This cross-sectional study investigates the association between metformin use and age-related macular degeneration.
Key Points
Question
What is the association of metformin with the development of age-related macular degeneration (AMD)?
Findings
In this cross-sectional study of a follow-up phase of a randomized clinical trial for prevention of diabetes, evaluation of retinal photographs 16 years posttrial from 549 participants taking metformin, 514 undergoing lifestyle intervention, and 512 in the placebo arm, metformin was not associated with prevalence of AMD.
Meaning
Long-term use of metformin and lifestyle intervention may not be associated with development of AMD.
Abstract
Importance
Age-related macular degeneration (AMD) is a leading cause of blindness with no treatment available for early stages. Retrospective studies have shown an association between metformin and reduced risk of AMD.
Objective
To investigate the association between metformin use and age-related macular degeneration (AMD).
Design, Setting, and Participants
The Diabetes Prevention Program Outcomes Study is a cross-sectional follow-up phase of a large multicenter randomized clinical trial, Diabetes Prevention Program (1996-2001), to investigate the association of treatment with metformin or an intensive lifestyle modification vs placebo with preventing the onset of type 2 diabetes in a population at high risk for developing diabetes. Participants with retinal imaging at a follow-up visit 16 years posttrial (2017-2019) were included. Analysis took place between October 2019 and May 2022.
Interventions
Participants were randomly distributed between 3 interventional arms: lifestyle, metformin, and placebo.
Main Outcomes and Measures
Prevalence of AMD in the treatment arms.
Results
Of 1592 participants, 514 (32.3%) were in the lifestyle arm, 549 (34.5%) were in the metformin arm, and 529 (33.2%) were in the placebo arm. All 3 arms were balanced for baseline characteristics including age (mean [SD] age at randomization, 49 [9] years), sex (1128 [71%] male), race and ethnicity (784 [49%] White), smoking habits, body mass index, and education level. AMD was identified in 479 participants (30.1%); 229 (14.4%) had early AMD, 218 (13.7%) had intermediate AMD, and 32 (2.0%) had advanced AMD. There was no significant difference in the presence of AMD between the 3 groups: 152 (29.6%) in the lifestyle arm, 165 (30.2%) in the metformin arm, and 162 (30.7%) in the placebo arm. There was also no difference in the distribution of early, intermediate, and advanced AMD between the intervention groups. Mean duration of metformin use was similar for those with and without AMD (mean [SD], 8.0 [9.3] vs 8.5 [9.3] years; P = .69). In the multivariate models, history of smoking was associated with increased risks of AMD (odds ratio, 1.30; 95% CI, 1.05-1.61; P = .02).
Conclusions and Relevance
These data suggest neither metformin nor lifestyle changes initiated for diabetes prevention were associated with the risk of any AMD, with similar results for AMD severity. Duration of metformin use was also not associated with AMD. This analysis does not address the association of metformin with incidence or progression of AMD.
Introduction
Age-related macular degeneration (AMD) is one of the most prevalent age-related eye conditions and a leading cause of blindness in the US.1,2 Early and intermediate stages of AMD can progress to 2 forms of late AMD: geographic atrophy and neovascular AMD.3 Hallmark features of early and intermediate AMD include drusen and pigment changes and are evaluated using various retinal imaging modalities.4,5 The natural history of AMD is progression from early AMD to the intermediate stage and eventual geographic atrophy and may be accompanied by the development of neovascular AMD.6
Currently approved therapies for AMD address only the neovascular stage, with intravitreal anti–vascular endothelial growth factor injections being the standard of care treatment. There is no treatment available for the other advanced form of AMD, geographic atrophy. To date, the Age-Related Eye Disease Study (AREDS) 2 supplements are the only treatment options available for patients with large drusen to prevent progression to late AMD.7 Retrospective studies have suggested that metformin, a widely used drug in the treatment of diabetes, may have a role in treatment of AMD and may provide a protective effect against progression to late AMD.8,9,10 Studies so far have been based on retrospective electronic medical records data with inconclusive results.11
The Diabetes Prevention Program Outcomes Study (DPPOS) is a follow-up phase of a large multicenter randomized clinical trial, Diabetes Prevention Program (DPP), to investigate the effects of treatment with metformin or an intensive lifestyle modification (lifestyle) compared with placebo on preventing the onset of type 2 diabetes in a population at high-risk of developing diabetes.12 Per the DPP protocol, eligibility criteria included age 25 years or older, a body mass index (calculated as weight in kilograms divided by height in meters squared) of 24 or higher (22 or higher in Asian individuals), and a plasma glucose concentration of 95 to 125 mg/dL (to convert to millimoles per liter, multiply by 0.0555) in the fasting state (≤125 mg/dL in the American Indian clinics) and 140 to 199 mg/dL 2 hours after a 75-g oral glucose load.
