Background:
Patients undergoing lumbar spine surgery usually suffer from moderate to severe acute pain. Erector spinae plane block (ESPB) has been applied to relieve acute pain in various surgeries and improve postoperative outcomes. This study aimed to further identify the efficacy and safety of erector spinae plane block in patients undergoing lumbar spine surgery. This study also evaluates the outcomes of the erector spinae plane block compared with other regional blocks.
Methods:
We searched PubMed, Web of Science, Cochrane library, Embase, and CINAHL databases to identify all randomized controlled trials evaluating the effects of ESPB on postoperative pain after lumbar spine surgery. The primary outcome is postoperative total opioid consumption in 24 hours. The secondary outcomes are postoperative pain scores, intraoperative opioid consumption, time to first rescue analgesia, number of patients requiring rescue analgesia, first time to ambulation after surgery, length of hospital stay, patients’ satisfaction score, and postoperative side effects such as postoperative nausea and vomiting, itching.
Results:
A total of 19 randomized controlled trials are included in the final analysis. Compared with no/sham block, ultrasound-guided erector spinae plane block can decrease perioperative opioid consumption including intraoperative opioid consumption: standardized mean difference (SMD) = −3.04, 95% confidence interval (CI) (−3.99, −2.09), P < .01, and opioid consumption postoperatively: (SMD = −2.80, 95% CI [−3.61, −2.00], P < .01); reduce postoperative pain at 2, 6, 12, 24, and 48 hours both at rest and movement; meanwhile shorten time to hospital length of stay: (SMD = −1.01, 95% CI [−1.72, 0.30], P = .006), decrease postoperative nausea and vomiting (RR = 0.35, 95% CI [0.27, 0.46], P < .00001), and improve patient satisfaction (SMD = −2.03, 95% CI [−0.96, 3.11], P = .0002). But ultrasound-guided ESPB doesn’t shorten the time to ambulation after surgery (SMD = −0.56, 95% CI [−1.21, 0.08], P = .09). Additionally, ESPB is not superior to other regional blocks (e.g., thoracolumbar interfascial plane/midtransverse process to pleura block).
Conclusion:
This meta-analysis demonstrates that ultrasound-guided ESPB can provide effective postoperative analgesia in patients undergoing lumbar spine surgery and improve postoperative outcomes, and it deserves to be recommended as an analgesic adjunct in patients undergoing lumbar spine surgeries.
Keywords: erector spinae plane block, lumbar spine surgery, meta-analysis, postoperative analgesia, short-term outcome, ultrasound-guided
1. Introduction
More and more lumbar spine surgeries have been performed in the past few decades. Postoperative moderate to severe pain is frequently encountered in patients following lumbar spine surgery and the acute pain after surgery was often controlled poorly.[1–3] Postoperative pain is associated with delayed postoperative ambulation, venous thrombosis, and an increased incidence of pulmonary and cardiac complications, which may delay postoperative recovery, and even prolong the length of hospital stays.[4] In addition, inadequate acute pain treatment may lead to postoperative persistent pain.[5] Therefore, it is imperative to effectively manage postsurgical pain following lumbar spine surgery. Patient-controlled intravenous analgesia (PCIA) with opioid analgesics is the most common analgesic method for kinds of surgeries and can provide excellent pain relief. However, high-dose opioids are limited by side effects like nausea, vomiting, respiratory inhibiting, pruritus, addiction, and other opioid-related side effects.[6] Furthermore, the opioid crisis has been a public health challenge.[7]
Multimodal analgesia has been the recognized mode for postoperative analgesia and is the direction of future research. Ultrasound-guided fascial plane blocks have as become an essential part of multimodal analgesia because of the simplicity of operation and fewer complications.[8] The erector spinae plane block (ESPB) was first used in chronic thoracic neuropathic pain by Forero in 2016.[9] Over the past several years, it was widely used in different types of surgeries, such as breast surgery,[10] thoracic surgery,[11] and laparoscopic cholecystectomy,[12] and achieved good analgesic effectiveness. Recently, several systematic reviews and meta-analyses reported that ESPB can provide effective analgesia in lumbar surgery. But most of the studies included a small number of randomized controlled trials (RCTs) and all of them did not compare ESPB with other types of blocks. For example, Liu’s meta-analysis revealed that ESPB was effective and safe for postoperative analgesia, but only six RCTs were included and it did not pay attention to some important outcomes, such as pain score at rest and length of hospital stay.[13] Additionally, Oh’s review incorporated some abstracts and letters without sufficient RCTs.[14] This study, therefore, aimed to further clarify the efficacy and safety of ESPB on postoperative analgesia outcomes in patients undergoing lumbar spine surgery. It also evaluated the analgesic effect of erector spinae plane block compared with other regional block techniques.
2. Methods and Materials
The present study is a systematic review and meta-analysis of previously published RCTs, so ethical approval was not required. This study was conducted and reported in line with preferred reporting items for systematic review and meta-analysis guidelines.[15] PROSPERO registration number: CRD42021284430.
2.1. Search strategy and study selection
The following databases were searched (from inception to March 2022): PubMed, Web of Science, Cochrane library, Embase, and CINAHL Keywords including “lumbar spine surgery,” “decompression,” “spondylolisthesis,” “lumbar spinal stenosis,” “ESP block,” “ESPB,” “erector spinae plane block” and “erector spinae block” were used with no limits on language. To prevent missing any relative research, references to relevant articles or reviews and meta-analyses were also screened and checked. We included published full-text randomized controlled trials involving adults undergoing lumbar spine operations following ESPB with no block, sham block, or other regional blocks. Case reports, ongoing trials, articles without completed results, abstracts, letters, comments, or editorials were not considered for inclusion. Non-ultrasound-guided regional blocks were also excluded.
2.2. Data extraction
Firstly, two authors browsed the title and abstract to exclude irrelevant literature. Then we further reviewed the full text of the literature that initially met the inclusion criteria. Any inconsistencies were discussed with a third author. Two reviewers extracted separately the data using a data collection table. The following items were recorded: the first author, publication year, age, sample size, surgery description, the use of local anesthetics, time, methods of postoperative analgesia, and related outcomes. The primary outcome is postoperative opioid consumption in the first 24 hours. Secondary outcomes include pain score at rest and movement at 2, 6, 12, 24, and 48 hours after surgery, time to first rescue analgesia, the number of patients who requires rescue analgesia, time to ambulation after surgery, length of hospital stay, satisfaction score, opioid-related adverse effects such as incidence of postoperative nausea and vomiting (PONV), itching, etc, and complications associated with blocks.
2.3. Quality assessment
Two authors independently assessed the risk of bias in each reviewed study. Any disagreements were resolved by discussion with a third author. The Cochrane Collaboration risk of bias assessment was used to assess the quality of each study.[16] The risk of bias was classified as low, unclear, or high risk. We evaluated the quality of evidence of primary outcome using the Grading of Recommendations, Assessment, Development, and Evaluation guidelines.[17]
2.4. Statistical analysis
Statistical analysis was conducted using Review Manager (RevMan 5.3). For continuous data, standardized mean differences (SMD) with a 95% confidence interval (CI) were calculated. The dichotomous data, Risk ratios (RR) with 95% CI were calculated. If the data were shown as median, minimum to maximum or inter-quartile ranges, we converted them to mean and standard deviation.[18,19] When data were presented as a graph, we extracted the information with digitizing software (GetData Graph Digitizer 2.26). We tried to contact the corresponding author to obtain the raw data if data could not be found in a study. Random-effects modeling was used to pool data. When a P value < .05, it was considered statistically significant. We used I2 statistics to check the heterogeneity of each study and I2 > 50% meant significant heterogeneity. We performed sensitivity analysis and subgroup analysis to evaluate the stability of the results and explored the possible sources of heterogeneity. The Egger test by Stata 12.0 was used to assess potential publication bias.
