Abstract
Vaginal microbiome is mutually beneficial to the host and has a significant impact on health and disease. Candida species, including Candida albicans, are part of the mucosal flora of most healthy women. Under suitable conditions, they can live in the vulvovaginal mucosa, resulting in symptomatic vulvovaginal candidiasis (VVC). Based on the analysis of 16S ribosomal RNA gene sequences, great progress has been made in exploring the composition and structure of vaginal bacterial community. Moreover, researchers have conducted several studies on whether vaginal microbiome will change during VVC infection. In addition, it has been reported that vaginal colonization of probiotics in vaginal microorganisms, especially Lactobacillus, can effectively reduce the risk of VVC and treat VVC. This review aims to summarize the changes of vaginal microflora during VVC infection, and further point out the possibility of using lactic acid bacteria as probiotics to treat VVC, so as to reduce the adverse consequences of VVC infection and reduce the expensive treatment cost.
Keywords: vulvovaginal candidiasis, vaginal infection, microbial changes, Lactobacillus, treatment
Introduction
Bacteria are the largest group of all organisms, which first appeared about 3.8 billion years ago, and nucleolus is one of their most obvious signs (Mojzsis et al., 1996). Fungi have true nuclei and complete organelles, so they are also called eukaryotic cell type microorganisms (Lopez-Garcia and Moreira, 2020). The human microbiome is made up of archaea, bacteria, viruses, and fungi that form a highly complex web of interactions with each other and their hosts (Wang et al., 2015; Wu et al., 2020). The host and the microbial community have co-evolved an immune system to prevent the colonization of the foreign microorganisms in the body (Wu et al., 2019). Researchers have used a variety of methods, including metabolomics, proteomics, transcriptomics and metagenomics, to verify that microbial communities are dynamic, interactive and complex organic whole (Chen et al., 2021; Xiang et al., 2022).
The impact of microbial flora in the human body has aroused extensive concern. It not only plays an important role in the intestine, which is widely studied nowadays, but also gradually functions in other organ systems (Champer et al., 2018; Khanna et al., 2022). Nowadays, researchers pay more and more attention to women’s health, especially the vaginal microbiota, since the vagina has a huge microecosystem containing billions of species of microorganisms (Witkin and Forney, 2020). In recent years, tremendous advances have been made in exploring the composition and structure of vaginal bacterial community using the method based on analysis of 16S ribosomal RNA (rRNA) gene sequences (Chen et al., 2017). In the case of bacterial or fungal infections, changes in the vaginal microbiome have also been reported (Onderdonk et al., 2016; Chen et al., 2021).
In addition to the widely studied bacterial vaginosis (BV), vulvovaginal candidiasis (VVC) has been a hot topic, which is a multifactor infectious disease of women’s lower reproductive tract, mainly caused by Candida albicans, resulting in pathological inflammation (Farr et al., 2021). In a study that collected 649 separate strains of VVC patients in China, researchers found that Candida albicans was the dominant pathogen of VVC, but the proportion of non-Candida albicans infection was also increasing (Pang et al., 2022).
This review aims to describe changes in the vaginal microbiome during VVC, propose the possible associations between VVC and vaginal microbiome changes, and summarize the possibility of using this association to treat and defend VVC to reduce adverse health outcomes and treatment costs.
Vaginal microbiota in healthy women
The native microbiota in the vaginal environment is thought to be symbiotic with the host (Ma et al., 2012). The technology for assessing human microbial diversity has gradually progressed. Nowadays, scientists have successfully identified different bacterial communities in the vagina of women of four races using advanced high-throughput methods and analyzed species composition through 16S rRNA gene sequencing (Di Bella et al., 2013). The vaginal bacterial communities of these women can be roughly divided into five types: the first four are mainly composed of Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners, and Lactobacillus jensenii, while the fifth bacterial community is relatively specific, showing a low proportion of Lactobacillus and a high proportion of strictly anaerobic organisms (Ravel et al., 2011). Therefore, Lactobacillus are considered the most common microorganisms isolated from the vagina of healthy people, including Lactobacillus iners, Lactobacillus crispatus, Lactobacillus gasseri and Lactobacillus jensenii (Chee et al., 2020). In several studies, these vaginal Lactobacillus can be used to prevent the invasion of pathogens (Petrova et al., 2017; Kalia et al., 2020).
In addition, fungi, especially Candida, are believed to exist in the vaginal mucosa as symbiotes, forming a complex vaginal ecosystem with other bacteria (Gow and Hube, 2012; Hall and Noverr, 2017). The defense function of fungi plays a role through various mechanisms, including lowering vaginal pH, producing bioactive compounds, competing for nutrients and adhesion sites, and regulating host immune responses (Parolin et al., 2015; Calonghi et al., 2017; Parolin et al., 2018; Younes et al., 2018).