As part of an annual follow-up visit at year 16 of DPPOS, retinal images were acquired and AMD was evaluated. The DPPOS provides a unique opportunity to study the association of metformin and lifestyle changes with preventing or delaying the development of AMD.
Methods
The Diabetes Prevention Program (1996-2001) was a multicenter controlled clinical trial in patients at risk for diabetes. The study included 3 randomized arms with 3234 participants: an intensive lifestyle intervention focused on achieving at least 150 minutes physical activity weekly and 7% or more body weight loss; metformin, 850 mg, twice daily with standard diet and exercise recommendations; or a double-blinded placebo twice daily with standard diet and exercise recommendations, as previously described.13 Written informed consent was obtained from all participants before screening, consistent with the Declaration of Helsinki14 and institutional review board approval was obtained from participating clinical sites. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline was followed.
The study was stopped prematurely in 2001 when the primary outcome was achieved; both metformin and lifestyle intervention were effective in reducing the incidence of diabetes in those at high risk. The DPPOS (2002-ongoing) is a follow-up study to understand the long-term association of the DPP interventions with outcomes, with 2776 of participants continuing the study. At the end of DPP/beginning of DPPOS, the treatment groups were unmasked, the placebo arm was discontinued, and all participants were offered lifestyle sessions. Those randomized to metformin continued to receive the study drug at the same dose. Many participants who developed type 2 diabetes were treated with metformin by their outside health care professional, and this was tracked with medication inventories at study visits. Thus, use of metformin was categorized as any metformin, in-study metformin, and out-of-study metformin. All participants receive an honorarium at scheduled visits in recognition of the time and effort spent in the DPPOS.
As part of the DPPOS study, fundus photographs and spectral-domain optical coherence tomography (SD-OCT) images were collected at the follow-up visit in 2018, termed year-16 visit. The imaging protocol included 7-field stereoscopic color photographs. SD-OCT images included volume scans from either Zeiss Cirrus or Heidelberg Spectralis. All images were obtained by certified technicians using a standardized image acquisition protocol.
AMD was evaluated from color photographs using the AREDS AMD classification system with inclusion of SD-OCT features.4 In brief, AMD severity was determined based on color photographs and SD-OCT images and classified as no AMD, early AMD, intermediate AMD, or advanced AMD (central geographic atrophy or neovascular AMD). Early AMD included many small drusen or few medium drusen in the absence of pigment changes. Intermediate AMD included many medium drusen or 1 or more large drusen with or without pigment changes. Geographic atrophy was identified using typical features from color photographs or evidence of complete retinal pigment epithelial and outer retinal atrophy on SD-OCT.15 Neovascular AMD was identified by presence of pigment epithelial detachment with or without retinal fluid on color photographs or SD-OCT. If the AMD severity differed between the 2 eyes, the more severe stage of AMD was assigned for the participant.
The primary analysis was to study the association between treatment arm and prevalence of AMD. The variability in the treatment during the follow-up period (as mentioned in the Methods) was dealt with using intent-to-treat analysis. The original treatment assignments were used for all analysis. For the secondary analysis studying the association of observed metformin usage with AMD, we adjusted for the treatment arms. Baseline characteristics were compared between the 3 arms using Pearson χ2 test or Fisher exact when numbers are small for categorical variables and analysis of variance test or Wilcoxon/Kruskal-Wallis when distribution is not normal for continuous variables. A logistic regression model adjusting for age, sex, self-reported race and ethnicity, and treatment groups was used to assess the association of metformin and lifestyle interventions with the risk of AMD. Other candidate risk factors associated with AMD were evaluated individually in logistic regression models adjusted for age, sex, race and ethnicity, and DPP randomization groups. These included education, BMI, fasting glucose level, 2-hour glucose level, hemoglobin A1c, and dietary protein and weight at baseline DPP visit in addition to diabetes, smoking status, duration of use of antidiabetic drugs during the cumulative years up to DPPOS year 16. Two-sided α values were statistically significant at .05. All analyses were completed in SAS statistical software version 9.4 (SAS Institute). Analysis took place between October 2019 and May 2022.
