Abstract
Mounting evidence suggests that childhood health is an important predictor of wellness as an adult. Indigenous peoples worldwide suffer worse health outcomes compared to settler populations. No study comprehensively evaluates surgical outcomes for Indigenous pediatric patients. This review evaluates inequities between Indigenous and non-Indigenous children globally for postoperative complications, morbidities, and mortality. Nine databases were searched for relevant subject headings including “pediatric”, “Indigenous”, “postoperative”, “complications”, and related terms. Main outcomes included postoperative complications, mortality, reoperations, and hospital readmission. A random-effects model was used for statistical analysis. The Newcastle Ottawa Scale was used for quality assessment. Fourteen studies were included in this review, and 12 met inclusion criteria for meta-analysis, representing 4793 Indigenous and 83,592 non-Indigenous patients. Indigenous pediatric patients had a greater than twofold overall (OR 2.0.6, 95% CI 1.23–3.46) and 30-day postoperative mortality (OR 2.23, 95% CI 1.23–4.05) than non-Indigenous populations. Surgical site infections (OR 1.05, 95% CI 0.73–1.50), reoperations (OR 0.75, 95% CI 0.51–1.11), and length of hospital stay (SMD = 0.55, 95% CI − 0.55–1.65) were similar between the two groups. There was a non-significant increase in hospital readmissions (OR 6.09, 95% CI 0.32–116.41, p = 0.23) and overall morbidity (OR 1.13, 95% CI 0.91–1.40) for Indigenous children. Indigenous children worldwide experience increased postoperative mortality. It is necessary to collaborate with Indigenous communities to promote solutions for more equitable and culturally appropriate pediatric surgical care.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00383-023-05377-2.
Keywords: Indigenous, Surgical outcomes, Health equity
Introduction
Safe and appropriate surgical care is an integral component of an effective and resilient healthcare system [1]. Surgical care is a growing need globally, with surgical conditions accounting for over 33% of the global burden of disease [2]. Substantial health inequities continue to negatively impact the health of Indigenous populations worldwide, notably in the Americas and Oceania [3, 4, 5, 6]. These inequities are driven by healthcare systems, practitioner factors and by socioeconomic and connectivity deficits related to colonization, globalization, loss of culture, racism, and disconnection from their traditional land [3, 7]. Minority pediatric populations face unique challenges due to both their age and vulnerability and, consequently, Indigenous children and adolescents living in settler-governed countries face some of the largest health inequities worldwide [8]. The consequences of racism on Indigenous children within healthcare institutions have been compared to the impacts of adverse childhood experiences (ACEs) such as abuse or neglect. Both have lifelong negative impacts on a child’s mental and physical health [8, 9]. On an international stage, pediatric Indigenous health has been recognized by calls from the United Nations Declaration on the Rights of Indigenous Peoples and Canada’s 2015 Truth and Reconciliation Commission for institutions to address health inequalities at the familial level [10, 11, 12].
Current data on postoperative outcomes in pediatric Indigenous patients remain limited and of poor quality [13]. Mapping the evidence that exists in the published literature regarding barriers to adequate surgical quality of care for pediatric Indigenous populations is necessary to understand the extent of the inequities in postoperative outcomes that exist worldwide. This scoping review aims to assess if and which inequities exist between surgical outcomes in pediatric Indigenous and non-Indigenous peoples on the American and Oceanic continents by comprehensively reviewing the existing literature and meta-analyzing the results.
Methods
This scoping review and meta-analysis portion was registered in Open Science Framework (osf.io/qs3vz) and reported in accordance with Preferred Reporting Items for Systematic Review and Meta-Analysis-Extension for Scoping Reviews (PRISMA-ScR) and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines (see supplementary data) [14, 15].
Data sources and searches
A search strategy was developed in conjunction with a professional librarian. Comprehensive electronic database searches were undertaken in MEDLINE, Embase, Global Health, Cochrane Library, PsycInfo, SOCIndex, Web of Science, and ProQuest Dissertations & Theses Global from inception to December 25, 2022, using key MeSH terms. No restriction was placed on language. Complete search strategies of databases can be found in supplementary documents. Grey literature, reference lists of reviews and retrieved articles, and consultations with experts were also conducted to identify additional relevant studies.
Study selection and criteria
Two reviewers independently screened titles, abstracts, and full texts (R.L. and G.F., C.B., K.S., or S.M.). Discrepancies were resolved via group consensus. Studies were included if they were experimental or observational studies and excluded if they were book chapters, conference abstracts, or non-peer reviewed articles. Regional differences exist with regards to the definition of “pediatric patients”. For the purpose of this study, multiple definitions of “pediatric” were included, as defined by the specific study. Studies were excluded if they focused on Indigenous populations outside of the Americas or Oceania, if they lacked a non-Indigenous comparator group, or if they included adult patients. Geography was restricted to these continents as they share similar European colonial settler histories and consequent displacement and oppression of native peoples to those lands different than those in Asian, African, European, and Middle Eastern Indigenous groups [16]. Studies describing minor interventions and procedures conducted by interventional radiologist, pulmonologists, gastroenterologists, hematologists, or interventional cardiologists, including angiography, bronchoscopy, colonoscopy, gastroscopy, bone marrow biopsies, and percutaneous procedures were excluded. If studies only described pre-operative or intraoperative outcomes, they were excluded.
Data extraction and quality assessment
One reviewer (R.L.) completed data extraction and quality assessment (QA), while another reviewer (K.S., C.B., or S.M.) verified the extracted data and QA findings. The following data were extracted from included studies using Microsoft Excel (Microsoft Corporation, Version 16.60): authors’ name, journal, year of publication, age category, population sizes, sex, type of study, surgery specialty and operations performed, outcomes of interest, and study conclusions. Studies were included in data extraction if they reported the surgical procedure performed and at least one outcome of interest resulting from the procedure. Studies reporting on two separate Indigenous groups had data extracted independently for each unique group. Quality of studies and risk-of-bias assessment was conducted using the Newcastle–Ottawa Scale (NOS), adapted for observational studies [17]. To assess the risk of publication bias, the effect odds ratio (OR) for each of the included studies was plotted against their standard error on a logarithmic scale to produce a funnel plot. Funnel plots were assessed for asymmetry to indicate possibility of publication bias. Disagreements between reviewers regarding data extraction and QA ratings were resolved through consensus.
