Abstract
Human precision-cut lung slices (hPCLS), considered a highly relevant ex vivo model of the lung, offer native architecture and cells of the lung tissue including respiratory parenchyma, small airways, and immune competent cells. However, the irregular availability of donor lungs has limited the accessibility of this system. As described here, thousands of hPCLS can be created from 1 lung, cryopreserved, and used “on demand” by applying slicing and cryopreservation methodology improvements. Fresh and cryopreserved (∼7 and ∼34 weeks; F&C) hPCLS from 1 donor lung were cultured for up to 29 days and evaluated for biomass, viability, tissue integrity, and inflammatory markers in response to lipopolysaccharide (LPS; 5 µg/ml) and Triton X-100 (TX100; 0.1%) challenge (24 h) at days 1, 8, 15, 22, and 29 following culture initiation. The F&C hPCLS retained biomass, viability, and tissue integrity throughout the 29 days and demonstrated immune responsiveness with up to ∼30-fold LPS-induced cytokine increases. Histologically, more than 70% of normal cytomorphological features were preserved in all groups through day 29. Similar retention of tissue viability and immune responsiveness post cryopreservation (4–6 weeks) and culture (up to 14 days) was observed in hPCLS from additional 3 donor lungs. Banking cryopreserved hPCLS from various donors (and disease states) provides a critical element in researching human-derived pulmonary tissue. The retention of viability and functional responsiveness (≥4 weeks) allows evaluation of long-term, complex endpoints reflecting key events in Adverse Outcome Pathways and positions hPCLS as a valuable human-relevant model for use in regulatory applications.
Keywords: human lung, nonanimal testing, new approach methodology (NAM), precision-cut slices, cryopreservation and long-term storage, long-term ex vivo culture
Historically, respiratory research has primarily been conducted using animals, despite poor physiological and anatomical concordance with the human lung (BeruBe et al., 2009; Clippinger et al., 2018; Miller and Spence, 2017; Williams and Roman, 2016). Varied efforts have focused on developing reliable and human-relevant in vitro and ex vivo pulmonary test systems that model human in vivo responses and replace animal use (Hubrecht and Carter, 2019). Models, such as 3-dimensional reconstructed human airway epithelium grown at the air-liquid-interface (ALI), organoids, and lung microphysiological systems (Cao et al., 2021; Gkatzis et al., 2018; Li et al., 2019), have been shown to be useful. In addition, human precision-cut lung slices (hPCLS) have the advantage of representing the complete architecture and cell composition of lung tissue and can be used to assess key endpoints associated with human lung disease progression (Tanner and Single, 2020).
Pharmacology and toxicology studies using precision-cut tissue slices have been reported for many years (de Kanter et al., 2002; Parrish et al., 1995). hPCLS offer 3-dimensional, native lung architecture (containing small airways and respiratory parenchyma), making it one of the most physiologically relevant models of the human lower lung. While hPCLS is a closed system and does not provide access to the recruitable immune system (one of the shortcomings of ex vivo models), it still offers the advantage of containing all the cells present in the tissue core at the time of slicing, including type I and II alveolar cells, bronchial epithelial cells, endothelial cells, and immune cells such as alveolar macrophages and dendritic cells (Alsafadi et al., 2020; Banerjee et al., 2019; Liu et al., 2019; Morin et al., 2013). Therefore, hPCLS provide a realistic interpretation of complex human pulmonary responses to various exposures, and have gained attention as a highly relevant ex vivo model for respiratory research (Behrsing, 2020; Herbert and Gow, 2020; Sewald and Braun, 2013).
hPCLS have proven useful in many applications, including exposure-induced toxicity (Fisher et al., 1994), airway contractility (Jude et al., 2016, 2019), fibrosis (Alsafadi et al., 2017; Cedilak et al., 2019; Westra et al., 2013), metabolism (Henjakovic et al., 2008; Lauenstein et al., 2014; Sewald and Braun, 2013; Switalla et al., 2010; Temann et al., 2017; Yilmaz et al., 2019), inflammation and immunotoxicity (Henjakovic et al., 2008; Lauenstein et al., 2014; Sewald and Braun, 2013; Switalla et al., 2010; Temann et al., 2017), transcriptomics (Stegmayr et al., 2021), and vaccine exposure response (Neuhaus et al., 2013). Although difficulties associated with long-term hPCLS culture have been reported in the past (BeruBe et al., 2009; Liu et al., 2019), more recent studies have indicated 2–3 week cultures are possible (Bailey et al., 2020; Neuhaus et al., 2017; Temann et al., 2017), and now 4-week or longer cultures have been described using hPCLS (Patel et al., 2021; Preuß et al., 2022). These reports of multi-week culture longevity and complex responses have positioned hPCLS as a candidate model to evaluate key events associated with lung disease progression that may manifest over time.
However, with infrequent donor tissue availability, low throughput slice generation, and lack of reliable preservation techniques, hPCLS have been difficult to employ in research studies that required readily available test systems, were large scale, and/or required repetition with the same donor tissue. Further, laboratories that were able to create higher numbers of slices had to immediately use all the slices or discard them since they could not be preserved. Such constraints provided rationale to develop reliable hPCLS preservation techniques that are able to deliver a high-quality tissue performance (ie, retaining tissue integrity and functionality) post long-term storage.
In 2016, an article addressing this need described the cryopreservation and successful use of hPCLS for acute studies (Bai et al., 2016). In this proof-of-concept study, we aim to assess the cryopreservation and culture method by comparing fresh versus short- and long-term cryopreserved (F&C) hPCLS using several performance metrics (viability, retained biomass and cytomorphological features, and functional response to challenge) over up to 4-week culture period.
Materials and methods
Reagents
The basal medium used for hPCLS culture was DMEM:F-12 (equal volumes) with HEPES (15 mM), glucose (3.151 g/l), and l-glutamine (Lonza, Basel, Switzerland [cat. no. 12-719F]). The culture medium consisted of basal medium supplemented with 1% insulin-transferrin-selenium (ITS-G, 100X; Gibco, Waltham, MA [cat. no. 41400045]) and 1% antibiotic-antimycotic (100X; Gibco [cat. no. 15240062]). The acclimation medium consisted of culture medium supplemented with hydrocortisone-water soluble (2 µM; Sigma Aldrich, St. Louis, Missouri [cat. no. H0396]) and 2-phospho-l-ascorbic acid trisodium salt (10 µg/ml; Sigma Aldrich [cat. no. 49752]). The lipopolysaccharides (LPS) from Escherichia coli O55: B5 was purchased from Sigma Aldrich (cat. no. L6529) and the Triton X-100 (TX100) was purchased from Thermo Fisher (Waltham, Massachusetts [cat. no. BP151-100]). The slicing buffer consisted of Belzer UW cold storage solution (Bridge to Life, Northbrook, Illinois) supplemented with 2-phospho-l-ascorbic acid trisodium salt (10 µg/ml), antibiotic-antimycotic (1%), and l-glutathione reduced (0.9 g/l; Sigma Aldrich [cat. no. G6013]). The filling buffer consisted of a 0.8% Agarose I (Molecular Biology Grade; bioWORLD, Dublin, Ohio [cat. no. 40100164-3]) dissolved in Hank’s Balanced Salt Solution (Thermo Fisher [cat. no. 14025076]) and after cooling to ∼40°C, an equal volume of DMEM:F-12 supplemented with ITS-G (1%), antibiotic-antimycotic (1%), 2-phospho-l-ascorbic acid trisodium salt (10 µg/ml), and 2 mM GlutaMAX (100X; Thermo Fisher [cat. no. 35050061]) was added. The lysis buffer consisted of 0.5% TX100 in Dulbecco’s phosphate-buffered saline (Thermo Fisher [cat. no. 14190144]). The cryopreservation buffer was developed at Institute for In Vitro Sciences (IIVS), is proprietary, and therefore not further disclosed.
Human lung procurement
Nontransplantable lungs were obtained from human donors through the procurement agencies International Institute for the Advancement of Medicine (IIAM; Edison, New Jersey) or Novabiosis, Inc. (Durham, North Carolina). Donor lungs were procured through the Organ Procurement Organizations using the United Network for Organ Sharing identification guidelines, with authorization obtained from the donor’s next of kin and maintaining patient confidentiality.