3. Results
3.1. Literature search, study characteristics, and quality assessment
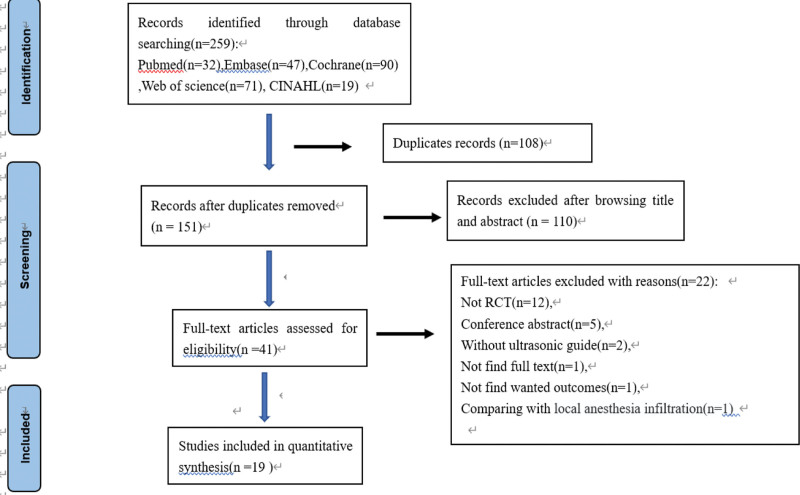
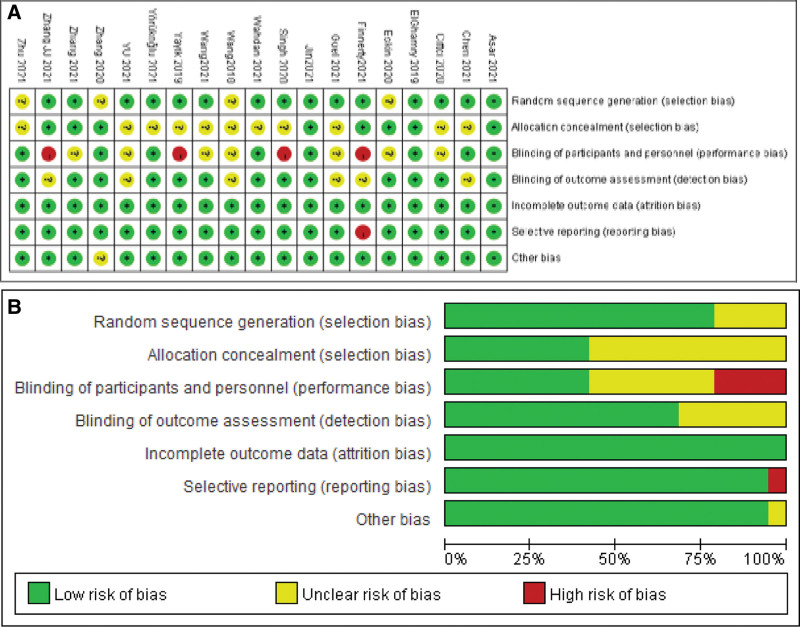
The initial search identified 259 studies. After removing 108 duplicated studies, we excluded irrelevant studies by browsing titles and abstracts. Subsequently, 41 full-text articles were further acquired and evaluated. Then 20 randomized controlled trials (RCTs) were assessed for eligibility and one article was excluded because of a comparison with local anesthetic infiltration.[20] Finally, 19 articles were included in this systematic review and meta-analysis.[21–39] The detailed screening process and selection results are shown in Figure 1. The characteristics of the included 19 RCTs enrolling in 1561 patients are shown in Table 1. Nineteen trials compared ESPB with no block or sham block, among two trials compared ESPB both to no block and thoracolumbar interfascial plane (TLIP) block,[26,29] and one trial[22] compared ESPB both to no block and midtransverse process to pleura (MTP) block. We could not get access directly to the primary data in the study of Eskin et al,[22] so we got in touch with the authors to get the relevant data. ESPB is performed by ultrasound guidance with a single injection of local anesthetics in all studies. Bupivacaine or levobupivacaine were administered in ten studies,[21–24,29–33,35] ropivacaine was administered in eight studies,[25–28,34,37–39] and the mixture of bupivacaine and lidocaine solution was administered in one study.[36] The risk of bias for each included study is summarized in Figure 2. Fifteen studies reported specific randomization methods and allocation concealment is adequate in only eight studies. The risk of performance bias was classified as high in four RCTs and most studies were judged to be at low risk for detection bias, selective reporting, and other biases. Quality of evidence for the opioid consumption in the first 24 hours after surgery was downgraded to low due to the high heterogeneity and risk of bias. Egger test by Stata 12.0 was performed to evaluate publication bias and revealed there was no obvious publication bias (P = .186 > .05).
Figure 1.
Flow diagram of study searching and selection process. RCT = randomized controlled trial.
Table 1.
Characteristics of the included studies.
| Study and year | Age (yr) | Group and number | Procedures | Treatments | Time | Perioperative analgesia | Postoperative analgesia | Rescue analgesia |
|---|---|---|---|---|---|---|---|---|
| 1. Ciftci 2020[29] | 18–65 | ESPB/mTLIP/control (30/30/30) | Single-level lumbar discectomy and hemilaminectomy surgery | Ultrasound-guided ESPB group: 20 mL of 0.25% bupivacaine (each side) Ultrasound-guided mTLIP group: 20 mL of 0.25% bupivacaine (each side) Control group: no block. |
After the induction of anesthesia | Remifentanl | Paracetamol + PCIA (fentanyl) | Meperidine iv (VAS ≥ 4) |
| 2. Eskin 2020[22] | 18–80 | ESPB/MTP/control (40/40/40) | Elective lumbar decompression surgery | Ultrasound-guided ESPB group: 20 mL of 0.25% bupivacaine (each side) MTP block: 20 mL of 0.25% bupivacaine (each side) Control group: no block |
After the induction of anesthesia | Remifentanil | Paracetamol + dexketoprofen + PCIA (Tramadol) | Pethidine iv (VAS > 3) |
| 3. Zhang 2020[25] | 18–80 | ESPB/control (30/30) | Open posterior lumbar decompression surgery | Ultrasound-guided ESPB group: 25 mL of 0.3% ropivacaine (each side) Control group: no block |
Before the induction of anesthesia | Sufentanil | PCIA (Morphine) | |
| 4. Yayik 2019[24] | 18–65 | ESPB/control (30/30) | Open lumbar decompression surgery | Ultrasound-guided ESPB group: 20 mL of 0.25% bupivacaine (each side) Control group: no block |
Before the induction of anesthesia | Remifentanil | PCIA (Tramadol) | Pethidine (VAS ≥ 4) |
| 5. Singh 2020[23] | 18–65 | ESPB/control (20/20) | Prolapsed lumbar intervertebral disk, lumbar stenosis or laminectomy | Ultrasound-guided ESPB group: 20 mL of 0.5% bupivacaine (each side) Control group: no block |
Before the induction of anesthesia | Fentanyl | Intravenous diclofenac | Morphine (NRS ≥ 4). |
| 6. Ghamry 2019[21] | 18–60 | ESPB/control (30/30) | Elective PLIF | Ultrasound-guided ESPB group: 20 mL of 0.25% bupivacaine (each side) Control group: 20 mL normal saline (each side) |
Before the induction of anesthesia | Fentanyl | Intravenous paracetamol + ketorolac | Morphine iv (VAS > 30) |
| 7. Zhang 2021[34] | 20–75 | ESPB/control (30/30) | Open posterior lumbar spinal fusion surgery | Ultrasound-guided ESPB group: 20 mL 0.4% ropivacaine (each side) Control group: Sham blocks (subcutaneous infiltration: 1% lidocaine 1 mL on each side) |