Results
The DPPOS included 2776 participants with 935 from the intensive lifestyle arm, 926 from the metformin arm, and 915 from the placebo arm. In total, 2051 participants attended the annual checkup at DPPOS year 16 with 664 from the lifestyle group, 696 from metformin group, and 691 from the placebo group (overall mean [SD] age at randomization, 49 [9] years; 464 [29%] female and 1128 [71%] male; 335 [21%] African American, 126 [8%] American Indian, 81 [5%] Asian, 266 [17%] Hispanic, and 784 [49%] White). All participants were invited for retinal image acquisition. Overall retinal imaging was available in 1592 participants (77.6%). Reasons for missing images included difficulty in availability of camera or photographer, participant refusal for imaging, and remote visit. After excluding 5 participants with undetermined AMD status, retinal imaging was gradable in 1587 participants distributed as 513 (32.3%), 546 (34.4%), and 528 (33.2%) in the lifestyle, metformin, and placebo groups, respectively. Table 1 shows the baseline demographics by treatment arm in the subgroup with retinal imaging. There was no significant difference in age at randomization, sex, race and ethnicity, smoking status, or BMI between the 3 treatment arms in the retinal imaging subgroup. Of note, it was nearly 22 years after randomization, which resulted in the mean age at the DPPOS year-16 visit around 70 years. As expected and compatible with the primary results of DPP, there was a difference in the prevalence of type 2 diabetes with a higher proportion in the placebo arm. The median (IQR) number of years of any metformin use (in study or out of study) in the lifestyle, metformin, and placebo arms was 0 (0-7), 18.5 (9.5-20.3), and 1.5 (0-8.5), respectively. The corresponding mean (SD) values are 3.59 (4.81), 14.67 (6.96), and 4.35 (5.17), respectively (P < .001).
Table 1. Baseline and Follow-up Characteristics and AMD Severity Status by Treatment Arms for the Subgroup With Retinal Imaging (N = 1592).
| Characteristics | Risk factor characteristics by treatment group, No. (%) | P value | ||
|---|---|---|---|---|
| Lifestyle (n = 513) | Metformin (n = 546) | Placebo (n = 528) | ||
| Age, mean (SD), y | ||||
| At randomization | 49.2 (9.5) | 49.3 (8.8) | 48.6 (8.8) | .28 |
| At AMD | 69.2 (9.4) | 69.2 (8.8) | 68.6 (8.8) | .42 |
| Sex | ||||
| Female | 144 (28) | 170 (31) | 148 (28) | .44 |
| Male | 369 (72) | 376 (69) | 380 (72) | |
| Race and ethnicity | ||||
| African American | 104 (20) | 123 (22) | 106 (20) | .45 |
| American Indian | 41 (8) | 40 (7) | 45 (9) | |
| Asian | 34 (7) | 19 (4) | 28 (5) | |
| Hispanic | 91 (18) | 88 (16) | 85 (16) | |
| Non-Hispanic White | 243 (47) | 276 (51) | 264 (50) | |
| Smoking status at baseline | ||||
| Never | 317 (62) | 349 (64) | 326 (62) | .34 |
| Current | 24 (5) | 27 (5) | 38 (7) | |
| Ever | 172 (34) | 170 (31) | 164 (31) | |
| Baseline BMI, mean (SD) | 33.5 (6.0) | 33.3 (6.2) | 34.1 (6.6) | .16 |
| Type 2 diabetes status at AMD assessment | ||||
| No | 228 (45) | 245 (45) | 196 (37) | .02a |
| Yes | 285 (55) | 301 (55) | 332 (73) | |
| Years with any metformin, median (IQR) | 0 (0-7) | 18.5 (9.5-20.25) | 1.5 (0-8.5) | <.001a |
| AMD status | ||||
| Absent | 361 (70) | 381 (70) | 366 (69) | .09 |
| Early | 87 (17) | 78 (14) | 64 (12) | |
| Intermediate | 56 (11) | 78 (14) | 84 (16) | |
| Advanced | 9 (2) | 9 (2) | 14 (3) | |
Abbreviations: AMD, age-related macular degeneration; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Significant association using P < .05 threshold.
Using multimodal imaging, AMD was identified in 479 participants (30%): early-stage AMD in 229 (14.4%), intermediate AMD in 218 (13.7%), and advanced AMD in 32 (2%). Central geographic atrophy was seen in 14 (0.9%) and neovascular AMD in 18 (1.1%). There was agreement for AMD severity between color photography and SD-OCT in 2875 eyes (92.9%); the κ agreement statistic is 0.79, generally considered to be excellent (eTable 1 in Supplement 1). Of 217 disagreements, 191 were in the early and intermediate AMD stages detected on color photographs and classified as absent or questionable on SD-OCT. In contrast, more eyes were detected to have central geographic atrophy and neovascular AMD based on SD-OCT compared with color photographs.