The donor demographics and hPCLS information are provided in Table 1.
Table 1.
Donor demographics and hPCLS
| Donor number | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age | 62 | 66 | 66 | 59 |
| Race | Caucasian | Caucasian | Hispanic/Latino | Hispanic/Latino |
| Height | 183 cm | 172.7 cm | 157 cm | 163 cm |
| Weight | 106.5 kg | 77.6 kg | 61.7 kg | 88.6 kg |
| Sex | M | M | M | M |
| Lung health status | Normal | COPD | Normal | Normal |
| Mean slice thicknessa | 552 ± 22 µm | 548 ± 65 µm | 424 ± 35 µm | 437 ± 51 µm |
| Cryopreservation length | ∼7 or ∼34 weeks | ∼4 weeks | ∼5 weeks | ∼6 weeks |
N = 5 slices per lung.
Note: This proof-of-concept study focuses on the method and ability to cryopreserve hPCLS with retention of viability/immune responsiveness. Initially, only 1 donor lung (donor 1) was used to show that the cryopreserved hPCLS retain viability/immune responsiveness following cryopreservation and long-term culture. The data from additional lungs (donors 2, 3, and 4, with different cryopreservation batches, culture lengths, and the immune markers assessed) were included to show the robustness of the conclusions made in this study. The donor-to-donor variability in immune responses and the effects of the donor lung condition (eg, disease state) on the performance of slices in culture and the endpoints of interest should be considered while choosing cryopreserved hPCLS for research. Performance and quality check for each batch of cryopreserved hPCLS are necessary to assist in the selection of an appropriate hPCLS batch based on study design.
hPCLS preparation and cryopreservation
Upon receipt, the lungs were visually inspected to ascertain quality and usable tissue (free of gross lesions or other abnormalities that would preclude use) prior to coring and slicing. The lungs were inflated with warm (38–42°C) filling buffer using a balloon catheter. After gelling of the agarose (∼45 min under cold conditions [eg, on ice or 2–8°C]), 1–1.5-cm-thick sections of the lungs were cut using a scalpel and placed in cold slicing buffer until cored. Cylindrical cores (8 mm diameter) were created from the sections using a MD2300 low speed coring press (Alabama Research and Development, Munford, Alabama) and stored in cold slicing buffer until used for slicing. The cores were used to generate slices using Krumdieck MD4000 tissue slicer (Alabama Research and Development). The hPCLS were collected and stored in cold slicing buffer until placed into culture or cryopreserved.
The hPCLS to be preserved for later use were carefully placed into cryovials containing 1 ml of cryopreservation buffer (proprietary; IIVS, Gaithersburg, Maryland) and stored in freezing containers at less than or equal to −60°C for 4–72 h. The cryovials were then transferred to liquid nitrogen tank and stored until use.
hPCLS culture
The hPCLS designated for “fresh” evaluation were incubated (on day −3) in acclimation medium for 3 days using standard cell culture inserts (12 mm diameter; PET membrane) in 12-well plates with 1 ml medium (0.9 ml in basal compartment + 0.1 ml in apical compartment). For donor 1, the cryopreserved hPCLS designated for freeze-thaw evaluation were thawed after ∼7 weeks (Cryo 1) or ∼34 weeks (Cryo 2) on day −1 and acclimated in acclimation medium for 24 h. For remaining donors, the cryopreserved slices were thawed after 4–6 weeks (Table 1). The hPCLS were cultured at ALI. After the acclimation period, the acclimation medium was replaced with culture medium or designated test material. The culture medium was replaced 3 times per week (every 2–3 days) and the hPCLS were maintained at standard culture conditions (SCCs; ie, 37 ± 1°C, 5% CO2, and 90% relative humidity) throughout the culture period.
hPCLS treatment and harvest
Donor 1
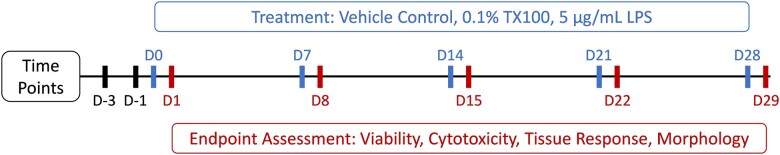
At the end of acclimation period, the hPCLS were assigned to the 3 treatment groups (N = 24/group) including LPS (5 µg/ml in culture medium), TX100 (0.1% in culture medium), and vehicle control (VC; culture medium only). At days 0, 7, 14, 21, and 28 during culture, medium of 6 hPCLS in each group was replaced with the designated test material for 24 h (0.9 ml in basal compartment + 0.1 ml in apical compartment). At the end of the 24-h treatment period (ie, on days 1, 8, 15, 22, and 29, respectively), all hPCLS in each treatment group were evaluated for viability (WST-8 assay) and then half the number of hPCLS in each group were either lysed (N = 3/group) using lysis buffer or fixed (N = 3/group) using 10% neutral-buffered formalin (NBF) (Azer Scientific, Morgantown, Pennsylvania). The lysate supernatants were aliquoted with 1 aliquot stored at 2–8°C for use in LDH assay and remaining aliquot(s) at less than or equal to −60°C for protein content analysis. The medium samples were aliquoted with 1 aliquot stored at 2–8°C for use in LDH assay and remaining aliquot(s) at less than or equal to −60°C for additional cytokine/biomarker analysis. The time course for all 3 groups is illustrated in Figure 1.
Figure 1.
Experimental timeline. After hPCLS acclimation, slices were exposed to 0.1% TX100 or 5 µg/ml LPS challenge at various time points during culture, for 24 h prior to endpoint assessments.
Donors 2–4
At the end of acclimation period, the hPCLS were assigned to 2 treatment groups (N = 12/group) including LPS (5 µg/ml in culture medium) and VC (culture medium only). At days 6 and 13 during culture, medium of 3 hPCLS in each group was replaced with the designated test material for 24 h (0.9 ml in basal compartment + 0.1 ml in apical compartment). At the end of the 24 h treatment period (ie, on days 7 and 14), the hPCLS in each treatment group were evaluated for viability (WST-8 assay) and then lysed using lysis buffer. The lysate supernatants were aliquoted and stored at less than or equal to −60°C for protein content analysis. The medium samples were aliquoted and stored at less than or equal to −60°C for cytokine/biomarker analysis.
Tissue viability
The hPCLS viability was assessed by measuring dehydrogenase activity using the cell counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Rockville, Maryland [cat. no. CK04]) containing a water-soluble tetrazolium salt, WST-8. Viability was assessed at multiple time points throughout the culture period (Donor 1: days 1, 8, 15, 22, and 29; Donors 2–4: days 7 and 14). CCK-8 solution was mixed with assay medium at a 1:10 ratio and the hPCLS were transferred to multi-well plates containing 500 µl of CCK-buffer. After 2 h incubation at SCC, 100 µl of the CCK-buffer (incubated with hPCLS) was transferred to a 96-well plate and absorbance was measured at 450 nm using a VersaMax microplate reader (Molecular Devices, San Jose, California).
Note: For the Donor 1 fresh and ∼7 weeks cryopreserved (Cryo 1) hPCLS, all tissues designated for treatments at later time points were also assessed for viability on days 4, 11, 18, and 25. Following viability assessment, the hPCLS were rinsed with culture medium and put back into culture at SCC until next viability assessment or treatment time point. After confirming no loss of viability occurred for Fresh and Cryo 1, the number of viability sampling days was reduced for Cryo 2.
Tissue lysis
The hPCLS were placed in 1.5 ml microcentrifuge tubes containing 2 metal beads and 500 µl of lysis buffer. The hPCLS were then disrupted using the TissueLyser II (Qiagen, Germantown, Maryland) for 2 min. Following disruption, homogenates were centrifuged at 10°000×g, 4°C for 10 min, and the supernatants were aliquoted. One aliquot was stored at 2–8°C for use in LDH assay and remaining aliquot(s) at less than or equal to −60°C for additional analysis.