Before the induction of anesthesia | Remifentanil | PCIA (sufentanil) + flurbiprofen | Intravenous sufentanil bolus via PCA (NRS ≥ 4) |
| 8. Wahdan 2021[31] | 18–65 | ESPB/control (70/70) | Elective lumbar spine surgeries. | Ultrasound-guided ESPB group: 20 mL of 0.25% levobupivacaine (each side) Control group: 20 mL normal saline (each side) |
After the induction of anesthesia | Morphine sulfate | Ketorolac + PCIA (morphine) | Morphine iv (VAS ≥ 4) |
| 9. Finnerty 2021[30] | >18 | ESPB/control (30/30) | Open thoracolumbar vertebral decompression for degenerative stenosis or trauma | Ultrasound-guided ESPB group: 20 mL of 0.25% levobupivacaine (each side) Control group: no block |
After the induction of anesthesia | Oxycodone | Paracetamol + ibuprofen + oxycodone | Intravenous aliquots of oxycodone (VRS > 2) |
| 10. YU 2021[32] | 26–67 | ESPB/control (40/40) | Posterior internal fixation for single-level lumbar fracture | Ultrasound-guided ESPB group: 30 mL of 0.25% bupivacaine (each side) Control group: normal saline30 mL (each side) |
After the induction of anesthesia | Remifentanil | PCIA (sufentanil + flurbiprofen) | Pethidine hydrochloride im (NRS > 4) |
| 11. Yörükoğlu 2021[33] | 18–65 | ESPB/control (28/26) | Elective single-level lumbar microdiscectomy | Ultrasound-guided ESPB group: at the surgical level, 20 mL of 0.25% bupivacaine (each side) Control group: sham block (normal saline 20 mL each side) |
Before the induction of anesthesia | No describe | PCIA (morphine) | Tenoxicam 20 mg iv (NRS > 3) |
| 12. Zhu 2021[27] | 45–70 | ESPB/control (20/20) | Posterior lumbar fusion surgery | Ultrasound-guided ESPB group: L2 level, 20 mL of 0.375% ropivacaine (each side) Control group: sham block (normal saline 20 mL each side) |
Before the induction of anesthesia | Remifentanil | PCIA (oxycodone) | 5 μg sufentanil iv (VAS ≥ 4) |
| 13. Zhang JJ 2021[28] | 18–80 | ESPB/control (30/29) | Lumbar spine surgery | Ultrasound-guided ESPB group: 25 mL of 0.3% ropivacaine (each side) Control: no block. |
Before the induction of anesthesia | Sufentanil | PCIA (morphine) | |
| 14. Wang 2021[26] | / | ESPB/TLIP/control (102/102/102) | Lumbar spine fusion surgery | Ultrasound-guided ESPB group: 30 mL of 0.375% ropivacaine (each side) Ultrasound-guided TLIP group: 30 mL of 0.375% ropivacaine (each side) Control group: no block |
After the induction of anesthesia | Remifentanil | PCIA (sufentanil + flurbiprofen) | Sufentanil 5 µg iv (NRS > 5) |
| 15. Wang 2018[39] | 18-70 | ESPB/control (30/30) | Posterior lumbar surgery | Ultrasound-guided ESPB group: 15 mL of 0.5% ropivacaine (each side) Control group: no block |
Before the induction of anesthesia | Remifentanil | PCIA (sufentanil) | Parecoxib sodium 40 mg im (VAS > 4) |
| 16. Asar 2021[36] | 18–75 | ESPB/control (35/35) | Spinal surgery with instrumentation involving single or multi levels in the lumbar or thoracic regions | Ultrasound-guided ESPB group: 20 mL volume consisting of 10 mL of 0.5% bupivacaine, 5 mL of 2% lidocaine, and 5 mL of 0.9% NaCl Control group: no block |
At the end of surgery | Remifentanil | PCIA (tramadol) + paracetamol | 75 mg diclofenac sodiumim (VAS > 4) |
| 17. Chen 2021[38] | 30–65 | ESPB/control (20/20) | Posterior lumbar interbody fusion | Ultrasound-guided ESPB group: L2 level, 20 mL of 0.375% ropivacaine (each side) Control group: sham block (NS 20 mL each side) |
Before the induction of anesthesia | Remifentanil | PCIA (oxycodone) | Sufentanil 5 µg iv (VAS > 4) |
| 18. Goel 2021[35] | 18–78 | ESPB/control (50/50) | Single-level transforaminal lumbar inter-body fusion surgery | Ultrasound-guided ESPB group: 20 mL of 0.25% bupivacaine (each side) Control group: no block |
After the induction of anesthesia | Fentanyl | Multimodal analgesia: Paracetamol + ketorolac + pregabalin capsule | Fentanyl (NRS > 5) |
| 19. Jin 2021[37] | / | ESPB/control (30/32) | Lumbar laminoplasty | Ultrasound-guided ESPB group: 20 mL of 0.375% ropivacaine (each side) Control group: no block |
After the induction of anesthesia | Sufentanil/remifentanil | PCIA (sufentanil, dezocine, dex-medetomidine and palonosetron) | Sodium parecoxib and intramuscular pethidine |
ESPB = erector spinae plane block, MTP = midtransverse process to pleura, NRS = numerical rating scale, PCIA = patient-controlled intravenous analgesia, TLIP = thoracolumbar interfascial plane, VAS = visual analogue scale, VRS = Verbal Rating Scale.
Figure 2.
(A) Risk of bias summary: review authors’ judgements about each risk of bias item for each included study; (B) Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
3.2. Primary outcome
For the primary outcome, total opioid consumption in the first 24 hours after surgery was reported in 18 trials that included 1325 patients.