Of course, it cannot be ignored that the vaginal microbiome is variable. Vaginal microbiome varies among individuals, and these differences are caused by differences in sexual habits, menstrual hygiene habits (Noyes et al., 2018), flushing habits (Schwebke et al., 1999), chronic stress (Culhane et al., 2002), geography (Gupta et al., 2017), socioeconomic status, psychosocial pressure, community characteristics (Paul et al., 2008), and other factors. Moreover, vaginal pH also varies due to the different compositions in certain populations. Recent studies have found that a small percentage of asymptomatic healthy women have low levels of Lactobacillus in their vaginas, but they include a variety of facultative or strictly anaerobic bacteria with a slightly higher pH (5.3-5.5), which is similar to the fifth bacterial community described above. When these women did not have the disease, their vaginal bacterial community was considered normal, while the abnormal type of vaginal microbiome may be strongly associated with symptomatic bacterial vaginosis (Zhou et al., 2007; Zhou et al., 2010; Denning et al., 2018). Furthermore, vaginal microorganisms are a dynamic ecosystem of more than 200 bacteria, and the same individuals may also be significantly different from their previous performance (Chen et al., 2017). There is some evidence that the vaginal microbiome changes throughout a woman’s life and therefore has an important impact on the quality of life from newborn to post-menopausal age.
The changes of the vaginal microbiome include the decrease in the abundance of Lactobacillus and the increase in the abundance of facultative and anaerobic organisms, which can make the host vulnerable to a variety of diseases, such as low birth weight and increased bacterial infection risk (Jayaram et al., 2020). Therefore, the physiological state of the vaginal environment is of great significance for the health and reproduction of the host (Amabebe and Anumba, 2018).
To sum up, based on the variable and important characteristics of vaginal microbiome, we strongly advocate a more precise definition of the bacterial community in healthy women, which should fully consider the differences among individuals. The precise definition of a healthy vaginal microbiome can be used to diagnose disease faster and more accurately when the vaginal microbiome changes.
Vaginal microbial changes during VVC
The disruption of vaginal ecosystem balance can cause pathogen overgrowth, which will lead to more complex vaginal infections, such as BV, sexually transmitted infections (STIs), and VVC (Chee et al., 2020). Among them, VVC is defined as a symptom of inflammation and excessive overgrowth of Candida, especially Candida albicans (Sobel, 2007). Candida albicans is a major species in premenopausal, pregnant, and acute VVC women (Farr et al., 2021). VVC is one of the most common infectious vaginitis, second only to BV, and it is estimated that about 75% of women have been infected at least once in their lifetime. In addition, recurrent VVC can affect nearly 8% of women in the world (Sobel, 2016; Matheson and Mazza, 2017; Denning et al., 2018). Many experiments have shown that vaginal microbiota changes, including species and metabolites, occur during VVC infection.
Some experiments showed no significant change in the vaginal microbiota during VVC infection. As early as 2009, studies showed that no new bacteria were found in women who frequently suffered from VVC. This study evaluated the vaginal microbial species composition of 42 women with and without frequent VVC, and evaluated microbial community diversity on this basis. The results showed no significant differences in the vaginal microbiome between the two groups. This study also showed that the vaginal flora of most women in the two groups was dominated by Lactobacillus, which was similar to the vaginal microbiota of most healthy women mentioned above. Therefore, it failed to provide evidence to prove the change or unusual presence of the vaginal bacterial communities in women with frequent VVC compared to those without frequent VVC (Zhou et al., 2009).
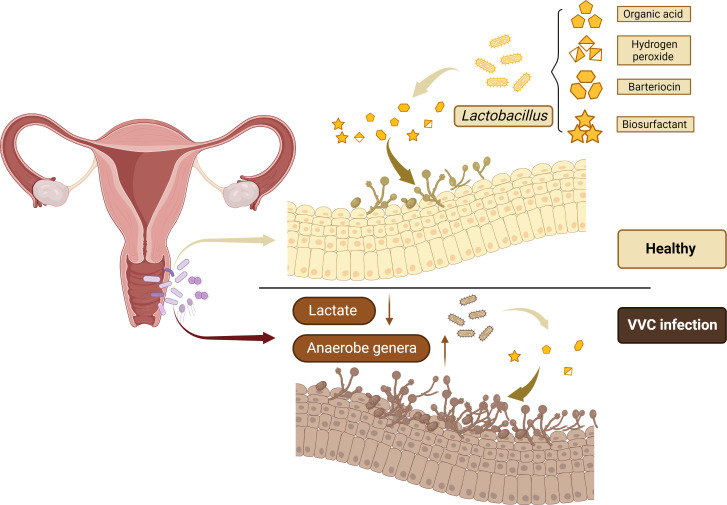
However, there are still many studies to support the theory that the vaginal microbiome of VVC patients is different from that of the normal population ( Figure 1 ). In 1980, researchers studied Candida albicans in the vaginal microbiome. The results obtained from 340 vaginal specimens showed in the absence of Candida albicans, all microbiome increased, especially Gram-negative bacteria (Auger and Joly, 1980). Liu et al. measured the vaginal microbial communities in patients with BV and VVC. The results showed that VVC patients had high changes in their vaginal microbiome (Liu et al., 2013). The healthy vaginal microbiome is dominated by Lactobacillus crispatus, however, when turning from health to Chlamydia trachomatis infection (CT), VVC, and BV, Lactobacillus crispatus is gradually replaced by Lactobacillus iners. Studies have shown that CT, VVC and BV are mainly characterized by Gardnerella, Prevotella, Megasphaera, Roseburia, and Atopobium. At the same time, changes in bacterial community during genital infection will lead to significant changes in the composition of vaginal metabolites. The production of lactic acid is highly conserved in the vaginal microorganisms among different women, while the decline of lactic acid is a common sign of all the above pathological conditions (Ceccarani et al., 2019).