Total protein content
Total protein content (as an indicator of biomass) in the hPCLS lysates was measured using Pierce BCA protein assay kit (ThermoFisher Scientific, Waltham, Massachusetts [cat. no. 23227]). Briefly, 25 μl lysate samples and standards were added to a 96-well plate in duplicates. In total, 200 μl of BCA working reagent was added to each well and the plate was shaken briefly on an orbital shaker. The plate was incubated for 30 min at SCC followed by measurement of absorbance at 562 nm using the VersaMax microplate reader.
LDH content
LDH content in the hPCLS medium and lysate samples was determined using CytoScan LDH Cytotoxicity Assay (G-Biosciences, St. Louis, Missouri [cat. no. 786-210]). In total, 50 μl of the samples (medium or lysates) were transferred to a 96-well plate and 50 µl of reaction mixture was added to each well. The plate was gently agitated by hand and then incubated (covered or in the dark) for 30 min at room temperature. Following incubation, the absorbance was measured at 490 nm using the VersaMax microplate reader. Percent cytotoxicity was calculated by dividing the OD490 of the test sample (medium), by the combined lysate + medium OD490 signals from the same treatment group (N = 3/group).
Cytokine/biomarker analysis
The cytokine/biomarker panels consisted of common markers of LPS-induced inflammatory responses to assess the immune responsiveness of the hPCLS. While each marker may have different down-stream effect(s), these were not taken into consideration during the selection of the panel. The following analyte panels included the following cytokines/biomarkers.
Donor 1
Interleukin (IL)-1β, IL-6, CXCL-8 (formerly IL-8), CCL-2 (monocyte chemoattractant protein-1), CXCL-1 (growth-regulated protein [GRO]-α), and tissue inhibitor of metalloproteinases (TIMP)-1.
Donors 2–4
IL-6, tumor necrosis factor alpha (TNF-α), and matrix metalloproteinase (MMP)-3.
The levels of each analyte in medium samples were assessed using Luminex assay kit (R&D Systems, Minneapolis, Minnesota [cat. no. LXSAHM]). The samples were prepared as instructed in the kit manual and run on Luminex MAGPIX system (Luminex Corporation, Austin, Texas). The data were analyzed using the Luminex xPONENT software.
Histological evaluation
The hPCLS were fixed in 10% NBF for 18–72 h and then stored in 70% ethanol. The fixed samples were then sent, processed further, and stained at a histology facility (StageBio, Mt. Jackson, Virginia). Briefly, the paraffin sections (approximately 5 µm thick) of hPCLS were stained with hematoxylin and eosin (H&E), or subjected to immunohistochemical (IHC) staining for CD86 (cat. no. 91882S, diluted 1:500; Cell Signaling Technology, Danvers, Massachusetts), aquaporin 5 (AQ5) (cat. no. PA599403, diluted 1:200; Thermo Fisher), or prosurfactant protein C (pSPC) (cat. no. AB3786, diluted 1:1000; Sigma Aldrich) using standard protocols.
The stained tissue sections were evaluated (blinded) by a pathologist with emphasis on tissue architecture, cellular viability, and metaplastic changes. For H&E slides, semiquantitative histology scores (increments of 10, 0–100) were based on the appearance of the evaluated section and cell viability, relative to “normal” lung tissue (ie, “90” represents a score reflecting 90% viability and retention of architecture compared with normal/healthy tissue).
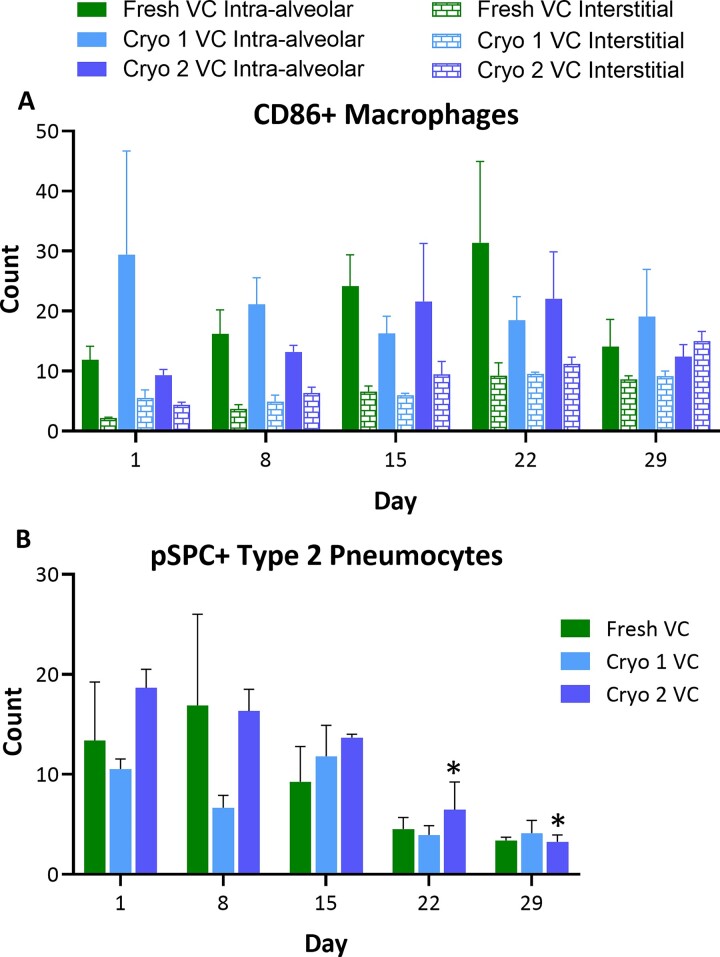
Macrophages (CD86 positive cells) were counted separately within the alveolar septae (interstitium) and the alveolar spaces. The count was performed in 5 high-power (×40) representative microscopic fields with highest concentration of positive cells after scanning the entire slice section on low magnification. Similarly, pSPC positive cells lining the alveoli and small airways were counted in 5 high-power fields. A mean score was then calculated for each slice. For AQ5, semiquantitative analysis of staining, modeled after the H-score, was performed for each of the slice section using the following equation: 1 × (% cells 1+) + 2 × (% cells 2+) + 3 × (% cells 3+) = range 0–300.
Statistical analyses
The data are presented as the mean (AVE) ± standard error of mean (SEM) or standard deviation (SD) and statistically compared using 2-way ANOVA with Tukey’s post multiple comparisons test (GraphPad Prism). A P-value < .05 was considered statistically significant compared with respective VC groups at indicated time points and marked using “*”. Data generated that demonstrated significant tissue changes over time, with respect to culture longevity (and in a comparative manner across groups) were marked appropriately in presented datasets.
Results
Evaluation of hPCLS health from single donor
Total protein content
The protein content of hPCLS (fresh, Cryo 1, and Cryo 2 groups) was determined using the BCA protein assay at days 1, 8, 15, 22, and 29, post culture initiation. Mean total protein content per slice varied within and across time points in each group (Figure 2). With an approximate mean thickness of 500–600 µm per slice, a protein measurement of ∼200 µg/slice is expected (from historical data; not shown). The protein content in VC groups ranged from 121–333, 93–225, and 142–359 µg/slice, for Fresh, Cryo 1, and Cryo 2, respectively. The highest protein values (and group variability) were seen at D29 for Fresh (where one slice had high protein content) and Cryo 2 (where two slices had high protein content). Treatment with LPS did not reduce protein content (in any groups and at any time point) but the irritant (0.1% TX100) did elicit severe damage (as expected) to all exposed tissues and a concomitant reduction in hPCLS protein content is observed. No significant differences were seen between F&C slices.
Figure 2.
