Key Points
-
•
Hepatocyte TfR1 requires HFE for its role in hepcidin regulation and iron homeostasis.
-
•
Hepatocyte TfR1 restrains hepcidin induction by serum iron and promotes hepcidin suppression and iron overload in β-thalassemia.
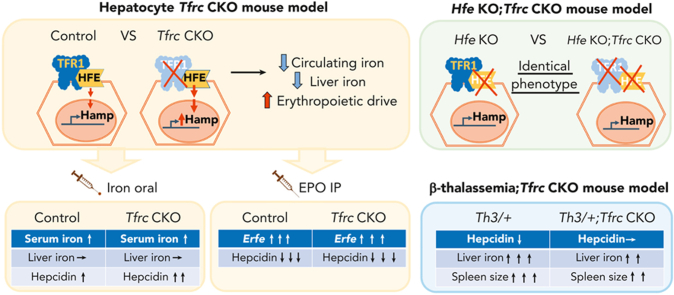
Visual Abstract
Abstract
Transferrin receptor 1 (TfR1) performs a critical role in cellular iron uptake. Hepatocyte TfR1 is also proposed to influence systemic iron homeostasis by interacting with the hemochromatosis protein HFE to regulate hepcidin production. Here, we generated hepatocyte Tfrc knockout mice (Tfrcfl/fl;Alb-Cre+), either alone or together with Hfe knockout or β-thalassemia, to investigate the extent to which hepatocyte TfR1 function depends on HFE, whether hepatocyte TfR1 impacts hepcidin regulation by serum iron and erythropoietic signals, and its contribution to hepcidin suppression and iron overload in β-thalassemia. Compared with Tfrcfl/fl;Alb-Cre− controls, Tfrcfl/fl;Alb-Cre+ mice displayed reduced serum and liver iron; mildly reduced hematocrit, mean cell hemoglobin, and mean cell volume; increased erythropoietin and erythroferrone; and unchanged hepcidin levels that were inappropriately high relative to serum iron, liver iron, and erythroferrone levels. However, ablation of hepatocyte Tfrc had no impact on iron phenotype in Hfe knockout mice. Tfrcfl/fl;Alb-Cre+ mice also displayed a greater induction of hepcidin by serum iron compared with Tfrcfl/fl;Alb-Cre− controls. Finally, although acute erythropoietin injection similarly reduced hepcidin in Tfrcfl/fl;Alb-Cre+ and Tfrcfl/fl;Alb-Cre− mice, ablation of hepatocyte Tfrc in a mouse model of β-thalassemia intermedia ameliorated hepcidin deficiency and liver iron loading. Together, our data suggest that the major nonredundant function of hepatocyte TfR1 in iron homeostasis is to interact with HFE to regulate hepcidin. This regulatory pathway is modulated by serum iron and contributes to hepcidin suppression and iron overload in murine β-thalassemia.
Hepcidin is the master regulator of systemic iron homeostasis. Using genetically manipulated murine models, Xiao and colleagues reveal that hepatocyte transferrin receptor 1 (TfR1) restrains hepcidin induction by serum iron and promotes hepcidin suppression and iron overload in β-thalassemia. The authors prove that TfR1 action is dependent upon HFE, the product of the most commonly mutated gene in hereditary hemochromatosis, adding important detail to our knowledge of iron regulation.
Introduction
Iron is an essential nutrient. Upon dietary absorption or release from body stores, iron is loaded onto transferrin for transportation to all cells in the body. Iron-bound transferrin binds to the ubiquitously expressed transferrin receptor 1 (TfR1) to deliver iron via receptor-mediated endocytosis. In addition to cellular iron uptake, hepatocyte TfR1 is also proposed to contribute to systemic iron homeostasis regulation by influencing the expression of the iron hormone, hepcidin.1,2 The master regulator of systemic iron homeostasis, hepcidin, is produced by hepatocytes in response to serum and tissue iron status, erythropoietic drive, and inflammation.2 Hepcidin binds to the iron exporter, ferroportin, to inhibit iron export and to induce ferroportin degradation, thereby blocking the entry of iron into circulation from dietary sources and body stores.3, 4, 5 Serum iron, liver iron, and erythropoietic drive all regulate hepcidin expression by influencing the activity of the bone morphogenetic protein (BMP)- small mothers against decapentaplegic (SMAD) signaling pathway.6
Hepatocyte TfR1 has been proposed to influence hepcidin expression through its interaction with homeostatic iron regulator, HFE, the most commonly mutated gene in the iron overload disorder, hereditary hemochromatosis.7 HFE functions as a positive regulator of hepcidin expression by enhancing the activation of the BMP-SMAD signaling cascade,8 possibly by interacting with the components of the BMP receptor complex.9,10 TfR1 binds directly to HFE at a binding site that overlaps with transferrin.11,12 Genetic mouse models globally expressing mutant TfR1 that either favors or precludes HFE binding, suggest that HFE activates hepcidin when not sequestered by TfR1.1
Recently, mice with a hepatocyte conditional knockout of Tfrc were generated to elucidate the role of hepatocyte TfR1 in iron homeostasis in vivo.13 Liver iron levels were lower in hepatocyte Tfrc knockout mice than littermate controls, which the authors partly attributed to reduced iron uptake via TfR1. Although reduced liver iron levels should suppress hepcidin, absolute hepcidin levels did not differ between hepatocyte Tfrc knockout mice and controls, suggesting that hepcidin levels were inappropriately high. The authors proposed that hepatocellular iron loading influences hepcidin expression by reducing hepatocyte TfR1 expression via the iron regulatory protein system, thereby releasing HFE to activate hepcidin. However, this study did not test whether hepatocyte TfR1 function depended on HFE nor assess the relative importance of hepatocyte TfR1’s iron uptake function vs its hepcidin regulatory function. Moreover, a functional role of hepatocyte TfR1 in hepcidin regulation by serum iron and erythropoietic drive was not demonstrated.
In addition to liver iron, serum iron, presumably in the form of transferrin-bound iron, is an independent regulator of hepcidin expression.14,15 Although TfR1 homologue transferrin receptor 2 (TfR2) is thought to be the major sensor of circulating iron to regulate hepcidin,16, 17, 18 a role for hepatocyte TfR1 has also been proposed.1 In this model, increases in circulating transferrin-bound iron would enhance transferrin binding to hepatocyte TfR1, thereby displacing HFE to induce hepcidin, possibly via an interaction with TfR2.1,16 However, direct experimental evidence of hepatocyte TfR1’s contribution to hepcidin regulation by serum iron has not been demonstrated in vivo.
When erythropoiesis increases, proliferating erythroblasts secrete erythroferrone (ERFE), which suppresses liver hepcidin production by sequestering BMP ligands.19, 20, 21 This increases the availability of iron to support erythropoiesis in the context of anemia, but excess activation of this pathway contributes to iron overload in the disorders of ineffective erythropoiesis.19,22 A prototypical disorder of ineffective erythropoiesis is β-thalassemia, resulting from defective β-globin synthesis, characterized by microcytic anemia, extramedullary hematopoiesis, splenomegaly, ERFE excess, hepcidin deficiency, and iron overload.23 Notably, phlebotomy or concomitant β-thalassemia still lowered hepcidin expression in Erfe knockout mice, albeit to a lesser extent, suggesting additional ERFE-independent mechanisms for hepcidin suppression.19,22 A previous study proposed that a reduction in circulating diferric transferrin owing to increased uptake by erythropoietic cells, may be sensed by the liver as an additional mechanism of hepcidin suppression by erythropoietic drive.24 However, this model has not been definitively proven experimentally.