Figure 1.
Lactobacillus is the most frequently isolated microorganism from the healthy women vagina to prevent the colonization and overgrowth of pathogens by excreting metabolic by-products, including organic acid, hydrogen peroxide, bacteriocin, and biosurfactant.
The microbiome of many VVC patients receiving treatment is similar to the abnormal vaginal microbiome of healthy women, suggesting that the abnormal vaginal microbiome may constitute a transitional state between disease and health, especially since many women have asymptomatic infections with Candida (Liu et al., 2013). Therefore, in addition to focusing on women with abnormal vaginal microbiome during VVC, we should also pay attention to women with asymptomatic infections with Candida, and women who appear healthy but have abnormal vaginal microbiome.
Lactobacillus treatment and defense against VVC
Over the past few years, the investigation of the vaginal microbiome has grown exponentially. These studies together found that a dominant microflora was observed in healthy vaginas: Lactobacillus. Therefore, it is proposed that vaginal colonization of Lactobacillus can reduce the risk of VVC.
It has been proved that the bacterial microbiome in the mucosal layer can achieve defense function through acidic pH regulation, release antifungal peptides and physiological control of ecological disorders. The important role of bacterial microorganisms, especially Lactobacillus, in maintaining vaginal health can promote their application as potential therapeutic methods for VVC, and alleviate the symptoms of VVC (Balakrishnan et al., 2022). Lactobacillus are thought to prevent the colonization and overgrowth of pathogens by excreting metabolic by-products and acidification of the vaginal microenvironment, helping maintain body balance. Metabolites of Lactobacillus, including organic acid, hydrogen peroxide, bacteriocin and biosurfactant, can contribute to antifungal effect (Wang et al., 2017; Fuochi et al., 2019). Researchers are also exploring whether Lactobacillus can prevent pathogens from colonizing the body, and whether it can be used to treat VVC in women. In recent years, several studies on the influence of Lactobacillus on VVC have drawn different conclusions, mainly regarding the role of Lactobacillus in preventing Candida colonization and treating VVC.
In vitro experiments have shown that some Lactobacillus strains can inhibit the adhesion and growth of Candida albicans, which will provide new insights into the prevention and treatment of VVC ( Table 1 ). As early as 1999, it was reported that Lactobacillus pentosus TV35b, isolated from the vaginal posterior fornix secretion of prenatal patients, produced a bacteriocin like peptide to inhibit Candida albicans (Okkers et al., 1999). In 2001, Osset et al. found that 8 of the 15 Lactobacillus significantly blocked the adhesion of Candida albicans Y18 to vaginal cells. In liquid assays, some Lactobacillus had a certain degree of inhibition to Candida albicans Y17 (Osset et al., 2001). In 2005, Strus et al. found that Lactobacillus delhalis produced a large amount of H2O2, which more strongly inhibited Candida albicans growth faster than other strains isolated from healthy women vaginas, and Lactobacillus plantarum without H2O2 showed the longest inhibitory activity after 24 h (Strus et al., 2005). In 2016, researchers evaluated the in vitro probiotic potential of 23 Lactobacillus isolated from the vaginal ecosystem of healthy women for BV and VVC treatment. In vitro experiments have shown that all these strains had excellent adhesion properties, which can aggregate with Gardnerella vaginalis and Candida albicans, producing a large amount of hydrogen peroxide and lactic acid. These results suggested that these strains have promising probiotic potential for the prevention and treatment of BV and VVC (Santos et al., 2016). In 2017, Wang et al. found that Lactobacillus crispatus showed significant antimicrobial activity. Seven kinds of cell-free supernatants from the Lactobacillus crispatus reduced the growth of Candida albicans. It was shown that Lactobacillus crispatus is a dominant Lactobacillus genus, which is associated with a healthy vagina and strongly inhibits the growth and hyphal formation of Candida albicans (Wang et al., 2017). In 2019, Li et al. investigated the therapeutic effects and mechanisms of Lactobacillus crispatus and Lactobacillus delbrueckii on VVC caused by Candida albicans in a Sprague-Dawley rat model. In vitro results demonstrated that two Lactobacillus strains showed inhibitory activity against Candida colony forming unit counts, indicating that Lactobacillus crispatus and Lactobacillus delbrueckii may become potential adjuvants of VVC, especially in patients with antifungal drug resistance, adverse reactions or contraindications (Li et al., 2019). Some Lactobacillus species can produce small molecules under laboratory conditions that can block Candida albicans yeast-to-filament transition, which is an important virulence trait. In 2021, relevant results showed that the 1-acetyl-β-carboline produced by Lactobacillus can prevent the yeast-to-filament transition of Candida albicans by inhibiting Yak1 (MacAlpine et al., 2021). Researchers examined the inhibitory activity of bacteriocin-like inhibitory substances (BLISs) from Lactobacillus and Streptococci on Candida albicans and non-Candida albicans isolated from patients with VVC. Using agar pore diffusion test, BLISs can inhibit both Candida albicans and non-Candida albicans (Hefzy et al., 2021). The bactericidal effect of Lactobacillus casei on the main VVC pathogenic species Candida albicans, Candida tropicalis, Clostridium novigen and Paracandida was investigated by calculating the colony forming units after co-cultivation. Lactobacillus casei had an inhibitory effect on all tested Candida genera, and Lactobacillus casei could reduce the formation of Candida albicans mycelia and early biofilm, showing a strong anti-Candida effect (Paniagua et al., 2021).