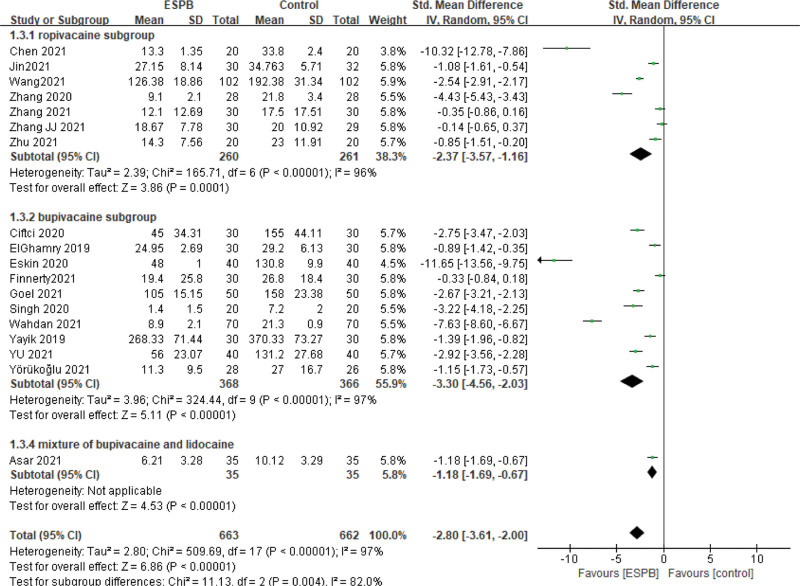
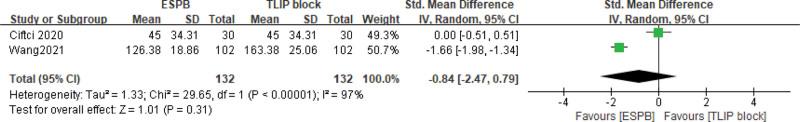
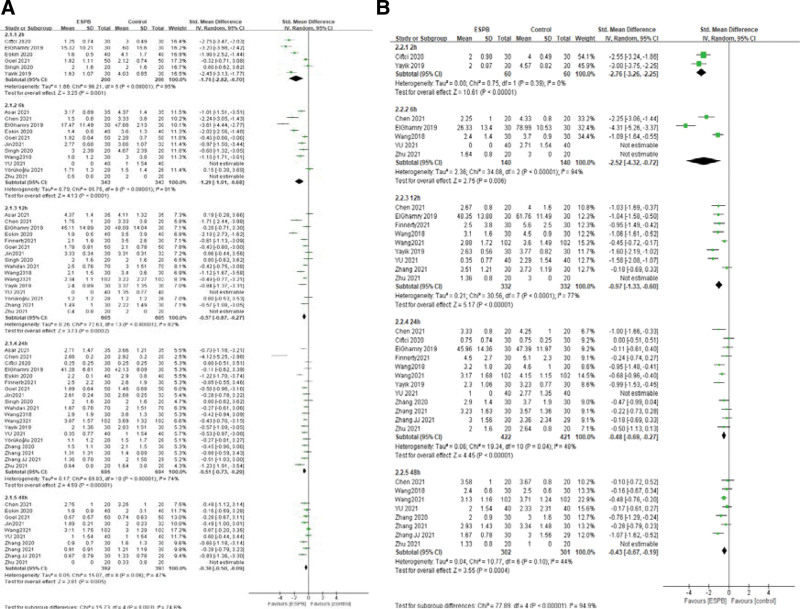
Patients receiving ESPB had significantly decreased postoperative opioid consumption in 24 hours than patients who received no or sham block (SMD = −2.80, 95% CI [−3.61, −2.00], I2 = 97%, P < .00001; Fig. 3). Subgroup analysis was performed according to the type of local anesthetics. In the bupivacaine subgroup, ten studies and 734 participants were analyzed (SMD = −3.30, 95% CI [−4.56, −2.03], I2 = 97%, P < .00001), and in the ropivacaine subgroup, seven studies and 521 patients were included in the analysis (SMD = −2.37, 95% CI = [−3.57, −1.16], I2 = 96%, P < .0001). One study[36] used a mixture of bupivacaine and lidocaine solution and showed opioid consumption was lower in the ESPB group (6.21 ± 3.28) compared to the control group (10.12 ± 3.29) (P = .000). According to our sensitivity analysis, there was no change between the primary and sensitivity results after removing one study in turn. So the results were relatively robust and are presented in Table 2. Two studies of ESPB versus TLIP block including 264 patients reported there was no difference in postoperative opioid consumption in 24 hours between the two groups (SMD = −0.84, 95% CI [−2.47, −0.79], I2 = 97%, P = .31; Fig. 4). One study compared ESPB with MTP block and found ESPB can reduce opioid consumption in 24 hours after surgery compared with MTP group (48.0 ± 1.0 vs 84.9 ± 4.0 mg).
Figure 3.
Forest plot for the comparison of postoperative opioid consumption in 24 hours in study groups receiving ESPB compared with no/ sham block. CI = confidence interval, ESPB = erector spinae plane block, SD = standard deviation.
Table 2.
Sensitivity analysis of the primary outcome.
| Removed study | SMD | 95% CI lower limit | 95% CI upper limit | z value | P value |
|---|---|---|---|---|---|
| Asar 2021[36] | −2.92 | −3.78 | −2.06 | 6.68 | <.00001 |
| Chen 2021[38] | −2.49 | −3.28 | −1.71 | 6.26 | <.00001 |
| Ciftci 2020[29] | −2.81 | −3.65 | −1.98 | 6.59 | <.00001 |
| Ghamry 2019[21] | −2.94 | −3.79 | −2.09 | 6.77 | <.00001 |
| ESkin 2020[22] | −2.36 | −3.09 | −1.62 | 6.29 | <.00001 |
| Finnerty 2021[30] | −2.96 | −3.80 | −2.13 | 6.95 | <.00001 |
| Goel 2021[35] | −2.82 | −3.67 | −1.98 | 6.54 | <.00001 |
| Jin 2021[37] | −2.93 | −3.78 | −2.07 | 6.72 | <.00001 |
| Singh 2020[23] | −2.78 | −3.61 | −1.96 | 6.59 | <.00001 |
| Wahdan 2021[31] | −2.45 | −3.16 | −1.74 | 6.79 | <.00001 |
| Wang 2021[26] | −2.84 | −3.72 | −1.97 | 6.40 | <.00001 |
| Yayik 2019[24] | −2.91 | −3.76 | −2.05 | 6.67 | <.00001 |
| YU 2021[32] | −2.80 | −3.64 | −1.97 | 6.57 | <.00001 |
| Yörükoğlu 2021[33] | −2.92 | −3.77 | −2.07 | 6.73 | <.00001 |
| Zhang 2020[25] | −2.70 | −3.51 | −1.89 | 6.53 | <.00001 |
| Zhang 2021[34] | −2.96 | −3.80 | −2.13 | 6.94 | <.00001 |
| Zhang JJ 2021[28] | −2.97 | −3.80 | −2.14 | 7.03 | <.00001 |
| Zhu 2021[27] | −2.93 | −3.77 | −2.09 | 6.83 | <.00001 |
CI = confidence interval, SMD = standardized mean differences.
Figure 4.
Forest plot of total opioid consumption in first 24 hours after surgery in study groups receiving ESPB compared with TLIP block. CI = confidence interval, ESPB = erector spinae plane block, SD = standard deviation, TLIP = thoracolumbar interfascial plane.
3.3. Secondary outcome: pain score
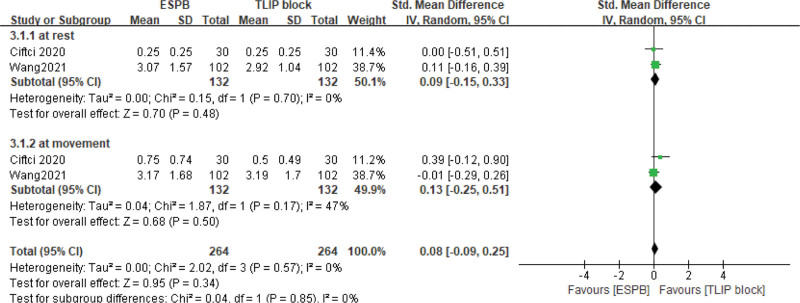
We analyzed pain scores at rest and movement at five-time points in the postoperative period: 2, 6, 12, 24, and 48 hours. Different pain assessment tools were used in eligible studies. Visual analogue scale (VAS, 0–10 scale) was applied to assessed pain score in eight studies,[22,24,27,29,31,37–39] one study used 0–100 VAS,[21] nine studies used 0–10 numerical rating scale,[23,25,26,28,32–34] and one study used a 0–10 Verbal Rating Scale.[30] Postoperative pain scores at rest at 2, 6, 12, 24, and 48 hours was reported in six, eleven, sixteen, nineteen, and ten articles, respectively. ESPB significantly reduced postoperative pain intensity at rest compared with no/sham block: at 2 hours: (SMD = −1.76, 95% CI = [−2.82, −0.70], I2 = 95%, P = .001), 6 hours: (SMD = −1.29, 95% CI [−1.91, −0.68], I2 = 91%, P < .0001), 12 hours: (SMD = −0.57, 95% CI [−0.87, −0.27], I2 = 82%, P = .0002), 24 hours: (SMD = −0.51, 95% CI [−0.73, −0.29], I2 = 74%, P < .00001), 48 hours: (SMD = −0.30, 95% CI [−0.50, −0.09], I2 = 47%, P = .005; Fig. 5A). Postoperative pain scores at movement at 2, 6, 12, 24, and 48 hours were reported in two, five, nine, twelve, and eight articles, respectively. The polled results show that patients with ESPB had lower postoperative pain scores at movement compared with no/sham block: at 2 hours: (SMD = −2.76, 95% CI [−3.26, −2.25], I2 = 0%, P < .00001);6 hours: (SMD = −2.52, 95% CI [−4.32, −0.72], I2 = 94%, P = .006); 12 hours: (SMD = −0.97, 95% CI[−1.33, −0.6], I2 = 77%, P < .00001); 24 hours: (SMD = −0.48, 95% CI [−0.69, −0.27], I2 = 48%, P < .00001); 48 hours: (SMD = −0.43, 95% CI [−0.67, −0.19], I2 = 44%, P = .0004; Fig. 5B). We also analyzed the pain score of ESPB versus TLIP block. The results show pain score at 24 hours postoperatively had no significant difference whether at rest or movement (SMD = 0.08, 95% CI [−0.09, 0.25], I2 = 0%, P = .34, Fig. 6). We did not get the pooled results of other time points due to the lack of enough studies. Ciftci et al[29] reported pain score at 2 hours at rest postoperatively was lower in ESPB group, but it was not different at movement. No study reported pain score at 6 hours postoperatively. Wang et al[26] evaluated pain score at 12 and 48 hours postoperatively, and the scores in the rest of Group ESPB was lower than those in Group TLIP block. However, there was no difference in movement between the two groups. Eskin et al[22] reported VAS scores were lower in group ESPB than group MTP block at postoperative 2, 6, 8, and 12 hours, but there was no difference at postoperative 24, 48 hours.
