant era, between January and August 2022.17
We found that 3.2% of patients had persistent symptoms, and the incidence was higher among older children. The incidence and clinical characteristics of persistent symptoms of COVID-19 in children in other countries have been reported. A large prospective cohort study in the United Kingdom revealed that in 77 (4.4%) of 1734 children with COVID-19, the illness lasted at least 28 days, and the common symptoms experienced during this period were fatigue, headache, and anosmia.18 According to a cohort study conducted in Switzerland, 4 (3.7%) of 109 SARS-CoV-2–seropositive children had at least 1 symptom that lasted >12 weeks and common symptoms were fatigue, difficulty concentrating, and increased need for sleep.19 We believe that our finding in Japan is consistent with the results previously reported.18,19 However, several studies have reported that the frequency of persistent symptoms after SARS-CoV-2 infection did not differ between SARS-CoV-2 test positive and negative children.19,20 Further investigation is needed to assess the impact of COVID-19 on persistent symptoms in children.
Information about risk factors for persistent symptoms of COVID-19 in children and adolescents is limited. A prospective cohort study of 518 children with COVID-19 in Russia revealed that older age and a history of allergic diseases were risk factors for symptom persistence for >5 months.21 Our findings also indicated that older age was associated with the persistence of symptoms at least 28 days after COVID-19 onset in children and adolescents. Furthermore, we found that the presence of fever or dysosmia during the acute phase of the illness was also a risk factor. The results indicate that clinicians should monitor pediatric patients with COVID-19 who have had such symptoms.
This study had several limitations. First, patient enrollment in the JPS COVID-19 registry is voluntary, and only 0.3% (5621/2,066,410) of the nationwide pediatric COVID-19 cases <20 years old in Japan were enrolled in this survey.22 Second, it is assumed that the clinical manifestations of our survey population were comparatively more severe than those of the average pediatric patients in Japan because most of the responding pediatricians whose data were used for the survey belonged to secondary or tertiary hospitals which accept and manage more severe cases than the clinics. Because of these limitations, the generalizability of our findings is limited. Third, the patients’ data in the JPS COVID-19 registry did not include information about viral strains, and the effects of variant strains on symptom persistence have not been assessed directly. Last, the survey for postacute clinical manifestations included patients in whom the disease was diagnosed before the omicron VOC period. Dysgeusia and dysosmia, the most common persistent symptoms of COVID-19 in our cohort, have been observed less commonly during the omicron VOC period23; therefore, the generalizability of our results regarding postacute clinical manifestations may be limited regarding the omicron VOC.
CONCLUSION
In Japan, the rate of symptomatic COVID-19 cases, including fever, seizure, vomiting, or complications with croup syndrome, increased gradually in some age groups, especially during the Omicron variant predominant period. In addition, some children with COVID-19, especially in older age, had symptoms even at 28 days during the whole survey period. These results suggest that certain pediatric COVID-19 patients may have severe or prolonged outcomes. Further studies are needed to analyze the influence of the new SARS-CoV-2 variants on pediatric COVID-19 cases.
ACKNOWLEDGMENTS
We thank all Japan Pediatric Society members who participated in this survey.
Footnotes
This work was supported by the Health and Labor Sciences Research Grants from the Ministry of Health, Labour, and Welfare in Japan (Grant No. 20CA2035 and 21DA2003).
The authors have no conflicts of interest to disclose.
T.K., Y.A., and K.S. Have contributed equally to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Yuta Aizawa, Email: ya_nifr@yahoo.co.jp.
Kensuke Shoji, Email: shoji-k@ncchd.go.jp.
Naoki Shimizu, Email: naoki.shimizu@marianna-u.ac.jp.
Kenji Okada, Email: okadak@college.fdcnet.ac.jp.
Takashi Nakano, Email: nakano@med.kawasaki-m.ac.jp.
Hajime Kamiya, Email: hakamiya@nih.go.jp.
Kiyoko Amo, Email: kiyokoamo@ybb.ne.jp.
Naruhiko Ishiwada, Email: naruhikoishiwada@gmail.com.
Satoshi Iwata, Email: siwata@ncc.go.jp.
Makoto Oshiro, Email: mtkn046@gctv.ne.jp.
Nobuhiko Okabe, Email: okabenobu46@gmail.com.
Seigo Korematsu, Email: kseigo@saitama-med.ac.jp.
Shigeru Suga, Email: suga.shigeru.ke@mail.hosp.go.jp.
Takeshi Tsugawa, Email: tsugawat@sapmed.ac.jp.
Naoko Nishimura, Email: naon@konan.jaaikosei.or.jp.
Haruka Hishiki, Email: hishiki.haruka.mv@teikyo-u.ac.jp.
Masashi Fujioka, Email: fjok@silver.ocn.ne.jp.
Mitsuaki Hosoya, Email: mhosoya@fmu.ac.jp.
