Background:
Daridorexant is a novel dual orexin receptor antagonist that has shown efficacy as a treatment for insomnia in multiple randomized clinical trials. However, the efficacy and safety of daridorexant for treatment of insomnia disorder has not been characterized comprehensively in the literature. Therefore, we performed a meta-analysis of available studies. We performed a meta-analysis to systematically evaluate the efficacy and safety of daridorexant for treatment of insomnia disorder.
Methods:
MEDLINE, Embase, Cochrane Library, and Clinicaltrials.gov for randomized controlled trials were systematically searched up to February 2022. Relative risk and standard mean difference were used to evaluate clinical outcomes.
Results:
We pooled 2271 patients from 4 randomized clinical trials, and evaluated efficacy endpoints. We found that 50 mg of daridorexant was superior to placebo for 4 efficacy outcomes including wake time after sleep onset, latency to persistent sleep, subjective total sleep time, and Insomnia Daytime Symptoms and Impacts Questionnaire domain score (P < .05). In addition, there were no significant differences (P > .05) in adverse events between daridorexant and placebo.
Conclusions:
Different dosages of daridorexant were tested for treatment of insomnia; however, 5 and 10 mg are not available because of issues of suboptimal effectiveness. Daridorexant showed better efficacy and safety for treatment of insomnia disorder at doses of 25 and 50 mg.
Keywords: daridorexant, efficacy, insomnia disorder, meta-analysis, safety
1. Introduction
Insomnia includes sleep difficulties including difficulty falling asleep, difficulty maintaining sleep, waking early, and feeling tired during the daytime.[1] Generally, insomnia is divided into acute or short-term sleep disorders (<3 months) and chronic sleep disorders (more than 3 months).[2] The prevalence of insomnia has been estimated to be 2% to 48% due to the differences in criteria used to define insomnia and differences in populations.[3,4] The prevalence of insomnia is higher in older individuals than in younger individuals. As the global population ages, sleep difficulties will become more prevalent.[5] Insomnia is a major public health issue that places a large burden on society. A previous study suggested that the costs of untreated insomnia are significantly greater than the direct costs associated with treatment of insomnia.[6] Quality sleep helps to preserve physical and mental health, resulting in better overall quality of life.
Cognitive behavioral therapy is the first-line therapy for all patients with insomnia, but pharmacotherapy is also a widely used treatment strategy.[7] The spectrum of drugs for treatment of insomnia is remarkably diverse, and includes benzodiazepines, non-benzodiazepine receptor agonists, melatonin receptor agonists, orexin receptor antagonists, and tricyclic antidepressants.[1] However, drugs used to treat insomnia are associated with various side effects, which highlights the need for novel therapeutic options[8] Dual orexin receptor antagonists (DORAs), which act as competitive inhibitors of orexin A and orexin B, can promote sleep and mitigate insomnia by selectively binding to orexin receptors 1 and 2. Orexins are 2 neuropeptides produced exclusively in the lateral hypothalamus that act on 2 specific receptors that are widely distributed throughout the brain and are involved in myriad neurophysiological functions[9] A large systematic review and meta-analysis incorporating data from 13 studies provided strong support that DORAs were superior to placebo in terms of efficacy and safety.[10]
Orexin receptors 1 and 2 are promising targets for treatment of insomnia, and drug development is ongoing. Daridorexant, a novel dual orexin receptor antagonist, was recently approved by the Food and Drug Administration for treatment of insomnia disorder. Studies have shown that daridorexant significantly improved sleep onset and sleep maintenance, resulting in improved daytime function.[11,12] No previous studies have systematically evaluated the overall effects of daridorexant. In this study, we performed a meta-analysis to systematically evaluate the efficacy and safety of daridorexant compared to placebo for treatment of insomnia in the adult and elderly population.
2. Methods
2.1. Protocol and registration
The meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.[13] Our study has not been registered.
2.2. Search strategy
MEDLINE, Embase, Cochrane Library, and Clinicaltrials.gov were searched using the following terms: [(“Daridorexant and insomnia disorder”) (“ACT-541468 and insomnia disorder”)] until February 2022. After removing duplicates and irrelevant studies, 2 investigators manually screened each possible manuscript by reading the reference lists.
2.3. Ethical review and informed consent of patients
This research is a systematic review, so the content does not involve ethical review and unethical projects.
2.4. Study selection
The study inclusion criteria were as follows: randomized clinical trials (RCTs); enrolled participants diagnosed with insomnia disorder; study used daridorexant as the intervention; and participants were over 18 years old. The study exclusion criteria were as follows: retrospective studies, cohort studies, case reviews, and case reports; active control without placebo was used (a known, effective treatment instead of comparison to an experimental treatment).
