Background:
Insulin resistance and hepatogenic diabetes are common complications in patients with liver cirrhosis. Previous studies have shown that reducing the fasting phase by supplying a late evening snack (LES) is a potential intervention to improve substrate utilization and liver function. However, the underlying mechanisms need to be further elucidated. The purpose of current meta-analysis is to evaluate effects of LES on glucose homeostasis in cirrhotic patients.
Methods:
Electronic databases including PubMed, Web of Science, and major scientific conference sessions were searched without language restriction and carried out on March 1, 2022 with an additional manual search of bibliographies of relevant articles. A total of 4145 studies were identified, and 10 studies were eligible for the meta-analysis, comprising 631 patients (319 in the LES group and 312 in the non-LES group). Subgroup analyses were performed to investigate the effect of LES on cirrhotic patients with or without diabetes.
Results:
Analysis showed that LES intervention had significant effects in cirrhotic patients for glycemic parameters on fasting plasma glucose, fasting insulin, and glycosylated hemoglobin respective effect sizes of −8.7, −0.86, and −0.76. Subgroup result revealed that the effect of LES on fasting plasma glucose is higher in cirrhotic patients with diabetes group than cirrhotic patients without diabetes group, and long-term LES supplementation (>2 months) was more beneficial to maintain glucose homeostasis in cirrhotic patients than that of short-term supplementation (<2 months). LES also had significant effect on nutritional metabolic parameters like including albumin and non-protein respiratory quotient.
Conclusion:
Meta-analysis indicated that LES not only improved malnutrition in cirrhotic patients with or without diabetes but also maintain glucose homeostasis in cirrhotic patients with diabetes. LES is a promising and simple intervention that beneficial to maintain glucose homeostasis in cirrhotic patients.
Keywords: glucose homeostasis, hepatogenic diabetes, late evening snack, liver cirrhosis
1. Introduction
Liver cirrhosis (LC) has been a serious health problem with high morbidity and mortality rates across the world.[1] As an important organ responsible for energy and glucose metabolism in the human body, the liver plays an important role in glucose homeostasis.[2] Approximately 70% of cirrhotic patients are diagnosed with diabetes, 25% of patients have impaired glucose tolerance and only 4% have normal glucose tolerance.[3] Hepatogenic diabetes (HD)[4,5] and malnutrition[6] are common complications of LC. It is often ignored that chronic liver disease can cause diabetes mellitus known as HD, which refers to the state of impaired glycemic regulation in patients with cirrhosis of liver as a result of the loss of liver function.[5]
Although the first-line therapy for type 2 diabetes mellitus is lifestyle modifications, including a hypocaloric diet and regular physical exercise, these are not always applicable in patients with HD. Most cirrhotic patients have various grades of malnutrition that prevents them from consuming a hypocaloric diet, regularly. The characteristic of LC is accelerated hunger, and in the fasted state, the patient’s primary energy source changes from glucose to lipids. LES is recommended according to the European Society for Clinical Nutrition and Metabolism guidelines.[7] In China, it is recommended that cirrhotic patients should not fast for >12 hours, including overnight fasting. Patients with LC should eat 4 to 6 meals a day (3 meals + 3 extra meals, including 1 at night).[8] This treatment can promote protein and energy absorption in the body and help to prevent sarcopenia.[9,10] Therefore, LES deserves in-depth study as a potential meal strategy. Researchers have previously demonstrated LES was the benefit for liver function and malnutrition in cirrhotic patients.[11,12] However, the efficacy of LES supplementation on blood glucose in cirrhotic patients with impaired insulin resistance (IR) or even HD is yet to be fully confirmed. Furthermore, existing guidelines do not provide a consensus on the dietary requirements for HD. Most previous studies were performed by adopting a single group pretest/posttest crossover analysis with a limited number of samples[13] and a lack of a randomized controlled group.[14] Therefore, the evidence provided by these studies is not reliable. In the present study, we conducted a meta-analysis to elucidate the control of LES on glucose homeostasis in cirrhotic patients.
2. Materials and methods
The protocol used for this review complies with PRISMA’s recommendation for meta-analysis. This study was registered with the PROSPERO database (http://www.crd.york.ac.uk/PROSPERO), an international prospective register of systematic reviews. The registration number is CRD42022310854.
2.1. Search strategy
Potentially relevant studies were identified by searching PubMed, Web of Science, China National Knowledge Infrastructure, China Biology Medicine disc, China Science and Cochrane and Technology Journal Database to identify articles published up to March 1, 2022 using edictal medical subject heading terms and non-medical subject heading terms terms related to the topic. The key terms included “liver diseases,” “liver cirrhosis,” “hepatocellular carcinoma,” “liver failure,” “liver dysfunction,” “liver dysfunctions,” “late evening snack,” “nocturnal snack,” “nocturnal meal,” “evening meal,” “nocturnal,” “evening,” and “late-evening snack.” Moreover, all the databases were searched with no language or date limitation. The references of the included studies were also checked to identify possible additional studies (Fig. 1).
Figure 1.
Flowchart of literature search and trial selection.
The outcome measures for meta-analysis were liver biochemical data and blood glucose data related to LC, including albumin (ALB), non-protein respiratory quotient (npRQ), fasting plasma glucose (FPG), glycosylated hemoglobin (HbA1c), fasting serum insulin (FINS) and homeostasis model assessment method for IR (HOMA-IR).
