Highlights
Novel insights on recent advances in nanotechnology-based agro seed treatment formulations.
Details on reducing the environmental impact of seed treatment by using nanoagrochemicals.
Applications of potential of nanopesticides and nanofertilizers for sustainable seed treatments.
Described scope of possible next-generation nanomaterials for seed treatment formulations with associated challenges and risks assessment methodologies.
Keywords: Agro seeds, Environmental seed stressors, Nanoagrochemicals, Toxicological implications, Risk regulations
Abstract
Agro seeds are vulnerable to environmental stressors, adversely affecting seed vigor, crop growth, and crop productivity. Different agrochemical-based seed treatments enhance seed germination, but they can also cause damage to the environment; therefore, sustainable technologies such as nano-based agrochemicals are urgently needed. Nanoagrochemicals can reduce the dose-dependent toxicity of seed treatment, thereby improving seed viability and ensuring the controlled release of nanoagrochemical active ingredients However, the applications of nanoagrochemicals to plants in the field raise concerns about nanomaterial safety, exposure levels, and toxicological implications to the environment and human health. In the present comprehensive review, the development, scope, challenges, and risk assessments of nanoagrochemicals on seed treatment are discussed. Moreover, the implementation obstacles for nanoagrochemicals use in seed treatments, their commercialization potential, and the need for policy regulations to assess possible risks are also discussed. Based on our knowledge, this is the first time that we have presented legendary literature to readers in order to help them gain a deeper understanding of upcoming nanotechnologies that may enable the development of future generation seed treatment agrochemical formulations, their scope, and potential risks associated with seed treatment.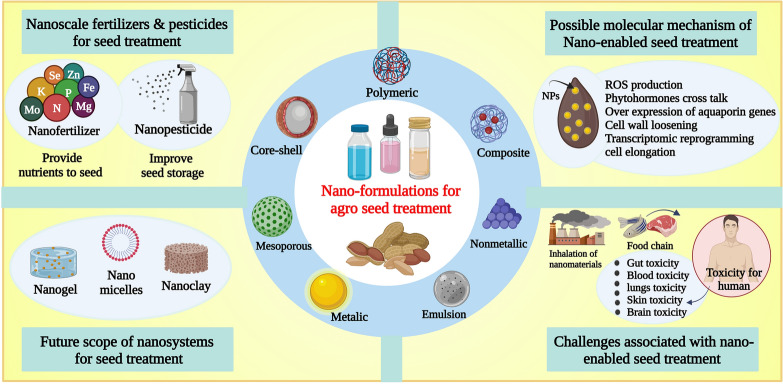
Introduction
The agricultural industry plays a significant role in developing economies and provides food for a rapidly growing world population of nearly 7.5 billion people [1, 2]. Since 90% of food crops are grown from seed, the seed is a vital input for sustainable agricultural productivity and production. A healthy agro seed produces healthier, more viable, and vigorous seedlings, contributing to effective agricultural practices. In the current scenario, agriculture faces a wide range of challenges, including changing environmental conditions like salinity, drought, heavy metal accumulation in soil and climate changes etc., which can adversely affect seed germination, seedling development, and ultimately, crop production [3–5]. The quality of seeds may also be reduced by seed-borne diseases or destroyed by insects and other pests [6]. This can lead to abnormal seed dormancy, non-viability, and reduced water absorption, negatively impacting crop production and final yield. Therefore, maintaining the seed quality is crucial for germination, seedling establishment, and crop growth. Agrochemical-based seed treatment can prevent these issues and enhance seed quality by protecting agro seeds from biotic and abiotic stresses. In order to address and prevent various pests, diseases, and nutritional deficiencies, various agrochemicals are used separately and in combination with each other for seed treatments [7–12]. They include fungicides, insecticides, fertilizers, and fertilizer enhancers. Nevertheless, these chemicals are costly, toxic for health, toxic leaching occurs in soil, seed pathogens are showing resistance, and the chemicals reach the water sources like rivers or sea, causing eutrophication, reducing soil fertility, reducing beneficial microbial activity, and altering the pH of the soil. The abundant use of conventional agrochemicals and runoff of their wastes also contributes to nutrient and food chain imbalances in ecosystems, leading to pollution of the environment and soil [13–15]. For this reason, it is imperative to implement sustainable agricultural practices to protect seeds from pests and insects while maintaining the agro-ecosystem. Conventional agrochemicals are discouraged as they are not contributing to sustainable agriculture seed treatment practices due to the issues of leaching, degradation, hydrolysis of agrochemicals. New technologies which are safe and economic, and based on green chemistry approaches are urgently needed to reduce environmental burden on soil [13–15].
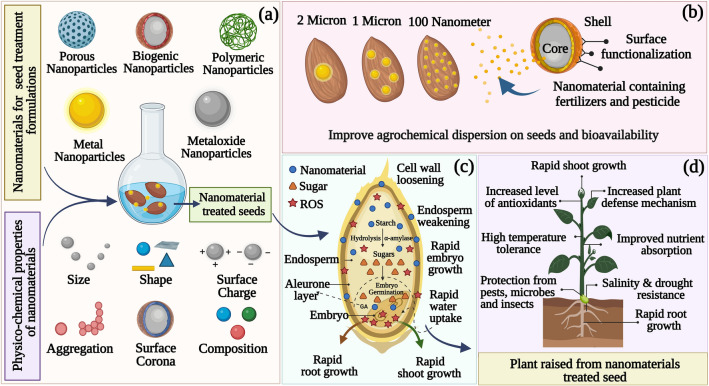
New techniques and strategies are constantly evolving to address these pertinent issues with agro seeds. To revolutionize modern agriculture practices, nanomaterial-based products are being introduced. The high surface area-to-volume ratio and novel physicochemical properties of these materials enable them to meet increasing demand due to their high reactivity [16]. Nanotechnology deals with materials with a size ranging from 1 to 100 nm [17–25]. Moreover, by increasing the surface area per mass of a material, a more significant amount of the nanomaterial can contact surrounding materials, influencing its reactivity [26]. The surface area of nanomaterials is much larger than that of similar masses of larger-scale materials [27]. The application of nanotechnology to seed treatment is a relatively new area of research. Nanoagrochemicals for seed treatment can achieve popularity today because they are more effective than conventional agrochemicals, making them economically viable and environmentally friendly. Nanotechnology can significantly contribute to the sustainable development of nanoscale agrochemicals for seed treatment and can enhance the efficiency of agricultural inputs. There has been evidence that nanoparticles increased seed germination and biomass yield on seeds. The nanoparticles have also increased the seed’s resistance to several biotic and abiotic stresses. The biological functions of seeds depend on molecular events. There has been little progress at the molecular level regarding nanoparticle-induced mechanisms and seeds, which is an important step in evaluating potential mechanisms. To understand seed’s underlying mechanisms and responses toward nanoparticles and the changes in gene expression through molecular approaches, it is crucial to understand how seeds respond to nanoparticles. In the last decade, nanomaterials have demonstrated extensive and beneficial chemical interactions with agro seed systems, ranging from seed disease management and yield improvement to environmental safety [17, 28–31]. As shown in Fig. 1a, nanomaterials can be surface engineered to provide desirable properties and functions for particular seed treatment. This will primarily allow them to target the correct locations within the seed or seed coat and offer smart release and delivery strategies (Fig. 1b). Recent studies have found that seeds treated with nanomaterials can activate several genes during germination [32–34]. It has been shown that nanomaterials promote seed germination by forming nanopores in seed coats, introducing reactive oxygen species (ROS), increasing enzyme activity at starch-degrading sites, and introducing ROS to the seed coat (Fig. 1c). A variety of signaling molecules regulate seed germination, including ROS and phytohormones. ROS regulate gene expression and phytohormone signaling, and they maintain a balance between abscisic acid, gibberellins, auxins, and ethylene [8]. In contrast, excessive ROS levels hamper seed germination by causing extensive oxidative damage. Therefore, ROS levels should be controlled spatiotemporally so they can be enclosed in the so-called oxidative window, ensuring proper germination. A significant physiological effect on seed germination appears to be caused by nanoparticles, although the exact mechanism is unknown. Some studies revealed that, ROS can be generated by nanoparticles by triggering the production of •OH radicals. As a result of soaking seeds in nanomaterial containing solutions for a certain period of time, the •OH radicals produced by bound nanoparticles would loosen cell walls, thereby stimulating seedling growth [8]. Using nanoagrochemicals to treat seeds is an efficient method of altering seed metabolism and signaling pathways, which significantly impacts germination and establishment of plant’s overall life cycle (Fig. 1d). By applying nanomaterials to seeds, we can protect them during storage, enhance germination, synchronize germination, improve growth early on, and significantly reduce the amount of pesticides and fertilizers that need to be applied [35].
Fig. 1.
Application of organic–inorganic nanomaterials in seed germination and plant development. a Nanoparticles’ properties (such as size, shape, surface charges, composition, and concentration) affecting the seed interaction. b Surface-engineered nanoparticles with the desirable properties for seed treatments. c Nanoparticles induced seed metabolism. d Nanoparticles effect for the improved growth and establishment of plants
The literature search has shown that nanomaterials such as silver, gold, copper, palladium, selenium, zinc oxide, magnesium oxide, titanium dioxide, and iron oxide have been proven to promote seed germination and improve crop yields [10, 19–21, 23, 24, 28, 29, 31]. The revolution of next-generation seed agrochemicals will be driven by porous, biogenic, metallic, metal oxide, and polymeric nanomaterials [36–38]. Apart from promoting seeds germination, nanomaterials can serve as seed protectors as well. They can protect seeds from bacteria, fungi, and pests. In some exceptional cases, nanomaterials have been observed to have size- and concentration-dependent toxicity, such as reducing germination rates and causing phytotoxicity to seedlings [39–41]. Nanoparticle toxicity can be reduced by controlling their physicochemical properties, such as size, shape, surface charges, composition, and concentration, which determine their biological response. Using nanoagrochemicals for seed treatment raises the possibility of their release into the ecosystem and soil. Applying these nanoagrochemicals in actual field situations raises concerns about their safety, exposure levels, and toxicological consequences for the environment and human health [42–44]. Depending on their nature and the presence of organic and inorganic constituents, nanoagrochemicals may undergo physical, chemical, and biological transformations once they enter the environment. When the nanoagrochemicals are transformed or aggregated, their stability, reactivity, toxicity, and selectivity may be affected, and their target may be altered [45].
Nanoagrochemicals for seed treatment need to be evaluated more closely to determine their fate in the environment. There is no comprehensive review of seed treatment-specific nanomaterials development, application, safety, and regulation in the literature. The present discussion aims to provide readers with up-to-date information on the latest organic and inorganic nanomaterials used for sustainable seed treatments. Furthermore, this review provides a detailed overview of the safety of nanomaterials in the environment when used for seed treatment. In developing nano-based agrochemicals, some nanomaterials would be excellent candidates for seed-specific treatments and for developing agro products nanoformulations for seed treatment. Nanomaterials-enabling technology and products have the potential to provide one of the most effective and environmentally friendly seed treatment options.
Nanotechnology Toward Sustainable Seed Treatments
In order to ensure a sustainable future for agriculture, there is an urgent need for sustainable seed treatment practices. Sustainable seed treatment practices ensure profitability, environmental health, social equity, and profitability of existing and future generations. As part of sustainable seed treatment practices, agrochemical usage is to be getting control, since they can contaminate the soil, water, turf, and other vegetation, as well as harm nontarget organisms such as plants, birds, animals, and fish. Agrochemicals which are used for seed treatment are absorbed by surrounding land and water bodies, entering the food chain and accumulating in body [13–15]. As far as their effects on crops are concerned, excessive application of these chemicals generates significant residues. Agrochemical residues contribute to nutrient imbalance and quality reduction of agricultural products. Furthermore, these over use of agrochemicals can adversely affect the environment by causing abnormal climate change, damaging biodiversity, polluting groundwater and soil, destroying natural resources, violating waste management laws, and creating noise pollution and air pollution [13–15]. Therefore, it is necessary to develop sustainable agricultural practices to overcome agrochemical issues. We need smart agrochemicals for sustainable seed treatment in order to achieve this. This approach proposes formulations and products that fulfill the needs for chemicals that provide sustainable nutrient delivery systems that maximize agricultural crop yield and minimize environmental impact [46].
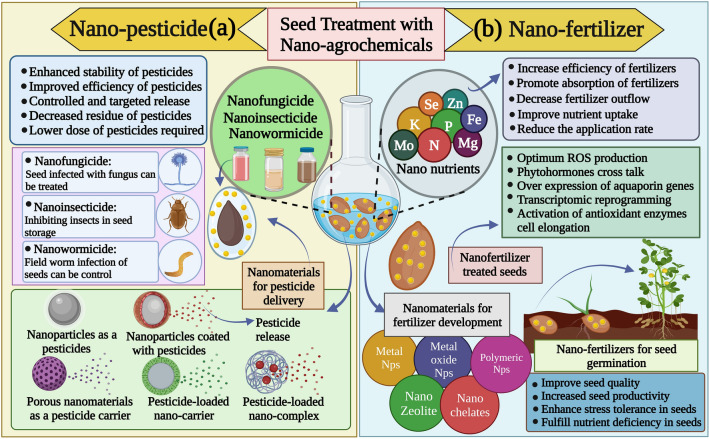
The use of nanoscale agrochemicals as smart chemicals for seed treatment, such as nanofertilizers, nanopesticides and nanofungicides [47–51], has transformed traditional agriculture practices to become more sustainable and efficient (Fig. 2). Using nanomaterials and nanotechnology in agrochemicals overcomes several disadvantages of conventional agrochemicals, including poor solubility, low bioavailability, easy photolysis, organic solvent pollution and excess toxicity. Nanomaterials have been used successfully in the development of seed treatment over the past few years [52, 53]. The potential applications of nanomaterials for seed treatment can be categorized into active nanoparticles and sustained release nanocarrier systems [54]. The active nanoparticle is a nanoparticle that can cause a biological effect. Active nanoparticles can act as stimulants, anti-pathogens, or both. Active nanoparticles include multi-walled carbon nanotubes that act as an effective agrochemical carrier, metal oxide-based nanoparticles for encouraging germination, nanosilver as antimicrobial agents, nanotitanium oxide for photocatalytic activity and nanosilica to deliver pesticides and fertilizers due to its large surface areas [18, 37, 55, 56]. As depicted in Fig. 2, several metal nanoparticles, including iron, zinc, manganese, selenium, etc., are critical nutrients for seed germination and development. Other studies have used nanopolymers and liposomes as renewable, biodegradable, and environmentally friendly carriers to encapsulate essential oils, pesticides, nutrients, and fertilizers [35, 57–59]. A sustained release nanocarrier encapsulates an active ingredient (biological or synthetic) and delivers this compound continuously over time instead of releasing it all at once.
Fig. 2.
Role of nanoagrochemicals and nanofertilizers in seed treatment. a Characteristics of nanopesticides, such as enhanced stability, control, and targeted delivery of agrochemicals, assist the seed in effectively protecting itself from pathogens and pests during germination. b Nanofertilizers compositions (Se, Zn, N, P, K, Mo, etc.) providing the nutrient-rich element for enhanced seed protection, enhanced stress tolerance, and fulfilling nutrient deficiency in the soil for the effective seed germination
More than 99.9% of pesticides fail to reach their targets and leave harmful impacts on soil, water, and air health while increasing pathogenic resistance and reducing biodiversity. Using a controlled release nano-system for targeted seed treatment is an efficient route to upgrading and advancing it sustainably. It has been shown that stimuli-response release nano-systems can be observed using photosensitive polymers. In this way, nanocomposite-based stimuli can intelligently react to the stimulation produced by the target or adjacent environment that ultimately triggers the release of agrochemicals to regulate the seed disease effectively. The controlled release nano-system offers several advantages over conventional chemical applications; the controlled release nano-systems allow more efficient delivery of pesticides and fertilizers more quickly into seeds, resulting in a decline in the concentration of agrochemicals used [60, 61]. Various nanoparticle-based products and smart agrochemical delivery systems using nanocomposites are constantly being developed for seed treatment (Table 1). The potential of nanocomposite as a nanofertilizer, nanoherbicide, nanofungicide, and nanoinsecticide for the next-generation treatment for seed treatments offer a variety of advantages, including durability, effectiveness, wettability, good dispersibility, less toxicity, good biodegradability in soil and environment, and photogenerated nature with the least amount of residues compared to conventional chemicals [53].
Table 1.
An overview of the latest advances in the application of nanomaterials for the treatment of seeds with nanofertilizers and nanopesticides
| Type of agrochemicals | Type of nanomaterials used | Type of seeds | Dose concentration | Key features | References |
|---|---|---|---|---|---|
| Nanofertilizers | Zinc | Peanut (A. hypogaea L.) | 100–500 ppm | An improvement in morphological, yield, and biochemical traits | [212] |
| Sorghum (S. bicolor L.) | 6 mg/kg soil | Increased yields and growth, increased nitrogen and potassium uptake, improved grain nutrient profile | [213] | ||
| Maize (Z. mays L.) | 50–2000 ppm | Improved seed germination, seedling vigor index, biomass, productivity, and zinc accumulation in grains | [214] | ||
| Iron | Soybean (G. max L.) | 25–1 M | Seed weight is increased in comparison with normal plants | [215] | |
| Peanut (A. hypogaea L.) | 2–1000 ppm | Growth characteristics, root morphology, and productivity were all improved | [75] | ||
| Maize (Z. mays L.) | 3–5 ppm | The seed germination frequency, the germination time, and the early growth were positively increased | [216] | ||
| Titanium oxide | Barley (Hordeum vulgare L.) | 500–1000 mg kg−1 soil | Nanoparticles are found to enhance plant growth by increasing germination | [217] | |
| Wheat (T. vulgare L.) | 5–40 ppm | Plant performance is not affected significantly | [218] | ||
| Titanium oxide activated carbon | Tomato (S. lycopersicum L.) and mungbean (Vigna radiates L.) | 0–500 ppm | It is possible to enhance tomato and mungbean seed germination rates using the appropriate concentration of nanoparticles | [219] | |
| Silver | Soybean [G. max (L.) Mell.] | 31.2–62.5 mg/kg soil | Affects seed development and nitrogen fixation negatively | [220] | |
| Silica | Fababean (V. faba L.) | 1–3 mM | The seeds germinated more rapidly and grew longer, produced more biomass, improved seed quality, and produced more nitrogen, phosphorous, potassium, calcium and sodium | [221] | |
| Nanopesticide | Silver | Soybean [G. max (L.) Mell.] | 70 ppm | Pesticidal silver nanoparticle-based treatment for Callosobruchus chinensis provided the least amount of seed damage, a minimum seed weight loss, and total mortality | [222] |
| Alumina | Wheat (T. vulgare L.) | 62.5–500 ppm | The nanostructured alumina has been shown to exhibit potential insecticidal effects against S. oryzae, which may help improve wheat kernels | [223] | |
| Silica | Maize (Z. mays L.) | 0.0031–10 g/kg | For better storage of maze seeds, Nanosilica showed insecticidal effects against S. oryzae, Rhizoper thadominica, Tribolium castaneum and O. philussurinamenisis | [224] | |
| French bean (P. vulgaris) | 400 mg/kg | Insects (O. surinamensis, Stegobium paniceum, T. confusum) are controlled during seed storage with Nanosilica as an effective insecticide | [225] | ||
| Peanut (A. hypogaea L.) | 0.67 mg/kg and 1.7 mg/kg | In groundnut storage, silica nanoparticles showed strong defensive properties against Caryedon serratus. Mortality rates increased with nanoparticle exposure time | [226] | ||
| Aluminum oxide | Wheat (T. vulgare L.) | 8000 mg/kg | A significant decrease in the number of S. oryzae offspring was a dose-dependent phenomenon | [227] | |
| Zinc Oxide | Mung bean (V. radiata) | 200 ppm | Nanozinc oxides showed the highest mortality (100% of C. maculatus) and the lowest egg production in green gram storage | [228] |
Nanofertilizer for Seed Germination
The nanofertilizers are considered to be promising candidates for the fertilizer industry, and they hold the promise of improving nutrient retention in seed and crop growth [62]. Seeds contain ample food reserves that support germination and seedling growth. For seed germination and seedling establishment, the starchy endosperm is the major tissue that accumulates seed reserve food material. The results of several studies have demonstrated that nanofertilizers can assist seeds in germinating in a way that conventional fertilizers cannot. In light of these studies, it is clear that nanofertilizers penetrate seed coats due to their nanosize and increase water absorption by upregulating aquaporin genes, thereby enhancing seed germination and reducing adverse effects of salinity, drought, and heavy metal stresses. Most importantly, many studies have demonstrated that nanofertilizer is not directly applied to the seeds with soil, pre-sowing treatment is beneficial with nanofertilizer, so seeds are in direct contact with nanoparticles in aqueous medium, and seed germination gets induced. Here, nanofertilizer acts as a nanocatalyst for enhancing starch degradation enzyme activity, acting as a mild stress inducer or ROS generator, and creating nanopores in the seed coat. The nanofertilizers also bring positive effect in the microbial communities around seeds, therefore, indirectly contribute to the seed germination [63]. Compared with conventional fertilizers, nanofertilizers are a more effective means of absorbing and utilizing nutrients. This is due to a considerable reduction in leaching and volatilization losses [63]. Unlike chemical fertilizers, the nanofertilizers diffuse freely through soil structures. As nanofertilizers have considerably smaller losses, they can be applied in smaller quantities. This is in contrast to synthetic fertilizers, which require greater quantities in order to compensate for their significant loss through leaching and emission [63]. Nanofertilizers with polymeric coats avoid premature contact with soil and water. Thus, loss becomes negligible, and nutrient contents of nanofertilizers become available when plants are in a position to internalize the released nutrients [64].