Data analysis
A random-effects model was used to define all pooled outcome measures and the OR was estimated with its variance and 95% confidence interval (CI). The prevailing heterogeneity between ORs for the comparable outcomes between different studies was calculated using the I-squared inconsistency test that depicts the percentage of total variation across studies and reflects heterogeneity rather than chance. The absence of statistical heterogeneity was indicated by a value of 0%, whereas larger values indicate increasing heterogeneity. Studies were only eligible for inclusion in meta-analysis if data were reported which summary associations (ORs or RRs) and their 95% CIs could be calculated or these summary associations were provided in the study itself. All meta-analyses were carried out using Review Manager, Version 5.4 (Cochrane Collaboration, 2020).
Outcomes from studies were separated into categories of postoperative morbidity, postoperative mortality, or increased health system interactions. Morbidity included surgical infections (superficial and deep surgical site infections, anastomotic dehiscence), hematologic (postoperative anemia, hematoma, hemorrhage), pulmonary (pneumonia, aspiration), and immunologic (graft rejection, graft failure) postoperative complications. Mortality was divided into two categories: (1) in-hospital and 30-day mortality and (2) greater than 30-day mortality, which included overall mortality and survival. Increased health system interactions included readmission, reoperation, and length of hospital stay. Subgroup analyses were conducted based on surgical speciality, surgery performed, and geographic location. Sensitivity analysis compared fixed-effects to random-effects models to test the assumption that the random-effects method was the most appropriate choice for the analysis.
Results
Study selection and characteristics
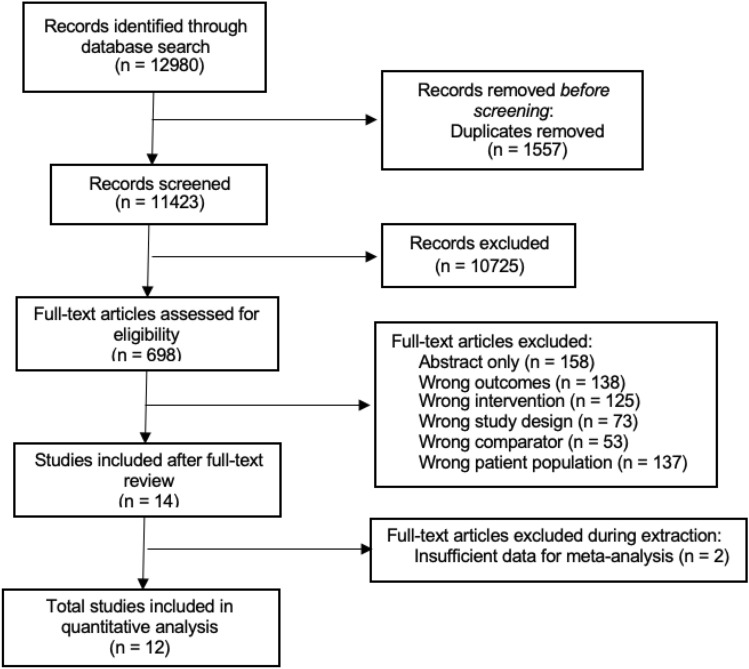
A PRISMA flow diagram outlining the scoping review process is presented in Fig. 1. The initial search resulted in a total of 11,423 non-duplicate studies, of which 698 were included in full-text review after title and abstract review. Following full-text review and gray literature search, 14 unique studies met inclusion criteria, of which 12 were included in the final meta-analysis.
Fig. 1.
PRISMA flow diagram of study selection process, inclusions, and exclusions
Of the 14 studies that met our inclusion criteria, all were retrospective studies, eight of which (57.1%) were retrospective cross-sectional cohort studies. A comprehensive summary of findings and characteristics of all included studies are presented in Table 1. The studies were published between 2009 and 2021, with research conducted from 1985 to 2016. A total of seven studies were based in Oceania (four New Zealand, three Australia), seven in NA (four USA, three Canada), and none were based in SA. Surgical outcomes were reported for 147,861 patients across six pediatric surgical specialties, including general surgery (n = 4), urology (n = 2), neurosurgery (NS) (n = 2), ear nose and throat (ENT) (n = 2), cardiac (n = 2), and ophthalmology (n = 1).
Table 1.