2.2. Selection of trials
Articles employed in this study that conformed to the following: study design: comparisons between LES and non-LES, study population: cirrhotic patients, with or without diabetes, and illustrated at least one of the outcome measures (FPG, HbA1c, blood glucose level). Studies were excluded from the analysis as follows: trials that did not provide original data, or the outcomes of interest were not reported; letters, expert opinion, animal experiments, book sections, leading articles, and case reports; interventions were not LES; and experiment type was not a randomized controlled trial.
2.3. Data extraction
Two reviewers examined the eligibility and quality of the selected studies in an independent manner according to the inclusion and exclusion criteria.
2.4. Statistical analysis
Data analysis was conducted using the Review Manager, version 5.3 statistical package (Cochrane Collaboration, Oxford, UK). A meta-analysis was performed according to the recommendations reported in the PRISMA guidelines. For continuous outcomes, the results were presented as mean difference (MD) with a 95% confidence interval (CI) for dichotomous outcomes using risk ratios as the summary statistic.
The overall effects were measured using a Z score with significance set at P < .05. A standardized MD of approximately (P > .05) indicated no statistical significance between the intervention group and control group, while standardized MD values deviating from 0 (P < .05) were determined to be statistically significant. Statistical heterogeneity was evaluated using χ2 and I2 tests with significance set at P ≤ .1. Values of P ≤ .1 and I2 > 50% were significantly heterogeneous. The random effects method was used to combine results if significant heterogeneity was observed; otherwise, the fixed effect method was used. Preplanned subgroup analyses were carried out according to combined HD (or not), intervention periods and LES content. To assess sources of potential bias, sensitivity analyses were performed when required.
3. Results
3.1. Study selection and characteristics of the included studies
Ten studies[15–24] were employed in the meta-analysis and are summarized in Table 1. Ten studies were included in this analysis that consisted of 631 patients (319 in the LES group and 312 in the non-LES group). All 10 eligible studies were randomized controlled trial and 374 of the 631 cirrhotic patients were diagnosed with diabetes.
Table 1.
Characteristics of the studies included in this meta-analysis.
| Study | Year | Region | Diseases | Group | N | Age (yr) | Male/Female | Study type |
|---|---|---|---|---|---|---|---|---|
| Xie | 2020 | China | LC with HD | LES control | 43 | 54.4 ± 4.6 | 34/9 | RCT |
| 42 | 54.4 ± 4.6 | 34/8 | ||||||
| Takeshita | 2009 | Japan | LC with HCC | LES control | 28 | 69.1 ± 8.2 | 19/9 | RCT |
| 28 | 70.6 ± 9.8 | 21/7 | ||||||
| Nakaya | 2007 | Japan | LC | LES control | 19 | 67.0 ± 9.0 | 13/6 | RCT |
| 19 | 67.0 ± 8.0 | 7/12 | ||||||
| Ma | 2020 | China | LC with HD | LES control | 68 | 68.0 ± 4.1 | 40/28 | RCT |
| 67 | 67.0 ± 4.1 | 35/32 | ||||||
| Lin | 2020 | China | LC with HD | LES control | 64 | 68.6 ± 2.6 | 38/26 | RCT |
| 65 | 68.4 ± 2.2 | 40/25 | ||||||
| Ichikawa | 2010 | Japan | LC | LES control | 12 | 66.2 ± 8.2 | 5/7 | RCT |
| 9 | 67.4 ± 9.9 | 5/4 | ||||||
| Hou | 2019 | China | LC | LES control | 39 | 51.0 ± 10.3 | NA | RCT |
| 40 | 50.2 ± 11.7 | NA | ||||||
| Hou | 2021 | China | LC | LES control | 20 | 50.4 ± 9.02 | 16/4 | RCT |
| 20 | 51.8 ± 11.4 | 17/3 | ||||||
| Harima | 2010 | Japan | LC with HCC | LES control | 13 | 64.5 ± 9.5 | 11/2 | RCT |
| 10 | 66.4 ± 12.8 | 8/2 | ||||||
| Dong | 2019 | China | LC with HD | LES control | 13 | 57.2 ± 7.6 | 11/2 | RCT |
| 12 | 54.2 ± 6.4 | 10/2 |
HCC = , HD = hepatogenic diabetes, LC = liver cirrhosis, LES = late evening snack, RCT = randomized controlled trial.
3.2. LES administration
3.2.1. Duration of LES administration.
The characteristics of the LES administered in the 10 studies are listed in Table 2. In most cases, LES was administered between 10:00 PM and 1 hour before bedtime. As shown in Table 2, the duration of LES administration was 2 consecutive weeks in 1 study, >2 months in most studies, and up to 6 months in 1 study.
Table 2.
Late evening snack: type of supplement, calories and nutritional composition in each study.
| Study | Type | Total calories | Protein | Carbohydrate | Treatment period | Snack time |
|---|---|---|---|---|---|---|
| Xie | Original food | 210 kcal | NA | NA | 2 wk | 1 h before bedtime |
| Takeshita | BCAA | 210 kcal | NA | NA | 3 mo | 10:00 PM |
| Nakaya | BCAA | 210 kcal | 13.5 g | NA | 5 wk | 10:00 PM |
| Ma | Original food | 225 kcal | NA | 50.1 g | 2 mo | 1 h before bedtime |
| Lin | Original food | NA | NA | 50.1 g | 6 mo | 1 h before bedtime |
| Ichikawa | BCAA | 210 kcal | 13.5g | NA | 3 mo | 10:00 PM |
| Hou | Lotus root starch | 200 kcal | 7 g | 50.1 g | 1 mo | No statement |
| Hou | Low glycemic index food | 200 kcal | 0.1 g | 26.7 g | 2 mo | Before bedtime |
| Harima | BCAA | 210 kcal | 13.5 g | NA | 3 mo | 10:00 PM |
| Dong | High dietary fiber food | 200 kcal | 5.5 g | 39.5 g | 3 mo | 1 h before bedtime |
BCAA = branched-chain amino acid.
