Background:
Asymptomatic SARS-CoV-2 infections have raised concerns for public health policies to manage epidemics. This systematic review and meta-analysis aimed to estimate the age-specific proportion of asymptomatic SARS-CoV-2 infected persons globally by year of age.
Methods:
We searched PubMed, Embase, medRxiv and Google Scholar on September 10, 2020, and March 1, 2021. We included studies conducted during January to December 2020, before routine vaccination against COVID-19. Because we expected the relationship between the asymptomatic proportion and age to be nonlinear, multilevel mixed-effects logistic regression (QR decomposition) with a restricted cubic spline was used to model asymptomatic proportions as a function of age.
Results:
A total of 38 studies were included in the meta-analysis. In total, 6556 of 14,850 cases were reported as asymptomatic. The overall estimate of the proportion of people who became infected with SARS-CoV-2 and remained asymptomatic throughout infection was 44.1% (6556/14,850, 95% CI: 43.3%–45.0%). The predicted asymptomatic proportion peaked in children (36.2%, 95% CI: 26.0%–46.5%) at 13.5 years, gradually decreased by age and was lowest at 90.5 years of age (8.1%, 95% CI: 3.4%–12.7%).
Conclusions:
Given the high rates of asymptomatic carriage in adolescents and young adults and their active role in virus transmission in the community, heightened vigilance and public health strategies are needed among these individuals to prevent disease transmission.
Keywords: asymptomatic proportion, SARS, CoV, 2 infection, systematic review, meta, analysis
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic causing coronavirus disease 2019 (COVID-19) has had a profound impact on public health, our daily life and economies around the world. Asymptomatic infections have raised concerns about public health policies for managing epidemics because they are a potential source of transmission of the virus and a challenge for controlling the pandemic.1,2 A large number of systematic reviews have been conducted to determine the contribution of asymptomatic infection to SARS-CoV-2 transmission.1,3–21 Previous researchers attempted to synthesize the best available evidence in different age groups such as children, adults and elderly.5,7,11,13,16 None, however, have investigated the proportion of asymptomatic SARS-CoV-2 infections throughout the course of infection by age. This review, therefore, aims to (1) identify, assess and synthesize the evidence on the proportion of people infected with SARS-CoV-2 who were asymptomatic throughout the course of infection, and (2) to estimate asymptomatic proportion by age. Although our research was conducted up to March 2021, studies included in this systematic review were completed during January to December 2020, before routine vaccination against COVID-19 and the emergence of the alpha, delta or omicron variants.
MATERIALS AND METHODS
Study Design
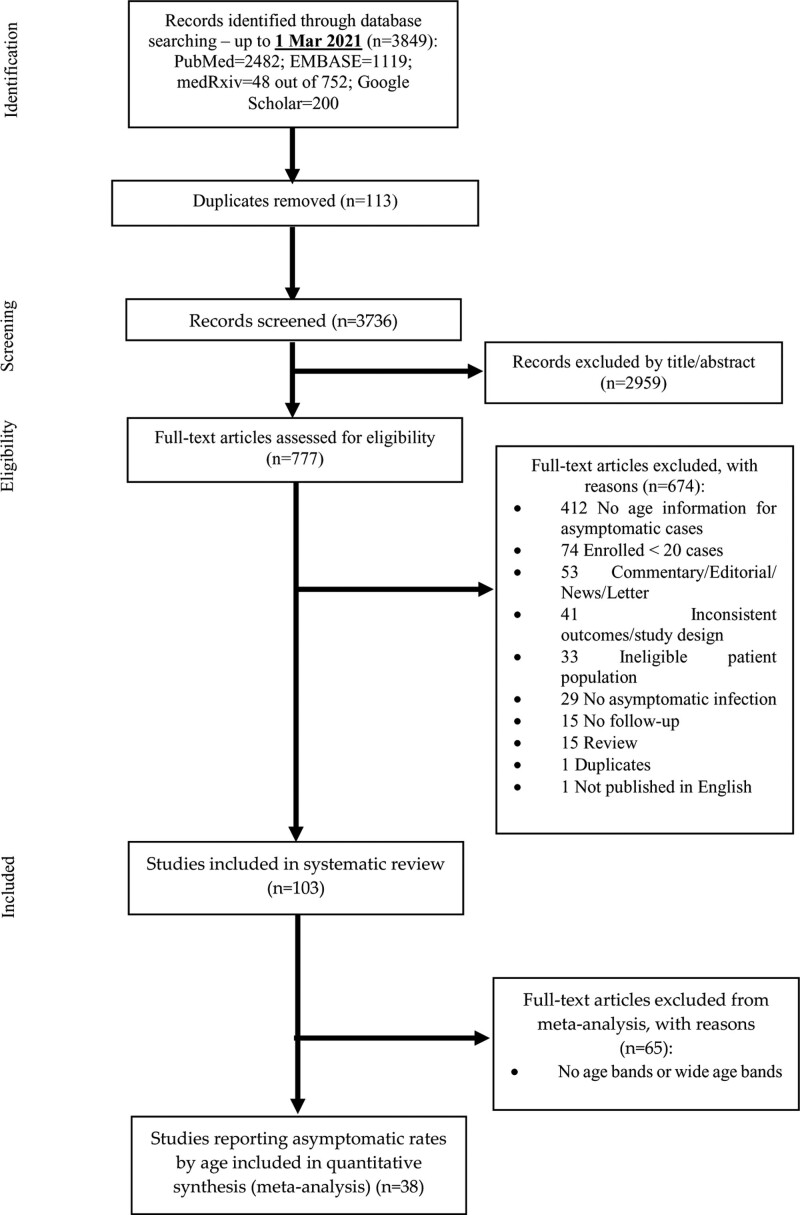
We conducted this systematic review and meta-analysis based on the statement of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines22 (Fig. 1). The study protocol was registered in the International Prospective Register for Systematic Reviews (PROSPERO, registration number: CRD42020209419).