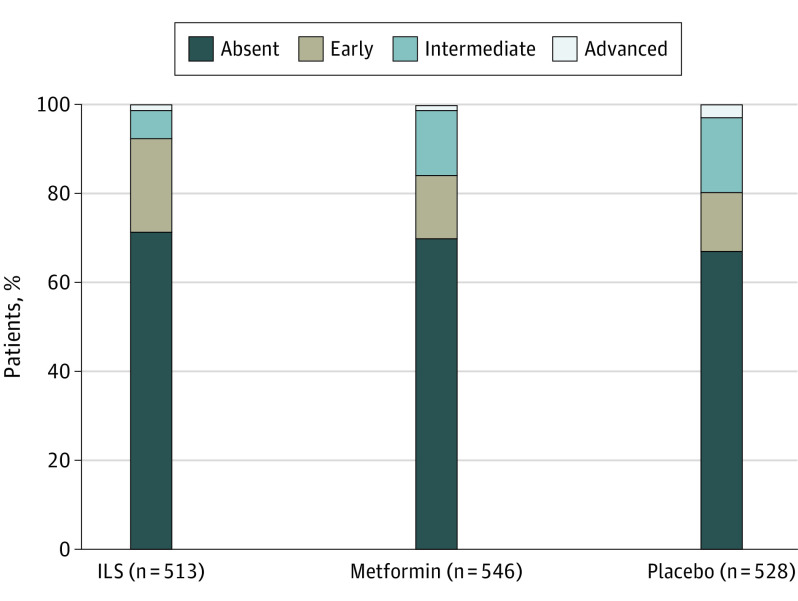
The prevalence of AMD was 152 (29.6%) in the lifestyle arm, 165 (30.2%) in the metformin arm, and 162 (30.7%) in the placebo arm. Distribution of AMD severity stages across the 3 study arms is shown in Table 1 and Figure 1. There was no difference in AMD presence or severity between the 3 treatment arms.
Figure 1. Distribution of Age-Related Macular Degeneration Severity in the 3 Study Arms.
There was no significant difference in age-related macular degeneration presence or severity between intensive lifestyle (ILS), metformin, and placebo arms.
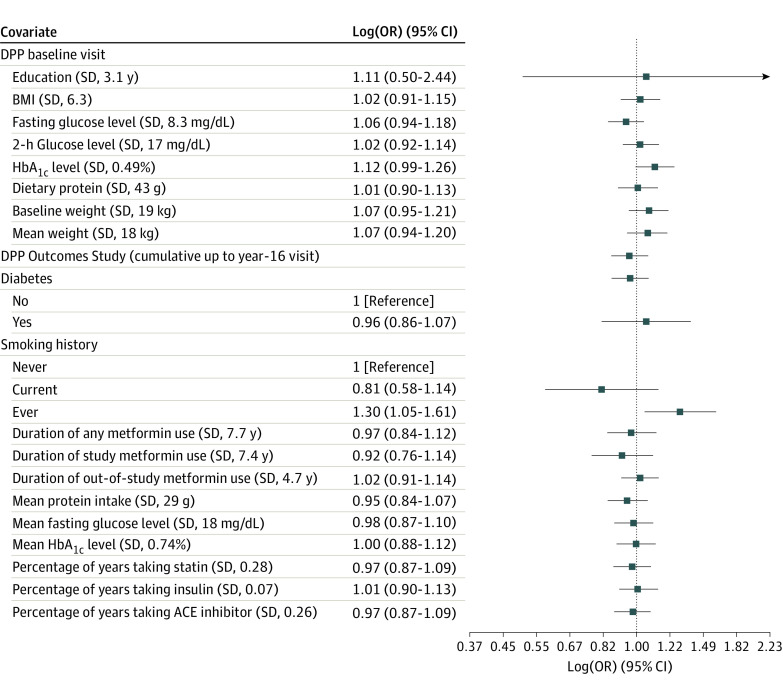
The association of baseline DPP and year-16 DPPOS characteristics with prevalence of AMD was assessed in models adjusting for age, sex, race and ethnicity, and treatment group (Table 2). Comparing participants with AMD and those free of AMD, age at randomization was significantly higher in the AMD group (mean [SD] age, 51.4 [9.6] vs 48 [8.6] years; P < .001). Smoking had a complex association with AMD, which was present in 273 of 997 individuals who had never smoked (28%), 185 of 506 of those who had ever smoked (37%), but only 21 of 89 current smokers (24%) (P = .001). In the model adjusted for age, sex, race and ethnicity and treatment arms, smoking (ever) was the only variable significantly associated with prevalence of AMD (odds ratio, 1.3; 95% CI, 1.1-1.6; Figure 2). Associations with other variables such as education level and baseline BMI were also not significant.
