Abstract
The purpose of this study was to assess the demographic data, clinical manifestations, cerebrospinal fluid (CSF), hematology, brain magnetic resonance imaging, electroencephalograms, and therapy and prognosis related to anti-gamma-aminobutyric acid B (anti-GABABR) encephalitis. We retrospectively examined the demographic data, clinical manifestations, laboratory results, brain magnetic resonance imaging, electroencephalograms, and therapy and prognosis of 6 patients with anti-GABABR encephalitis. We used the clinical data of patients with anti-GABABR encephalitis admitted to the Department of Neurology of Mianyang Central Hospital obtained from January 2017 to September 2020. Six patients with anti-GABABR encephalitis were included. Generalized tonic-clonic seizure was the first clinical symptom in 5 patients, while 1 patient first showed behavior disorder. After the first clinical symptom attack, 2 patients developed a memory deficit, 4 cases showed cognitive decline, 3 cases showed behavior disorder, 1 patient developed status epilepticus and only 1 patient returned to normal. CSF testing indicated normal intracranial pressure in 5 patients and elevated pressure in only 1 patient. Additionally, the cerebrospinal fluid tests revealed slight leukocytosis in all patients and elevated protein levels in 5 patients. The anti-GABABR antibody was positive in both serum and CSF in all patients. Brain magnetic resonance imaging showed limbic system lesions in 4 patients. Long-term electroencephalograms revealed abnormal waves in half of the patients. All patients were treated with high dosages of methylprednisolone, which was combined with intravenous immunoglobulin in 2 patients; symptoms were improved in 4 patients, 1 patient showed no significant change and 1 patient with status epilepticus died of severe pneumonia during hospitalization. Epilepsy is the most common initial symptom in patients of anti-GABABR encephalitis. Many patients are also affected by tumors. Early immunotherapy can achieve excellent effects, the long-term prognosis is good for most patients.
Keywords: anti-GABABR encephalitis, methylprednisolone, prognosis, seizure
1. Introduction
Anti-gamma-aminobutyric acid B (anti-GABAB) receptor encephalitis is a rare form of limbic encephalitis whose clinical features consist of seizures, ataxia, and opsoclonus-myoclonus and memory and behavioral changes. Half of the patients with anti-GABABR encephalitis have small cell lung cancer. As a treatment, early immunotherapy can achieve excellent effects. At present, corticosteroids, and intravenous immunoglobulin (IVIg) and plasma exchange are the first-line treatment strategies for anti-GABABR encephalitis.[1] However, the long-term prognosis is determined by the presence of an underlying malignancy.[2] Here, we retrospectively examined the demographic data, clinical manifestations, laboratory results, brain magnetic resonance imaging, electroencephalograms, and therapy and prognosis of 6 patients with anti-GABABR encephalitis to improve the diagnostics and treatment for the future.
2. Methods
2.1. Patients
In this study, we collected the data of all patients with anti-GABABR encephalitis at the Department of Neurology, Mianyang Central Hospital, from January 2017 to September 2020. All patients met the following diagnostic criteria:[3]
(1) Acute onset of seizures, memory deficits, cognitive decline, behavior disorder, psychiatric symptoms or focal neurological signs;
(2) Positive results for anti-GABABR antibodies in cerebrospinal fluid and/or serum by indirect immunofluorescence assays on human embryonic kidney (293) cells (Euroimmun, Luebeck, Germany);
(3) Age > 18 years.
2.2. Research methods
We retrospectively examined the patients’ clinical characteristics, including general demographic characteristics (age and sex), clinical manifestations, past medical history, auxiliary examination characteristics (laboratory examination, electroencephalogram and brain magnetic resonance imaging [MRI]), and therapy and prognosis. The prognosis of the patients was evaluated by examining the modified Rankin scale (mRS), which was measured at admission, discharge and 6 months after onset. An mRS score of 0 to 2 was defined as a good outcome.[4]
3. Results
3.1. Demographic and clinical symptoms
The demographic data, clinical symptoms and other information are presented in Table 1. From January 2017 to September 2020, 6 Chinese patients with anti-GABABR encephalitis were included, comprising 4 males and 2 females, aged 49 to 76 years, with an average age of 65 years.
Table 1.
