Abstract
Background
Long-term data concerning the efficacy of different polymer-coating strategies of new-generation drug-eluting stents (DES) in patients with coronary artery calcification (CAC) are scant.
Aims
We aimed to investigate 10-year outcomes by degree of CAC after new-generation DES implantation with different polymer-coating strategies.
Methods
We analysed individual patient and lesion characteristics of patients randomised to treatment with polymer-free sirolimus-eluting, biodegradable-polymer sirolimus-eluting and permanent-polymer zotarolimus- or everolimus-eluting stents. Endpoints of interest at 10 years were all-cause mortality, myocardial infarction (MI), target lesion revascularisation (TLR) and definite or probable stent thrombosis (ST) according to the degree of CAC (no, mild, moderate or severe) and coating strategy (polymer-free vs biodegradable-polymer vs permanent-polymer).
Results
A total of 4,953 patients with 6,924 lesions were included. No, mild, moderate or severe CAC was present in 24.5%, 41.8%, 25.8% and 8.0% of patients, respectively. At 10-year follow-up, overall event rates were high, with an incremental increase according to the degree of CAC (all-cause mortality: no 25.3%, mild 32.1%, moderate 41.7%, severe CAC 46.5%; adjusted [adj.] p=0.004; TLR: no 17.4%, mild 16.5%, moderate 19.8%, severe CAC 28.7%; adj. p=0.001; MI: no 4.9%, mild 5.9%, moderate 6.0%, severe CAC 10.5%; adj. p=0.02; and ST: no 1.3%, mild 1.4%, moderate 1.8%, severe CAC 3.6%; adj. p=0.57). In patients with moderate-severe CAC, event rates were comparable, regardless of the DES polymer-coating strategy.
Conclusions
At 10 years after PCI with new-generation DES, there was an incremental increase in adverse events by degree of coronary calcification. These detrimental effects do not seem to be impacted by different polymer-coating strategies.
Introduction
Coronary artery calcification (CAC) is common in patients treated with percutaneous coronary intervention (PCI), and its prevalence is expected to increase further1. Previous studies have demonstrated that moderate or severe CAC is associated with elevated rates of periprocedural complications and adverse clinical outcomes in patients undergoing PCI2,3,4.
New-generation drug-eluting stents (DES) have been shown to improve both safety and efficacy long-term outcomes as compared to early-generation DES3,5. Nevertheless, clinical event rates remain remarkable during extended follow-up to 10 years6. This is specifically true for high-risk patients and certain lesion subsets, such as calcified lesions7,8,9. In patients with moderate or severe CAC, limited data are available regarding the long-term efficacy of new-generation DES in general and the comparative efficacy of different available polymer-coating strategies in particular. Notably, long-term data concerning the comparative efficacy of permanent-polymer, biodegradable-polymer and polymer-free new-generation DES remain poorly defined.
We therefore sought to investigate the impact of CAC degree (no, mild, moderate or severe) on long-term outcomes at 10 years after PCI with polymer-free (PF) sirolimus-eluting, biodegradable-polymer (BP) sirolimus-eluting or permanent-polymer (PP) zotarolimus- or everolimus-eluting DES in the setting of the randomised ISAR-TEST 4 (Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting STents) and 5 (Intracoronary Stenting and Angiographic Results: Test Efficacy of Sirolimus- and Probucol- and Zotarolimus- Eluting Stents) trials.
Methods
Study population, study design and devices
Individual patient level data from the ISAR-TEST 4 and 5 randomised trials were pooled for this analysis. Both trials have been described previously5,6,10,11,12,13. In brief, ISAR-TEST 4 randomised patients with ischaemic symptoms or evidence of myocardial ischaemia in the presence of ≥50% de novo stenosis located in native coronary vessels to receive a new-generation biodegradable-polymer sirolimus-eluting stent (BP-SES; Yukon Choice PC; Translumina), a new-generation permanent-polymer everolimus-eluting stent (PP-EES) (XIENCE; Abbott Vascular) or an early-generation PP-SES (CYPHER; Cordis Corporation) in a 2:1:1 allocation between September 2007 and August 2008. The early-generation PP-SES, which has not been commercially available since 2011, was excluded from the current analysis. ISAR-TEST 5 was a trial with similar inclusion and exclusion criteria to ISAR-TEST 4 and randomised patients to treatment with new-generation polymer-free sirolimus- and probucol-eluting stents or permanent-polymer zotarolimus-eluting stents in a 2:1 allocation. The polymer-free sirolimus- and probucol-eluting stents consist of a premounted, sand-blasted, 316L stainless steel microporous thin-strut (87 μm) stent, which is coated with a mixture of sirolimus, probucol and shellac resin. This coating strategy is currently available in 2 devices: VIVO ISAR (Translumina) and Coroflex ISAR NEO (B. Braun). The permanent-polymer zotarolimus-eluting stent (Resolute; Medtronic) consists of a cobalt-chrome, thin-strut (91 μm) stent platform. The polymer-coating system consists of 3 different polymers: a hydrophobic C10 polymer, a hydrophilic C19 polymer and polyvinylpyrrolidone. The principal characteristics of these new-generation DES are depicted in Supplementary Figure 1.
Patients for both trials were enrolled in 2 centres in Munich, Germany. All patients provided written informed consent for participation before receiving the assigned treatment. Both trials were conducted in accordance with the provisions of the Declaration of Helsinki and with the International Conference on Harmonization Good Clinical Practices. Analysis of data from extended follow-up, which was not prespecified in the original trial protocol, was approved by the ethics committee responsible for the participating centres. All events were adjudicated and classified by an independent event adjudication committee who were blinded to treatment allocation.
Endpoints and definitions
Endpoints of interest in this analysis were all-cause mortality, myocardial infarction (MI), target lesion revascularisation (TLR) and definite or probable stent thrombosis (ST) according to the degree of CAC (severe vs no, mild or moderate CAC) and the polymer-coating strategy (PF vs BP vs PP) at 10 years after PCI with DES implantation. An additional endpoint of interest was binary angiographic restenosis (BAR) and late luminal loss at 6- to 8-month angiographic follow-up.
Patients were systematically evaluated at 1 and 12 months and annually up to 10 years after the index PCI. Extended follow-up was performed in the setting of routine care by either telephone calls or visits at the outpatient clinic of the participating centres. In both trials, follow-up angiography was performed systematically at 6-8 months.
Coronary angiograms were digitally recorded and assessed offline in the quantitative coronary angiographic (QCA) core laboratory (ISAResearch Center, Munich, Germany) using an automated edge detection system (QAngio XA version 7.3; Medis Medical Imaging Systems) by independent personnel who were unaware of the treatment allocation. CAC was graded according to the angiographic classification of Mintz et al14. Qualitative morphological lesion characteristics were characterised by standard criteria.