3.2.2. Composition and dosage of the LES.
As shown in Table 2, LES was investigated with regard to branched-chain amino acid (BCAA) in 4 studies, original food in 3 studies, lotus root starch in 1 study, low glycemic index foods in 1 study, and high dietary fiber foods in 1 study. The total of calories provided by LES were 200 to 210 kcal in most studies, only 1 study provided up to 225 kcal, and 1 study did not provide the quantity of calories.
3.3. Study quality
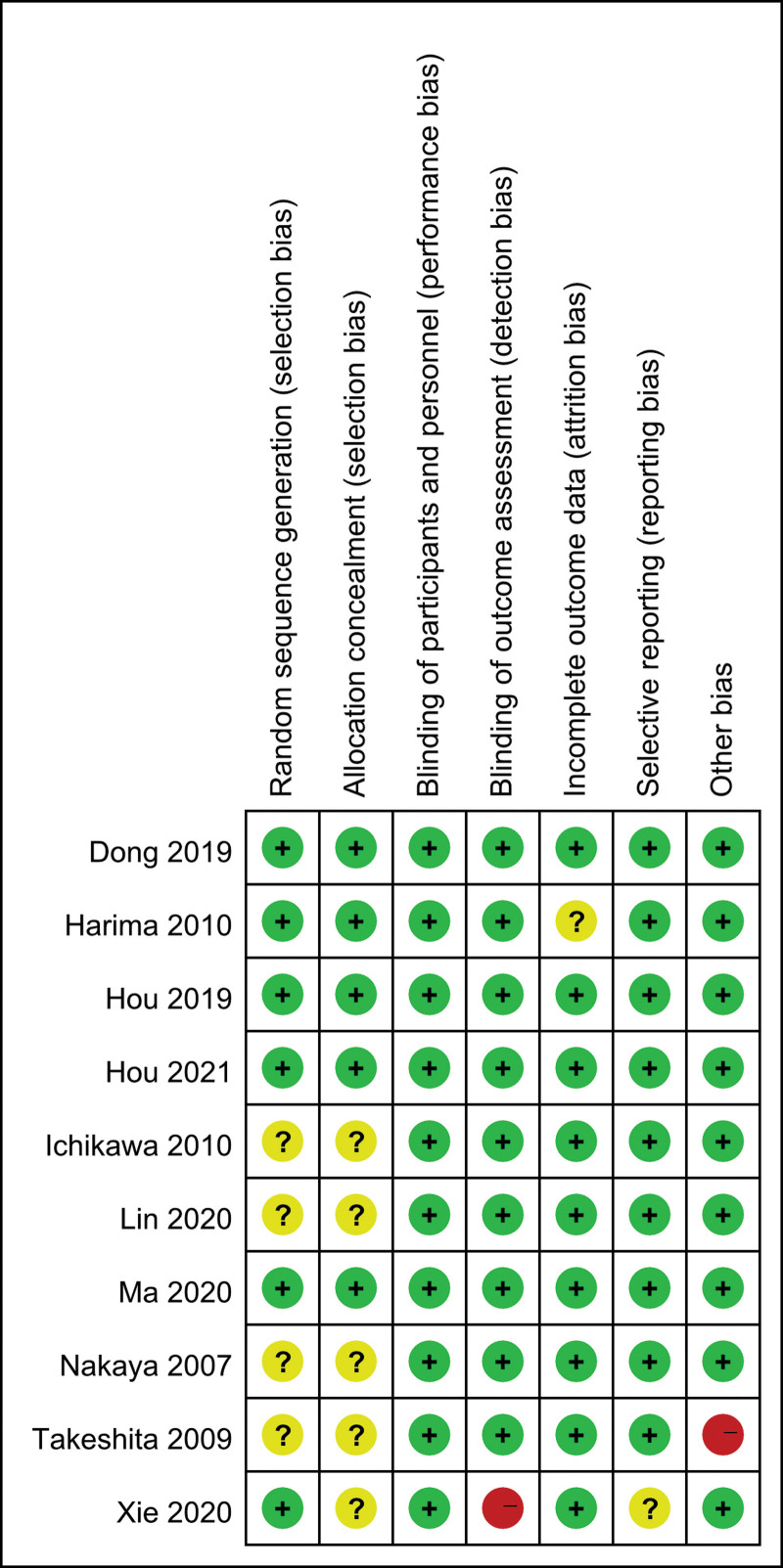
The quality of these studies was assessed using the risk of bias method recommended by the Cochrane Collaboration. Several domains were assessed: allocation generation, allocation concealment, the blinding of participants, personnel, and outcome assessors, the completeness of outcome data, freedom from selective reporting, and freedom from other biases (Fig. 2).
Figure 2.
Risk of bias summary of all studies.