Table 1.
In vitro experiments.
| Authors | Time | Results | References |
|---|---|---|---|
| Okkers et al. | 1999 | Lactobacillus pentosus TV35b produced a bacteriocin like peptide to inhibit Candida albicans | (Okkers et al., 1999) |
| Osset et al. | 2001 | Some Lactobacillus had a certain degree of inhibition to Candida albicans Y17. | (Osset et al., 2001) |
| Strus et al. | 2005 | Lactobacillus delhalis produced a large amount of H2O2 and inhibited Candida albicans growth. Lactobacillus plantarum without H2O2 showed the longest inhibitory activity after 24 h | (Strus et al., 2005) |
| Santos et al. | 2016 | Lactobacillus strains had excellent adhesion properties, which can aggregate with Gardnerella vaginalis and Candida albicans, producing a large amount of hydrogen peroxide and lactic acid. | (Santos et al., 2016) |
| Wang et al. | 2017 | 7 kinds of cell-free supernatants from the Lactobacillus crispatus reduced the growth of Candida albicans. | (Wang et al., 2017) |
| Li et al. | 2019 | Lactobacillus strains showed inhibitory activity against Candida colony forming unit counts, indicating that Lactobacillus crispatus and Lactobacillus delbrueckii may become potential adjuvants of VVC. | (Li et al., 2019) |
| MacAlpine et al. | 2021 | 1-acetyl-β-carboline produced by Lactobacillus can prevent the yeast-to-filament transition of Candida albicans by inhibiting Yak1. | (MacAlpine et al., 2021) |
| Hefzy et al. | 2021 | Bacteriocin-like inhibitory substances can inhibit both Candida albicans and non-Candida albicans. | (Hefzy et al., 2021) |
| Paniagua et al. | 2021 | Lactobacillus casei had an inhibitory effect on all tested Candida genera, and Lactobacillus casei could reduce the formation of Candida albicans mycelia and early biofilm. | (Paniagua et al., 2021) |
Lactobacillus strains and their products can inhibit the growth of Candida, and clinical studies are also in progress ( Table 2 ). In 2001, a trial of 64 healthy women was conducted in which oral capsules of Lactobacillus rhamnosus GR-1 and Lactobacillus fermentum RC-14 were taken daily. The results showed that the combination of Lactobacillus rhamnosus GR-1 and Lactobacillus fermentum RC-14 can not only be safely used for daily use in healthy women, but also reduce the potential pathogenic bacteria and yeast colonization in vagina (Reid et al., 2003). De Seta et al. also evaluated the effect of the application of Lactobacillus plantarum P17630 on the recovery of vaginal microbiota and prevention of recurrence in women with acute VVC. These results confirmed the role of Lactobacillus plantarum P17630 as a potential empirical preventive agent, which can reduce vaginal discomfort after routine treatment of acute VVC and improve vaginal pH (De Seta et al., 2014). In 2019, Russo et al. found that the combination of a Lactobacillus mixture with lactoferrin is a safe and effective auxiliary method to reduce VVC symptoms and recurrence (Russo et al., 2019). Based on in vitro evaluation, Oerlemans et al. selected three strains from the Lactobacillus genus complex (Lactobacillus rhamnosus GG, Lactobacillus pentosus KCA1, and Lactobacillus plantarum WCFS1) and prepared them with gel for vaginal use. The gel was evaluated in 20 patients suffering from acute VVC, whose fungal concentrations were similar to those of women treated with fluconazole. These results pointed out the important aspects of choosing Lactobacillus for VVC treatment in the future (Oerlemans et al., 2020). Besides, the safety and antimicrobial activity of biosurfactants (BS) isolated from Lactobacillus vaginalis strains on Candida genus were studied. The results showed that BS from Lactobacillus crispatus BC1 can interfere with the adherence of Candida in vivo and in vitro, indicating its potential as a preventive measure against Candida mucosal damage during VVC (De Gregorio et al., 2020).
Table 2.
Clinical studies.
| Authors | Time | Results | References |
|---|---|---|---|
| Reid et al. | 2001 | The combination of Lactobacillus rhamnosus GR-1 and Lactobacillus fermentum RC-14 can not only be used for daily use in healthy women, but also reduce the potential pathogenic bacteria and yeast colonization in vagina. | (Reid et al., 2003) |
| De Seta et al. | 2014 | Lactobacillus plantarum P17630 can reduce vaginal discomfort after routine treatment of acute VVC and improve vaginal pH. | (De Seta et al., 2014) |
| Russo et al. | 2019 | The combination of a Lactobacillus mixture with lactoferrin is a safe and effective auxiliary method to reduce VVC symptoms and recurrence. | (Russo et al., 2019) |
| Oerlemans et al. | 2020 | Lactobacillus can be used for VVC treatment in the future. | (Oerlemans et al., 2020) |
| De Gregorio et al. | 2020 | Biosurfactants from Lactobacillus crispatus BC1 can interfere with the adherence of Candida in vivo and in vitro, indicating its potential as a preventive measure against Candida mucosal damage during VVC. | (De Gregorio et al., 2020) |
The above experiments focused on the possibility of Lactobacillus to prevent VVC. In view of the great potential of Lactobacillus, whether other probiotics can be used for the treatment and defense of VVC deserves further research. In addition, it has been shown that certain biological components or subcomponents can inhibit the growth of Candida. de Freitas et al. used F2 and sub-fraction F2.4 tannins from Stryphnodendron adstringens stem bark to treat mice with vaginal infection with Candida albicans and analyzed vaginal histopathology and fungal load. The results showed that F2 and F2.4 have efficacy in controlling candidiasis in mouse models (de Freitas et al., 2018).