Nanofertilizers containing nitrogen, phosphorous, and potassium have been found to improve plant development and crop production [65]. Nitrogen is the principal mineral element required in the biosynthesis of amino acids, proteins, nucleic acids, enzymes, hormones, vitamins, secondary metabolites, etc. Nitrogen plays a key role in photosynthesis as it is the main constituent of chlorophyll. For plant growth, phosphorous is the second most abundant nutrient. It plays a critical role in the synthesis of nucleic acids, phospholipids, and phosphor-proteins. Furthermore, it is the main component of the metabolic energy source adenosine triphosphate. Among the primary nutrients, potassium is the third-most important and controls a number of metabolic processes, such as transport, opening and closing of stomata, controlling cytoplasmic pH, and activating more than 60 enzymes. Potassium is known to enhance the defense mechanism of a plant. Synthetic fertilizers release their nutrients in 4–10 days, whereas nanofertilizers release them in 40–50 days [66]. Furthermore, nanofertilizer increases the tolerance of seeds to both biotic and abiotic stresses by triggering many molecular mechanisms [67]. Badran and Savin [68] successfully studied the seed germination and early stages of bitter almond seedlings' growth under saline conditions using nanofertilizer (nanourea modified with hydroxyapatite nanoparticles). A copper oxide-based tenorite nanofertilizer was effectively developed by Esper Neto et al. [69], for the growth of corn seedlings. Using the seed priming technique (pre-soaking of seeds in colloidal solution of nanoparticles), Abdel-Aziz et al. [70], investigated the effects of engineered nanomaterials alone or in combination with nitrogen, phosphorous, and potassium on the growth and productivity of French beans. Kumar Das et al. [71] developed a sustainable design for rice production using nitrogen, phosphorous, and potassium fertilizer equivalent nano-pyrite seed dressing. Using zerovalent iron nanofertilizer, Titir Guha et al. [72] improved the germination of aromatic rice (Oryza sativa cv. Gobindabhog L.). Kubavat et al. [73] synthesized a chitosan-based sustained release nanofertilizer formulation to improve the biomass production of Zea mays. In the study by Abdel-Aziz et al. [70] engineered carbon nanotubes nitrogen, phosphorous, potassium and chitosan nanoparticles nitrogen, phosphorous, potassium fertilizer was effectively tested on the growth of French beans (Phaseolus vulgaris). A novel nanofertilizer synthesized by Yusefi-Tanha et al. [74] has been evaluated for its influence on soybean seed yield (Glycine max cv. Kowsar). A study by Rui et al. [75] suggested using iron oxide nanoparticles as a potential iron fertilizer for peanuts (Arachis hypogea). These are a few recent examples of nanofertilizers that were used to promote seed germination and seedling growth. A very limited amount of research is being done in the area of nanofertilizers for seed germination and development. More research is needed in this area.
Nanopesticides for Seed Protection
Using nanotechnology to enhance pesticide delivery could improve pesticide utilization and reduce runoff into the environment, thus reducing environmental pollution and negative impacts caused by pesticides [76]. On plant surfaces, nanopesticide formulations can improve droplet adhesion, increasing the dispersion and bioactivity of active ingredients. Therefore, nanopesticides are more effective at controlling crop pests than conventional pesticides. Nanopesticides not only improve pesticide dispersion but also enhance their bioavailability by accelerating the delivery of their beneficial ingredients. Consequently, nanopesticides are widely used to reduce the shortcomings of conventional pesticides, such as their low efficacy and large doses. In contrast to conventional pesticide formulations, nanopesticides release the active ingredient slowly at a predetermined rate to achieve their desired efficacy and longevity. By encapsulating pesticides in nanopesticides, the active ingredients of pesticides are protected from premature degradation and direct release to mankind. Unlike conventional pesticides, nanopesticides have a large surface area, which increases their ability to interact with target pests at a lower concentration [77]. Thus, the application of nanopesticides can be a sustainable option for increasing the crop productivity. Nanopesticides are pesticides formulated in the nanoscale for agricultural applications, whether pesticides are fixed on a nanomaterials, encapsulated in a matrix or embedded in enzyme- or stimuli-triggered nanocarriers [78]. The term nanopesticide refers to any nanochemical that kills pests, including weeds, insects, bacteria, and fungi [79]. Nanomaterials like silver, gold, iron oxide, titanium oxide, copper oxide, and zinc oxide are antimicrobial and anti-insecticidal, so they are ideal for use as nanopesticides. Despite their nanostructure and small size, nanomaterials can penetrate cell membranes and attach to cell organelles, causing abnormal oxygen species to form and causing an alteration to normal cell functioning. A normal level of ROS is necessary for important physiological processes, such as cell signaling, gene expression, and protein redox regulation; however, high levels of ROS cause anomalies and interfere with normal functioning, which results in cell death. Another molecular mechanism of nanopesticides for seed protection involves inhibiting cell wall synthesis, depolarising the cell membrane, inhibiting protein synthesis, inhibiting amino acid synthesis, and inhibiting metabolic pathways in pests and microorganisms [80].
Nanopesticide-mediated ROS not only kill seed pathogens, but also enhance seed and plant defense by activating antimicrobial peptides and secondary metabolites in plants raised from nanotreated seeds. Plant secondary metabolites play an important role in their defense, communication, and adaptation, but their secondary metabolism in response to nanoparticles is not completely understood. Several studies [81, 82] demonstrate that nanoparticles trigger ROS production significantly across plant species, which result in the synthesis of antimicrobial secondary metabolites; however, their exact molecular mechanisms are unknown. Although it is clear that ROS are involved in transcriptional regulation of antimicrobial secondary metabolites, there is also a link between ROS and secondary signaling messengers [81]. Thus, ROS generated by nanoparticle interactions may interfere with plant secondary metabolism and cause plants to produce antimicrobial secondary metabolites to defend themselves from pathogens. A nanofungicide made of iron nanorods was successfully used to inhibit the growth and fabricated zinc oxide nanoparticles as a tool for controlling soybean seed-borne phytopathogenic fungi was studied by Lakshmeesha et al. (2021) [83]. Almaary et al. [84] explored the application of seed-borne Penicillium duclauxii to the synthesis of silver nanoparticles. The comparative pot studies of chitosan and chitosan-metal nanocomposites as nanofungicides were conducted by Kaur et al. [85] against fusarium wilt of chickpea (Cicer arietinum L.) using triazolyl dithiocarbamate. A potent antifungal nanosilver agent has been developed by Sharma et al. [86] against bakanae disease of rice. The novel study was delivering pesticides to plant parasitic nematodes using tobacco mild green mosaic virus as a nanocarrier was carried out by Chariou et al. [87]. Sankar and Abideen [88] investigated the nanopesticidal effects of silver and lead nanoparticles against the pest Sitophilus oryzae. To protect the faba bean (Vicia faba) from insects, Thabet et al. [89] investigated silica nanoparticles as potential nanopesticides. A nanoformulation of thiosemicarbazone has been developed by Spadola et al. [90] to control fungus Aspergillus flavus infection in grains. The above-mentioned nanopesticides-based studies highlighted their potential for use in seed science and technology. As seen in these reports, a substantial amount of research can be conducted in the near future to develop nanopesticides for seed-borne infection and storage.
Nanoparticles: Potential Tool for Seed Treatment
The nanoparticles in nanoformulations are the only constant component, and they keep changing with the type of product. Nanoparticles are classified into inorganic and organic nanomaterials according to their chemical composition. Inorganic and organic nanoparticles are used as promising agents for seed priming, coating, and pelleting (Table 2). Listed below are some examples of most effective nanoparticles that can be used for the designing of seed-specific nanoformulations.
Table 2.
An overview of the nanoparticle systems used in seed priming and coating, their characteristics, and their main effects on different seed species
| Type of nanomaterial | Size in nanometers | Concentrations for seed treatment | Seed type | Key findings | References |
|---|---|---|---|---|---|
| Zinc oxide | 20–30 nm | 25–100 ppm | Wheat (T. aestivum L.) | Reduce cadmium uptake | [105] |
| 15–52 nm | 5–200 ppm | Rice (O. sativa L.) | Improved biofortification | [229] | |
| 35–40 nm | 750–1250 mg/kg | Chili (C. annuum L.) | High antimicrobial activity | [230] | |
| 40 and 60 nm | 1–5000 ppm | Common bean (P. vulgaris L.) | Improved biomass | [98] | |
| 21.3 nm | 20–60 mg/L | Lupin (Lupini stermis L.) | High salinity resistance | [99] | |
| 32 nm | 50–500 ppm | Pearl millet (Pennisetum glaucum L.) | Antimicrobial resistance | [100] | |
| Iron | 50 nm | 10–500 ppm | Sorghum (S. bicolor (L.) Moench) | Increased water content in leaves | [91] |
| 19–30 nm | 20–160 ppm | Watermelon (Citrullus lanatus (Thunb.) Matsum and Nakay varieties) | Increased the activity of plant growth regulator | [231] | |
| 6–20 nm | 30 µg/mL | Rice (O. sativa L.) | High antimicrobial activity | [93] | |
| 80 nm | 25–1000 µg/mL | Wheat (T. aestivum L.), types WL711 (low-iron genotype) and IITR26 (high-iron genotype) | Increased harvest yield | [95] | |
| 33.8 ± 3.59 nm | 10–160 mg/L | Rice (O. sativa L.) | Improved water uptake | [72] | |
| 25–100 nm | 50 µg/mL | Rice (O. sativa L.) | Improved enzymatic activity | [71] | |
| 20–30 nm | 20–40 ppm | Wheat (T. aestivum L.) seeds of varieties galaxy-13, Pakistan-13, and NARC-11 | It develops abiotic stress resistance in wheat | [103] | |
| Manganese (III) oxide | 50 nm | 0.1–1 mg/mL | Jalapeño (C. annuum L.) | Salinity resistance development | [105] |
| Copper | 25, 40, and 80 nm | 1–1000 mg/L | Common bean (P. vulgaris L.) | High concentrations showed toxic effects on seed germination | [102] |
| 15–30 nm | 20–40 ppm | Wheat (T. aestivum L.) seeds of varieties galaxy-13, Pakistan-13, and NARC-11 | Abiotic stress resistance development | [102] | |
| Platinum | 3.2 ± 0.8 nm | Concentrated solution at 1.0 mM | Pea (P. sativum L.) | Decreased microorganism’s colonization | [198] |
| Carbon | 13–14 nm | 70 µg/mL | Wheat (T. aestivum L.) | Improved harvest | [232] |
| Molybdenum | 35–50 nm | 10 mg/L | Chickpea (C. arietinum L.) | Increased antioxidant enzymes and harvest | [233] |
| Silver | 6–26 nm | 10 and 20 mg/mL | Rice seeds (O. sativa L. cv. KDML 105) | Increased aquaporin gene expression | [119] |
| 11.6 ± 2.40 nm | 31.3 µg/mL | Onion (A. cepa L.) | Potentially increased biochemical activity | [120] | |
| 10–35 nm | 0–50 mg/L | Wheat seeds (T. aestivum L.) | Increased seed and seedlings vigor | [121] | |
| 5–30 nm | 10–50 µg/mL | Soybean (G. max (L.) Merr.) | Potential antimicrobial activity | [123] | |
| Gold | 10–30 nm | 5–15 ppm | Maize (Z. mays L.) | Improved seed and seedlings vigor | [124] |
| 93.68 ± 2.06 nm | 31.3 µg/mL | Onion (A. cepa L.) | Improved seed and seedlings vigor | [66] | |
| Silica | 90 nm | 300–1200 ppm | Wheat (T. aestivum L.) | Reduced cadmium uptake | [234] |
| ~ 100 nm | 2 mg/mL | Pea seeds (P. sativum L.) | Improved seed and seedlings vigor | [18] | |
| Chitosan | 259.4 ± 4.7 nm | 1–100 µg/mL | Wheat (T. aestivum L.) | Increased plant growth regulator (auxin) | [235] |
| 95 ± 2 nm | 20 µg/L | Common bean (P. vulgaris L.) | Increased ROS levels | [69] | |
| 122 nm | 0.05–0.2% | Rice (O. sativa L.) | Potential antimicrobial activity | [129] | |
| 560 nm | 0.1% | Chickpea (C. arietinum L.) | Improved activity of plant growth regulator | [236] | |
| 450 ± 10 nm | 0.05–0.0005 mg/mL | Tomato (S. lycopersicum var. cerasiforme) | Improved harvest yield | [237] | |
| 400 nm | 250 mg/kg | Pearl millet (P. glaucum) | Improved plant growth regulators | [132] | |
| 374.3 ± 8.2 nm | 0.01–0.16% w/v | Maize seeds (Z. mays L.) | Improved seed and seedlings vigor | [133] | |
| 387.7 ± 4 nm | 0.01–0.16% w/v | Maize seeds (Z. mays L.) | Development of biotic resistance and improved harvest yield | [101] | |
| Lignin | 200–250 nm | 0.5, 1, and 1.5 mg/mL | Arugula (Erucavisicaria L.) Cav. subsp. sativa), tomato (S. lycopersicum L. cv. Ciliegino), and chickpea (C. arietinum L.) | Improved seed and seedlings vigor | [137] |
Inorganic Nanoparticles
Iron Oxide Nanoparticles
Iron oxide nanoparticles at low concentrations promoted the growth and development of seeds. A study by Maswada et al. [91] demonstrated the potential of nanoiron oxide to improve sorghum (Sorghum bicolor) germination and seedling growth using soaking and priming of seed under salinity conditions. Kasote et al. [92] used onion extract to synthesize iron oxide nanoparticles. It was shown that non-toxic iron oxide nanoparticles could be applied sustainably to watermelon seeds to increase anti-inflammatory properties and enhance defenses. Using aqua dispersed nanoparticles of ferrous sulfide, Ahuja et al. [93] assessed the phytopathological effects against the rice born fungus Fusarium verticillioides. Nanoiron treated rice seed showed significant seed germination and inhibition of fungus F. verticillioides [93, 94]. Sundaria et al. [95] suggested that biofortifying wheat with iron through seed priming could address anemia caused by iron deficiency. The given treatment showed a significant increase in grain iron content and higher accumulation. Guha et al. [72] reported the use of nanopriming using zerovalent iron to increase germination and growth in aromatic rice cultivars. Das et al. [71] proposed a seed dressing approach for rice, and their experiments revealed that the nano-pyrite seed dressing triggers nitrogen, phosphorous, potassium equivalent rice production without compromising yield. Thus, the above-mentioned iron oxide nanoparticles could be used as a platform for further asset delivery system development. Iron oxide nanoparticles can significantly reduce the presence of iron in the cotyledon, which inhibits the uptake and translocation of nanoparticles. Furthermore, these iron oxides are externally aggregated on seeds, making them ideal for seed priming and pest control applications.
Zinc Oxide Nanoparticles
Seeds require zinc for many physiological and biochemical processes. Many studies show that seed primed with zinc oxide nanoparticles has a higher zinc content, which contributes to a higher yield and higher growth rate. According to Rizwan et al. [96], zinc oxide nanoparticles positively affected wheat growth and decreased cadmium accumulation in wheat. Zinc nanoparticles enhanced zinc concentrations in roots, shoots, and grains. Overall, nanoparticles significantly increase wheat biomass, and nutrient retention, and decrease cadmium toxicity. In a study by Itroutwar et al. [97], biogenic nanozinc was synthesized for rice seeds using brown seaweed extract Turbinaria ornataas a priming agent, resulting in increased rice seed quality and crop yield as a result. Zinc nanoparticles were used by Savassa et al. [98] to enhance seed nutrition. This study evaluated the effects of different concentrations and sizes of zinc nanoparticles on bean (P. vulgaris) seed germination. Biotransformation of zinc oxide nanoparticles was detected using X-ray absorption spectroscopy. It was found that most of the zinc absorbed by seed coat was trapped there, while a small fraction entered cotyledons. The results demonstrate potential for using zinc nanoparticles as an agrochemical due to their properties, particularly the slow zinc release and lower toxicity than zinc sulfate. According to Latef et al. [99], nanozinc effectively prevented seed germination loss of Lupinus termis seeds grown under salinity stress. As a result, zinc nanoparticles may boost the growth and yield of plants growing in salinized soils. The mechanisms by which zinc oxide nanoparticles alleviate the adverse effects of salinity stress in seeds require further study. The use of biofabricated nanozinc as a potent seed priming agent for growth promotion and mildew control in pearl millet was recently reported by Nandhini et al. [100]. Chudhary et al. [101] fabricated the zinc–chitosan nanoparticles and assessed them via seed priming and foliar application in maize. Zinc–chitosan nanoparticles have been shown to act as antifungals and promote seedling growth. Above all, the studies showed that nanozinc could be utilized in agriculture, but a thorough understanding of their interactions with seeds is needed.
Copper Oxide Nanoparticles
A number of enzymes are activated by copper, which contributes to RNA synthesis and improves photosystems’ performance. A range of copper oxide nanoparticle sizes and concentrations influence P. vulgaris seedling germination and growth. According to Duran et al. [102], it was found that most copper was in its pristine form through X-ray absorption spectroscopy. Seed germination was not affected by copper nanoparticles, but seedling weight gain was promoted at low concentrations but inhibited at high concentrations. Biosynthesized copper oxide nanoparticles markedly induced and promoted antioxidant enzyme activities. The potential role of nanocopper in wheat yield was studied by Yasmeen et al. [103]. Size- and dose-dependent toxic activity was observed for the biosynthesized copper oxide nanoparticles against two wheat grain damaging insects, Sitophilus granarius and Rhyzopertha dominica. Findings suggested that copper oxide nanoparticles should be used at lower concentrations in agricultural fields as insecticides that will not inhibit wheat growth. A study by Wang et al. [104] examined the concentration-dependent effects of copper oxide nanoparticles on the germination, growth, and physiological responses of Brassica pekinensis L. In light of these results, nanocopper can serve as a powerful insecticide to facilitate the storage of a wide range of seeds and, at lower concentrations, it can significantly improve the seed germination rate.