3.4. Effects of LES on glucose homeostasis
3.4.1. FPG.
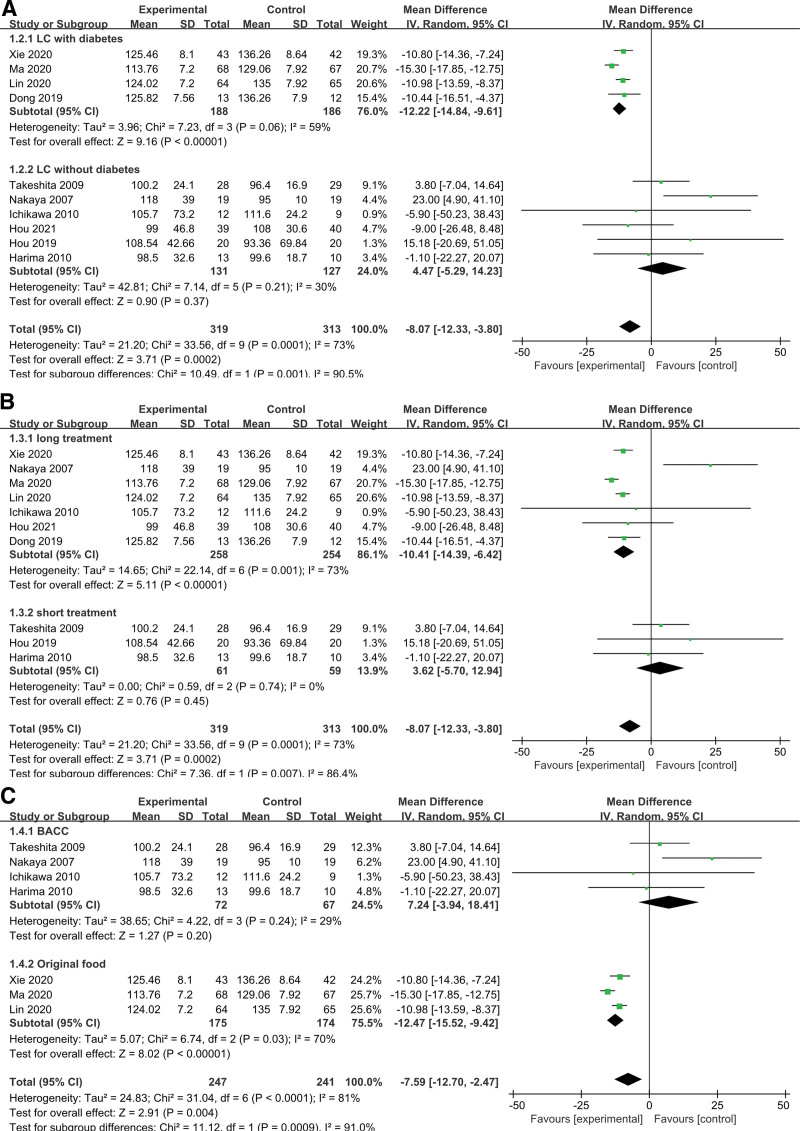
Effects analysis of LES on the FPG in cirrhotic patients revealed that there was significant heterogeneity among the 10 studies (χ2 = 33.56, P = .0002, I2 = 73%) (Fig. 3A). To identify causes of heterogeneity, subgroup analysis was performed according to diabetes (with or without), treatment period and LES content.
Figure 3.
Meta-analysis of the changes in glucose homeostasis. (A) Comparisons of FPG between LES and control groups. (B) Comparisons of HbA1c between LES and control groups. (C) Comparisons of FINS between LES and control groups. (D) Comparisons of HOMA-IR between LES and control groups. FINS = fasting serum insulin, FPG = fasting plasma glucose, HbA1c = glycosylated hemoglobin, HOMA-IR = homeostasis model assessment method for insulin resistance, LES = late evening snack.
3.4.1.1. Subgroup analysis of effects on FPG in LC (with and without diabetes).
Six studies involving cirrhotic patients without diabetes and 4 trials showcasing cirrhotic patients with diabetes were compared (Fig. 4A). After subgrouping, the former group’s heterogeneity remained considerable whereas there was no significant heterogeneity in the latter, proving that diabetes’ presence or absence was not one of the reasons for variability. The 95% CI of the combined effect size for the group with diabetes was (−14.84, −9.61) while the 95% CI of the combined effect size of the group without diabetes was (−5.29, 14.23). The 95% CI for the 2 groups did not overlap, thus representing a statistically significant difference and an interaction between grouping factors and the combined effect size. Additionally, the pooled results of the group with diabetes were statistically significant, with a risk ratios < 0, which was consistent with the overall pooled results, thus indicating that LES improved FPG in the subgroup of cirrhotic patients with diabetes but did not significantly improve FPG in the subgroup without diabetes.
Figure 4.
Meta-analysis of the changes in FPG in subgroups. (A) Subgroup analysis of the effects on FPG in LC with and without HD. (B) Subgroup analysis of the effects of different intervention periods on FPG. (C) Subgroup analysis of the effects of different LES contents on FPG. FPG = fasting plasma glucose, HD = hepatogenic diabetes, LC = liver cirrhosis, LES = late evening snack.
3.4.1.2. Subgroup analysis of the effects of different intervention periods on FPG.
Three of the studies featured short-term LES (<2 months) compared to long-term LES (>2 months) in the other studies. FPG in the long-term LES group was increased with high heterogeneity (I2 = 73%), although FPG in the short-term LES group was increased with no heterogeneity (I2 = 0%) (Fig. 4B). The differences between the groups was significant (P = .007). The changes in FPG following long-term LES outperformed those following short-term LES.
In the sensitivity analysis, the study of Hou[22] was excluded because the LES supplement time (6 months) was significantly longer than the other studies. The remaining studies had a significant level of sample homogeneity (χ2 = 20.661, P = .007, I2 = 86.2%). These results showed that long-term LES could significantly improve the FPG level in cirrhotic patients.