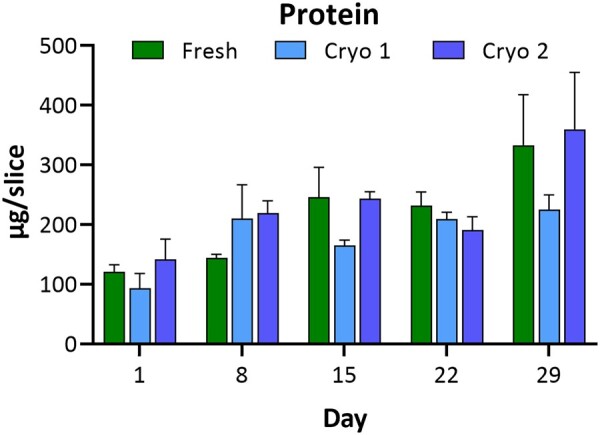
hPCLS protein content. Comparison of protein content in hPCLS indicates there is no loss of protein over 29 days in culture. Each group (Fresh, Cryo 1, and Cryo 2) exhibits protein content variability at some time points, but no trend in variability is evident for any group, nor is there an apparent distinction between Fresh versus Cryo 1 or Cryo 2. The data are presented as AVE ± SEM, N = 3/group per time point.
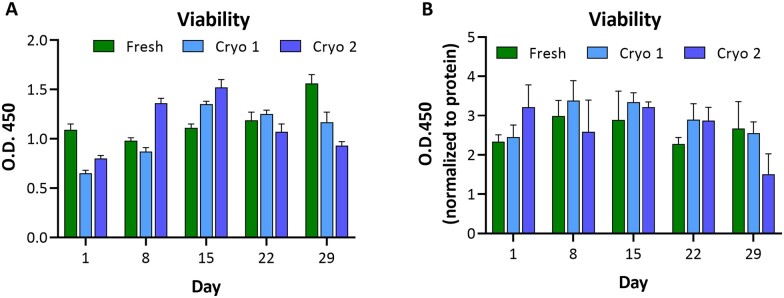
Viability
WST-8 conversion was used to indicate viability of hPCLS. While protein content was only performed on hPCLS lysates, WST-8 readings were compiled for all untreated and VC-treated groups over time until the terminal time point for each group of tissues (N = 6–60, depending on the time point), but with the exclusion of groups designated for LPS treatment.
Our previous work with fresh hPCLS indicates that 500 ± 100-µm-thick viable slices generated from a normal lung show WST-8 absorbance reads in the range of 0.8–1.5 (data not shown). Our current study data also showed the absorbance reads in a similar ranges in the fresh (0.98–1.56; Figure 3), Cryo 1 (0.6–1.35), and Cryo 2 (0.80–1.52) groups. The Cryo 1 and Cryo 2 groups showed lower reads on day 1 (0.65 and 0.80, respectively) compared with the fresh group; however, they recover at later time points. Overall, the hPCLS retained viability over the 29 day culture period in all 3 Fresh, Cryo 1, and Cryo 2 VC groups, indicative of an equivalence between fresh and both short-term and long-term stored hPCLS.
Figure 3.
hPCLS viability. Comparison of viability values in hPCLS indicates there is no loss over time. Each group (Fresh, Cryo 1, and Cryo 2) exhibits retained substrate conversion with some trends similar to protein content at distinct time points. The data are presented as AVE ± SEM O.D.450 (A) or O.D.450 normalized to protein (B), N = 6–78/group per time point.
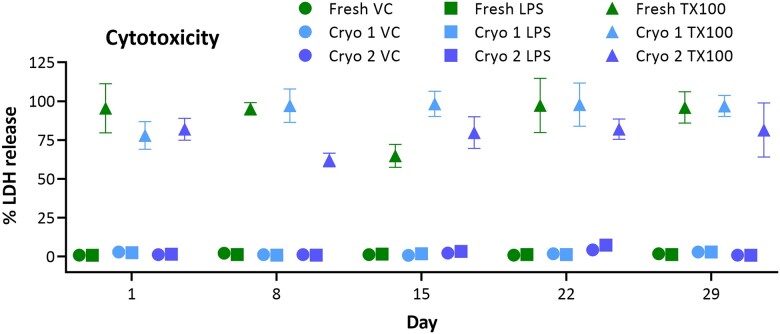
Tissue integrity
hPCLS were evaluated for baseline LDH release as a function of tissue integrity. Both Cryo 1 and Cryo 2 groups demonstrated negligible (baseline) LDH release into the culture medium (Figure 4), which was comparable to that in the Fresh group. This was evident in the VC groups as well as the LPS treatment group, which was not expected to cause tissue damage at the time point and concentration used (<10% release). Further, the hPCLS were challenged with TX100 (0.1%) for 24 h to evaluate tissue vulnerability/sensitivity (fresh vs cryopreserved) to an irritant exposure. All 3 groups (Fresh, Cryo 1, and Cryo 2) exhibited similar LDH release responses to the TX100 challenge, and the data indicate nearly complete destruction of tissues at most time points (80%–100% release) with low residual LDH signals retained in all tissue groups.
Figure 4.
Irritant-induced cytotoxicity. Comparison of baseline LDH release and irritant-induced cytotoxicity. Neither Fresh, Cryo 1, nor Cryo 2 groups exhibited differential release (in VC and LPS groups) indicating intact and comparable membrane integrity. Treatment of hPCLS with 0.1% TX100 for 24 h elicited similar responses from the same 3 groups and demonstrated equivalent response to irritant challenge. The data are presented as AVE ± SEM, N = 6/group per time point.
Histological evaluation of hPCLS
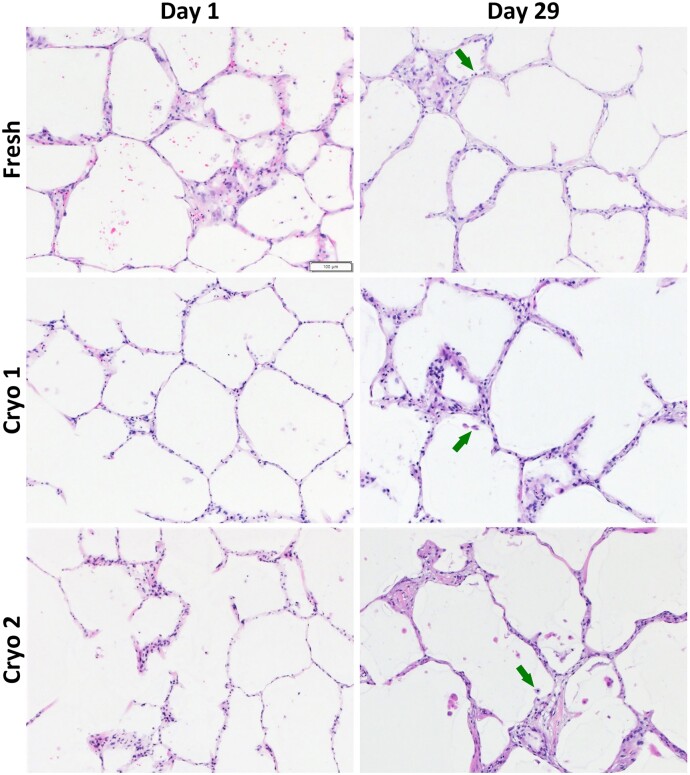
Morphology (H&E)
The hPCLS in each group (Fresh, Cryo 1, and Cryo 2) were stained with H&E for morphological evaluation. With the exception of the Cryo 2 group at day 1, all groups exhibited ≥75% of “normal” histological attributes for both VC and LPS treatments during the course of 29 days in culture (Table 2). The Cryo 2 day 1 group yielded suboptimal cytomorphological scores of <70% “normal,” and is believed to be an outlier (and possibly this tissue set had been handled slightly differently) since subsequent time point evaluations all yielded higher scores. Photomicrographs of VC tissues at day 1 and day 29 depict the retention of normal morphological features for both ∼7 weeks and ∼34 weeks cryopreserved groups as well as the Fresh group (Figure 5). Some nonuniform and patchy septal wall thickening was observed in all 3 groups that appeared to be more pronounced at later time points. Additionally, review of tissues (all 3 groups) suggested the presence of macrophages over the course of 4-week culture.
Table 2.