Included study descriptions and summary of findings
| First author, year | Study design; cohort years | Age group; participants* (n) | Surgical discipline; procedure(s) | M&M postoperative outcome category | Overall difference in M&M for IN | Overall quality assessment |
|---|---|---|---|---|---|---|
| North America | ||||||
| Canada | ||||||
| Beres 2010 [18] | Retrospective cohort; 2006–2008 | Pediatric (< 21); total n = 210; Northern Aboriginal n = 68; local n = 142 | Pediatric general surgery; appendectomy | Surgical site infections (wound infection/abscess); other morbidity: (perforation, ileus); postoperative readmission; LOS differences | No difference | Poor |
| Matsuda-Abedini 2009 [19] | Retrospective cohort; 1985–2005 | Pediatric (< 18); total n = 159; Aboriginal n = 24; non-Aboriginal n = 135 | Pediatric urology; renal transplantation | Immunologic complications (delayed-graft function, acute rejection, late rejection, eGFR at 5 years); > 30-day mortality (all-cause mortality) | Increased | Poor |
| Samuel 2011 [20] | Retrospective cross-sectional cohort; 1992–2007 | Pediatric (< 22); total n = 291; Aboriginal n = 35; White n = 256 | Pediatric urology; renal transplantation | Immunologic complications (first graft failure within 1-year) | Increased | Poor |
| USA | ||||||
| Attenello 2015 [21] | Retrospective cross-sectional; 2000, 2003, 2006, 2009 | Pediatric (< 21); total n = 16,941; Native American n = 155; white n = 16,786 | Pediatric neurosurgery; ventriculoatrial shunt, ventriculopleural shunt, ventriculoperitoneal shunt, replacement of ventricular shunt | 30-day mortality (30-day mortality, inpatient death); non-routine discharge | Increased | Good |
| Sherrod 2016 [22] | Retrospective cross-sectional cohort; 2012–2013 | Pediatric (< 18); total n = 7228; Native American n = 57; Pacific Islander n = 24; White n = 7204 | Pediatric neurosurgery; myelomeningocele repairs, spine procedures, craniectomy/craniotomy, shunt/ventricular catheter including revision/removal, skin lesions, other | Postoperative readmission (unplanned readmission) | Increased | Poor |
| Stone 2013 [23] | Retrospective cross-sectional; 2003, 2006 | Pediatric (< 21); total n = 59,185; Native American n = 474; White n = 58,711 | Pediatric general surgery; appendectomy, pyloromyotomy, intussusception, decortication, congenital diaphragmatic hernia repair, colonic resection for Hirschsprung's disease | 30-day mortality (postoperative); Overall Morbidity (postoperative morbidity (wound (mechanical), infection, urinary, pulmonary, gastrointestinal, cardiovascular, systemic, and procedural); LOS | No difference | Good |
| Zwintscher, 2014 [24] | Retrospective cross-sectional cohort; 2009 | Pediatrics (< 20); total n = 45,043; Native American n = 828; Caucasian n = 44,215 | Pediatric general surgery; appendectomy | Surgical site infections (wound, dehiscence, gangrene; hematologic complications (anemia, hemorrhage, hematoma); pulmonary complications (pneumonia, other respiratory); postoperative reoperation (including for incisional hernias and postoperative obstruction) other postoperative complications (foreign body, UTI, shock) | No difference | Poor |
| Oceania | ||||||
| Australia | ||||||
| Chinnaratha 2014 [25] | Retrospective cross-sectional cohort; 1985–2012 | Pediatric (< 16): total = 638; indigenous (Aboriginal Australian and Torres Strait Islander) n = 14; non-indigenous n = 622 | Pediatric general surgery; liver transplantation | Overall morbidity: (1, 3, 5, 10, 15-year graft survival) > 30 day mortality: (1, 3, 5, 10, 15-year overall survival) | No difference | Poor |
| Jassar 2009 [26] | Retrospective cohort; 1996–2004 | Pediatric (< 10); total n = 213; indigenous n = 111; non-indigenous n = 102 | Pediatric ENT; tympanostomy | Surgical site infections (postoperative infection) | No difference | Poor |
| Justo 2017 [27] | Retrospective cohort; 2008–2014 | Pediatric; total n = 1528; indigenous (Aboriginal Australian and Torres Strait Islander) n = 123; non-indigenous n = 1405 | Pediatric cardiac surgery; CHD surgery | 30-day mortality (30-day mortality, surgical survival 5 year unadjusted); LOS differences (ICU stay (days), hospital stay (days), surgical re-intervention) | Increased | Good |
| New Zealand | ||||||
| Campbell 2016 [28] | Retrospective cohort; 2004–2013 | Pediatric (< 15); total n = 153; Māori n = 34; European n = 119 | Pediatric general surgery; cholecystectomy | Overall morbidity: operative complications (bile leak, bowel injury, bowel obstruction, cholangitis, wound infection, hemorrhage, cardiorespiratory, pseudocyst) | Increased | Poor |
| Chong 2021 [29] | Retrospective cross-sectional cohort; 2005–2014 | Pediatrics (< 20); total n = 3414; Māori n = 698; PI n = 219; European n = 3195 | Pediatric ophthalmology; strabismus repairs | Postoperative reoperation (reoperation rates for surgical failures) | Increased | Poor |
| Cloete 2019 [30] | Retrospective cohort; 2006–2014 | Pediatric (< 18); total n = 144; Māori n = 38; PI n = 13; European n = 113 | Pediatric cardiac surgery; HLHS, Aortic coarctation, interrupted aortic arch, aortic valve/supravalvular anomalies repairs | 30-day mortality (postoperative mortality) | Increased | Poor |
| Johnston 2018 [31] | Retrospective cross-sectional cohort; 1996–2006 | Pediatric (< 10); total n = 11,941; Māori n = 2387; non-Māori n = 9554 | Pediatric ENT; Myringotomy + tube insertion | Postoperative Readmission (30-day readmission rate with surgical complication); postoperative reoperation; LOS differences. total number of readmissions for surgery | No difference | Poor |
CHD congenital heart disease, eGFR estimated glomerular filtration rate, ENT ears, nose, throat, HLHS hypoplastic left heart syndrome, ICU intensive care unit, IN indigenous, LOS length of (hospital) stay, M&M mortality and morbidity, PI Pacific Islander
*Participant populations are listed as reported in individual studies
The studies evaluated outcomes for numerous surgical procedures, the most common ones being appendectomy (n = 104,438 participants in three studies); NS procedures including shunt placement (ventricular, ventriculoatrial, ventriculopleural, and ventriculoperitoneal), myelomeningocele repair, craniectomy, craniotomy, spinal procedures, skin lesion procedures, and other neurosurgical operations (n = 24,169 participants in two studies); tympanostomy and myringotomy with tube insertion (n = 12,154 participants in two studies); renal transplantation (n = 450 participants in three studies); strabismus surgery (n = 3414 participants in one study); cardiac surgeries including surgery for congenital heart defects (CHD), acquired heart disease, hypoplastic left heart syndrome (HLHS), aortic coarctation, interrupted aortic arch, aortic valve/supravalvular anomalies repairs, and others (n = 1672 participants in two studies); liver transplantation (n = 638 participants in one study); and cholecystectomy (n = 149 participants in one study). Study population-specific sample sizes ranged from 13 (Pacific Islander patients receiving cardiac surgery) to 44,215 (non-Indigenous comparator population receiving appendectomies).