FIGURE 1.
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for article inclusion and exclusion.
The following study characteristics were considered when estimating the proportion of asymptomatic infections: study period, study population, country, SARS-CoV-2 infection definition, asymptomatic case definition and follow-up period.
Search Strategy
We searched PubMed, Embase, medRxiv and Google Scholar on September 10, 2020, and March 1, 2021, using keywords COVID-19, SARS-CoV-2, 2019-nCoV, coronavirus disease 2019 and asymptomatic. We only included studies conducted during January to December 2020, before routine vaccination against COVID-19 and the emergence of the alpha, delta or omicron variants. Additionally, this review did not identify or include any studies from regions with the β variant, which was first detected in South Africa in September 2020. Specific search terms suitable to the individual databases were developed. These search terms included combinations of Medical Subject Headings (MeSH)/Emtree and text words contained in the title and abstract. Preprints were searched using “covid asymptomatic” (match all words in the abstract or title) in medRxiv. There were 48 of 752 manuscripts were found to be relevant to asymptomatic SARS-CoV-2 infection. Google Scholar was searched using key words (covid|coronavirus|SARS-CoV-2+asymptomatic+proportion|prevalence|epidemiology) and the first 200 results saved and screened.
Selection Criteria
The article selection process occurred in 2 phases: (1) title and abstract screen: titles and abstracts of articles identified from the electronic databases and from Internet searches were reviewed; (2) full text review: the full text of articles selected at the title and abstract screen were obtained and reviewed for eligibility. The screening process was completed according to a predefined protocol (Supplemental Digital Content 1, http://links.lww.com/INF/E887). We included all studies reporting
Proportion of asymptomatic persons among all SARS-CoV-2 infected persons. The numerator includes all SARS-CoV-2 positive persons who were asymptomatic. The denominator includes all SARS-CoV-2 positive persons who tested positive.
Prevalence of asymptomatic SARS-CoV-2 positive persons among the defined general population. The numerator includes all SARS-CoV-2 positive persons who were asymptomatic. The denominator is the defined study population who were tested for SARS-CoV-2 (e.g., general population in the local community, healthcare workers, patients on hospital admission, nursing home residents).
Asymptomatic infection: a person with confirmed SARS-CoV-2 infection, who has no symptoms at the time of screening (including the first clinical assessment or laboratory test) and had no symptoms throughout the follow-up period. We aimed to include all studies enrolling infected individuals who were symptomatic and asymptomatic. At the beginning of the COVID-19 pandemic, respiratory symptoms and fever were considered consistent with symptomatic SARS-CoV-2 infection. As the pandemic evolved, a broader spectrum of clinical manifestations associated with COVID-19 have been recognized such as loss of the senses of smell (anosmia) and taste (ageusia). In some countries, chest imaging has been used to diagnose and monitor the disease for patients without respiratory symptoms. Consequently, some studies defined asymptomatic infection as no clinical symptoms and absence of abnormal chest imaging findings. In this review, we did not limit studies using different definitions of symptomatic disease or asymptomatic infection as absence of these symptoms. We only attempted to exclude patients who were previously asymptomatic and later became symptomatic, and therefore excluded studies without any follow-up.
This review aimed to fit a statistical model to the real-world data and estimate the proportion of asymptomatic infections by age. We acknowledged that isolation policies have constantly changed over time and significantly varied between countries. For example, in some countries, all SARS-CoV-2 infected persons were admitted to hospital for treatment or isolation whether they presented with symptoms. Universal COVID-19 testing programs have been implemented in some countries. We included all studies aiming to enroll infected individuals who were symptomatic and asymptomatic. We excluded studies that purposely selected SARS-CoV-2 infected cases and did not enroll cases consecutively. We did not exclude any studies enrolling cases in specific study populations, and only excluded
studies published in languages other than English.
comments, letters, editorials, consensus reports and reviews.
studies that did not report any age information (e.g., mean or median age) for asymptomatic infections.
studies that clearly stated that the SARS-CoV-2 infected persons were included without any follow up and did not distinguish between asymptomatic and presymptomatic infections.
studies that only tested and enrolled asymptomatic persons and mild cases.
case studies, case reports and case series with fewer than 20 SARS-CoV-2 infected persons.
case studies, case reports and case series that identified SARS-CoV-2 positive persons through contact tracing where only symptomatic persons were tested.
serology studies that did not check history of symptoms compatible with SARS-CoV-2 infection and enrolled cases confirmed with SARS-CoV-2 infection by use of IgM only.
Data Extraction
We did not assess study quality because the critical appraisal tools, which we planned to use are research design-specific, preclude comparison of the quality of different study designs, and cannot reflect heterogeneity of studies reporting proportions with asymptomatic infections. We did, however, consider several methodological factors in the inclusion/exclusion criteria such as the follow-up period and case identification method. Eight authors (BW, PA, SE, HM, ZL, AT, CB, SG) used an online form in Covidence or a Microsoft Excel spreadsheet to extract the following information: study design, setting, study period, study population (sample size, mean or median age, case definition, etc.), country, follow-up duration, and outcomes (number of people sampled/tested, total number of SARS-CoV-2 positive persons, number of asymptomatic SARS-CoV-2 positive persons).
