Industry-sponsored biomedical research has grown substantially over the past few decades and generated innovations that have materially and positively affected the diagnosis and treatment of myriad human diseases. Much of this research has been done collaboratively with academic investigators. Given the accelerated pace of therapeutic innovation arising from post-genomic biotechnology, the interface between industry and academe is likely to expand and become more complex in the years ahead.
On the other hand, industry's potential steering effects on the clinical literature have become a source of serious concern. Pharmaceutical review articles appear to be skewed in favour of specific drugs when authors have financial relationships with the relevant companies.1 As Montaner and colleagues have noted,2 the “epidemiology” of industry-sponsored clinical research differs from that of research sponsored by peer-reviewed agencies. This appears to reflect not inferior scientific quality but pre-selection of interventions and designs,3,4,5 probably reinforced by delayed publication of unfavourable results. Delayed publication in general could have as much to do with a misguided culture of “positive publication bias” as with actual sponsor interference. However, study contracts may carry clauses that allow sponsors to suppress studies,6 and there have now been several highly publicized incidents wherein industrial sponsors have litigated or otherwise attempted to interfere with the dissemination of findings that might be construed as adverse to their corporate interests.7,8,9,10 Such actions can intrude on researchers' clear-cut ethical obligations regarding the safety of patients enrolled in clinical studies. Furthermore, whether defined in terms of their scientific integrity, academic freedom, professional autonomy or duties to subjects who may have volunteered in hopes of advancing medical knowledge, researchers also have well-established rights and responsibilities to publish findings deemed valid after peer review.
These tensions at the academic–industry interface recently led the editors of major medical journals to issue guidelines designed to ensure the independence and integrity of clinical research studies sponsored by for-profit enterprises or co-authored by industry scientists or both.11 In Toronto, recognition of this trend and an intense local controversy led the Hospital for Sick Children in 1999–2000 to review and enhance its approach to management of clinical research involving industrial sponsors.12 Similarly, in early 2001, the University of Toronto Faculty of Medicine and all 8 University-affiliated teaching hospitals agreed on a set of principles governing clinical research contracts with third parties, as the first step in ensuring the independence and integrity of industry-sponsored research. Only by harmonizing standards across all hospitals in the immediate academic family could we provide a consistent framework for negotiations with industrial sponsors. What follows is a progress report on the first few months after implementation of this new approach to contract research.
The process was overseen by the Research Committee of the Toronto Academic Health Science Council, composed of senior representatives from the University and all the teaching hospitals. We established working groups to deal with 5 areas where harmonization of research policy and practices are logical, i.e., human subjects research review, clinical study agreements, ethical conduct of research, intellectual property and technology transfer, and animal care. The Clinical Study Agreements Working Group includes individuals at each hospital, some with legal training, who have the responsibility for the content of research contracts with industry and other sponsors.
As a starting point, 4 principles were adopted to guide negotiation of research contracts. First, agreements should not allow research sponsors to suppress or otherwise censor research results. As a corollary, investigators must not be precluded from retaining a copy of the relevant site-specific data. The rationale for this provision is obvious. Second, while agreements may allow sponsors time to protect intellectual property or to review and debate the interpretation of a given study's results, investigators should generally be able to submit work for publication within 6 months of sharing the findings with a sponsor. We allowed for delays of up to 12 months in exceptional circumstances, such as multi-centre trials. The cap on allowable delays was aimed at precluding de facto suppression by virtue of exceedingly slow response times on the part of an industry sponsor to a draft abstract or manuscript. In the event of non-agreement between the sponsor and researcher(s), the investigator retains rights to publish as he or she sees fit as per the first provision above. Third, researchers must retain the right to disclose immediately any safety concerns that arise during the study. This provision is grounded in long-standing ethical requirements and is tied to the process of regulatory approval for new drugs. A fourth provision for dispute resolution was subsequently re-assessed; see below.
These provisions were accepted across the hospitals by the spring of 2001. As a self-audit of our implementation progress, the managers of research contracts at 7 hospitals with contract-based clinical studies reviewed their files for clinical study agreements (CSAs) signed between April 1, 2001, and October 31, 2001. They focused on agreements for new clinical studies funded or supported by investor-owned corporations and also examined similar contracts involving government or foundations. They excluded contracts that were amendments to or extensions of pre-existing CSAs. We agreed that one institution could report on studies both received and signed in this period, because it had accumulated a backlog of previously negotiated but unsigned study contracts.