Statistical analysis
For the purpose of the analysis, the pooled patient population derived from the ISAR-TEST 4 and 5 randomised trials was divided into 4 groups, based on the presence of no, mild, moderate or severe CAC on QCA. Further dedicated analyses were performed in the subset of patients with moderate or severe CAC. Continuous data are presented as mean with standard deviation or median with interquartile range (IQR). Categorical variables are summarised as frequencies and proportions. Data distribution was tested for normality using the Kolmogorov-Smirnov test for goodness of fit. Depending on the data distribution, differences between groups were checked for significance using the Student’s t-test or the Wilcoxon rank-sum test (continuous data) or the chi-square or Fisher’s exact test, when the expected cell value was <5 (categorical variables), as appropriate. Survival was analysed by the Kaplan-Meier method. Hazard ratios (HR) and 95% confidence intervals (95% CI) were calculated using the Cox proportional hazards model after checking for fulfilment of the proportional hazards assumption with the Grambsch and Therneau method15. Conventional multivariable analysis was performed for all-cause mortality, TLR, MI and ST, with adjustment for the following baseline and procedural variables: DES type, age, gender, diabetes mellitus, arterial hypertension, smoking, hypercholesterolaemia, estimated glomerular filtration rate (eGFR), multivessel disease, clinical presentation, prior MI, prior coronary artery bypass grafting (CABG), body mass index (BMI), ejection fraction, target vessel, chronic total occlusion (CTO), lesion complexity, bifurcation lesion, ostial lesion, lesion length, vessel size, diameter stenosis, balloon-to-vessel ratio, maximal balloon pressure, total stent length, and number of stents. Additionally, sensitivity analysis was performed, comparing outcomes amongst the 3 different polymer-coating groups (PF vs BP vs PP). All tests were 2-sided and a p-value <0.05 was considered statistically significant. Statistical analyses were performed using R, version 3.6.1 (R Foundation for Statistical Computing).
Results
Baseline and procedural characteristics according to the degree of calcification in the entire cohort
A total of 4,953 patients with 6,924 lesions were included in this analysis. According to angiographic core laboratory analysis, no, mild, moderate or severe CAC was present in 24.5% (1,212/4,953), 41.8% (2,069/4,953), 25.8% (1,276/4,953) and 8.0% (396/4,953) of the entire cohort, respectively. Moderate or severe CAC was present in one-third of all patients with de novo coronary artery stenosis (33.8%).
Baseline characteristics according to the degree of CAC are displayed in Table 1. Notably, patients with severe CAC were older, differed with regard to clinical presentation and displayed a worse clinical risk profile with higher rates of diabetes mellitus, arterial hypertension, prior CABG and 3-vessel disease as well as a worse renal function compared to those with no, mild or moderate CAC.
Table 1. Baseline characteristics according to degree of coronary calcification.
|
No (n=1,212) |
Mild (n=2,069) |
Moderate (n=1,276) | Severe (n=396) | p-value | ||
|---|---|---|---|---|---|---|
| Age, yrs | 64.8±11.0 | 67.4±10.9 | 68.9±10.7 | 70.2±10.6 | <0.001 | |
| Male | 921 (76.0) | 1,570 (75.9) | 969 (75.9) | 320 (80.8) | 0.186 | |
| Body mass index, kg/m2 | 27.5±4.4 | 27.6±4.4 | 27.5±4.6 | 27.5±4.3 | 0.760 | |
| Diabetes mellitus | 280 (23.1) | 586 (28.3) | 422 (33.1) | 142 (35.9) | <0.001 | |
| Insulin-dependent diabetes | 94 (7.8) | 167 (8.1) | 160 (12.5) | 53 (13.4) | <0.001 | |
| Arterial hypertension | 771 (63.6) | 1,375 (66.5) | 912 (71.5) | 283 (71.5) | <0.001 | |
| Hyperlipidaemia | 774 (63.9) | 1,335 (64.5) | 847 (66.4) | 242 (61.1) | 0.244 | |
| Current smoker | 237 (19.6) | 334 (16.1) | 186 (14.6) | 69 (17.4) | 0.008 | |
| eGFR*, ml/min | 79.0±20.6 | 74.9±21.4 | 72.2±23.0 | 71.3±21.5 | <0.001 | |
| Prior myocardial infarction | 345 (28.5) | 608 (29.4) | 380 (29.8) | 115 (29.0) | 0.905 | |
| Prior coronary artery bypass grafting | 77 (6.4) | 185 (8.9) | 155 (12.1) | 65 (16.4) | <0.001 | |
| Clinical presentation | Acute myocardial infarction | 279 (23.0) | 346 (16.7) | 210 (16.5) | 69 (17.4) | <0.001 |
| Unstable angina | 251 (20.7) | 507 (24.5) | 299 (23.4) | 81 (20.5) | ||
| Stable angina | 682 (56.3) | 1,216 (58.8) | 767 (60.1) | 246 (62.1) | ||
| Ejection fraction**, % | 53.7±10.7 | 52.8±11.7 | 52.4±12.0 | 51.1±12.6 | 0.002 | |
| 1-vessel disease | 282 (23.3) | 316 (15.3) | 133 (10.4) | 28 (7.1) | <0.001 | |
| 2-vessel disease | 350 (28.9) | 599 (29.0) | 299 (23.4) | 62 (15.7) | ||
| 3-vessel disease | 580 (47.9) | 1,154 (55.8) | 844 (66.1) | 306 (77.3) | ||
| Number of lesions | 1.26±0.54 | 1.38±0.63 | 1.56±0.74 | 1.57±0.74 | <0.001 | |
| Type of polymer coating | Biodegradable-polymer DES | 470 (38.8) | 525 (25.4) | 245 (19.2) | 59 (14.9) | <0.001 |
| Polymer-free DES | 388 (32.0) | 859 (41.5) | 578 (45.3) | 177 (44.7) | ||
| Permanent-polymer DES | 354 (29.2) | 685 (33.1) | 453 (35.5) | 160 (40.4) | ||
| Values are n (%) or mean±standard deviation unless otherwise indicated. *available in n=4,562 patients, **available in n=4,893 patients. DES: drug-eluting stent; eGFR: estimated glomerular filtration rate | ||||||
Ten-year follow-up was complete in all but 793 patients (16.0%), with no difference among the degrees of calcification (p=0.39). In patients without complete follow-up to 10 years, the median follow-up interval was 5.3 (4.1-6.9) years.
Procedural characteristics according to the degree of CAC are shown in Table 2 and were analysed at lesion level. Lesions with severe CAC were more often complex (B2/C) with higher rates of CTO and more often involved bifurcation or ostial lesions. Moreover, lesions were longer and diameter stenosis was higher in lesions with severe CAC. Furthermore, the balloon-to-vessel ratio, maximum balloon pressure, number of stents and total stent length were different, as compared to any other degree of calcification. Of note, rotational atherectomy was only used in a minority of cases (0.39% of all lesions).