Histological assessment of hPCLS
| hPCLS type | Treatment | D1 |
D8 |
D15 |
D22 |
D29 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AVE±SD | AVE±SD | AVE±SD | AVE±SD | AVE±SD | |||||||||||||
| H&E | Fresh | VC | 87 | ± | 3 | 78 | ± | 3 | 82 | ± | 3 | 80 | ± | 0 | 73 | ± | 3 |
| LPS | 80 | ± | 9 | 87 | ± | 6 | 83 | ± | 6 | 80 | ± | 0 | 77 | ± | 6 | ||
| Cryo 1 | VC | 83 | ± | 8 | 85 | ± | 5 | 80 | ± | 5 | 80 | ± | 0 | 82 | ± | 8 | |
| LPS | 75 | ± | 9 | 83 | ± | 3 | 80 | ± | 5 | 83 | ± | 3 | 75 | ± | 5 | ||
| Cryo 2 | VC | 60 | ± | 10 | 78 | ± | 3 | 82 | ± | 3 | 80 | ± | 5 | 78 | ± | 3 | |
| LPS | 68 | ± | 8 | 75 | ± | 5 | 75 | ± | 0 | 77 | ± | 6 | 78 | ± | 3 | ||
| AQ5 | Fresh | VC | 197 | ± | 6 | 190 | ± | 0 | 185 | ± | 5 | 177 | ± | 32 | 165 | ± | 18 |
| LPS | 177 | ± | 25 | 203 | ± | 6 | 203 | ± | 6 | 187 | ± | 25 | 178 | ± | 8 | ||
| Cryo 1 | VC | 160 | ± | 42 | 197 | ± | 6 | 197 | ± | 12 | 197 | ± | 6 | 160 | ± | 20 | |
| LPS | 177 | ± | 15 | 217 | ± | 6 | 190 | ± | 26 | 213 | ± | 12 | 173 | ± | 12 | ||
| Cryo 2 | VC | 133 | ± | 25 | 193 | ± | 15 | 203 | ± | 12 | 217 | ± | 6 | 203 | ± | 25 | |
| LPS | 137 | ± | 12 | 197 | ± | 23 | 207 | ± | 6 | 207 | ± | 15 | 177 | ± | 15 | ||
Notes: Evaluation of H&E- and AQ5-stained sections at days 1, 8, 15, 22, and 29 indicates comparable results throughout the ∼4-week culture period. All groups (Fresh, Cryo 1, and Cryo 2) yielded ≥75% “normal” morphological characteristics (H&E) for both VC- and LPS-treated hPCLS, with the exception of Cryo 2 at day 1. A general concordance with WST-8 viability (Figure 3) was seen. Similar results were observed for AQ5 levels with no significant differences among the groups, N = 3/group per time point.
Figure 5.
hPCLS demonstrate retained morphological features over 4 weeks in culture. Photomicrographs (×10) taken at day 1 post acclimation (4 days following slice creation for fresh, and 2 days post thaw for Cryo 1 and Cryo 2) show normal morphology, regardless of group. At the final harvest time point (day 29) of the 4-week culture period all 3 groups again show a highly preserved lung architecture and cellular viability as seen in healthy lung tissue. Note the cells morphologically compatible with macrophages (arrows) in the intra-alveolar spaces at day 29. N = 3/group per time point.
Prosurfactant protein C, AQ5, and CD86 (IHC)
The hPCLS were stained for AQ5 (membrane protein that plays a crucial role in water and small solute transport across epithelial and endothelial barriers), pSPC (marker of type 2 pneumocytes), and CD86 (marker of antigen presenting cells such as macrophages and dendritic cells).
The AQ5 expression remained stable throughout the ∼4-week culture period in all the groups (Table 2). The type 2 pneumocyte marker pSPC progressively decreased over time for all 3 groups, with statistical significance achieved for Cryo 2 D29 group compared with respective D1 group. For all time points, the levels were comparable between the F&C slices (Figure 6B). The CD86 staining indicated that the number of intra-alveolar and interstitial macrophages were stable with no statistical difference over time in all 3 groups (Figure 6A). Representative photomicrographs of IHC-stained VC tissues at D1 and D29 are provided in Supplementary Figures S1–S3.
Figure 6.
Levels of intra-alveolar and interstitial CD86+ macrophages and type 2 pneumocytes. The hPCLS were stained with IHC stains for CD86 (A) or pSPC (B). The stained cells were counted in 5 high-powered fields. Changes in the levels of interstitial macrophages and type 2 pneumocytes were observed over the course of culture period. The scores are presented as AVE ± SEM, N = 3/group per time point.
Functional response to proinflammatory challenge
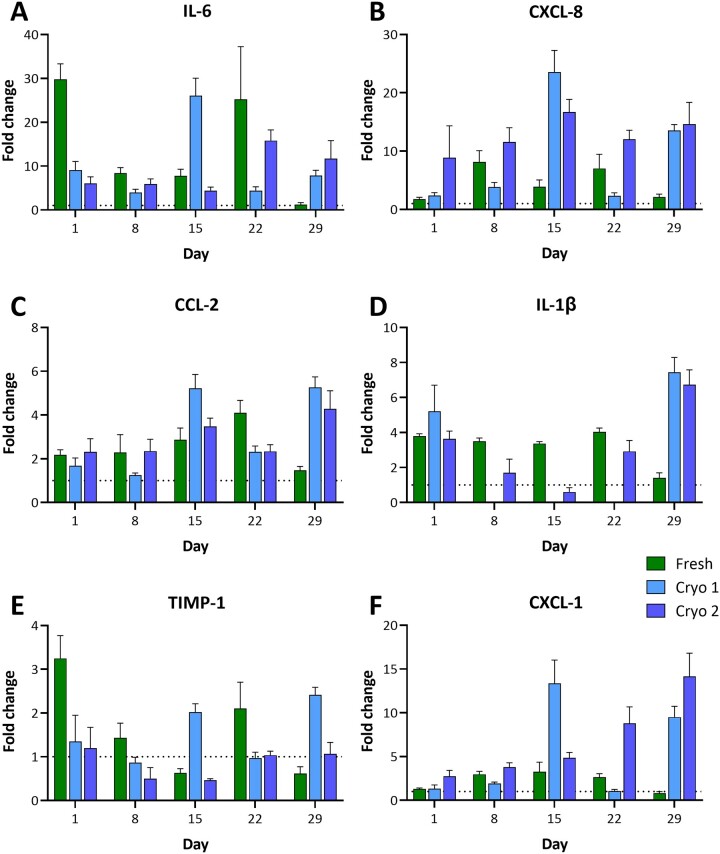
The hPCLS were challenged with 5 µg/ml LPS at days 0, 7, 14, 21, and 28 for 24 h and some of the common markers of LPS-induced inflammatory responses (IL-6, CXCL-8, CCL-2, TIMP-1, IL-1β, and CXCL-1) were quantified in supernatants. All groups (F&C) demonstrated responsiveness to the LPS challenge (Figure 7 and Supplementary Table S1), and with different markers exhibiting varying levels of fold change versus VC. Overall, LPS-induced increases were seen in IL-6 (up to 29.8-fold in Fresh, 26-fold in Cryo 1, and 15.8-fold in Cryo 2), CXCL-8 (up to 8.1-fold in Fresh, 23.5-fold in Cryo 1, and 16.7-fold in Cryo 2), CCL-2 (up to 4.1-fold in Fresh, 5.3-fold in Cryo 1, and 4.3-fold in Cryo 2), and CXCL-1 (up to 3.2-fold in Fresh, 13.3-fold in Cryo 1, and 14.2-fold in Cryo 2) at all time points. Increases in IL-1β levels were also observed (up to 4-fold in Fresh, 7.5-fold in Cryo 1, and 6.8-fold in Cryo 2), however, the diluted sample concentrations were below the standard curve range. TIMP-1 did not show any major LPS-induced increases.
Figure 7.
LPS-induced responses in cryopreserved hPCLS. Following cryopreservation, the hPCLS were thawed and cultured at ALI using DMEM:F-12 medium for up to 29 days. During the culture period, a subset of hPCLS were treated with 0.1% TX100, 5 µg/ml LPS or VC (culture medium) at days 0, 7, 14, 21, and 28. After 24 h of treatment, the medium samples were collected for determination of analytes IL-6 (A), CXCL-8 (B), CCL-2 (C), IL-1β (D), TIMP-1 (E), and CXCL-1 (F). The data from LPS-treated groups are presented as AVE ± SEM fold change relative to the VC groups at indicated time points and condition (fresh vs cryopreserved), N = 6/group per time point.