A total of 88,385 patients were included in the meta-analysis; 4793 (5.4%) were Indigenous and 83,592 (94.6%) were non-Indigenous. Indigenous populations consisted of 3157 (65.9%) Māori, 1040 (21.7%) Native American, 248 (5.2%) Aboriginal Australians and Torres Strait Islander, 256 (5.3%) Pacific Islander Peoples, and 92 (1.9%) Indigenous Canadians.
Overall postoperative morbidity and mortality for Indigenous children in comparison to non-Indigenous children was increased in 8/14 studies (57.1%) and there was no significant difference in 6/14 studies. A decrease in morbidity and mortality for Indigenous populations in comparison to non-Indigenous patients was not reported in any studies within this review.
Risk-of-bias assessment
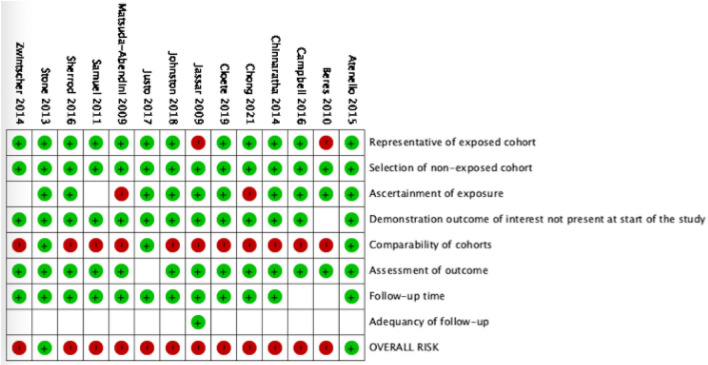
The majority of studies (n = 12, 85.7%) were high risk of bias and low quality, while two (14.3%) studies were low risk of bias and good quality [21, 23]. The low quality of studies was mainly attributed to failure of studies to control for significant differences, such as age, pre-existing co-morbidities, and/or sex in their analysis between Indigenous and non-Indigenous groups (n = 11, Fig. 2). Funnel plots for each outcome were generated; however, due to the inherent heterogeneity of the study and the low overall number of events for each outcome, asymmetry could not be reliably assessed.
Fig. 2.
Risk of bias and quality assessment of included studies using the Newcastle Ottawa Scale. Red: High risk/low quality; white: unclear risk/fair quality study; green: low risk/good quality study
Postoperative morbidity
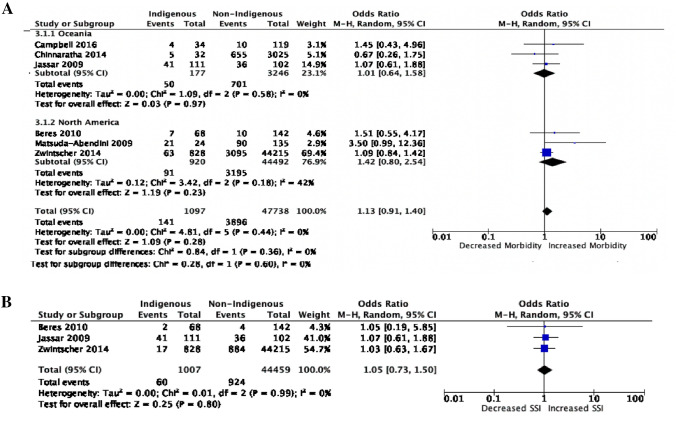
Seven studies addressed postoperative morbidity, of which six studies were included in the morbidity meta-analysis, representing 141 Indigenous patients and 3,896 non-Indigenous patients with postoperative complications [18, 19, 24, 25, 26, 28]. Overall, no significant difference was observed in postoperative morbidity between Indigenous and non-Indigenous pediatric patients (OR 1.13, 95% CI 0.91–1.40, p = 0.28; Fig. 3A). Studies were homogeneous in nature with an I-squared of 0%. When analyzed by geographic location, overall morbidity between Indigenous and non-Indigenous patients remained non-significant in both Oceania (OR 1.01, CI 0.64–1.58, p = 0.64; Fig. 3A) and North America (OR 1.42, CI 0.80–2.54, p = 0.23; Fig. 3A).
Fig. 3.
A Overall postoperative morbidity with sub-group analysis by geographic location and B postoperative surgical site infection (SSI) rate differences between Indigenous and non-Indigenous pediatric patients. Overall morbidity includes any postoperative complications, such as infectious, cardiac, respiratory, thromboembolic, bleeding, and immunologic complications
Surgical site infections
Three studies described postoperative SSIs including wound infections, wound dehiscence, abscesses, sepsis, and necrotizing infections [18, 24, 26]. A total of 60 Indigenous and 924 non-Indigenous children were included in this analysis who experienced postoperative surgical infections. There was no significant difference between the two groups with regards to the odds of experiencing a postoperative surgical infection (OR 1.05, 95% CI 0.73–1.50, p = 0.80; Fig. 3B).
Hematologic complications
One study provided data on hematologic postoperative complications, including hematomas, hemorrhages, and/or anemia from blood loss [24]. The study found that 0.8% (n = 7) of Native American children versus 0.5% (n = 221) of non-Indigenous children undergoing appendectomies experienced hematologic postoperative complications. However, no statistics on differences between the groups were presented in this study.
Pulmonary complications
One study provided data on postoperative pulmonary complications [24]. These complications included pneumonia and other, unspecified respiratory complications (including pneumothorax, respiratory distress, and/or aspiration). In this study, 1.4% (n = 12) of Native American children experienced pulmonary complications, compared to only 0.7% (n = 12) of non-Indigenous patients following appendectomies. Similar to above, no statistics on differences between the groups were presented in this study.
Immunologic complications
Three studies provided data on postoperative graft function and rejection after solid organ transplantation [19, 20, 25]. One of the studies did not find a significant difference in short-term outcomes post-transplantation including delayed-graft function and acute rejection between Indigenous and non-Indigenous children in Canada [19]. However, Indigenous children were found to have significantly poorer long-term graft survival than non-Indigenous children [19]. In the other study that examined immunologic complications, Indigenous children had a three times higher risk of renal graft failure compared to non-Indigenous children in Canada (HR 3.26, CI 1.51–7.03) [20]. The one study examining post-liver transplant graft survival in Aboriginal Australians and Torres Strait Islander children did not find a significant difference in graft survival between the Indigenous and non-Indigenous Australian children [25].