2.5. Data extraction
The data were extracted independently by 2 investigators (F.J. and H.L.), and any disagreements were settled through discussion. Basic information for the included trials (first author, year, number of NCT, countries, centers, publication, and treatment group), patient characteristics (age, race, gender, and body-mass index), and main outcome events were used to extract the data (Tables 1 and 2). Additional details are provided in the Tables 1 and 2, Supplemental Digital Content, http://links.lww.com/MD/I359, which illustrate the efficacy of different dosage of daridorexant and the safety of different dosage of daridorexant respectively.
Table 1.
Characteristics of the included studies and outcome events.
| Study | NCT number | Study type | Countries | Centers | Publication | Total number of participants | Treatment group | Main outcome events |
|---|---|---|---|---|---|---|---|---|
| Mignot et al 2022 | NCT03545191 | RCT | 10 | 75 | Lancet Neurology | 930 | ACT-541468: 25 mg, 50 mg vs Placebo | a b c d |
| NCT03575104 | RCT | 11 | 81 | Lancet Neurology | 924 | ACT-541468: 10 mg, 25 mg vs Placebo | a b c d | |
| Dauvilliers et al 2020 | NCT02841709 | RCT | 6 | 38 | ANNALS of Neurology | 58 | ACT-541468: 5 mg, 10 mg, 25 mg vs Placebo | e f g |
| Zammit et al 2020 | NCT02839200 | RCT | 2 | 10 | Neurology | 360 | ACT-541468: 5 mg, 10 mg, 25 mg, 50 mg vs Placebo or 10 mg zolpidem | e f |
(a) Change from baseline to month 1 in Wake After Sleep Onset. (b) Change from baseline to month 3 in Wake After Sleep Onset. (c) Change from baseline to month 1 in Latency to Persistent Sleep. (d) Change from baseline to month 3 in Latency to Persistent Sleep. (e) Change in Wake After Sleep Onset from Baseline to days 1 and 2. (f) Change in latency to persistent sleep from baseline to days 1 and 2. (g) Change in subjective wake after sleep onset and subjective latency to sleep onset from baseline recording to week 4 average.
Table 2.
Baseline of participants of the included studies.
| Daridorexant 50 mg (n = 429) | Daridorexant 25 mg (n = 737) | Daridorexant 10 mg (n = 423) | Daridorexant 5 mg (n = 118) | Placebo (n = 736) | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 277 | 511 | 292 | 77 | 492 |
| Male | 152 | 226 | 131 | 41 | 244 |
| Age | 55.8 | 56.3 | 57.1 | 55.5 | 56.1 |
| Race | |||||
| White | 384 | 668 | 376 | 108 | 651 |
| Black or African American | 38 | 52 | 27 | 8 | 67 |
| Asian | 4 | 14 | 14 | 0 | 13 |
| Other | 3 | 3 | 6 | 2 | 5 |
| Body-mass index, kg/m2 | 26.0 | 26.2 | 25.9 | 25.3 | 26.2 |
| Nighttime efficacy variables | |||||
| WASO, min | 98* | 102.8† | 105.5‡ | 106.7§ | 105.4∥ |
| LPS, min | 65.8* | 69.3† | 68.1‡ | 74.2§ | 69.9∥ |
| Self-reported total sleep time, min | 311.9* | 309.3† | 307.4‡ | 309.8§ | 311.9∥ |
| Total sleep time, min | 312.7* | 315.5† | 314.4‡ | 306.8§ | 312.1∥ |
| Insomnia Severity Index score | 19.7 | 19.5 | 20.2 | 20.7 | 19.6 |
LPS = latency to persistent sleep, WASO = wake time after sleep onset.
n = 427.
n = 734.
n = 419.
n = 116.
n = 732.
2.6. Outcomes
The efficacy outcomes were changes from baseline in wake time after sleep onset (WASO) and latency to persistent sleep (LPS) at months 1 and 3. In addition, subjective total sleep time (sTST), Insomnia Daytime Symptoms and Impacts Questionnaire (IDSIQ) domain score, WASO, and LPS at days 1 and 2 were also included. Adverse events (AEs) were chosen as the safety endpoint.
2.7. Subgroup analysis
Subgroup analyses were performed to answer specific questions, such as the effects of types of interventions for different ages. The I2 statistic was used to address heterogeneity between studies.