Manganese Oxide Nanoparticles
It was known that manganese helps enhance the seed germination rate. It has been found that nanoscale manganese is less phytotoxic and more effective at minimizing abiotic stresses compared to conventional bulk or ionic manganese compounds. There is little information available regarding the physiological and toxicological effects of manganese nanoparticles on agricultural crops. Manganese oxide nanoparticles were studied as a nanopriming agent by Ye et al. [105] to alleviate salinity stress in the Capsicum annuum during germination. According to the study, the surface charge plays an important role in the behavior of nanomanganese oxide. Manganese oxide nanoparticles have been used as seed priming agents to improve chlorophyll and antioxidant profiles in watermelon seedlings by Kasote et al. [106]. Similarly, bio-engineered magnesium oxide nanoparticles [107] were shown to enhance green gram seedling strength. However, more research is needed to determine the exact effect of seed priming with manganese on agricultural output, including its role in enhancing abiotic and biotic tolerance.
Cobalt Nanoparticles
The micronutrient cobalt is another essential one. Cobalt concentrations influence the response of seeds. It promotes plant growth in low concentrations but can cause phytotoxicity at higher concentrations. Hong et al. [108] investigated the effect of nanoscale zerovalent cobalt on soybean growth. Soybean growth and development were positively influenced by zerovalent cobalt at nanoscales. Krishnamoorthy et al. [109] investigated hexa-amino-cyclotriphosphazene and cobalt nanoparticles incorporating polyvinylpyrrolidone seed coatings for improving cowpea seed germination. Cobalt-coated seeds showed higher imbibition rates, which could help reduce drought stress. Nanocobalt-based seed coating given to a seedling could increase germination rates and enhance stand establishment.
Carbon Nanoparticles
Using multi-walled carbon nanotubes as a nanopriming agent, Joshi et al. [110] developed a new nanopriming agent. The use of multi-walled carbon nanotubes in wheat significantly increases seed yield. The effects of carbon-based nanomaterials on seed germination under salt stress were investigated by Pandey et al. [111], and nanocarbon has been described as promising seed germination and plant growth product. Carbon nanomaterials are promising seed germination promoters and plant growth regulators. Baz et al. [112] reported that carbon nanoparticles might enhance seed germination and post-germination growth of lettuce under salinity stress. The study showed that soluble nanoparticles improved lettuce seed germination under salt stress, which provides fundamental evidence about the potential of nanoparticles in agricultural applications to improve crop yield and quality.
Graphene Oxide Nanoparticle
A graphene oxide is a unique material that consists of a single monomolecular layer of graphite with epoxide, carbonyl, carboxyl, and hydroxyl groups containing oxygen functionality. Recently, graphene oxide nanoparticles have been investigated as seed stimulators in agriculture for improving seed germination, seedling growth, and further development. According to Yin et al. [113], graphene oxide significantly impacted the germination and growth of seeds, as well as the uptake of cadmium in solution cultures. As a result of this study, graphene oxide can inhibit the adverse effects of cadmium on seed germination, seedling growth, and uptake of cadmium in solution, and positively stimulates the seed germination. In another study, Kim et al. [114] demonstrate that silver–graphene oxide nanoparticles can significantly affect the early growth of seeds depending on the species. In rice seeds, Li et al. (2020) demonstrated the significant effects of graphene oxide nanosheets on rice seed growth. Based on these findings, graphene oxide nanosheets were found to inhibit the adverse effects of cadmium on rice seed germination and to reduce cadmium uptake and accumulation in rice seedling roots and shoots, helping to determine cadmium’s fate and ecotoxicity [115]. As a result, graphene oxide nanoparticles can be utilized as a potential seed stimulant.
Silicon Nanoparticles
Silicon decreased malondialdehyde levels in radicles under stress, indicating a decreased lipid peroxidation rate. Exogenous silicon increased antioxidant defense in bud seedlings, improving seed germination and alleviating oxidative stress. Cadena et al. [116] demonstrated the enhancement of cinnamon essential oil activity using nanoparticle encapsulation to control seed pathogens. To combat seed-borne diseases, cinnamaldehyde-mesoporous silica nanoparticles were incorporated into a sodium alginate seed coating. Hussen et al. (2019) [117] determined the effects of nanosilica on wheat growth, yield, and cadmium accumulation. Rahimi et al. (2021) [118] investigated the potential role of silicon nanoparticles in affecting seed germination and vigor of calendula (Calendula officinalis L) under drought stress induced by polyethylene glycol.
Silver Nanoparticles
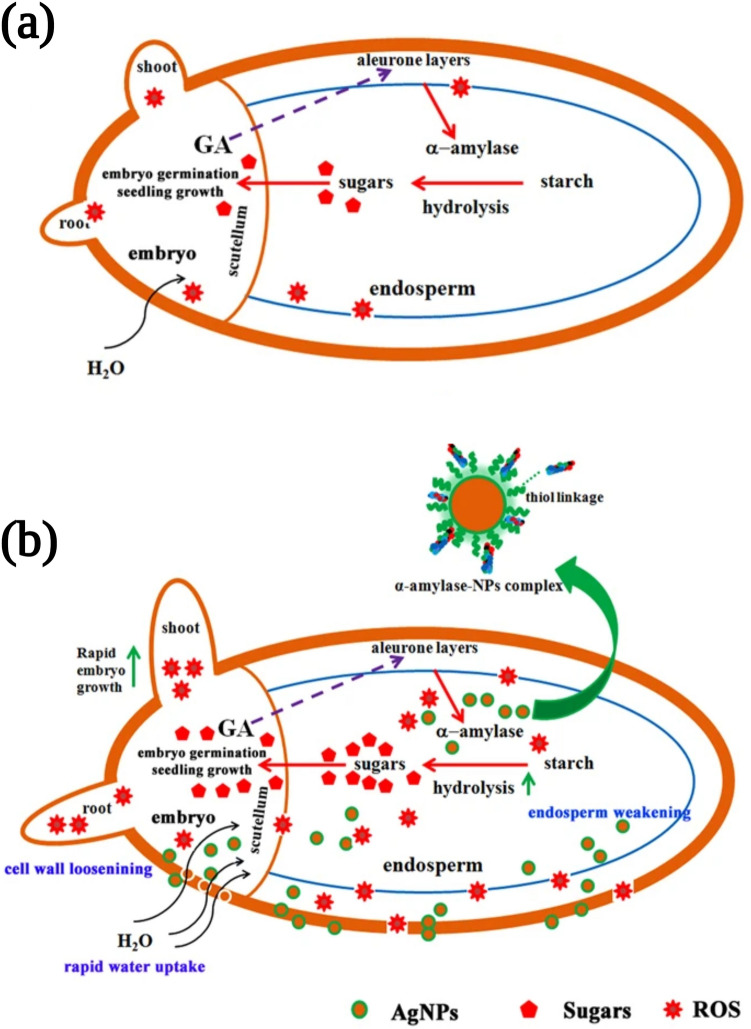
A wide range of nanomaterials are used in agriculture research, including silver nanoparticles. According to Mahakhham et al. (2017) [119], the mechanism of silver nanoparticles promoting seed germination has been hypothesized as (i) functioning as a nanocatalyst for enhancing starch degradation enzyme activity, (ii) acting as a mild stress inducer or ROS generator, and (iii) creating nanopores in the seed coat. Silver nanoparticles can be used as a nanopriming agent for enhancing seed germination and starch metabolism of rice-aged seeds. As depicted in Fig. 3, the nanopriming of silver could cause an increase in the activity of amylase, leading to a higher content of soluble sugars that would support seedling growth. As a result of nanopriming treatment, amylase activity increased, resulting in a higher concentration of soluble sugars. As sugar concentrations increase in the cells, the osmotic potential and water potential decrease. As a result, the difference (gradient) between the water potential outside and inside the tissues increases, allowing water to move into the seeds through osmosis. Due to the increased soluble sugar content and amylase activity in nanoprimed seeds, the increase in water uptake may also be due to the change in internal osmotic potential caused by soluble sugars (solutes). In Mahakhham et al. (2017) [119] study, ROS, including hydroxyl radicals, were shown to be important for cell wall loosening, testa, and endosperm weakening, which is necessary for radicle protrusion.
Fig. 3.
Phytosynthesized silver nanoparticles enhance aged rice seeds’ germination and starch metabolism. a Seeds without silver nanoparticle priming treatment have lower metabolic activity because of slow water uptake, and starch is hydrolyzed slowly; as a result, sugar levels are low in the initial stage of imbibition, resulting in slow seed germination and growth. b Silver nanoparticle seed priming enhances seed germination. This is a reprinted image of Ref. [119] with permission
The nanopriming treatment produced higher ROS levels in germinating seeds than the unprime control or other priming treatments, suggesting both ROS and aquaporins contribute to seed germination. There was evidence that nanosilver can internalize seed coats and support water uptake inside seeds, thus promoting seed germination and starch metabolism. Several mechanisms have been proposed for nanopriming-induced seed germination, including the creation of nanopores that enhance water absorption, rejuvenation of ROS/antioxidant systems in seeds, and the generation of hydroxyl radicals that loosen the cell wall. As seed nanopriming maintains ROS levels within the oxidative window that promotes seed germination, seed nanopriming increases seed germination. Nanoparticles can reduce the level of ROS in seeds under stress conditions, as they increase the activity of enzymes like superoxidase dismutase, catalases, and guaiacol-peroxidase. This reduces seed cell damage. According to the study of Mahakhham et al. (2017), silver nanoparticles penetrated seed coats and created small pores, which resulted in increased water uptake and increased expression of aquaporin genes involved in water uptake. Acharya et al. (2019) [120] synthesized nanosilver from onion extract, which was internalized by onion seeds. Several greenhouse and field studies have demonstrated that seeds germination, growth, and yield are significantly enhanced. The study by Kannaujia et al. [121] demonstrated the potential role of biogenic nanosilver on wheat growth as a growth promoter without any toxic effect typically associated with chemically synthesized nanosilver. Using agro-industrial by-products, Acharya et al. (2020) [122] synthesized silver nanoparticles and used them as nanopriming agents for diploid and triploid watermelon seeds. Seed treatment with nanosilver has been demonstrated to improve seed germination, growth, and fruit quality. Spagnolettia et al. (2019) [123] obtained stable silver nanoparticles from the exudate of the soil fungus Macrophomina phaseolina using a low-cost, green synthesis process. The effect of silver nanoparticle dosage on soybean seed germination was also studied to test its potential applicability as a seed protection agent.
Gold Nanoparticles
The properties of gold nanoparticles make them attractive candidates for seed priming applications since they have a small size, good biocompatibility, low toxicity, easy surface chemistry, and easy surface modification. According to Mahakhham et al. (2016) [124], a nanogold solution was used to elicit seedling growth and germination in aged maize seeds. A study showed that the nanopriming approach minimized gold translocation from seeds into plant vegetative organs. Gopinath et al. (2014) [125] studied the effects of gold nanoparticles produced from fruit extract of Terminalia arjuna on Gloriosa superba seed germination. It was found that nanogold significantly affected seed germination and vegetative growth of Gloriosa superba. A study by et al. (2017) [126] examined the effect of green synthesized gold nanoparticles on rice germination and root growth. Overall, the results indicated that gold nanoparticles synthesized by Tiliacora triandra can enhance seed vigor and are biocompatible. Brassica juncea growth and seed yield are enhanced by gold nanoparticles, as demonstrated by Arora et al. (2012) [127].
Organic Nanoparticles
Nanoparticles made from natural or synthetic organic molecules are called organic nanoparticles. An organic nanoparticle can be made from a polysaccharide, lipids, or proteins that are biodegradable, biocompatible, and able to react to various environmental stimuli like pH, temperature, etc. [128]. Organic nanoparticles can be effective carriers of seed health-promoting compounds when applied as seed coatings or seed dressings material. A wide selection of chemicals can be loaded into these nanoparticles, including fungicides, essential oils, plant growth regulators, and fertilizers.
Chitosan Nanoparticles
Nanoparticles made of chitosan are biodegradable, more stable, less toxic, and biocompatible. Li et al. (2018) [129] examined the effect and mechanism of chitosan nanoparticles on wheat germination and seedling growth. As a result of the higher adsorption of chitosan nanoparticles on the surface of wheat seeds at low concentrations, chitosan nanoparticles provide beneficial effects to the growth of wheat seeds. A chitosan guar nanoparticle was prepared by Sathiyabama et al. (2020) [129] with high antimicrobial activity as a bioprotectant against rice phytopathogens. According to this study, chitosan guar nanoparticles can be used as an antimicrobial agent to combat rice blast and blight disease. Nanochitosan loaded with nitrogen, phosphorous, and potassium is tested as a fertilizer for french beans by Azizi et al. (2019) [69]. According to the obtained results, nanochitosan loaded with nitrogen, phosphorous, and potassium might be used to improve seed germination. A study by Divya et al. [130] optimized the synthesis of chitosan nanoparticles and investigated their potential use as a germination elicitor for rice seeds. Rice seeds treated with chitosan nanoparticles remained effective when stored at room temperature for seven months. Using nanoalginate–chitosan and nanochitosan–tripolyphosphate containing gibberellic acid, Anderson do Espirito Santo Pereira et al. (2019) [131] designed a seed treatment that improved the growth and productivity of Solanum lycopersicum under field conditions. Using chitosan nanoparticles, Siddaiah et al. [132] investigated the effectiveness of the nanoparticles against downy mildew in pearl millet. A study showed that chitosan nanoparticles increased pearl millet germination and seedling vigor after seed treatment with chitosan nanoparticles. The effect of copper–chitosan nanoparticles on physiological and biochemical changes during maize seedling growth was investigated by Saharan et al. [133]. According to studies, copper–chitosan nanoparticles promote seedling growth through better mobilization of reserved food, such as starch, through increased levels of α-amylase.
Cellulose Nanofibers
The cellulose nanoparticles are easy to process, cost-effective, biodegradable, and have good solubility in many organic solvents. Biodegradable cellulose biopolymer-based nanofiber seed coatings were used by Xu et al. [134] to enhance agrochemical delivery and seedling development. The greenhouse studies found that nano-enabled seed coatings effectively deliver agrochemicals at the right place while consuming a minimum number of agrochemicals. Zhang et al. [135] developed cellulose anionic hydrogels based on cellulose nanofibers for significant seed germination and seedling growth. The present study provided an easy and effective method for fabricating cellulose anionic hydrogel and evaluated its application in agriculture.
Lignin Nanoparticles
Lignin nanoparticles have excellent antibacterial and antioxidative properties due to their surface chemistry and shape. Kacsó et al. [136] used zein and lignin-based nanoparticles to treat soybean seeds. A seed treatment containing azoxystrobin-loaded lignin nanoparticles provided almost complete antifungal protection for soybean against fungus Rhizoctonia solani. The results indicate that nanozein and lignin are safe and effective delivery systems for active compounds in seed treatments. Falsini et al. [137] synthesized lignin nanocapsules, which were used as potential vectors for the delivery of bioactive compounds to tomato and miller seeds; it was found that lignin nanocapsules enhanced seed growth and development of tomato and miller seeds. It is necessary to conduct further studies to determine the precise mechanisms responsible for the differential effects of nanolignin on seeds.
Possible Next-generation Nanoscale Architectures for Future Seed Treatment Formulations
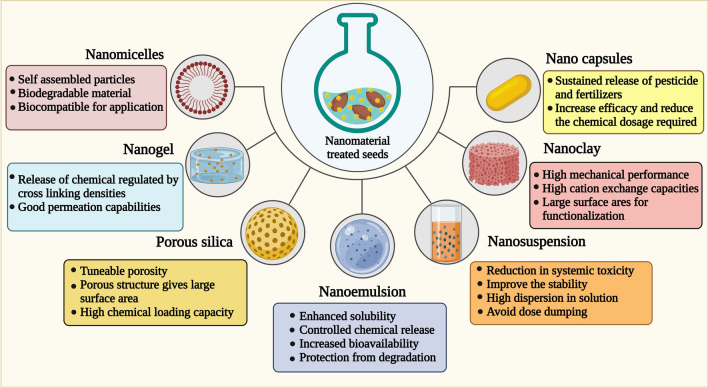
Nanoscale systems of the next generation can control stability, solubility, and bioavailability and provide controlled release of bioactive for seed treatment [138]. Liquid and solid nanoscale systems are the two main types of designing next-generation nanoscale formulations. Using these systems, as depicted in Fig. 4, next-generation seed treatment formulations can be developed for highly effective seed treatments.
Fig. 4.
Next-generation nanoagrochemicals for enhancing seed germination (nanoagrochemicals with a wide range of morphologies and structures such as nanomicelles, nanogels, porous silica nanoemulsion, nanosuspension, nanoclay providing controlling stability, solubility, bioavailability, and controlled release of agrochemicals for enhanced seed germinations)
Polymeric Nanocapsules and Nanospheres
The polymeric nanocapsule and nanospheres can be fabricated from preformed polymers or by polymerizing monomers [139]. The nanocapsule and spheres consist of a vesicular or reservoir-type structure with an inner cavity surrounded by a polymer coating or membrane. It may be possible to prepare seed treatments with pesticide/fertilizer-loaded nanocapsules using several different techniques, including nanoprecipitation, emulsion-solvent diffusion, emulsion-solvent evaporation, layer-by-layer self-assembly, ionic gelation, polyelectrolyte complexation, and melt-dispersion techniques [30]. In a double emulsion technique, Kumara et al. (2014) [140] prepared alginate nanocapsules containing imidacloprid and neonicotinoid insecticide. A successful field study on crop pests was conducted to evaluate the effectiveness of imidacloprid nanoformulation. The hydrophilic carbamate insecticide methomyl has been encapsulated in an elegant way by Chuxiang et al. [141]. Nanoencapsulation of methomyl is necessary to prevent early degradation. Chen et al. [142] developed leaf-adhesive pesticide nanocapsules with pH-responsive release to enhance crop leaf retention and improve utilization efficiency. The dual-functionalized pesticide nanocapsule delivery system with improved spreading behavior and enhanced bioactivity was developed by Cui [143]. In contrast to nanocapsules, nanospheres are homogenous, monolithic systems in which the bioactive element is evenly dispersed throughout the polymer matrix. Aza-loaded polymeric nanospheres have been prepared both as suspensions and as powders by da Costa et al. [144]. Using freeze-drying, the colloidal suspension was transformed into powders that provide the best protection against ultraviolet light-induced degradation of the aza in the neem product. Jiang et al. [145] developed lignin–xylan hybrid nanospheres with enzyme-mediated release properties as pesticide carriers. Pectin nanospheres were prepared by Li et al. [146], and their potential impact on wheat seed germination and growth was studied. Here are a few examples of nanocapsules and nanospheres that are effective in their applications. By experimenting with nanocapsules and nanospheres, it is possible to create future formulations that preserve seeds or improve seed storage.
Nanomicelles
Nanomicelles are self-assembling colloidal particles formed by amphiphilic block copolymers in water. During the formation of a micellar core surrounded by a hydrophilic corona, hydrophobic interactions are developed that drive self-assembly. Zhang et al. [147] developed polyethylene glycosylated-camptothecin nanomicelles to control pesticide combinations. According to this study, micelles could be effective carriers for pesticide combination control. Adak et al. [148] developed nanosized micellar aggregates from amphiphilic copolymers to make controlled release formulations of imidacloprid using aqueous media self-assemble into micellar aggregates upon contacting water. Dong et al. [149] developed pH-responsive ultrasonic self-assembly spinosad-loaded nanomicelles and studied their antifungal activity against Fusarium oxysporum. Nanomicelles have not yet been extensively studied for their potential applications in agrochemical delivery. Researchers have the opportunity to explore this nanoarchitecture to design an effective seed treatment system. Biodegradable and environmentally friendly techniques have proven effective seed treatment strategies.
Nanogels
“Nanogel” generally refers to a water-swollen network of nanoscale polymers, such as hydrophilic or amphiphilic chains that swell without water dissolving. A large surface area of nanogels facilitates multivalent bio-conjugation, and a strong interior network facilitates the incorporation of biomolecules. The transient antiviral activity of chloroinconazide was enhanced by alginate-based nanogel, and its effect on plant growth was studied by Lv et al. [150]. Lv et al. [150] demonstrated the antiviral activity and growth promotion of small molecule pesticides using nanogel carriers for the first time. The composition of the nanogel can easily be applied to the spray-based delivery of pesticides, representing a novel strategy for preparing new pesticide preparations and using multifunctional pesticides to improve seed germination. Ziaee et al. [151] prepared the myristic acid–chitosan nanogel containing essential oil of Cuminum cyminum to manage stored product beetle pests effectively. By using this technique, it may be possible to overcome the limitations of essential oils in managing stored product insect infections. It is still necessary to conduct more experiments to optimize nanogels and clarify their toxicity in various commodities, environmental conditions, and insects. However, nanogel has not been fully explored for seed technology, but it could be used to deliver pesticides as it provides pesticidal effects. Nanogel is suitable for use in the initial stages of seed germination and seedling development. Nanogel can be used to coat the seeds with nutrients and maintain moisture while the seeds germination.