Interestingly, previous studies showed that global Tfrc haploinsufficiency or apotransferrin administration ameliorated hepcidin suppression and iron overload in a mouse model of β-thalassemia.25,26 This was postulated to result from a reduced erythroid TfR1 expression, leading to iron-restricted erythropoiesis, and thereby resulting in improvements in ineffective erythropoiesis, splenomegaly, and ERFE excess. It was not investigated whether a reduction in hepatocyte TfR1 also contributed to the improvement.
Here, we generated hepatocyte-specific Tfrc knockout mice and littermate Cre− controls, either alone or together with a global knockout of Hfe or a heterozygous B1/B2 globin gene deletion (the Hbbth3/+ model of β-thalassemia intermedia). We used these mice to test whether and to what extent hepatocyte TfR1 function is dependent on HFE and investigate the role of hepatocyte TfR1 in hepcidin regulation by serum iron and erythropoietic signals. Additionally, we explored the contribution of hepatocyte TfR1 to hepcidin suppression and iron overload in β-thalassemia.
Methods
Mice
Animal experiments were approved by the Institutional Animal Care and Use Committee at Massachusetts General Hospital. Tfrcfl/fl female mice (B6.129S[Cg]-Tfrctm3.1Nca/J, Jackson 028363) were crossed with male mice expressing Cre recombinase driven by an albumin promoter (B6.Cg-Speer6-ps1Tg(Alb-cre)21Mgn/J, Jackson 003574, hereafter termed as Alb-Cre+) to generate Tfrcwt/fl;Alb-Cre+ and Tfrcwt/fl;Alb-Cre− mice, which were further intercrossed to generate hepatocyte-specific Tfrc knockout mice (Tfrcfl/fl;Alb-Cre+) and littermate controls (Tfrcfl/fl;Alb-Cre−). Tfrcfl/fl;Alb-Cre+ mice were crossed with Hfe knockout mice (Hfe−/−, provided by Nancy Andrews27) in a C57BL/6J background to generate Hfe+/−;Tfrcwt/fl;Alb-Cre+ and Hfe+/−;Tfrcwt/fl;Alb-Cre− mice, which were further crossed to generate Hfe and hepatocyte Tfrc double knockout mice (Hfe−/−;Tfrcfl/fl;Alb-Cre+) and Hfe single knockout littermates (Hfe−/−;Tfrcfl/fl;Alb-Cre−). β-thalassemia Hbbth3/+ mice with heterozygous deletions of both Hbb-b1 and Hbb-b2 alleles (B6;129P2-Hbb-b1tm1Unc Hbb-b2tm1Unc/J, Jackson 003253) were crossed with Tfrcfl/fl;Alb-Cre+ mice to generate Hbbth3/+;Tfrcwt/fl;Alb-Cre+ mice, which were further crossed with Tfrcfl/fl mice to generate double-mutant hepatocyte Tfrc knockout β-thalassemia mice (Hbbth3/+;Tfrcfl/fl;Alb-Cre+), β-thalassemia mice (Hbbth3/+;Tfrcfl/fl;Alb-Cre−), and littermate controls (Hbb+/+;Tfrcfl/fl;Alb-Cre−). All mice had ad libitum access to water and a house diet (Prolab 5P75 Isopro RMH 3000 containing 380 ppm iron) unless otherwise indicated. When indicated, 6-week-old Tfrcfl/fl;Alb-Cre+ mice and littermate Tfrcfl/fl;Alb-Cre− controls received an intraperitoneal injection of epoetin alfa (Amgen) at 200 U per mouse or phosphate-buffered saline and were euthanized after 9 or 15 hours. Seven-week-old Tfrcfl/fl;Alb-Cre+ mice and littermate Tfrcfl/fl;Alb-Cre− controls were fed a low-iron diet (2-6 ppm; Harlan #TD.80396) for 12 days, orally gavaged with ferrous sulfate containing 2 mg/kg elemental iron or distilled water and euthanized after 5 hours. Four-week-old Hfe−/−;Tfrcfl/fl;Alb-Cre+ and Hfe−/−;Tfrcfl/fl;Alb-Cre− mice were fed a low-iron diet (2-6 ppm; Harlan #TD.80396) for 3 weeks before euthanasia.
RNA isolation, reverse transcription, and quantitative reverse transcription-polymerase chain reaction (PCR)
Total liver or bone marrow RNA was isolated using QIAshredder and PureLink RNA mini kits (Invitrogen). First-strand complementary DNA was synthesized from RNA (1 μg) using the High-Capacity RNA-to-cDNA Kit (Applied Biosystems). PCR was performed using the PowerUp SYBR Green Master Mix on the QuantStudio3 Real-Time PCR system (Applied Biosystems) using primers as shown in supplemental Table 1, available on the Blood website. Transcript levels were determined as previously described using the standard curve method and normalized to Rpl19.28
Complete blood count (CBC) and iron analysis
Serum iron and unsaturated iron binding capacity were measured by colorimetric assay (Pointe Scientific) to calculate transferrin saturation according to the manufacturer’s instructions. CBC and tissue-nonheme iron concentrations (μg/g wet weight) were determined as described previously.28,29
Immunoblot
Liver lysates were prepared and immunoblots performed as described previously28 using mouse anti-TfR1 (1:2000; 13-6800; Thermo Fisher Scientific), rabbit anti-TfR2 (1:1000; TFR21; Alpha Diagnostic), goat antiferritin light chain (1:1000; NBP1-06986; Novus Biologicals), rabbit antiphosphorylated SMAD5 (pSMAD5; 1:500; ab92698; Abcam), rabbit anti-SMAD5 (1:1000; ab40771; Abcam), or mouse antiactin (1:20 000; MAB1501; Millipore) antibodies. Immunoreactivity was visualized by chemiluminescence (SuperSignal West Pico, Thermo Fisher Scientific) and the G:box mini digital darkroom (Syngene). Quantification was performed using ImageJ 1.46v. Protein expression was normalized to actin or total SMAD5 (for pSMAD5).
Statistics
Statistical significance was determined by using a 2-tailed Student t test or two-way analysis of variance with Tukey post hoc test for pairwise multiple comparisons using Prism 8 (GraphPad). The value of P < .05 was considered significant.