3.4.1.3. Subgroup analysis of the effects of different LES contents on FPG.
Effects of BCAA and original food on FPG were compared among studies. As showed in Figure 4C, FPG in the original food group was increased with high heterogeneity (I2 = 70%), while FPG in the BCAA group was increased with low heterogeneity (I2 = 29%), there was significant difference between the 2 groups (P = .0009). Moreover, there was a significant increasing of FPG in the original food group (P < .00001).
Sensitivity analysis was conducted to evaluate the robustness of the effect, and the result showed the sensitivity was high (MD = −7.59, 95% CI [−12.70, −2.47]), the 95% CI for all articles was −8.07 (−12.33, −3.80).
3.4.2. HbA1c.
Analysis of the effect of LES on the HbA1c in cirrhotic patients revealed significant homogeneity among the 4 studies (χ2 = 8.37, P < .00001, I2 = 76%). Funnel plots and Egger tests showed that no publication bias existed among these studies (P = .53). MD fixed effect model analysis was −0.76 (95% CI [−0.85, −0.67]), these results indicated that LES substantially improved the HbA1c of patients with LC (Fig. 3B).
In the sensitivity analysis, the study by Dong[24] were excluded because the LES content was different from the other studies. The remaining studies had a significant level of sample homogeneity (χ2 = 2.18, P = .34, I2 = 8%), the MD from fixed effect model analysis was −7.6 (95% CI [−0.86, −0.66]). These data further confirmed that LES significantly improved the HbA1c levels in these patients.
3.4.3. FINS.
Analysis of FINS data showed that the 4 included studies exhibited a high degree of sample heterogeneity (χ2 = 8.52, P = .04, I2 = 65%). Random effects model analysis showed that the MD was −0.86 (95% CI [−1.31, −0.41]) (Fig. 3C). In the sensitivity analysis, the study by Harima[23] was excluded, because cirrhotic patients in this study had liver cancer. The remaining studies had a significant level of sample homogeneity (χ2 = 1.41, P = .49, I2 = 0%). The MD was −0.87 (95% CI [−1.07, −0.68]) in fixed effects model analysis. This analysis showed that the administration of LES significantly improved the FINS of patients with LC.
3.4.4. HOMA-IR.
Analysis of the HOMA-IR data showed that 2 of the included studies exhibited a high degree of sample heterogeneity (Q = 5.84, P = .02, I2 = 83%). In the random effects model analysis, the MD was 0.14 (95% CI [−1.65, 1.93]) (Fig. 3D). These analyses showed that the administration of LES did not significantly reduce the HOMA-IR of patients with LC. However, the number of studies was small and there was some deviation.
3.5. Effects of LES on metabolism-related biochemistry
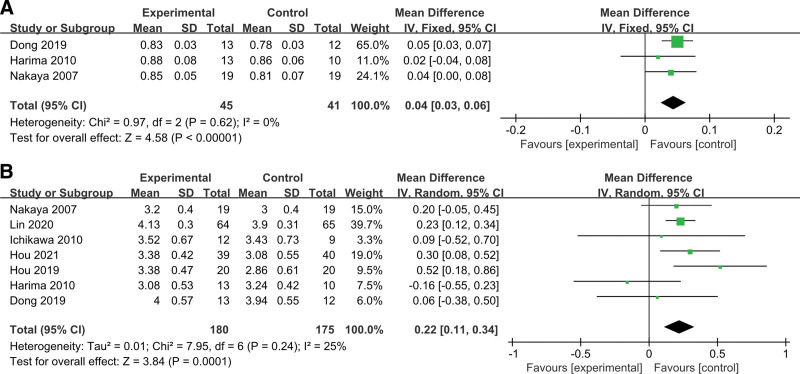
3.5.1. npRQ.
Three studies compared npRQ levels between patients who were treated with or without LES. There was no significant sample heterogeneity in these studies (χ2 = 0.97, P = .62, I2 = 0%). Fixed effect analysis demonstrated the npRQ level in the LES group was significantly higher than that of the control group (MD = 0.04, 95% CI [0.03, 0.06]). These results indicated that the administration of LES significantly increased the RQ levels in patients with cirrhosis (Fig. 5A).
Figure 5.
Meta-analysis of the changes in nutrient metabolism. (A) Comparisons of npRQ between LES and control groups. (B) Comparisons of ALB between LES and control groups. ALB = albumin, LES = late evening snack, npRQ = non-protein respiratory quotient.
3.5.2. ALB.
Analysis of ALB showed that the 7 studies had a significant level of sample heterogeneity (χ2 = 25.31, P = .0003, I2 = 76%) (Fig. 5B). This result indicated that the administration of LES significantly increased ALB levels in patients with LC. In the sensitivity analysis, the study by Harima[23] was excluded because cirrhotic patients had liver cancer. The remaining studies had a significant level of sample homogeneity (χ2 = 10.2, P = .07, I2 = 51%). MD was 2.65 (95% CI [1.21, 4.09]) in the random effects model analysis. This analysis showed that the administration of LES significantly improved the ALB of patients with LC.