Nanofibers
Nanofibers are fibers that are nanometers in diameter. Fiber diameters ranged from 200 to 400 nm. The nanofibers can be fabricated from various polymers and therefore have a variety of properties and applications. Nanofibers can be synthesized using natural polymers such as collagen, cellulose, silk fibroin, keratin, gelatine, and polysaccharides such as chitosan and alginate [152]. Several methods are available to create nanofibers, including drawing, electrospinning, self-assembly, template synthesis, and thermally induced phase separation. Nanofibers are most commonly generated through electrospinning due to the simplicity of the setup, the ability to synthesize continuous nanofibers from various polymers, and the ability to control the fibers’ diameter, composition, and orientation [152]. Farias et al. [153] used electrospun polymer nanofibers in seed coatings for crop protection. According to Farias et al., localized pesticide delivery can be achieved by coating seeds with cellulose diacetate nanofibers containing abamectin or fluopyram. It is found that nanofibrous coatings electrospun on soybean seeds do not reduce seed germination regardless of coating thickness or uniformity. The in vitro fungal assay performed with fluopyram-loaded nanofibers consistently inhibited the growth of Alternaria lineariae [154]. Combining sustained release profiles with moisture stability suggest that nanofibrous seed coatings can act as a unique platform to control nematodes and fungi in seeds. Enhancing agrochemical delivery and seedling development with biodegradable, tunable, biopolymer-based nanofiber seed coatings was developed by Xu et al. [134]. Nanofiber-coated seeds (tomato and lettuce) were studied in greenhouse experiments in the presence and absence of a fungal pathogen (Fusarium sp) to determine how they germinate and grow over time [155]. This seed nanocoating approach may increase yields in pathogen-infested soil conditions as a result of the precise delivery of agrichemical at the right place while using a relatively small amount of agrochemical. Greenhouse experiments suggest that such nano-enabled seed coating approaches may be useful in pathogen-infested soil conditions [156]. The environment friendly effective seed coat was developed by Krishnamoorthy et al. [157] using electrospun polyvinyl pyrrolidone incorporated with urea and cobalt nanoparticles for use as seed coatings on cowpeas. This new nanofiber seed coating method offers precision in agrochemical delivery and significantly improves germination and seedling biomass for model seeds over conventional film coating methods, due to its unique nanofiber structure and controlled release mechanism.
Porous Silica Nanoparticles
Agrochemicals can be transported or encapsulated in porous silica nanoparticles because of their biocompatibility, high load capacity and tuneable porosity. Since these nanoporous particles are used in various applications, they can be a viable option for designing nanoformulations for developing seed treatment. Porous silica nanoparticles allow for sustained release of pesticides and fertilizers, intended to increase efficacy and reduce doses required to achieve desired seed effects. Research is underway to establish the potential application of porous silica for effective seed treatments. Sun et al. [158] investigated mesoporous silica nanoparticles ability to enhance wheat and lupin seedling growth and photosynthesis. A dramatic increase in growth was observed in wheat and lupine exposed to mesoporous silica nanoparticles. Furthermore, mesoporous silica nanoparticles localized to chloroplasts in leaves, while photosynthetic activity was markedly increased. The growth and physiological responses of maize to porous silica nanoparticles in soil were studied by Rangaraj et al. [159]. Using nanoscale silica in maize is more effective than bulk silica, thus enabling sustainable agriculture of maize crops as an alternative source of silica fertilizer. Using mesoporous silica nanoparticles, Sattary et al. [160] tested the potential antifungal properties of lemongrass and clove oil against wheat’s take-all disease. In this study, essential oils-mesoporous silica nanoparticles were a safe product to control take-all diseases in wheat crops. Cadena et al. [116] reported improving cinnamon essential oil activity by encapsulating it in mesoporous silica nanoparticles for controlling seed pathogens. Study findings showed that mesoporous silica nanoparticles could be encapsulated to enhance the antimicrobial activity of plant products, thus allowing for the use of volatile biocides, such as essential oils, at very low concentrations to treat and prevent microbial diseases in crops. Study findings showed that mesoporous silica nanoparticles could be encapsulated to enhance the antimicrobial activity of plant products, thus allowing for the use of volatile biocides, such as essential oils, at very low concentrations to treat and prevent microbial diseases in crops. To promote rice seedling growth by regulating amino acid metabolism, Zhao et al. [161] developed mesoporous silica nanoparticles containing fungicides. By regulating amino acid metabolic pathways, fungicide-loaded mesoporous silica nanoparticles protect plants from the negative effects of fungicides [162]. In all of these studies, porous silica nanoparticles have demonstrated that they could potentially be used as novel delivery systems for active ingredients such as pesticides or fertilizers. There is huge scope to explore this material for seed-protecting treatments since very few studies have been done in this field. This material can be a next-generation candidate for developing seed treatment nanoformulations.
Nanoemulsions
Nanoemulsions are emulsions comprised of droplets with a size on the nanometer scale. They are kinetically stable but are not thermodynamically unstable. Thus, nanoemulsions are metastable systems whose stability depends on the preparation method. Either high-energy or low-energy emulsification methods can prepare nanoemulsions. High-energy methods utilize high-shear stirring, high-pressure homogenizers, and ultrasonic generators, whereas low-energy methods take advantage of the stored energy in the system to produce small droplets. Few reports have been published in which nanoemulsions have been applied in seed treatment. The physic mechanical and antifungal properties of neem oil nanoemulsion for soybean seed coating were studied by Silva et al. [163]. A neem oil emulsion inhibited the growth of fungus A. flavus and Penicillium citrinum [164–166]. Soybean seeds coated with this nanoemulsion showed positive results in the germination process. These new materials have the potential to be used as seed coatings because of their fungicidal properties derived from Neem oil nanoemulsions. Acharya et al. [122] developed a turmeric oil nanoemulsion. This nanoemulsion was successfully used to prime the watermelon seeds of two types of diploids (riverside) and three types of triploids (maxima) using agro-industrial by-products. A new nanoemulsion of eucalyptus oil was developed by Adak et al. [167] to treat two major storage insects (S. oryzae (L.) and Tribolium castaneum (Herbst) of rice. Compared to eucalyptus oil, their nanoemulsions were superior, and they can be recommended as a safe, non-toxic alternative to harmful chemical pesticides. This small number of studies prompted a willingness to study nanoemulsion development more closely, to design future seeds that contain pesticides or fertilizers.
Nanoclay
The nutrient-rich nature of clay-containing soils and their ability to retain water make them valuable soils. A nanoclay, also known as layered silicates, is a widely used and studied nanoagent to prepare nanocomposites. A few of the advantages of nanoclays are their widespread availability, easy processing ability, high performance, and low cost. Nanoclay is prepared through a mixing technique in which nanoflakes are dispersed in aqueous media under laminar and turbulent flow conditions. Nanoclay helps deliver micronutrients for crop improvement; it is also used to encapsulate pesticides in nanomaterials for controlled release, stabilizing biopesticides with nanomaterials [168]. Nanoclay has been shown to reduce water usage by up to 50%. Nuruzzaman et al. (2021) [169] studied the ability of organically modified montmorillonite nanoclay to deliver imidacloprid. Taking into account the imidacloprid release pattern from the montmorillonite nanoclay, it can be used as a component in a formulation for a slow-release pesticide where the nanoclay will minimize the instantaneous release of total pesticide. Wang et al. [170] improved the dispersion of nanoclay by incorporating biochar and biosilica to reduce the loss of pesticides. Prepared nanoclay, when added to the pesticide, could effectively increase its adhesion, resulting in a decreased loss of pesticide and reduced pollution risk. Nanoclay application for seed treatment has not received much attention from the scientific community. The potential use of nanoclay in seed coating or pesticide delivery in seeds needs to be explored.
Nanosuspension
Nanosuspension forms from the dispersion of crystalline or amorphous nanoparticles of the active ingredients in a liquid medium. Nanosuspension preparation has been carried out using several methods, including wet milling, high-pressure homogenization, emulsification, and solvent evaporation. The nanosuspension enhances the solubility and bioavailability of nonsoluble compounds. For this reason, nanosuspension is being considered a possible seed treatment agent in the near future [171]. Zhu et al. (2021) [172] developed a simple method to prepare agrochemical nanosuspensions that combined high concentrations, eco-friendly excipients, and an intensified preparation process to enhance potency. This study shows that flash nanoprecipitation significantly increases the biological potency of agrochemical nanosuspension and reduces their dosage, demonstrating a considerable benefit over traditional preparation methods [173]. Corrias et al. [174] evaluated nanosuspention of zoxamide to improve the solubility of zoxamide and reduce the accumulation and its retention of zoxamide in tomato seeds. The results clearly suggest that nanosuspensions may represent a promising alternative to using poorly soluble pesticides in agriculture. Cui et al. (2019) [175] presented an easy-to-use method of constructing pesticide nanosuspensions through wet milling that improved pesticide (abamectin) bioavailability. Using the highly effective nanoformulation will improve pesticide efficacy, reduce pesticide dosage and reduce environmental pollution. As a demonstration of the potential applications of this system, a formulation was made with carbofuran, a poorly soluble crystalline insecticide. It was found that the nanosuspension system was physically and chemically stable after two years. In all the reports, the effectiveness of nanosuspension in the development of agrochemicals has been demonstrated. The liquid nature of nanosuspension makes it useful for seed priming, seed soaking, and seed coating [176]. Nanosuspension should be explored for seed treatment, as it may benefit seed storage and germination.
Challenges and Risk Assessment of Nanomaterials-based Agrochemicals
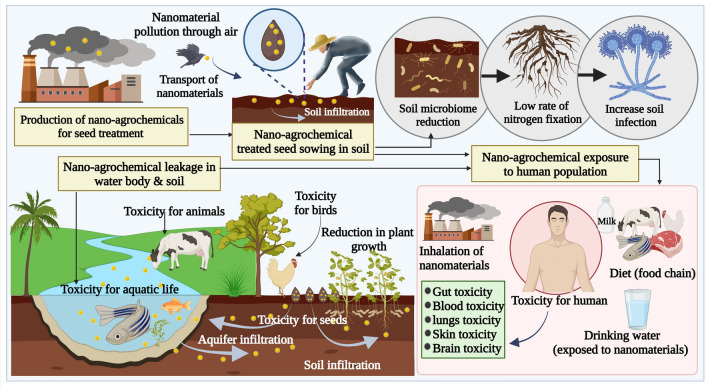
Nanoparticle concentration is key in determining the cytotoxic and genotoxic effects in seed treatment studies, which may vary by agro seed species. Various nanoparticle diameters can enter the nucleus via nuclear pores, indicating that nanoparticles interact with cell components based on their size. Nanoparticles might also disrupt cell cycle checkpoints, enter the cell through mechanical or chemical contact with enzymes that generate reactive oxygen species (ROS), or interfere with cell division mechanisms by binding to proteins and inhibiting protein synthesis. While designing seed treatments using nanomaterials, it is important to consider the properties of nanomaterials, such as size, doses, exposure times, surface chemistry, structures, immune responses, accumulations, and other effects to control the toxicity [177]. Nanomaterials can also enter the environment and soil systems through seed treatment strategies (Fig. 5). Therefore, nanoparticle exposure to seed treatment must be critically assessed and managed in the nanoagricultural field. Research in human and eco-toxicology can provide insight into the complex relationship between the agroenvironment, nanoscale agrochemicals, and human exposure levels [177]. There is an impasse in nanotoxicology regarding how best to assess the risk of nanomaterials-based agrochemicals for environmental monitoring and human health [178]. Toxicology testing can be performed on live (in vivo) organisms, such as microcrustaceans, fishes, mice, other animals and plant models or on cell cultures (in vitro) [179, 180]. Moreover, computational models like quantitative structure–activity relationship provide a lot of potential for understanding the possible toxicity effects of nanomaterial-based agrochemicals on humans and the environment [181, 182]. However, until now, the toxicity of nanomaterial-based agrochemicals is not evaluated according to any standards, making it difficult to compare the results and reach a consensus on their toxicity [78]. There are no standard methods for evaluating nanomaterial-based agrochemicals, as they can present a range of physicochemical properties. So far, most studies have been adapted from standard methods for other substances like drugs, synthetic chemicals, etc. Various assays have been proposed, but there is still no standard protocol. As of now, governments of all nations have not adopted a fixed toxicity regulation strategy for nanoparticle used to assess human and animal health, safety, and ecological impacts.
Fig. 5.
Possible risks associated with nanoagrochemicals-based seed treatments (nanomaterials which made their way into wastewater and soil can contaminate water resource and increases soil pollution. A wide range of soil microbiomes and their nitrogen fixation, mineralization, and plant growth-promoting processes may adversely impact by nanomaterials. A nanomaterial can enter the body of an aquatic organism and livestock can seep into water bodies and enter the food chain and ultimately effecting the human health)
In the present state of knowledge, the mechanisms of toxicity of nanomaterial-based agrochemicals to plants are largely unknown [183], and little information exists about how nanoagrochemicals might enter seeds, plants and where they may end up in the food chain [184]. However, a few studies have tried to unravel the toxicity effects and mechanisms of different nanoparticle treatments on seeds and plants. A phytotoxicity evaluation method was conducted on seeds of Allium cepa, Z. mays (maize), Cucumis sativus (cucumber) and Lycopersicum esculentum (tomato) exposed to nanoparticles [185]. In vitro studies on seeds and seedlings were conducted to determine cytotoxicological effects such as mitotic index, chromosomal aberrations, vagrant chromosomes, sticky chromosomes, disturbed metaphase, breaks, apoptosis, and micronuclei formation. Phytotoxicity endpoints can be evaluated through in vitro and in vivo studies that expose seed systems to different nanoparticle dispersion concentrations to evaluate germination rate, root/shoot length, adsorption, accumulation, and translocation of the nanoparticles [186, 187]. The toxicity of many nanoparticles was assessed using biochemical studies on protein expression analysis, DNA consistency after nanoparticle treatment, and enzyme levels in seeds, seedlings and plants [188]. Seed structure and toxicity effects can be studied with advanced microscopic techniques like electron microscopy. They can reveal nanotoxicity by using focused electron beams rather than visible light. These approaches can better understand the risks associated with nanomaterials and their products before using them directly on the field for seed treatment. Plant model organisms such as Arabidopsis are useful for genetic experiments because it has several important characteristics such as a short generation time, small size, and prolific seed production through self-pollination. Arabidopsis is useful in toxicity testing of nanomaterials to analyze the mutations, accumulation and translocation, physiological responses, and oxidative stress generation during the interaction of nanomaterials [189]. An assessment of nanotoxic effects of nanoagrochemicals on seed germination and plant growth can be performed with the help of phytotoxicity assays and advanced microscopic techniques.
A plant or seed treated with nanomaterials may produce antimicrobial secondary metabolites as a defense against various plant pathogens. Plants can benefit from agronanochemicals to an extent because they can improve their overall disease resistance. There is a possibility that plants raised with nanomaterial-treated seeds will produce phytoestrogens. A phytoestrogen is a plant-produced bioactive secondary metabolite that plays an integral role in plant defenses and accumulates during times of stress or infection. This phytoestrogen is highly toxic to the human body as considered as endocrine disruptors. It interferes with hormone regulation in the reproductive system, leading to infertility and abnormal estrus cycles in women. In addition, there is little evidence that phytoestrogens have harmful effects on humans, but the use of risk assessment strategies to monitor crops raised from nanoagrochemical treated seeds is necessary before its consumption. As a safety precaution, phytoestrogens can be detected using a Sciex API III heated nebulizer atmospheric pressure chemical ionization interface coupled with tandem mass spectrometry.
It is well established that nanomaterials considerably impact the soil microbiome, including their abundance, diversity, and essential microbial processes, such as nitrogen fixation, mineralization, and plant growth-promoting activities [190, 191]. Studying the nanomaterial’s toxicity in soil and the influence of soil properties can also provide insight into the role of nanomaterials in soil pollution. An adverse effect of nanomaterials on soil microbiota can occur when they interact with the soil. The negative effects of most nanoparticles in soil are due to higher doses and mainly affect the enzymatic activities of soil microorganisms. Nanoparticles can inhibit critical steps in nutrient recycling such as ammonification, denitrification, nitrogen fixation, phosphate solubilization, and plant growth-promoting activities, all crucial for maintaining soil fertility and the ecosystem [192]. Zhang et al. [193] observed that silver nanoparticles changed the pH of soil and adversely affected soil microorganisms involved in nitrogen, carbon, and phosphorus cycles. Another study by Li et al. [194] found that soils amended with silver nanoparticles reduced seed development and caused silver bioaccumulation. Nanoparticles of other metallic metals, such as titanium and zinc, have been found to have adverse effects on the soil microbiota based on concentration and exposure duration [195, 196]. In a study by Rahman et al. [197], platinum nanoparticles stabilized with poly(vinylpyrrolidone) caused adverse effects on pea root microbiota, with a decrease in mycorrhizal fungi and rhizobia [198]. Using the soil metagenomic technique, it is possible to uncover the interactions and toxicity of different nanomaterials with the soil microbiome. In soil metagenomics, a nanomaterial-treated soil cultivation-independent molecular approach can be used to explore and exploit the immense diversity of soil microbial communities [191]. In this technology, soil microbiome DNA is isolated, and clone libraries are produced and screened. The nitrogen, carbohydrate, and phosphorus cycles are of high environmental importance, and nanomaterials appear to interfere with them. Nitrogen is fixed by urease, which converts urea into carbon dioxide and ammonia. Nanomaterials have been reported to reduce enzyme activity in several studies. An assessment of urease activity and its reduction in response to nanomaterials can also indicate reduced rhizospheric microbes [199].
It is possible for nanomaterial-based agrochemicals can seep into water bodies and enter the food chain (bioaccumulation) [40]. Many industries are interested in scaling up nanomaterials and nanomaterial-based agriproducts. Effluents from industrial plants contain nanomaterials that can contaminate water bodies. A nanomaterial may enter the body of an aquatic organism if it is disposed of in water and interfere with its physiology and feeding. A toxicology test can be used to evaluate the effects of nanomaterials or nanomaterial-based products on aquatic organisms. A toxicology test can be used to assess the potential for damage to aquatic environments and provide a database of information that can be used to assess the risk associated with using nanomaterials in a specific situation [42]. A common standard test species like fathead minnow (Pimephales promelas), the daphnids (Daphnia magna, D. pulex, D. pulicaria and C. dubia), the rainbow trout (Oncorhynchus mykiss), the sheepshead minnow (Cyprinodon variegatu), the zebra fish (Danio rerio), mysids are used commonly as a model organism to assess the risk of nanomaterials to examine the aquatic toxicity for in vivo studies [200–202]. Studying the genotoxicity effects and physiological responses of aquatic animals exposed to nanomaterials is possible through in vivo assays. Assays in vivo can provide information about hemocompatibility, immune response, histological profiling, protein analysis, and gene expression. In vitro assays (Cytotoxicity and cell viability assays) can also be used to determine the acute toxicity of nanomaterials on fish hepatoma and juvenile rainbow trout cell lines [203]. This approach will allow us to assess nanoagrochemical’s toxicity on aquatic organisms. Another novel, a new generation of nano-quantitative structure–activity/structure–property relationship tools for toxicity assessment, is examined. These tools are analyzed, including modeling methods and validation procedures, to evaluate whether these tools meet the current requirements for approving nanoformulations.