Table 2. Procedural characteristics according to degree of coronary calcification.
|
Lesions with no CAC (n=1,811) |
Lesions with mild CAC (n=2,977) |
Lesions with moderate CAC (n=1,667) | Lesions with severe CAC (n=469) | p-value | ||
|---|---|---|---|---|---|---|
| Target vessel | Left anterior descending artery | 849 (46.9) | 1,297 (43.6) | 750 (45.0) | 210 (44.8) | 0.121 |
| Left circumflex artery | 431 (23.8) | 791 (26.6) | 444 (26.6) | 108 (23.0) | ||
| Right coronary artery | 531 (29.3) | 889 (29.9) | 473 (28.4) | 151 (32.2) | ||
| Complex morphology (B2/C) | 1,168 (64.5) | 2,036 (68.4) | 1,431 (85.8) | 446 (95.1) | <0.001 | |
| Chronic total occlusion | 59 (3.3) | 153 (5.1) | 117 (7.0) | 46 (9.8) | <0.001 | |
| Bifurcation | 404 (22.3) | 730 (24.5) | 566 (34.0) | 131 (27.9) | <0.001 | |
| Ostial | 329 (18.2) | 521 (17.5) | 356 (21.4) | 107 (22.8) | 0.001 | |
| Lesion length, mm | 14.7±8.4 | 15.7±9.3 | 17.2±9.9 | 18.4±11.0 | <0.001 | |
| Vessel size, mm | 2.8±0.5 | 2.8±0.5 | 2.8±0.5 | 2.8±0.5 | 0.084 | |
| Diameter stenosis, % | Before procedure | 67.0±16.8 | 65.8±15.8 | 67.6±15.3 | 68.9±16.1 | <0.001 |
| After procedure | 11.3±7.5 | 11.5±6.6 | 12.5±8.5 | 13.2±8.1 | <0.001 | |
| Type of polymer coating | Biodegradable-polymer DES | 651 (35.9) | 671 (22.5) | 295 (17.7) | 66 (14.1) | <0.001 |
| Polymer-free DES | 596 (32.9) | 1,320 (44.3) | 783 (47.0) | 213 (45.4) | ||
| Permanent-polymer DES | 564 (31.1) | 986 (33.1) | 589 (35.3) | 190 (40.5) | ||
| Maximum balloon diameter, mm | 3.11±0.52 | 3.08±0.50 | 3.06±0.53 | 3.08±0.53 | 0.075 | |
| Balloon-to-vessel ratio | 0.53±0.56 | 0.39±0.54 | 0.31±0.51 | 0.31±0.50 | <0.001 | |
| Maximum balloon pressure, bar | 14.9±3.0 | 15.4±3.0 | 16.0±3.2 | 17.2±3.6 | <0.001 | |
| Number of stents | 1.63±0.64 | 1.73±0.64 | 1.82±0.64 | 1.96±0.83 | <0.001 | |
| Total stent length, mm | 23.3±10.5 | 25.1±11.5 | 27.2±12.5 | 30.3±14.6 | <0.001 | |
| Rotational atherectomy | 3 (0.2) | 3 (0.1) | 6 (0.4) | 15 (3.2) | <0.001 | |
| Values are n (%) or mean±standard deviation unless otherwise indicated. CAC: coronary artery calcification; DES: drug-eluting stent | ||||||
Baseline and procedural characteristics according to the polymer-coating strategy in patients with moderate or severe CAC
In patients with moderate or severe CAC, baseline and procedural characteristics according to the polymer-coating strategy are displayed in Supplementary Table 1 and Supplementary Table 2, respectively. In this subset of patients, 18.2% (304/1,672) were treated with BP-DES, 45.2% (755/1,672) with PF-DES and 36.7% (613/1,672) with PP-DES. There were significant differences with regards to BMI (p=0.029), clinical presentation (p<0.001) and the number of lesions (p=0.014) (Supplementary Table 1). Moreover, lesion length (p=0.001), number of stents (p=0.017) and total stent length (p=0.003) differed significantly (Supplementary Table 2).
Angiographic outcome at 6- to 8-month follow-up according to the degree of coronary calcification in the entire cohort
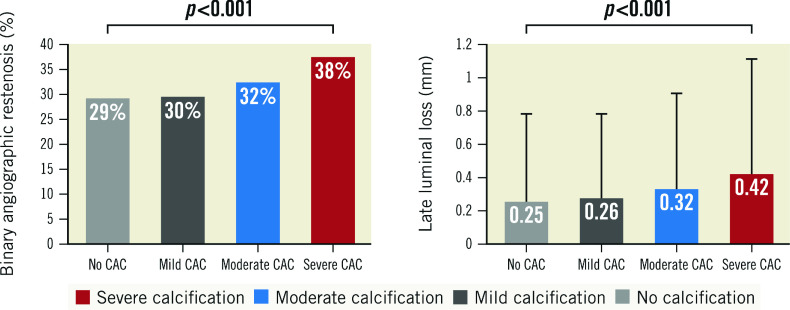
Angiographic follow-up at 6-8 months was available in 76.8% of all patients. Angiographic outcome variables at 6-8 months were analysed at lesion level. BAR was more frequent in lesions with severe CAC compared to those with no, mild or moderate CAC (38% vs 29%, 30% and 32%, respectively; p<0.001) (Figure 1). Likewise, mean late luminal loss was higher in lesions with severe CAC compared to those with no, mild or moderate CAC (0.42±0.72 vs 0.25±0.54, 0.26±0.54 and 0.32±0.60, respectively; p<0.001) (Figure 1).
Figure 1. Angiographic outcome at 6-8 months according to the degree of coronary calcification.
CAC: coronary artery calcification
Angiographic outcome at 6- to 8-month follow-up according to the polymer-coating strategy in patients with moderate or severe CAC
In patients with moderate or severe CAC, angiographic outcome at 6-8 months was further analysed according to the polymer-coating strategy. BAR was similar across all 3 treatment groups (BP-DES: 19% vs PF-DES: 21% vs PP-DES: 19%; p=0.576) (Supplementary Figure 2). Of note, mean late luminal loss was lower with BP-DES compared with PF-DES and PP-DES (0.26±0.62 vs 0.37±0.65 vs 0.33±0.62; p=0.034) (Supplementary Figure 2).