As shown in Table 1, 152 CSAs were reviewed. No CSA allowed sponsors indefinite suppression or ultimate censorship or approval of the final study reports. For an explicit cap on sponsor-driven delays in submission of abstracts and manuscripts, compliance was 93%. The caps were generally set at 6 months or less, with a smaller number extending to 12 months. Non-compliance in 2 centres occurred either in the early phase of the new dispensation or in the context of multi-centre trials where premature single-site publication was deemed scientifically undesirable (see below).
Table 1
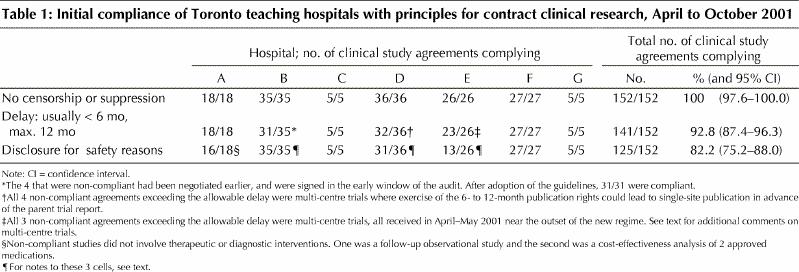
Compliance with the requirement for a clause stipulating researchers' rights to disclose safety concerns was only 82%, largely because of low compliance in Centre E. The relevant manager at Centre E assumed that, absent bans on disclosure, this provision was assured by default given usual and customary practices for reporting study adverse events to regulators and to the Research Ethics Board (REB) for patient notification and, as appropriate, notification of other sites. More recent contracts in Centre E all contain specific provision for notification.
In Centre B, all agreements permitted disclosure to subjects or guardians, to the REB (which could notify other REBs) and to regulatory authorities. However, explicit provision for disclosure to REBs of other participating centres in multi-site trials was not consistently specified. This has been rectified. In Centre D, 4 of 5 instances of non- compliance dated from the early phase of implementation (April–May 2001).
These findings indicate that a co-operative and solid foundation has been laid for continued ethical enhancement of contract clinical research in the Toronto academic health science complex. Compliance with the agreed principles across multiple hospitals has improved as managers of CSAs in the various institutions have met regularly to compare strategies for negotiation and wording of the relevant clauses. Non-compliance in recent months appears to be exceedingly uncommon. Although each manager made her or his own determination of compliance, and full standardization of audit criteria was impossible, we have no reason to believe that the findings overstate compliance with the provisions. The contracts themselves are confidential, rendering external audit difficult. However, in the future, it may be feasible to abstract the relevant clauses from agreements excluding any proprietary or identifying information and ask local REBs to review a sampling of contracts.
Most industrial sponsors have responded very reasonably to these principles, but some have resisted changes in standardized contracts and negotiations have occasionally become protracted. In only 2 instances have sponsors outright refused to sign agreements because of our insistence on these principles.
The relevant working groups are now fully engaged in ongoing discussion, revision and elaboration of the original principles. Actual application to contracts has highlighted a number of challenges and the need for greater clarity about both the intended goals of, and implementation mechanisms for, these protections for researchers and patients. For example, the original wording indicated that, in the event of patient safety concerns, the investigator had the right to make those concerns “public.” This vague wording leaves investigators vulnerable to legal debate about the definition of “public.” Conversely, sponsors may object to an implication that the investigator could simply release his or her concerns to the mass media without peer review or any discussion with the sponsor. Research managers have already clarified this provision in practice. They expect agreements to permit the disclosure of research results to study subjects or their guardians, sponsors, REBs at the site and at other participating study sites, and regulators, if and when the investigator deems disclosure necessary to protect the health of study participants. Provision for disclosure must also be made so as to obtain and maintain informed consent. The issue of single-site publication of adverse effects observed in multi-centre trials is covered below.
As noted earlier, the University and hospital administrations originally agreed that all contracts with sponsors should contain provisions for the effective resolution of disagreements between the sponsor and the researchers. Many of those directly involved in negotiations and dispute resolution have deemed this provision unhelpful, because the most effective dispute resolution mechanism depends meaningfully on the nature of the disagreement. There has also since been agreement across all institutions that binding arbitration is unacceptable as a dispute resolution mechanism for matters related to patient safety. In that instance binding arbitration could leave the investigators with an ethical conundrum (e.g., if an arbitrator rules in favour of a sponsor, proscribing the revelation to patients of side effects that the investigators believe are clinically important). Thus, we are now actively revisiting the question of dispute resolution.