Results
Hepatocyte Tfrc knockout mice exhibit hypoferremia, reduced liver iron, reduced hematocrit, and microcytosis
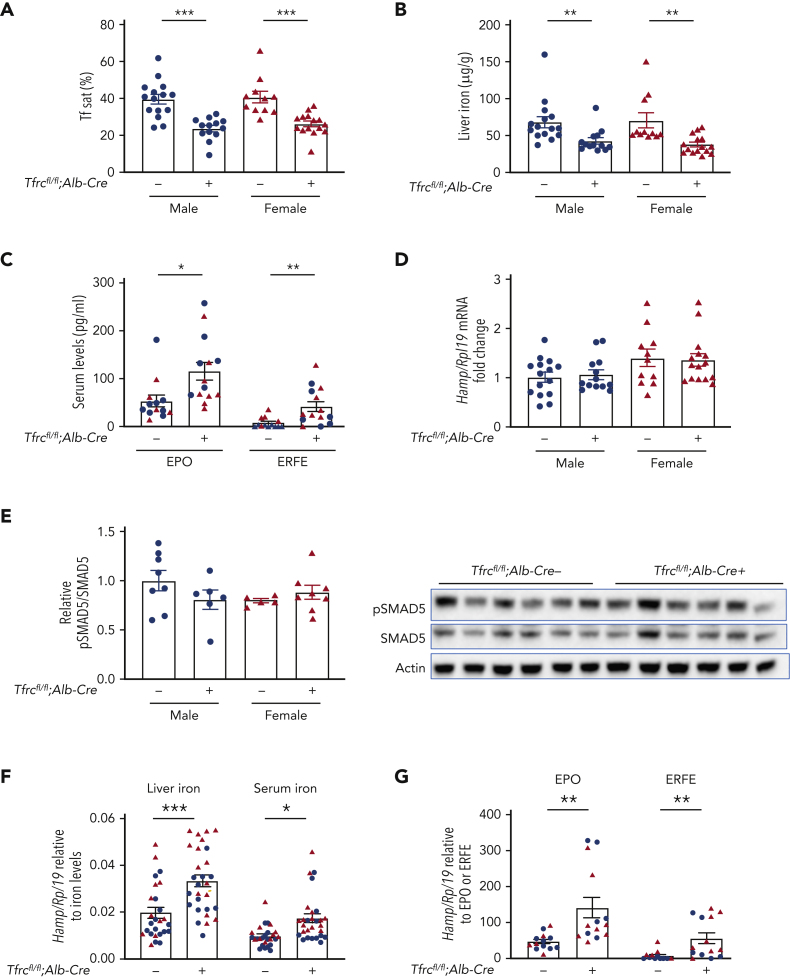
Hepatocyte-specific Tfrc knockout mice (Tfrcfl/fl;Alb-Cre+) and littermate controls (Tfrcfl/fl;Alb-Cre−) were generated as described (refer to “Methods”). We previously validated the efficacy and specificity of this Cre model for causing recombination in liver hepatocytes, but not endothelial cells, Kupffer cells, or stellate cells using Cre reporter mice and immunostaining.30 Like previously reported hepatocyte Tfrc knockout mice generated using a different floxed allele and background strain,13 whole liver Tfrc messenger RNA (mRNA) and TfR1 protein were reduced by more than 90% and 87% respectively in Tfrcfl/fl;Alb-Cre+ mice compared with Tfrcfl/fl;Alb-Cre− controls (supplemental Figure 1A). Moreover, loss of TfR1 did not cause a compensatory upregulation of TfR2, but rather slightly decreased liver TfR2 protein levels in female Tfrcfl/fl;Alb-Cre+ mice, with a similar trend in males (supplemental Figure 1B). Tfrcfl/fl;Alb-Cre+ mice also exhibited reduced serum iron and transferrin saturation without any impact on the total iron binding capacity, reduced liver nonheme iron and liver ferritin expression, and unchanged spleen iron compared with sex-matched Tfrcfl/fl;Alb-Cre− mice (Figure 1A-B and supplemental Figure 1C-F). Reduced transferrin saturation may account for reduced TfR2 protein levels in Tfrcfl/fl;Alb-Cre+ mice because TfR2 protein stability is enhanced by diferric transferrin.17,18
Figure 1.
Hepatocyte Tfrc knockout mice (Tfrcfl/fl;Alb-Cre+) exhibit hypoferremia, reduced liver iron, and increased erythropoietic drive, without appropriate hepcidin suppression. Eight-week-old male (blue) and female (red) hepatocyte Tfrc knockout mice (Tfrcfl/fl;Alb-Cre+) and littermate controls (Tfrcfl/fl;Alb-Cre−) were analyzed for (A) serum transferrin saturation and (B) liver iron levels by colorimetric assays. (C) Serum EPO and ERFE protein levels were quantified by ELISA. (D) Livers were analyzed for Hamp relative to Rpl19 mRNA by qRT-PCR. (E) Livers were analyzed for pSMAD5 relative to total SMAD5 and actin protein by immunoblot and chemiluminescence quantitation. A representative immunoblot is shown. For panels D-E, the average of the control male mice was set to 1. (F) Hamp/Rpl19 mRNA was divided by liver iron content or serum iron content to normalize for the degree of iron overload and, (G) Hamp/Rpl19 mRNA was multiplied by serum EPO or ERFE levels to normalize for the degree of increased erythropoietic signals. For all graphs, individual data points are shown, and bars represent mean ± SEM. ∗P < .05, ∗∗P < .01, ∗∗∗ P < .001 relative to sex-matched Tfrcfl/fl;Alb-Cre− mice by the Student t test. ELISA, enzyme-linked immunosorbent assay; qRT, quantitative reverse transcription; SEM, standard error of the mean.
In contrast to the previously reported hepatocyte Tfrc knockout mice, Tfrcfl/fl;Alb-Cre+ mice exhibited slightly, but significantly, decreased hematocrit compared with Tfrcfl/fl;Alb-Cre− controls. Hemoglobin was also significantly reduced in Tfrcfl/fl;Alb-Cre+ males, with a similar trend in females (Table 1). Tfrcfl/fl;Alb-Cre+ mice also exhibited reduced mean cell volume, reduced mean cell hemoglobin, and increased red cell distribution width (Table 1). In accordance with their reduced hematocrit, Tfrcfl/fl;Alb-Cre+ mice had increased serum erythropoietin (EPO) and ERFE levels compared with Alb-Cre− controls (Figure 1C), consistent with increased erythropoietic drive. Together, these data demonstrate that Tfrcfl/fl;Alb-Cre+ mice have a deficiency of both circulating and stored iron, leading to iron-restricted erythropoiesis.
Table 1.
Hepatocyte Tfrc knockout mice exhibit iron-restricted erythropoiesis
| Sex | Genotype | HCT (%) | Hgb (g/dL) | MCV (fL) | MCH (pg) | RDW (%) |
|---|---|---|---|---|---|---|
| Male | Tfrcfl/fl;Alb-Cre− | 40.78 ± 0.81 | 13.56 ± 0.27 | 46.92 ± 0.37 | 15.61 ± 0.10 | 16.58 ± 0.12 |
| Tfrcfl/fl;Alb-Cre+ | 37.46 ± 0.61∗∗ | 12.58 ± 0.23∗ | 44.06 ± 0.29∗∗∗ | 14.80 ± 0.11∗∗∗ | 17.76 ± 0.31∗∗ | |
| Female | Tfrcfl/fl;Alb-Cre− | 40.71 ± 0.53 | 13.66 ± 0.24 | 46.84 ± 0.25 | 15.71 ± 0.08 | 16.45 ± 0.10 |
| Tfrcfl/fl;Alb-Cre+ | 38.69 ± 0.56∗ | 13.07 ± 0.24† | 44.53 ± 0.21∗∗∗ | 15.04 ± 0.10∗∗∗ | 17.44 ± 0.14∗∗∗ |
Data represent means ± SEM; n = 11 to 14 mice per group (8 weeks old).
HCT, hematocrit; Hgb, hemoglobin; MCV, mean cell volume; MCH, mean cell hemoglobin; RDW, red cell distribution width; SEM, standard error of the mean.
P < .05 vs sex-matched Tfrcfl/fl;Alb-Cre− mice by Student's t test.
P < .01 vs sex-matched Tfrcfl/fl;Alb-Cre− mice by Student's t test.
P < .001 vs sex-matched Tfrcfl/fl;Alb-Cre− mice by Student's t test.
P = .09 vs female Tfrcfl/fl;Alb-Cre− mice by Student t test.
Despite lower serum iron, lower liver iron, and higher ERFE levels, each of which suppress BMP-SMAD signaling and hepcidin transcription,6 hepcidin (Hamp) mRNA expression was unchanged in Tfrcfl/fl;Alb-Cre+ mice compared with Tfrcfl/fl;Alb-Cre− controls (Figure 1D). Similarly, there was no difference in pSMAD5 protein levels or the BMP-SMAD target transcript Id1 (Figure 1E and supplemental Figure 1G). These data suggest that hepcidin levels are inappropriately high relative to serum iron, liver iron, and ERFE levels in Tfrcfl/fl;Alb-Cre+ mice. Indeed, when normalized to liver iron, serum iron, or ERFE, hepcidin was significantly higher in Tfrcfl/fl;Alb-Cre+ mice than Tfrcfl/fl;Alb-Cre− controls (Figure 1F-G).