Ten-year clinical outcomes according to the degree of coronary calcification in the entire cohort
At 10-year follow-up, event rates were overall high with incremental increase according to the degree of CAC (all-cause mortality: no 25.3%, mild 32.1%, moderate 41.7% or severe CAC 46.5%; adjusted [adj.] p=0.004; TLR: no 17.4%, mild 16.5%, moderate 19.8% or severe CAC 28.7%; adj. p=0.001; MI: no 4.9%, mild 5.9%, moderate 6.0% or severe CAC 10.5%; adj. p=0.02; and ST: no 1.3%, mild 1.4%, moderate 1.8% or severe CAC 3.6%; adj. p=0.57) (Table 3).
Table 3. Ten-year clinical outcomes according to degree of coronary calcification in the entire cohort.
| No (n=1,212) | Mild (n=2,069) | Moderate (n=1,276) | Severe (n=396) | adj. HR | adj. p-value | |
|---|---|---|---|---|---|---|
| All-cause mortality | 282 (25.3) | 602 (32.1) | 483 (41.7) | 166 (46.5) | severe vs no: 1.3 (1.1-1.7) severe vs mild: 1.3 (1.1-1.6) severe vs moderate: 1.1 (0.9-1.3) | 0.004 |
| Target lesion revascularisation | 201 (17.4) | 325 (16.5) | 239 (19.8) | 109 (28.7) | severe vs no: 1.5 (1.1-2.0) severe vs mild: 1.7 (1.3-2.2) severe vs moderate: 1.4 (1.1-1.9) | 0.001 |
| Myocardial infarction | 57 (4.9) | 116 (5.9) | 74 (6.0) | 41 (10.5) | severe vs no: 1.9 (1.2-3.0) severe vs mild: 1.7 (1.1-2.5) severe vs moderate: 1.8 (1.2-2.8) | 0.02 |
| Definite or probable stent thrombosis | 15 (1.3) | 28 (1.4) | 23 (1.8) | 14 (3.6) | severe vs no: 1.6 (0.7-4.0) severe vs mild: 1.7 (0.8-3.8) severe vs moderate: 1.4 (0.7-3.1) | 0.57 |
| Data are number of events and incidence (%) with Kaplan-Meier estimates. Adjusted hazard ratios (95% CI) and adjusted p-values derived from conventional multivariable analysis. CI: confidence interval; HR: hazard ratio | ||||||
In detail, severe CAC as compared with no, mild or moderate CAC was associated with a significantly increased risk of all-cause mortality (adjusted hazard ratio [aHR] 1.3 [1.1-1.7], 1.3 [1.1-1.6] or 1.1 [0.9-1.3]; adj. p=0.004), TLR (aHR 1.5 [1.1-2.0], 1.7 [1.3-2.2] or 1.4 [1.1-1.9]; adj. p=0.001) and MI (aHR 1.9 [1.2-3.0], 1.7 [1.1-2.5] or 1.8 [1.2-2.8]; adj. p=0.02). The risk of ST in severe CAC as compared with no, mild or moderate CAC was comparable (aHR 1.6 [0.7-4.0], 1.7 [0.8-3.8] or 1.4 [0.7-3.1]; adj. p=0.57) (Table 3, Figure 2). Kaplan-Meier curves and results of the landmark analysis at 0-1 and 1-10 years for each clinical outcome are provided in Supplementary Figure 3A-Supplementary Figure 3D.
Figure 2. Comparison of adjusted probability of ten-year clinical outcomes by degree of coronary calcification.
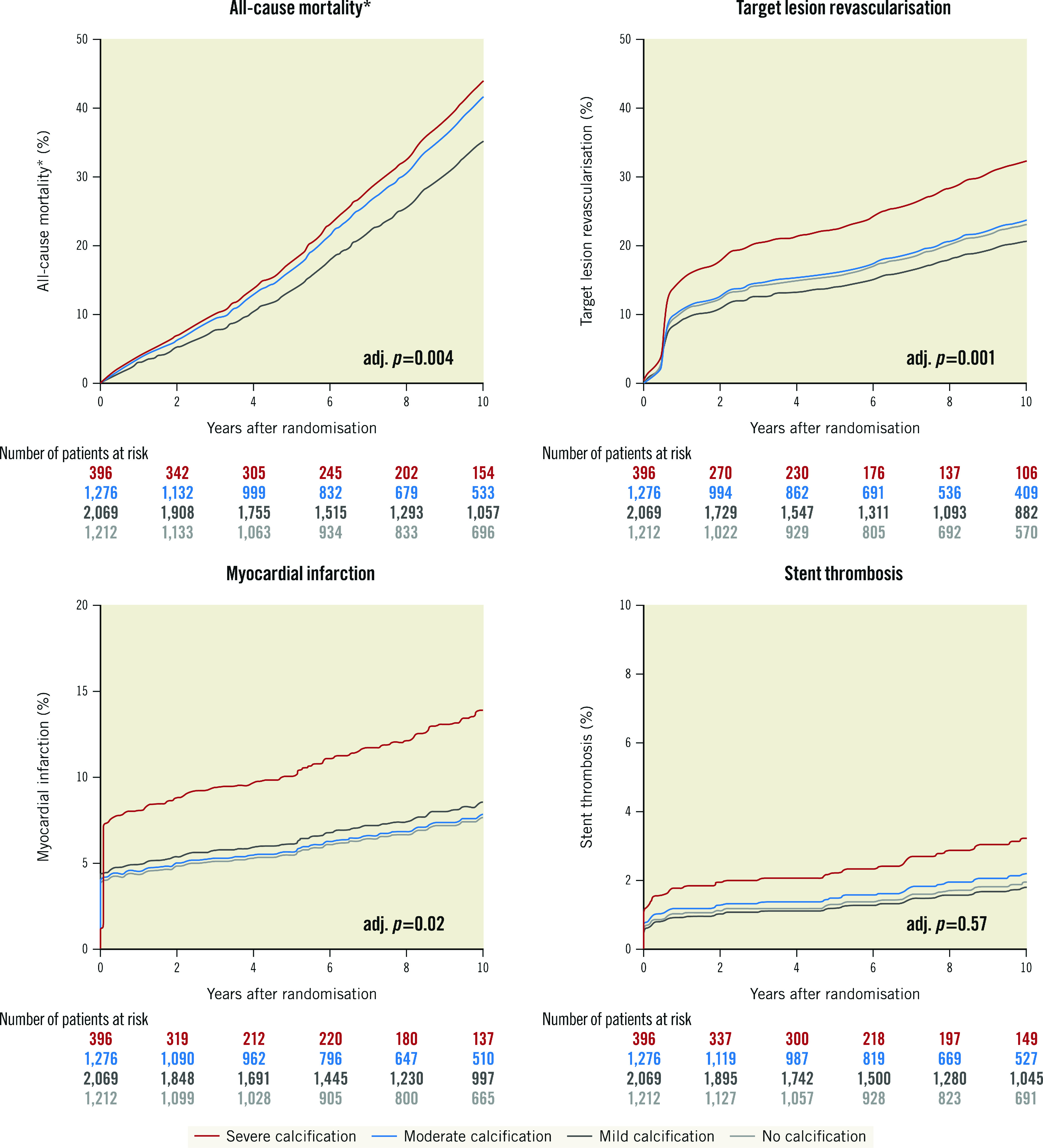
*The adjusted curves for all-cause mortality for no calcification (light grey) and mild calcification (dark grey) are overlapping. adj: adjusted
Ten-year clinical outcomes according to the polymer-coating strategy in patients with moderate or severe CAC
Clinical outcomes of patients with moderate or severe CAC according to different polymer-coating strategies of new-generation DES at 10 years are shown in Table 4 and Supplementary Figure 4. Long-term clinical event rates were comparable in patients treated with BP-DES, PF-DES or PP-DES, including all-cause mortality (41.6% vs 43.5% vs 42.7%; adj. p=0.82), TLR (25.0% vs 26.1% vs 25.0%; adj. p=0.29), MI (8.7% vs 6.5% vs 8.4%; adj. p=0.05) and probable or definite ST (2.0% vs 1.8% vs 3.3%; adj. p=0.76).