The contracts managers have recently recommended a clearer and more stringent approach to publication delays, capturing the distinctions between single-site and multi-site studies. For single-site studies, where the investigator will submit a manuscript to the sponsor at any time after the study has been completed, the sponsor should have no more than 90 days (exceptionally up to 6 months) to respond with concerns and suggested revisions. At that point the investigator can exercise his or her right to submit an abstract or manuscript for presentation or publication. These timelines are moving steadily toward those recommended in the recent medical editors' statement.11
For multi-site studies, the publication policy must balance the right of an individual investigator to publish site-specific results against the right of the multi-site steering committee to publish the overall results of the study in full. Single-site publications will generally be under-powered statistically, may usurp work done by the steering committee in the design and organization of the overall study and can lead to double-counting13 when multiple trials are aggregated in a meta-analysis. Under the original policy, a teaching hospital is in the difficult position of negotiating the rights of local investigators to publish within 6 to 12 months of study completion, when the opposition to such publication may arise as much from the multi-site steering committee as from the sponsor.
Conversely, we have no way of knowing whether the overall CSA for the parent study contains principles that compromise the independence of the steering committee. In the event that (for any reason) the study steering committee does not publish a joint report, this may be the only way in which an investigator is able to disseminate important study data. Furthermore, if a site withdraws from a study because of safety concerns, the investigator should be able not only to notify subjects, REBs and regulators, but also to publish those concerns.
The ability of the steering committee to publish the overall results of the study in a timely fashion is therefore a matter of importance to the teaching hospitals and the University. Although this position has not been taken by other academic centres and may be seen by some sponsors and colleagues as overreaching, we believe the steering committee's publication rights should be confirmed prior to execution of the site-specific CSA. Hence, on a trial basis, we are asking sponsors for assurance that the parent CSA contains protections of publication rights (e.g., submission may usually proceed within 6 months of the date that the draft multi-site manuscript is submitted to the sponsor). If those assurances are provided, single-site publication still should be protected in specific circumstances. For example, a site should be able to proceed if there is unreasonable delay in submitting the primary manuscript (e.g., more than 12 months from completion of the study in all sites), or if it has withdrawn because of safety concerns, in which case standard single-site publication rights should be exercisable. We are still debating the contentious issue of publication rights for ancillary studies mounted on a single-site basis within multi-centre studies.14
In conclusion, the Toronto experience demonstrates that it is feasible to implement upgraded and consistent standards for management of clinical research contracts across a multi-institutional academic health science complex. This is simply the first step in what will be a continuing initiative that must address other challenges, such as the process to be followed when a sponsor unilaterally withdraws funding from a study. Montaner and colleagues have commented that “the alliance between academic and industry, despite its inevitable tensions, is a valuable one that should be nurtured and perfected.”2 Although we agree with the sentiment, it is clear that there are no perfect policies to govern that alliance and that no group has a monopoly on wisdom and morality in these matters. Our experience demonstrates instead that policies and practices pertaining to the academia–industry interface must be reviewed and improved on a continuing basis. What is extraordinary is not that tensions have arisen concerning industry-sponsored research but rather that so little has been done in the past to build in systematic protections to balance the legitimate interests of industrial sponsors with the academic responsibilities and ethical obligations of clinical researchers. We do not know how many other Canadian hospitals and universities have implemented similar policies and processes, but it is surely reasonable for all to do so, and for mechanisms to be established for sharing relevant ideas and experiences on a national basis.
Footnotes
Contributors: C. David Naylor drafted the manuscript.
The following members of the Research Committee of the Toronto Academic Health Science Council provided input into the guidelines and their implementation, authorized or oversaw local reviews or both, and commented on the manuscript: Manuel Buchwald, Chief of Research, The Hospital for Sick Children; Michael Julius, Vice President – Research, Sunnybrook and Women's College Health Sciences Centre; Stephen Lye, Interim Director, Samuel Lunenfeld Research Institute, Mt. Sinai Hospital; Christopher Paige, Vice President – Research, University Health Network; Arthur Slutsky, Vice President – Research, St. Michael's Hospital; Donald Stuss, Vice President – Research, Baycrest Centre for Geriatric Care. Franco Vaccarino, Vice President – Research, Centre for Addiction and Mental Health, provided input into the guidelines and their implementation and authorized and oversaw local reviews. J. Ivan Williams, Vice President – Research, Toronto Rehabilitation Institute, provided input into the guidelines and their implementation and commented on the manuscript.