Statistical Analysis
Most studies reporting prevalence of asymptomatic persons among the tested population were cross-sectional community screening studies without regular follow-up of SARS-CoV-2 positive persons. We excluded screening studies which clearly stated in the Methods or Discussion sections that they did not follow up any cases or could not distinguish between asymptomatic and presymptomatic cases. The number of screening studies, which were eligible and included in our review, was small (n = 3). Therefore, the percentage of asymptomatic cases among the tested population was not assessed in the meta-analysis. We only assessed percentage of asymptomatic infections among the confirmed population with laboratory confirmed/clinically diagnosed SARS-CoV-2 infections based on history of exposure to SARS-CoV-2 infection and suggestive clinical symptoms of pneumonia (Fig. 1).
We used a published method to assess the effect of age on proportion of asymptomatic SARS-CoV-2 positive persons.21,22 We anticipated the relationship between the asymptomatic proportion and age in years to be nonlinear based on previous reviews.23,24 A multilevel mixed-effects logistic regression (QR decomposition) model with a restricted cubic spline was performed to model the asymptomatic proportion as a function of age in years. The restricted cubic spline with 5 knots placed at the ages of 1.4, 13.5, 33.3, 54.5 and 80.4 years was applied, based on Harrell’s recommended percentiles.25 Studies were nested within region/country as nested random effects. The model allows for multilevels of nested clusters of random effects on the assumption that observations within the same cluster are correlated. The outcome measure was the number of asymptomatic persons observed in the study population recorded in binomial form, with the number of SARS-CoV-2 positive persons in the study population as the denominator. When the mean age was not available, we estimated the mean age for each age group using the midpoint of the age band. Any studies reporting the proportion of asymptomatic infections using an age band wider than 20 years were excluded from the meta-analysis. Additional efforts were made to contact the first authors for more information where needed. All analyses were performed using Stata 16.1.26
RESULTS
There were 114 eligible studies. Because a few narrative review and subgroup analyses by age group (e.g., children and adults) have previously been published,5,7,11,13,16 only the results of the meta-analysis are discussed here (Fig. 1).
Study Characteristics
A total of 38 studies involving 14,850 persons were included in the meta-analysis (see Table, Supplemental Digital Content 2, http://links.lww.com/INF/E888) including 13 pediatric studies (n = 2729), 8 studies with adults only (n = 1156), and 17 studies with children and adults (n = 10,965), with an age range of 0 to 100 years. Gender was reported in 37 studies including 8931 (61.2%) males and 5574 (38.4%) females. Of 14,850 SARS-CoV-2 positive persons included in the meta-analysis, 5498 were from China, 3643 from India, 1519 from Saudi Arabia, 1255 from Bangladesh, 539 from the United States, 417 from Kuwait, 230 from Croatia, 220 from Nepal, 213 from Italy, 203 from Greece, 1113 from the rest of the world. Study settings ranged from community screening to hospital treatment/isolation. In 27 studies, SARS-CoV-2 positive patients were enrolled and followed up in the hospital setting.27–53 Only 3 community/international traveler/repatriation screening studies54–56 were included as those studies reported follow-up outcomes. In addition, 7 disease surveillance studies were included with follow-up outcomes presented in the publication57–61 or correspondence with authors.32,61 One online survey reporting follow-up outcomes was also included.62 In total, 14 studies including the retrospective online survey,31,36,38,40,41,45,52,53,57,60–64 which did not clearly state the follow-up period but presented follow-up outcomes, were included in the meta-analysis. The remaining studies followed up patients during the defined follow-up period or during hospital admission. Most SARS-CoV-2 infections were confirmed by RT-PCR. Five patients tested negative by RT-PCR and were diagnosed with SARS-CoV-2 infection, based on a known clinical exposure to a positive household contact and suggestive clinical symptoms.38,41,49 Only 1 patient had a negative SARS-CoV-2 test result and was diagnosed according to the clinical diagnostic criteria.49 There were 17 patients diagnosed by serology testing.40,54 The proportion with asymptomatic infection ranged from 0 to 91.0% with an overall proportion of 44.1%.
Meta-Analysis
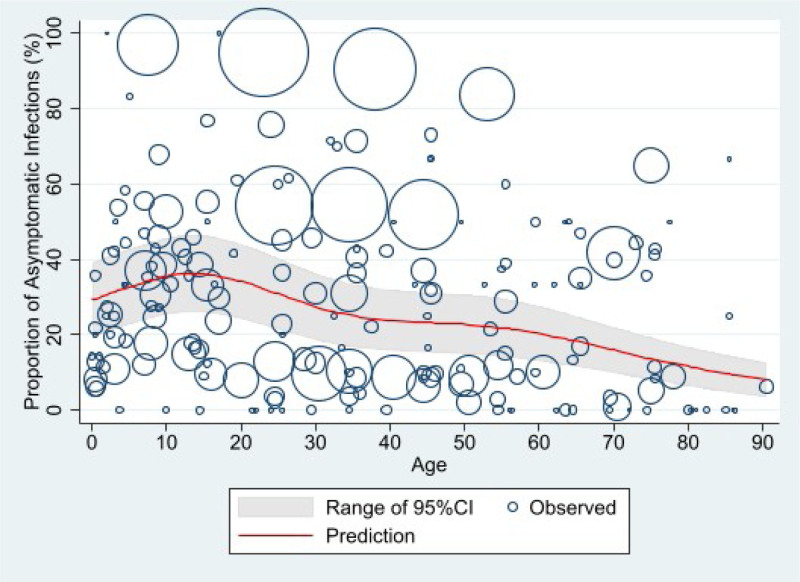
In total, 6556 of 14,850 persons (44.1%, 95% CI: 43.3%–45.0%) were reported as asymptomatic throughout the course of infection. The predicted asymptomatic proportion peaked (36.2%, 95% CI: 26.0%–46.5%) at 13.5 years of age, then gradually decreased, leveling out in adults 40–50 years old, before dropping to 8.1% (95% CI: 3.4%–12.7%) by 90.5 years (Fig. 2).