Deletion of hepatocyte Tfrc does not alter iron phenotype in Hfe knockout mice
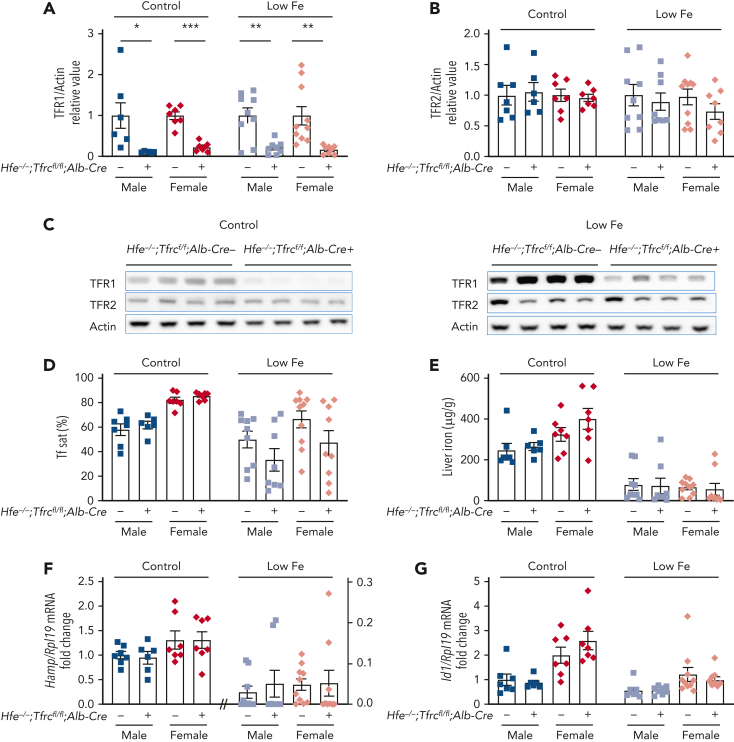
To determine which aspects of hepatocyte TfR1 function depend on HFE, we compared the iron phenotype of mice with a double global Hfe knockout and hepatocyte Tfrc knockout (Hfe−/−;Tfrcfl/fl;Alb-Cre+) vs littermate single Hfe knockout mice (Hfe−/−;Tfrcfl/fl;Alb-Cre−). Both genotypes were maintained on a standard rodent diet. Total liver Tfrc mRNA and TfR1 protein expression was significantly reduced in double Hfe−/−;Tfrcfl/fl;Alb-Cre+ knockout mice vs single Hfe−/−;Tfrcfl/fl;Alb-Cre− knockout mice, and there was no compensatory upregulation of TfR2 (supplemental Figure 2A and Figure 2A-C, left panels). In contrast to the impact of hepatocyte Tfrc knockout on wildtype mice (Figure 1 and supplemental Figure 1; Table 1), serum transferrin saturation and liver iron levels were not reduced in double Hfe−/−;Tfrcfl/fl;Alb-Cre+ knockout mice compared with single Hfe−/−;Tfrcfl/fl;Alb-Cre− knockout littermates (Figure 2D-E, left panels). Moreover, CBC parameters and red blood cell indices were unchanged (Table 2). Consistent with the lack of change in iron parameters, liver Hamp and Id1 expression were similar in both genotypes (Figure 2F-G, left panels).
Figure 2.
Deletion of hepatocyte Tfrc in Hfe knockout mice does not alter iron phenotype compared with Hfe single knockout mice. Four to 5-week-old male (blue) and female (red) double global Hfe knockout; hepatocyte Tfrc knockout mice (Hfe−/−;Tfrcfl/fl;Alb-Cre+) and littermate single Hfe knockout mice (Hfe−/−;Tfrcfl/fl;Alb-Cre−) were either maintained on a standard diet (control, ∼380 ppm iron) or low-iron diet (low Fe, 2-6 ppm iron) for 3 weeks. Livers were analyzed for (A,C) TFR1 and (B-C) TFR2 relative to total actin protein by immunoblot and chemiluminescence quantitation. For panels A-B, the average of the Hfe−/−;Tfrcfl/fl;Alb-Cre− mice for each sex and diet was set to 1. Representative immunoblots are shown. (D) Serum transferrin saturation and (E) liver iron levels were analyzed by colorimetric assays. Livers were analyzed for (F) Hamp and (G) Id1 relative to Rpl19 mRNA by qRT-PCR. For panels F-G, the average of the Hfe−/−;Tfrcfl/fl;Alb-Cre− male mice on the standard diet was set to 1. For all graphs, individual data points are shown, and bars represent mean ± SEM. ∗P < .05, ∗∗P < .01, ∗∗∗ P < .001 relative to diet- and sex-matched Hfe−/−;Tfrcfl/fl;Alb-Cre− mice by the Student t test. qRT, quantitative reverse transcription; SEM, standard error of the mean.
Table 2.
Deletion of hepatocyte Tfrc in Hfe knockout mice does not change red blood cell parameters compared with Hfe single knockout mice
| Sex | Diet | Genotype | HCT (%) | Hgb (g/dL) | MCV (fL) | MCH (pg) | RDW (%) |
|---|---|---|---|---|---|---|---|
| Male | Con | Hfe−/−;Tfrcfl/fl;Alb-Cre− | 41.7 ± 0.6 | 13.5 ± 0.2 | 49.4 ± 0.3 | 15.9 ± 0.1 | 15.9 ± 0.1 |
| Hfe−/−;Tfrcfl/fl;Alb-Cre+ | 41.0 ± 0.7 | 13.4 ± 0.2 | 49.2 ± 0.3 | 16.1 ± 0.1 | 15.8 ± 0.1 | ||
| Low Fe | Hfe−/−;Tfrcfl/fl;Alb-Cre− | 42.4 ± 1.5 | 13.7 ± 0.4 | 49.0 ± 0.3 | 15.9 ± 0.1 | 16.1 ± 0.1 | |
| Hfe−/−;Tfrcfl/fl;Alb-Cre+ | 40.7 ± 1.4 | 13.1 ± 0.5 | 48.1 ± 0.5 | 15.4 ± 0.3 | 17.2 ± 0.5 | ||
| Female | Con | Hfe−/−;Tfrcfl/fl;Alb-Cre− | 40.3 ± 0.5 | 13.1 ± 0.1 | 49.1 ± 0.3 | 16.0 ± 0.2 | 15.7 ± 0.2 |
| Hfe−/−;Tfrcfl/fl;Alb-Cre+ | 41.3 ± 0.9 | 13.6 ± 0.3 | 49.8 ± 0.3 | 16.4 ± 0.1 | 15.8 ± 0.1 | ||
| Low Fe | Hfe−/−;Tfrcfl/fl;Alb-Cre− | 43.9 ± 1.1 | 14.1 ± 0.3 | 49.5 ± 0.3 | 15.9 ± 0.2 | 16.2 ± 0.1 | |
| Hfe−/−;Tfrcfl/fl;Alb-Cre+ | 42.3 ± 1.2 | 13.5 ± 0.5 | 47.8 ± 1.1 | 15.3 ± 0.5 | 17.9 ± 1.3 |
Data represent means ± SEM; n = 3 to 10 mice per group (7 weeks old). No significant differences were observed between sex- and diet-matched genotypes by the Student t test.
Abbreviations are explained in Table 1.