Postoperative mortality and survival
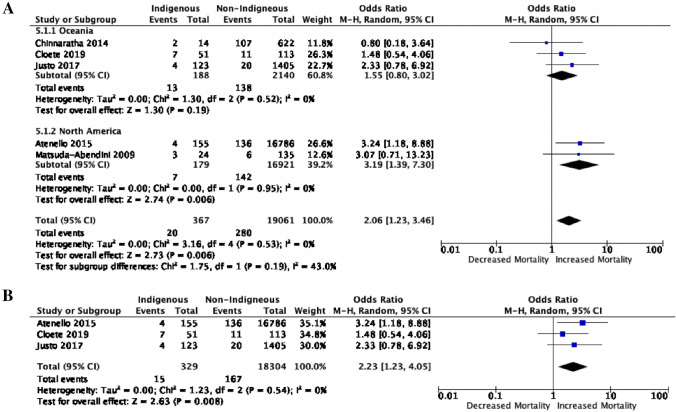
Six studies addressed postoperative mortality, of which five studies were included in the mortality meta-analysis, reflecting 367 Indigenous and 19,061 non-Indigenous postoperative deaths [19, 21, 25, 27, 30]. The excluded study did not present appropriate data for inclusion in this meta-analysis (HR only) [23]. Three studies provided data on in-hospital and 30-day mortality [21, 27, 30] and two provided data for greater than 30-day mortality [19, 25]. Overall mortality was significantly higher for Indigenous patients compared to non-Indigenous patients (OR 2.06, 95% CI 1.23–3.46, p = 0.006; Fig. 4A). Similarly, 30-day mortality was significantly increased for Indigenous patients compared to non-Indigenous patients (OR 2.23, 95% CI 1.23–4.05, p = 0.008; Fig. 4B). When stratified by geographic location, Indigenous children in North America had greater than 300 × the odds of postoperative mortality (OR 3.19, CI 1.39–7.30, p = 0.006; Fig. 4A). In contrast, Indigenous children from Oceania did not have a significant increase in surgical mortality compared to non-Indigenous patients (OR 1.55, 95% CI 0.80–43.02, p = 0.19; Fig. 4A. In a study that examined 5-year surgical survival, Indigenous children in Canada had 50% lower survival than their non-Indigenous counterparts following renal transplantation (n = 3, p = 0.03) [19]. Deaths in the Indigenous patients were attributed to a cardiac event (n = 1), respiratory failure (n = 1), and unknown cause (n = 1). In the non-Indigenous group, the causes of death were due to a cardiac event (n = 1), malignancy (n = 1), CVA (n = 1), and unknown causes (n = 3). Conversely, in a similar study comparing post-liver transplantation survival for Aboriginal and Torres Strait Islander children to non-Indigenous Australians, there was no significant difference in 1- to 15-year survival between the two groups [25].
Fig. 4.
A Overall mortality with geographic-specific sub-group analysis and B in-hospital and 30-day postoperative mortality rates between Indigenous and non-Indigenous pediatric patients
Healthcare system interactions
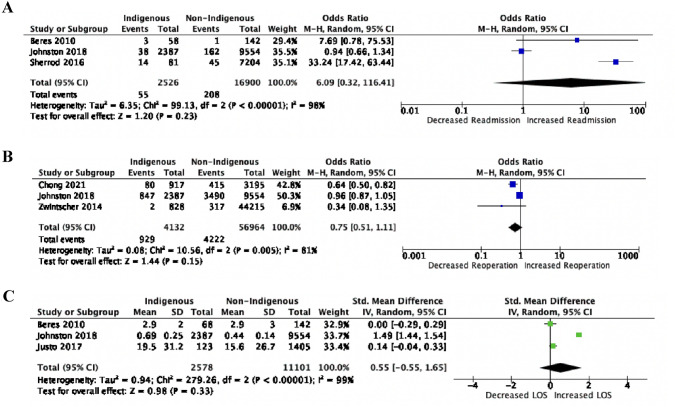
Six studies were included in the meta-analysis on reoperation rates, readmission rates, and average length of hospital stay [18, 22, 24, 27, 29, 31]. Indigenous patients had a non-significant increase in hospital readmissions post-operatively (OR 6.09, 95% CI 0.32–116.41, p = 0.23) and an increased average hospital LOS (SMD = 0.55, 95% CI − 0.55–1.65, p = 0.33) (Fig. 5A, 5C). There was no significant difference in reoperation rates between Indigenous and non-Indigenous patients (OR 0.75, 95% CI 0.51–1.11, p = 0.15) (Fig. 5B). Of note, one of the studies reporting on reoperation rates noted that Māori and Pacific Islander children received significantly less reoperations following failed strabismus repairs compared to non-Indigenous children [29].
Fig. 5.
A Postoperative readmission rates B reoperation rates and C length of hospital stay (days) differences between Indigenous and non-Indigenous pediatric patients
Sensitivity and sub-group analyses
We performed a sensitivity analysis to test the assumption that a random-effects method was the most appropriate choice for the analysis. No noticeable change in the direction of the effect with a fixed-effects method was appreciated. A secondary sensitivity analysis was planned based on quality of studies; however, as only one study was good quality, this analysis was not possible. There were no enough studies on specific surgeries to conduct a surgical procedure-specific sub-group analysis.