Figure 5.
A–B: Forest plot of postoperative pain scores at rest and movement in study groups receiving ESPB compared with no/sham block. (A) Postoperative pain scores at rest in the first 48 hours. (B) Postoperative pain scores at movement in the first 48 hours. CI = confidence interval, ESPB = erector spinae plane block, SD = standard deviation.
Figure 6.
Forest plot of pain score at 24 hours in study groups receiving ESPB compared with TLIP block. CI = confidence interval, ESPB = erector spinae plane block, SD = standard deviation, TLIP = thoracolumbar interfascial plane.
3.4. Other outcomes
Other outcomes of the identified trials are reported in Table 3. Intraoperative opioid consumption was reported in twelve studies that included 452 patients in ESPB groups and 453 patients in the control (no/sham block) groups. Patients undergoing ESPB had lower intraoperative opioid requirements than patients with no/sham block (SMD = −3.04, 95% CI [−3.99, −2.09], I2 = 96%, P < .00001). Two articles reported intraoperative opioid consumption for ESPB versus TLIP block. The results showed no statistical differences between the two groups at intraoperative opioid consumption (SMD = −0.03, 95% CI [−0.21, 0.27], I2 = 0%, P = .79).
Table 3.
Other outcomes data of RCTs included in the meta-analysis.
| Outcomes (ESPB vs no/sham block) | Studies included | RR or Std. mean difference [95% CI] | P value for statistical significance | P value for heterogeneity | I2 test for heterogeneity |
|---|---|---|---|---|---|
| Intraoperative opioid consumption | 12 | 3.04 [−3.99, −2.09] | <.00001 | <.00001 | 96% |
| Time to first rescue analgesic postoperatively | 5 | 7.77 [5.25, 10.29] | <.00001 | <.00001 | 94% |
| Number of patients requiring rescue analgesia | 8 | 0.33 [0.25, 0.43] | <.00001 | .55 | 0% |
| First time to ambulation after surgery | 4 | −0.56 [−1.21, 0.08] | .09 | <.00001 | 87% |
| Hospital length of stay | 8 | −1.01 [−1.72, −0.30] | .006 | <.00001 | 95% |
| PONV | 13 | 0.35 [0.27, 0.46] | <.00001 | .12 | 33% |
| Itching | 6 | 0.48 [0.27, 0.84] | .01 | .18 | 34% |
| Satisfaction score | 4 | 2.03 [0.96, 3.11] | .0002 | <.00001 | 94% |
| Outcomes (ESPB vs TLIP block) | Studies included | RR or Std.mean differance [95% CI] | P value for statistical significance | P value for heterogeneity | I2 test for heterogeneity |
| Intraoperative opioid consumption | 2 | 0.03 [−0.21, 0.27] | .79 | .51 | 0% |
| Number of patients requiring rescue analgesia | 2 | 0.90 [0.51, 1.61] | .73 | .28 | 14% |
| PONV | 2 | 1 [0.56, 1.77] | 0.99 | 0.70 | 0% |
CI = confidence interval, ESPB = erector spinae plane block, PONV = postoperative nausea and vomiting, RCT = randomized controlled trial, RR = risk ratios, TLIP = thoracolumbar interfascial plane.
For erector spinae plane block versus no/sham block, five studies including 380 patients reported time to first rescue analgesic. The results indicated ESPB significantly prolonged the time to first rescue analgesic (SMD = 7.77, 95% CI [−5.25, 10.29], I2 = 94%, P < .00001). The number of patients requiring rescue analgesia was reported in eight studies including 578 patients and was significantly lower in the erector spinae plane block groups compared to no/sham block group (49 patients vs 150 patients, RR = 0.33, 95% CI [0.25, 0.43], I2 = 0%, P < .00001). One article[22] reported time to first rescue analgesic and showed that patients receiving ESPB prolonged time to first rescue analgesic compared to MTP block group (14.2 ± 1.6 hour vs 0.8 ± 0.4 hour).
Two studies of ESPB versus TLIP block reported the number of patients requiring rescue analgesia and there was no significant difference between the two groups (21 patients vs 24 patients, P = .73). One article[22] recorded ESPB compared with MTP block and reported the number of patients requiring rescue analgesia was significantly lower in the group ESPB(7 patients) than the group MTP block (22 patients).
Five studies including 309 patients recorded first time to ambulation after surgery and there was no significant difference between patients who received ESPB and those who received no/sham block (SMD = −0.56, 95% CI [−1.21, 0.08], I2 = 87%, P = .09).
Eight studies that included 367 patients in ESPB groups and 369 patients in no/sham block groups measured the hospital length of stay and it was significantly shorter in patients with ESPB (SMD = −1.01, 95% CI [−1.72, 0.30], I2 = 95%, P = .006). One article[26] reported that there was a similar hospital stay between ESPB and TLIP block.
Fifteen studies that included 1006 patients compared the incidence of PONV in patients receiving ESPB with no/sham block and found the ESPB significantly reduced the incidence of PONV compared with no/sham block (RR = 0.35, 95% CI [0.27, 0.46], I2 = 33%, P < .00001). Two studies including 264 patients of ESPB versus TLIP block showed there was no difference in the incidence of PONV (RR = 1.00, 95% CI [0.56, 1.77], I2 = 0%, P = .99). One article[22] reported the incidence of PONV was lower in patients with ESPB compared to MTP block (1(2.5%) vs 4 (10%)).Six studies compared the incidence of itching in patients receiving ESPB with no/sham block. ESPB reduced the incidence of itching after surgery compared with no/sham block (RR = 0.48, 95% CI [0.27, 0.84], I2 = 34%, P = .01). One study[29] reported patients receiving TLIP block were lower in the rate of itching compared with those receiving ESPB (7 [23.3%] vs 4 [13.3%]). The study of Eskin et al[22] reported that fewer patients in the group ESPB suffered from itching compared to group MTP block (1 [2.5%] vs 3 [7.5%]). Four studies including 360 patients compared satisfaction scores of patients receiving ESPB with no/sham block. Patients receiving ESPB had higher postoperative satisfaction scores (SMD = −2.03, 95% CI [−0.96, 3.11], I2 = 94%, P = .0002). Eskin et al[22] reported patient satisfaction scores were higher in the group ESPB than the group MTP block (8.2 ± 1.4 vs 7.1 ± 0.9). No complications associated with nerve blocks such as pneumothorax, infection, and local anesthetic toxicity were reported in all included studies.