Clinical symptoms and long-term follow-up results of 6 patients with anti-GABABR encephalitis.
| Assessment | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|---|---|---|---|---|---|
| Sex | M | F | F | M | M | M |
| Age | 76 | 66 | 49 | 56 | 70 | 74 |
| Date to admitting | 11 | 15 | 7 | 8 | 6 | 3 |
| Previous history | BPH and COPD | No | No | No | No | BPH and COPD |
| Personal history | Smoking | No | No | Smoking Alcohol consumption | No | Smoking |
| Initial symptom | Epilepsy | Epilepsy | Epilepsy | Epilepsy | Epilepsy | Behavior disorder |
| Other symptom | Memory deficit | No | Memory deficit Cognitive decline | Behavior disorder Cognitive decline | Behavior disorder Cognitive decline | Status epilepticus Cognitive decline |
| CSF Pressure (mmH2O)* | 123 | 108 | 158 | 162 | 150 | 250 |
| Leukocyte in CSF† | 16 *106/L | 17 *106/L | 35 *106/L | 56 *106/L | 13 *106/L | 15 *106/L |
| Protein in CSF‡ | 510 mg/L | 840 mg/L | 290 mg/L | 480 mg/L | 530 mg/L | 550 mg/L |
| CSF antibody titer | 1:100 | 1:32 | 1:320 | 1:32 | 1:32 | 1:32 |
| Serum antibody titer | 1:32 | 1:10 | 1:32 | 1:10 | 1:32 | 1:10 |
| Other antibody in CSF or Serum | Anti-Ma 2 antibody in Serum | No | No | No | No | No |
| D-dimmer§ | 1.1 mg/L | 1.72 mg/L | 0.51 mg/L | 0.42 mg/L | 0.31 mg/L | 0.54 mg/L |
| Tumor maker | No | No | No | No | No | No |
| Tumors | Suspected testicular cancer | small cell cancer | No | small cell lung cancer | Esophagus cancer | No |
| Lesions on brain MRI | Left frontal lobe, guide lobe, temporal lobe and hippocampus | Bilateral guide lobe, frontal parietal lobe and basal ganglia | No lesions | Right hippocampus | Bilateral hippocampus | unchecked |
| EEG | Normal | Abnormal | Abnormal | Normal | Normal | Abnormal |
| Corticosteroid pulse therapy | Yes | Yes | Yes | Yes | Yes | Yes |
| IVIg | No | No | No | No | Yes | Yes |
| AEDs treatment in hospital | VPA and LEV | VPA and LEV | VPA | VPA and LEV | LEV | VPA and LEV |
| mRS score at admission | 4 | 3 | 3 | 4 | 4 | 5 |
| mRS score at discharge | 1 | 2 | 0 | 0 | 4 | 6# |
| mRS score at 6 mo after onset | 0 | 0 | 0 | 0 | 3 | NA |
| Residual symptoms at discharge | No | No | No | No | Cognitive decline | NA |
| treatment after discharge | Prednisone and LEV | No | No | Prednisone and LEV | Prednisone and LEV | NA |
AEDs = antiepileptic drug, anti-GABAB = Anti-gamma-aminobutyric acid B, BPH = benign prostatic hyperplasia, COPD = chronic obstructive pulmonary disease, CSF = cerebrospinal fluid, EEG = electroencephalography, IVIg = intravenous immunoglobulin, LEV = levetiracetam, MRI magnetic resonance imaging, mRS = modified Rankin scores, NA = not available, VPA = valproic acid.
normal range of CSF Pressure: 80-180 mmH2O,
normal range of CSF leukocyte count: 0-6 × 106/L,
normal range of CSF proteins: 150-450 mg/L,
normal range of D-dimmer: 0-0.55 mg/L FEU,
# death.
The course of the disease was 3 to 15 days before treatment, with an average of 8 days. Two patients (patients 1 and 6) had a history of benign prostatic hyperplasia and chronic obstructive pulmonary disease, 3 male patients (patients 1, 4, and 6) had a history of smoking and 1 patient (patient 4) had a long history of alcohol consumption. Generalized tonic-clonic seizure was the first clinical symptom in 5 patients (patients 1, 2, 3, 4, and 5), while 1 patient (patient 6) first showed behavior disorder. However, after the behavior disorder, status epilepticus soon occurred in patient 6. Furthermore, after the first clinical symptom attack, 2 patients (patients 1 and 3) developed a memory deficit, 4 cases showed cognitive decline (patients 3, 4, 5, and 6), 3 cases showed behavior disorder (patients 4, 5 and 6), 1 patient (patient 6) developed status epilepticus and only 1 patient (patient 2) returned to normal.