2.8. Summary measures and synthesis of results
Review Manager 5.4 was used to analyze the data. Estimated standard mean differences and estimated risk ratio (standard mean difference [SMD] or risk ratio [RR]; 95% confidence interval [CI]) were calculated using a random-effects model. According to conventional interpretations, SMD was classified as negligible (<0.2), small (0.2–0.4), moderate (0.4–0.8), or large (>0.8).The I2 statistic was used to estimate statistical heterogeneity as follows: I2 < 30% represents “low heterogeneity,” 30% < I2 < 50% represents “moderate heterogeneity,” and I2 > 50% represents “substantial heterogeneity.” Results were considered statistically significant when P < .05. All tests were 2-tailed. The Grading Recommendations Assessment, Development and Evaluation was used to show the quality of evidence for each outcome. The summary of findings table was formulated using Guideline Development Tool.
2.9. Risk of bias
Review Manager 5.4 software was used to create the risk of bias plot for individual studies. The Cochrane collaboration uniform criteria were used for assessing the risk of bias of RCTs. Selection bias, performance bias, detection bias, attrition bias, reporting bias, and other possible biases were included in the criteria.
3. Results
3.1. Search results
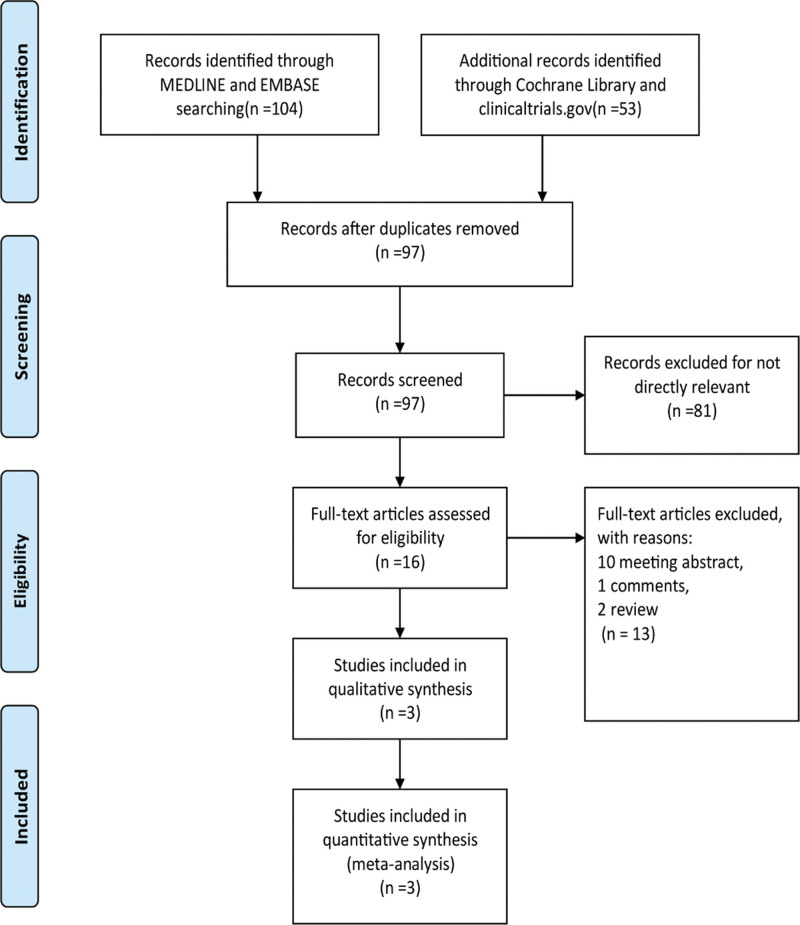
A total of 157 manuscripts and abstracts from MEDLINE, Embase, the Cochrane Library, and clinicaltrials.gov were identified. After removing duplicates and uncorrelated titles, 16 articles were directly related to the topic of this study. From these articles, 13 were excluded because there were 10 conference abstracts, 1 comment, and 2 reviews. Finally, 4 RCTs (one included article has 2 RCTs) containing 2271 patients were included in our meta-analysis. The complete search process is detailed in Figure 1.
Figure 1.
The study search, selection, and inclusion process.