Table 4. Ten-year clinical outcomes in patients with moderate-severe coronary calcification according to polymer coating strategies.
| BP-DES (n=304) | PF-DES (n=755) | PP-DES (n=613) | Adj. p-value | |
|---|---|---|---|---|
| All-cause mortality | 112 (41.6) | 297 (43.5) | 240 (42.7) | 0.82 |
| Target lesion revascularisation | 60 (25.0) | 161 (26.1) | 127 (25.0) | 0.29 |
| Myocardial infarction | 24 (8.7) | 44 (6.5) | 47 (8.4) | 0.05 |
| Definite or probable stent thrombosis | 6 (2.0) | 13 (1.8) | 18 (3.3) | 0.76 |
| Data are shown as crude event rates (Kaplan-Meier estimates as percentages), p-values derived from Cox proportional hazard models. BP: biodegradable-polymer; DES: drug-eluting stent; PF: polymer-free; PP: permanent-polymer | ||||
Discussion
This is a pooled analysis of individual patient data from the randomised ISAR-TEST 4 and 5 trials. Both the impact of the degree of coronary calcification on long-term outcomes at 10-year follow-up after PCI with new-generation DES implantation and the respective performance of different polymer-coating strategies in the subset of patients with moderate or severe CAC were investigated. The main findings can be summarised as follows:
core laboratory-assessed moderate or severe CAC was present in one-third of patients with de novo coronary artery stenosis;
adverse clinical event rates were overall high during the extended follow-up to 10 years after PCI with an incremental increase according to the degree of coronary calcification;
detrimental effects did not differ with different polymer-coating strategies of new-generation drug-eluting stents in patients with moderate or severe CAC.
The prevalence of moderate or severe CAC in approximately one-third of patients with de novo coronary artery stenosis located in native coronary vessels is consistent with reports from previous randomised trials3,4 and is expected to further increase in the future with an ageing population1. In our study, approximately 10% of patients exhibited severe CAC and this subset of patients was older and displayed a worse clinical baseline risk profile with higher rates of chronic kidney disease, diabetes mellitus, arterial hypertension, and 3-vessel disease. This indicates that the severity of coronary calcification is likely a marker of a higher extent of prognostically relevant comorbidities, as illustrated by the mortality curves (Figure 2).
In previous studies, moderate or severe CAC was associated with higher rates of periprocedural adverse cardiovascular events in patients undergoing PCI with DES implantation. The higher event rates have been attributed to the extent of comorbidities, the larger plaque burden of coronary artery lesions and incomplete revascularisation4,16,17. New-generation DES have been shown to improve both safety and efficacy outcomes as compared to early-generation DES, including for patients with coronary artery calcification3,5. In a recent analysis including 18 randomised trials with almost 20,000 patients, the benefits of second-generation DES were present for up to 5 years of follow-up in patients with moderate or severe CAC; however, adverse clinical event rates remained remarkably high3. Our study yielded similar results in a large cohort of patients with overall high event rates during extended follow-up at 10 years after PCI with new-generation DES, and an incremental increase in event rates according to the degree of CAC. Patients with severe CAC had significantly higher rates as well as elevated adjusted hazards of all-cause mortality, target lesion revascularisation and myocardial infarction
Several mechanisms have been attributed to explain the elevated adverse event rates in this specific subset, including an increased technical complexity of PCI procedures with impaired stent delivery and deployment as well as impaired drug elution in calcified lesions18,19. To date, data regarding the differential performance of available polymer-coating strategies of new-generation DES, which have replaced early-generation DES, are lacking. In this analysis, including almost 5,000 patients treated in 2 prospective, randomised trials, adverse clinical event rates at 10 years were overall high and comparable with any polymer-coating strategy. Although polymer coating has become the key component of different new-generation DES platforms, the investigated devices did not only differ with regard to their coating strategy, but also with regard to the type, amount and release kinetics of antiproliferative drugs as well as stent backbones (cobalt-chrome, stainless steel) and varying strut thicknesses (Supplementary Figure 1). Therefore the relative efficacy of DES included in this trial may not be attributed to the polymer strategy alone, but rather to the composition of different independent iterations of each device.
Of note, restenosis rates as well as late luminal loss at angiographic follow-up at 6-8 months after index PCI were already significantly higher in lesions with severe CAC as compared with any other severity grade of CAC, indicating the importance of optimal immediate procedural results in this subset of lesions. Key procedural aspects, such as the balloon-to-vessel ratio or residual diameter stenosis, were already unfavourable during index PCI in patients with severe CAC indicating that optimal lesion preparation is a prerequisite for successful stent delivery and implantation, for both intermediate and long-term outcomes, especially in a population predisposed to stent failure3. These angiographic findings are mirrored by an excess in clinical event rates during index PCI and the first year after DES implantation in patients with severe CAC as compared to any other severity degree of CAC. In detail, the vast majority of thrombotic events, including myocardial infarction and stent thrombosis, occurred during the index procedure or within the first 30 days after the procedure. Although this finding is in line with a large body of evidence from previous randomised trials20,21,22, the relative increased risk of myocardial infarction in patients with severe CAC remains noteworthy. On the other hand, results concerning angiographic and clinical efficacy deserve further consideration. First, revascularisation rates during the first year are obviously increased due to follow-up angiography and should be interpreted with caution. These are displayed in Supplementary Figure 3B. Nevertheless, revascularisation rates of the target lesion continue to increase up to 10 years, particularly in patients with severe CAC. Second, interestingly, in patients with moderate or severe CAC, the significant benefit concerning angiographic outcomes in patients treated with BP-SES with respect to late lumen loss, which also results in numerically lower TLR rates during the first and second year, seems to be fully attenuated at 10 years. A potential explanation for these favourable early findings might be the shorter lesions in this subgroup resulting in shorter stent lengths and most importantly, a lower number of stents implanted along with potentially less stent-overlap; these procedural characteristics have been previously reported to impact DES efficacy23. This may be specifically true in this cohort of highly complex lesions, resulting in a favourable efficacy in short-intermediate outcomes. This finding, however, appears to be completely attenuated during long-term follow-up with reported stent failure up to 10 years in every fifth patient. This is in line with comparable results concerning revascularisation rates and thrombotic events in patients with moderate-severe CAC at 10 years, regardless of the polymer-coating strategy. Mortality rates >40% in this subgroup remain noteworthy, mirroring the high morbidity of this cohort.