Table 2. Association of Baseline and Year-16 Visit Characteristics With AMD: Unadjusted Model and Adjusted Model for Age, Sex, Race and Ethnicity, and Treatment Arms.
| Characteristic | No. | AMD, mean (SD) | P value | ||
|---|---|---|---|---|---|
| Without (n = 1108) | With (n = 479) | Unadjusted | Adjusteda | ||
| Age at randomization, y | 1587 | 48.0 (8.6) | 51.4 (9.6) | <.001 | NA |
| Sex, No. (%) | |||||
| Female | 1125 | 801 (71) | 324 (29) | .06 | NA |
| Male | 462 | 307 (66) | 155 (34) | ||
| Race and ethnicity, No. (%) | |||||
| African American | 333 | 241 (72) | 92 (28) | .50 | NA |
| American Indian | 126 | 90 (71) | 36 (29) | ||
| Asian | 81 | 52 (64) | 29 (36) | ||
| Hispanic | 264 | 177 (67) | 87 (33) | ||
| Non-Hispanic White | 783 | 548 (70) | 235 (30) | ||
| Education level, y | 1587 | 10.9 (3.0) | 10.6 (3.4) | .19 | .80 |
| Baseline BMI | 1587 | 33.7 (6.1) | 33.6 (6.7) | .25 | .71 |
| Baseline fasting plasma glucose, mg/dL | 1587 | 106 (8.1) | 106 (8.8) | .49 | .34 |
| Baseline 2-h plasma glucose, mg/dL | 1587 | 164 (16.5) | 165 (18.0) | .74 | .68 |
| Baseline HbA1c, % | 1583 | 5.9 (0.5) | 5.9 (0.5) | .01 | .07 |
| Baseline dietary protein intake, median (IQR), g | 1553 | 81.0 (60.8-108.4) | 78.2 (59.5-105.2) | .22 | .91 |
| Baseline weight, kg | 1587 | 92.8 (18.3) | 92.2 (19.2) | .43 | .24 |
| DPPOS variables at AMD assessment | |||||
| Follow-up weight, kg | 1587 | 91.3 (17.8) | 89.9 (19.2) | .14 | .31 |
| Follow-up HbA1c, % | 1587 | 6.1 (0.8) | 6.1 (0.7) | .50 | .72 |
| Diabetes at assessment, No. (%) | |||||
| No | 669 | 456 (68) | 213 (32) | Reference | Reference |
| Yes | 918 | 652 (71) | 266 (29) | .22 | .47 |
| Diabetic retinopathy, No. (%) | |||||
| No | 1319 | 910 (69) | 409 (31) | Reference | Reference |
| Yes | 268 | 198 (74) | 70 (26) | .11 | .07 |
| Smoking status, No. (%) | |||||
| Never | 992 | 719 (72) | 273 (28) | .001 | Reference |
| Ever | 506 | 321 (63) | 185 (37) | .02 | |
| Current | 89 | 68 (76) | 21 (24) | .23 | |
| Years with any metformin | 1587 | 8.5 (9.3) | 7.9 (9.3) | .33 | .69 |
| Years with study metformin | 1587 | 2.9 (7.0) | 2.90 (7.0) | .95 | .37 |
| Years with out-of-study metformin | 1587 | 2.9 (4.8) | 2.78 (4.8) | .23 | .71 |
Abbreviations: AMD, age-related macular degeneration; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DPPOS, Diabetes Prevention Program Outcomes Study; HbA1c, hemoglobin A1c, NA, not applicable.
SI conversion factors: To convert glucose to millimoles per liter, multiply by 0.0555; hemoglobin to proportion of total hemoglobin, multiply by 0.01.
For categorical variables, the unadjusted P values are calculated from Pearson χ2 tests, while the adjusted P values are calculated from logistic regressions comparing each level with the reference level. For continuous variables, the unadjusted P values are calculated from 2-sample t test or Wilcoxon rank sum test (baseline dietary protein intake), while the adjusted P values are calculated from their coefficients in the logistic regression using AMD as the outcome.
Figure 2. Association Between DPP Baseline Characteristics and Diabetes Prevention Program Outcomes Study Retinal Imaging Visit Characteristics With Prevalence of Age-Related Macular Degeneration.
SI conversion factors: To convert glucose to millimoles per liter, multiply by 0.0555; hemoglobin to proportion of total hemoglobin, multiply by 0.01. Forest plot of logistic regression adjusting for demographic information and treatment. Log (odds ratio [OR]) vs reference category or per sample SD change for continuous measures. ACE indicates angiotensin-converting enzyme; BMI, body mass index, DPP, Diabetes Prevention Program; HbA1c, hemoglobin A1c.