4. Discussion
Our study demonstrates that ultrasound-guided erector spinae plane block preoperatively is a feasible and effective adjunct for acute pain control after lumbar spine surgery. Compared with no/sham block, erector spinae plane block preoperatively not only reduces intraoperative and postoperative opioid consumption in 24 hours but also decreases postoperative pain scores at all measured time points up to 48 hours. In addition, ESPB can reduce opioid-related side effects, improve patient satisfaction, and shorten hospital stays. However, there was no sufficient evidence to support ultrasound-guided ESPB was better than other regional blocks.
Currently, the mechanisms of ESPB have not yet been clarified fully. Most clinical studies thought ESPB provides analgesia by blocking the ventral and dorsal ramus of the spinal nerves, or the local anesthetic may diffuse into the paravertebral space even epidural space.[40] The reasons for widely using the ESPB: on the one hand, ultrasound-guided ESPB is easily performed and has fewer complications compared with epidural or paravertebral nerve block; on the other hand, the range of blocking is narrower, therefore it has the advantages of lighter inhibition on respiratory and circulatory function.
The important finding of this meta-analysis is that ESPB preoperatively decreased intraoperative and postoperative opioid consumption in patients with lumbar procedures, which may be beneficial to the long-term outcome for people after lumbar surgery. Perioperative opioid consumption has been associated with tolerance, hyperalgesia, poor pain outcomes, and even more complications.[41] The higher dose and the longer use of postoperative opioids lead to lower satisfaction, a higher risk of prolonged opioid use, and disability after lumbar spine surgery.[42] Therefore, reducing the opioid dosage as far as possible could promote cessation of opioids, which may improve patient postoperative outcomes. Several studies have reported that ESPB had efficacy in the treatment of persistent chronic pain,[43–46] referring to stubborn thoracic neuropathic pain, chronic shoulder pain, and post-herpetic neuralgia. But most of these studies are case reports, case series, and retrospective analyses, lacking high-quality evidence. Further, large sample studies about ESPB are needed to evaluate its efficacy in chronic pain. As we know, preoperative ESPB can prevent the afferent noxious stimulus in advance and reduce central sensitization, thereby reducing intraoperative and postoperative opioid consumption. However, postoperative ESPB does not reduce intraoperative opioids. Additionally, using PCIA or not also impact the consumption of opioids after surgery.
Our study found that ESPB can decrease postoperative pain scores within 48 hours, while previous meta-analyses only analyzed pain scores within 24 hours. A study about freehand ESPB reported there was no difference in pain scores at 24 hours between ESPB group and the control group. But several case series found that there was still a low pain score in 72 hours after surgery. At present, the effect of ESPB on postoperative pain score varies greatly among different studies, possibly because of the type of local anesthetic, dose, adjuvant, and indwelling catheter or not. Ropivacaine and bupivacaine are the main local anesthetics drugs, the concentration of ropivacaine is 0.2% or 0.375%, and bupivacaine is 0.25% or 0.5%. Subgroup analysis showed both drugs were effective analgesics. Furthermore, the duration of postoperative analgesia may be affected by the time of performing the erector spinae plane block(before/after surgery). Most of the included studies performed ESPB before surgery except Asar et al[36] at the end of surgery. Performing erector spinae plane block before surgical stimulation can prevent peripheral and central sensitivity caused by pain and inflammatory stimulation by blocking the dorsal branch of spinal nerves, and play a role in advanced analgesia.
Although our meta-analysis doesn’t report block-related complications, some studies have found that erector spinae plane block is not safe absolutely. Yawata et al[47] reported a case of local anesthetic poisoning after ESPB with a total of 30 mL of 0.5% levobupivacaine. Even though the local drug did not exceed the limit in this case, local drug poisonings occurred. Another study reported transient postoperative paraplegia occurred in a patient with ESPB and it may be due to the local anesthetic diffusing forward into the epidural space.[48] Recently, a retrospective study of 342 consecutive cases analyzed complications associated with ultrasound-guided erector spinae plane block and didn’t find other complications related to ESPB except for one unilateral pneumothorax.[49] Thus, ultrasound-guided ESPB is a relatively safe analgesic method for patients undergoing lumbar spine surgery. However, we should always be alert to related complications such as local anesthetic intoxication, muscle weakness, infection, and hematoma when performing erector spinae plane block or other regional nerve blocks. Moreover, further studies are needed to investigate the changes in plasma concentration of local anesthetics after erector spinae plane block to improve safety. Our meta-analysis also didn’t find some evidence that ESPB reduced postoperative ambulation time. The probable reason is most doctors still recommend complete bed rest to reduce the burden of the lumbar spine for patients undergoing lumbar surgery. The previous meta-analysis about ESPB did not report this important outcome. Our meta-analysis shows that erector spinae plane block shortens the length of the patient’s hospital stay with high heterogeneity. Shortening hospital length of stay is a valuable indicator to evaluate the effectiveness of ESPB and fast recovery, and it also can improve patient satisfaction and reduce financial burden. Less or no opioids, lower postoperative pain scores, and fewer complications are all helpful to shorten the postoperative hospital stay and promote the rapid recovery of patients.
Erector spinae plane block reduces postoperative opioid-related side effects such as nausea and vomiting, and itching, and PONV is a commonly occurring problem after surgical procedures. Although some patients undergoing spinal surgery received appropriate antiemetic therapy, PONV still occurred in about 60% of patients.[50] It not only causes patients to feel unpleasant but also leads to various complications, such as wound breakdown, aspiration pneumonia, bleeding, and hematomas, thus leading to prolonged residence time in the postanesthesia care unit and delay of discharge, even increasing health care costs.[51] The opioid is one of the risk factors for PONV in adults and increases the risk for PONV with a dose-dependent. A meta-analysis by Frauenknecht et al[52] showed that intraoperative opioids correlated with an increased risk of PONV compared with opioid- free anesthesia. Performing of ESPB decreases the rate of PONV, possibly because of the decrease in opioid consumption in our study and our conclusion is consistent with other meta-analyses about ESPB.[13,53]
Our meta -analysis also compared erector spinae plane block with other regional blocks, such as TLIP block and MTP block. TLIP block is a new fascial plane block that can block the dorsal branch of the thoracolumbar spinal nerve.[54] The analgesic effect of MTP block is achieved due to the anesthetic drug is deposited at the mid-point between the transverse process and pleura and diffused the paravertebral space to block the spinal nerve root.[55] TLIP block and MTP block both have been shown to reduce postoperative opioid consumption and improve pain in patients with lumbar spine surgeries.[22,56] There are few studies comparing ESPB with other nerve blocks, so the benefits of ESPB compared to other regional techniques remain unclear.
Our pooled results have high heterogeneity. There is still significant heterogeneity after sensitivity analysis is performed for the primary outcome. Several probable reasons can explain the significant heterogeneity: The types, volume, and concentrations of anesthetic drugs are different among including studies (e.g., bupivacaine 0.25%, 0.5%, 20/30 volume; ropivacaine 0.3%, 0.4%, 0.375%, 0.5%, 15/20/25/30 volume). ESPB is performed after induction of anesthesia in ten studies, so dermatomal sensory testing of the block could not be done to exclude possible block failure. Besides, sensory testing of the block was performed before induction of anesthesia only in six studies, the effectiveness of ESPB was uncertain in the rest studies. The detailed surgical methods are different. For example, lumbar spinal surgeries localized one or two levels in most research, but more segments were involved in a few studies. The greater surgical trauma and longer operation time may cause more severe postoperative pain, which leads to a difference in pain scores. Outcomes of some included studies are presented with the median, the first and third quartiles, the range, and even graphs. Conversion of these data to means and standard deviation may introduce errors that could lead to heterogeneity between studies.