3.2. Characteristics of cerebrospinal fluid (CSF) and hematological specimens
The characteristics of cerebrospinal fluid (CSF) and hematological specimens are presented in Table 1. CSF testing indicated normal intracranial pressure in 5 patients (patients 1, 2, 3, 4, and 5) and elevated pressure in only 1 patient (patient 6). Cerebrospinal fluid tests revealed slight leukocytosis in all patients in the range from 13*106 to 56*106/L, with an average of 25*106/L, as well as elevated protein levels in 5 patients (patients 1, 2, 4, 5, and 6) in the range of 290 to 840 mg/L, with an average of 533 mg/L. The anti-GABABR antibody was positive in both CSF and serum in all patients, the titer in CSF was higher than that in serum in 5 patients (patients 1, 2, 3, 4, and 6). In addition, anti-Ma 2 antibody was positive in the serum but negative in the CSF of a 76-year-old man with suspected testicular cancer (patient 1). Meanwhile, tumor markers (CA125, AFP, CEA, CA19-9, CA15-3, CA24-2, PSA, and NSE) were tested in all patients, and all of them were normal. Imaging and electroencephalogram results
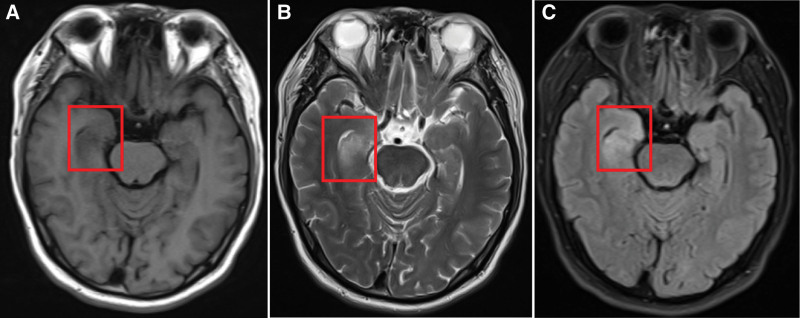
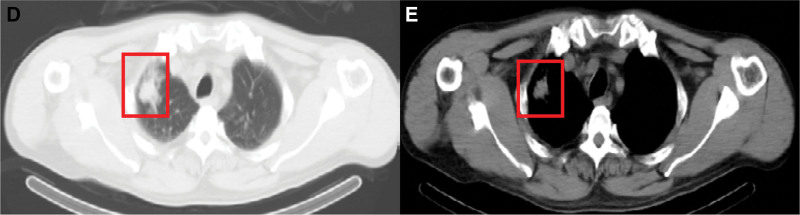
The characteristics of imaging and electroencephalogram results are presented in Table 1. We performed brain MRI on 5 patients, and only 1 patient (patient 6) was not checked due to their serious medical condition at admission. The brain MRI demonstrated encephalitis lesions in 66.7% of patients; the brain lesions were located in the bilateral/unilateral frontal lobe, guide lobe, temporal lobe, and hippocampus and basal ganglia. None of the lesions showed significant enhancement (Fig. 1). Furthermore, no lesions were found in 1 patient (patient 3) without tumors. In addition, we found definite tumors in 3 patients (patients 2, 4, and 5) and suspected testicular cancer in 1 patient (patient 1) (Fig. 2). Long-term electroencephalograms revealed abnormal waves in half of the patients. These abnormalities are mostly typical of sharp waves, indicating epileptic seizures.
Figure 1.
Brain magnetic resonance imaging (MRI) findings in patients with anti-GABABR encephalitis. anti-GABAB = Anti-gamma-aminobutyric acid B, MRI = magnetic resonance imaging.
Figure 2.
Chest computed tomography (CT) findings in patients with anti-GABABR encephalitis. anti-GABAB = Anti-gamma-aminobutyric acid B.