An important limitation of this model is that Hfe deletion causes iron overload in mice on a standard rodent diet. Iron overload results in increased circulating nontransferrin-bound iron (NTBI), which may load hepatocytes via the NTBI transporter ZIP14,31 therefore bypassing the need of TfR1 for hepatocyte iron uptake. To minimize the impact of iron overload, double Hfe−/−;Tfrcfl/fl;Alb-Cre+ and single Hfe−/−;Tfrcfl/fl;Alb-Cre− knockout mice were also examined after receiving a low-iron diet (2-6 ppm iron) starting at 4 weeks of age. The low-iron diet abrogated the iron overload of the standard diet in both the single and double knockout mice, with mean liver iron reduced from a range of 246 to 400 μg/g down to 55 to 80 μg/g (Figure 2E), and mean serum transferrin saturation reduced from a range of 58% to 85% down to 33% to 67% (Figure 2D). Mean liver iron and serum transferrin saturation in single and double knockout mice on the low-iron diet approached that of the Tfrcfl/fl;Alb-Cre− control mice on a standard diet (70 μg/g and 40% respectively, Figure 1A-B), albeit with more variability. Even on a low-iron diet, there were no significant differences in serum iron, liver iron, spleen iron, CBC, or RBC indices, Hamp or Id1 mRNA expression, or TFR2 levels in double Hfe−/−;Tfrcfl/fl;Alb-Cre+ knockout mice compared with single Hfe−/−;Tfrcfl/fl;Alb-Cre− knockout mice (Figure 2 and supplemental Figure 2 right panels; Table 2). Together, these data suggest that the nonredundant function of hepatocyte TfR1 in hepcidin and iron homeostasis regulation is fully dependent on HFE.
Increases in serum iron induce higher hepcidin expression in Tfrcfl/fl;Alb-Cre+ mice
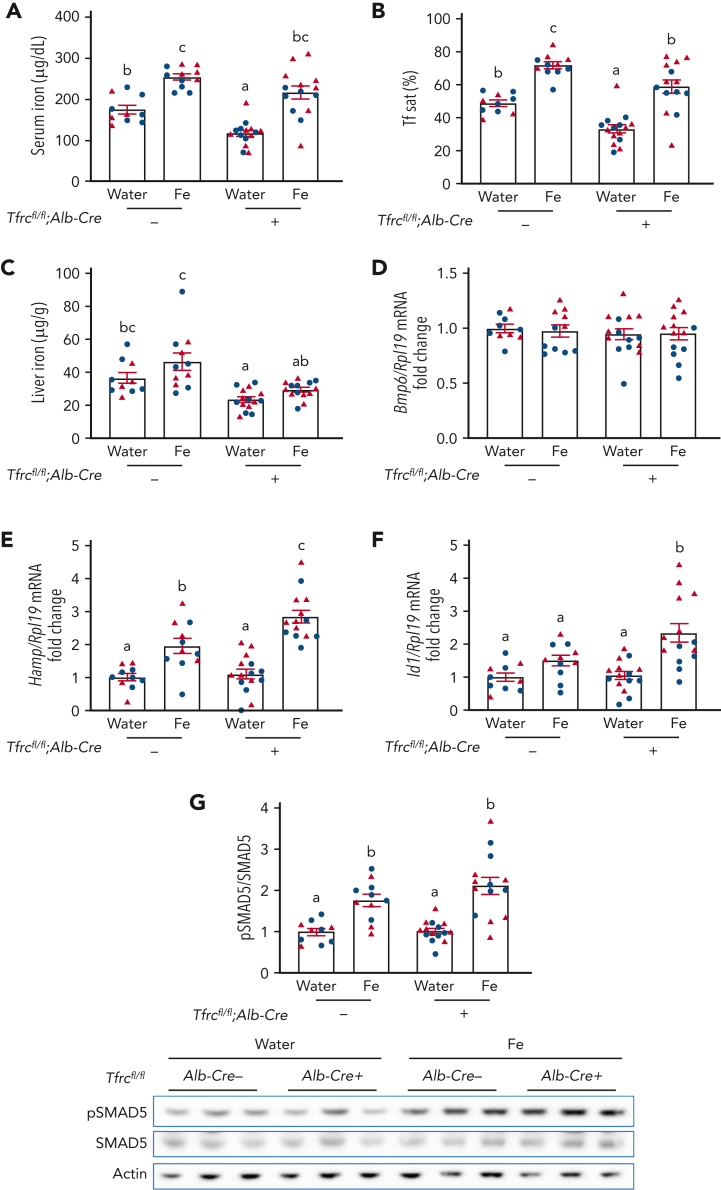
A previous study reported that hepatocyte TfR1 plays a role in fine-tuning the hepcidin response to hepatocellular iron loading.13 Because hepcidin levels in Tfrcfl/fl;Alb-Cre+ mice were not only inappropriately high relative to liver iron, but also serum iron, we investigated whether hepatocyte TfR1 also contributes to serum iron-mediated hepcidin regulation. Tfrcfl/fl;Alb-Cre+ mice and Tfrcfl/fl;Alb-Cre− controls received a single dose of 2 mg/kg elemental iron as ferrous sulfate by oral gavage to increase serum iron without impacting liver iron levels. Oral gavage of iron similarly increased serum iron and transferrin saturation in both Tfrcfl/fl;Alb-Cre+ and Tfrcfl/fl;Alb-Cre− mice relative to water gavage, without impacting the total iron binding capacity (Figure 3A-B and supplemental Figure 3). However, oral gavage of iron did not significantly increase liver iron (Figure 3C) or Bmp6 mRNA (Figure 3D), which is regulated by liver iron,14 in either genotype. Expectedly, the increased serum iron and transferrin saturation significantly induced hepcidin expression in Tfrcfl/fl;Alb-Cre− mice (Figure 3E). Interestingly, the hepcidin induction was significantly greater in Tfrcfl/fl;Alb-Cre+ mice than Tfrcfl/fl;Alb-Cre− mice (Figure 3E). In addition, there was significantly greater Id1 induction and a trend to higher pSMAD5 induction in Tfrcfl/fl;Alb-Cre+ mice than Tfrcfl/fl;Alb-Cre− mice (Figure 3F-G), suggesting that stronger SMAD-mediated transcription is likely responsible for the more robust hepcidin induction in Tfrcfl/fl;Alb-Cre+ mice. Together, these results suggest that hepatocyte TfR1 functions to restrain hepcidin induction in response to serum iron by sequestering HFE to limit SMAD signaling and thereby hepcidin transcription.
Figure 3.
Acute serum iron loading by oral gavage of iron induces higher hepcidin expression in Tfrcfl/fl;Alb-Cre+mice compared with Tfrcfl/fl;Alb-Cre−mice. Seven-week-old male (blue) and female (red) Tfrcfl/fl;Alb-Cre+ mice and littermate controls (Tfrcfl/fl;Alb-Cre−) were maintained on a low-iron diet (2-6 ppm iron). After 12 days, mice were orally gavaged with 2 mg/kg elemental iron (as ferrous sulfate) or distilled water. After 5 hours, (A) serum iron, (B) serum transferrin saturation, and (C) liver iron levels were analyzed by colorimetric assays. Livers were analyzed for (D) Bmp6, (E) Hamp, and (F) Id1 relative to Rpl19 mRNA by qRT-PCR. (G) Liver pSMAD5 relative to total SMAD5 and actin protein were analyzed by immunoblot and chemiluminescence quantitation. A representative immunoblot is shown. For panels D-G, the average of the water-treated control mice was set to 1. For all graphs, individual data points are shown, and bars represent mean ± SEM. Data were analyzed by two-way analysis of variance with Tukey post hoc test. Means without a common superscript differ significantly (P < .05). qRT, quantitative reverse transcription; SEM, standard error of the mean.