3.2. Efficacy and safety of dosing regimens
3.2.1. Efficacy.
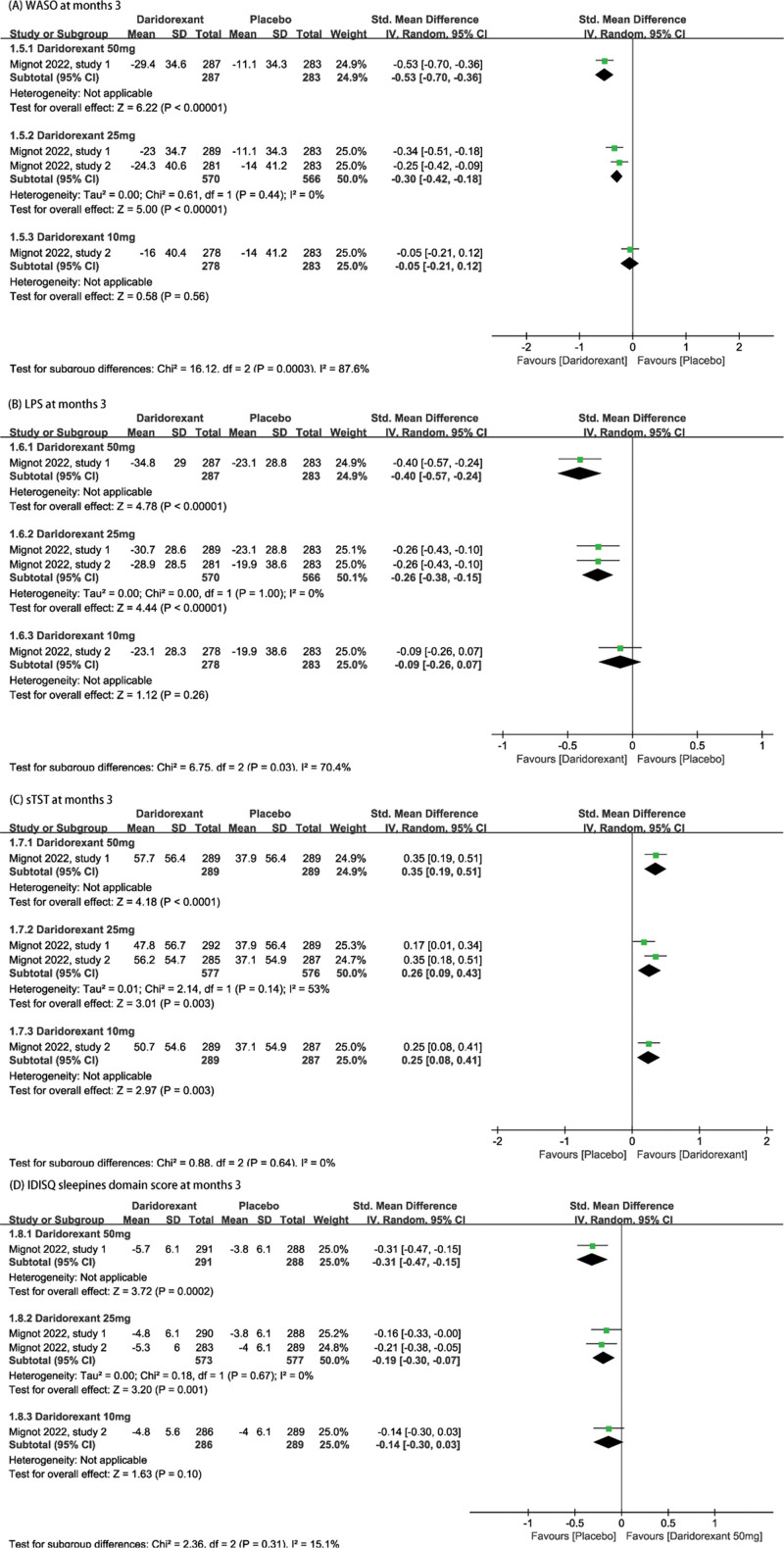
The efficacy outcomes included change from baseline in WASO, and change from baseline in LPS, sTST, and IDSIQ sleepiness domain scores (IDSIQ only in Mignot 2022) at different time points. According to the summary effect sizes, daridorexant was significantly more effective than placebo for all efficacy outcomes. In particular, 25 and 50 mg doses of daridorexant were the most effective.
Treatment with 25 mg or 50 mg of daridorexant had significant long-term effects, as all improvements were sustained at month 3. The results showed that WASO was significantly reduced from baseline among participants who received 50 mg of daridorexant (SMD = −0.53, 95% CI: [−0.70, −0.36], P < .001) or 25 mg of daridorexant (SMD = −0.30, 95% CI: [−0.42, −0.16], P < .001) compared with that in the placebo group. In addition, LPS was also significantly reduced from baseline (50 mg daridorexant, SMD = −0.53, 95% CI: [−0.70, −0.36], P < .001, 25 mg daridorexant SMD = −0.30, 95% CI: [−0.42, −0.16], P < .001). Compared with placebo, self-reported total sleep time was significantly increased from baseline in participants who received 25 mg or 50 mg of daridorexant (50 mg daridorexant, SMD = 0.35, 95% CI: [0.19, 0.51], P < .001, 25 mg daridorexant, SMD = 0.26, 95% CI: [0.09, 0.43], P = .003). Significant differences in IDSIQ sleepiness domain score were observed at month 3 among participants who received 50 mg (SMD = −0.31, 95% CI: [−0.47, −0.15], P < .001) or 25 mg (SMD = −0.19, 95% CI: [−30, −0.07], P = .001) of daridorexant. Forest plots of each outcome are listed in Figure 2.