Because of crossover use of metformin in other arms, we analyzed the pooled as-treated metformin users, irrespective of study arm. The AMD prevalence among those who used any metformin, irrespective of duration, across all study groups was 321 of 1115 (28.8%) and among those who never used metformin was 158 of 472 (33.5%). There was no association between use of metformin and AMD in this pooled analysis also. In addition, there was no difference in the mean number of years of metformin use in participants with and without AMD (mean [SD], 8.0 [9.3] vs 8.5 [9.3] years; P = .33). The odds ratio for the likelihood of AMD associated with number of years of metformin use was 1.0 (95% CI, 0.9-1.2; P = .69). eTable 2 in Supplement 1 shows distribution of years with any metformin use up to the study visit, years with in-study metformin use, and years with out-of-study metformin use across AMD severity stages. There was no difference in the distribution of all uses of metformin across all AMD severity levels.
The DPPOS also provides an opportunity to study the association between diabetes and AMD. Prevalence of AMD in those with and without diabetes was 29% (266 of 921) vs 32% (213 of 671). The distribution of AMD severity stages in participants with and without diabetes was 71.0% (652 of 918) vs 68.2% (456 of 669) for no AMD, 13.4% (123 of 918) vs 15.8% (106 of 669) for early AMD, 12.4% (123 of 918) vs 14.2% (95 of 669) for intermediate AMD, and 2.2% (20 of 918) vs 1.8% (12 of 669) for advanced AMD, respectively. There was no association of diabetes with either prevalence of AMD or AMD severity. Since all participants had diabetic retinopathy evaluated using color photographs, we investigated the association between diabetic retinopathy and AMD. Of the 268 participants with diabetic retinopathy, 70 (26.1%) had AMD compared with 409 of 1319 (31%) without diabetic retinopathy. In eyes with and without diabetic retinopathy, the distribution of AMD stages was 13% (36 of 268) vs 15% (193 of 1319) for early AMD, 12% (31 of 268) vs 14% (187 of 1319) for intermediate AMD, and 1% (3 of 268) vs 2% (29 of 1319) for advanced AMD. There was no association between diabetic retinopathy and severity of AMD.
Discussion
In the DPPOS, there was no difference in AMD presence or severity between metformin, intensive lifestyle intervention, and placebo arms at median time of 21 years since randomization. Using masked reading center interpretation of images, the DPPOS cohort is unique in its ability to examine the role of metformin in protecting against development of AMD due to both the randomization intervention arm and the duration of follow-up with known exposure to metformin. In addition, we assessed associations of metformin use with AMD severity. We found no association of metformin use with the presence or severity of AMD, nor did we find an association between any use of metformin or number of years of metformin with AMD. Lastly, we found no association between in-study and out-of-study metformin use and AMD.
The association between AMD and metformin has been studied using electronic medical records and insurance databases.8,10,16 The largest such report included a case-control study using the IBM Marketscan database with 624 780 records of patients with and without AMD based on eye examinations and was matched for risk factors.9 The authors found reduced odds of developing AMD (0.94; 95% CI, 0.92-0.96) for those using metformin. This association was dose dependent, with low to moderate doses of metformin showing the greatest potential benefit, and the association was preserved in a subgroup analysis of those with diabetes. Analysis from the Rotterdam study found metformin was associated with a lower risk of AMD (odds ratio, 0.69; 95% CI, 0.49-0.98). Although this was a large prospective population-based cohort, the number included in the AMD subgroup was small (n = 215) and limited to participants with diabetes taking metformin compared with untreated diabetes.17 A meta-analysis of 5 retrospective studies found no statistical significant association between metformin use and AMD (pooled adjusted odds ratio, 0.8; 95% CI, 0.54-1.05; I2 = 98.8%).11 There is one clinical trial underway on the association of metformin with geographic atrophy (NCT02684578). The nonadvanced forms of AMD (early and intermediate stages) have minimal association with vision and are mostly an incidental finding for those who visit ophthalmologists. Patients seeking frequent eye examinations are likely to have vision-threatening diseases such as advanced forms of AMD or diabetic retinopathy and can introduce bias into the sample. In addition, AMD detection in these studies is either from insurance billing codes, ophthalmologist’s records, or International Classification of Diseases codes. The lack of AMD phenotyping, misdiagnosis, and underreporting of early stages are major limitations of these studies. More importantly, comprehensive patient demographics, which serve as major confounders, are unavailable in insurance databases.