In order to overcome some of the hurdles associated with stent failure and to achieve optimal (procedural) results in calcified coronary lesions, the use of available technologies for adequate vessel preparation prior to drug-eluting stent implantation, including debulking-, ablative- or balloon-based techniques as well as intravascular imaging, are encouraged in accordance with current expert recommendations to enhance the proficiency of PCI procedures24,25. As severely calcified lesions are challenging to cross and dilate with conventional balloons, an armamentarium of special devices including rotational or orbital atherectomy, modified balloons (cutting or scoring), super high-pressure balloons and intravascular lithotripsy has been increasingly advocated over the last years, and the comparative performance of these different concepts for lesion preparation has been investigated in recent randomised trials7,8,9,26,27. Although these trials were not powered to address clinical endpoints, overall short-term rates of adverse clinical events were low, even in the context of complex calcific coronary artery disease7,8. Whether these dedicated strategies with improved angiographic short- and mid-term results translate into improved clinical long-term outcomes of patients with calcified lesions needs to be investigated in future, large-scale trials.
Limitations
This study has several limitations which deserve attention. First, this is a pooled analysis of individual patient data from 2 randomised trials. The data pooling was justified by similar inclusion and exclusion criteria, the common standard operating procedures for interventional procedures and patient management, as well as for the acquisition and analysis of angiographic data at enrolling institutions. Nevertheless, the analyses of this prespecified subgroup were post hoc and therefore remain hypothesis-generating. Second, lesion preparation, using dedicated techniques, as recommended by current guidelines28, was done in a minority of cases (<1%), using rotational atherectomy prior to DES implantation. Third, we did not have any information on the rate of intravascular imaging use, which is generally recommended in this setting of complex percutaneous coronary procedures. Moreover, we did not have data on the medical treatment and control of cardiovascular risk factors of the patients included in this analysis up to 10 years.
Conclusions
Coronary artery calcification is common in patients undergoing PCI with DES implantation, and there was an incremental increase in adverse events by the degree of coronary calcification at 10-year follow-up after PCI with new-generation DES. These detrimental effects do not seem to be impacted by different polymer-coating strategies.
Impact on daily practice
Coronary artery calcification is increasingly encountered in patients undergoing PCI with DES implantation. Long-term data up to 10 years demonstrate that there was incremental increase in adverse cardiovascular events at 10-year follow-up, regardless of the polymer-coating strategy of new-generation DES in patients with moderate or severe calcification. Whether dedicated devices for lesion preparation of calcified lesions improve long-term outcomes needs to be further investigated.
Supplementary data
Baseline characteristics according to polymer coating strategy in patients with moderate-severe coronary calcification.
Procedural characteristics according to polymer coating strategy in patients with moderate-severe coronary artery calcification.
Overview of principal characteristics of selected new-generation drug-eluting stents.
Angiographic outcomes at 6-8 months according to polymer coating strategy in patients with moderate-severe coronary calcification.
Kaplan-Meier curves and landmark analyses.
Ten-year clinical outcomes according to different polymer coating strategies in patients with moderate-severe coronary calcification.
Acknowledgments
Conflict of interest statement
T. Rheude received speaker fees from AstraZeneca and SIS Medical; and travel support from SIS Medical. M. Joner reports institutional grant support from Boston Scientific, Cardiac Dimensions, Edwards Lifesciences, and Infraredx; consulting fees from Biotronik, TriCares, Veryan, and Shockwave; speaker fees from Abbott, AstraZeneca, Biotronik, Boston Scientific, Cardiac Dimensions, Edwards Lifesciences, ReCor Medical, and Shockwave; participation on a Steering Committee of Biotronik and Edwards Lifesciences; travel support from Boston Scientific, Cardiac Dimensions, Edwards Lifesciences, and SIS Medical. J. Wiebe reports speaker fees from AstraZeneca and Abbott Vascular; and grants to the institution from Abbott Vascular.S. Cassese reports lecture/proctoring honoraria from SIS Medical, AstraZeneca, Boston Scientific, and Teleflex; and grants to the institution from SIS Medical, Boston Scientific, and Abbott Vascular. H. Schunkert reports grants and personal fees from AstraZeneca; and personal fees from Vifor Pharma, Boehringer Ingelheim, Brahms, Medtronic, Sanofi‐Aventis, MSD, Bristol‐Myers Squibb, Servier, Bayer Vital GmbH, Daiichi Sankyo, AMGEN, Novartis, Synlab, and Pfizer, outside the submitted work. A. Kastrati reports personal payments from CRF, New York, for participation on the DSMB Microport TARGET IV trial. S. Kufner reports speaker and consulting fees from AstraZeneca and Bristol-Myers Squibb, not related to the current work; speaker fees from Translumina; and participation on an advisory board of Bristol-Myers Squibb. The other authors have no relevant conflicts of interest to declare.
Abbreviations
- BAR
binary angiographic restenosis
- BP-DES
biodegradable-polymer DES
- CAC
coronary artery calcification
- DES
drug-eluting stents
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- PF-DES
polymer-free DES
- PP-DES
permanent-polymer DES
- ST
stent thrombosis
- TLR
target lesion revascularisation
Contributor Information
Tobias Rheude, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany, Germany.
Tobias Koch, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany, Germany.
Michael Joner, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany, Germany; DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany.
Tobias Lenz, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany, Germany.
Erion Xhepa, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany, Germany.
Jens Wiebe, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany, Germany.
J.J. Coughlan, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany, Germany.
Alp Aytekin, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany, Germany.
Salvatore Cassese, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany, Germany.
Karl-Ludwig Laugwitz, DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany; Klinik und Poliklinik Innere Medizin I, Klinikum rechts der Isar, Technische Universität München, Munich, Germany.
Heribert Schunkert, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany, Germany; DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany.
Adnan Kastrati, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany, Germany; DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany.