Figure 2.
The pooled SMD of efficacy outcomes in different doses compared with placebo. (A) WASO at months 3. (B) LPS at months 3. (C) sTST at months 3. (D) IDSIQ sleepiness domain score at months 3. CI = confidence interval, IDSIQ = Insomnia Daytime Symptoms and Impacts Questionnaire, LPS = latency to persistent sleep, sTST = subjective total sleep time, WASO = wake time after sleep onset.
In addition, daridorexant showed significant short-term effects. Treatment with 50 mg of daridorexant improved WASO (SMD = −0.62, 95% CI: −0.85, −0.39], P < .001), LPS (SMD = −0.36, 95% CI: [−0.50, −0.21], P < .001), sTST (SMD = 0.45, 95%CI: [0.31, 0.60], P < .001), and IDSIQ sleepiness domain score (SMD = −0.37, 95% CI: [−0.53, −0.21], P < .001) in comparison with placebo at month 1. Treatment with 25 mg of daridorexant also was superior to placebo for all outcomes (WASO: SMD = −0.32, 95% CI: [−0.43, −0.21], P < .001; LPS: SMD = −0.23, 95% CI: [−0.34, −0.13], P < .001; sTST: SMD = 0.27, 95% CI: [0.16, 0.38], P < .001; and IDSIQ sleepiness domain score: SMD = −0.15, 95% CI: [−0.26, −0.04], P = .009). Forest plots for each outcome are listed in Figure S1, Supplemental Digital Content, http://links.lww.com/MD/I360, which demonstrates WASO, LPS, sTST, and IDSIQ sleepiness domain score at month 1. In addition, treatment with 10, 25, or 50 mg of daridorexant resulted in improvements in WASO and LPS at days 1 and 2. Therefore, an initial dose greater than 5 mg/d is necessary to provide therapeutic effects. Only the 25 and 50 mg doses as these are the only ones available for treatment of insomnia. Detailed outcomes and forest plots are listed in Figure S2, Supplemental Digital Content, http://links.lww.com/MD/I361, which demonstrates WASO and LPS at days 1 and 2. Furthermore, it is worth mentioning that the effect of 50 mg of daridorexant was relatively better compared to 25 mg (Forest plots for all efficacy outcome were in favor of treatment with 50 mg.).
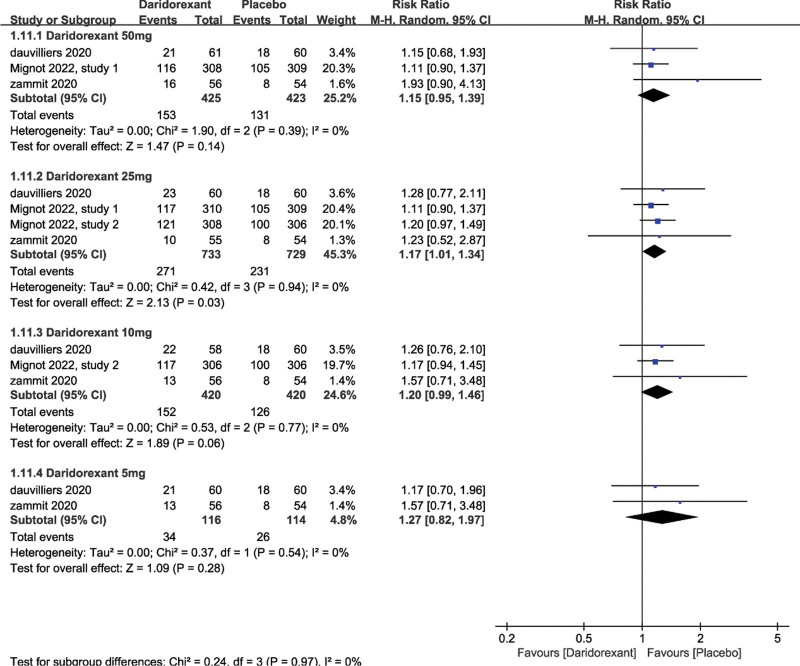
3.2.2. Safety.
AEs were the primary outcomes to determine safety. Only the total number of AEs was analyzed because the AEs were complex and varied greatly among participants and studies. We studied the number of participants with ≥1 AE. The most common AEs were nasopharyngitis, fatigue, and headache. Daridorexant was well tolerated at all doses investigated (50 mg daridorexant: RR = 1.15, 95% CI: [0.95, 1.39], P = .14; 10 mg daridorexant: RR = 1.20, 95% CI: [0.99, 1.46], P = .06; 5 mg daridorexant: RR = 1.27, 95% CI: [0.82, 1.97], P = .28; 25 mg daridorexant: RR = 1.17, 95% CI: [1.01, 1.34], P = .03, Fig. 3).