3.3. Treatment and prognosis
In our research series, all patients were treated with corticosteroid pulse therapy (methylprednisolone 1 g/day for 5 days, 0.5 g/day for 3 days, 0.25 g/day for 3 days, 0.125 g/day for 3 days, and subsequent oral administration), which was combined with intravenous gamma globulin treatment (0.4 g/kg/day for 5 days) in 2 patients with severe symptoms (patients 5 and 6). All patients received antiepileptic drugs. After treatment, 4 patients (patients 1, 2, 3, and 4) showed complete or partial neurological relief with improved mRS scores (range: 0–2). One patient (patient 5) showed no significant change, and 1 patient (patient 6) died of severe pneumonia during hospitalization. At discharge, 4 patients (patients 1, 2, 3, and 4) showed good outcomes. At follow-up after 6 months, 4 patients (patients 1, 2, 3, and 4) had recovered completely, and only 1 patient (patient 5) still had residual cognitive decline.
4. Discussion
Metabotropic gamma-aminobutyric acid type B receptor (GABABR) is found at the end of synapses in the central nervous system and plays a vital role in inhibitory neurotransmission.[5] The GABABR is a G-protein-coupled receptor that acts slowly and maintains the inhibitory tone.[6] Research has shown that the GABABR is associated with brain and behavioral diseases, including epilepsy, anxiety, and spasticity and neuropathic pain.[7] In 2010, Lancaster et al[8] first reported 15 patients who were positive for anti-GABABR antibodies in CSF and analyzed the clinical characteristics, treatment and prognosis of this encephalitis. In the following 10 years, dozens of other series and/or case reports related to this encephalitis were published in various medical journals (Table 2). In these reports, epilepsy, psychiatric symptoms and memory dysfunction were the most prevalent conditions, and some patients even had persistent epilepsy or refractory epilepsy. Some patients had confusion, disorientation and abnormal behavior. Few patients were affected by speech disorder, opsoclonus-myoclonus, and gait ataxia and orofacial twitching. In addition, some studies have shown that some patients have symptoms of infectious encephalitis such as headache and fever before the diagnosis of autoimmune encephalitis.[9–19] In our cohort, all patients were positive for anti-GABABR antibody in cerebrospinal fluid and serum, and the titer in cerebrospinal fluid was higher than that in serum. In addition, there are also many case reports, which have shown a portion of patients who were positive for a combination of other antibodies, such as anti-GAD, anti-Hu, anti-VGCC, anti-CRMP5/CV2, and anti-IgLON5 and anti-SOX1.[20–24] As in the previous reports, our study confirmed that all patients had seizures. In addition, behavior disorder, memory deficit and cognitive decline also appeared in a few patients. The encephalitis associated with GABABR antibodies usually develops as limbic encephalitis.[8] Cerebrospinal fluid examination and brain MRI play an important role in limbic encephalitis. Some patients with anti-GABABR encephalitis have a slightly elevated level of white blood cells and proteins in their cerebrospinal fluid, but this is not distinguishable from viral or other infectious encephalitis types. Therefore, detecting antineuronal antibodies from cerebrospinal fluid and/or serum is of great value. These antibodies should include cell surface antibodies and classical paraneoplastic antibodies, such as NMDAR, AMPAR 1, AMPAR 2, LGI 1, Caspr 2, GABABR, Hu, Yo, Ri, CV 2, Ma 2, amphiphysin, ANNA-3, Tr, and PCA-2 and GAD.[25] Detecting the specific types of antibody can allow for distinguishing from other autoimmune encephalitis types. Many studies in the past have shown that unilateral or bilateral hyperintense lesions in the medio-temporal lobes, hippocampus and amygdala were found by the brain MRI.[12] These abnormal signals in brain MRI can explain the symptoms of epilepsy, cognitive impairment, and mental abnormalities and other manifestations in patients with anti-GABABR encephalitis. In a small number of patients, abnormalities may be found outside the limbic system.[17] In our series, brain MRI examination confirmed intracranial lesions in 4 patients and a normal state in 1 patient. These lesions may appear in patients with other types of limbic encephalitis. Therefore, we consider that brain MRI has no specificity for the diagnosis of anti-GABABR encephalitis. Although epileptic seizures are a major clinical manifestation of anti-GABAB receptor encephalitis, the EEG features are usually nonspecific. In patients with anti-GABABR encephalitis, about 63% to 100% patients show abnormalities with long-term EEG monitoring. Such abnormalities include ictal or postepileptic state.[18] In addition, patients with anti-GABABR encephalitis have a high proportion of tumors, especially small cell lung cancer.[26] Therefore, cancer screening is essential for every patient, especially in the respiratory system, and digestive system and genitourinary system.
Table 2.