Discussion
To the best of the authors’ knowledge, this scoping review and meta-analysis is the first to comprehensively analyze postoperative outcomes in pediatric Indigenous populations in the Americas and Oceania. Our work showed that Indigenous children have a greater than twofold increase in 30-day and long-term postoperative mortality compared to non-Indigenous children. Additionally, while overall postoperative morbidity rates were not found to be significantly different, several studies indicated that Indigenous patients face greater postoperative morbidity than non-Indigenous patients, including increases in hospital readmission rates, decreased rates of reoperations for failed surgeries, and lower rates of graft survival following renal transplantation. Thus, we present evidence that Indigenous pediatric patients suffer from inequitable postoperative outcomes worldwide. The evidence presented here strengthens findings in the literature that indicate the dire need to remodel current surgical systems in a manner that directly reduces inequities and barriers for Indigenous children in accessing safe and effective surgical care [13, 32].
While the reasons as to why increased postoperative morbidity and mortality exist among pediatric Indigenous versus non-Indigenous populations is out of the scope of this review, several important determinants are worthy of note. Poorer health outcomes experienced by Indigenous peoples are often attributed in large part to the rurality and remoteness of areas in which many Indigenous populations live, based on the assumption that this delays access to surgical care and increases late-stage disease presentation. However, an Australian study from 2016 that stratified Indigenous versus non-Indigenous patients by rurality status demonstrated that both rural and urban Indigenous patients experienced significantly higher rates of adverse post-renal transplantation outcomes compared to non-Indigenous rural and urban populations [33]. Similarly, other studies that control for rurality when assessing postoperative mortality and morbidity between Indigenous and non-Indigenous patients in North America and Oceania also conclude that Indigenous status alone, not distance from urban centers, portends worse surgical outcomes [34, 35, 36]. However, when rurality and remoteness do affect health outcomes, it is important to acknowledge that until the past few decades, governmental policies were created and enforced to purposely exclude Indigenous peoples from urban centers, which likewise continues to influence the lives of rural and remote-dwelling Indigenous peoples today [3].
Factors other than rurality and remoteness must also be explored as they contribute significantly to the discrepancy in surgical outcomes for Indigenous children. In their hallmark review, McVicar et al. describe how potential contributors to increased mortality for Canadian Indigenous populations may include late-stage disease at presentation, national surgical referral patterns, and inadequate systems for transition to follow-up care [32]. Undeniably, the ongoing effects of colonization and systemic racism continue to greatly affect the health of pediatric Indigenous populations worldwide. These inequities, driven by culturally insensitive healthcare systems, care teams, and socioeconomic factors [3, 7], must be recognized for their potential to augment traumatic morbidity and increase risk of death following surgery in children. To truly improve a surgical system, it is essential to continue to explore, address, and tackle these complex and intertwining factors.
In addition to describing the increased postoperative mortality and morbidity for Indigenous children globally, this study also highlights the lack of high-quality studies on surgical outcomes in Indigenous children, including the paucity of studies in Canada and the complete absence of data from Central and South America. This is particularly disappointing given the fact that both North and South America have high gaps in life expectancy for their Indigenous populations, with Indigenous Peoples having up to a 16-year less life-expectancy than non-Indigenous people [37]. When adjusted for geographic location, only the North American studies continued to demonstrate significantly increased post-surgical mortality for Indigenous children, indicating differences in postoperative mortality among the diverse Indigenous populations across America and Oceania.
In recent years, scrutiny and increased attention has been directed at the inequitable social determinants of health faced by Indigenous Peoples worldwide, which has resulted in efforts to address these inequities such as the implementation of cultural safety training programs and the development of focused political and health policy agendas [38, 39]. This review is inclusive to the end of 2021; however, the most recent cohort year included in the meta-analysis is 2014. While concrete evidence of positive change is yet to be seen, as further research emerges on this topic, it will be interesting to explore if policy and cultural changes in the last decade have created meaningful improvements in the health of Indigenous children.
The results of this scoping review are consistent with the United Nations Mandate on Indigenous Peoples Health which describes how Indigenous Peoples across the globe face overall poorer health outcomes, reduced quality of life, and higher rates of disability compared to non-Indigenous peoples [37]. Ultimately, we present the disheartening but critically important finding that these inequities in health outcomes begin in infancy and childhood, manifesting as steeply elevated postoperative mortality rates and increased risk of complications following surgery for Indigenous children.
Based on this study’s findings, we propose the need for further research and health systems reforms that are appropriate for the Indigenous context and enshrine in their models the need for multi-pronged solutions that respect the unique cultures, experiences, and needs of diverse Indigenous communities worldwide. Further research is required to investigate rates of and structural factors influencing inequities in postoperative morbidity and mortality in Indigenous pediatric populations, especially in the Americas. The pediatric context must be recognized as unique and treated accordingly in both research and practice to ensure their right to equitable and safe healthcare is upheld. These research initiatives should involve a clear methodology that is developed in collaboration with Indigenous leaders, communities, and healers, with specific care taken to incorporate and accommodate all of the diverse Indigenous groups of a given region. End goals must emphasize the transformation of research into practice by reforming various steps in the surgical care pathway in manners that radically improve access, safety, cultural-appropriateness, and outcomes for Indigenous children.
Limitations
Heterogeneity present in the research question, including the diversity of Indigenous populations, surgeries performed, surgical procedures, and reported outcomes, makes it difficult to pool the results for this meta-analysis in a manner that reflects nuances between the study populations, while also providing homogeneity in pooled odds. The studies included in this review were also primarily of poor quality and retrospective in nature. Furthermore, the majority of studies included in this review did not explicitly define or provide separate outcome numbers for unique Indigenous groups of a specific geographic region, labeling their populations strictly as “Indigenous” and grouping together several geographically, culturally, and ethnically diverse Indigenous groups. This homogenization of Indigenous groups consequently does not allow for analysis of unique experiences and barriers for these distinct groups.