It should also be noted that there exist differences in the vaginal microbiome among women of different races, such as the body’s own immune system, the different amounts and composition of vaginal secretions. Therefore, it needs to be further studied whether treatment and defense of Lactobacillus and other microbiota against VVC are also related to the host’s genetic factors, behavior, and cultural differences (Denning et al., 2018).
Conclusion
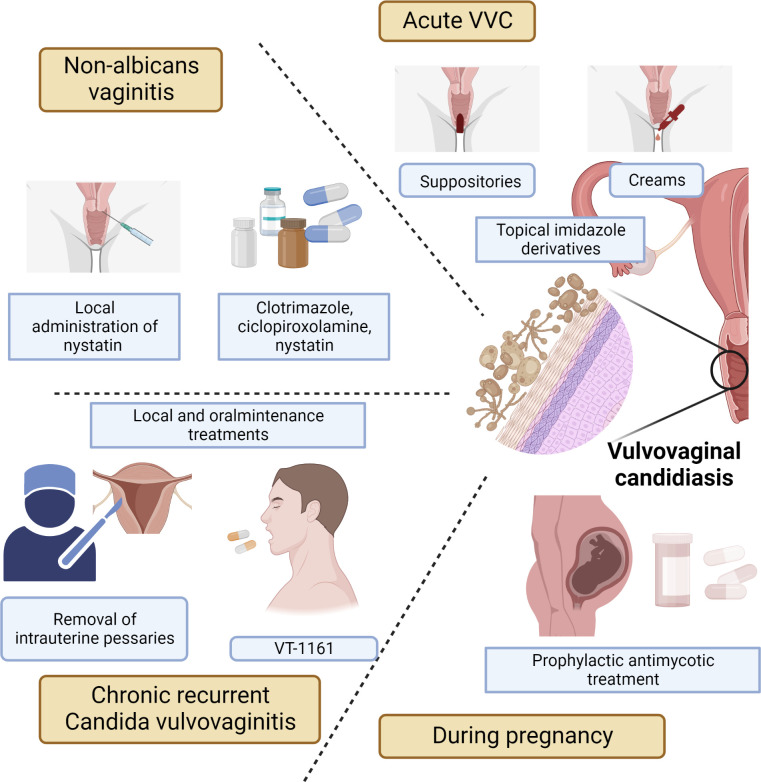
VVC is a complex disease, and its symptoms are affected by host physiology, fungal biology and immune response. Currently, there are many treatments for VVC, mainly including prescription oral dosage forms, over-the-counter topical preparations, and vaginal suppositories ( Figure 2 ).
Figure 2.
Treatments for acute VVC or recurrent VVC, including prescription oral formulations, over-the-counter topical preparations, and vaginal suppositories.
Given the projected global prevalence and economic burden of VVC in the next decade, high-income countries need better solutions and improved quality of care for affected women. With the increasing awareness of human microorganisms, probiotic treatment has been a hot topic in recent years. In many in vivo and in vitro experiments, Lactobacillus have been shown to have a certain effect on the prevention and treatment of VVC, but clinical data are still scarce and need to be further explored. It is believed that with the advancement of technology, the composition of vaginal microbiome and the preventive and therapeutic effects of vaginal microbiome on VVC can be further elucidated.