Summary of Findings of studies included for anti-GABABR encephalitis.
| Author/Year | No.Of patients | Mean age | Sex ratio (% males) | Clinical presentation | CSF Abnormality | MRI(abnormality, commonest areas) | EEG abnormality | Cancer rate(%) | Outcome (mRS 0–2) |
|---|---|---|---|---|---|---|---|---|---|
| Kim et al, 2014 | 5 | 63.6 | 3/2 (67) | Confusion and disorientation 100% Psychiatric symptoms 100% Seizures 60% | 80% | 40%, MTL | 5/5 (100) | 4/5 (80) | 5/5 (100) |
| Guan et al, 2015 | 18 | 56.4 | 13/5 (77) | Memory dysfunction 67% Psychiatric symptoms 61% Seizures 94% | NA | 56%, MTL | 12/14 (86) | 6/18 (33) | 11/18 (61) |
| Qiao et al, 2016 | 7 | 51.2 | 6/1 (86) | Memory dysfunction 100% Psychiatric symptoms 100% Seizures 100% | 100% | 57%, Hippocampus, MTL | 7/7 (100) | 4/7 (57) | 3/7 (43) |
| Chen et al,. 2017 | 11 | 51 | 8/3 (72) | Memory dysfunction 90% Psychiatric symptoms 90% Seizures 100% | 100% | 63%, MTL | 7/11 (63) | 3/11 (27) | 7/11 (63) |
| Cui et al, 2018 | 11 | 63 | 7/4 (64) | Memory dysfunction 100% Psychiatric symptoms 46% Seizures 91% | 73% | 36%, Hippocampus, MTL | 10/11 (91) | 5/11 (45) | 8/11 (73) |
| Si et al, 2019 | 5 | 41.2 | 3/2 (60) | Confusion and disorientation 60% Psychiatric symptoms 100% Seizures 80% | 0% | 60%, Hippocampus, MTL | 5/5 (100) | 0/5 (0) | 5/5 (100) |
| Lin et al, 2019 | 28 | 53 | 17/11 (61) | Memory dysfunction 93% Psychiatric symptoms 86% Seizures 96% | NA | 25%, Hippocampus, MTL | 18/24 (75) | 9/28 (32) | 16/28 (57) |
| Zhao et al,.2020 | 12 | 65 | 9/3 (75) | Memory dysfunction 91% Psychiatric symptoms 58% Seizures 91% | 100% | 33%, MTL | 11/12 (91%) | 7/12 (58) | 7/12 (58) |
| Zeng et al, 2020 | 7 | 44.7 | 4/3 (57) | Memory dysfunction 86% Psychiatric symptoms 71% Seizures 100% | 29% | 72%, Hippocampus, MTL | 6/6 (100) | 3/7 (42) | 4/7 (57) |
| Zhang et al, 2020 | 19 | 58.3 | 10/9 (53) | Memory dysfunction 95% Psychiatric symptoms 89% Seizures 90% | NA | 72%, Hippocampus, MTL | 17/19 (89) | 5/19 (26) | 11/19 (58) |
| Zhu et al, 2020 | 14 | 52 | 9/5 (64) | Memory dysfunction 79% Psychiatric symptoms 64% Seizures 100% | NA | 57%, Hippocampus, temporal lobe | 10/14 (71) | 3/14 (21) | NA |
Data are expressed as proportion (%) unless otherwise specified. Age is expressed in years.
anti-GABAB = Anti-gamma-aminobutyric acid B, CSF = cerebrospinal fluid, EEG = electroencephalogram, MRI = magnetic resonance imaging, mRS = modified rankin scores, MTL = medial temporal lobe, NA = not available.