This meta-analysis also has some shortcomings. Firstly, some of the included studies lack the double-blind design and detailed allocation concealment, which weaken the quality of the study. Secondly, certain results are associated with significant heterogeneity and sensitivity analysis did not identify the source of heterogeneity. Thirdly, few studies compare ESPB with other types of blocks, so we cannot conclude whether the ESPB was superior to other types of blocks (e.g., TLIP block, MTP block). Fourthly, the number of patients was relatively small in most of the included studies, and more RCTs with larger sample sizes are required to explore this issue further. Finally, some important outcomes such as other complications (hemorrhage, arrhythmia, postoperative respiratory), and postoperative chronic pain are not investigated based on currently limited data and more RCTs should be performed to explore these outcomes.
5. Conclusion
Ultrasound-guided erector spinae plane block preoperatively significantly improves the quality of analgesia, decreases opioid consumption, reduces the incidence of PONV, and improves patient satisfaction following lumbar surgery. In summary, the erector spinae plane block could be a valuable adjunct for relieving pain after lumbar surgery. Further studies need to compare the analgesic effect of ESPB with other regional nerve blocks, and pay more attention to the effect of erector spinal block on postoperative chronic pain.
Author contributions
Conceptualization: Hui Liu.
Data curation: Jing Zhu, Jing Wen.
Formal analysis: Jing Wen.
Software: Hui Liu, Jing Zhu.
Writing – original draft: Hui Liu.
Writing – review & editing: Qiang Fu.
Abbreviations:
- CI
- confidence interval
- ESPB
- erector spinae plane block
- MTP
- midtransverse process to pleura
- PCIA
- patient-controlled intravenous analgesia
- PONV
- postoperative nausea and vomiting
- RCT
- randomized controlled trial
- RR
- risk ratios
- SMD
- standardized mean differences
- TLIP
- thoracolumbar interfascial plane
- VAS
- visual analogue scale
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Liu H, Zhu J, Wen J, Fu Q. Ultrasound-guided erector spinae plane block for postoperative short-term outcomes in lumbar spine surgery: A meta-analysis and systematic review. Medicine 2023;102:7(e32981.
Contributor Information
Jing Zhu, Email: 3426696235@qq.com.
Jing Wen, Email: 3426696235@qq.com.
Qiang Fu, Email: 3093897685@qq.com.
References
- [1].Gerbershagen HJ, Aduckathil S, van Wijck AJ, et al. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118:934–44. [DOI] [PubMed] [Google Scholar]
- [2].Kraiwattanapong C, Arnuntasupakul V, Kantawan R, et al. Effect of multimodal drugs infiltration on postoperative pain in split laminectomy of lumbar spine: a randomized controlled trial. Spine (Phila Pa 1976). 2020;45:1687–95. [DOI] [PubMed] [Google Scholar]
- [3].Ntalouka MP, Brotis AG, Bareka MV, et al. Multimodal analgesia in spine surgery: an umbrella review. World Neurosurg. 2021;149:129–39. [DOI] [PubMed] [Google Scholar]
- [4].Alian MFB, Ver MR, Ramos MRD, et al. Postoperative single-shot epidural fentanyl and bupivacaine for postoperative analgesia after lumbar decompression: a prospective, double-blind randomized study. Spine (Phila Pa 1976). 2020;45:1017–23. [DOI] [PubMed] [Google Scholar]
- [5].Lovich-Sapola J, Smith CE, Brandt CP. Postoperative pain control. Surg Clin North Am. 2015;95:301–18. [DOI] [PubMed] [Google Scholar]
- [6].Bajwa SJ, Haldar R. Pain management following spinal surgeries: an appraisal of the available options. J Craniovertebr Junction Spine. 2015;6:105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rieder TN. Solving the opioid crisis isn’t just a public health challenge-it’s a bioethics challenge. Hastings Cent Rep. 2020;50:24–32. [DOI] [PubMed] [Google Scholar]
- [8].Chin KJ, McDonnell JG, Carvalho B, et al. Essentials of our current understanding: abdominal wall blocks. Reg Anesth Pain Med. 2017;42:133–83. [DOI] [PubMed] [Google Scholar]
- [9].Forero M, Adhikary SD, Lopez H, et al. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41:621–7. [DOI] [PubMed] [Google Scholar]
- [10].Leong RW, Tan ESJ, Wong SN, et al. Efficacy of erector spinae plane block for analgesia in breast surgery: a systematic review and meta-analysis. Anaesthesia. 2021;76:404–13. [DOI] [PubMed] [Google Scholar]
- [11].Huang W, Wang W, Xie W, et al. Erector spinae plane block for postoperative analgesia in breast and thoracic surgery: a systematic review and meta-analysis. J Clin Anesth. 2020;66:109900. [DOI] [PubMed] [Google Scholar]
- [12].Koo CH, Hwang JY, Shin HJ, et al. The effects of erector spinae plane block in terms of postoperative analgesia in patients undergoing laparoscopic cholecystectomy a meta-analysis of randomized controlled trials. J Clin Med. 2020;10:2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu MJ, Zhou XY, Yao YB, et al. Postoperative analgesic efficacy of erector spinae plane block in patients undergoing lumbar spinal surgery: a systematic review and meta-analysis. Pain Ther. 2021;10:333–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oh SK, Lim BG, Won YJ, et al. Analgesic efficacy of erector spinae plane block in lumbar spine surgery: a systematic review and meta-analysis. J Clin Anesth. 2022;78:110647. [DOI] [PubMed] [Google Scholar]
- [15].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Higgins JP, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guyatta G, Oxman AD, Akl EA, et al. GRADE guidelines:1. Introduction GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94. [DOI] [PubMed] [Google Scholar]
- [18].Wan X, Wang WQ, Liu JM, et al. Estimating the sample mean and standard deviation from the sample size, median, range and or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Luo DH, Wang X, Liu JM, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and or mid-quartile range. Stat Methods Med Res. 2018;27:1785–805. [DOI] [PubMed] [Google Scholar]
- [20].Vergari A, Muro M, Nestorini RA, et al. Bilateral lumbar Ultrasound-guided Erector Spinae. Minerva Anestesiol. 2022;88:465–71. [DOI] [PubMed] [Google Scholar]
- [21].Ghamry ME, Elgebaly A, Anwar A, et al. Ultrasound-guided erector spinae plane block for acute pain management in patients undergoing posterior lumbar interbody fusion under general anaesthesia. South Afr J Anaesth Analg. 2019;25:26–31. [Google Scholar]
- [22].Eskin MB, Ceylyan A, Özhan MO, et al. Ultrasound-guided erector spinae block versus midtransverse process to pleura block for postoperative analgesia in lumbar spinal surgery. Anaesthesist. 2020;69:742–50. [DOI] [PubMed] [Google Scholar]
- [23].Singh S, Choudhary NK, Lalin D, et al. Bilateral ultrasound-guided erector spinae plane block for postoperative analgesia in lumbar spine surgery: a randomized control trial. J Neurosurg Anesthesiol. 2020;32:330–4. [DOI] [PubMed] [Google Scholar]
- [24].Yayik AM, Cesur S, Ozturk F, et al. Postoperative analgesic efficacy of the ultrasound-guided erector spinae plane block in patients undergoing lumbar spinal decompression surgery: a randomized controlled study. World Neurosurg. 2019;126:e779–85. [DOI] [PubMed] [Google Scholar]