Figure 3.
The pooled RR of adverse events (the number of participants with ≥1 adverse event). CI = confidence interval, RR = relative risk.
3.3. Subgroup analysis
We performed subgroup analyses of elderly participants (≥65 years-of-age) and other adults (<65 years-of-age) to compare safety of treatment with 25 mg of daridorexant compared to placebo. No significant differences were observed (see Figure S3, Supplemental Digital Content, http://links.lww.com/MD/I362, which demonstrates the Subgroup of safety analysis of age ≥ 65 years and age ≤ 64 years), which indicated that daridorexant was well-tolerated and safe at all doses.
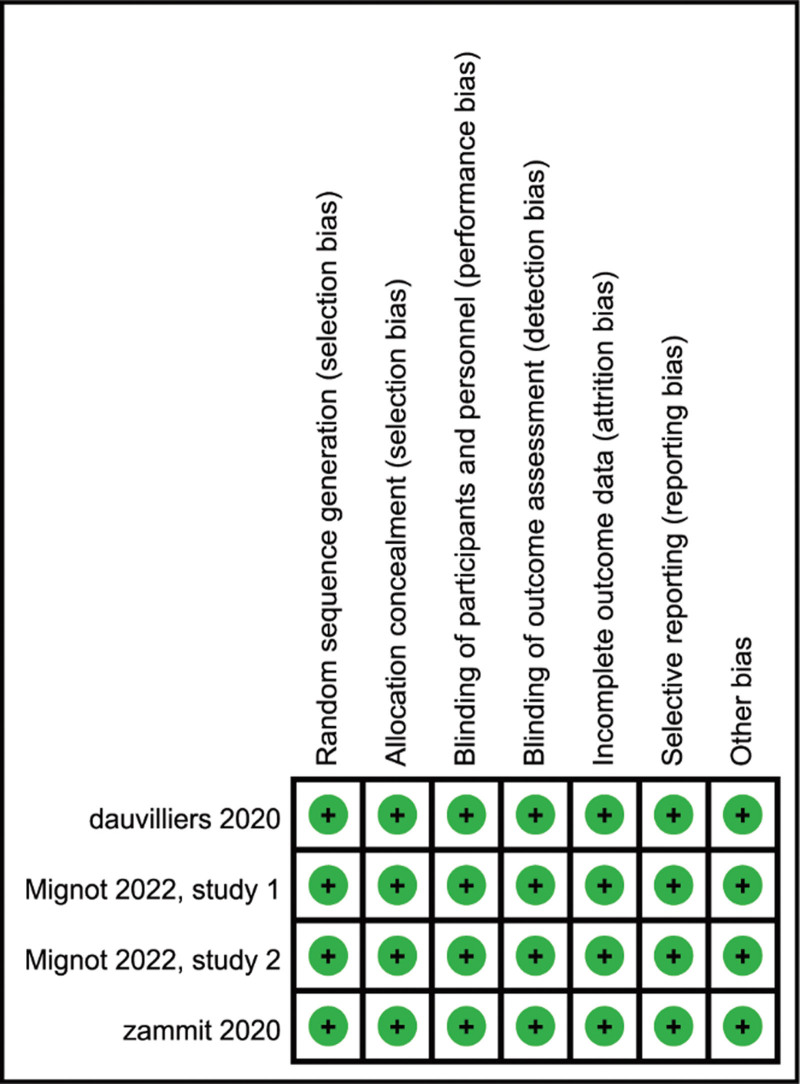
3.4. Risk of bias in the included studies
Details of the risk bias for all studies are shown in Figure 4. All risks for bias were low in each of the 4 RCTs analyzed.
Figure 4.
Risk of bias: a summary table for each risk of bias item for each study.
4. Discussion
We performed a meta-analysis of 4 RCTs (one included article has 2 RCTs) that included 2271 patients. A key strength of these 4 studies was that they included assessments of most components of insomnia, as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria. The results of our study can be used as a reference to aid in decisions regarding drug selection and dosing. Five and 10 mg are not available for treatment due to poor efficacy. The recommended initial dose of daridorexant is 50 mg/d. The dose of 50 mg, in particular, may not only provide better nighttime sleep for patients with chronic insomnia, including the time and duration of sleep onset, but may also improve daytime function. If intolerable, the dose of 25 mg may be considered at first.