FIGURE 2.
Predicted proportion of asymptomatic SARS-CoV-2 infection by age*. *The size of each circle is proportional to the total number of SARS-CoV-2 positive persons reported in each age group in individual studies, with larger circles indicating a larger sample size.
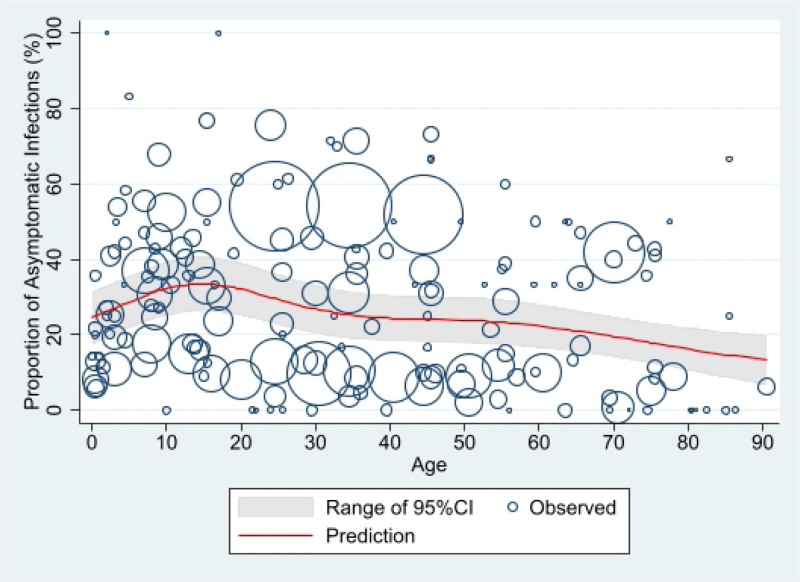
In the sensitivity analysis, 3 studies with a very low or high proportion of asymptomatic infection were excluded to investigate the effect of outliers. In a family cluster study conducted in China,46 although the proportion of asymptomatic infection was 0, 20 of 22 cases had mild symptoms with only 2 having moderate to severe clinical manifestations. In this study, a case without any symptoms during the whole course of the disease had a CT chest scan suggestive of pathological changes in both lungs. In a disease surveillance study conducted in Korea,64 of the total 18,303 COVID-19 tests performed between 16 January 2020 and 24 March 2020 in Busan, 108 had positive PCR results (positive test rate, 0.6%). Contacts with high exposure levels, such as family members, were instructed to get tested regardless of the presence or absence of symptoms. The authors were contacted and provided updated information on clinical outcomes. Among the asymptomatic patients (n = 12) at diagnosis, 4 patients were completely asymptomatic (proportion of asymptomatic infections, 3.7%) and 8 patients developed symptoms during the follow up period. Cough and fever were the most common symptoms in symptomatic patients. In another disease surveillance study conducted in India,63 among the 3404 SARS-CoV-2 positive cases, 3096 (91%) were asymptomatic. Asymptomatic cases were defined as those with a positive PCR test in the absence of symptoms. The study included all cases diagnosed with SARS-CoV-2 infection in the Karnataka state, reported from March 8 to May 31, 2020. In this disease surveillance study, the authors reported Karnataka state had better contact tracing in the country during the early stage of the pandemic by detecting 47.4 contacts per confirmed COVID-19 case. During the study period, the Karnataka state was testing approximately 4377 per million people, with a positivity rate of 1.1%. The authors also stated that all the cases during the lockdown period were hospitalized and were under medical observation for 14 days, and therefore the chance of misclassification is unlikely. After removing those 3 studies, the peak predicted value was lower (33.5% [95% CI: 26.2%–40.8%] at 14.5 years old) than that of the primary analysis, but the overall trend was still like the primary analysis (Fig. 3).
FIGURE 3.
Predicted proportion of asymptomatic SARS-CoV-2 infection by age after removing 3 studies with a very low or high proportion of asymptomatic infection*. *The size of each circle is proportional to the total number of SARS-CoV-2 positive persons reported in each age group in individual studies, with larger circles indicating a larger sample size.