The prevalence of AMD in the DPPOS cohort was 28.2% for nonadvanced AMD (early and intermediate). In a systematic review of all population-based studies where AMD was determined using reading center procedures similar to the DPPOS, the pooled global prevalence of any AMD was 8.69% (95% CI, 4.26%-17.4%) in those older than 40 years.18 AMD prevalence increased with age to 17.61% (95% CI, 12.53%-23.87%) in those who were aged 70 to 79 years and 24.96% (95% CI, 18.01%-33.25%) in those aged 80 to 84 years. Considering that most of the DPPOS population were aged 60 to 80 years at the time of image evaluation, the prevalence is as expected for age. The slightly higher prevalence could be explained by the addition of SD-OCT imaging, which improves the accuracy of detecting advanced AMD. Of the 39 eyes with advanced AMD on SD-OCT, only 17 (43.6%) were identified on color photographs (eTable 1 in Supplement 1). In contrast, color photography serves as a better tool for identifying early/intermediate AMD. Of 2581 eyes classified as no AMD on SD-OCT, 191 (7.4%) were identified as early or intermediate on color photographs. Difficulty in identifying small and medium drusen due to resolution, interscan density in high-speed SD-OCT volume scans and limited field of view are some reasons for lower detection. In addition, the DPPOS population included patients at risk of developing diabetes, with BMI more than 24 and impaired glucose tolerance. It is possible that some of these characteristics made this population also high risk for AMD.
The association between diabetes and AMD has been reported in many studies with conflicting results.19 Analysis of risk factors for incident AMD from 3 large population-based studies with retinal imaging found diabetes was not associated with AMD.20 While baseline hemoglobin A1c was close to significance (P < .07), follow-up hemoglobin A1c was not associated with concurrent AMD. In addition, there was no association between diabetes at retinal image visit and AMD. In this analysis of the DPPOS study, stringent criteria were followed for classification of both diabetic retinopathy and AMD. While statistically not significant, the adjusted model showed that association of AMD with diabetic retinopathy approached significance (P < .07). On further analysis, we were not able to find any AMD severity–related associations. There was no association of diabetic retinopathy with the nonexudative nor exudative forms of AMD.
The study did not find association between lifestyle intervention and AMD. While there is overall agreement between studies that physical activity has protective effect against AMD, the results are variable with regards to stage of AMD, gender, and type of activity.21,22,23 The lifestyle intervention was intensive only during DPP, after which group lifestyle was offered to all participants.
Strengths and Limitations
The study has numerous strengths. The DPPOS is a well-characterized longitudinal study with rigorous and regular ascertainment of study measures with long-term metformin intervention. In addition, AMD was evaluated using multimodal retinal imaging by a central reading center.
Despite being one of the best data sets to study the association between metformin and AMD, the study has some limitations. This is a cross-sectional analysis of images available at a single visit and cannot assess the relationship between metformin and incidence or progression of AMD. All participants including those in placebo arm were offered lifestyle intervention and if needed, out-of-study metformin. This crossover, out-of-study use of metformin in DPPOS may have impacted the ability to detect the true association of the metformin intervention with outcomes. The timing of metformin intervention and AMD diagnosis could be reversed in some participants, eg, participants may have had AMD during the DPP study period and crossed over to out-of-study metformin later during the follow-up period. Data were collected from participants to the best possible extent and both in-study and out-of-study metformin was reported. The DPPOS population is an overall healthy population and participants followed lifestyle interventions. In addition, the inclusions of only adults at high risk of developing diabetes limits generalizability.
Conclusions
To summarize, in the DPPOS cohort with 1592 participants randomized to metformin for over 20 years, there was no association between metformin use and prevalence of AMD. There was also no association with stages of AMD classified from multimodal imaging, nor was there an association with duration of metformin use. Until randomized data are available, the DPPOS provides strong evidence that does not support the use of metformin in the treatment of any stage of AMD.