The applications of nanoagrochemicals also face major challenge due lack of fields data. The increasing reports on the applications of nanoagrochemicals have not encouraged the translation of laboratory data on field data. The reason can be the inadequate level of knowledge about nanoagrochemicals to enable reliable assessments of their risk. Several studies have investigated the fate of nanoagrochemicals, but hazard consequences cannot be determined adequately using protocols developed for other chemicals [204, 205]. Data insufficiencies result in some significant uncertainties related to environmental and consumer safety, essential to creating public confidence in products. It is paramount to answer several critical questions regarding the current state of uncertainty before being tried for field trials [205]. However, it is important to note that due to the uncertainty of regulatory frameworks as well as differing opinions globally, nanoagrochemical-based products for agricultural benefits are not flourishing and facing difficulties in reaching the market for field trials. The applications of nanoagrochemicals face major challenges due to emphasis on the sustainable development. Considering the United Nations Sustainable Development Goals 2030, it becomes increasingly important to use sustainable nanoagrochemicals in agricultural crop production to ensure compliance with regulation and consumer acceptance. The objective of sustainable nanoagrochemicals is to maximize their functional and economic performance while minimizing their adverse effects on the environment and human health [206]. Sustainable nanoagrochemicals (nanofertilizers, nanopesticides, nanoinsecticides etc.,) were prepared using green chemistry principles and encapsulated or coated by natural or biodegradable polymeric materials [207]. In order to achieve sustainability, it is crucial to integrate fundamental science (e.g., materials synthesis, characterization, and modeling) with engineering research (e.g., system design, fabrication, and testing) [208]. Hence, the application of sustainable nanoagrochemicals in the field cannot be judged based on a few studies, and to maximize their potential, sustainability nanoagrochemicals must be integrated into larger interdisciplinary research programs and/or government-funded research and development centers.
The successful application of nanotechnology in agro seed treatment products requires a comprehensive and effective strategy and coordinated risk management. It is important to consider the toxicological assessment of nanomaterials in seed treatment as a fundamental step toward identifying hazards related to applications of nanomaterials in the agro seed sector. A number of agronano products (Table 3) are now available on the commercial market [209], and efforts are being made worldwide to address and regulate the production and use of nanomaterials, either through legislation or by recommendations and guidance [76]. Governments and scientific organizations widely recognize the importance of nanomaterials-risk management in the agrochemical sector. Nanoagrochemicals can be considered a milestone in collaborative discussion, and information exchange forums are needed to ensure threat mitigation [210]. The combined efforts of governmental organizations, scientists, and social communities are required to prevent the adverse effects of nanoagrochemicals on humans and the environment. To successfully implement this nanotechnology within the profit margins for seed treatment, they must be capable of balancing system costs and benefits. For nanomaterials to be commercially useful, they must undergo extensive screening and optimization processes according to different seed types. A nanoscale seed treatment chemical must be developed with a simple handling process, low cost, sharp release system, and a high degradation rate. Seed treatments must overcome the major barriers to commercialization, poor demonstration of nanoscale products in field conditions, cost-effectiveness, consumer acceptance, and technical feasibility. A lack of public awareness campaign, inconsistency of the legal framework, and inconsistency of the regulatory framework affect the marketing of such nanoagrochemical products. Regulatory guidelines and frameworks are becoming increasingly important to resolve emerging issues associated with nanoscale agrochemicals. Nanoscale agrochemicals can undoubtedly alleviate many concerns caused by implementing routine agrochemicals, but more testing is necessary to lower agroecological risks. Innovative monitoring applications make it impossible for sustainable seed development and pollution control to be improved without creating nanoparticle contamination as a new hazard [211].
Table 3.
An overview of the number of nanotechnology-enabled products, manufacturing firms, and countries involved in the plant-protection industry (Ref. [238] and https://product.statnano.com)
| Type of nanoagrochemicals | Nanomaterial used | Nano-inspired products | Forming company | Key features |
|---|---|---|---|---|
| Nanofertilizer | Phosphorus and potassium | Fosvit K30 | Kimitec Group, Spain | It is systemic and penetrates easily into plants, it facilitates potassium distribution |
| Silver | Nano-Ag answer® | Urth Agriculture, USA | It helps plants and soil microorganisms survive and fix the organic matter | |
| Zinc | Nanozinc chelate fertilizer | Afme Trading Group, UK | It helps plants grow and develop by providing them with zinc metal element necessary for nutrient uptake by plants | |
| Zinc | Nanozinc | Alert Biotech, India | Zinc deficiency related crop issues are treated by this product | |
| Boron | Nanobor | Alert Biotech, India | The treatment is suitable for regions with high rainfall and acidic and sandy soils with poor organic matter | |
| Silica | Nanonutrients | NanoL and Baltic, Lithuania | By spraying it on leaf surfaces, all nutrients are delivered directly to chlorophyll and photosynthesis is enhanced | |
| Calcium | Nubiotek® Ultra Ca | Bioteksa, Mexico | It effectively helps crops endure climatic stress, pests, diseases, and aggressive conditions | |
| Iron and magnesium | Nubiotek® Hyper | Bioteksa, Mexico | The compound improves vegetative development by acting as an activator | |
| Titanium dioxide | Nanofertilizer | SilvertechKimya Sanayi veTicaret Ltd, Turkey | Plants grown from nano-TiO2 treated seeds showed significantly higher dry weight, photosynthetic rate, and chlorophyll-a formation | |
| Manganese molybdenum, zinc | Nanovec TSS 80 | Laboratórios Bio-Médicin, Brazil | A defensive agent for seed treatment increases germination and leguminous plants’ emergence | |
| Potassium | Groagro 4: super kalium catalyst + T.E | Microwell Bio Solutions SdnBhd, Malaysia | Accelerating plant growth with improved nutritional value | |
| Potassium | Nano-potassium | Kanak Biotech | It helps plants develop roots and fruit, and improves their drought resistance | |
| Phosphorous | Nano-phosphorous | Kanak Biotech | Reduced tillering in cereals and poor seed development | |
| Urea | Nanourea (liquid) fertilizer | Indian Farmers Fertiliser Cooperative Limited | Nanourea contains nitrogen which effectively meets the crop’s nitrogen needs. Nanourea is more efficient than conventional urea in its use. It increases crop productivity, soil health, and nutritional quality | |
| Nanopesticide | Silver | Nanosept® Aqua | Nanosept, Hungary | Seed treatments can benefit from its potential disinfectant properties |
| Copper | Nanocu | BioNano Technology, Egypt | To improve root vigor while acting as an antifungal agent | |
| Silver | Blue lagoon UV-block | Dennerle, Germany | For the prevention of algal infection and growth in agriculture ponds | |
| Sulfur | Nanosulf foliar spray | Alert Biotech, India | Nanosulf is composed of elemental sulfur on nanosize particles, which dissolve completely in water. Nasosulf can also be used as a plant fertilizer, fungicide, and insecticide |
Conclusions and Future Perspective
Nanotechnology can be applied to nanoagrochemical delivery to improve the efficacy of seeds, reduce environmental pollution, support the sustainability of agricultural systems, and improve food security. By reducing the concentration of pesticides and fertilizers applied to the land, nanoagrochemical seed treatments increase the precision and effectiveness of seed protection products. In agro seed treatment, nanocarriers with controlled delivery can offer several advantages over conventional chemical delivery systems for sustainable agriculture, including biocompatibility, biosorption rate, low synthesis costs, thermo-plasticity, and ease of biodegradation. Developing nanoscale materials like nanocapsules, nanogels, nanofibers, nanoclay, and nanosuspensions can be used to design next-generation seed treatment strategies. More international and national risk assessment and management strategies need to be developed to achieve successful implementation. At last, we can conclude that using nanoscale products or formulations for agro seed treatments is essential for raising crops, and they have a pivotal role in sustainable crop production that cannot be ignored.
It is unclear what molecular mechanisms are responsible for the changes affecting seeds during and after treatment with nanoagroproducts. To understand the mechanism, further research is needed in the areas of genomic, proteomic, and metabolic to unreveal the effect of nanoformulations on seed responses. If we learn more about how nanoscale seed treatment works, we might be able to develop a viable seed processing technology. Future research should consider several factors to develop nanoagrochemicals for an advanced seed treatment; agrochemical parameters at the nanoscale should be optimized to achieve a reproducible and beneficial effect from seed treatment. Delivering the nanoagrochemicals to the right place and calculating the correct dose are challenging. When the parameters of nanoagrochemicals are optimized, it is possible to apply them to seeds so that significant treatment effects can occur. An appropriately responsive strategy and coordinated risk management are required to achieve nanotechnology’s positive effects in agro seed products. To identify the risks associated with nanomaterials used in seed treatment, conducting a toxicological assessment of those nanomaterials is crucial to answer their environmental issues. Research involving humans and eco-toxicology can help us understand how agroecosystems, nanoscale agrochemicals, exposure levels, and human beings interact. Nanoagrochemicals must be further investigated for agroecological toxicity and their mechanistic application.
Acknowledgements
R.P. and A.S. sincerely acknowledge Savitribai Phule Pune University (SPPU) (Formerly Pune University), Pune, for providing facilities. Figures and graphics were created with “BioRender.com” using a purchased copy of the software-2022.
Author Contributions
AS and RP were involved in conceptualization; AS and AVS helped in data curation. AS and RP contributed to writing—original draft preparation; AS, SN, DR, JB, GK, and RP were involved in writing—review and editing; AS and AVS helped in graphic design and visualization; JX, RP, and MC contributed to supervision; AS, RP, SN, and MC were involved in project administration; SN and GK helped in funding acquisition.
Funding
Open access funding provided by Shanghai Jiao Tong University. This work was funded by The Key Science and Technology Projects of Breeding New Varieties of Agriculture in Zhejiang Province (2021C02074-3), National Young Qihuang Scholars Training Program, National “Ten-thousand Talents Program” for Leading Talents of Science and Technology Innovation in China, Zhejiang Provincial Ten Thousands Program for Leading Talents of Science and Technology Innovation (2018R52050), Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents, Research Project of Zhejiang Chinese Medical University (2021JKZDZC06), the Opening Project of Zhejiang Provincial Preponderant and Characteristic Subject of Key University (Traditional Chinese Pharmacology), Zhejiang Chinese Medical University [ZYAOXYB2019009]. We appreciate the experimental support from the Public Platform of Medical Research Centre, Academy of Chinese Medical Science, Zhejiang Chinese Medical University.
Contributor Information
Shivraj Hariram Nile, Email: nileshivraj@yahoo.com.
Manohar Chaskar, Email: chaskarmanohar@gmail.com.
Guoyin Kai, Email: guoyinkai1@126.com.
Rajendra Patil, Email: rpatil@unipune.ac.in.
References
- 1.Berners-Lee M, Kennelly C, Watson R, Hewitt CN. Current global food production is sufficient to meet human nutritional needs in 2050 provided there is radical societal adaptation. Elem. Sci. Anthrop. 2018;6:52. doi: 10.1525/elementa.310. [DOI] [Google Scholar]
- 2.Porter J, Xie L, Challinor A, Chhetri N, Nepal U, et al. Food security and food production systems. In: Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, et al., editors. Climate Change—Impacts, Adaptation and Vulnerability: Part A: Global and Sectoral Aspects: Working Group II Contribution to the IPCC Fifth Assessment Report. Cambridge: Cambridge University Press; 2014. pp. 485–534. [Google Scholar]
- 3.Yadav S, Modi P, Dave A, Vijapura A, Patel D, et al. (2020) Effect of abiotic stress on crops. In: Hasanuzzaman M, Filho MCMT, Fujita M, Nogueira TAR, et al., editors. Sustainable Crop Production. London: IntechOpen; 2020. [Google Scholar]
- 4.Imran QM, Falak N, Hussain A, Mun B-G, Yun B-W. Abiotic stress in plants; stress perception to molecular response and role of biotechnological tools in stress resistance. Agronomy. 2021;11:1579. doi: 10.3390/agronomy11081579. [DOI] [Google Scholar]
- 5.He M, He C-Q, Ding N-Z. Abiotic stresses: general defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 2018;9:1771. doi: 10.3389/fpls.2018.01771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A, Kumar R. Management of seed-borne diseases: an integrated approach. In: Kumar R, Gupta A, editors. Seed-Borne Diseases of Agricultural Crops: Detection, Diagnosis and Management. Singapore: Springer; 2020. [Google Scholar]
- 7.Vojvodić M, Bažok R. Future of insecticide seed treatment. Sustainability. 2021;13(16):8792. doi: 10.3390/su13168792. [DOI] [Google Scholar]
- 8.Nile SH, Thiruvengadam M, Wang Y, Samynathan R, Shariati MA, et al. Nano-priming as emerging seed priming technology for sustainable agriculture-recent developments and future perspectives. J. Nanobiotechnol. 2022;20(1):254. doi: 10.1186/s12951-022-01423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayesha MS, Suryanarayanan TS, Nataraja KN, Prasad SR, Shaanker RU. Seed treatment with systemic fungicides: time for review. Front. Plant Sci. 2021;12:654512. doi: 10.3389/fpls.2021.654512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajwade JM, Chikte RG, Paknikar KM. Nanomaterials: new weapons in a crusade against phytopathogens. Appl. Microbiol. Biotechnol. 2020;104(4):1437–1461. doi: 10.1007/s00253-019-10334-y. [DOI] [PubMed] [Google Scholar]
- 11.Mravlje J, Regvar M, Vogel-Mikuš K. Development of cold plasma technologies for surface decontamination of seed fungal pathogens: present status and perspectives. J. Fungi. 2021;7(8):650. doi: 10.3390/jof7080650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mancini V, Romanazzi G. Seed treatments to control seedborne fungal pathogens of vegetable crops. Pest Manag. Sci. 2014;70(6):860–868. doi: 10.1002/ps.3693. [DOI] [PubMed] [Google Scholar]
- 13.Sharma A, Kumar V, Shahzad B, Tanveer M, Sidhu GPS, et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019;1(11):1446. doi: 10.1007/s42452-019-1485-1. [DOI] [Google Scholar]
- 14.Abhilash PC, Singh N. Pesticide use and application: an Indian scenario. J. Hazard. Mater. 2009;165(1):1–12. doi: 10.1016/j.jhazmat.2008.10.061. [DOI] [PubMed] [Google Scholar]
- 15.Sharma BM, Bharat GK, Tayal S, Nizzetto L, Čupr P, et al. Environment and human exposure to persistent organic pollutants (pops) in india: a systematic review of recent and historical data. Environ. Int. 2014;66:48–64. doi: 10.1016/j.envint.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Chan KK, Yap SHK, Yong K-T. Biogreen synthesis of carbon dots for biotechnology and nanomedicine applications. Nano Micro Lett. 2018;10(4):72. doi: 10.1007/s40820-018-0223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neme K, Nafady A, Uddin S, Tola YB. Application of nanotechnology in agriculture, postharvest loss reduction and food processing: food security implication and challenges. Heliyon. 2021;7(12):e08539. doi: 10.1016/j.heliyon.2021.e08539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mittal D, Kaur G, Singh P, Yadav K, Ali SA. Nanoparticle-based sustainable agriculture and food science: recent advances and future outlook. Front. Nanotechnol. 2020;2:579954. doi: 10.3389/fnano.2020.579954. [DOI] [Google Scholar]
- 19.Shang Y, Hasan MK, Ahammed GJ, Li M, Yin H, et al. Applications of nanotechnology in plant growth and crop protection: a review. Molecules. 2019;24(14):2558. doi: 10.3390/molecules24142558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasad R, Bhattacharyya A, Nguyen QD. Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives. Front. Microbiol. 2017;8:1014. doi: 10.3389/fmicb.2017.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Usman M, Farooq M, Wakeel A, Nawaz A, Cheema SA, et al. Nanotechnology in agriculture: current status, challenges and future opportunities. Sci. Total Environ. 2020;721:137778. doi: 10.1016/j.scitotenv.2020.137778. [DOI] [PubMed] [Google Scholar]
- 22.Fincheira P, Tortella G, Seabra AB, Quiroz A, Diez MC, et al. Nanotechnology advances for sustainable agriculture: current knowledge and prospects in plant growth modulation and nutrition. Planta. 2021;254(4):66. doi: 10.1007/s00425-021-03714-0. [DOI] [PubMed] [Google Scholar]
- 23.Kamle M, Mahato DK, Devi S, Soni R, Tripathi V, et al. Nanotechnological interventions for plant health improvement and sustainable agriculture. 3 Biotech. 2020;10(4):168. doi: 10.1007/s13205-020-2152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C, Zhou H, Zhou J. The applications of nanotechnology in crop production. Molecules. 2021;26(23):7070. doi: 10.3390/molecules26237070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guha T, Gopal G, Kundu R, Mukherjee A. Nanocomposites for delivering agrochemicals: a comprehensive review. J. Agric. Food Chem. 2020;68(12):3691–3702. doi: 10.1021/acs.jafc.9b06982. [DOI] [PubMed] [Google Scholar]