Hepatocyte TfR1 is not required for acute EPO-mediated hepcidin suppression in mice
A reduction in circulating diferric transferrin has been proposed to contribute to EPO-mediated hepcidin suppression.24 Because hepatocyte TfR1 plays a role in hepcidin regulation by serum iron, and hepcidin levels were inappropriately high relative to EPO levels in Tfrcfl/fl;Alb-Cre+ mice, we tested whether hepatocyte TfR1 plays a role in hepcidin suppression by erythropoietic drive. We injected recombinant EPO or phosphate-buffered saline into 6-week-old Tfrcfl/fl;Alb-Cre+ mice and Tfrcfl/fl;Alb-Cre− controls for 9 or 15 hours. Time points were chosen to optimize the ability to detect ERFE-independent (9 hours) and -dependent (15 hours) hepcidin suppression.19,24 EPO successfully induced bone marrow Erfe expression in both Tfrcfl/fl;Alb-Cre− and Tfrcfl/fl;Alb-Cre+ mice at 9 and 15 hours (supplemental Figure 4A,C). Hepcidin was similarly suppressed by EPO in Tfrcfl/fl;Alb-Cre− mice compared with Tfrcfl/fl;Alb-Cre+ mice after both 9 hours (67.5% vs 64.5%) and 15 hours (75.2% vs 70.1%) (supplemental Figure 4B,D). These data suggest that hepatocyte TfR1 is not required for hepcidin suppression by an acute EPO injection, at least under the experimental conditions tested.
Deletion of hepatocyte Tfrc ameliorates hepcidin suppression and liver iron overload in β-thalassemia mice
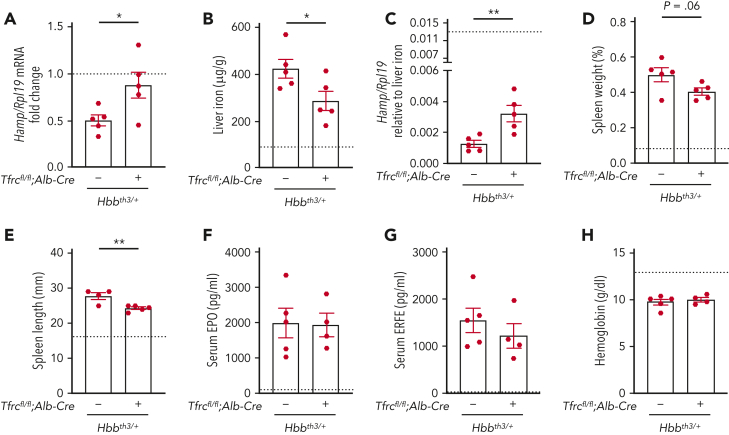
Global TfR1 haploinsufficiency was previously demonstrated to ameliorate ineffective erythropoiesis and reduce iron overload in β-thalassemia mice.25 To investigate whether the loss of hepatocyte TfR1 contributes to the improvement, we evaluated the impact of hepatocyte Tfrc knockout in an Hbbth3/+ mouse model of β-thalassemia intermedia (Hbbth3/+;Tfrcfl/fl;Alb-Cre+) compared with littermate thalassemia mice (Hbbth3/+;Tfrcfl/fl;Alb-Cre−) and nonthalassemic controls. As previously described,22 β-thalassemia mice (Hbbth3/+;Tfrcfl/fl;Alb-Cre−) exhibited reduced hepcidin levels, liver iron overload, splenomegaly, elevated EPO and ERFE levels, and anemia compared with nonthalassemic controls (Figure 4, left columns, nonthalassemic controls represented by dotted line). In double mutant Hbbth3/+;Tfrcfl/fl;Alb-Cre+ mice, hepcidin levels were increased and liver iron loading was reduced compared with β-thalassemia mice, although there were no significant changes in serum or spleen iron (Figure 4A-B and supplemental Figure 5A-B). Hepcidin levels were even more significantly increased in the double mutant mice when considered relative to the reduced liver iron loading of these mice compared with β-thalassemia mice (Figure 4C). There was a small reduction in spleen length and a tendency to reduction in spleen weight in double mutant mice relative to β-thalassemia mice (Figure 4D-E). However, there were no significant differences in serum EPO or ERFE levels, hemoglobin levels, mean cell volume, or other makers of splenic extramedullary hematopoiesis in double mutant mice compared with β-thalassemia mice (Figure 4F-H and supplemental Figure 5C-I). Together, these data suggest that hepatocyte TfR1 contributes to hepcidin deficiency and iron overload in β-thalassemia but does not significantly impact ineffective erythropoiesis and anemia.
Figure 4.
Deletion of hepatocyte Tfrc ameliorates hepcidin suppression and liver iron overload in β-thalassemic mice. Six-week-old littermate female double-mutant hepatocyte Tfrc knockout thalassemia mice (Hbbth3/+;Tfrcfl/fl;Alb-Cre+), thalassemia mice (Hbbth3/+;Tfrcfl/fl;Alb-Cre−), and nonthalassemic controls (Hbb+/+;Tfrcfl/fl;Alb-Cre−, represented by dotted line) were analyzed for (A) Hamp relative to Rpl19 mRNA by qRT-PCR. The average of the nonthalassemic control mice was set to 1. (B) Liver iron levels were analyzed by colorimetric assay. (C) Hamp/Rpl19 mRNA was divided by liver iron content to normalize for the degree of iron overload. (D-E) Spleens were measured for (D) weight divided by total body weight and (E) length. (F) Serum EPO and (G) ERFE protein levels were quantified by ELISA. (H) Hemoglobin levels were analyzed by CBC. For all graphs, the average of the nonthalassemic group is shown as a dotted line. For other genotypes, individual data points are shown, and bars represent mean ± SEM. ∗P < .05, ∗∗P < .01 for Hbbth3/+;Tfrcfl/fl;Alb-Cre+ mice relative to Hbbth3/+;Tfrcfl/fl;Alb-Cre− mice by the Student t test. ELISA, enzyme-linked immunosorbent assay; qRT, quantitative reverse transcription; SEM, standard error of the mean.
Discussion
TfR1 has an essential role in cellular iron uptake in many cell types and has been proposed to contribute to systemic iron homeostasis regulation in hepatocytes. However, many aspects of hepatocyte TfR1 function remain uncertain, including its functional dependence on HFE, role in serum iron and erythropoietic-mediated hepcidin regulation, and role in β-thalassemia. Here, we generated hepatocyte Tfrc knockout mice to understand the functional role of hepatocyte TFR1 in vivo.
We have shown that Tfrcfl/fl;Alb-Cre+ mice have reduced liver and serum iron levels, mild hypochromic microcytic anemia, and increased EPO/ERFE levels compared with Cre− controls. A similar phenotype was previously reported by Fillebeen et al13 in another hepatocyte Tfrc knockout mouse model. However, in the previous study, baseline hematocrit was not reduced in hepatocyte Tfrc knockout mice when fed a standard diet, although the mice were more prone to develop anemia when fed a low-iron diet. The more pronounced iron deficiency phenotype of our mice comparable with the one reported by Fillebeen et al13 may be because of different background strains, different diets or other housing conditions, and/or a different strategy for Tfrc deletion. Nevertheless, the overall consistent phenotype in the 2 studies, provides strong evidence of a functional role for hepatocyte TfR1 in contributing to systemic iron homeostasis.
Reduced liver iron, reduced serum iron, and increased ERFE levels in Tfrcfl/fl;Alb-Cre+ mice should each suppress hepcidin to increase iron availability, thereby ameliorating iron deficiency and anemia.6 However, hepcidin levels were unchanged in Tfrcfl/fl;Alb-Cre+ mice compared with Cre− controls, suggesting that hepcidin levels were inappropriately elevated relative to liver iron, serum iron, and ERFE in the absence of hepatocyte TfR1. These data suggest that hepatocyte TfR1 plays an important role in controlling hepcidin responses to 1 or more of these signals and that inappropriately high hepcidin has a causal role in the iron deficiency and iron-restricted erythropoiesis phenotype of the mice. Fillebeen et al13 reported similar inappropriately elevated hepcidin levels relative to liver iron in their hepatocyte Tfrc knockout mice. They postulated that hepatocyte TfR1 has a dual role to (1) contribute to hepatocyte iron uptake and (2) control HFE availability for regulating hepcidin in response to hepatocellular iron. However, they did not experimentally validate the role of HFE in hepatocyte TfR1 function, nor determine how much of the phenotype of hepatocyte Tfrc knockout mice was owing to the iron uptake function of TfR1 vs its hepcidin regulatory role.