Observations in hPCLS from additional donor lungs
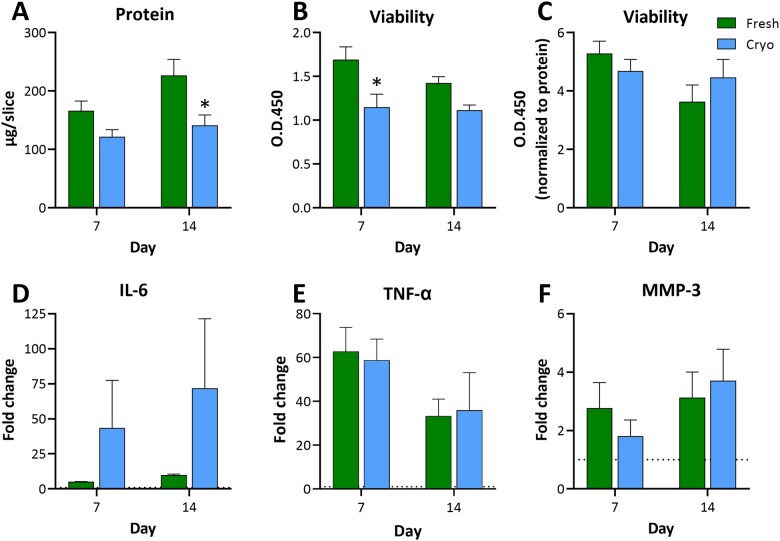
The main purpose of this study was to compare fresh slices with short-term (∼7 weeks) and long-term (∼34 weeks) cryopreserved hPCLS. However, 3 more donor lungs were procured (Table 1), processed and standard quality control assays were performed at days 7 and 14 in F&C slices to confirm the observations in slices from donor 1. The hPCLS (from the 3 donors) were cultured fresh or after cryopreservation for up to 2 weeks and the tissue viability and biomass were assessed at days 7 and 14. The LPS-induced responses (following a 24-h exposure) were also assessed by measuring IL-6, TNF-α, and MMP-3. Similar to the first donor, variability was observed in the data at different time points. The slices in both F&C groups were viable throughout the 2-week culture period (only VC data shown, Figure 8B and C), albeit with lower reads in the cryopreserved groups compared with that in fresh groups. It was noted that the protein content in the cryopreserved slices from donor numbers 3 and 4 was lower than that in the fresh slices (only VC data shown, Figure 8A), however, it did not seem to affect the immune responsiveness of the cryopreserved slices. While the finite pictogram per milliliter values in cryopreserved slices were lower, induced values relative to the negative control (fold change) were maintained, suggesting biomass-equivalent functionality. The LPS-induced increases were seen in IL-6 (up to 9.8-fold in Fresh and 71.8-fold in Cryo), TNF-α (up to 62.7-fold in Fresh and 58.7-fold in Cryo), and MMP-3 (up to 3.1-fold in Fresh and 3.7-fold in Cryo) at both the time points (Figure 8D–F, respectively) compared with the respective VC groups. The IL-6 levels in several VC and LPS groups (from donor numbers 3 and 4, in both F&C hPCLS) were higher than the standard curve range and could not be extrapolated by the Luminex xPONENT software, and high donor-to-donor variability was observed. The LPS-induced TNF-α increase was lower on day 14 (∼34-fold) compared with that on day 7 (∼60-fold), in both F&C hPCLS. Overall, the tissue viability and immune responsiveness were retained after cryopreservation in all donor slices.
Figure 8.
Retained viability and immune responsiveness in cryopreserved hPCLS from additional donor lungs. The F&C hPCLS were cultured at ALI using DMEM:F-12 medium for up to 14 days. During the culture period, a subset of hPCLS were treated with 5 µg/ml LPS or VC (culture medium) at days 6 and 13. After 24 h of treatment, the lysate and medium samples were collected for determination of protein content (A), tissue viability (B, C), IL-6 (D), TNF-α (E), and MMP-3 (F). The data are presented as AVE ± SEM, N = 3/group per time point.
Discussion
Recent reports have described the multi-week culture of hPCLS (Bailey et al., 2020; Neuhaus et al., 2017; Preuß et al., 2022; Temann et al., 2017) including a recommended method of culture and preferred medium that allows hPCLS to remain functional for 4 or more weeks (Patel et al., 2021). While this work highlighted optimized methods for multi-week cultures, the longstanding issue of donor lung availability, maximizing tissue utilization, and the ability to access the same donor tissue for “on-demand” use remained. Recent work by Bai et al. (2016) showed successful cryopreservation and use of hPCLS with responsive airways and functional immune cells at an acute (<24 h) time point. In this proof-of-concept study, hPCLS from the same donor were evaluated to assess the performance of short-term (∼7 weeks) and long-term (∼34 weeks) cryopreserved tissues against fresh hPCLS. Several markers of tissue integrity were used to monitor the health and functional status of hPCLS over time in a 4-week culture. The main aim of this work was to assess the improved method and demonstrate the proof-of-concept of long-term culture after cryopreservation using only 1 donor. However, to address potential effect of donor-to-donor variability in tissue quality and performance following cryopreservation, hPCLS from additional 3 donors were used to confirm the retention of viability and immune responsiveness over a modified 2-week culture post short-term cryopreservation.
Total protein content
hPCLS were created using Krumdieck slicers and some variability is expected due to the technical aspects of slicing and inherent variability of tissue density, thickness, and even diameter. Differences are also expected based on the amount of airway presence versus hPCLS with primarily alveolar space represented. No notable differences were seen across the 3 F&C groups at any time points, for any donor.
Viability
The maintenance of tissue health was monitored using the viability (enzymatic activity) assay, as well as membrane integrity (LDH release) of the hPCLS. As measurements of enzymatic activity, these markers may be subject to fluctuation due to tissue heterogeneity (as indicated previously), but significant changes are expected when membrane integrity or enzymatic activity is compromised. The lower initial enzymatic activity in Cryo groups was not unexpected as the slices typically exhibit increasing metabolic activity after thaw at the earlier time points. As noted with protein content, no trend in loss of viability or lysate LDH content was observed for VC- or LPS-treated groups. All F&C groups appeared to perform similarly, without cryopreserved hPCLS demonstrating any predisposition to challenge. The data from additional donors confirmed that the hPCLS, irrespective of donor-to-donor variability in tissue quality, retained viability for at least 2 weeks in culture post short-term cryopreservation. While the performance of the hPCLS from additional donors after long-term cryopreservation and culture beyond 2 weeks was not tested, it is not expected to be affected by the laboratory procedures used. However, the performance depends on the tissue quality and state (eg, diseased) at the time of procurement.
Cytokine/biomarker analysis
Despite the health markers showing equivalent results for the F&C groups, subpopulations of less prevalent cell types (eg, immune cells) may be susceptible to freeze/thaw and/or long-term storage and culture (Kadic et al., 2017; Venkataraman, 1994). Hence, proinflammatory immune responses upon LPS challenge were assessed throughout the culture period. Cytokine induction was detected in all groups and at all time points, albeit highly variable. Similar to other endpoints, the variability is expected to be influenced by the tissue substructure variability. The LPS-induced concentrations of IL-6 observed in the current study (F&C groups) were similar to, while that of IL-8 were lower than that reported in fresh hPCLS by Temann et al. (2017). Such differences may be due to several methodological differences between the 2 studies [eg, different hPCLS generation (use of 1.5% agarose) and culture (submerged conditions) methods, LPS concentration, multiplex platform (Meso Scale Discovery), etc. used by the Temann group] and donor-to-donor variability.