- 26.Nile SH, Baskar V, Selvaraj D, Nile A, Xiao J, et al. Nanotechnologies in food science: applications, recent trends, and future perspectives. Nano Micro Lett. 2020;12(1):45. doi: 10.1007/s40820-020-0383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Chen L, Li X, Sun D, Zhang H. Recent progress on asymmetric carbon- and silica-based nanomaterials: from synthetic strategies to their applications. Nano Micro Lett. 2022;14(1):45. doi: 10.1007/s40820-021-00789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pramanik P, Krishnan P, Maity A, Mridha N, Mukherjee A, et al. Application of nanotechnology in agriculture. In: Dasgupta N, Ranjan S, Lichtfouse E, et al., editors. Environmental Nanotechnology Volume 4. Environmental Chemistry for a Sustainable World. Cham: Springer; 2020. pp. 317–348. [Google Scholar]
- 29.Jiang M, Song Y, Kanwar MK, Ahammed GJ, Shao S, et al. Phytonanotechnology applications in modern agriculture. J. Nanobiotechnol. 2021;19(1):430. doi: 10.1186/s12951-021-01176-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chanu NB, Singh MC. Applications of nanotechnology in precision agriculture. In: Ghorbanpour M, Shahid MA, editors. Nano-enabled Agrochemicals in Agriculture. Cambridge: Academic Press; 2022. p. 175. [Google Scholar]
- 31.Pulizzi F. Nano in the future of crops. Nat. Nanotechnol. 2019;14(6):507–507. doi: 10.1038/s41565-019-0475-1. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Dimkpa C, Deng CY, Elmer WH, Gardea-Torresdey J, et al. Impact of engineered nanomaterials on rice (Oryza sativa L.): a critical review of current knowledge. Environ. Pollut. 2022;297:118738. doi: 10.1016/j.envpol.2021.118738. [DOI] [PubMed] [Google Scholar]
- 33.Szőllősi R, Molnár Á, Kondak S, Kolbert Z. Dual effect of nanomaterials on germination and seedling growth: stimulation vs. phytotoxicity. Plants. 2020;9(12):1745. doi: 10.3390/plants9121745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandrasekaran U, Luo X, Wang Q, Shu K. Are there unidentified factors involved in the germination of nanoprimed seeds? Front. Plant Sci. 2020;11:00832. doi: 10.3389/fpls.2020.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao C, Zhao H, Wang P. Recent development in functional nanomaterials for sustainable and smart agricultural chemical technologies. Nano Converg. 2022;9(1):11. doi: 10.1186/s40580-022-00302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.do Espirito Santo Pereira A, Caixeta Oliveira H, Fernandes Fraceto L, Santaella C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials. 2021;11(2):267. doi: 10.3390/nano11020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rani Sarkar M, Rashid MH-O, Rahman A, Kafi MA, Hosen MI, et al. Recent advances in nanomaterials based sustainable agriculture: an overview. Environ. Nanotechnol. Monit. Manag. 2022;18:100687. doi: 10.1016/j.enmm.2022.100687. [DOI] [Google Scholar]
- 38.Agrawal S, Kumar V, Kumar S, Shahi SK. Plant development and crop protection using phytonanotechnology: a new window for sustainable agriculture. Chemosphere. 2022;299:134465. doi: 10.1016/j.chemosphere.2022.134465. [DOI] [PubMed] [Google Scholar]
- 39.Rienzie R, Adassooriya N. Toxicity of nanomaterials in agriculture and food. In: Rai M, Biswas J, editors. Nanomaterials: Ecotoxicity, Safety, and Public Perception. Cham: Springer; 2018. [Google Scholar]
- 40.Murali M, Gowtham HG, Singh SB, Shilpa N, Aiyaz M, et al. Fate, bioaccumulation and toxicity of engineered nanomaterials in plants: current challenges and future prospects. Sci. Total Environ. 2022;811:152249. doi: 10.1016/j.scitotenv.2021.152249. [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Dhungana B, Cobb GP. Environmental behavior, potential phytotoxicity, and accumulation of copper oxide nanoparticles and arsenic in rice plants. Environ. Toxicol. Chem. 2018;37(1):11–20. doi: 10.1002/etc.3945. [DOI] [PubMed] [Google Scholar]
- 42.Iavicoli I, Leso V, Beezhold DH, Shvedova AA. Nanotechnology in agriculture: opportunities, toxicological implications, and occupational risks. Toxicol. Appl. Pharmacol. 2017;329:96–111. doi: 10.1016/j.taap.2017.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajput V, Minkina T, Mazarji M, Shende S, Sushkova S, et al. Accumulation of nanoparticles in the soil-plant systems and their effects on human health. Ann. Agric. Sci. 2020;65(2):137–143. doi: 10.1016/j.aoas.2020.08.001. [DOI] [Google Scholar]
- 44.Sun Y, Liang J, Tang L, Li H, Zhu Y, et al. Nano-pesticides: a great challenge for biodiversity? Nano Today. 2019;28:100757. doi: 10.1016/j.nantod.2019.06.003. [DOI] [Google Scholar]
- 45.Hotze E, Phenrat T, Lowry G. Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment. J. Environ. Quality. 2010;39:1909–1924. doi: 10.2134/jeq2009.0462. [DOI] [PubMed] [Google Scholar]
- 46.Meena RS, Kumar S, Datta R, Lal R, Vijayakumar V, et al. Impact of agrochemicals on soil microbiota and management: a review. Land. 2020;9(2):34. doi: 10.3390/land9020034. [DOI] [Google Scholar]
- 47.Sharma B, Lakra U, Sharma R, Sharma SR. A comprehensive review on nanopesticides and nanofertilizers—a boon for agriculture. In: Ghorbanpour M, Shahid MA, editors. Nano-enabled Agrochemicals in Agriculture. Cambridge: Academic Press; 2022. pp. 273–290. [Google Scholar]
- 48.Fazelian N, Yousefzadi M. Nano-biofertilizers for enhanced nutrient use efficiency. In: Ghorbanpour M, Shahid MA, editors. Nano-enabled Agrochemicals in Agriculture. Cambridge: Academic Press; 2022. pp. 145–158. [Google Scholar]
- 49.Dziergowska K, Michalak I. The role of nanoparticles in sustainable agriculture. In: Chojnacka K, Saeid A, editors. Smart Agrochemicals for Sustainable Agriculture. Cambridge: Academic Press; 2022. pp. 225–278. [Google Scholar]
- 50.Shalaby TA, Bayoumi Y, Eid Y, Elbasiouny H, Elbehiry F, et al. Can nanofertilizers mitigate multiple environmental stresses for higher crop productivity? Sustainability. 2022;14(6):3480. doi: 10.3390/su14063480. [DOI] [Google Scholar]
- 51.Wang D, Saleh NB, Byro A, Zepp R, Sahle-Demessie E, et al. Nano-enabled pesticides for sustainable agriculture and global food security. Nat. Nanotechnol. 2022;17:347–360. doi: 10.1038/s41565-022-01082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shelar A, Singh AV, Dietrich P, Maharjan RS, Thissen A, et al. Emerging cold plasma treatment and machine learning prospects for seed priming: a step towards sustainable food production. RSC Adv. 2022;12(17):10467–10488. doi: 10.1039/D2RA00809B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shelar A, Singh AV, Maharjan RS, Laux P, Luch A, et al. Sustainable agriculture through multidisciplinary seed nanopriming: prospects of opportunities and challenges. Cells. 2021;10(9):2428. doi: 10.3390/cells10092428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun Y, Zheng L, Yang Y, Qian X, Fu T, et al. Metal–organic framework nanocarriers for drug delivery in biomedical applications. Nano Micro Lett. 2020;12(1):103. doi: 10.1007/s40820-020-00423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siddiqi KS, Husen A. Plant response to engineered metal oxide nanoparticles. Nanoscale Res. Lett. 2017;12(1):92. doi: 10.1186/s11671-017-1861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo H, Liu Y, Chen J, Zhu Y, Zhang Z. The effects of several metal nanoparticles on seed germination and seedling growth: a meta-analysis. Coatings. 2022;12(2):183. doi: 10.3390/coatings12020183. [DOI] [Google Scholar]
- 57.An C, Sun C, Li N, Huang B, Jiang J, et al. Nanomaterials and nanotechnology for the delivery of agrochemicals: strategies towards sustainable agriculture. J. Nanobiotechnol. 2022;20(1):11. doi: 10.1186/s12951-021-01214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vurro M, Miguel-Rojas C, Pérez-de-Luque A. Safe nanotechnologies for increasing the effectiveness of environmentally friendly natural agrochemicals. Pest Manag. Sci. 2019;75(9):2403–2412. doi: 10.1002/ps.5348. [DOI] [PubMed] [Google Scholar]
- 59.Mishra RK, Ha SK, Verma K, Tiwari SK. Recent progress in selected bio-nanomaterials and their engineering applications: an overview. J. Sci. Adv. Mater. Dev. 2018;3(3):263–288. doi: 10.1016/j.jsamd.2018.05.003. [DOI] [Google Scholar]
- 60.Kalia A, Sharma SP, Kaur H, Kaur H. Novel nanocomposite-based controlled-release fertilizer and pesticide formulations: prospects and challenges. In: Abd-Elsalam KA, editor. Micro and Nano Technologies, Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems. Amsterdam: Elsevier; 2020. pp. 99–134. [Google Scholar]
- 61.Kumar A, Choudhary A, Kaur H, Mehta S, Husen A. Smart nanomaterial and nanocomposite with advanced agrochemical activities. Nanoscale Res. Lett. 2021;16(1):156. doi: 10.1186/s11671-021-03612-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tarafder C, Daizy M, Alam MM, Ali MR, Islam MJ, et al. Formulation of a hybrid nanofertilizer for slow and sustainable release of micronutrients. ACS Omega. 2020;5(37):23960–23966. doi: 10.1021/acsomega.0c03233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nongbet A, Mishra AK, Mohanta YK, Mahanta S, Ray MK, et al. Nanofertilizers: a smart and sustainable attribute to modern agriculture. Plants. 2022;11(19):2587. doi: 10.3390/plants11192587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iqbal MA. Nano-fertilizers for sustainable crop production under changing climate: a global perspective. In: Hasanuzzaman M, Filho MCMT, Fujita M, Nogueira TAR, editors. Sustainable Crop Production. London: IntechOpen; 2019. [Google Scholar]
- 65.Bhardwaj AK, Arya G, Kumar R, Hamed L, Pirasteh-Anosheh H, et al. Switching to nanonutrients for sustaining agroecosystems and environment: the challenges and benefits in moving up from ionic to particle feeding. J. Nanobiotechn. 2022;20(1):19. doi: 10.1186/s12951-021-01177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo H, White JC, Wang Z, Xing B. Nano-enabled fertilizers to control the release and use efficiency of nutrients. Curr. Opin. Environ. Sci. Health. 2018;6:77–83. doi: 10.1016/j.coesh.2018.07.009. [DOI] [Google Scholar]
- 67.Seleiman MF, Almutairi KF, Alotaibi M, Shami A, Alhammad BA, et al. Nano-fertilization as an emerging fertilization technique: Why can modern agriculture benefit from its use? Plants. 2020;10(1):2. doi: 10.3390/plants10010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Badran A, Savin I. Effect of nano-fertilizer on seed germination and first stages of bitter almond seedlings’ growth under saline conditions. BioNanoScience. 2018;8:1–10. doi: 10.1007/s12668-018-0531-6. [DOI] [Google Scholar]
- 69.Esper Neto M, Britt D, Jackson K, Braccini A, Inoue T, et al. Early development of corn seedlings primed with synthetic tenorite nanofertilizer. J. Seed Sci. 2020;42:e202042040. doi: 10.1590/2317-1545v42240979. [DOI] [Google Scholar]
- 70.Abdel-Aziz HMM, Hasaneen MNA, Omer AM. Impact of engineered nanomaterials either alone or loaded with npk on growth and productivity of French bean plants: seed priming vs. foliar application. South. Afr. J. Bot. 2019;125:102–108. doi: 10.1016/j.sajb.2019.07.005. [DOI] [Google Scholar]
- 71.Kumar Das C, Jangir H, Kumar J, Verma S, Mahapatra S, et al. Nano-pyrite seed dressing: a sustainable design for npk equivalent rice production. Nanotechnol. Environ. Eng. 2018;3:14. doi: 10.1007/s41204-018-0043-1. [DOI] [Google Scholar]
- 72.Guha T, Ravikumar KVG, Mukherjee A, Mukherjee A, Kundu R. Nanopriming with zero valent iron (nzvi) enhances germination and growth in aromatic rice cultivar (Oryza sativa cv. Gobindabhog L.) Plant Physiol. Biochem. 2018;127:403–413. doi: 10.1016/j.plaphy.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 73.Kubavat D, Trivedi K, Pradip V, Prasad K, Gopalakrishnan VA, et al. Characterization of chitosan based sustained release nano-fertilizer formulation as a soil conditioner whilst improving biomass production of Zea mays .L. Land Degrad. Dev. 2020;31:2734–2746. doi: 10.1002/ldr.3629. [DOI] [Google Scholar]
- 74.Yusefi-Tanha E, Fallah S, Rostamnejadi A, Pokhrel LR. Zinc oxide nanoparticles (ZnONPs) as a novel nanofertilizer: influence on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar) Sci. Total Environ. 2020;738:140240. doi: 10.1016/j.scitotenv.2020.140240. [DOI] [PubMed] [Google Scholar]
- 75.Rui M, Ma C, Hao Y, Guo J, Rui Y, et al. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea) Front. Plant Sci. 2016;7:815. doi: 10.3389/fpls.2016.00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh H, Sharma A, Bhardwaj SK, Arya SK, Bhardwaj N, et al. Recent advances in the applications of nano-agrochemicals for sustainable agricultural development. Environ. Sci. Process Impacts. 2021;23(2):213–239. doi: 10.1039/D0EM00404A. [DOI] [PubMed] [Google Scholar]
- 77.Shekhar S, Sharma S, Kumar A, Taneja A, Sharma B. The framework of nanopesticides: a paradigm in biodiversity. Mater. Adv. 2021;2(20):6569–6588. doi: 10.1039/D1MA00329A. [DOI] [Google Scholar]
- 78.Chaud M, Souto EB, Zielinska A, Severino P, Batain F, et al. Nanopesticides in agriculture: benefits and challenge in agricultural productivity, toxicological risks to human health and environment. Toxics. 2021;9(6):131. doi: 10.3390/toxics9060131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zobir SAM, Ali A, Adzmi F, Sulaiman MR, Ahmad K. A review on nanopesticides for plant protection synthesized using the supramolecular chemistry of layered hydroxide hosts. Biology. 2021;10(11):1077. doi: 10.3390/biology10111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roseline TA, Murugan M, Sudhakar MP, Arunkumar K. Nanopesticidal potential of silver nanocomposites synthesized from the aqueous extracts of red seaweeds. Environ. Techn. Innov. 2019;13:82–93. doi: 10.1016/j.eti.2018.10.005. [DOI] [Google Scholar]
- 81.El-Shetehy M, Moradi A, Maceroni M, Reinhardt D, Petri-Fink A, et al. Silica nanoparticles enhance disease resistance in arabidopsis plants. Nat. Nanotechn. 2021;16(3):344–353. doi: 10.1038/s41565-020-00812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tripathi D, Singh M, Pandey-Rai S. Crosstalk of nanoparticles and phytohormones regulate plant growth and metabolism under abiotic and biotic stress. Plant Stress. 2022;6:100107. doi: 10.1016/j.stress.2022.100107. [DOI] [Google Scholar]
- 83.Lakshmeesha TR, Murali M, Ansari MA, Udayashankar AC, Alzohairy MA, et al. Biofabrication of zinc oxide nanoparticles from melia azedarach and its potential in controlling soybean seed-borne phytopathogenic fungi. Saudi J. Biol. Sci. 2020;27(8):1923–1930. doi: 10.1016/j.sjbs.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Almaary KS, Sayed SRM, Abd-Elkader OH, Dawoud TM, El Orabi NF, et al. Complete green synthesis of silver-nanoparticles applying seed-borne Penicillium duclauxii. Saudi J. Biol. Sci. 2020;27(5):1333–1339. doi: 10.1016/j.sjbs.2019.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaur P, Duhan J, Thakur R. Comparative pot studies of chitosan and chitosan-metal nanocomposites as nano-agrochemicals against fusarium wilt of chickpea (Cicer arietinum L.) Biocatal. Agric. Biotechnol. 2018;14:466–471. doi: 10.1016/j.bcab.2018.04.014. [DOI] [Google Scholar]
- 86.Sharma A, Sidhu A, Manchanda P, Ahuja R. 1,2,4-triazolyldithiocarbamate silver nano conjugate: potent seed priming agent against bakanae disease of rice (Oryzae sativa) Eur. J. Plant. Pathol. 2022;162:825–841. doi: 10.1007/s10658-021-02439-w. [DOI] [Google Scholar]
- 87.Chariou PL, Steinmetz NF. Delivery of pesticides to plant parasitic nematodes using tobacco mild green mosaic virus as a nanocarrier. ACS Nano. 2017;11(5):4719–4730. doi: 10.1021/acsnano.7b00823. [DOI] [PubMed] [Google Scholar]
- 88.Sankar M, Abideen A. Pesticidal effect of green synthesized silver and lead nanoparticles using Avicennia marina against grain storage pest Sitophilus oryzae. Dig. J. Nanomater. Biostruct. 2015;5:32–39. [Google Scholar]
- 89.Thabet A, Boraei H, Galal O, El-Samahy M, Mousa K, et al. Silica nanoparticles as pesticide against insects of different feeding types and their non-target attraction of predators. Sci. Rep. 2021;11:14484. doi: 10.1038/s41598-021-93518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spadola G, Sanna V, Bartoli J, Carcelli M, Pelosi G, et al. Thiosemicarbazone nano-formulation for the control of aspergillus flavus. Environ. Sci. Pollut. Res. 2020;27(16):20125–20135. doi: 10.1007/s11356-020-08532-7. [DOI] [PubMed] [Google Scholar]
- 91.Maswada HF, Djanaguiraman M, Prasad PVV. Seed treatment with nano-iron (iii) oxide enhances germination, seeding growth and salinity tolerance of sorghum. J. Agro. Crop Sci. 2018;204(6):577–587. doi: 10.1111/jac.12280. [DOI] [Google Scholar]
- 92.Kasote D, Lee J, Jayaprakasha G, Patil B. Seed priming with iron oxide nanoparticles modulate antioxidant potential and defense linked hormones in watermelon seedlings. ACS Sustain. Chem. Eng. 2019;7(5):5142–5151. doi: 10.1021/acssuschemeng.8b06013. [DOI] [Google Scholar]
- 93.Ahuja R, Sidhu A, Bala A. Synthesis and evaluation of iron(ii) sulfide aqua nanoparticles (FeS-NPs) against fusarium verticillioides causing sheath rot and seed discoloration of rice. Eur. J. Plant. Pathol. 2019;155(1):163–171. doi: 10.1007/s10658-019-01758-3. [DOI] [Google Scholar]
- 94.Haris M, Hussain T, Mohamed HI, Khan A, Ansari MS, et al. Nanotechnology - a new frontier of nano-farming in agricultural and food production and its development. Sci. Total Environ. 2023;857(3):159639. doi: 10.1016/j.scitotenv.2022.159639. [DOI] [PubMed] [Google Scholar]
- 95.Sundaria N, Singh M, Upreti P, Chauhan RP, Jaiswal JP, et al. Seed priming with iron oxide nanoparticles triggers iron acquisition and biofortification in wheat (Triticum aestivum L.) grains. J. Plant Growth Regul. 2019;38(1):122–131. doi: 10.1007/s00344-018-9818-7. [DOI] [Google Scholar]
- 96.Rizwan M, Ali S, Ali B, Adrees M, Arshad M, et al. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere. 2019;214:269–277. doi: 10.1016/j.chemosphere.2018.09.120. [DOI] [PubMed] [Google Scholar]
- 97.Itroutwar P, Govindaraju K, Selvaraj T, Kannan M, Raja K, et al. Seaweed-based biogenic ZnO nanoparticles for improving agro-morphological characteristics of rice (Oryza sativa L.) J. Plant Growth Regul. 2020;39:717–728. doi: 10.1007/s00344-019-10012-3. [DOI] [Google Scholar]
- 98.Savassa SM, Duran NM, Rodrigues ES, de Almeida E, van Gestel CAM, et al. Effects of ZnO nanoparticles on Phaseolus vulgaris germination and seedling development determined by x-ray spectroscopy. ACS Appl. Nano Mater. 2018;1(11):6414–6426. doi: 10.1021/acsanm.8b01619. [DOI] [Google Scholar]