To assess the requirement of HFE for hepatocyte TfR1 function, we tested the impact of hepatocyte Tfrc knockout in Hfe knockout mice. Notably, hepatocyte Tfrc knockout did not have any functional impact on the liver or systemic iron levels or hepcidin expression in Hfe knockout mice, even when mice were fed a low-iron diet. These data provide direct experimental evidence that the major nonredundant function of hepatocyte TfR1 in iron homeostasis is to functionally interact with HFE to regulate hepcidin. Although hepatocyte TfR1 has been reported to contribute to hepatocyte iron uptake,13 liver iron levels were not reduced in mice lacking both HFE and hepatocyte TfR1, even when fed a low-iron diet. These findings indicate that TfR1 is dispensable for hepatocyte iron uptake, and suggest that the low liver iron levels in single Tfrcfl/fl;Alb-Cre+ mice are predominantly caused by hepcidin excess rather than impaired hepatocyte iron uptake. Multiple other iron uptake pathways have been demonstrated in hepatocytes, including transferrin-mediated iron uptake by TfR2 and NTBI uptake by ZIP14,13,31,32 and we hypothesize that these pathways compensate for the loss of TfR1 in hepatocyte iron uptake in Tfrcfl/fl;Alb-Cre+ mice.
Serum iron and liver iron are distinct signals to induce hepcidin.14,15 Liver iron induces hepcidin at least in part by inducing liver endothelial cell Bmp6 mRNA expression.14,15 Serum iron induces hepcidin by activating SMAD signaling downstream or independent of an induction in Bmp6.14 Our finding that oral gavage of iron, which increased serum iron without increasing liver iron, more robustly induced SMAD signaling and hepcidin in Tfrcfl/fl;Alb-Cre+ mice compared with Cre− controls, provides the first direct experimental evidence that hepatocyte TfR1 has a functional role in hepcidin regulation by serum iron in vivo. These results support a model wherein HFE is normally sequestered by TfR1 to restrain SMAD signaling and hepcidin induction by serum iron and may be released over time by competitive holo-transferrin binding. Conversely, in Tfrcfl/fl;Alb-Cre+ mice, HFE is already free and poised to induce SMAD signaling and hepcidin more robustly. In the study by Fillebeen et al, no difference in the hepcidin response to acute holotransferrin injection vs apotransferrin injection was reported in their hepatocyte Tfrc knockout mice compared with control mice.13 However, this study was limited by the very poor hepcidin induction response to holotransferrin vs apotransferrin (∼0.25-fold). The use of apotransferrin as a control may also have complicated data interpretation because apotransferrin itself can influence iron homeostasis parameters through multiple mechanisms.25,26,33 Although our findings clearly establish a functional role for hepatocyte TfR1 in regulating hepcidin in response to serum iron, it does not negate the important role of TfR2. Stabilized by binding to holotransferrin,17,18 hepatocyte TfR2 contributes to hepcidin induction in response to serum iron by activating the SMAD signaling cascade,34 possibly via a physical interaction with HFE.2,16
The increased ERFE levels coupled with inappropriately high hepcidin in Tfrcfl/fl;Alb-Cre+ mice suggest that hepatocyte TfR1 may contribute to erythropoietic drive-mediated hepcidin suppression. One model for how hepatocyte TFR1 may detect enhanced erythropoiesis is through reduced circulating diferric transferrin caused by increased erythrocyte uptake.24 However, hepcidin expression was similarly suppressed by acute EPO injection in both Tfrcfl/fl;Alb-Cre+ and Tfrcfl/fl;Alb-Cre− mice, suggesting that hepatocyte TfR1 does not play a significant role in acute EPO-mediated hepcidin suppression, at least under the experimental conditions tested. Nevertheless, this does not rule out a role for hepatocyte TfR1 under more chronic conditions of an increased erythropoietic drive. Indeed, we found that hepatocyte Tfrc knockout ameliorated hepcidin deficiency and reduced liver iron overload in a mouse model of β-thalassemia intermedia, despite similar levels of EPO and ERFE excess. We hypothesize that the decreased iron overload in these mice is a consequence of increased HFE-mediated hepcidin induction, which counteracts to some degree the hepcidin suppression by ERFE excess and other potential erythroid hepcidin suppressors. The relevance of these findings for β-thalassemia patients, in whom transferrin saturation tends to be higher than in mice, remains to be demonstrated. Nevertheless, our results suggest that some of the beneficial effects previously reported for global Tfrc haploinsufficiency and apotransferrin injection for ameliorating hepcidin suppression and decreasing iron overload in β-thalassemia mice25,26 are likely a consequence of reducing hepatocyte TfR1 in addition to erythroid TfR1. Interestingly, other studies have demonstrated that increasing hepcidin by providing hepcidin mimetics, inhibiting Tmprss6, or inhibiting Erfe not only decreased iron overload, but also ameliorated splenomegaly and, in some cases, anemia in thalassemia mouse models.21,22,35, 36, 37, 38 This is hypothesized to result from systemic iron restriction, thereby reducing the iron supply to erythroid precursors and improving ineffective erythropoiesis. However, we saw only a marginal reduction in splenomegaly and no significant improvement in markers of ineffective erythropoiesis or anemia in hepatocyte Tfrc knockout thalassemia mice. This is likely owing to a less profound induction of hepcidin and systemic iron restriction in our model compared with these other models.
In conclusion, our data demonstrate that the major function of hepatocyte TfR1 in iron homeostasis is to interact with HFE to regulate hepcidin. Hepatocyte TfR1 influences hepcidin regulation by serum iron in addition to its previously described role in fine-tuning hepcidin regulation by hepatocellular iron. Although it does not impact hepcidin suppression by acute erythropoietin injection, hepatocyte TfR1 does contribute to hepcidin suppression and liver iron overload in murine β-thalassemia.
Conflict-of-interest disclosure: J.L.B. has been a consultant for Incyte Corporation and Alnylam Pharmaceuticals, and owns equity in Ferrumax Pharmaceuticals, a company focused on targeting RGM proteins (including hemojuvelin) and bone morphogenetic protein (BMP/TGF-β) superfamily signaling as hepcidin modulating agents for the treatment of anemia and other iron disorders. Her interests were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict-of-interest policies. The remaining authors declare no competing financial interests.
Acknowledgments
This work was supported by Cooley’s Anemia Foundation Research Fellowship (X.X.) and National Institutes of Health grants R01-DK087727 and R01-DK128068 and the Patricia and Scott Eston Massachusetts General Hospital Research Scholar Award (J.L.B.).
Authorship
Contribution: X.X. designed and performed experiments, interpreted data, and wrote the manuscript; G.A.M., Y.X., A.L.F., V.M.A.-M., S.D. and C.-Y.W. helped with mouse studies and edited the manuscript; J.L.B. conceived and oversaw the study, interpreted data, and wrote the manuscript.