For the current study, the data were reviewed from several perspectives: (A) overt cytokine induction differences between F&C groups, (B) relative fold-induction versus time point-specific VC, and (C) relative induction versus fresh day 1 for all time points assayed (ie, to determine if a loss of induction or change in basal levels occurs over time). A high level review of cytokine induction suggests LPS-induced cytokine expression of majority of the markers evaluated (IL-6, CXCL-8, CCL-2, IL-1β, and CXCL-1). Regardless of the group, fold-expression levels varied across time points, and the Fresh group did not demonstrate a higher induction than cryopreserved groups. In fact, while at some time points the Cryo groups may have expressed a lesser induction, they often delivered a greater induction than that measured in the Fresh group. Comparison of all the VC groups to VC Fresh day 1 group showed similar basal levels of the cytokines post-cryopreservation and over the 4-week culture period, with the exception of Fresh day 29 group that showed slightly higher levels. Similar observations were made in F&C slices from additional donors cultured for up to 2 weeks. The LPS-induced IL-6 levels in the F&C groups were similar to that seen in slices from donor 1, however, the fold induction was greater in the cryopreserved groups. The LPS-induced TNF-α induction seemed to be decreasing over time, and similar results were reported in fresh slices by the Temann’s group (Temann et al., 2017). In summary, while the variability of cytokine response complicated the evaluation of cytokine induction consistency over the 4-week period, the cryopreservation of hPCLS did not impair the immune responsiveness of tissues. Our laboratory is currently exploring potential methods of data normalization that, if successful, may mitigate the variability commonly seen across hPCLS datasets.
Histological evaluation
A primary benefit of utilizing hPCLS for research of the deep lung is the native architecture and presence of native cell types. Blinded evaluation of H&E-stained hPCLS sections verified viability data from biochemical assays that indicated all 3 groups (F&C) retain a high level of tissue integrity and health over a 4-week culture period. Some thickening of the septal wall was observed that appeared to be more pronounced at later time points; however, it was not uniform and somewhat patchy in some of the slices. Possible explanations for this occurrence can be the migration of macrophages (CD86+ cells) in the alveolar septal region (Supplementary Figure S3), deposition of collagenous tissue over time, change in the alveolar lining cells phenotype over time to more plump and cuboidal as they adapt to new environment, and/or variable degree of alveolar inflation during lung processing so that the slices obtained from the slightly over inflated areas will have thinner septa. Importantly, viable innate immune cells, such as alveolar macrophages, were observed in all 3 F&C groups until the day 29 time point. This observation supports the robust LPS-induced immune responses seen over the 4-week culture period. Overall, all 3 F&C groups demonstrated a high degree of normal lung tissue morphology and consistency.
The number of intra-alveolar CD86+ macrophages and AQ5 levels remained stable throughout the culture period. The type 2 pneumocyte marker pSPC progressively decreased over time, indicating change in phenotype from type 2 to type 1 pneumocytes. Similar observations have been reported in primary culture of human alveolar epithelial cells (Fuchs et al., 2003). Additionally, a successive but not statistically significant increase in the interstitial CD86+ macrophages was observed throughout the 4-week culture period. These findings are compelling and may be utilized to elucidate certain disease processes but additional studies are required to evaluate relevant pathophysiologic mechanisms. Importantly, these changes were similar in both F&C slices.
Conclusion
hPCLS have recently enjoyed some technological and performance advances that have positioned this test system of the respiratory parenchyma as more accessible (with increased utility) to research labs. Studies employing hPCLS can deliver complex results over short- or long-term culture durations due to the varied complement of cell types and longevity over time. A major challenge of the fresh hPCLS model has been the infrequent availability of quality donor lungs for research and a lack of ability to store tissues for future use. With the development of a cryopreservation method that demonstrates a high retention of tissue integrity and viability, post thaw and culture, exciting new possibilities for this model can now be realized. The results presented from this proof-of-concept study demonstrate a favorable scenario where cryopreserved hPCLS can be used for the evaluation of long-term, complex endpoints. Donor-to-donor and slice-to-slice variability in immune responses and the effects of the donor lung condition (eg, disease state) on the performance of slices in culture and the endpoints of interest should be considered while choosing cryopreserved slices for research projects. Performance and quality check for each batch of cryopreserved hPCLS are necessary to assist in the selection of an appropriate hPCLS batch for research. Additional work is needed to further explore the capabilities of cryopreserved hPCLS for use in respiratory research and improve the quality of data generated, including metabolic profiling and data normalization. These results suggest efforts are warranted to generate a standardized approach for the use of hPCLS (F&C) across laboratories so data comparisons can be made and this test system can be applied in a regulatory setting that evaluates pulmonary exposure risk to human health.
Declaration of conflicting interests
Work described was conducted (in part) by authors who are employees of IIVS, a not-for-profit contract research organization that offers assays using in vitro and ex vivo models referred to in this manuscript.
Supplementary data
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
Contributor Information
Vivek S Patel, Institute for In Vitro Sciences, Inc., Gaithersburg, Maryland 20878, USA.
Khalid Amin, Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, Minnesota 55455, USA.
Adam Wahab, Institute for In Vitro Sciences, Inc., Gaithersburg, Maryland 20878, USA.
Méry Marimoutou, Institute for In Vitro Sciences, Inc., Gaithersburg, Maryland 20878, USA.
Lindsey Ukishima, Institute for In Vitro Sciences, Inc., Gaithersburg, Maryland 20878, USA.
Jose Alvarez, Institute for In Vitro Sciences, Inc., Gaithersburg, Maryland 20878, USA.
Kelley Battle, Institute for In Vitro Sciences, Inc., Gaithersburg, Maryland 20878, USA.
Andreas O Stucki, PETA Science Consortium International e.V., Stuttgart 70499, Germany.
Amy J Clippinger, PETA Science Consortium International e.V., Stuttgart 70499, Germany.
Holger P Behrsing, Institute for In Vitro Sciences, Inc., Gaithersburg, Maryland 20878, USA.
Acknowledgments
The authors thank Dr. Pooja Naik for her contributions to the initial laboratory phases of this work.
Funding
The work described was funded in part by PETA Science Consortium International e.V.
References
- Alsafadi H. N., Staab-Weijnitz C. A., Lehmann M., Lindner M., Peschel B., Konigshoff M., Wagner D. E. (2017). An ex vivo model to induce early fibrosis-like changes in human precision-cut lung slices. Am. J. Physiol. Lung Cell. Mol. Physiol. 312, L896–L902. [DOI] [PubMed] [Google Scholar]
- Alsafadi H. N., Uhl F. E., Pineda R. H., Bailey K. E., Rojas M., Wagner D. E., Konigshoff M. (2020). Applications and approaches for three-dimensional precision-cut lung slices. Disease modeling and drug discovery. Am. J. Respir. Cell Mol. Biol. 62, 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Krishnamoorthy N., Patel K. R., Rosas I., Sanderson M. J., Ai X. (2016). Cryopreserved human precision-cut lung slices as a bioassay for live tissue banking. A viability study of bronchodilation with bitter-taste receptor agonists. Am. J. Respir. Cell Mol. Biol. 54, 656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K. E., Pino C., Lennon M. L., Lyons A., Jacot J. G., Lammers S. R., Konigshoff M., Magin C. M. (2020). Embedding of precision-cut lung slices in engineered hydrogel biomaterials supports extended ex vivo culture. Am. J. Respir. Cell Mol. Biol. 62, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S. K., Huckuntod S. D., Mills S. D., Kurten R. C., Pechous R. D. (2019). Modeling pneumonic plague in human precision-cut lung slices highlights a role for the plasminogen activator protease in facilitating type 3 secretion. Infect. Immun. 87, e00175-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrsing H., Erin H., Curren R. (2020). Human precision-cut lung slices can provide important information to toxicologists. Appl. Vitro Toxicol. 6, 75–76. [Google Scholar]
