Abstract
The objective of this work was to investigate whether impaired insulin secretion can be restored by lifestyle intervention in specific subphenotypes of prediabetes. We assigned 1,045 participants from the Prediabetes Lifestyle Intervention Study (PLIS) to six recently established prediabetes clusters. Insulin secretion was assessed by a C-peptide–based index derived from oral glucose tolerance tests and modeled from three time points during a 1-year intervention. We also analyzed the change of glycemia, insulin sensitivity, and liver fat. All prediabetes high-risk clusters (cluster 3, 5, and 6) had improved glycemic traits during the lifestyle intervention, whereas insulin secretion only increased in clusters 3 and 5 (P < 0.001); however, high liver fat in cluster 5 was associated with a failure to improve insulin secretion (Pinteraction < 0.001). Thus, interventions to reduce liver fat have the potential to improve insulin secretion in a defined subgroup of prediabetes.
Introduction
Prediabetes is a heterogenous condition comprising subphenotypes with different risks of diabetes and its complications (1). From its two key features, insulin resistance and impaired insulin secretion, insulin resistance can be clearly improved by lifestyle intervention (LI); however, it is not known whether LI can improve insulin secretion in specific subphenotypes of reduced insulin secretion (2). Recently, we described six clusters of prediabetic metabolism (1). Two of these clusters (clusters 3 and 5) have a high risk of progression to diabetes. Another group (cluster 6) has an intermediate risk of diabetes as these individuals are capable of compensating insulin resistance via hyperinsulinemia over years. In this study, we retrospectively stratified participants of a large multicenter study into these novel clusters of prediabetic metabolism (1) and investigated whether LI improved their insulin secretion and other glycemic traits.
Research Design and Methods
Study Population
The Prediabetes Lifestyle Intervention Study (PLIS) is a randomized, controlled, multicenter trial testing the efficacy of different intervention intensities in individuals with prediabetes (ClinicalTrials.gov identifier NCT01947595) (2). Participants with prediabetes were divided into a group with a low risk or a group with a high risk for diabetes progression. The low-risk group was then randomized to a control or conventional LI, whereas the high-risk group was randomized to a conventional or intensive LI for 1 year. While the control arm had only one 30-min consultation session with a dietitian, participants in the conventional and intensified arms received 8 or 16 recurring counseling sessions, respectively. The intention of counseling was to decrease body mass by 5% through reducing fat and increasing fiber intake. Participants with the conventional and intensified intervention were also motivated to perform 3 and 6 h of exercise per week, respectively. Postprandial glucose (glucose at 120 min after a glucose challenge) was the primary end point of the study. Secondary end points were insulin sensitivity, liver fat, and insulin secretion.
Assignment to Metabolic Clusters
Participants in the intention-to-treat analysis were assigned to metabolic clusters based on several variables: area under the curve (AUC)0–120 glucose, insulin sensitivity, insulin secretion (ratio of AUC0–30C-peptide to AUC0–30glucose), HDL-cholesterol, visceral fat volume, subcutaneous fat volume, liver fat content, and type 2 diabetes polygenic risk score, as described previously (1). Insulin and C-peptide were measured using the ADVIA Centaur XP Immunoassay System. Liver fat content was assessed with 1H MRS, as described previously (2). Missing variables were imputed using multivariable imputation with chained equations (3), and complete cases set for the required variables was achieved for N = 1,045 (see Supplementary Table 1).
Outcome Measures
Standardized 75-g oral glucose tolerance tests (OGTTs) were performed at baseline after 6 months and 12 months of LI. Insulin sensitivity was assessed by the Matsuda index from 5-point insulin and glucose measurements (4). Results of the per-protocol analysis of the low-risk and high-risk groups of PLIS did not show an effect of LI on insulin secretion measured by an insulin-based index. We measured C-peptide as a post hoc analysis of the study to assess insulin secretion by ratio of AUC0–120C-peptide to AUC0–120glucose, as this index had a lower coefficient of variation while still achieving high discrimination (5).
Statistics
Computations were performed with R 3.6.1 software. The change of outcome measures during LI was modeled with generalized linear mixed models applying the participant as a random effect using the lme4 library. Fixed-effects covariates comprised the intervention group and its interaction term with time, sex, age, BMI, and time (since randomization). Insulin sensitivity was log-transformed when analyzed as an outcome. For insulin secretion, further adjustment was performed for insulin sensitivity. To test how liver fat affects the change in insulin secretion, we fitted the interaction of time and liver fat, measured at the beginning and at the end of the trial, on insulin secretion in different prediabetes clusters. All tests were two-sided with an α level of 0.05. According to simulations performed with the simr package (6), the statistical power to detect the change of insulin secretion in cluster 5 (β = 10, Ngroups = 213, Nmeasurements = 594) was 76% (95% CI 66–84).