Footnotes
Data are available on request from the corresponding author, Jodie L. Babitt (babitt.jodie@mgh.harvard.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Supplementary Material
References
- 1.Schmidt PJ, Toran PT, Giannetti AM, Bjorkman PJ, Andrews NC. The transferrin receptor modulates Hfe-dependent regulation of hepcidin expression. Cell Metab. 2008;7(3):205–214. doi: 10.1016/j.cmet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang CY, Babitt JL. Liver iron sensing and body iron homeostasis. Blood. 2019;133(1):18–29. doi: 10.1182/blood-2018-06-815894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 4.Aschemeyer S, Qiao B, Stefanova D, et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood. 2018;131(8):899–910. doi: 10.1182/blood-2017-05-786590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billesbolle CB, Azumaya CM, Kretsch RC, et al. Structure of hepcidin-bound ferroportin reveals iron homeostatic mechanisms. Nature. 2020;586(7831):807–811. doi: 10.1038/s41586-020-2668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao X, Alfaro-Magallanes VM, Babitt JL. Bone morphogenic proteins in iron homeostasis. Bone. 2020;138 doi: 10.1016/j.bone.2020.115495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feder JN, Gnirke A, Thomas W, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13(4):399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 8.Corradini E, Garuti C, Montosi G, et al. Bone morphogenetic protein signaling is impaired in an HFE knockout mouse model of hemochromatosis. Gastroenterology. 2009;137(4):1489–1497. doi: 10.1053/j.gastro.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Alessio F, Hentze MW, Muckenthaler MU. The hemochromatosis proteins HFE, TfR2, and HJV form a membrane-associated protein complex for hepcidin regulation. J Hepatol. 2012;57(5):1052–1060. doi: 10.1016/j.jhep.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Wu XG, Wang Y, Wu Q, et al. HFE interacts with the BMP type I receptor ALK3 to regulate hepcidin expression. Blood. 2014;124(8):1335–1343. doi: 10.1182/blood-2014-01-552281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett MJ, Lebron JA, Bjorkman PJ. Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor. Nature. 2000;403(6765):46–53. doi: 10.1038/47417. [DOI] [PubMed] [Google Scholar]
- 12.Giannetti AM, Bjorkman PJ. HFE and transferrin directly compete for transferrin receptor in solution and at the cell surface. J Biol Chem. 2004;279(24):25866–25875. doi: 10.1074/jbc.M401467200. [DOI] [PubMed] [Google Scholar]
- 13.Fillebeen C, Charlebois E, Wagner J, et al. Transferrin receptor 1 controls systemic iron homeostasis by fine-tuning hepcidin expression to hepatocellular iron load. Blood. 2019;133(4):344–355. doi: 10.1182/blood-2018-05-850404. [DOI] [PubMed] [Google Scholar]
- 14.Corradini E, Meynard D, Wu Q, et al. Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice. Hepatology. 2011;54(1):273–284. doi: 10.1002/hep.24359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramos E, Kautz L, Rodriguez R, et al. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology. 2011;53(4):1333–1341. doi: 10.1002/hep.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9(3):217–227. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson MB, Enns CA. Diferric transferrin regulates transferrin receptor 2 protein stability. Blood. 2004;104(13):4287–4293. doi: 10.1182/blood-2004-06-2477. [DOI] [PubMed] [Google Scholar]
- 18.Robb A, Wessling-Resnick M. Regulation of transferrin receptor 2 protein levels by transferrin. Blood. 2004;104(13):4294–4299. doi: 10.1182/blood-2004-06-2481. [DOI] [PubMed] [Google Scholar]
- 19.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678–684. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang CY, Xu Y, Traeger L, et al. Erythroferrone lowers hepcidin by sequestering BMP2/6 heterodimer from binding to the BMP type I receptor ALK3. Blood. 2020;135(6):453–456. doi: 10.1182/blood.2019002620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arezes J, Foy N, McHugh K, et al. Antibodies against the erythroferrone N-terminal domain prevent hepcidin suppression and ameliorate murine thalassemia. Blood. 2020;135(8):547–557. doi: 10.1182/blood.2019003140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kautz L, Jung G, Du X, et al. Erythroferrone contributes to hepcidin suppression and iron overload in a mouse model of beta-thalassemia. Blood. 2015;126(17):2031–2037. doi: 10.1182/blood-2015-07-658419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivella S. Iron metabolism under conditions of ineffective erythropoiesis in beta-thalassemia. Blood. 2019;133(1):51–58. doi: 10.1182/blood-2018-07-815928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirciov CSG, Wilkins SJ, Hung GCC, Helman SL, Anderson GJ, Frazer DM. Circulating iron levels influence the regulation of hepcidin following stimulated erythropoiesis. Haematologica. 2018;103(10):1616–1626. doi: 10.3324/haematol.2017.187245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Choesang T, Bao W, et al. Decreasing TfR1 expression reverses anemia and hepcidin suppression in beta-thalassemic mice. Blood. 2017;129(11):1514–1526. doi: 10.1182/blood-2016-09-742387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Rybicki AC, Suzuka SM, et al. Transferrin therapy ameliorates disease in beta-thalassemic mice. Nat Med. 2010;16(2):177–182. doi: 10.1038/nm.2073. [DOI] [PubMed] [Google Scholar]
- 27.Levy JE, Montross LK, Cohen DE, Fleming MD, Andrews NC. The C282Y mutation causing hereditary hemochromatosis does not produce a null allele. Blood. 1999;94(1):9–11. [PubMed] [Google Scholar]
- 28.Wang CY, Core AB, Canali S, et al. Smad1/5 is required for erythropoietin-mediated suppression of hepcidin in mice. Blood. 2017;130(1):73–83. doi: 10.1182/blood-2016-12-759423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher AL, Wang CY, Xu Y, et al. Functional role of endothelial transferrin receptor 1 in iron sensing and homeostasis. Am J Hematol. 2022;97(12):1548–1559. doi: 10.1002/ajh.26716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canali S, Zumbrennen-Bullough KB, Core AB, et al. Endothelial cells produce bone morphogenetic protein 6 required for iron homeostasis in mice. Blood. 2017;129(4):405–414. doi: 10.1182/blood-2016-06-721571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenkitkasemwong S, Wang CY, Coffey R, et al. SLC39A14 is required for the development of hepatocellular iron overload in murine models of hereditary hemochromatosis. Cell Metab. 2015;22(1):138–150. doi: 10.1016/j.cmet.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawabata H, Yang R, Hirama T, et al. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem. 1999;274(30):20826–20832. doi: 10.1074/jbc.274.30.20826. [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Choesang T, Li H, et al. Increased hepcidin in transferrin-treated thalassemic mice correlates with increased liver BMP2 expression and decreased hepatocyte ERK activation. Haematologica. 2016;101(3):297–308. doi: 10.3324/haematol.2015.127902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corradini E, Rozier M, Meynard D, et al. Iron regulation of hepcidin despite attenuated Smad1,5,8 signaling in mice without transferrin receptor 2 or Hfe. Gastroenterology. 2011;141(5):1907–1914. doi: 10.1053/j.gastro.2011.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casu C, Oikonomidou PR, Chen H, et al. Minihepcidin peptides as disease modifiers in mice affected by beta-thalassemia and polycythemia vera. Blood. 2016;128(2):265–276. doi: 10.1182/blood-2015-10-676742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nai A, Pagani A, Mandelli G, et al. Deletion of TMPRSS6 attenuates the phenotype in a mouse model of beta-thalassemia. Blood. 2012;119(21):5021–5029. doi: 10.1182/blood-2012-01-401885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt PJ, Toudjarska I, Sendamarai AK, et al. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe(-/-) mice and ameliorates anemia and iron overload in murine beta-thalassemia intermedia. Blood. 2013;121(7):1200–1208. doi: 10.1182/blood-2012-09-453977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo S, Casu C, Gardenghi S, et al. Reducing TMPRSS6 ameliorates hemochromatosis and beta-thalassemia in mice. J Clin Invest. 2013;123(4):1531–1541. doi: 10.1172/JCI66969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.