Author contributions
ZS, XG and BQ had the idea for the article. ZX and CJ performed the literature search and data analysis. JW and YL drafted and critically revised the work. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Amabebe E., Anumba D. O. C. (2018). The vaginal microenvironment: The physiologic role of lactobacilli. Front. Med. (Lausanne) 5. doi: 10.3389/fmed.2018.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger P., Joly J. (1980). Microbial flora associated with candida albicans vulvovaginitis. Obstetrics Gynecol. 55 (3), 397–401. doi: 10.1097/00006250-198003000-00029 [DOI] [PubMed] [Google Scholar]
- Balakrishnan S. N., Yamang H., Lorenz M. C., Chew S. Y., Than L. T. L. (2022). Role of vaginal mucosa, host immunity and microbiota in vulvovaginal candidiasis. Pathogens 11 (6), 618. doi: 10.3390/pathogens11060618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calonghi N., Parolin C., Sartor G., Verardi L., Giordani B., Frisco G., et al. (2017). Interaction of vaginal lactobacillus strains with HeLa cells plasma membrane. Benef Microbes 8 (4), 625–633. doi: 10.3920/BM2016.0212 [DOI] [PubMed] [Google Scholar]
- Ceccarani C., Foschi C., Parolin C., D'Antuono A., Gaspari V., Consolandi C., et al. (2019). Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 9 (1), 14095. doi: 10.1038/s41598-019-50410-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champer M., Wong A. M., Champer J., Brito I. L., Messer P. W., Hou J. Y., et al. (2018). The role of the vaginal microbiome in gynaecological cancer. BJOG 125 (3), 309–315. doi: 10.1111/1471-0528.14631 [DOI] [PubMed] [Google Scholar]
- Chee W. J. Y., Chew S. Y., Than L. T. L. (2020). Vaginal microbiota and the potential of lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact 19 (1), 203. doi: 10.1186/s12934-020-01464-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Lu Y., Chen T., Li R. (2021). The female vaginal microbiome in health and bacterial vaginosis. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.631972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Song X., Wei W., Zhong H., Dai J., Lan Z., et al. (2017). The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 8 (1), 875. doi: 10.1038/s41467-017-00901-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culhane J. F., Rauh V., McCollum K. F., Elo I. T., Hogan V. (2002). Exposure to chronic stress and ethnic differences in rates of bacterial vaginosis among pregnant women. Am. J. Obstet Gynecol 187 (5), 1272–1276. doi: 10.1067/mob.2002.127311 [DOI] [PubMed] [Google Scholar]
- de Freitas A. L. D., Kaplum V., Rossi D. C. P., da Silva L. B. R., Melhem M. S. C., Taborda C. P., et al. (2018). Proanthocyanidin polymeric tannins from stryphnodendron adstringens are effective against candida spp. isolates and for vaginal candidiasis treatment. J. Ethnopharmacol. 216, 184–190. doi: 10.1016/j.jep.2018.01.008 [DOI] [PubMed] [Google Scholar]
- De Gregorio P. R., Parolin C., Abruzzo A., Luppi B., Protti M., Mercolini L., et al. (2020). Biosurfactant from vaginal lactobacillus crispatus BC1 as a promising agent to interfere with candida adhesion. Microb. Cell Fact 19 (1), 133. doi: 10.1186/s12934-020-01390-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning D. W., Kneale M., Sobel J. D., Rautemaa-Richardson R. (2018). Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect. Dis. 18 (11), e339–e347. doi: 10.1016/S1473-3099(18)30103-8 [DOI] [PubMed] [Google Scholar]
- De Seta F., Parazzini F., De Leo R., Banco R., Maso G. P., De Santo D., et al. (2014). Lactobacillus plantarum P17630 for preventing candida vaginitis recurrence: a retrospective comparative study. Eur. J. Obstet Gynecol Reprod. Biol. 182, 136–139. doi: 10.1016/j.ejogrb.2014.09.018 [DOI] [PubMed] [Google Scholar]
- Di Bella J. M., Bao Y., Gloor G. B., Burton J. P., Reid G. (2013). High throughput sequencing methods and analysis for microbiome research. J. Microbiol. Methods 95 (3), 401–414. doi: 10.1016/j.mimet.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Farr A., Effendy I., Frey Tirri B., Hof H., Mayser P., Petricevic L., et al. (2021). Guideline: Vulvovaginal candidosis (AWMF 015/072, level S2k). Mycoses 64 (6), 583–602. doi: 10.1111/myc.13248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuochi V., Cardile V., Petronio Petronio G., Furneri P. M. (2019). Biological properties and production of bacteriocins-like-inhibitory substances by lactobacillus sp. strains from human vagina. J. Appl. Microbiol. 126 (5), 1541–1550. doi: 10.1111/jam.14164 [DOI] [PubMed] [Google Scholar]
- Gow N. A., Hube B. (2012). Importance of the candida albicans cell wall during commensalism and infection. Curr. Opin. Microbiol. 15 (4), 406–412. doi: 10.1016/j.mib.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Gupta V. K., Paul S., Dutta C. (2017). Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. A., Noverr M. C. (2017). Fungal interactions with the human host: exploring the spectrum of symbiosis. Curr. Opin. Microbiol. 40, 58–64. doi: 10.1016/j.mib.2017.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefzy E. M., Khalil M. A. F., Amin A. A. I., Ashour H. M., Abdelaliem Y. F. (2021). Bacteriocin-like inhibitory substances from probiotics as therapeutic agents for candida vulvovaginitis. Antibiotics (Basel) 10 (3), 306. doi: 10.3390/antibiotics10030306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram P. M., Mohan M. K., Konje J. (2020). Bacterial vaginosis in pregnancy - a storm in the cup of tea. Eur. J. Obstet Gynecol Reprod. Biol. 253, 220–224. doi: 10.1016/j.ejogrb.2020.08.009 [DOI] [PubMed] [Google Scholar]