DISCUSSION
COVID-19 vaccines are highly effective at preventing severe illness, hospitalizations, and death, and have been critical to controlling the COVID-19 pandemic to restore normal social and economic life.65 The COVID-19 vaccine rollout has been extended to children from 6 months old of age in many countries including the United States, Australia and Europe. Newly authorized Bivalent COVID-19 booster vaccines have become available in the United States, United Kingdom and Canada.66 COVID-19 Vaccination has been shown to contribute to reducing deaths and severe illness from COVID-19, and to reduce the transmission of COVID-19.67 Children and young people have been infected with the Delta or Omicron variants resulting from high transmissibility and as they remain an undervaccinated group. Previous reviews and meta-analyses5,7,11,13,16 have demonstrated that children have the highest proportion of asymptomatic infections, which may jeopardize efforts to prevent transmission within a community. However, these reviews only investigated the proportion with asymptomatic infection across wide age ranges such as adults and children. Our review and meta-analysis are the first to calculate more granular estimates of asymptomatic SARS-CoV-2 infection across the age range.
There have been many systematic reviews conducted including narrative reviews and meta-analyses.1,3–21 However, all meta-analyses used random-effect models to estimate the pooled percentages of asymptomatic infections and presented Forest plots. Our analysis fits a statistical model to the real-world data and used age to predict the proportion of asymptomatic infections. Age was a continuous variable in our model to better demonstrate effect of age (in years) on proportion of asymptomatic SARS-CoV-2 positive persons, but not an ordered factor tied to specific age ranges. Since previous meta-analyses only estimated the pooled percentage of asymptomatic infection for specific age ranges of SARS-CoV-2 positive persons (e.g., <20 years, 20–39 years, 40–59 year, ≥60 years), we were not able to compare our results with previous studies. We found a high proportion of asymptomatic infections in children and young people consistent with previous reviews, which reported the highest proportions in children, and lower in adults, especially older adults.5,7,11,13,16 A recent review estimated the pooled percentage of asymptomatic infection to be 41%,11 which is like our study result of 44%. Four other reviews reported that at least one-third of SARS-CoV-2 infections were asymptomatic.4,12,15,18 Systematic reviews which were conducted during the early stages of the COVID-19 pandemic,5–7,9,10,17,19 however, found that the proportion with asymptomatic infection was much lower than our estimates (13–24%), except for 1 review13 reporting a pooled percentage of asymptomatic cases of 48%. This review13 included 16 studies in total. Four of the 16 studies68–71 enrolled only patients with asymptomatic SARS-CoV-2 infection. Another review also found that the reported proportion of asymptomatic infections was lower before February 2020 (10%) than after (34%).16 This may be caused by changes in testing practices, mitigation measures and dynamics of different circulating variants over time.
Our systematic review aimed to use the real-world data and estimate the proportion of asymptomatic infection globally, rather than in a well-controlled environment with a single definition of symptomatic and asymptomatic infection, COVID-19 contract tracing and isolation policy, healthcare setting, COVID-19 testing policy and surveillance system. The ideal study design would be a longitudinal cohort study ensuring the study sample is representative, with asymptomatic infections followed over a well-defined follow up period. Because we attempted to include all studies aiming to enroll infected individuals with or without symptoms, high heterogeneities were found in the studies included in our meta-analysis. Testing and isolation policies, study settings, follow-up period and definition of SARS-CoV-2 infection and asymptomatic cases varied between studies. The study settings ranged from hospital admission to universal screening. COVID-19 disease control policies varied between countries. In some countries such as China and Korea, most infected individuals were hospitalized for treatment or isolation regardless of being symptomatic or asymptomatic. In most other countries, asymptomatic cases have only been required to isolate at home. In studies involving hospital admission, the case notes of hospitalized patients were retrospectively reviewed and proportions of asymptomatic cases reported. Almost two-thirds of studies included in the review were studies involving hospitalized patients. Consequently, the proportion of asymptomatic infections may be underestimated if symptomatic patients were more likely to be admitted to hospital. Only a small number of community screening studies were identified in our review, mainly because they were cross-sectional and did not follow up asymptomatic individuals. In the inpatient/outpatient screening studies, patients were admitted to hospital for non-COVID-19 conditions such as obstetric admission, dialysis and elective surgery, which may not represent the broader population in terms of infection and transmission risk. Additionally, asymptomatic infection rates may be overestimated in COVID-19 testing clinics and outbreak settings such as passengers of cruise ships and airplanes.
The follow up period varied significantly between studies. Each publication was meticulously scrutinized, and studies were excluded where asymptomatic infections could not adequately be distinguished from presymptomatic infections. In the hospital admission studies, cases were frequently followed up. Duration of follow-up varied from a predefined period of days after test positivity or until 2 negative PCR tests at least 24 hours apart. Some studies, however, did not specify the duration of follow-up in the publication.
One of the limitations of our review is that the definitions of SARS-CoV-2 infection and asymptomatic infection were inconsistently used across studies. At the beginning of the pandemic, mainly respiratory symptoms were considered. Over the past 3 years, a broad range of clinical symptoms have been recognized. Diagnosis criteria have also been changed over time. We appreciate that knowledge gaps existed at the early stage of the pandemic, and therefore did not limit studies based on their definition of symptomatic and asymptomatic infection. Some studies defined SARS-CoV-2 infections using clinical diagnostic criteria including radiology findings without a positive PCR/serology test. Although most patients included in our meta-analysis were diagnosed on PCR testing, 23 patients were diagnosed based on serology testing, history of exposure to SARS-CoV-2 infection and suggestive clinical symptoms of pneumonia but without a positive PCR test. Because the number was small, those patients were included.