Until now, there was no standard treatment for limbic encephalitis. Common first-line immunotherapeutic agents include corticosteroids, IVIg and plasma exchange.[1,3] Despite a lack of evidence, corticosteroids are frequently the first choice in patients with anti-GABABR encephalitis.[27] Of course, corticosteroids with either IVIg or plasma exchange are also recommended to treat limbic encephalitis. However, plasma exchange after IVIg is not recommended. If these treatments do not respond well, second-line therapies such as rituximab and cyclophosphamide should be performed.[28,29] Tocilizumab and low-dose interleukin-2 (aldesleukin) are used sometimes.[30,31] Azathioprine and mycophenolate mofetil are also used occasionally.[1]
In our research series, all patients were treated with corticosteroids and antiepileptic drugs, which were combined with IVIg in 2 patients. After treatment, 4 patients symptoms were improved, 1 patient showed no significant change and 1 patient died of severe pneumonia. At discharge, 3 patients continued to take oral prednisone and levetiracetam. Early immunotherapy can help to achieve a good prognosis for anti-GABABR encephalitis patients without tumors.[16] Anti-tumor therapy is highly necessary for anti-GABABR encephalitis patients with cancer, in addition to immunotherapy. Of course, recurrent anti-GABABR encephalitis has been reported in some studies. Wu et al[32] reported that the recurrence rate was 9% (1/11), but the sample size was small and the clinical symptoms during the patient recurrence were less severe than in the first onset. Although this disease has a good prognosis, some patients still die after treatment, with severe pneumonia being the main cause of death, regardless of whether there is a tumor. Deep vein thrombosis is another cause of death. postepileptic coma or persistent epilepsy can increase the risk of lung infections and deep vein thrombosis.[15] Therefore, antiepileptic, and antibacterial and anticoagulant therapy is necessary for anti-GABABR encephalitis patients.
Conclusion
Epilepsy is the most common initial symptom in patients of anti-GABABR encephalitis. Many patients are also affected by tumors. Early immunotherapy can achieve excellent effects, and the long-term prognosis is good for most patients.
Author contributions
Data curation: Qiang Li, Xianwen Zhang, Jingfeng Duan
Formal analysis: Jingfeng Duan
Funding acquisition: Ting Zeng
Investigation: Qiang Li, Yufeng Tang, Xianwen Zhang, Bufan Yang
Project administration: Yufeng Tang
Resources: Qiang Li, Yufeng Tang, Bufan Yang
Software: Qiang Li, Ting Zeng
Validation: Xianwen Zhang
Abbreviations:
- anti-GABAB =
- anti-gamma-aminobutyric acid B
- CSF
- cerebrospinal fluid
- IVIg
- intravenous immunoglobulin
- MRI
- magnetic resonance imaging
- mRS
- modified Rankin scale
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
The study was approved by the local Ethics Committee of the Mianyang Central Hospital. Consent was obtained from all individual participants included in the study.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Li Q, Zhang X, Zeng T, Yang B, Duan J, Tang Y. Clinical characteristics and prognosis of anti-GABABR encephalitis: A single-center experience. Medicine 2023;102:7(e32956).
Contributor Information
Qiang Li, Email: lidcq2683@163.com.
Xianwen Zhang, Email: 675061951@qq.com.
Ting Zeng, Email: 876322181@qq.com.
Bufan Yang, Email: ml15030572791@163.com.
Jingfeng Duan, Email: duancs@126.com.
References
- [1].Shin YW, Lee ST, Park KI, et al. Treatment strategies for autoimmune encephalitis. Ther Adv Neurol Disord. 2018;11:1756285617722347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hoftberger R, Titulaer MJ, Sabater L, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology. 2013;81:1500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Neurology Branch of Chinese Medical Association. Chinese expert consensus on the diagnosis and management of autoimmune encephalitis. Chin J Neurol. 2017;50:91–8. [Google Scholar]
- [4].Van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–7. [DOI] [PubMed] [Google Scholar]
- [5].Bowery NG, Hill DR, Hudson AL, et al. Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980;283:92–4. [DOI] [PubMed] [Google Scholar]
- [6].Bettler B, Kaupmann K, Mosbacher J, et al. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–67. [DOI] [PubMed] [Google Scholar]
- [7].Froestl W. Chemistry and pharmacology of GABAB receptor ligands. Adv Pharmacol. 2010;58:19–62. [DOI] [PubMed] [Google Scholar]
- [8].Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim TJ, Lee ST, Shin JW, et al. Clinical manifestations and outcomes of the treatment of patients with GABAB encephalitis. J Neuroimmunol. 2014;270:45–50. [DOI] [PubMed] [Google Scholar]