Other DORAs besides daridorexant have shown the ability to improve sleep quality. Suvorexant, almorexant, filorexant, and lemborexant were well-tolerated by patients in RCTs.[14–17] Interest in DORAs has increased considerably, and the US Food and Drug Administration recently approved suvorexant and lemborexant for treatment of insomnia.[18] Almorexant development has been discontinued due to its side effect profile.[19] Daridorexant was approved by the Food and Drug Administration in 2022 for treatment of insomnia. Studies have shown that DORAs target excessive wakefulness in insomnia and improve sleep variables without the side effects of GABA receptor agonists.[20,21] Therefore, daridorexant improves sleep by inhibiting orexin rather than by inducing global sedation. The benefits of daridorexant include fast absorption, optimal half-life (8 hours), no bio-accumulation over time, and no active metabolites.[22,23] Furthermore, daridorexant did not induce any AEs that suggested drug misuse might have occurred, as evidenced by lack of withdrawal symptoms or rebound insomnia.[12] In contrast, suvorexant and lemborexant include warnings about dependency.[24,25] A recent study (NCT03657355) demonstrated that daridorexant at the highest phase-3 dose of 50 mg showed less dose-related drug-liking compared to supratherapeutic doses of suvorexant and zolpidem.[26] Daridorexant was well-tolerated and induced dose-dependent improvements in sleep metrics, which were statistically significant for WASO and LPS at doses of ≥10 mg. Based on our analyses, 25 and 50 mg doses of daridorexant are recommended based on safety and efficiency from a statistical perspective.
Our study had many strengths. First, all studies included in this meta-analysis were high-quality and low-risk studies based on Cochrane risks bias assessment. Secondly, our study was the first meta-analysis of clinical use of daridorexant for treatment of insomnia disorder. Meta-analysis is a highly efficient research strategy that uses predefined steps to perform quantitative analyses to synthesize the results of multiple studies.[27] In addition, we evaluated the efficacy and safety of daridorexant for short-term and long-term treatment of insomnia. Finally, we investigated the clinical relevance of treatment with daridorexant.
Our study suffered from the following limitations. The number of RCTs included was small, which may limit the generalizability of the findings. In addition, the variation in study designs and inclusion/exclusion criteria may have contributed to heterogeneity. For instance, the insufficient information about the effect of daridorexant on Asian population. Furthermore, evaluation of AEs was a challenge. Finally, we only evaluated efficacy and safety of daridorexant at month 1, month 3, and at days 1 and 2. Therefore, further studies are needed to characterize the long-term safety and efficacy of daridorexant.
5. Conclusion
In conclusion, daridorexant was an effective and safe treatment for insomnia disorder when compared to placebo. More studies on daridorexant are needed to further develop therapeutic strategies for insomnia disorder using daridorexant.
Author contributions
Formal analysis: Hang Li.
Methodology: Hang Li, Haifeng Lu.
Supervision: Jianqiang Ni, Gang Chen.
Validation: Yanting Chen.
Writing – original draft: Feiyu Jiang.
Supplementary Material
Abbreviations:
- AEs
- adverse events
- CI
- confidence interval
- DORAs
- dual orexin receptor antagonists
- IDSIQ
- Insomnia Daytime Symptoms and Impacts Questionnaire
- LPS
- latency to persistent sleep
- RCTs
- randomized controlled trials
- RR
- risk ratio
- SMD
- standard mean difference
- sTST
- subjective total sleep time
- WASO
- wake time after sleep onset
This work was supported by the Suzhou Science and Technology Program Project (SKJY2021059 and KJS2031).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
Supplemental Digital Content is available for this article.
How to cite this article: Jiang F, Li H, Chen Y, Lu H, Ni J, Chen G. Daridorexant for the treatment of insomnia disorder: A systematic review and meta-analysis of randomized controlled trials. Medicine 2023;102:7(e32754).
Contributor Information
Feiyu Jiang, Email: 1297419371@qq.com.
Hang Li, Email: neurosurgeryli@163.com.
Yanting Chen, Email: nju_neurosurgery@163.com.
Haifeng Lu, Email: lu.haifeng110@163.com.
Gang Chen, Email: nju_neurosurgery@163.com.