The following members of the Clinical Study Agreements Working Group of the Toronto Academic Health Science Council provided input into the guidelines and their implementation, carried out local contract reviews and commented on the manuscript: Pat Clark, Manager, Grant and Contract Services, University Health Network; Michelle Moldofsky, Contract Manager, Research Institute, Hospital for Sick Children; Margaret Kerr, Counsel to the Research Institute, Sunnybrook and Women's College Health Sciences Centre; Paul MacPherson, Research Business Services Coordinator, St. Michael's Hospital. Tamara Birkenheier, Research Contracts Specialist, Samuel Lunenfeld Research Institute, and Klara Vichnevetski, Research Contracts and Licensing Officer, Centre for Addiction and Mental Health, provided input into the guidelines and their implementation and carried out local contract reviews.
Lydia D'Emilio, Financial Analyst, Grants Coordinator, Baycrest Centre for Geriatric Care, carried out local review.
Acknowledgement: We thank Peter Munsche, Assistant Vice President – Technology Transfer, University of Toronto, for his input into the original draft guidelines for industry-sponsored research.
Competing interests: None declared.
Correspondence to: Dr. C. David Naylor, Dean, Faculty of Medicine, University of Toronto, c/o MSB 2109, 1 King's College Circle, Toronto ON M5S 1A8; fax 416 978-1774
References
- 1.Stelfox HT, Chau G, O'Rourke K, Detsky A. Conflict of interest in the debate over calcium-channel antagonists. N Engl J Med 1998; 338:101-6. [DOI] [PubMed]
- 2.Montaner JSG, O'Shaughnessy, Schechter, MT. Industry-sponsored clinical research: a double-edged sword. Lancet 2001;358:1893-5. [DOI] [PubMed]
- 3.Djulbegovic B, Lacevic M, Cantor A, Fields KK, Bennett CL, Adams JR, et al. The uncertainty principle and industry-sponsored research. Lancet 2000;356:635-8. [DOI] [PubMed]
- 4.Rochon PA, Gurwitz JH, Simms RW, Fortin PR, Felson DT, Minaker KL, et al. A study of manufacturer-supported trials of nonsteroidal anti-inflammatory drug in the treatment of arthritis. Arch Intern Med 1994;154:157-63. [PubMed]
- 5.Johansen H, Gotzsche P. Problems in the design and reporting of trials of antifungal agents encountered during meta-analysis. JAMA 1999;282:1752-9. [DOI] [PubMed]
- 6.Kiln M. Industry-sponsored research. Lancet 2001;357:1209-10. [DOI] [PubMed]
- 7.Vogel G. Long-suppressed study finally sees light of day. Science 1997; 276: 525-6. [DOI] [PubMed]
- 8.Roush W. Secrecy dispute pits Brown researcher against company. Science 1997;276:523-4. [DOI] [PubMed]
- 9.Rennie D. Thyroid storm. JAMA 1997;277:1238-43. [PubMed]
- 10.Nathan DG, Weatherall DJ. Academia and industry: lessons from the unfortunate events in Toronto. Lancet 1999;353:771-2. [DOI] [PubMed]
- 11.Davidoff F, DeAngelis CD, Drazen JM, Hoey J, Hojgaard L, Horton R, et al. Sponsorship, authorship, and accountability. JAMA 2001;286:1232-4. [DOI] [PubMed]
- 12.The Hospital for Sick Children Research Policy Review Task Force report. Available: www.sickkids.ca/taskforcereport/default.asp (accessed 2002 Jan 20).
- 13.Huston P, Moher D. Redundancy, disaggregation, and the integrity of medical research. Lancet 1996;347:1024-6. [DOI] [PubMed]
- 14.Sackett DL, Naylor CD. Should there be early publication of ancillary studies prior to the first primary report of an unblinded randomized clinical trial? J Clin Epidemiol 1993;46:395-402. [DOI] [PubMed]