- [10].Guan HZ, Ren HT, Yang XZ, et al. Limbic encephalitis associated with anti- γ- aminobutyric acid B receptor antibodies: a case series from China. Chin Med J (Engl). 2015;128:3023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Qiao S, Zhang YX, Zhang BJ, et al. Clinical, imaging, and follow-up observations of patients withanti-GABABR encephalitis. Int J Neurosci. 2017;127:379–85. [DOI] [PubMed] [Google Scholar]
- [12].Chen X, Liu F, Li JM, et al. Encephalitis with antibodies against the GABAB receptor: seizures as the most common presentation at admission. Neurol Res. 2017;39:973–80. [DOI] [PubMed] [Google Scholar]
- [13].Cui J, Bu H, He J, et al. The gamma-aminobutyric acid-B receptor (GABAB) encephalitis: clinical manifestations and response to immunotherapy. Int J Neurosci. 2018;128:627–33. [DOI] [PubMed] [Google Scholar]
- [14].Si Z, Wang A, Liu J, et al. Typical clinical and imaging manifestations of encephalitis with anti-γ-aminobutyric acid B receptor antibodies: clinical experience and a literature review. Neurol Sci. 2019;40:769–77. [DOI] [PubMed] [Google Scholar]
- [15].Lin J, Li C, Li A, et al. Encephalitis with antibodies against the GABAB receptor: high mortality and risk factors. Front Neurol. 2019;10:1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhao XH, Yang X, Liu XW, et al. Clinical features and outcomes of Chinese patients with anti- γ-aminobutyric acid B receptor encephalitis. Exp Ther Med. 2020;20:617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zeng W, Cao L, Zheng J, et al. Clinical characteristics and long-term follow-up of seven cases of anti-GABABR encephalitis in patients of Han Chinese descent. Neurol Sci. 2020;41:373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang X, Lang Y, Sun L, et al. Clinical characteristics and prognostic analysis of anti-gamma-aminobutyric acid-B (GABA-B) receptor encephalitis in Northeast China. BMC Neurol. 2020;20:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhu F, Shan W, Lv R, et al. Clinical characteristics of anti-GABA-B receptor encephalitis. Front Neuro. 2020;11:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Boronat A, Sabater L, Saiz A, et al. GABA(B) receptor antibodies in limbic encephalitis and anti-GAD-associated neurologic disorders. Neurology. 2011;76:795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cho JJ, Wymer JP. Paraneoplastic lambert-eaton myasthenic syndrome with limbic encephalitis: clinical correlation with the coexistence of anti-VGCC andanti-GABABR antibodies. J Clin Neuromuscul Dis. 2017;19:84–8. [DOI] [PubMed] [Google Scholar]
- [22].Li H, Zhang A, Hao Y, et al. Coexistence of Lambert–Eaton myasthenic syndrome and autoimmune encephalitis with anti-CRMP5/CV2 andanti-GABABR antibodies in small cell lung cancer: a case report. Medicine (Baltim). 2018;97:e0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chung HY, Wickel J, Voss A, et al. Autoimmune encephalitis with anti-IgLON5 and anti-GABAB-receptor antibodies: a case report. Medicine (Baltim). 2019;98:e15706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Qin W, Wang X, Yang J, Hu W. Coexistence of anti-SOX1 and Anti-GABAB receptor antibodies with autoimmune encephalitis in small cell lung cancer: a case report. Clin Interv Aging. 2020;15:171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Leypoldt F, Armangue T, Dalmau J. Autoimmune encephalopathies. Ann N Y Acad Sci. 2015;1338:94–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nosadini M, Mohammad SS, Ramanathan S, et al. Immune therapy in autoimmune encephalitis: a systematic review. Expert Rev Neurother. 2015;15:1391–419. [DOI] [PubMed] [Google Scholar]
- [28].Lee WJ, Lee ST, B JI, et al. Rituximab treatment for autoimmune limbic encephalitis in an institutional cohort. Neurology. 2016;86:1683–91. [DOI] [PubMed] [Google Scholar]
- [29].Wiseman AC. Immunosuppressive medications. Clin J Am Soc Nephrol. 2016;11:332–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lee WJ, Lee ST, Moon J, et al. Tocilizumab in autoimmune encephalitis refractory to rituximab: an institutional cohort study. Neurotherapeutics. 2016;13:824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lim JA, Lee ST, Moon J, et al. New feasible treatment for refractory autoimmune encephalitis: low-dose interleukin-2. J Neuroimmunol. 2016;299:107–11. [DOI] [PubMed] [Google Scholar]
- [32].Wu H, Wang Y, Wei K, et al. Clinical characteristics and elevated ProGRP and positive oligoclonal bands of 13 Chinese cases with anti-GABABR encephalitis. Int J Dev Neurosci. 2021;81:492–501. [DOI] [PubMed] [Google Scholar]