4. Discussion
There is a strong association between liver diseases and diabetes. As research increasing, the mechanisms of the interaction between liver disease and diabetes have been identified. On the one hand, LC could impair the metabolism of insulin and leads to secondary IR and hyperinsulinemia. The increase of glucagon, growth hormone, insulin-like growth factors, and free fatty acids could exacerbate hyperinsulinemia. These abnormalities also affect IR in peripheral tissues and pancreatic β cells.[25] On the other hand, patients with impaired glucose tolerance and consequent diabetes can also cause further damage of liver function.[26] IR is a risk factor of the progression of liver fibrosis,[27] the development of hepatocellular carcinoma,[28] and leads to a reduction in survival time.[29] Indeed, in addition to its specialized role in glucose metabolism, insulin can also influence the production of cytokines,[30] growth factors[31] and inflammatory mediators.[32] Besides, insulin can influence the function of non-parenchymal hepatocytes, fibrogenesis[33,34] and the progression of liver disease.[35] Therefore, IR is an important therapeutic target in patients at any stage of chronic liver disease.[36]
As a result of impaired liver function, HD usually exhibits specific clinical characteristics and is closely linked to hypoglycemic episodes, but has a less association with risk factors such as age, body mass index and a family history of diabetes. This is due to the fact that chronic liver illness, rather than a genetic predisposition, caused the diabetic state. HD is caused by both β-cell dysfunction and decreased insulin sensitivity. Additionally, it was discovered that cirrhotic patients caused by hepatitis B had decreased insulin secretion.[37]
The key factors contributing to a poor prognosis in cirrhotic patients include altered nutrition, energy, and glucose metabolism, including elevated levels of FPG and fat oxidation, decreased glucose oxidation, and protein-energy malnutrition. In addition to improving the metabolic imbalance of degraded protein and fat oxidative energy supply in cirrhotic patients, LES reduces the physiological fasting time at night, which also prevents hypoglycemia the next morning. However, it is unclear that whether this LES approach is appropriate for HD patients.
The aim of this meta-analysis was to investigate whether LES helps cirrhotic patients maintain glucose homeostasis. This is the first study that utilizes a systematic review and meta-analysis to investigate how LES affects blood glucose management in cirrhotic patients. Results showed that taking LES significantly reduced FPG levels in patients with HD. It’s probable that LES prevented a single diet from exerting more pressure than the islet B cells could handle, keeping blood glucose levels in check. It was possible to prevent hypoglycemia symptoms and increase patients’ energy metabolism when blood glucose levels dropped in late night fasting. In cirrhotic patients without diabetes, FPG levels did not significantly improve, this could be because dysglycemia was not yet developed. It is possible that LES avoided the burden of a single diet exceeding the work of islet B cells, so that blood glucose was not too high. When blood glucose decreased, prolonged fasting was avoided, thus avoiding hypoglycemic reactions and could improve energy metabolism in patients. FPG levels were not significantly improved in cirrhotic patients without diabetes, this may be due to the fact that dysglycemia had yet to develop. In addition, FINS and HbA1c levels were shown to be reduced in cirrhotic patients after taking LES. This demonstrates that LES can improve abnormalities in glucose tolerance and glucose homeostasis in cirrhotic patients.
Protein-energy malnutrition is often observed in cirrhotic patients. When the energy metabolism of cirrhotic patients is measured using indirect calorime npRQ decreases as the severity of LC increases.[38] NpRQ refers to the ratio of the amount of CO2 released by the patient during breathing to the amount of O2 absorbed per unit time, that is, the respiratory quotient measured when sugars and lipids are substrates for oxidative decomposition, npRQ are able to reflect the oxidative energy supply of proteins when sugars and lipids are insufficiently supplied.[39] The present meta-analysis showed that npRQ improved better in the LES group than that of the non-LES group after the intervention. It is suggested that supplying LES shorten the fasting time at night, prolong the energy supply time of carbohydrates and lipids, improve the metabolic imbalance of decomposed protein and fat oxidation energy supply, and thus steadily maintain glucose homeostasis of cirrhotic patients.
In the present study, it was found that nutrient supplement with 200 to 210 kcal was both rich and optimal as a LES for cirrhotic patients. With regards to the formula and dosages of LES, this meta-analysis could not provide sufficient evidence to support the application of LES that are rich in BCAA. In previous studies, BCAA was more suitable for LES supplementation to improve muscle mass and the major cirrhosis-related events of cirrhotic patients.[40,41] However, in this study, original food was significantly superior to BCAA in terms of improving FPG for cirrhotic patients with diabetes. Subgroup result provided that a long-term supplementation intervention. Results showed that long-term administration is preferable to short-term administration.
Present study demonstrated the impact of LES administration on glucose homeostasis in cirrhotic patient with or without diabetes. Some suggestions are recommended for the clinical healthcare system. It is important for healthcare providers to establish a diet plan for patients with liver diseases, especially patients with LC, and the progression of glucose homeostasis should be regularly followed up. A multidisciplinary team will be useful to carry out a comprehensive healthcare plan of nutrition for patients with liver diseases. It is also important to develop education programs for patients and caregivers with regards to eating times and food content. However this meta-analysis may have been influenced by the ethnicity of the pooled population (derived mainly from Japanese and Chinese patients). Furthermore, the major etiology of LC was hepatitis C virus infection for Japanese, while hepatitis B virus infection for Chinese. The effects of LES can be influenced by additional factors which should be investigated further in future.
5. Conclusion
LES can effectively maintain glucose homeostasis in cirrhotic patients with diabetes and improve malnutrition in cirrhotic patients. Long-term LES supplementation (>2 months) is more beneficial for glucose homeostasis in cirrhotic patients than that of short-term supplementation (<2 months). In addition, LES of original food is a better complement than LES of BCAA with regards to controlling glucose homeostasis.
Author contributions
Conceptualization: Ni Chen, Xinze Qiu, Huaqiang Ruan, Shiquan Liu.
Data curation: Ni Chen.
Formal analysis: Ni Chen.
Funding acquisition: Shiquan Liu.
Investigation: Ni Chen.
Methodology: Ni Chen, Shiquan Liu.
Project administration: Ni Chen, Shiquan Liu.
Resources: Ni Chen.
Software: Ni Chen.