Yumi Mizuno, Email: yumi-mi@mvc.biglobe.ne.jp.
Isao Miyairi, Email: miyairi@hama-med.ac.jp.
Chiaki Miyazaki, Email: t.miyazaki01@fc-jigyoudan.org.
Tsuneo Morishima, Email: tmorishima@aichi-med-u.ac.jp.
Tetsushi Yoshikawa, Email: tetsushi@fujita-hu.ac.jp.
Kazunobu Ouchi, Email: ouchipapa3@gmail.com.
Hiroyuki Moriuchi, Email: hiromori@nagasaki-u.ac.jp.
Keiko Tanaka-Taya, Email: ktaya@niid.go.jp.
Akihiko Saitoh, Email: asaitoh@med.niigata-u.ac.jp.
REFERENCES
- 1.Liguoro I, Pilotto C, Bonanni M, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179:1029–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katsuta T, Shimizu N, Okada K, et al. The clinical characteristics of pediatric coronavirus disease 2019 in 2020 in Japan. Pediatr Int. 2022;64:e14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoji K, Akiyama T, Tsuzuki S, et al. Clinical characteristics of hospitalized COVID-19 in children: report from the COVID-19 registry in Japan. J Pediatric Infect Dis Soc. 2021;10:1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmermann P, Pittet LF, Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J. 2021;40:e482–e487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aizawa Y, Takanashi S, Ogimi C. Updates on coronavirus disease 2019 in children in Japan. Pediatr Infect Dis J. 2022;41:e461–e467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bova SM, Serafini L, Serati I, et al. Seizures may be an early sign of acute COVID-19, and the Omicron variant could present a more epileptogenic profile. Acta Paediatr. 2022;111:1814–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludvigsson JF. Convulsions in children with COVID-19 during the Omicron wave. Acta Paediatr. 2022;111:1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurd M, Hashavya S, Benenson S, et al. Seizures as the main presenting manifestation of acute SARS-CoV-2 infection in children. Seizure. 2021;92:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman DL, Aldrich ML, Hagmann SHF, et al. Compassionate use of remdesivir in children with severe COVID-19. Pediatrics. 2021;147:e2020047803. [DOI] [PubMed] [Google Scholar]
- 10.Gilead Sciences I. Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of Remdesivir (GS-5734™) in Participants From Birth to < 18 Years of Age With Coronavirus Disease 2019 (COVID-19) (CARAVAN). 2020. Available at: https://www.clinicaltrials.gov/ct2/show/NCT04431453. Accessed November 2, 2022.
- 11.Mendez-Echevarria A, Perez-Martinez A, Gonzalez Del Valle L, et al. Compassionate use of remdesivir in children with COVID-19. Eur J Pediatr. 2021;180:1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi SH, Choi JH, Yun KW. Therapeutics for the treatment of coronavirus disease 2019 in children and adolescents. Clin Exp Pediatr. 2022;65:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. 2022. Available at: https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/remdesivir/. Accessed November 2, 2022. [PubMed]
- 14.Brewster RC, Parsons C, Laird-Gion J, et al. COVID-19-associated croup in children. Pediatrics. 2022;149:e2022056492. [DOI] [PubMed] [Google Scholar]
- 15.Tunҫ EM, Koid Jia Shin C, Usoro E, et al. Croup during the coronavirus disease 2019 omicron variant surge. J Pediatr. 2022;247:147–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dasdemir S, Uysal Yazici M, Gudeloglu E, et al. Croup as a previously unrecognized symptom of COVID-19 in infants. Pediatr Infect Dis J. 2022;41:e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institute of Infectious Diseases. Epidemiology of COVID-19 fatality cases under 20 years of age. 2022. Available at: https://www.niid.go.jp/niid/ja/2019-ncov/2559-cfeir/11480-20-2022-8-31.html. Accessed November 2, 2022. (in Japanese)
- 18.Molteni E, Sudre CH, Canas LS, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health. 2021;5:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radtke T, Ulyte A, Puhan MA, et al. Long-term symptoms After SARS-CoV-2 infection in children and adolescents. JAMA. 2021;326:869–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zavala M, Ireland G, Amin-Chowdhury Z, et al. Acute and persistent symptoms in children with polymerase chain reaction (PCR)-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection compared with test-negative children in England: active, prospective, national surveillance. Clin Infect Dis. 2022;75:e191–e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osmanov IM, Spiridonova E, Bobkova P, et al. ; and the Sechenov StopCOVID Research Team. Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: a prospective cohort study. Eur Respir J. 2022;59:2101341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ministry of Health Labour and Welfare. Epidemiology of COVID-19 in Japan. 2022. Available at: https://www.mhlw.go.jp/content/10906000/000934908.pdf. Accessed November 2, 2022. (in Japanese)
- 23.Menni C, Valdes AM, Polidori L, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399:1618–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]