- 99.Abdel Latef AAH, Abu Alhmad MF, Abdelfattah KE. The possible roles of priming with zno nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J. Plant Growth Regul. 2017;36(1):60–70. doi: 10.1007/s00344-016-9618-x. [DOI] [Google Scholar]
- 100.Nandhini M, Rajini SB, Udayashankar AC, Niranjana SR, Lund OS, et al. Biofabricated zinc oxide nanoparticles as an eco-friendly alternative for growth promotion and management of downy mildew of pearl millet. Crop Prot. 2019;121:103–112. doi: 10.1016/j.cropro.2019.03.015. [DOI] [Google Scholar]
- 101.Choudhary RC, Kumaraswamy RV, Kumari S, Sharma SS, Pal A, et al. Zinc encapsulated chitosan nanoparticle to promote maize crop yield. Int. J. Biol. Macromol. 2019;127:126–135. doi: 10.1016/j.ijbiomac.2018.12.274. [DOI] [PubMed] [Google Scholar]
- 102.Duran NM, Savassa SM, Lima RG, de Almeida E, Linhares FS, et al. X-ray spectroscopy uncovering the effects of Cu based nanoparticle concentration and structure on phaseolus vulgaris germination and seedling development. J. Agric. Food Chem. 2017;65(36):7874–7884. doi: 10.1021/acs.jafc.7b03014. [DOI] [PubMed] [Google Scholar]
- 103.Yasmeen F, Raja NI, Razzaq A, Komatsu S. Proteomic and physiological analyses of wheat seeds exposed to copper and iron nanoparticles. Biochim Biophys Acta (BBA) Proteins Proteom. 2017;1865(1):28–42. doi: 10.1016/j.bbapap.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 104.Wang W, Ren Y, He J, Zhang L, Wang X, et al. Impact of copper oxide nanoparticles on the germination, seedling growth, and physiological responses in Brassica pekinensis L. Environ. Sci. Pollut. Res. Int. 2020;27(25):31505–31515. doi: 10.1007/s11356-020-09338-3. [DOI] [PubMed] [Google Scholar]
- 105.Ye Y, Cota-Ruiz K, Hernández-Viezcas JA, Valdés C, Medina-Velo IA, et al. Manganese nanoparticles control salinity-modulated molecular responses in Capsicum annuum L. through priming: a sustainable approach for agriculture. ACS Sustain. Chem. Eng. 2020;8(3):1427–1436. doi: 10.1021/acssuschemeng.9b05615. [DOI] [Google Scholar]
- 106.Kasote DM, Lee JHJ, Jayaprakasha GK, Patil BS. Manganese oxide nanoparticles as safer seed priming agent to improve chlorophyll and antioxidant profiles in watermelon seedlings. Nanomaterials. 2021;11(4):1016. doi: 10.3390/nano11041016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vijai Anand K, Anugraga AR, Kannan M, Singaravelu G, Govindaraju K. Bio-engineered magnesium oxide nanoparticles as nano-priming agent for enhancing seed germination and seedling vigour of green gram (Vigna radiate L.) Mater Lett. 2020;271:127792. doi: 10.1016/j.matlet.2020.127792. [DOI] [Google Scholar]
- 108.Hong DD, Anh HTL, Tam LT, Show PL, Leong HY. Effects of nanoscale zerovalent cobalt on growth and photosynthetic parameters of soybean Glycine max (L.) merr. Dt26 at different stages. BMC Energy. 2019;1(1):6. doi: 10.1186/s42500-019-0007-4. [DOI] [Google Scholar]
- 109.Krishnamoorthy V, Rajiv S. Potential seed coatings fabricated from electrospinning hexaaminocyclotriphosphazene and cobalt nanoparticles incorporated polyvinylpyrrolidone for sustainable agriculture. ACS Sustain. Chem. Eng. 2017;5:146–152. doi: 10.1021/acssuschemeng.6b01088. [DOI] [Google Scholar]
- 110.Joshi A, Kaur S, Singh P, Dharamvir K, Nayyar H, et al. Tracking multi-walled carbon nanotubes inside oat (Avena sativa L.) plants and assessing their effect on growth, yield, and mammalian (human) cell viability. Appl. Nanosci. 2018;8:1399–1414. doi: 10.1007/s13204-018-0801-1. [DOI] [Google Scholar]
- 111.Pandey K, Lahiani MH, Hicks VK, Hudson MK, Green MJ, et al. Effects of carbon-based nanomaterials on seed germination, biomass accumulation and salt stress response of bioenergy crops. PLoS ONE. 2018;13(8):e0202274. doi: 10.1371/journal.pone.0202274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baz H, Creech M, Chen J, Gong H, Bradford K, et al. Water-soluble carbon nanoparticles improve seed germination and post-germination growth of lettuce under salinity stress. Agronomy. 2020;10(8):1192. doi: 10.3390/agronomy10081192. [DOI] [Google Scholar]
- 113.Yin L, Wang Z, Wang S, Xu W, Bao H. Effects of graphene oxide and/or Cd2+ on seed germination, seedling growth, and uptake to Cd2+ in solution culture. Water Air Soil Pollut. 2018;229(5):151. doi: 10.1007/s11270-018-3809-y. [DOI] [Google Scholar]
- 114.Kim MJ, Kim W, Chung H. Effects of silver-graphene oxide on seed germination and early growth of crop species. Peer J. 2020;8:e8387. doi: 10.7717/peerj.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li J, Wu F, Fang Q, Wu Z, Duan Q, et al. The mutual effects of graphene oxide nanosheets and cadmium on the growth, cadmium uptake and accumulation in rice. Plant Physiol. Biochem. 2020;147:289–294. doi: 10.1016/j.plaphy.2019.12.034. [DOI] [PubMed] [Google Scholar]
- 116.Bravo Cadena M, Preston GM, Van der Hoorn RAL, Flanagan NA, Townley HE, et al. Enhancing cinnamon essential oil activity by nanoparticle encapsulation to control seed pathogens. Ind. Crops Prod. 2018;124:755–764. doi: 10.1016/j.indcrop.2018.08.043. [DOI] [Google Scholar]
- 117.Hussain A, Rizwan M, Ali S, Rehman MZU, Qayyum MF, et al. Combined use of different nanoparticles effectively decreased cadmium (cd) concentration in grains of wheat grown in a field contaminated with cd. Ecotoxicol. Environ. Saf. 2021;215:112139. doi: 10.1016/j.ecoenv.2021.112139. [DOI] [PubMed] [Google Scholar]
- 118.Rahimi S, Hatami M, Ghorbanpour M. Silicon-nanoparticle mediated changes in seed germination and vigor index of marigold (Calendula officinalis L.) compared to silicate under peg-induced drought stress. Gesunde Pflanz. 2021;73:1–15. doi: 10.1007/s10343-021-00579-x. [DOI] [Google Scholar]
- 119.Mahakham W, Sarmah AK, Maensiri S, Theerakulpisut P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017;7(1):8263. doi: 10.1038/s41598-017-08669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Acharya P, Jayaprakasha GK, Crosby K, Jifon J, Patil B. Green-synthesized nanoparticles enhanced seedling growth, yield, and quality of onion (Allium cepa L.) ACS Sustain. Chem. Eng. 2019;7(17):14580–14590. doi: 10.1021/acssuschemeng.9b02180. [DOI] [Google Scholar]
- 121.Kannaujia R, Srivastava CM, Prasad V, Singh BN, Pandey V. Phyllanthus emblica fruit extract stabilized biogenic silver nanoparticles as a growth promoter of wheat varieties by reducing ros toxicity. Plant Physiol. Biochem. 2019;142:460–471. doi: 10.1016/j.plaphy.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 122.Acharya P, Jayaprakasha GK, Crosby KM, Jifon JL, Patil BS. Nanoparticle-mediated seed priming improves germination, growth, yield, and quality of watermelons (Citrullus lanatus) at multi-locations in texas. Sci. Rep. 2020;10(1):5037. doi: 10.1038/s41598-020-61696-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Spagnoletti FN, Spedalieri C, Kronberg F, Giacometti R. Extracellular biosynthesis of bactericidal Ag/AgCl nanoparticles for crop protection using the fungus Macrophomina phaseolina. J. Environ. Manage. 2019;231:457–466. doi: 10.1016/j.jenvman.2018.10.081. [DOI] [PubMed] [Google Scholar]
- 124.Mahakham W, Theerakulpisut P, Maensiri S, Phumying S, Sarmah AK. Environmentally benign synthesis of phytochemicals-capped gold nanoparticles as nanopriming agent for promoting maize seed germination. Sci. Total Environ. 2016;573:1089–1102. doi: 10.1016/j.scitotenv.2016.08.120. [DOI] [PubMed] [Google Scholar]
- 125.Gopinath K, Gowri S, Karthika V, Arumugam A. Green synthesis of gold nanoparticles from fruit extract of Terminalia arjuna, for the enhanced seed germination activity of Gloriosa superba. J. Nanostruc. Chem. 2014;4(3):115. doi: 10.1007/s40097-014-0115-0. [DOI] [Google Scholar]
- 126.Tsi Ndeh N, Maensiri S, Maensiri D. The effect of green synthesized gold nanoparticles on rice germination and roots. Adv. Nat. Sci: Nanosci. Nanotechnol. 2017;8(3):035008. doi: 10.1088/2043-6254/aa724a. [DOI] [Google Scholar]
- 127.Arora S, Sharma P, Kumar S, Nayan R, Khanna P, et al. Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regul. 2012;66:303–310. doi: 10.1007/s10725-011-9649. [DOI] [Google Scholar]
- 128.Fang F, Li M, Zhang J, Lee C-S. Different strategies for organic nanoparticle preparation in biomedicine. ACS Mater. Lett. 2020;2(5):531–549. doi: 10.1021/acsmaterialslett.0c00078. [DOI] [Google Scholar]
- 129.Li R, He J, Xie H, Wang W, Bose S, et al. Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.) Int. J. Biol. Macromol. 2018;126:91–100. doi: 10.1016/j.ijbiomac.2018.12.118. [DOI] [PubMed] [Google Scholar]
- 130.Sathiyabama M, Muthukumar S. Chitosan guar nanoparticle preparation and its in vitro antimicrobial activity towards phytopathogens of rice. Int. J. Biol. Macromol. 2020;153:297–304. doi: 10.1016/j.ijbiomac.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 131.Divya K, Vijayan S, Nair SJ, Jisha MS. Optimization of chitosan nanoparticle synthesis and its potential application as germination elicitor of Oryza sativa L. Int. J. Biol. Macromol. 2019;124:1053–1059. doi: 10.1016/j.ijbiomac.2018.11.185. [DOI] [PubMed] [Google Scholar]
- 132.Siddaiah CN, Prasanth KVH, Satyanarayana NR, Mudili V, Gupta VK, et al. Chitosan nanoparticles having higher degree of acetylation induce resistance against pearl millet downy mildew through nitric oxide generation. Sci. Rep. 2018;8(1):2485. doi: 10.1038/s41598-017-19016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saharan V, Kumaraswamy RV, Choudhary RC, Kumari S, Pal A, et al. Cu-chitosan nanoparticle mediated sustainable approach to enhance seedling growth in maize by mobilizing reserved food. J. Agric. Food Chem. 2016;64(31):6148–6155. doi: 10.1021/acs.jafc.6b02239. [DOI] [PubMed] [Google Scholar]
- 134.Xu T, Ma C, Aytac Z, Hu X, Ng KW, et al. Enhancing agrichemical delivery and seedling development with biodegradable, tunable, biopolymer-based nanofiber seed coatings. ACS Sustain. Chem. Eng. 2020;8(25):9537–9548. doi: 10.1021/acssuschemeng.0c02696. [DOI] [Google Scholar]
- 135.Zhang H, Yang M, Luan Q, Tang H, Huang F, et al. Cellulose anionic hydrogels based on cellulose nanofibers as natural stimulants for seed germination and seedling growth. J. Agric. Food Chem. 2017;65(19):3785–3791. doi: 10.1021/acs.jafc.6b05815. [DOI] [PubMed] [Google Scholar]
- 136.Kacsó T, Hanna EA, Salinas F, Astete CE, Bodoki E, et al. Zein and lignin-based nanoparticles as soybean seed treatment: translocation and impact on seed and plant health. Appl. Nanosci. 2022;12:1557–1569. doi: 10.1007/s13204-021-02307-3. [DOI] [Google Scholar]
- 137.Falsini S, Clemente I, Papini A, Tani C, Schiff S, et al. When sustainable nanochemistry meets agriculture: lignin nanocapsules for bioactive compound delivery to plantlets. ACS Sustain. Chem. Eng. 2019;7(24):19935–19942. doi: 10.1021/acssuschemeng.9b05462. [DOI] [Google Scholar]
- 138.Sampathkumar K, Tan KX, Loo SCJ. Developing nano-delivery systems for agriculture and food applications with nature-derived polymers. iScience. 2020;23(5):101055. doi: 10.1016/j.isci.2020.101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shakiba S, Astete CE, Paudel S, Sabliov CM, Rodrigues DF, et al. Emerging investigator series: polymeric nanocarriers for agricultural applications: synthesis, characterization, and environmental and biological interactions. Environ. Sci. Nano. 2020;7(1):37–67. doi: 10.1039/C9EN01127G. [DOI] [Google Scholar]
- 140.Kumar S, Bhanjana G, Sharma A, Sidhu M, Dilbaghi N. Synthesis, characterization and on field evaluation of pesticide loaded sodium alginate nanoparticles. Carbohydr. Polym. 2014;101:1061–1067. doi: 10.1016/j.carbpol.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 141.Chuxiang S, Ke S, Wei W, Zhao Y, Tian L, et al. Encapsulation and controlled release of hydrophilic pesticide in shell cross-linked nanocapsules containing aqueous core. Int. J. Pharm. 2014;463:108–114. doi: 10.1016/j.ijpharm.2013.12.050. [DOI] [PubMed] [Google Scholar]
- 142.Chen H, Zhi H, Liang J, Yu M, Cui B, et al. Development of leaf-adhesive pesticide nanocapsules with pH-responsive release to enhance retention time on crop leaves and improve utilization efficiency. J. Mater. Chem. B. 2021;9(3):783–792. doi: 10.1039/D0TB02430A. [DOI] [PubMed] [Google Scholar]
- 143.Cui J, Sun C, Wang A, Wang Y, Zhu H, et al. Dual-functionalized pesticide nanocapsule delivery system with improved spreading behavior and enhanced bioactivity. Nanomaterials. 2020;10(2):220. doi: 10.3390/nano10020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.da Costa JT, Forim MR, Costa ES, De Souza JR, Mondego JM, et al. Effects of different formulations of neem oil-based products on control Zabrotes subfasciatus (boheman, 1833) (coleoptera: Bruchidae) on beans. J. Stored Prod. Res. 2014;56:49–53. doi: 10.1016/j.jspr.2013.10.004. [DOI] [Google Scholar]
- 145.Jiang Y, Chen Y, Tian D, Shen F, Wan X, et al. Fabrication and characterization of lignin–xylan hybrid nanospheres as pesticide carriers with enzyme-mediated release property. Soft Matter. 2020;16(39):9083–9093. doi: 10.1039/D0SM01402H. [DOI] [PubMed] [Google Scholar]
- 146.Li R, Li M, He J, Xie H, Wang W, et al. Preparation of pectin nanospheres and its effect on wheat (Triticum aestivum L.) seed germination and growth. J. Plant Growth Regul. 2022;41:3197–3207. doi: 10.1007/s00344-021-10505-0. [DOI] [Google Scholar]
- 147.Zhang Z-J, Shang X-F, Yang L, Shi Y-B, Liu Y-Q, et al. Engineering of peglayted camptothecin into nanomicelles and supramolecular hydrogels for pesticide combination control. Front. Chem. 2020;7:922. doi: 10.3389/fchem.2019.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Adak T, Kumar J, Shakil NA, Walia S. Development of controlled release formulations of imidacloprid employing novel nano-ranged amphiphilic polymers. J. Environ. Sci. Health B. 2012;47(3):217–225. doi: 10.1080/03601234.2012.634365. [DOI] [PubMed] [Google Scholar]
- 149.Dong E, Li P, Zhou C, Wang C, Li S, et al. PH-responsive ultrasonic self-assembly spinosad-loaded nanomicelles and their antifungal activity to fusarium oxysporum. React. Funct. Polym. 2019;141:123–132. doi: 10.1016/j.reactfunctpolym.2019.05.004. [DOI] [Google Scholar]
- 150.Lv X, Yuan M, Pei Y, Liu C, Wang X, et al. The enhancement of antiviral activity of chloroinconazide by aglinate-based nanogel and its plant growth promotion effect. J. Agric. Food Chem. 2021;69(17):4992–5002. doi: 10.1021/acs.jafc.1c00941. [DOI] [PubMed] [Google Scholar]
- 151.Ziaee M, Moharramipour S, Mohsenifar A. MA-chitosan nanogel loaded with Cuminum cyminum essential oil for efficient management of two stored product beetle pests. J. Pest Sci. 2014;87:691–699. doi: 10.1007/s10340-014-0590-6. [DOI] [Google Scholar]
- 152.Meraz-Dávila S, Pérez-García CE, Feregrino-Perez AA. Challenges and advantages of electrospun nanofibers in agriculture: a review. Mater. Res. Express. 2021;8(4):042001. doi: 10.1088/2053-1591/abee55. [DOI] [Google Scholar]
- 153.Farias BV, Pirzada T, Mathew R, Sit TL, Opperman C, et al. Electrospun polymer nanofibers as seed coatings for crop protection. ACS Sustain. Chem. Eng. 2019;7(24):19848–19856. doi: 10.1021/acssuschemeng.9b05200. [DOI] [Google Scholar]
- 154.Noruzi M. Electrospun nanofibres in agriculture and the food industry: A review. J. Sci. Food Agric. 2016;96(14):4663–4678. doi: 10.1002/jsfa.7737. [DOI] [PubMed] [Google Scholar]
- 155.Pirzada T, de Farias BV, Mathew R, Guenther RH, Byrd MV, et al. Recent advances in biodegradable matrices for active ingredient release in crop protection: towards attaining sustainability in agriculture. Curr. Opin. Colloid Interface Sci. 2020;48:121–136. doi: 10.1016/j.cocis.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Adisa IO, Pullagurala VLR, Peralta-Videa JR, Dimkpa CO, Elmer WH, et al. Recent advances in nano-enabled fertilizers and pesticides: a critical review of mechanisms of action. Environ. Sci. Nano. 2019;6(7):2002–2030. doi: 10.1039/C9EN00265K. [DOI] [Google Scholar]
- 157.Krishnamoorthy V, Elumalai G, Rajiv S. Environment friendly synthesis of polyvinylpyrrolidone nanofibers and their potential use as seed coats. New J. Chem. 2016;40:3268–3276. doi: 10.1039/C5NJ03008K. [DOI] [Google Scholar]
- 158.Sun D, Hussain HI, Yi Z, Rookes JE, Kong L, et al. Mesoporous silica nanoparticles enhance seedling growth and photosynthesis in wheat and lupin. Chemosphere. 2016;152:81–91. doi: 10.1016/j.chemosphere.2016.02.096. [DOI] [PubMed] [Google Scholar]
- 159.Rangaraj S, Gopalu K, Periasamy P, Venkatachalam R, Kannan N. Growth and physiological responses of maize (Zea mays L.) to porous silica nanoparticles in soil. J. Nanopart. Res. 2012;14:1294. doi: 10.1007/s11051-012-1294-6. [DOI] [Google Scholar]
- 160.Sattary M, Amini J, Hallaj R. Antifungal activity of the lemongrass and clove oil encapsulated in mesoporous silica nanoparticles against wheat's take-all disease. Pestic. Biochem. Physiol. 2020;170:104696. doi: 10.1016/j.pestbp.2020.104696. [DOI] [PubMed] [Google Scholar]
- 161.Zhao P, Wang C, Zhang S, Zheng L, Li F, et al. Fungicide-loaded mesoporous silica nanoparticles promote rice seedling growth by regulating amino acid metabolic pathways. J. Hazard Mater. 2022;425:127892. doi: 10.1016/j.jhazmat.2021.127892. [DOI] [PubMed] [Google Scholar]
- 162.Zhu F, Liu X, Cao L, Cao C, Li F, et al. Uptake and distribution of fenoxanil-loaded mesoporous silica nanoparticles in rice plants. Int. J. Mol. Sci. 2018;19:2854. doi: 10.3390/ijms19102854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Silva P, Pereira L, Lago A, Reis M, Rezende É, et al. Physical-mechanical and antifungal properties of pectin nanocomposites/neem oil nanoemulsion for seed coating. Food Biophys. 2019;14:456–466. doi: 10.1007/s11483-019-09592-0. [DOI] [Google Scholar]
- 164.N. Iqbal, D. Hazra, A. Purkait, N. Kumar, J. Kumar, Bioengineering of neem colloidal nano-emulsion formulation with adjuvant for better surface adhesion and long term activity in insect control. PREPRINT (Version 1) available at Research Square, (2020). 10.21203/rs.3.rs-116370/v1
- 165.Kumar S, Singh N, Devi LS, Kumar S, Kamle M, et al. Neem oil and its nanoemulsion in sustainable food preservation and packaging: Current status and future prospects. J. Agric. Food Res. 2022;7:100254. doi: 10.1016/j.jafr.2021.100254. [DOI] [Google Scholar]
- 166.Silva P, Pereira L, Rezende É, Reis M, Lago A, et al. Production and efficacy of neem nanoemulsion in the control of Aspergillus flavus and Penicillium citrinum in soybean seeds. Eur. J. Plant. Pathol. 2019;155:1105–1116. doi: 10.1007/s10658-019-01838-4. [DOI] [Google Scholar]