Because the onset of the SARS-CoV-2 pandemic, multiple new variants of concern have emerged, including the Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B.1.1.529 and BA.2). In December 2020, the United Kingdom was first country to start the COVID-19 vaccine rollout followed by other countries around the world. The Delta variant first detected in India in October 2020 and became the dominant variant globally until the Omicron variant emerged and spread rapidly across the world in November 2021. The population immunity gained through a combination of infection and vaccination has increased over time. Both variants are more transmissible than previously circulating strains and Delta has been shown to cause more severe disease in adults compared with Alpha.72 It is difficult to determine whether Omicron intrinsically causes milder disease than previous variants of concern. The proportion of asymptomatic infections with Omicron is estimated to be much higher,73 which may facilitate more rapid transmission in addition to the variant’s ability to invade both natural and vaccine-induced immunity.74 Our review included studies published before March 1, 2021, and only included from prevaccine studies era. The proportion of asymptomatic infections reported in our review does not reflect current epidemiological features of the Delta or Omicron variant. The epidemiology of early variants is different to Omicron.
Our findings are consistent with these previous reviews and support the concept that proportions of asymptomatic infection in children and adolescents were higher than adults. However, childhood SARS-CoV-2 infection in children is not without its complications, including Multisystem Inflammatory Syndrome in Children associated with COVID-19.75 We need to continue to monitor this group closely, especially given that vaccine recommendations differ widely across the globe.
ACKNOWLEDGMENTS
We express our appreciation to Ms. Natalie Dempster for her generous support and assistance with the literature search. We also thank Professor Chang-Hoon Kim at Busan Center for infectious Disease Control and Prevention, Korea, and Dr Guang Han at Hubei Cancer Hospital, China, for kindly providing additional information
Supplementary Material
Footnotes
H.S.M. is an investigator on vaccine trials sponsored by the GSK group of companies, GlaxoSmithKline, Pfizer, Sanofi, and Merck. H.S.M.’s, B.W.’s, P.A.’s, and H.M.’s institution receives funding for investigator-led studies from industry, including Pfizer and Sanofi Pasteur; H.S.M., B.W., P.A., and H.M. receive no personal payments from industry. Others have no potential conflicts of interest. The authors did not receive funding for this project.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Prabha Andraweera, Email: prabha.andraweera@adelaide.edu.au.
Salenna Elliott, Email: salenna.elliott@uq.edu.au.
Hassen Mohammed, Email: hassen.mohammed@adelaide.edu.au.
Zohra Lassi, Email: zohra.lassi@adelaide.edu.au.
Ashley Twigger, Email: ashley.twigger@sa.gov.au.
Chloe Borgas, Email: Chloe.Borgas@sa.gov.au.
Shehani Gunasekera, Email: Shehani.Gunasekera@sa.gov.au.
Shamez Ladhani, Email: shamez.ladhani@phe.gov.uk.
Helen Siobhan Marshall, Email: helen.marshall@adelaide.edu.au.
REFERENCES
- 1.Kronbichler A, Kresse D, Yoon S, et al. Asymptomatic patients as a source of COVID-19 infections: a systematic review and meta-analysis. Int J Infect Dis. 2020;98:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller CP. Do asymptomatic carriers of SARS-COV-2 transmit the virus? Lancet Reg Health Eur. 2021;4:100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Sadeq DW, Nasrallah GK. The incidence of the novel coronavirus SARS-CoV-2 among asymptomatic patients: a systematic review. Int J Infect Dis. 2020;98:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alene M, Yismaw L, Assemie MA, et al. Magnitude of asymptomatic COVID-19 cases throughout the course of infection: a systematic review and meta-analysis. PLoS One. 2021;16:e0249090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buitrago-Garcia D, Egli-Gany D, Counotte MJ, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med. 2020;17:e1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byambasuren O, Cardona M, Bell K, et al. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. Official J Assoc Med Microbiol Infect Dis Canada. 2020;5:223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Huang Z, Wang J, et al. Ratio of asymptomatic COVID-19 cases among ascertained SARS-CoV-2 infections in different regions and population groups in 2020: a systematic review and meta-analysis including 130 123 infections from 241 studies. BMJ Open. 2021;11:e049752e049752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Z, Xu Y, Sun C, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 2021;54:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He J, Guo Y, Mao R, et al. Proportion of asymptomatic coronavirus disease 2019: a systematic review and meta-analysis. J Med Virol. 2021;93:820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoang A, Chorath K, Moreira A, et al. COVID-19 in 7780 pediatric patients: a systematic review. EClinicalMedicine. 2020;24:100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Q, Liu J, Liu Q, et al. Global Percentage of asymptomatic SARS-CoV-2 infections among the tested population and individuals with confirmed COVID-19 diagnosis: a systematic review and meta-analysis. JAMA Network Open. 2021;4:e2137257e21372–e2137257e257-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sah P, Fitzpatrick MC, Zimmer CF, et al. Asymptomatic SARS-CoV-2 infection: a systematic review and meta-analysis. Proc Natl Acad Sci USA. 2021;118:e2109229118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Syangtan G, Bista S, Dawadi P, et al. Asymptomatic SARS-CoV-2 carriers: a systematic review and meta-analysis. Front Public Health. 2021;8:1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanes-Lane M, Winters N, Fregonese F, et al. Proportion of asymptomatic infection among COVID-19 positive persons and their transmission potential: a systematic review and meta-analysis. PLoS One. 2020;15:e0241536e0241536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oran DP, Topol EJ. The Proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review. Ann Intern Med. 2021;174:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C, Zhu C, Yan D, et al. The epidemiological and radiographical bing.wang@adelaide.edu.au.characteristics of asymptomatic infections with the novel coronavirus (COVID-19): a systematic review and meta-analysis. Int J Infect Dis. 2021;104:458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beale S, Hayward A, Shallcross L, et al. A rapid review and meta-analysis of the asymptomatic proportion of PCR-confirmed SARS-CoV-2 infections in community settings [version 1; peer review: 1 approved with reservations]. Wellcome Open Res. 2020;5:266. [Google Scholar]
- 18.Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection. Ann Intern Med. 2021;174:286–287. [DOI] [PubMed] [Google Scholar]
- 19.Cui X, Zhao Z, Zhang T, et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J Med Virol. 2021;93:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravindra K, Malik VS, Padhi BK, et al. Asymptomatic infection and transmission of COVID-19 among clusters: systematic review and meta-analysis. Public Health. 2022;203:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buitrago-Garcia D, Ipekci AM, Heron L, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: update of a living systematic review and meta-analysis. PLoS Med. 2022;19:e1003987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 23.Wang B, Santoreneos R, Giles L, et al. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine. 2019;37:2768–2782. [DOI] [PubMed] [Google Scholar]
- 24.Christensen H, May M, Bowen L, et al. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:853–861. [DOI] [PubMed] [Google Scholar]
- 25.Harrell FE. General aspects of fitting regression models. Regression modeling strategies. Springer; 2015:13–44. [Google Scholar]
- 26.Statistical Software: Release 16.1 (StataCorp, 2019) [Internet]. Available at: https://www.stata.com. Accessed November 27, 2022.