Supervision: Shiquan Liu.
Validation: Xinze Qiu, Jiean Huang, Shiquan Liu.
Visualization: Xinze Qiu, Huaqiang Ruan, Shiquan Liu.
Writing – original draft: Ni Chen.
Writing – review & editing: Ni Chen, Xinze Qiu, Huaqiang Ruan, Jiean Huang, Shiquan Liu.
Abbreviations:
- ALB
- albumin
- BCAA
- branched-chain amino acid
- CI
- confidence interval
- FINS
- fasting serum insulin
- FPG
- fasting plasma glucose
- HbA1c
- glycosylated hemoglobin
- HD
- hepatogenic diabetes
- HOMA-IR
- homeostasis model assessment method for IR
- IR
- insulin resistance
- LC
- liver cirrhosis
- LES
- late evening snack
- MD
- mean difference
- npRQ
- non-protein respiratory quotient
NC and XQ contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (81460380), the Natural Science Foundation of Guangxi Province (2020CXNSFAA159056), and the Development and Promotion Project of Suitable Technology of Traditional Chinese Medicine in Guangxi (GZSY21-56).
Ethical approval is not required for this study. This review would be disseminated in a peer-reviewed journal or conference presentations.
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
校对报告
当前使用的样式是 [Academic Medicine]
当前文档包含的题录共46条
有0条题录存在必填字段内容缺失的问题
所有题录的数据正常
How to cite this article: Chen N, Qiu X, Ruan H, Huang J, Liu S. Effects of late evening snacks on glucose homeostasis in cirrhotic patients: A meta-analysis. Medicine 2023;102:7(e32805).
Contributor Information
Ni Chen, Email: nezumi27@163.com.
Xinze Qiu, Email: qiuxinze@hotmail.com.
Huaqiang Ruan, Email: juicy_aran@163.com.
Jiean Huang, Email: hjagxmu@163.com.
References
- [1].Merli M, Berzigotti A, Zelber-Sagi S, et al. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Elkrief L, Rautou PE, Sarin S, et al. Diabetes mellitus in patients with cirrhosis: clinical implications and management. Liver Int. 2016;36:936–48. [DOI] [PubMed] [Google Scholar]
- [3].Holstein A, Hinze S, Thiessen E, et al. Clinical implications of hepatogenous diabetes in liver cirrhosis. J Gastroenterol Hepatol. 2002;17:677–81. [DOI] [PubMed] [Google Scholar]
- [4].Picardi A, D’Avola D, Gentilucci UV, et al. Diabetes in chronic liver disease: from old concepts to new evidence. Diabetes Metab Res Rev. 2006;22:274–83. [DOI] [PubMed] [Google Scholar]
- [5].Nath P, Anand AC. Hepatogenous diabetes: a primer. J Clin Exp Hepatol. 2021;11:603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kalafateli M, Mantzoukis K, Choi YY, et al. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J Cachexia Sarcopenia Muscle. 2017;8:113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Plauth M, Cabré E, Riggio O, et al. ESPEN guidelines on enteral nutrition: liver disease. Clin Nutr. 2006;25:285–94. [DOI] [PubMed] [Google Scholar]
- [8].[Clinical guidelines on nutrition in end-stage liver disease]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:330–42. [DOI] [PubMed] [Google Scholar]
- [9].Verboeket-van DVW, Westerterp KR, van Hoek B, et al. Energy expenditure and substrate metabolism in patients with cirrhosis of the liver: effects of the pattern of food intake. Gut. 1995;36:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Plank LD, Gane EJ, Peng S, et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology. 2008;48:557–66. [DOI] [PubMed] [Google Scholar]
- [11].Chen CJ, Wang LC, Kuo HT, et al. Significant effects of late evening snack on liver functions in patients with liver cirrhosis: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2019;34:1143–52. [DOI] [PubMed] [Google Scholar]
- [12].Yao J, Han W, Ren X, et al. Improvement of energy substrate metabolism by late evening snack supplementation in patients with liver cirrhosis: a meta-analysis. Ther Clin Risk Manag. 2019;15:659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yamauchi M, Takeda K, Sakamoto K, et al. Effect of oral branched chain amino acid supplementation in the late evening on the nutritional state of patients with liver cirrhosis. Hepatol Res. 2001;21:199–204. [DOI] [PubMed] [Google Scholar]
- [14].Hiraoka A, Michitaka K, Kiguchi D, et al. Efficacy of branched-chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2017;29:1416–23. [DOI] [PubMed] [Google Scholar]
- [15].Xie QL, Wang JY, Guo JY, et al. Effect of adding meal 1 hour before bedtime on energy metabolism and blood glucose level in patients with hepatitis B cirrhosis complicated with diabetes mellitus. Shan Dong Yi Xue Gao Deng Zhuan Ke Xue Xiao Xue Bao. 2020. [Google Scholar]
- [16].Takeshita S, Ichikawa T, Nakao K, et al. A snack enriched with oral branched-chain amino acids prevents a fall in albumin in patients with liver cirrhosis undergoing chemoembolization for hepatocellular carcinoma. Nutr Res. 2009;29:89–93. [DOI] [PubMed] [Google Scholar]
- [17].Nakaya Y, Okita K, Suzuki K, et al. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition. 2007;23:113–20. [DOI] [PubMed] [Google Scholar]
- [18].Ma X, Zhu J. Effect of adding meal 1 hour before bedtime on glucose metabolism and liver function in elderly patients with hepatitis B cirrhosis and diabetes mellitus. Lin Chuang Hu Li Zha Zhi. 2020. [Google Scholar]