Sebastian Kufner, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany, Germany.
References
- De Maria, Scarsini R, Banning AP. Management of Calcific Coronary Artery Lesions: Is it Time to Change Our Interventional Therapeutic Approach? JACC Cardiovasc Interv. 2019;12:1465–78. doi: 10.1016/j.jcin.2019.03.038. [DOI] [PubMed] [Google Scholar]
- Généreux P, Madhavan M, Mintz GS, Maehara A, Palmerini T, Lasalle L, Xu K, McAndrew T, Kirtane A, Lansky AJ, Brener SJ, Mehran R, Stone GW. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS. J Am Coll Cardiol. 2014;63:1845–54. doi: 10.1016/j.jacc.2014.01.034. [DOI] [PubMed] [Google Scholar]
- Guedeney P, Claessen BE, Mehran R, Mintz GS, Liu M, Sorrentino S, Giustino G, Farhan S, Leon MB, Serruys PW, Smits PC, von Birgelen, Ali ZA, Généreux P, Redfors B, Madhavan M, Ben-Yehuda O, Stone GW. Coronary Calcification and Long-Term Outcomes According to Drug-Eluting Stent Generation. JACC Cardiovasc Interv. 2020;13:1417–28. doi: 10.1016/j.jcin.2020.03.053. [DOI] [PubMed] [Google Scholar]
- Hemetsberger R, Abdelghani M, Toelg R, Mankerious N, Allali A, Garcia-Garcia HM, Windecker S, Lefèvre T, Saito S, Slagboom T, Kandzari D, Koolen J, Waksman R, Richardt G. Impact of Coronary Calcification on Clinical Outcomes After Implantation of Newer-Generation Drug-Eluting Stents. J Am Heart Assoc. 2021;10:e019815. doi: 10.1161/JAHA.120.019815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufner S, Joner M, Thannheimer A, Hoppmann P, Ibrahim T, Mayer K, Cassese S, Laugwitz KL, Schunkert H, Kastrati A, Byrne RA ISAR-TEST 4 (Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents) Investigators. Ten-Year Clinical Outcomes From a Trial of Three Limus-Eluting Stents With Different Polymer Coatings in Patients With Coronary Artery Disease. Circulation. 2019;139:325–33. doi: 10.1161/CIRCULATIONAHA.118.038065. [DOI] [PubMed] [Google Scholar]
- Kufner S, Ernst M, Cassese S, Joner M, Mayer K, Colleran R, Koppara T, Xhepa E, Koch T, Wiebe J, Ibrahim T, Fusaro M, Laugwitz KL, Schunkert H, Kastrati A, Byrne RA ISAR-TEST-5 Investigators. 10-Year Outcomes From a Randomized Trial of Polymer-Free Versus Durable Polymer Drug-Eluting Coronary Stents. J Am Coll Cardiol. 2020;76:146–58. doi: 10.1016/j.jacc.2020.05.026. [DOI] [PubMed] [Google Scholar]
- Rheude T, Rai H, Richardt G, Allali A, Abdel-Wahab M, Sulimov DS, Mashayekhi K, Ayoub M, Cuculi F, Bossard M, Kufner S, Xhepa E, Kastrati A, Fusaro M, Joner M, Byrne RA, Cassese S. Super high-pressure balloon versus scoring balloon to prepare severely calcified coronary lesions: the ISAR-CALC randomised trial. EuroIntervention. 2021;17:481–8. doi: 10.4244/EIJ-D-20-01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Wahab M, Toelg R, Byrne RA, Geist V, El-Mawardy M, Allali A, Rheude T, Robinson DR, Abdelghani M, Sulimov DS, Kastrati A, Richardt G. High-Speed Rotational Atherectomy Versus Modified Balloons Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Lesions. Circ Cardiovasc Interv. 2018;11:e007415. doi: 10.1161/CIRCINTERVENTIONS.118.007415. [DOI] [PubMed] [Google Scholar]
- Kereiakes DJ, Di Mario, Riley RF, Fajadet J, Shlofmitz RA, Saito S, Ali ZA, Klein AJ, Price MJ, Hill JM, Stone GW. Intravascular Lithotripsy for Treatment of Calcified Coronary Lesions: Patient-Level Pooled Analysis of the Disrupt CAD Studies. JACC Cardiovasc Interv. 2021;14:1337–48. doi: 10.1016/j.jcin.2021.04.015. [DOI] [PubMed] [Google Scholar]
- Byrne RA, Kastrati A, Kufner S, Massberg S, Birkmeier KA, Laugwitz KL, Schulz S, Pache J, Fusaro M, Seyfarth M, Schömig A, Mehilli J Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4) Investigators. Randomized, non-inferiority trial of three limus agent-eluting stents with different polymer coatings: the Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4) Trial. Eur Heart J. 2009;30:2441–9. doi: 10.1093/eurheartj/ehp352. [DOI] [PubMed] [Google Scholar]
- Kufner S, Byrne RA, Valeskini M, Schulz S, Ibrahim T, Hoppmann P, Schneider S, Laugwitz KL, Schunkert H, Kastrati A Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST4) Investigators. Five-year outcomes from a trial of three limus-eluting stents with different polymer coatings in patients with coronary artery disease: final results from the ISAR-TEST 4 randomised trial. EuroIntervention. 2016;11:1372–9. doi: 10.4244/EIJY14M11_02. [DOI] [PubMed] [Google Scholar]
- Kufner S, Sorges J, Mehilli J, Cassese S, Repp J, Wiebe J, Lohaus R, Lahmann A, Rheude T, Ibrahim T, Massberg S, Laugwitz KL, Kastrati A, Byrne RA ISAR-TEST-5 Investigators. Randomized Trial of Polymer-Free Sirolimus- and Probucol-Eluting Stents Versus Durable Polymer Zotarolimus-Eluting Stents: 5-Year Results of the ISAR-TEST-5 Trial. JACC Cardiovasc Interv. 2016;9:784–92. doi: 10.1016/j.jcin.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Massberg S, Byrne RA, Kastrati A, Schulz S, Pache J, Hausleiter J, Ibrahim T, Fusaro M, Ott I, Schömig A, Laugwitz KL, Mehilli J Intracoronary Stenting and Angiographic Results: Test Efficacy of Sirolimus- and Probucol-Eluting Versus Zotarolimus- Eluting Stents (ISAR-TEST 5) Investigators. Polymer-free sirolimus- and probucol-eluting versus new generation zotarolimus-eluting stents in coronary artery disease: the Intracoronary Stenting and Angiographic Results: Test Efficacy of Sirolimus- and Probucol-Eluting versus Zotarolimus-eluting Stents (ISAR-TEST 5) trial. Circulation. 2011;124:624–32. doi: 10.1161/CIRCULATIONAHA.111.026732. [DOI] [PubMed] [Google Scholar]
- Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Chuang YC, Ditrano CJ, Leon MB. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91:1959–65. doi: 10.1161/01.cir.91.7.1959. [DOI] [PubMed] [Google Scholar]
- Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- Généreux P, Redfors B, Witzenbichler B, Arsenault M-P, Weisz G, Stuckey TD, Rinaldi MJ, Neumann FJ, Christopher Metzger, Henry TD, Cox DA, Duffy PL, Mazzaferri EL, Francese DP, Marquis-Gravel G, Mintz GS, Kirtane AJ, Maehara A, Mehran R, Stone GW. Two-year outcomes after percutaneous coronary intervention of calcified lesions with drug-eluting stents. Int J Cardiol. 2017;231:61–7. doi: 10.1016/j.ijcard.2016.12.150. [DOI] [PubMed] [Google Scholar]
- Bourantas C, Zhang YJ, Garg S, Iqbal J, Valgimigli M, Windecker S, Mohr FW, Silber S, Vries Td, Onuma Y, Garcia-Garcia HM, Morel MA, Serruys PW. Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention: a patient-level pooled analysis of 7 contemporary stent trials. Heart. 2014;100:1158–64. doi: 10.1136/heartjnl-2013-305180. [DOI] [PubMed] [Google Scholar]
- Kuriyama N, Kobayashi Y, Yamaguchi M, Shibata Y. Usefulness of rotational atherectomy in preventing polymer damage of everolimus-eluting stent in calcified coronary artery. JACC Cardiovasc Interv. 2011;4:588–9. doi: 10.1016/j.jcin.2010.11.017. [DOI] [PubMed] [Google Scholar]
- Buiten RA, Ploumen EH, Zocca P, Doggen CJM, van Houwelingen, Danse PW, Schotborgh CE, Stoel MG, Scholte M, Linssen GCM, de Man, von Birgelen. Three contemporary thin-strut drug-eluting stents implanted in severely calcified coronary lesions of participants in a randomized all-comers trial. Catheter Cardiovasc Interv. 2020;96:E508–15. doi: 10.1002/ccd.28886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes RD, Leonardi S, Wojdyla DM, Vora AN, Thomas L, Storey RF, Vinereanu D, Granger CB, Goodman SG, Aronson R, Windecker S, Thiele H, Valgimigli M, Mehran R, Alexander JH. Stent Thrombosis in Patients With Atrial Fibrillation Undergoing Coronary Stenting in the AUGUSTUS Trial. Circulation. 2020;141:781–3. doi: 10.1161/CIRCULATIONAHA.119.044584. [DOI] [PubMed] [Google Scholar]
- Tada T, Byrne RA, Simunovic I, King LA, Cassese S, Joner M, Fusaro M, Schneider S, Schulz S, Ibrahim T, Ott I, Massberg S, Laugwitz KL, Kastrati A. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Interv. 2013;6:1267–74. doi: 10.1016/j.jcin.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Stefanini GG, Byrne RA, Serruys PW, de Waha, Meier B, Massberg S, Jüni P, Schömig A, Windecker S, Kastrati A. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. 2012;33:1214–22. doi: 10.1093/eurheartj/ehs086. [DOI] [PubMed] [Google Scholar]
- Coughlan JJ, Aytekin A, Koch T, Wiebe J, Lenz T, Cassese S, Joner M, Koppara T, Xhepa E, Ibrahim T, Fusaro M, Laugwitz KL, Schunkert H, Kastrati A, Kufner S. Long-term clinical outcomes after drug eluting stent implantation with and without stent overlap. Catheter Cardiovasc Interv. 2022;99:541–51. doi: 10.1002/ccd.29944. [DOI] [PubMed] [Google Scholar]
- Barbato E, Shlofmitz E, Milkas A, Shlofmitz R, Azzalini L, Colombo A. State of the art: evolving concepts in the treatment of heavily calcified and undilatable coronary stenoses - from debulking to plaque modification, a 40-year-long journey. EuroIntervention. 2017;13:696–705. doi: 10.4244/EIJ-D-17-00473. [DOI] [PubMed] [Google Scholar]
- Räber , L, Mintz , GS, Koskinas , KC, Johnson , TW, Holm , NR, Onuma , Y, Radu , MD, Joner , M, Yu , B, Jia , H, Meneveau , N, de la Torre Hernandez , JM, Escaned , J, Hill , J, Prati , F, Colombo , A, Di Mario , C, Regar , E, Capodanno , D, Wijns , W, Byrne , RA, Guagliumi , G Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2018;14:656–77. doi: 10.4244/EIJY18M06_01. [DOI] [PubMed] [Google Scholar]
- Rheude T, Toelg R, Byrne RA, Allali A, Wiebe J, Sulimov DS, Voll F, Cassese S, Richardt G, Kastrati A, Abdel-Wahab M. Outcomes of rotational atherectomy versus modified balloon angioplasty in severely calcified coronary lesions based on target lesion location: a post hoc analysis of the PREPARE-CALC randomised trial. EuroIntervention. 2020;16:e322–4. doi: 10.4244/EIJ-D-19-00488. [DOI] [PubMed] [Google Scholar]
- Rheude T, Fitzgerald S, Allali A, Mashayekhi K, Gori T, Cuculi F, Kufner S, Hemetsberger R, Sulimov DS, Rai H, Ayoub M, Bossard M, Xhepa E, Fusaro M, Toelg R, Joner M, Byrne RA, Richardt G, Kastrati A, Cassese S, Abdel-Wahab M. Rotational Atherectomy or Balloon-Based Techniques to Prepare Severely Calcified Coronary Lesions. JACC Cardiovasc Interv. 2022;15:1864–1874. doi: 10.1016/j.jcin.2022.07.034. [DOI] [PubMed] [Google Scholar]
- Riley RF, Henry TD, Mahmud E, Kirtane AJ, Brilakis ES, Goyal A, Grines CL, Lombardi WL, Maran A, Rab T, Tremmel JA, Truesdell AG, Yeh RW, Zhao DX, Jaffer FA. SCAI position statement on optimal percutaneous coronary interventional therapy for complex coronary artery disease. Catheter Cardiovasc Interv. 2020;96:346–62. doi: 10.1002/ccd.28994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics according to polymer coating strategy in patients with moderate-severe coronary calcification.
Procedural characteristics according to polymer coating strategy in patients with moderate-severe coronary artery calcification.
Overview of principal characteristics of selected new-generation drug-eluting stents.
Angiographic outcomes at 6-8 months according to polymer coating strategy in patients with moderate-severe coronary calcification.
Kaplan-Meier curves and landmark analyses.
Ten-year clinical outcomes according to different polymer coating strategies in patients with moderate-severe coronary calcification.