- 27.Alshukry A, Ali H, Ali Y, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) patients in Kuwait. PLoS One. 2020;15:e0242768e0242768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhandari S, Bhargava A, Sharma S, et al. Clinical profile of Covid-19 infected patients admitted in a tertiary care hospital in North India. J Assoc Physicians India. 2020;68:13–17. [PubMed] [Google Scholar]
- 29.Chaudhary A, Singh UN, Paudel P, et al. Characteristics and outcomes of hospitalized adults with COVID-19 in Nepal: a multicenter, prospective cohort study. J Infect Dev Ctries. 2022;16:469–477. [DOI] [PubMed] [Google Scholar]
- 30.Chua GT, Xiong X, Choi EH, et al. COVID-19 in children across three Asian cosmopolitan regions. Emerg Microbes Infect. 2020;9:2588–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grechukhina O, Greenberg V, Lundsberg LS, et al. Coronavirus disease 2019 pregnancy outcomes in a racially and ethnically diverse population. Am J Obstet Gynecol MFM. 2020;2:100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan S, Ali A, Shi H, et al. COVID-19: Clinical aspects and therapeutics responses. Saudi Pharm J. 2020;28:1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krajcar N, Stemberger Marić L, Šurina A, et al. Epidemiological and clinical features of Croatian children and adolescents with a PCR-confirmed coronavirus disease 2019: differences between the first and second epidemic wave. Croat Med J. 2020;61:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Thoon KC, Chong CY, et al. Comparative analysis of symptomatic and asymptomatic SARS-CoV-2 infection in children. Ann Acad Med Singap. 2020;49:530–537. [PubMed] [Google Scholar]
- 35.Liu S, Yuan C, Lin J, et al. Association between vaccinations and clinical manifestations in children with COVID-19. Transl Pediatr. 2021;10:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Lv J, Gan L, et al. Comparative analysis of clinical characteristics, imaging and laboratory findings of different age groups with COVID-19. Indian J Med Microbiol. 2020;38:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mannan A, Mehedi HMH, Chy N, et al. A multi-centre, cross-sectional study on coronavirus disease 2019 in Bangladesh: clinical epidemiology and short-term outcomes in recovered individuals. New Microbes New Infect. 2021;40:100838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcus N, Frizinsky S, Hagin D, et al. Minor Clinical Impact of COVID-19 Pandemic on Patients With Primary Immunodeficiency in Israel. Front Immunol. 2020;11:614086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martini F, D’Alessio A, Bracchi F, et al. On cancer, COVID-19, and CT scans: a monocentric retrospective study. J Clin Med. 2020;9:3935. doi:10.3390/jcm9123935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyts I, Bucciol G, Quinti I, et al. ; IUIS Committee of Inborn Errors of Immunity. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2021;147:520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panagiotakopoulos L, Myers TR, Gee J, et al. SARS-CoV-2 infection among hospitalized pregnant women: reasons for admission and pregnancy characteristics - Eight U.S. health care centers, March 1-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parri N, Lenge M, Cantoni B, et al. COVID-19 in 17 Italian pediatric emergency departments. Pediatrics. 2020;146:e20201235. doi:10.1542/peds.2020-1235. [DOI] [PubMed] [Google Scholar]
- 43.Peng X, Guo Y, Xiao H, et al. Overview of chest involvement at computed tomography in children with coronavirus disease 2019 (COVID-19). Pediatr Radiol. 2021;51:222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pongpirul WA, Wiboonchutikul S, Charoenpong L, et al. Clinical course and potential predictive factors for pneumonia of adult patients with Coronavirus Disease 2019 (COVID-19): A retrospective observational analysis of 193 confirmed cases in Thailand. PLoS NeglTrop Dis. 2020;14:e0008806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sahu AK, Dhar A, Aggarwal B. Radiographic features of COVID-19 infection at presentation and significance of chest X-ray: early experience from a super-specialty hospital in India. Indian J Radiol Imaging. 2021;31(Suppl 1):S128–S133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song R, Han B, Song M, et al. Clinical and epidemiological features of COVID-19 family clusters in Beijing, China. J Infect. 2020;81:e26–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun D, Zhu F, Wang C, et al. Children infected with SARS-CoV-2 from family clusters. Front Pediatr. 2020;8:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Temel H, Gündüz M, Arslan H, et al. Evaluation of the clinical features of 81 patients with COVID-19: an unpredictable disease in children. J Pediatr Infect Dis. 2021;16:047–052. [Google Scholar]