- Kalia N., Singh J., Kaur M. (2020). Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: a critical review. Ann. Clin. Microbiol. Antimicrob. 19 (1), 5. doi: 10.1186/s12941-020-0347-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna V., Lakshmi K., Khanna R., Verma S., Acharya V. (2022). Intestinal parasitic infections among diabetic patients in tertiary care hospital. Advanced Gut Microbiome Res. 2022. doi: 10.1155/2022/4829943 [DOI] [Google Scholar]
- Li T., Liu Z., Zhang X., Chen X., Wang S. (2019). Local probiotic lactobacillus crispatus and lactobacillus delbrueckii exhibit strong antifungal effects against vulvovaginal candidiasis in a rat model. Front. Microbiol. 10, 1033. doi: 10.3389/fmicb.2019.01033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. B., Xu S. R., He Y., Deng G. H., Sheng H. F., Huang X. M., et al. (2013). Diverse vaginal microbiomes in reproductive-age women with vulvovaginal candidiasis. PloS One 8 (11), e79812. doi: 10.1371/journal.pone.0079812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia P., Moreira D. (2020). The syntrophy hypothesis for the origin of eukaryotes revisited. Nat. Microbiol. 5 (5), 655–667. doi: 10.1038/s41564-020-0710-4 [DOI] [PubMed] [Google Scholar]
- MacAlpine J., Daniel-Ivad M., Liu Z., Yano J., Revie N. M., Todd R. T., et al. (2021). A small molecule produced by lactobacillus species blocks candida albicans filamentation by inhibiting a DYRK1-family kinase. Nat. Commun. 12 (1), 6151. doi: 10.1038/s41467-021-26390-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B., Forney L. J., Ravel J. (2012). Vaginal microbiome: rethinking health and disease. Annu. Rev. Microbiol. 66, 371–389. doi: 10.1146/annurev-micro-092611-150157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson A., Mazza D. (2017). Recurrent vulvovaginal candidiasis: A review of guideline recommendations. Aust. N Z J. Obstet Gynaecol. 57 (2), 139–145. doi: 10.1111/ajo.12592 [DOI] [PubMed] [Google Scholar]
- Mojzsis S. J., Arrhenius G., McKeegan K. D., Harrison T. M., Nutman A. P., Friend C. R. (1996). Evidence for life on earth before 3,800 million years ago. Nature 384 (6604), 55–59. doi: 10.1038/384055a0 [DOI] [PubMed] [Google Scholar]
- Noyes N., Cho K. C., Ravel J., Forney L. J., Abdo Z. (2018). Associations between sexual habits, menstrual hygiene practices, demographics and the vaginal microbiome as revealed by Bayesian network analysis. PloS One 13 (1), e0191625. doi: 10.1371/journal.pone.0191625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oerlemans E. F. M., Bellen G., Claes I., Henkens T., Allonsius C. N., Wittouck S., et al. (2020). Impact of a lactobacilli-containing gel on vulvovaginal candidosis and the vaginal microbiome. Sci. Rep. 10 (1), 7976. doi: 10.1038/s41598-020-64705-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkers D. J., Dicks L. M., Silvester M., Joubert J. J., Odendaal H. J. (1999). Characterization of pentocin TV35b, a bacteriocin-like peptide isolated from lactobacillus pentosus with a fungistatic effect on candida albicans. J. Appl. Microbiol. 87 (5), 726–734. doi: 10.1046/j.1365-2672.1999.00918.x [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Delaney M. L., Fichorova R. N. (2016). The human microbiome during bacterial vaginosis. Clin. Microbiol. Rev. 29 (2), 223–238. doi: 10.1128/CMR.00075-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osset J., Garcia E., Bartolome R. M., Andreu A. (2001). [Role of lactobacillus as protector against vaginal candidiasis]. Med. Clin. (Barc) 117 (8), 285–288. doi: 10.1016/s0025-7753(01)72089-1 [DOI] [PubMed] [Google Scholar]
- Pang Q., Liu W., Cui F., Kan S., Li X. (2022). Biased genotype distributions of candida albicans strains associated with 649 clinical vulvovaginal candidiasis in China. Mycopathologia 187 (5), 427–437. doi: 10.1007/s11046-022-00671-4 [DOI] [PubMed] [Google Scholar]
- Paniagua A. L., Correia A. F., Pereira L. C., de Alencar B. M., Silva F. B. A., Almeida R. M., et al. (2021). Inhibitory effects of lactobacillus casei shirota against both candida auris and candida spp. isolates that cause vulvovaginal candidiasis and are resistant to antifungals. BMC Complement Med. Ther. 21 (1), 237. doi: 10.1186/s12906-021-03405-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolin C., Frisco G., Foschis C., Giordani B., Salvo M., Vitali B., et al. (2018). Lactobacillus crispatus BC5 interferes with chlamydia trachomatis infectivity through integrin modulation in cervical cells. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.02630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolin C., Marangoni A., Laghi L., Foschi C., Palomino R. A. N., Calonghi N., et al. (2015). Isolation of vaginal lactobacilli and characterization of anti-candida activity. PloS One 10 (6), e0131220. doi: 10.1371/journal.pone.0131220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K., Boutain D., Manhart L., Hitti J. (2008). Racial disparity in bacterial vaginosis: the role of socioeconomic status, psychosocial stress, and neighborhood characteristics, and possible implications for preterm birth. Soc. Sci. Med. 67 (5), 824–833. doi: 10.1016/j.socscimed.2008.05.017 [DOI] [PubMed] [Google Scholar]