Data and Resource Availability
Data of the PLIS study are currently not publicly available. Making the data publicly available without additional consent or ethical approval might compromise participant privacy and the original ethical approval. The R code that supports this analysis is specific for the data set of the PLIS study and available upon request.
Results
All participants of the PLIS cohort met the criteria for prediabetes and ∼82% (856 of 1,045) were assigned to the previously described high-risk clusters 3, 5, or 6. The baseline characteristics of participants stratified for metabolic clusters are summarized in Supplementary Table 2. Owing to the low number of participants assigned to the metabolically healthy obese cluster (cluster 4, n = 8), this group was excluded from further analyses. There were no differences in renal function across the clusters that could impact assessment of insulin secretion through reduced C-peptide clearance (Supplementary Table 2). Before the intervention, cluster 3 had the lowest insulin secretion independent from insulin sensitivity (P < 3.69 ∗ 10−6) (see Supplementary Table 2), and cluster 5 had the lowest insulin sensitivity compared with all other clusters (see Supplementary Table 3).
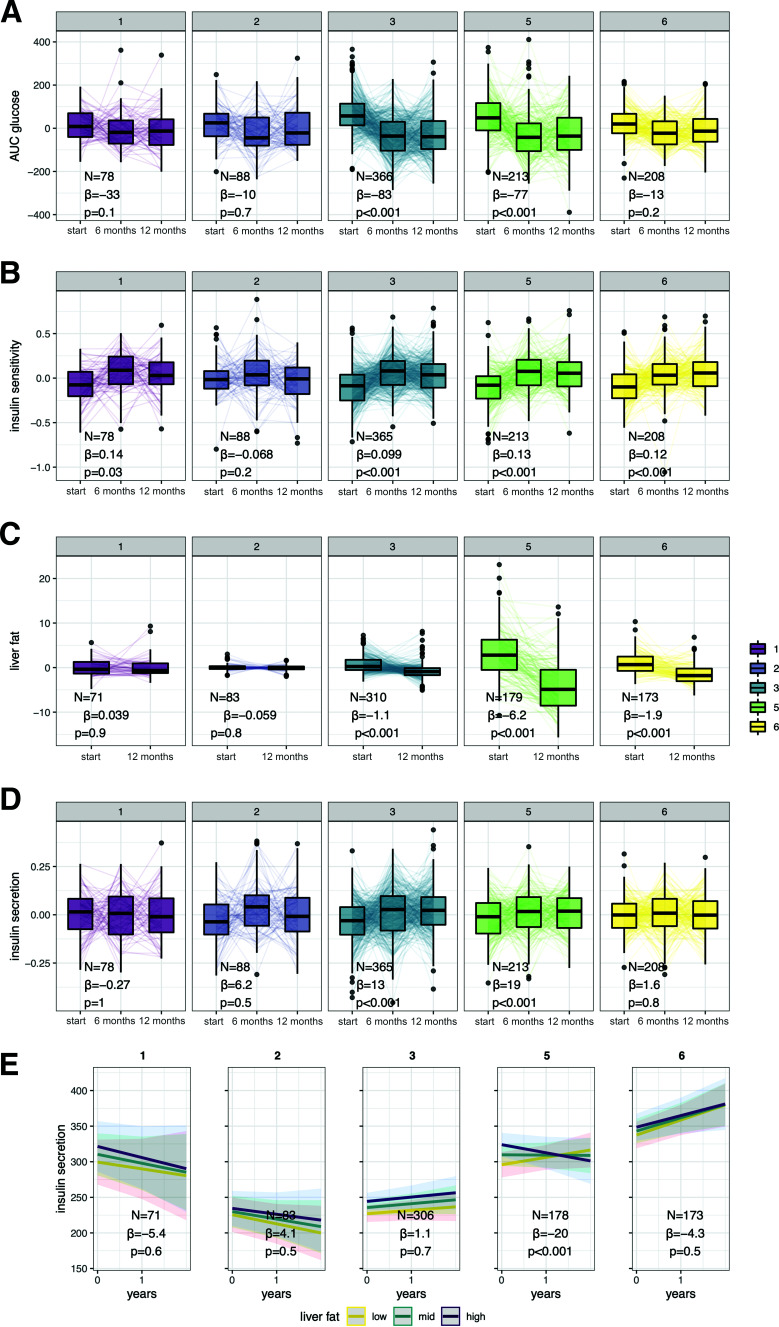
We analyzed the change in key glycemic traits during LI incorporating all evaluation points (baseline 6 months, except for liver fat, and 12 months). Glycemia, insulin sensitivity, and liver fat content were improved by LI in all three high-risk clusters (3, 5, and 6), independent from sex, age, BMI, and the type of LI (P < 0.001) (see Fig. 1A–C). Insulin secretion improved independently from insulin sensitivity and the above-mentioned covariates in clusters 3 and 5. However, insulin secretion did not change during LI in participants in cluster 6, which is characterized by hyperinsulinemia, (see Fig. 1D). Unadjusted insulin secretion also did not increase or decrease during the LI (P = 0.4).