- 49.Wu X, Sun R, Chen J, et al. Radiological findings and clinical characteristics of pregnant women with COVID-19 pneumonia. Int J Gynaecol Obstet. 2020;150:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu H, Liu E, Xie J, et al. A follow-up study of children infected with SARS-CoV-2 from Western China. Ann Transl Med. 2020;8:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan X, Han X, Peng D, et al. Clinical characteristics and prognosis of 218 patients with COVID-19: a retrospective study based on clinical classification. Front Med (Lausanne). 2020;7:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yayla BCC, Aykac K, Ozsurekci Y, et al. Characteristics and management of children With COVID-19 in a tertiary care hospital in Turkey. Clin Pediatr. 2021;60:170–177. [DOI] [PubMed] [Google Scholar]
- 53.Zhang S, Guo M, Wu F, et al. Factors associated with asymptomatic infection in health-care workers with severe acute respiratory syndrome coronavirus 2 infection in Wuhan, China: a multicentre retrospective cohort study. Clin Microbiol Infect. 2020;26:1670–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bailie CR, Franklin L, Nicholson S, et al. Symptoms and laboratory manifestations of mild COVID-19 in a repatriated cruise ship cohort. Epidemiol Infect. 2021;149:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lavezzo E, Franchin E, Ciavarella C, et al. ; Imperial College COVID-19 Response Team. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature. 2020;584:425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ren R, Zhang Y, Li Q, et al. Asymptomatic SARS-CoV-2 infections among persons entering China from April 16 to October 12, 2020. JAMA. 2021;325:489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alsofayan YM, Althunayyan SM, Khan AA, et al. Clinical characteristics of COVID-19 in Saudi Arabia: a national retrospective study. J Infect Public Health. 2020;13:920–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. doi:10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 59.Hurst JH, Heston SM, Chambers HN, et al. Severe acute respiratory syndrome coronavirus 2 infections among children in the biospecimens from respiratory virus-exposed kids (BRAVE Kids) study. Clin Infect Dis. 2021;73:e2875–e2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maltezou HC, Magaziotou I, Dedoukou X, et al. ; for Greek Study Group on SARS-CoV-2 Infections in Children. Children and adolescents with SARS-CoV-2 infection: epidemiology, clinical course and viral loads. Pediatr Infect Dis J. 2020;39:e388–e392. [DOI] [PubMed] [Google Scholar]
- 61.Mattar S, Martinez-Bravo C, Rivero R, et al. Epidemiological and viral features of a cohort of SARS-CoV-2 symptomatic and asymptomatic individuals in an area of the Colombian Caribbean. Ann Clin Microbiol Antimicrob. 2020;19:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hasan MJ, Chowdhury SM, Khan MAS, et al. Clinico-epidemiological characteristics of asymptomatic and symptomatic COVID-19-positive patients in Bangladesh. medRxiv. 2020:2020.08.18.20177089. doi:10.1101/2020.08.18.20177089. [PubMed] [Google Scholar]
- 63.Kumar N, Shahul Hameed SK, Babu GR, et al. Descriptive epidemiology of SARS-CoV-2 infection in Karnataka state, South India: transmission dynamics of symptomatic vs. asymptomatic infections. EClinicalMedicine. 2021;32:100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Son H, Lee H, Lee M, et al. Epidemiological characteristics of and containment measures for COVID-19 in Busan, Korea. Epidemiol Health. 2020;42:e2020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gostin LO, Salmon DA, Larson HJ. Mandating COVID-19 vaccines. JAMA. 2021;325:532–533. [DOI] [PubMed] [Google Scholar]
- 66.Dyer O. US and Canada to roll out the first omicron specific boosters within days. BMJ. 2022;378:o2144. [Google Scholar]
- 67.WHO. Statement for healthcare professionals: How COVID-19 vaccines are regulated for safety and effectiveness (Revised March 2022). Available at: https://www.who.int/news/item/17-05-2022-statement-for-healthcare-professionals-how-covid-19-vaccines-are-regulated-for-safety-and-effectiveness. Accessed September 19, 2022.
- 68.Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meng H, Xiong R, He R, et al. CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia at admission in Wuhan, China. J Infect. 2020;81:e33–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pan Y, Yu X, Du X, et al. Epidemiological and clinical characteristics of 26 asymptomatic severe acute respiratory syndrome coronavirus 2 carriers. J Infect Dis. 2020;221:1940–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y, Liu Y, Liu L, et al. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at hospital admission in Shenzhen, China. J Infect Dis. 2020;221:1770–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nyberg T, Ferguson NM, Nash SG, et al. ; COVID-19 Genomics UK (COG-UK) consortium. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murray CJL. COVID-19 will continue but the end of the pandemic is near. Lancet. 2022;399:417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pulliam JRC, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science. 2022;15:eabn4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Howard-Jones AR, Burgner DP, Crawford NW, et al. COVID-19 in children. II: Pathogenesis, disease spectrum and management. J Paediatr Child Health. 2022;58:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]