- BeruBe K., Aufderheide M., Breheny D., Clothier R., Combes R., Duffin R., Forbes B., Gaca M., Gray A., Hall I., et al. (2009). In vitro models of inhalation toxicity and disease. The report of a frame workshop. Altern. Lab. Anim. 37, 89–141. [PubMed] [Google Scholar]
- Cao X., Coyle J. P., Xiong R., Wang Y., Heflich R. H., Ren B., Gwinn W. M., Hayden P., Rojanasakul L. (2021). Invited review: human air–liquid–interface organotypic airway tissue models derived from primary tracheobronchial epithelial cells—overview and perspectives. Vitro Cell. Dev. Biol. Anim. 57, 104–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedilak M., Banjanac M., Belamarić D., Paravić Radičević A., Faraho I., Ilić K., Čužić S., Glojnarić I., Eraković Haber V., Bosnar M. (2019). Precision-cut lung slices from bleomycin treated animals as a model for testing potential therapies for idiopathic pulmonary fibrosis. Pulm. Pharmacol. Ther. 55, 75–83. [DOI] [PubMed] [Google Scholar]
- Clippinger A. J., Allen D., Jarabek A. M., Corvaro M., Gaça M., Gehen S., Hotchkiss J. A., Patlewicz G., Melbourne J., Hinderliter P., et al. (2018). Alternative approaches for acute inhalation toxicity testing to address global regulatory and non-regulatory data requirements: An international workshop report. Toxicol. Vitro 48, 53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kanter R., Monshouwer M., Meijer D. K., Groothuis G. M. (2002). Precision-cut organ slices as a tool to study toxicity and metabolism of xenobiotics with special reference to non-hepatic tissues. Curr. Drug Metab. 3, 39–59. [DOI] [PubMed] [Google Scholar]
- Fisher R. L., Smith M. S., Hasal S. J., Hasal K. S., Gandolfi A. J., Brendel K. (1994). The use of human lung slices in toxicology. Hum. Exp. Toxicol. 13, 466–471. [DOI] [PubMed] [Google Scholar]
- Fuchs S., Hollins A. J., Laue M., Schaefer U. F., Roemer K., Gumbleton M., Lehr C. M. (2003). Differentiation of human alveolar epithelial cells in primary culture: morphological characterization and synthesis of caveolin-1 and surfactant protein-c. Cell Tissue Res. 311, 31–45. [DOI] [PubMed] [Google Scholar]
- Gkatzis K., Taghizadeh S., Huh D., Stainier D. Y. R., Bellusci S. (2018). Use of three-dimensional organoids and lung-on-a-chip methods to study lung development, regeneration and disease. Eur. Respir. J. 52, 1800876. [DOI] [PubMed] [Google Scholar]
- Henjakovic M., Sewald K., Switalla S., Kaiser D., Muller M., Veres T. Z., Martin C., Uhlig S., Krug N., Braun A. (2008). Ex vivo testing of immune responses in precision-cut lung slices. Toxicol. Appl. Pharmacol. 231, 68–76. [DOI] [PubMed] [Google Scholar]
- Herbert J., Gow A. (2020). Precision cut lung slices as a model for 3R application in toxicology. Appl. Vitro Toxicol. 6, 47–48. [Google Scholar]
- Hubrecht R. C., Carter E. (2019). The 3Rs and humane experimental technique: implementing change. Animals (Basel) 9, 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jude J., Botelho D., Karmacharya N., Cao G. Y., Jester W., Panettieri R. A. Jr (2019). Salicylic acid amplifies carbachol-induced bronchoconstriction in human precision-cut lung slices. Respir. Res. 20, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jude J., Koziol-White C., Scala J., Yoo E., Jester W., Maute C., Dalton P., Panettieri R. Jr (2016). Formaldehyde induces rho-associated kinase activity to evoke airway hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 55, 542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadic E., Moniz R. J., Huo Y., Chi A., Kariv I. (2017). Effect of cryopreservation on delineation of immune cell subpopulations in tumor specimens as determinated by multiparametric single cell mass cytometry analysis. BMC Immunol. 18, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauenstein L., Switalla S., Prenzler F., Seehase S., Pfennig O., Forster C., Fieguth H., Braun A., Sewald K. (2014). Assessment of immunotoxicity induced by chemicals in human precision-cut lung slices (PCLS). Toxicol. Vitro 28, 588–599. [DOI] [PubMed] [Google Scholar]
- Li K., Yang X., Xue C., Zhao L., Zhang Y., Gao X. (2019). Biomimetic human lung-on-a-chip for modeling disease investigation. Biomicrofluidics 13, 031501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Betts C., Cunoosamy D. M., Aberg P. M., Hornberg J. J., Sivars K. B., Cohen T. S. (2019). Use of precision cut lung slices as a translational model for the study of lung biology. Respir. Res. 20, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. J., Spence J. R. (2017). In vitro models to study human lung development, disease and homeostasis. Physiology (Bethesda) 32, 246–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin J. P., Baste J. M., Gay A., Crochemore C., Corbiere C., Monteil C. (2013). Precision cut lung slices as an efficient tool for in vitro lung physio-pharmacotoxicology studies. Xenobiotica 43, 63–72. [DOI] [PubMed] [Google Scholar]
- Neuhaus V., Schaudien D., Golovina T., Temann U. A., Thompson C., Lippmann T., Bersch C., Pfennig O., Jonigk D., Braubach P., et al. (2017). Assessment of long-term cultivated human precision-cut lung slices as an ex vivo system for evaluation of chronic cytotoxicity and functionality. J. Occup. Med. Toxicol. 12, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus V., Schwarz K., Klee A., Seehase S., Forster C., Pfennig O., Jonigk D., Fieguth H. G., Koch W., Warnecke G., et al. (2013). Functional testing of an inhalable nanoparticle based influenza vaccine using a human precision cut lung slice technique. PLoS ONE 8, e71728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish A. R., Gandolfi A. J., Brendel K. (1995). Precision-cut tissue slices: applications in pharmacology and toxicology. Life Sci. 57, 1887–1901. [DOI] [PubMed] [Google Scholar]
- Patel V., Amin K., Allen D., Ukishima L., Wahab A., Grodi C., Behrsing H. (2021). Comparison of long-term human precision-cut lung slice culture methodology and response to challenge: an argument for standardisation. Altern. Lab. Anim. 49, 209–222. [DOI] [PubMed] [Google Scholar]
- Preuß E. B., Schubert S., Werlein C., Stark H., Braubach P., Höfer A., Plucinski E. K. J., Shah H. R., Geffers R., Sewald K., et al. (2022). The challenge of long-term cultivation of human precision-cut lung slices. Am. J. Pathol. 192, 239–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewald K., Braun A. (2013). Assessment of immunotoxicity using precision-cut tissue slices. Xenobiotica 43, 84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmayr J., Alsafadi H. N., Langwiński W., Niroomand A., Lindstedt S., Leigh N. D., Wagner D. E. (2021). Isolation of high-yield and -quality RNA from human precision-cut lung slices for RNA-sequencing and computational integration with larger patient cohorts. Am. J. Physiol. Lung Cell. Mol. Physiol. 320, L232–L240. [DOI] [PubMed] [Google Scholar]
- Switalla S., Lauenstein L., Prenzler F., Knothe S., Förster C., Fieguth H.-G., Pfennig O., Schaumann F., Martin C., Guzman C. A., et al. (2010). Natural innate cytokine response to immunomodulators and adjuvants in human precision-cut lung slices. Toxicol. Appl. Pharmacol. 246, 107–115. [DOI] [PubMed] [Google Scholar]
- Tanner L., Single A. B. (2020). Animal models reflecting chronic obstructive pulmonary disease and related respiratory disorders: translating pre-clinical data into clinical relevance. J. Innate Immun. 12, 203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temann A., Golovina T., Neuhaus V., Thompson C., Chichester J. A., Braun A., Yusibov V. (2017). Evaluation of inflammatory and immune responses in long-term cultured human precision-cut lung slices. Hum. Vaccin. Immunother. 13, 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman M. (1994). Effects of cryopreservation on immune responses: VII. Freezing induced enhancement of IL-6 production in human peripheral blood mononuclear cells. Cryobiology 31, 468–477. [DOI] [PubMed] [Google Scholar]
- Westra I. M., Pham B. T., Groothuis G. M., Olinga P. (2013). Evaluation of fibrosis in precision-cut tissue slices. Xenobiotica 43, 98–112. [DOI] [PubMed] [Google Scholar]
- Williams K., Roman J. (2016). Studying human respiratory disease in animals—role of induced and naturally occurring models. J. Pathol. 238, 220–232. [DOI] [PubMed] [Google Scholar]
- Yilmaz Y., Williams G., Walles M., Manevski N., Krahenbuhl S., Camenisch G. (2019). Comparison of rat and human pulmonary metabolism using precision-cut lung slices (PCLS). Drug Metab. Lett. 13, 53–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.