References
- [1].Sutton EL. Insomnia. Ann Intern Med. 2021;174:ITC33–48. [DOI] [PubMed] [Google Scholar]
- [2].Oh DY, Park SM, Choi SW. Daytime neurophysiological hyperarousal in chronic insomnia: a study of qEEG. J Clin Med. 2020;9:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liljenberg B, Almqvist M, Hetta J, et al. The prevalence of insomnia: the importance of operationally defined criteria. Ann Clin Res. 1988;20:393–8. [PubMed] [Google Scholar]
- [4].Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;22(Suppl 2):S347–53. [PubMed] [Google Scholar]
- [5].Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14:1017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Daley M, Morin CM, LeBlanc M, et al. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. 2009;32:55–64. [PMC free article] [PubMed] [Google Scholar]
- [7].Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- [8].Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jacobson LH, Hoyer D, de Lecea L. Hypocretins (orexins): the ultimate translational neuropeptides. J Intern Med. 2022;291:533–56. [DOI] [PubMed] [Google Scholar]
- [10].Xue T, Wu X, Chen S, et al. The efficacy and safety of dual orexin receptor antagonists in primary insomnia: a systematic review and network meta-analysis. Sleep Med Rev. 2022;61:101573. [DOI] [PubMed] [Google Scholar]
- [11].Treiber A, de Kanter R, Roch C, et al. The use of physiology-based pharmacokinetic and pharmacodynamic modeling in the discovery of the dual orexin receptor antagonist ACT-541468. J Pharmacol Exp Ther. 2017;362:489–503. [DOI] [PubMed] [Google Scholar]
- [12].Mignot E, Mayleben D, Fietze I, et al.; investigators. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol. 2022;21:125–39. [DOI] [PubMed] [Google Scholar]
- [13].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- [14].Black J, Pillar G, Hedner J, et al. Efficacy and safety of almorexant in adult chronic insomnia: a randomized placebo-controlled trial with an active reference. Sleep Med. 2017;36:86–94. [DOI] [PubMed] [Google Scholar]
- [15].Connor KM, Mahoney E, Jackson S, et al. A phase II dose-ranging study evaluating the efficacy and safety of the orexin receptor antagonist filorexant (MK-6096) in patients with primary insomnia. Int J Neuropsychopharmacol. 2016;19:pyw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13:461–71. [DOI] [PubMed] [Google Scholar]
- [17].Murphy P, Moline M, Mayleben D, et al. Lemborexant, A Dual Orexin Receptor Antagonist (DORA) for the treatment of insomnia disorder: results from a Bayesian, adaptive, randomized, double-blind, placebo-controlled study. J Clin Sleep Med. 2017;13:1289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Roch C, Bergamini G, Steiner MA, et al. Nonclinical pharmacology of daridorexant: a new dual orexin receptor antagonist for the treatment of insomnia. Psychopharmacology (Berl). 2021;238:2693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Richey SM, Krystal AD. Pharmacological advances in the treatment of insomnia. Curr Pharm Des. 2011;17:1471–5. [DOI] [PubMed] [Google Scholar]
- [20].Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2: e1918254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Herring WJ, Connor KM, Ivgy-May N, et al. Suvorexant in patients with insomnia: results from two 3-month randomized controlled clinical trials. Biol Psychiatry. 2016;79:136–48. [DOI] [PubMed] [Google Scholar]
- [22].Muehlan C, Brooks S, Zuiker R, et al. Multiple-dose clinical pharmacology of ACT-541468, a novel dual orexin receptor antagonist, following repeated-dose morning and evening administration. Eur Neuropsychopharmacol. 2019;29:847–57. [DOI] [PubMed] [Google Scholar]
- [23].Muehlan C, Heuberger J, Juif PE, et al. Accelerated development of the dual orexin receptor antagonist ACT-541468: integration of a microtracer in a first-in-human study. Clin Pharmacol Ther. 2018;104:1022–9. [DOI] [PubMed] [Google Scholar]
- [24].Eisai Inc. DAYVIGOTM (lemborexant) tablets, for oral use, revised: 12/2019. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212028s000lbl.pdf.
- [25].Merck Sharp & Dohme Corp. BELSOMRA® (suvorexant) tablets, for oral use, revised: 08/2014. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204569s000lbledt.pdf.
- [26].Ufer M, Kelsh D, Schoedel KA, et al. Abuse potential assessment of the new dual orexin receptor antagonist daridorexant in recreational sedative drug users as compared to suvorexant and zolpidem. Sleep. 2022;45:zsab224. [DOI] [PubMed] [Google Scholar]
- [27].Hernandez AV, Marti KM, Roman YM. Meta-analysis. Chest. 2020;158(1S):S97–102. [DOI] [PubMed] [Google Scholar]