Figure 1.
Cluster-specific change of glycemia (A), insulin sensitivity (B), liver fat (C), and insulin secretion (D) during LI with influence of liver fat on change of insulin secretion during LI (E). Subpanels 1–6 indicate the respective prediabetes clusters. Traits are shown as residuals from generalized linear mixed models adjusted for sex, age, BMI, time × intervention (control, conventional, or intensive), and in the case of insulin secretion, additionally for insulin sensitivity as fixed effects. Effect sizes (β) and P values are provided for the term time. Cluster-wise interactions between hepatic fat content and time in generalized linear mixed models are shown as marginal effects by plotting the modeled change of insulin secretion for low (mean − SD), mid (mean) and high (mean + SD) hepatic fat content. Effect sizes (β) and P values are provided for the interaction between liver fat and time.
We tested the hypothesis that the change of liver fat content—modeled as an interaction of time and MRS-derived hepatic fat content at study start and end—modulates insulin secretion using generalized linear mixed models with the fixed-effect terms: sex, age, BMI, insulin sensitivity, intervention, time × intervention, and time. There was a significant interaction between time and liver fat within cluster 5, but not within the other tested clusters. The results suggest that lower liver fat during LI was associated with an increase of insulin secretion, whereas higher liver fat levels inhibited this improvement of insulin secretion (Fig 1E). In contrast, with similarly constructed models, there was no interaction with BMI in any tested cluster, suggesting that BMI does not impact the change of insulin secretion in either metabolic cluster (Supplementary Fig. 1).
We also computed insulin secretion using the alternative index ratio of AUC0–30C-peptide to AUC0–30glucose that was used at the original cluster assignment. This index potentially involves hepatic insulin resistance (7) and yielded similar results (Supplementary Fig. 2).
Discussion
The data show that all previously defined high-risk clusters of prediabetes are amenable for improvement of glycemia, insulin sensitivity, and liver fat content through LI; however, insulin secretion only improves in clusters 3 and 5. These two clusters are characterized by low insulin secretion for their respective insulin sensitivity. Of note, cluster 3, with only moderate insulin resistance and insulinopenia, also improves insulin secretion. Insulin secretion did not increase in cluster 6 during LI, which aligns with the observation that individuals in this cluster already have prominent hyperinsulinemia. The lack of a decrease of insulin secretion (neither without nor with adjustment for insulin sensitivity) in cluster 6 suggests that hyperinsulinemia was not mitigated by LI. Further studies are needed to investigate the causes and therapeutic possibilities of hyperinsulinemia in this cluster.
Cluster 5 is characterized by insulin resistance, excessive liver fat content, and inadequate insulin secretion (1). Our data suggest that liver fat content, but not body weight loss, is an important modulator of β-cell function. Cell culture models show that a metabolic milieu characterized by a fatty liver and insulin resistance promotes inflammatory cytokine production in adipose tissue adjacent to the pancreatic islets, which is in turn detrimental to insulin secretion (8). Therefore, lowering liver fat has the potential to relieve compromised β-cell function in this prediabetes subphenotype.
Deterioration in β-cell function precedes the onset of type 2 diabetes (9), and increasing insulin secretion has been associated with lower diabetes risk in participants of the Diabetes Prevention Program (10). An improvement of β-cell function paralleling liver fat reduction has already been shown in patients with diabetes (11), but has not yet been reported in prediabetes (12). Therapeutic strategies addressing hepatic fat reduction could be pivotal in improving insulin secretion and thereby preventing hyperglycemia in a subset of patients on a trajectory toward type 2 diabetes.