- [25].Zhang TJ, Zhang JJ, Qu ZY, et al. Bilateral erector spinae plane blocks for open posterior lumbar surgery. J Pain Res. 2020;13:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang LZ, Wu Y, Dou LJ, et al. Comparison of two ultrasound-guided plane blocks for pain and postoperative opioid requirement in lumbar spine fusion surgery: a prospective, randomized, and controlled clinical trial. Pain Ther. 2021;10:1331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhu LJ, Wang MC, Wang XD, et al. Changes of opioid consumption after lumbar fusion using ultrasound-guided lumbar erector spinae plane block: a randomized controlled trial. Pain Physician. 2021;24:E161–8. [PubMed] [Google Scholar]
- [28].Zhang JJ, Zhang TJ, Qu ZY, et al. Erector spinae plane block at lower thoracic level for analgesia in lumbar spine surgery: a randomized controlled trial. World J Clin Cases. 2021;9:5126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ciftci B, Ekinci M, Celik EC, et al. Ultrasound-guided erector spinae plane block vs. modified-thoracolumbar interfascial plane block for lumbar discectomy surgery: a randomized, controlled study. World Neurosurg. 2020;144:e849–55. [DOI] [PubMed] [Google Scholar]
- [30].Finnerty D, Ní EA, Ahmed M, et al. A randomised trial of bilateral erector spinae plane block vs. no block for thoracolumbar decompressive spinal surgery. Anaesthesia. 2021;76:1499–503. [DOI] [PubMed] [Google Scholar]
- [31].Wahdan AS, Radwan TA, Mohammed MM, et al. Effect of bilateral ultrasound-guided erector spinae blocks on postoperative pain and opioid use after lumbar spine surgery: a prospective randomized controlled trial. Egypt J Anaesth. 2021;37:100–10. [Google Scholar]
- [32].Yu Y, Wang M, Ying H, et al. The analgesic efficacy of erector spinae plane blocks in patients undergoing posterior lumbar spinal surgery for lumbar fracture. World Neurosurg. 2021;147:e1–7. [DOI] [PubMed] [Google Scholar]
- [33].Yörükoğlu HU, İçli D, Aksu C, et al. Erector spinae block for postoperative pain management in lumbar disc hernia repair. J Anesth. 2021;35:420–5. [DOI] [PubMed] [Google Scholar]
- [34].Zhang Q, Wu Y, Ren F, et al. Bilateral ultrasound-guided erector spinae plane block in patients undergoing lumbar spinal fusion: a randomized controlled trial. J Clin Anesth. 2021;68:110090. [DOI] [PubMed] [Google Scholar]
- [35].Goel VK, Chandramohan M, Murugan C, et al. Clinical efficacy of ultrasound guided bilateral erector spinae block for single-level lumbar fusion surgery: a prospective, randomized, case-control study. Spine J. 2021;21:1873–80. [DOI] [PubMed] [Google Scholar]
- [36].Asar S, Sari S, Altinpulluk EY, et al. Efficacy of erector spinae plane block on postoperative pain in patients undergoing lumbar spine surgery. Eur Spine J. 2022;31:197–204. [DOI] [PubMed] [Google Scholar]
- [37].Jin Y, Zhao S, Cai J, et al. Erector Spinae plane block for perioperative pain control and short-term outcomes in lumbar laminoplasty: a randomized clinical trial. J Pain Res. 2021;14:2717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen LQ, Xu CF, Zhai ZX, et al. [Determination of the block range of ultrasound-guided lumbar erector spinal plane and its application in lumbar spine surgery]. Zhonghua Yi Xue Za Zhi. 2021;101:2993–8. [DOI] [PubMed] [Google Scholar]
- [39].Wang W, Liu YL, Zhang YY. Ultrasound-guided erector spine block as an adjuvant to general anesthesia and postoperative analgesia in patients undergoing lumbar spine surgery. J Clin Anesthestol. 2018;34:1172–5. [Google Scholar]
- [40].Chin KJ, El-Boghdadly K. Mechanisms of action of the erector spinae plane (ESP) block: a narrative review. Can J Anaesth. 2021;68:387–408. [DOI] [PubMed] [Google Scholar]
- [41].Weiner JA, Weiner JA, Snavely JE, et al. Impact of preoperative opioid use on postoperative patient-reported outcomes in lumbar spine surgery patients. Clin Spine Surg. 2021;34:E154–9. [DOI] [PubMed] [Google Scholar]
- [42].Hills JM, Carlile CR, Archer KR, et al. Duration and dosage of opioids after spine surgery: implications on outcomes at 1 year. Spine (Phila Pa 1976). 2020;45:1081–8. [DOI] [PubMed] [Google Scholar]
- [43].De Paolis V, De Sanctis F, Marinangeli F, et al. The association of erector spinae plane block and ultrasound guided dry needling could be a winning strategy for long-term relief of chronic musculoskeletal pain. Minerva Anestesiol. 2019;85:1138–9. [DOI] [PubMed] [Google Scholar]
- [44].Tulgar S, Selvi O, Senturk O, et al. Ultrasound-guided erector spinae plane block: indications, complications, and effects on acute and chronic pain based on a single-center experience. Cureus. 2019;11:e3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ueshima H, Otake H. An erector spinae plane block for chronic pain management after tissue expander insertion. J Clin Anesth. 2019;55:4. [DOI] [PubMed] [Google Scholar]
- [46].Urits I, Charipova K, Gress K, et al. Expanding role of the erector spinae plane block for postoperative and chronic pain management. Curr Pain Headache Rep. 2019;23:71. [DOI] [PubMed] [Google Scholar]
- [47].Yawata S, Imamachi N, Sakura S, et al. Local anesthetic systemic toxicity of levobupivacaine in erector spinae plane block. Korean J Anesthesiol. 2021;74:271–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Guna PK, Sonawane K, Rajasekaran S, et al. Transient paraplegia in lumbar spine surgery-a potential complication following erector spinae plane block. Eur Spine J. 2022;31:3719–23. [DOI] [PubMed] [Google Scholar]
- [49].Oezel L, Hughes AP, Onyekwere I, et al. Procedure specific complications associated with ultrasound-guided erector spinae plane block for lumbar spine surgery: a retrospective analysis of 342 consecutive cases. J Pain Res. 2022;15:655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kim EJ, Shim JK, Soh S, et al. Patient-controlled Analgesia with propacetamol-fentanyl mixture for prevention of postoperative nausea and vomiting in high-risk patients undergoing spine surgery: a randomized controlled trial. J Neurosurg Anesthesiol. 2016;28:316–22. [DOI] [PubMed] [Google Scholar]
- [51].Gan TJ, Belani KG, Bergese S, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2020;131:411–48. [DOI] [PubMed] [Google Scholar]
- [52].Frauenknecht J, Kirkham KR, Jacot-Guillarmod A, et al. Analgesic impact of intraoperative opioids vs. opioid-free anaesthesia: a systematic review and meta-analysis. Anaesthesia. 2019;74:651–62. [DOI] [PubMed] [Google Scholar]
- [53].Ma J, Bi Y, Zhang Y, et al. Erector spinae plane block for postoperative analgesia in spine surgery: a systematic review and meta-analysis. Eur Spine J. 2021;30:3137–49. [DOI] [PubMed] [Google Scholar]
- [54].Farag E, Seif J. Thoracolumbar interfascial block (TLIP): A new technique of interfascial plane blocks. J Clin Anesth. 2020;61:109640. [DOI] [PubMed] [Google Scholar]
- [55].Costache I, de Neumann L, Ramnanan CJ, et al. The mid-point transverse process to pleura (MTP) block: a new end-point for thoracic paravertebral block. Anaesthesia. 2017;72:1230–6. [DOI] [PubMed] [Google Scholar]
- [56].Chen K, Wang L, Ning M, et al. Evaluation of ultrasound-guided lateral thoracolumbar interfascial plane block for postoperative analgesia in lumbar spine fusion surgery a prospective, randomized, and controlled clinical trial. PeerJ. 2019;7:47967. [DOI] [PMC free article] [PubMed] [Google Scholar]