It is important to acknowledge that inequities in health outcomes between Indigenous and non-Indigenous populations impact patients at many stages of their treatment journey. While we compare inequity in surgical outcomes, the impact of contextualizing inequities leading up to surgery is outside the scope of this review. Questions such as: “Are Indigenous and non-Indigenous patients presenting with similar clinical pictures equally likely to receive appropriate surgical intervention?” and “Are negative surgical outcomes reported at equal rates in Indigenous and non-Indigenous populations?” remain. Furthermore, while this review did not find any studies from South or Central America, there is a possibility that this may have been influenced by our search strategy being limited to the English language.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors respectfully acknowledge that this review was conducted on Treaty 6 territory, the traditional land of the nêhiyawak (Cree), Anishinaabe (Saulteaux), Niitsitapi (Blackfoot), Métis, Dene, and Nakota Sioux peoples, the unceded territories of the xʷməθkʷəy̓əm (Musqueam), Sḵwx̱wú7mesh (Squamish), səlilwətaɬ (Tsleil-Waututh), and Tsimshian Nations, traditional territory of the Lheidli T’enneh, part of the Dakelh (Carrier) First Nation, as well as the traditional lands of the Sinixt, the Ktunaxa, the Secwepmec, and the Syilxsince since time immemorial.
Abbreviations
- ACE
Adverse childhood experiences
- CVA
Cerebrovascular accident
- ENT
Ears, nose, and throat surgery
- GS
General surgery
- ICU
Intensive care unit
- LOS
Length of stay
- M&M
Morbidity and mortality
- MI
Myocardial infarction
- MOOSE
Meta-analysis of observational studies in epidemiology
- NOS
Newcastle–Ottawa scale
- NS
Neurosurgery
- ORTHO
Orthopedic surgery
- PRISMA-ScR
Preferred reporting items for systematic review and meta-analysis-extension for scoping reviews
- RoB
Risk of bias
- SMD
Standard mean difference
- SSI
Surgical site infection
- QA
Quality assessment
- URO
Urology
- USA
United States of America
Author contributions
R.J.L, T.H, and E.Y. designed the study. R.J.L and C.B. designed the search strategy. R.J.L, G.F., K.S. C.B. and S.M. performed the literature review and study selection. R.J.L, G.F., K.S. C.B. and S.M. performed data analysis, prepared the figures, and wrote the article. T.H., S.J., and E.J. served as additional reviewers, made substantial contributions to the discussion, and critically revised the article for content. All authors read and approved the final article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no conflicts to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Meara JG, Leather AJM, Hagander L, et al. Global surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet. 2015;386(9993):569–624. doi: 10.1016/s0140-6736(15)60160-x. [DOI] [PubMed] [Google Scholar]
- 2.Shrime MG, Bickler SW, Alkire BC, Mock C. Global burden of surgical disease: an estimation from the provider perspective. Lancet Global Health. 2015;3:S8–S9. doi: 10.1016/s2214-109x(14)70384-5. [DOI] [PubMed] [Google Scholar]
- 3.King M, Smith A, Gracey M. Indigenous health part 2: the underlying causes of the health gap. Lancet. 2009;374(9683):76–85. doi: 10.1016/S0140-6736(09)60827-8. [DOI] [PubMed] [Google Scholar]
- 4.Young TK, Reading J, Elias B, O’Neil JD. Type 2 diabetes mellitus in Canada’s first nations: status of an epidemic in progress. CMAJ. 2000;163(5):561–566. [PMC free article] [PubMed] [Google Scholar]
- 5.Bourassa C, McKay-McNabb K, Hampton M. Racism, sexism and colonialism: the impact on the health of aboriginal women in canada. Can Women Stud. 2004;24(1):23–29. [Google Scholar]
- 6.Greenwood M, de Leeuw S, Lindsay N M (2018) Determinants of Indigenous Peoples’ health in Canada: Beyond the social. Canadian Scholar’s Press; Second Edition; 2018
- 7.Gurney J, McLeod M, Stanley J, et al. Disparities in post-operative mortality between Māori and non-Indigenous ethnic groups in New Zealand. N Z Med J. 2021;134(1542):15–28. [PubMed] [Google Scholar]
- 8.Morris MI, Sioui R, Sioui M. Conference proceeding from the annual meeting of the Canadian association of pediatric surgeons: “Caring for indigenous children: a CAPS perspective”. J Pediatr Surg. 2020;55(5):793–795. doi: 10.1016/j.jpedsurg.2020.01.029. [DOI] [PubMed] [Google Scholar]
- 9.Oh DL, Jerman P, Silvério Marques S, et al. Systematic review of pediatric health outcomes associated with childhood adversity. BMC Pediatr. 2018 doi: 10.1186/s12887-018-1037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UN General Assembly. United Nations Declaration on the Rights of Indigenous Peoples: resolution/adopted by the General Assembly. 2007. https://www.un.org/development/desa/indigenouspeoples/declaration-on-the-rights-of-indigenous-peoples.html. Accessed 3 July 2022
- 11.Truth and Reconciliation Commission of Canada. Calls to Action. Winnipeg, Manitoba 2015. https://www.nctr.ca/records/reports/. Accessed 4 July 2022
- 12.Patro N, Li B, Lee Y. Investigating disparities in surgical outcomes in Canadian indigenous populations. Can J Surg. 2021;64(6 Suppl 2):S100–S101. [Google Scholar]
- 13.Mcleod M, Signal V, Gurney J, Sarfati D. Postoperative mortality of indigenous populations compared with nonindigenous populations. JAMA Surg. 2020;155(7):636. doi: 10.1001/jamasurg.2020.0316. [DOI] [PubMed] [Google Scholar]
- 14.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 15.Brooke BS, Schwartz TA, Pawlik TM. MOOSE reporting guidelines for meta-analyses of observational studies. JAMA Surg. 2021;156(8):787–788. doi: 10.1001/jamasurg.2021.0522. [DOI] [PubMed] [Google Scholar]