- [19].Lin L. Effect of bedtime meals under nutrition risk screening in cirrhotic patients with diabetes. Shi Yong Zhong Xi Yi Jie He Lin Chuang. 2020. [Google Scholar]
- [20].Ichikawa T, Naota T, Miyaaki H, et al. Effect of an oral branched chain amino acid-enriched snack in cirrhotic patients with sleep disturbance. Hepatol Res. 2010;40:971–8. [DOI] [PubMed] [Google Scholar]
- [21].Hou W, Wang ZY, Dong JL, et al. Evaluation of the effect of enteral nutrition and late evening snacks in patients with liver cirrhosis. Lin Chuang Yao Wu Zhi Liao Za Zhi. 2019. [Google Scholar]
- [22].Hou W, Lv Z, Yang J, et al. Long-term carbohydrate-containing late-evening snack significantly improves the ratio of branched chain amino acids to aromatic amino acids in adults with liver cirrhosis due to Hepatitis B. Biomed Res Int. 2021;2021:1074565. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [23].Harima Y, Yamasaki T, Hamabe S, et al. Effect of a late evening snack using branched-chain amino acid-enriched nutrients in patients undergoing hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Hepatol Res. 2010;40:574–84. [DOI] [PubMed] [Google Scholar]
- [24].Dong JL, Jia L, Wang ZY, et al. Effect of late evening snacks on energy metabolism and blood glucose in patients with hepatitis B cirrhosis complicated with diabetes mellitus. Zhong Guo Quan Ke Yi Xue. 2019;22:4047–53. [Google Scholar]
- [25].Petrides AS, Groop LC, Riely CA, et al. Effect of physiologic hyperinsulinemia on glucose and lipid metabolism in cirrhosis. J Clin Invest. 1991;88:561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Judge A, Dodd MS. Metabolism. Essays Biochem. 2020;64:607–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Patel S, Jinjuvadia R, Patel R, et al. Insulin resistance is associated with significant liver fibrosis in chronic hepatitis C patients: a systemic review and meta-analysis. J Clin Gastroenterol. 2016;50:80–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Komura T, Mizukoshi E, Kita Y, et al. Impact of diabetes on recurrence of hepatocellular carcinoma after surgical treatment in patients with viral hepatitis. Am J Gastroenterol. 2007;102:1939–46. [DOI] [PubMed] [Google Scholar]
- [29].Kita Y, Mizukoshi E, Takamura T, et al. Impact of diabetes mellitus on prognosis of patients infected with hepatitis C virus. Metabolism. 2007;56:1682–8. [DOI] [PubMed] [Google Scholar]
- [30].Meroni M, Longo M, Erconi V, et al. mir-101-3p downregulation promotes fibrogenesis by facilitating hepatic stellate cell transdifferentiation during insulin resistance. Nutrients. 2019;11:2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Paradis V, Perlemuter G, Bonvoust F, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738–44. [DOI] [PubMed] [Google Scholar]
- [32].Jovanović SS, Martinović V, Bogojević D, et al. Modulation of diabetes-related liver injury by the HMGB1/TLR4 inflammatory pathway. J Physiol Biochem. 2018;74:345–58. [DOI] [PubMed] [Google Scholar]
- [33].Dai Y, Hao P, Sun Z, et al. Liver knockout YAP gene improved insulin resistance-induced hepatic fibrosis. J Endocrinol. 2021;249:149–61. [DOI] [PubMed] [Google Scholar]
- [34].Dongiovanni P, Meroni M, Baselli GA, et al. Insulin resistance promotes Lysyl Oxidase Like 2 induction and fibrosis accumulation in non-alcoholic fatty liver disease. Clin Sci (Lond). 2017;131:1301–15. [DOI] [PubMed] [Google Scholar]
- [35].Trombetta M, Spiazzi G, Zoppini G, et al. Review article: type 2 diabetes and chronic liver disease in the Verona diabetes study. Aliment Pharmacol Ther. 2005;22(Suppl 2):24–7. [DOI] [PubMed] [Google Scholar]
- [36].Lee H, Chien RN, Pao LH, et al. Decoupled glucose and lipid metabolic recovery after viral clearance in direct-acting antiviral-treated HCV patients: a 3-year prospective cohort study. Cells Basel. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang X, Shen W, Shen DM. [A clinical analysis of liver disease patients with abnormal glucose metabolism]. Zhonghua Gan Zang Bing Za Zhi. 2006;14:289–92. [PubMed] [Google Scholar]
- [38].Saito M, Seo Y, Yano Y, et al. Short-term reductions in non-protein respiratory quotient and prealbumin can be associated with the long-term deterioration of liver function after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. J Gastroenterol. 2012;47:704–14. [DOI] [PubMed] [Google Scholar]
- [39].Allison KC, Hopkins CM, Ruggieri M, et al. Prolonged, controlled daytime versus delayed eating impacts weight and metabolism. Curr Biol. 2021;31:650–657.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hernández-Conde M, Llop E, Gómez-Pimpollo L, et al. Adding branched-chain amino acids to an enhanced standard-of-care treatment improves muscle mass of cirrhotic patients with sarcopenia: a placebo-controlled trial. Am J Gastroenterol. 2021;116:2241–9. [DOI] [PubMed] [Google Scholar]
- [41].Park JG, Tak WY, Park SY, et al. Effects of branched-chain amino acid (BCAA) supplementation on the progression of advanced liver disease: a Korean nationwide, multicenter, prospective, observational, cohort study. Nutrients. 2020;12:1429. [DOI] [PMC free article] [PubMed] [Google Scholar]