eTable 1. Comparison of AMD severity status evaluated from color photographs and SDOCT
eTable 2. Distribution of number of years of metformin use by AMD severity
Nonauthor Collaborators. The Diabetes Prevention Program Research (DPPOS) Research Group members
References
- 1.Congdon N, O’Colmain B, Klaver CC, et al. ; Eye Diseases Prevalence Research Group . Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477-485. doi: 10.1001/archopht.122.4.477 [DOI] [PubMed] [Google Scholar]
- 2.Friedman DS, O’Colmain BJ, Muñoz B, et al. ; Eye Diseases Prevalence Research Group . Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564-572. doi: 10.1001/archopht.1941.00870100042005 [DOI] [PubMed] [Google Scholar]
- 3.Ferris FL III, Wilkinson CP, Bird A, et al. ; Beckman Initiative for Macular Research Classification Committee . Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844-851. doi: 10.1016/j.ophtha.2012.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danis RP, Domalpally A, Chew EY, et al. ; AREDS2 Study Group . Methods and reproducibility of grading optimized digital color fundus photographs in the Age-Related Eye Disease Study 2 (AREDS2 Report Number 2). Invest Ophthalmol Vis Sci. 2013;54(7):4548-4554. doi: 10.1167/iovs.13-11804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrity ST, Sarraf D, Freund KB, Sadda SR. Multimodal imaging of nonneovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2018;59(4):amd48-amd64. doi: 10.1167/iovs.18-24158 [DOI] [PubMed] [Google Scholar]
- 6.Klein ML, Ferris FL III, Armstrong J, et al. ; AREDS Research Group . Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115(6):1026-1031. doi: 10.1016/j.ophtha.2007.08.030 [DOI] [PubMed] [Google Scholar]
- 7.Age-Related Eye Disease Study 2 Research Group . Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005-2015. doi: 10.1001/jama.2013.4997 [DOI] [PubMed] [Google Scholar]
- 8.Brown EE, Ball JD, Chen Z, Khurshid GS, Prosperi M, Ash JD. The common antidiabetic drug metformin reduces odds of developing age-related macular degeneration. Invest Ophthalmol Vis Sci. 2019;60(5):1470-1477. doi: 10.1167/iovs.18-26422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blitzer AL, Ham SA, Colby KA, Skondra D. Association of metformin use with age-related macular degeneration: a case-control study. JAMA Ophthalmol. 2021;139(3):302-309. doi: 10.1001/jamaophthalmol.2020.6331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart JM, Lamy R, Wu F, Keenan JD. Relationship between oral metformin use and age-related macular degeneration. Ophthalmol Retina. 2020;4(11):1118-1119. doi: 10.1016/j.oret.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romdhoniyyah DF, Harding SP, Cheyne CP, Beare NAV. Metformin, a potential role in age-related macular degeneration: a systematic review and meta-analysis. Ophthalmol Ther. 2021;10(2):245-260. doi: 10.1007/s40123-021-00344-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Diabetes Prevention Program . The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22(4):623-634. doi: 10.2337/diacare.22.4.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 15.Sadda SR, Guymer R, Holz FG, et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of atrophy report 3. Ophthalmology. 2018;125(4):537-548. doi: 10.1016/j.ophtha.2017.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YY, Shen YC, Lai YJ, et al. Association between metformin and a lower risk of age-related macular degeneration in patients with type 2 diabetes. J Ophthalmol. 2019;2019:1649156. doi: 10.1155/2019/1649156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vergroesen JE, Thee EF, Ahmadizar F, et al. Association of diabetes medication with open-angle glaucoma, age-related macular degeneration, and cataract in the Rotterdam study. JAMA Ophthalmol. 2022;140(7):674-681. doi: 10.1001/jamaophthalmol.2022.1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106-e116. doi: 10.1016/S2214-109X(13)70145-1 [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Rong SS, Xu Q, et al. Diabetes mellitus and risk of age-related macular degeneration: a systematic review and meta-analysis. PLoS One. 2014;9(9):e108196-e108196. doi: 10.1371/journal.pone.0108196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology. 2001;108(4):697-704. doi: 10.1016/S0161-6420(00)00580-7 [DOI] [PubMed] [Google Scholar]
- 21.McGuinness MB, Karahalios A, Simpson JA, et al. Past physical activity and age-related macular degeneration: the Melbourne Collaborative Cohort Study. Br J Ophthalmol. 2016;100(10):1353-1358. doi: 10.1136/bjophthalmol-2015-307663 [DOI] [PubMed] [Google Scholar]
- 22.Knudtson MD, Klein R, Klein BEK. Physical activity and the 15-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Br J Ophthalmol. 2006;90(12):1461-1463. doi: 10.1136/bjo.2006.103796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauschitz MM, Schmitz MT, Verzijden T, et al. ; European Eye Epidemiology (E3) Consortium . Physical activity, incidence, and progression of age-related macular degeneration: a multicohort study. Am J Ophthalmol. 2022;236:99-106. doi: 10.1016/j.ajo.2021.10.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Comparison of AMD severity status evaluated from color photographs and SDOCT
eTable 2. Distribution of number of years of metformin use by AMD severity
Nonauthor Collaborators. The Diabetes Prevention Program Research (DPPOS) Research Group members