The assessment of a C-peptide–based insulin secretion index in this study allowed us to determine insulin secretion without interference from hepatic insulin clearance. However, even this OGTT-based index could inherently capture insulin resistance due to the physiologically intertwined nature of insulin secretion and insulin resistance. Also, we retrospectively analyzed data from an interventional study with different treatment arms, such that despite careful adjustment for treatment, a residual confounding might remain.
Our work identifies clusters of patients with prediabetes who respond to LI with better β-cell function and delineates a group with particular benefits from liver fat reduction. Therapeutic modalities reducing liver fat content should be prospectively tested in future studies for high-risk individuals to prevent diabetes and its complications.
Article Information
Acknowledgments. The authors thank our participants, whose dedication made this study possible. The authors are thankful for the excellent assistance and dedication of the study nurses, dietitians, and lifestyle advisors in all of the participating study sites. The authors acknowledge the authors of the following packages for the free statistical software R, which were used in the data analysis: tidyverse, Hmisc, emmeans, openxlsx, htmlTable, lme4, mice, patchwork, sjPlot, transplantr, and viridis.
Funding. The study was supported by the German Center for Diabetes Research (DZD). The DZD is funded by the Bundesministerium für Bildung und Forschung (German Federal Ministry for Education and Research) and the states where its partner institutions are located (01GI0925). Additional support was received from the state of Baden-Württemberg to R.W. and A.F. (325400/58/2, Forum Gesundheitsstandort Baden-Württemberg).
Duality of Interest. R.W. reports lecture fees from Novo Nordisk and Sanofi and travel grants from Eli Lilly. R.W. served on the advisory board of Akcea Therapeutics, Daiichi Sankyo, Sanofi, and Novo Nordisk. A.L.B. reports lecture fees and advisory board membership from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk. A.F. reports lecture fees and advisory board membership from Sanofi, Novo Nordisk, Eli Lilly, and AstraZeneca. M.H. reports research grants from Boehringer Ingelheim and Sanofi (both to the University Hospital of Tübingen) and lecture fees from Sanofi, Novo Nordisk, Eli Lilly, and Merck Sharp & Dohme. M.F.W. is a scientific consultant for Housey Pharmaceutical Research Laboratories. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. R.W. contributed to data collection, researched data, and wrote the manuscript. M.H., K.K., J.M., F.S., A.P., and L.F. contributed to data acquisition and discussion and reviewed and edited the manuscript. A.Sa., J.S., A.F.H.P., A.Sc., M.B., H.H., J.S., S.B., M.R., A.L.B., and M.F.W. contributed to discussion and edited and reviewed the manuscript. N.S., H.-U.H., and A.F. designed the study, researched data, and wrote and edited the manuscript. R.W. and A.F. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.21720137.
References
- 1. Wagner R, Heni M, Tabák AG, et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat Med 2021;27:49–57 [DOI] [PubMed] [Google Scholar]
- 2. Fritsche A, Wagner R, Heni M, et al. Different effects of lifestyle intervention in high- and low-risk prediabetes: results of the randomized controlled Prediabetes Lifestyle Intervention Study (PLIS). Diabetes 2021;70:2785–2795 [DOI] [PubMed] [Google Scholar]
- 3. van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw 2011;45:1–67 [Google Scholar]
- 4. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 5. Hudak S, Huber P, Lamprinou A, et al. Reproducibility and discrimination of different indices of insulin sensitivity and insulin secretion. PLoS One 2021;16:e0258476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Green P, MacLeod CJ. SIMR: an R package for power analysis of generalized linear mixed models by simulation. Methods Ecol Evol 2016;7:493–498 [Google Scholar]
- 7. Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 2007;30:89–94 [DOI] [PubMed] [Google Scholar]
- 8. Gerst F, Wagner R, Kaiser G, et al. Metabolic crosstalk between fatty pancreas and fatty liver: effects on local inflammation and insulin secretion. Diabetologia 2017;60:2240–2251 [DOI] [PubMed] [Google Scholar]
- 9. Lyssenko V, Almgren P, Anevski D, et al. Botnia study group . Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 2005;54:166–174 [DOI] [PubMed] [Google Scholar]
- 10. Kitabchi AE, Temprosa M, Knowler WC, et al. Diabetes Prevention Program Research Group . Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes 2005;54:2404–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011;54:2506–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmid V, Wagner R, Sailer C, et al. Non-alcoholic fatty liver disease and impaired proinsulin conversion as newly identified predictors of the long-term non-response to a lifestyle intervention for diabetes prevention: results from the TULIP study. Diabetologia 2017;60:2341–2351 [DOI] [PubMed] [Google Scholar]