- Petrova M. I., Reid G., Vaneechoutte M., Lebeer S. (2017). Lactobacillus iners: Friend or foe? Trends Microbiol. 25 (3), 182–191. doi: 10.1016/j.tim.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Ravel J., Gajer P., Abdo Z., Schneider G. M., Koenig S. S., McCulle S. L., et al. (2011). Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U.S.A. 108 Suppl 1, 4680–4687. doi: 10.1073/pnas.1002611107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G., Charbonneau D., Erb J., Kochanowski B., Beuerman D., Poehner R., et al. (2003). Oral use of lactobacillus rhamnosus GR-1 and l. fermentum RC-14 significantly alters vaginal flora: randomized, placebo-controlled trial in 64 healthy women. FEMS Immunol. Med. Microbiol. 35 (2), 131–134. doi: 10.1016/S0928-8244(02)00465-0 [DOI] [PubMed] [Google Scholar]
- Russo R., Superti F., Karadja E., De Seta F. (2019). Randomised clinical trial in women with recurrent vulvovaginal candidiasis: Efficacy of probiotics and lactoferrin as maintenance treatment. Mycoses 62 (4), 328–335. doi: 10.1111/myc.12883 [DOI] [PubMed] [Google Scholar]
- Santos C. M. A., Pires M. C. V., Leao T. L., Hernandez Z. P., Rodriguez M. L., Martins A. K. S., et al. (2016). Selection of lactobacillus strains as potential probiotics for vaginitis treatment. Microbiol. (Reading) 162 (7), 1195–1207. doi: 10.1099/mic.0.000302 [DOI] [PubMed] [Google Scholar]
- Schwebke J. R., Richey C. M., Weiss H. L. (1999). Correlation of behaviors with microbiological changes in vaginal flora. J. Infect. Dis. 180 (5), 1632–1636. doi: 10.1086/315065 [DOI] [PubMed] [Google Scholar]
- Sobel J. D. (2007). Vulvovaginal candidosis. Lancet 369 (9577), 1961–1971. doi: 10.1016/S0140-6736(07)60917-9 [DOI] [PubMed] [Google Scholar]
- Sobel J. D. (2016). Recurrent vulvovaginal candidiasis. Am. J. Obstet Gynecol 214 (1), 15–21. doi: 10.1016/j.ajog.2015.06.067 [DOI] [PubMed] [Google Scholar]
- Strus M., Brzychczy-Wloch M., Kucharska A., Gosiewski T., Heczko P. B. (2005). [Inhibitory activity of vaginal lactobacillus bacteria on yeasts causing vulvovaginal candidiasis]. Med. Dosw Mikrobiol 57 (1), 7–17. [PubMed] [Google Scholar]
- Wang S., Wang Q., Yang E., Yan L., Li T., Zhuang H. (2017). Antimicrobial compounds produced by vaginal lactobacillus crispatus are able to strongly inhibit candida albicans growth, hyphal formation and regulate virulence-related gene expressions. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.00564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wu J., Cao Y. (2015). The extended spectrum β-lactamases (ESBL) and virulence genes of intestinal enteroaggregative escherichia coli (EAEC) in healthy elderly individuals. Int. J. Clin. Exp. Med. 8 (11), 20953. [PMC free article] [PubMed] [Google Scholar]
- Witkin S. S., Forney L. J. (2020). The microbiome and women's health: perspectives and controversies. BJOG 127 (2), 127. doi: 10.1111/1471-0528.16010 [DOI] [PubMed] [Google Scholar]
- Wu J., Guo N., Zhang X., Xiong C., Liu J., Xu Y., et al. (2019). HEV-LFS: A novel scoring model for patients with hepatitis e virus-related liver failure. J. Viral Hepatitis 26 (11), 1334–1343. doi: 10.1111/jvh.13174 [DOI] [PubMed] [Google Scholar]
- Wu J., Huang F., Ling Z., Liu S., Liu J., Fan J., et al. (2020). Altered faecal microbiota on the expression of Th cells responses in the exacerbation of patients with hepatitis e infection. J. Viral Hepatitis 27 (11), 1243–1252. doi: 10.1111/jvh.13344 [DOI] [PubMed] [Google Scholar]
- Xiang Z., Li J., Lu D., Wei X., Xu X. (2022). Advances in multi-omics research on viral hepatitis. Front. Microbiol. 3365. doi: 10.3389/fmicb.2022.987324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes J. A., Lievens E., Hummelen R., van der Westen R., Reid G., Petrova M. I. (2018). Women and their microbes: The unexpected friendship. Trends Microbiol. 26 (1), 16–32. doi: 10.1016/j.tim.2017.07.008 [DOI] [PubMed] [Google Scholar]
- Zhou X., Brown C. J., Abdo Z., Davis C. C., Hansmann M. A., Joyce P., et al. (2007). Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 1 (2), 121–133. doi: 10.1038/ismej.2007.12 [DOI] [PubMed] [Google Scholar]
- Zhou X., Hansmann M. A., Davis C. C., Suzuki H., Brown C. J., Schutte U., et al. (2010). The vaginal bacterial communities of Japanese women resemble those of women in other racial groups. FEMS Immunol. Med. Microbiol. 58 (2), 169–181. doi: 10.1111/j.1574-695X.2009.00618.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Westman R., Hickey R., Hansmann M. A., Kennedy C., Osborn T. W., et al. (2009). Vaginal microbiota of women with frequent vulvovaginal candidiasis. Infect. Immun. 77 (9), 4130–4135. doi: 10.1128/IAI.00436-09 [DOI] [PMC free article] [PubMed] [Google Scholar]