- 16.Anderson I, Robson B, Conolly M, et al. Indigenous and tribal peoples’ health: the Lancet-Lowitja institute global collaboration: a population study. Lancet. 2016;388(10040):131–157. doi: 10.1016/S0140-6736(16)00345-7. [DOI] [PubMed] [Google Scholar]
- 17.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2000. [Google Scholar]
- 18.Beres A, Al-Abbad S, Puligandla PS. Appendicitis in northern aboriginal children: does delay in definitive treatment affect outcome? J Pediatr Surg. 2010;45(5):890–893. doi: 10.1016/j.jpedsurg.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda-Abedini M, Al-AlSheikh K, Hurley RM, et al. Outcome of kidney transplantation in Canadian aboriginal children in the province of British Columbia. Pediatr Transplant. 2009;13(7):856–860. doi: 10.1111/j.1399-3046.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 20.Samuel SM, Nettel-Aguirre A, Hemmelgarn BR, et al. Graft failure and adaptation period to adult healthcare centers in pediatric renal transplant patients. Transplantation. 2011;91(12):1380–1385. doi: 10.1097/TP.0b013e31821b2f4b. [DOI] [PubMed] [Google Scholar]
- 21.Attenello FJ, Ng A, Wen T, et al. Racial and socioeconomic disparities in outcomes following pediatric cerebrospinal fluid shunt procedures. J Neurosurg Pediatr. 2015;15(6):560–566. doi: 10.3171/2014.11.PEDS14451. [DOI] [PubMed] [Google Scholar]
- 22.Sherrod BA, Johnston JM, Rocque BG. Risk factors for unplanned readmission within 30 days after pediatric neurosurgery: a nationwide analysis of 9799 procedures from the American college of surgeons national surgical quality improvement program. J Neurosurg Pediatr. 2016;18(3):350–362. doi: 10.3171/2016.2.PEDS15604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone ML, LaPar DJ, Kane BJ, Rasmussen SK, McGahren ED, Rodgers BM. The effect of race and gender on pediatric surgical outcomes within the United States. J Pediatr Surg. 2013;48(8):1650–1656. doi: 10.1016/j.jpedsurg.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zwintscher NP, Steele SR, Martin MJ, Newton CR. The effect of race on outcomes for appendicitis in children: a nationwide analysis. Am J Surg. 2014;207(5):748–753. doi: 10.1016/j.amjsurg.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Chinnaratha MA, Chelvaratnam U, Stuart KA, et al. Liver transplantation outcomes for Australian aboriginal and torres strait islanders. Liver Transpl. 2014;20(7):798–806. doi: 10.1002/lt.23894. [DOI] [PubMed] [Google Scholar]
- 26.Jassar P, Sibtain A, Marco D, Jose J, Hunter G. Infection rates after tympanostomy tube insertion, comparing aboriginal and non-aboriginal children in the northern territory, Australia: a retrospective, comparative study. J Laryngol Otol. 2009;123(5):497–501. doi: 10.1017/S002221510800306X. [DOI] [PubMed] [Google Scholar]
- 27.Justo ER, Reeves BM, Ware RS, et al. Comparison of outcomes in Australian indigenous and non-indigenous children and adolescents undergoing cardiac surgery. Cardiol Young. 2017;27(9):1694–1700. doi: 10.1017/S1047951117000993. [DOI] [PubMed] [Google Scholar]
- 28.Campbell S, Richardson B, Mishra P, et al. Childhood cholecystectomy in New Zealand: a multicenter national 10 year perspective. J Pediatr Surg. 2016;51(2):264–267. doi: 10.1016/j.jpedsurg.2015.10.071. [DOI] [PubMed] [Google Scholar]
- 29.Chong C, Lawrence A, Allbon D. Addressing equity: a 10-year review of strabismus surgery in 0–19-year-olds in the New Zealand public health system. N Z Med J. 2021;134(1545):79–90. [PubMed] [Google Scholar]
- 30.Cloete E, Sadler L, Bloomfield FH, Crengle S, Percival T, Gentles TL. Congenital left heart obstruction: ethnic variation in incidence and infant survival. Arch Dis Child. 2019;104(9):857–862. doi: 10.1136/archdischild-2018-315887. [DOI] [PubMed] [Google Scholar]
- 31.Johnston J, McLaren H, Mahadevan M, Douglas RG. Surgical treatment of otitis media with effusion in Maori children. ANZ J Surg. 2018;88(11):1141–1144. doi: 10.1111/ans.14788. [DOI] [PubMed] [Google Scholar]
- 32.McVicar JA, Poon A, Caron NR, et al. Postoperative outcomes for indigenous peoples in Canada: a systematic review. CMAJ. 2021;193(20):E713–E722. doi: 10.1503/cmaj.191682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barraclough KA, Grace BS, Lawton P, McDonald SP. Residential location and kidney transplant outcomes in indigenous compared with nonindigenous Australians. Transplantation. 2016;100(10):2168–2176. doi: 10.1097/TP.0000000000001007. [DOI] [PubMed] [Google Scholar]
- 34.Cohen MM, Young TK, Hammarstrand KM. Ethnic variation in cholecystectomy rates and outcomes, Manitoba, Canada, 1972–84. Am J Public Health. 1989;79(6):751–755. doi: 10.2105/ajph.79.6.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivan E, Martinsen B, Igyarto Z, Sublett T, Nachimuthu S. Peripheral artery disease in vulnerable patient populations: outcomes of orbital atherectomy in native Americans compared to non-native Americans. A single-center experience in rural Oklahoma. Cardiovasc Revasc Med. 2021;22:71–77. doi: 10.1016/j.carrev.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Swift K, Thompson F, Roeder L, Choy KT, McDonald M, de Costa A. Appendicitis in far North Queensland: a new take on an old story. ANZ J Surg. 2022;92(1–2):114–120. doi: 10.1111/ans.17404. [DOI] [PubMed] [Google Scholar]
- 37.United Nations Mandate on Indigenous Peoples Health. United Nations. https://www.un.org/development/desa/indigenouspeoples/mandated-areas1/health.html. Accessed 4 July 2022
- 38.Muise GM. Enabling cultural safety in Indigenous primary healthcare. Healthc Manag Forum. 2019;32(1):25–31. doi: 10.1177/0840470418794204. [DOI] [PubMed] [Google Scholar]
- 39.Wylie L, Mcconkey S, Corrado AM. It’s a journey not a check box: indigenous cultural safety from training to transformation. Int J Indig Health. 2021 doi: 10.32799/ijih.v16i1.33240. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.