- 167.Adak T, Barik N, Patil N, Govindharaj G-P-P, Gowda Gadratagi B, et al. Nanoemulsion of eucalyptus oil: an alternative to synthetic pesticides against two major storage insects (Sitophilus oryzae (L.) and Tribolium castaneum (Herbst)) of rice. Ind. Crops Prod. 2019;143:111849. doi: 10.1016/j.indcrop.2019.111849. [DOI] [Google Scholar]
- 168.Saritha GNG, Anju T, Kumar A. Nanotechnology—big impact: How nanotechnology is changing the future of agriculture? J. Agric. Food Res. 2022;10:100457. doi: 10.1016/j.jafr.2022.100457. [DOI] [Google Scholar]
- 169.Nuruzzaman M, Liu Y, Ren J, Rahman MM, Zhang H, et al. Capability of organically modified montmorillonite nanoclay as a carrier for imidacloprid delivery. ACS Agric. Sci. Technol. 2022;2(1):57–68. doi: 10.1021/acsagscitech.1c00125. [DOI] [Google Scholar]
- 170.Wang L, Cai D, Zhang G, Ge C, Wu Z, et al. Improve the dispersion of nanoclay using biochar and biosilica-application to decrease the loss of pesticide. J. Nanosci. Nanotechnol. 2016;16(6):5869–5874. doi: 10.1166/jnn.2016.12065. [DOI] [PubMed] [Google Scholar]
- 171.Jahan N, Aslam S, rahman KU, Fazal T, Anwar F, et al. Formulation and characterisation of nanosuspension of herbal extracts for enhanced antiradical potential. J. Exp. Nanosci. 2016;11(1):72–80. doi: 10.1080/17458080.2015.1025303. [DOI] [Google Scholar]
- 172.Zhu Z, Shao C, Guo Y, Feng J, Chen C, et al. Facile pathway to generate agrochemical nanosuspensions integrating super-high load, eco-friendly excipients, intensified preparation process, and enhanced potency. Nano Res. 2021;14(7):2179–2187. doi: 10.1007/s12274-020-3177-y. [DOI] [Google Scholar]
- 173.Sasson Y, Levy-Ruso G, Toledano O, Ishaaya I. Nanosuspensions: emerging novel agrochemical formulations. In: Ishaaya I, Horowitz AR, Nauen R, editors. Insecticides Design Using Advanced Technologies. Berlin: Springer; 2007. pp. 1–39. [Google Scholar]
- 174.Corrias F, Melis A, Atzei A, Marceddu S, Dedola F, et al. Zoxamide accumulation and retention evaluation after nanosuspension technology application in tomato plant. Pest Manag. Sci. 2021;77(7):3508–3518. doi: 10.1002/ps.6404. [DOI] [PubMed] [Google Scholar]
- 175.Cui B, Lv Y, Gao F, Wang C, Zeng Z, et al. Improving abamectin bioavailability via nanosuspension constructed by wet milling technique. Pest Manag. Sci. 2019;75(10):2756–2764. doi: 10.1002/ps.5386. [DOI] [PubMed] [Google Scholar]
- 176.Chin C-P, Wu H-S, Wang S. New approach to pesticide delivery using nanosuspensions: research and applications. Ind. Eng. Chem. Res. 2011;50(12):7637–7643. doi: 10.1021/ie2001007. [DOI] [Google Scholar]
- 177.Sharma S, Shree B, Aditika, Sharma A, Irfan M, et al. Nanoparticle-based toxicity in perishable vegetable crops: molecular insights, impact on human health and mitigation strategies for sustainable cultivation. Environ. Res. 2022;212:113168. doi: 10.1016/j.envres.2022.113168. [DOI] [PubMed] [Google Scholar]
- 178.Ray PC, Yu H, Fu PP. Toxicity and environmental risks of nanomaterials: Challenges and future needs. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009;27(1):1–35. doi: 10.1080/10590500802708267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bahadar H, Maqbool F, Niaz K, Abdollahi M. Toxicity of nanoparticles and an overview of current experimental models. Iran Biomed. J. 2016;20(1):1–11. doi: 10.7508/ibj.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang H-J, Lee Y-H, Hsu Y-H, Liao C-T, Lin Y-F, et al. Current strategies in assessment of nanotoxicity: alternatives to in vivo animal testing. Int. J. Mol. Sci. 2021;22(8):4216. doi: 10.3390/ijms22084216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Burello E, Worth A. Computational nanotoxicology: predicting toxicity of nanoparticles. Nat. Nanotechnol. 2011;6:138–139. doi: 10.1038/nnano.2011.27. [DOI] [PubMed] [Google Scholar]
- 182.Tirumala MG, Anchi P, Raja S, Rachamalla M, Godugu C. Novel methods and approaches for safety evaluation of nanoparticle formulations: a focus towards in vitro models and adverse outcome pathways. Front. Pharmacol. 2021;12:1612659. doi: 10.3389/fphar.2021.612659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pérez-de-Luque A. Interaction of nanomaterials with plants: What do we need for real applications in agriculture? Front. Environ. Sci. 2017;5:12. doi: 10.3389/fenvs.2017.00012. [DOI] [Google Scholar]
- 184.Zia-ur-Rehman M, Qayyum MF, Akmal F, Maqsood MA, Rizwan M, et al. (2018) Recent progress of nanotoxicology in plants. In: Tripathi DK, Ahmad P, Sharma S, Chauhan DK, Dubey NK, et al., editors. Nanomaterials in Plants, Algae, and Microorganisms. Cambridge: Academic Press; 2018. pp. 143–174. [Google Scholar]
- 185.Kumari M, Ernest V, Mukherjee A, Chandrasekaran N. In vivo nanotoxicity assays in plant models. Methods Mol. Biol. 2012;926:399–410. doi: 10.1007/978-1-62703-002-1_26. [DOI] [PubMed] [Google Scholar]
- 186.Lin D, Xing B. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ. Pollut. 2007;150(2):243–250. doi: 10.1016/j.envpol.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 187.Savage DT, Hilt JZ, Dziubla TD. In vitro methods for assessing nanoparticle toxicity. In: Zhang Q, editor. Nanotoxicity. Methods in Molecular Biology. New York: Humana Press; 2019. pp. 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Verma D, Patel S, Kushwah K. Effects of nanoparticles on seed germination, growth, phytotoxicity and crop improvement. Agric. Rev. 2020;42:1–11. doi: 10.18805/ag.R-1964. [DOI] [Google Scholar]
- 189.Lee CW, Mahendra S, Zodrow K, Li D, Tsai Y-C, et al. Developmental phytotoxicity of metal oxide nanoparticles to arabidopsis thaliana. Environ. Toxicol. Chem. 2010;29(3):669–675. doi: 10.1002/etc.58. [DOI] [PubMed] [Google Scholar]
- 190.Patisaul HB. Endocrine disruption by dietary phyto-oestrogens: Impact on dimorphic sexual systems and behaviours. Proc. Nutri. Soci. 2017;76(2):130–144. doi: 10.1017/S0029665116000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Khan ST, Adil SF, Shaik MR, Alkhathlan HZ, Khan M, et al. Engineered nanomaterials in soil: their impact on soil microbiome and plant health. Plants. 2021;11(1):109. doi: 10.3390/plants11010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tian H, Kah M, Kariman K. Are nanoparticles a threat to mycorrhizal and rhizobial symbioses? A critical review. Front. Microbiol. 2019;10:1660. doi: 10.3389/fmicb.2019.01660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang H, Huang M, Zhang W, Gardea-Torresdey JL, White JC, et al. Silver nanoparticles alter soil microbial community compositions and metabolite profiles in unplanted and cucumber-planted soils. Environ. Sci. Technol. 2020;54(6):3334–3342. doi: 10.1021/acs.est.9b07562. [DOI] [PubMed] [Google Scholar]
- 194.Li M, Wang P, Dang F, Zhou D-M. The transformation and fate of silver nanoparticles in paddy soil: effects of soil organic matter and redox conditions. Environ. Sci. Nano. 2017;4(4):919–928. doi: 10.1039/C6EN00682E. [DOI] [Google Scholar]
- 195.Khan M, Khan MSA, Borah KK, Goswami Y, Hakeem KR, et al. The potential exposure and hazards of metal-based nanoparticles on plants and environment, with special emphasis on ZnO NPs, TiO2 NPs, and AgNPs: a review. Environ. Adv. 2021;6:100128. doi: 10.1016/j.envadv.2021.100128. [DOI] [Google Scholar]
- 196.Ameen F, Alsamhary K, Alabdullatif JA, Alnadhari S. A review on metal-based nanoparticles and their toxicity to beneficial soil bacteria and fungi. Ecotoxicol. Environ. Saf. 2021;213:112027. doi: 10.1016/j.ecoenv.2021.112027. [DOI] [PubMed] [Google Scholar]
- 197.Rahman MS, Chakraborty A, Mazumdar S, Nandi NC, Bhuiyan MNI, et al. Effects of poly(vinylpyrrolidone) protected platinum nanoparticles on seed germination and growth performance of Pisum sativum. Nano Struct. Nano Object. 2020;21:100408. doi: 10.1016/j.nanoso.2019.100408. [DOI] [Google Scholar]
- 198.Metch J, Burrows N, Murphy C, Pruden A, Vikesland P. Metagenomic analysis of microbial communities yields insight into impacts of nanoparticle design. Nat. Nanotech. 2018;13:253–259. doi: 10.1038/s41565-017-0029-3. [DOI] [PubMed] [Google Scholar]
- 199.Skjolding LM, Sørensen SN, Hartmann NB, Hjorth R, Hansen SF, et al. Aquatic ecotoxicity testing of nanoparticles-the quest to disclose nanoparticle effects. Angew. Chem. Int. Ed. 2016;55(49):15224–15239. doi: 10.1002/anie.201604964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Schultz A, Boyle D, Chamot D, Ong K, Wilkinson K, et al. Aquatic toxicity of manufactured nanomaterials: challenges and recommendations for future toxicity testing. Environ. Chem. 2014;11(3):207–226. doi: 10.1071/EN13221. [DOI] [Google Scholar]
- 201.Boros B-V, Ostafe V. Evaluation of ecotoxicology assessment methods of nanomaterials and their effects. Nanomaterials. 2020;10(4):610. doi: 10.3390/nano10040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hillegass JM, Shukla A, Lathrop SA, MacPherson MB, Fukagawa NK, et al. Assessing nanotoxicity in cells in vitro. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010;2(3):219–231. doi: 10.1002/wnan.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Weissig V, Pettinger TK, Murdock N. Nanopharmaceuticals (part 1): products on the market. Int. J. Nanomed. 2014;9:4357–4373. doi: 10.2147/IJN.S46900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lebre F, Chatterjee N, Costa S, Fernández-de-Gortari E, Lopes C, et al. Nanosafety: an evolving concept to bring the safest possible nanomaterials to society and environment. Nanomaterials. 2022;12(11):1810. doi: 10.3390/nano12111810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Phenny M. Risks, uncertainties, and ethics of nanotechnology in agriculture. In: Çelik Ö, editor. New Visions in Plant Science. London: IntechOpen; 2018. [Google Scholar]
- 206.Hutchison JE. The road to sustainable nanotechnology: challenges, progress and opportunities. ACS Sustain. Chem. Engin. 2016;4(11):5907–5914. doi: 10.1021/acssuschemeng.6b02121. [DOI] [Google Scholar]
- 207.Ying S, Guan Z, Ofoegbu PC, Clubb P, Rico C, et al. Green synthesis of nanoparticles: current developments and limitations. Environ. Techn. Innov. 2022;26:102336. doi: 10.1016/j.eti.2022.102336. [DOI] [Google Scholar]
- 208.Amorim MJ. The daunting challenge of ensuring sustainable development of nanomaterials. Int. J. Environ. Res. Public Health. 2016;13(2):245. doi: 10.3390/ijerph13020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Allan J, Belz S, Hoeveler A, Hugas M, Okuda H, et al. Regulatory landscape of nanotechnology and nanoplastics from a global perspective. Regul. Toxicol. Pharmacol. 2021;122:104885. doi: 10.1016/j.yrtph.2021.104885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Singh AV, Maharjan R-S, Kanase A, Siewert K, Rosenkranz D, et al. Machine-learning-based approach to decode the influence of nanomaterial properties on their interaction with cells. ACS Appl. Mater. Interfaces. 2021;13(1):1943–1955. doi: 10.1021/acsami.0c18470. [DOI] [PubMed] [Google Scholar]
- 211.Abdollahdokht D, Gao Y, Faramarz S, Poustforoosh A, Abbasi M, et al. Conventional agrochemicals towards nano-biopesticides: An overview on recent advances. Chem. Biol. Technol. Agric. 2022;9(1):13. doi: 10.1186/s40538-021-00281-0. [DOI] [Google Scholar]
- 212.Prasad TNVKV, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, et al. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012;35:905–927. doi: 10.1080/01904167.2012.663443. [DOI] [Google Scholar]
- 213.Dimkpa CO, White JC, Elmer WH, Gardea-Torresdey J. Nanoparticle and ionic zn promote nutrient loading of sorghum grain under low npk fertilization. J. Agric. Food Chem. 2017;65(39):8552–8559. doi: 10.1021/acs.jafc.7b02961. [DOI] [PubMed] [Google Scholar]
- 214.Subbaiah LV, Prasad TNVKV, Krishna TG, Sudhakar P, Reddy BR, et al. Novel effects of nanoparticulate delivery of zinc on growth, productivity, and zinc biofortification in maize (Zea mays L.) J. Agric. Food Chem. 2016;64(19):3778–3788. doi: 10.1021/acs.jafc.6b00838. [DOI] [PubMed] [Google Scholar]
- 215.Roghayyeh S, Mohammad S, Mehdi Tajbakhsh S, Rauf Seyed S. Effects of nano-iron oxide particles on agronomic traits of soybean. Not. Sci. Biol. 2010;2(2):112–113. doi: 10.15835/nsb224667. [DOI] [Google Scholar]
- 216.Hoang SA, Nguyen LQ, Nguyen NH, Tran CQ, Nguyen DV, et al. Metal nanoparticles as effective promotors for maize production. Sci. Rep. 2019;9(1):13925. doi: 10.1038/s41598-019-50265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Marchiol L, Mattiello A, Pošćić F, Fellet G, Zavalloni C, et al. Changes in physiological and agronomical parameters of barley (Hordeum vulgare) exposed to cerium and titanium dioxide nanoparticles. Int. J. Environ. Res. Public Health. 2016;13:332. doi: 10.3390/ijerph13030332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dağhan H, Gulmezoglu N, Köleli N, Karakaya B. Impact of titanium dioxide nanoparticles (TiO2-NPs) on growth and mineral nutrient uptake of wheat (Triticum vulgare L.) Biotech. Studies. 2020;29(2):69–76. doi: 10.38042/biost.2020.29.02.03. [DOI] [Google Scholar]
- 219.Singh P, Singh R, Borthakur A, Srivastava P, Srivastava N, et al. Effect of nanoscale TiO2-activated carbon composite on Solanum lycopersicum (L.) and Vigna radiata (L.) seeds germination. Energy Ecol. Environ. 2016;1(3):131–140. doi: 10.1007/s40974-016-0009-8. [DOI] [Google Scholar]
- 220.Ma C, Liu H, Chen G, Zhao Q, Guo H, et al. Dual roles of glutathione in silver nanoparticle detoxification and enhancement of nitrogen assimilation in soybean (Glycine max (L.) merrill) Environ. Sci. Nano. 2020;7(7):1954–1966. doi: 10.1039/D0EN00147C. [DOI] [Google Scholar]
- 221.Haghighi M, Pessarakli M. Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci. Hortic. 2013;161:111–117. doi: 10.1016/j.scienta.2013.06.034. [DOI] [Google Scholar]
- 222.Yerragopu PS, Hiregoudar S, Nidoni U, Ramappa KT, Sreenivas A, et al. Effect of plant-mediated synthesized silver nanoparticles on pulse beetle, Callosobruchus chinensis (L.) Int. J. Curr. Microbiol. Appl. Sci. 2019;8:1965–1972. doi: 10.1016/j.scienta.2013.06.034. [DOI] [Google Scholar]
- 223.Tadler TS, Arcía GPLÓG, Itto JGG, Uteler MB. Particulate nanoinsecticides: a new concept in insect pest management. In: Begum G, editor. Insecticides—Agriculture and Toxicology. London: IntechOpen; 2017. [Google Scholar]
- 224.El-Naggar ME, Abdelsalam NR, Fouda MMG, Mackled MI, Al-Jaddadi MAM, et al. Soil application of nano silica on maize yield and its insecticidal activity against some stored insects after the post-harvest. Nanomaterials. 2020;10(4):739. doi: 10.3390/nano10040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Belhamel C, Boulekbache-Makhlouf L, Bedini S, Tani C, Lombardi T, et al. Nanostructured alumina as seed protectant against three stored-product insect pests. J. Stored Prod. Res. 2020;87:101607. doi: 10.1016/j.jspr.2020.101607. [DOI] [Google Scholar]
- 226.Diagne A, Diop BN, Ndiaye PM, Andreazza C, Sembene M. Efficacy of silica nanoparticles on groundnut bruchid, Caryedon serratus (Olivier) (coleoptera, bruchidae) Afr Crop Sci. J. 2019;27:229. doi: 10.4314/acsj.v27i2.8. [DOI] [Google Scholar]
- 227.Ismail T, Salama MA, El-Ebiary M. Entomotoxic effects of synthesized aluminum oxide nanoparticles against sitophilus oryzae and their toxicological effects on albino rats. Toxicol. Ind. Health. 2021;37(10):594–602. doi: 10.1177/07482337211035000. [DOI] [PubMed] [Google Scholar]
- 228.Mohammed AM, Aswd SA. Effect of some nanoparticles on the stages biology of the southern cowpea beetle Callosobruchus maculatus (Fab.) (Coleoptera: Bruchidae) J. Edu. Sci. 2019;28(3):188–199. doi: 10.33899/edusj.2019.162956. [DOI] [Google Scholar]
- 229.Zhang H, Wang R, Chen Z, Cui P, Lu H, et al. The effect of zinc oxide nanoparticles for enhancing rice (Oryza sativa L.) yield and quality. Agriculture. 2021;11(12):1247. doi: 10.3390/agriculture11121247. [DOI] [Google Scholar]
- 230.Dileep Kumar G, Raja K, Natarajan N, Govindaraju K, Subramanian KS. Invigouration treatment of metal and metal oxide nanoparticles for improving the seed quality of aged chilli seeds (Capsicum annum L.) Mater. Chem. Phys. 2020;242:122492. doi: 10.1016/j.matchemphys.2019.122492. [DOI] [Google Scholar]
- 231.Waqas Mazhar M, Ishtiaq M, Maqbool M, Akram R, Shahid A, et al. Seed priming with iron oxide nanoparticles raises biomass production and agronomic profile of water-stressed flax plants. Agronomy. 2022;12(5):982. doi: 10.3390/agronomy12050982. [DOI] [Google Scholar]
- 232.Joshi A, Kaur S, Dharamvir K, Nayyar H, Verma G. Multi-walled carbon nanotubes applied through seed-priming influence early germination, root hair, growth and yield of bread wheat (Triticum aestivum L.) J. Sci. Food Agric. 2018;98(8):3148–3160. doi: 10.1002/jsfa.8818. [DOI] [PubMed] [Google Scholar]
- 233.Shcherbakova EN, Shcherbakov AV, Andronov EE, Gonchar LN, Kalenskaya SM, et al. Combined pre-seed treatment with microbial inoculants and mo nanoparticles changes composition of root exudates and rhizosphere microbiome structure of chickpea (Cicer arietinum L.) plants. Symbiosis. 2017;73(1):57–69. doi: 10.1007/s13199-016-0472-1. [DOI] [Google Scholar]
- 234.Hussain A, Rizwan M, Ali Q, Ali S. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. 2019;26(8):7579–7588. doi: 10.1007/s11356-019-04210-5. [DOI] [PubMed] [Google Scholar]
- 235.Saad AM, Alabdali AYM, Ebaid M, Salama E, El-Saadony MT, et al. Impact of green chitosan nanoparticles fabricated from shrimp processing waste as a source of nano nitrogen fertilizers on the yield quantity and quality of wheat (Triticum aestivum L.) cultivars. Molecules. 2022;27(17):5640. doi: 10.3390/molecules27175640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Muthukrishnan S, Murugan I, Selvaraj M. Chitosan nanoparticles loaded with thiamine stimulate growth and enhances protection against wilt disease in chickpea. Carbohydr. Polym. 2019;212:169–177. doi: 10.1016/j.carbpol.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 237.Mondal M, Puteh A, Dafader N. Foliar application of chitosan improved morpho-physiological attributes and yield in summer tomato (Solanum lycopersicum) Pak. J. Agric. Sci. 2016;53:339–344. doi: 10.21162/PAKJAS/16.2011. [DOI] [Google Scholar]
- 238.Rajput VD, Singh A, Minkina T, Rawat S, Mandzhieva S, et al. Nano-enabled products: challenges and opportunities for sustainable agriculture. Plants. 2021;10(12):2727. doi: 10.3390/plants10122727. [DOI] [PMC free article] [PubMed] [Google Scholar]