This cross-sectional study uses results from the National COVID Cancer Antibody Survey to evaluate whether spike protein antibody vaccine response following COVID-19 vaccination is associated with risk of SARS-CoV-2 breakthrough infection or hospitalization among patients with cancer in the UK.
Key Points
Question
After COVID-19 vaccination, is the risk of a breakthrough SARS-CoV-2 infection or hospitalization associated with spike protein antibody vaccine responses in patients with cancer?
Findings
In this UK national COVID-19 cancer cross-sectional study involving 4249 antibody test results from patients with cancer and 294 230 test results from the general population, an undetectable SARS-CoV-2 antibody response was associated with a significantly increased risk of breakthrough SARS-CoV-2 infection and hospitalization compared with those who had a positive response.
Meaning
This study’s findings suggest that SARS-CoV-2 antibody testing provides a good indication of risk of infection or hospitalization among patients with cancer.
Abstract
Importance
Accurate identification of patient groups with the lowest level of protection following COVID-19 vaccination is important to better target resources and interventions for the most vulnerable populations. It is not known whether SARS-CoV-2 antibody testing has clinical utility for high-risk groups, such as people with cancer.
Objective
To evaluate whether spike protein antibody vaccine response (COV-S) following COVID-19 vaccination is associated with the risk of SARS-CoV-2 breakthrough infection or hospitalization among patients with cancer.
Design, Setting, and Participants
This was a population-based cross-sectional study of patients with cancer from the UK as part of the National COVID Cancer Antibody Survey. Adults with a known or reported cancer diagnosis who had completed their primary SARS-CoV-2 vaccination schedule were included. This analysis ran from September 1, 2021, to March 4, 2022, a period covering the expansion of the UK’s third-dose vaccination booster program.
Interventions
Anti–SARS-CoV-2 COV-S antibody test (Elecsys; Roche).
Main Outcomes and Measures
Odds of SARS-CoV-2 breakthrough infection and COVID-19 hospitalization.
Results
The evaluation comprised 4249 antibody test results from 3555 patients with cancer and 294 230 test results from 225 272 individuals in the noncancer population. The overall cohort of 228 827 individuals (patients with cancer and the noncancer population) comprised 298 479 antibody tests. The median age of the cohort was in the age band of 40 and 49 years and included 182 741 test results (61.22%) from women and 115 737 (38.78%) from men. There were 279 721 tests (93.72%) taken by individuals identifying as White or White British. Patients with cancer were more likely to have undetectable anti-S antibody responses than the general population (199 of 4249 test results [4.68%] vs 376 of 294 230 [0.13%]; P < .001). Patients with leukemia or lymphoma had the lowest antibody titers. In the cancer cohort, following multivariable correction, patients who had an undetectable antibody response were at much greater risk for SARS-CoV-2 breakthrough infection (odds ratio [OR], 3.05; 95% CI, 1.96-4.72; P < .001) and SARS-CoV-2–related hospitalization (OR, 6.48; 95% CI, 3.31-12.67; P < .001) than individuals who had a positive antibody response.
Conclusions and Relevance
The findings of this cross-sectional study suggest that COV-S antibody testing allows the identification of patients with cancer who have the lowest level of antibody-derived protection from COVID-19. This study supports larger evaluations of SARS-CoV-2 antibody testing. Prevention of SARS-CoV-2 transmission to patients with cancer should be prioritized to minimize impact on cancer treatments and maximize quality of life for individuals with cancer during the ongoing pandemic.
Introduction
The SARS-CoV-2 pandemic remains a health care issue despite high rates of COVID-19 vaccinations and increasing rates of prior infection. Levels of immunity and protection from SARS-CoV-2 differ in the general population, and some groups are at disproportionate risk. Immunocompromised individuals, such as those with cancer, have a reduced ability to fight infections, and robust evidence of poor immunological response to COVID-19 vaccines and boosters exists.1,2,3,4,5,6,7,8 There is an unmet need to accurately identify groups with the lowest levels of protection from SARS-CoV-2 infection or severe COVID-19, and the next logical step is to determine whether poor immunological responses raise the risk of worse outcomes. This determination is important considering the issue of waning immunity following vaccination, the heterogeneous effectiveness of COVID-19 vaccine boosters, and the cumulative risks from infections and reinfections.9,10,11,12 However, outcomes of patients with cancer have been slowly improving as a result of better treatments and different coronavirus (CoV) variants such as B.1.1.529 Omicron.13,14 These groups would benefit from tailored interventions, such as early treatment programs or preexposure prophylaxis strategies. It remains unclear whether clinical factors alone (demographic, diagnoses, and treatments) are sufficient for identification or whether there is a further role for diagnostic testing, such as antibody testing. SARS-CoV-2 antibody testing gives a quantitative assessment of antibodies to either anti-N or anti-S (CoVS) antibodies. Anti-N presence denotes the presence of antibodies against the SARS-CoV-2 nucleocapsid protein and suggests previous infection. Anti-S presence denotes antibodies generated against the spike protein and suggests previous infection and/or response to vaccination. To date, no studies, to our knowledge, have demonstrated that antibody responses following vaccination are associated with future SARS-CoV-2 breakthrough infections or hospitalization in at-risk groups.15
To our knowledge, this National COVID Cancer Antibody Survey is the largest SARS-CoV-2 antibody study in a cancer cohort using the CoVS antibody test. This analysis assessed whether antibody responses are associated with patient demographic characteristics, time since booster, and cancer subtype. In addition, to our knowledge, this is the first evaluation of the use of antibody testing for estimating future SARS-CoV-2 infection and hospitalization.
Methods
Study Setting and Ethical Review
The UK Coronavirus Cancer Programme is part of the UK’s COVID-19 cancer pandemic response to safeguard, evaluate, and protect patients with cancer.16 This project, a cross-sectional population-based study of antibody testing in patients with cancer, was conducted from September 1, 2021, to March 4, 2022. It was run as part of our National COVID Cancer Antibody Survey.17 The study was designed as a public health surveillance analysis to support rapid clinical decision-making in accordance with the UK Policy Framework for Health and Social Care Research. The study was supported by the Department of Health and Social Care, UK Health Security Agency, University of Oxford, University of Southampton, University of Birmingham, and Blood Cancer UK, with ethical approval from the Public Health England Research Ethics and Governance of Public Health Practice group. Informed written or emailed consent was obtained for participation in this study. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Design and Population
The cancer cohort comprised individuals contained within Public Health England’s rapid registration national cancer data set (between January 1, 2018, and April 30, 2021) who had SARS-CoV-2 antibody test results from the pillar 3 antibody data set.18 During the study period, antibody testing was also made available to essential workers, including education, health care, and social care staff, who formed the population control (unless individuals were contained within the national cancer data set). During the evaluation period, patients with cancer could request an antibody test at any point following vaccination as part of this cross-sectional study. The study inclusion criteria included completion of their primary vaccination course (ie, received at least 2 doses; eFigure 1 in Supplement 1) and participation in SARS-CoV-2 antibody testing. Individuals who were being treated with anticoagulants were excluded due to an increased risk of bleeding during home sampling. SARS-CoV-2 antibody sampling was performed using capillary blood sampling as part of the UK Health Security Agency Home Antibody Testing Service. Sample analysis was performed centrally at accredited laboratories using an anti–SARS-CoV-2 S test (Elecsys; Roche). The assay provides quantification of SARS-CoV-2 spike protein antibodies with a saturation value of 25 000 U/mL. All individuals received their antibody test results in a positive/negative/void format. A manufacturer-specified negative result was issued if the result was less than 0.8 U/mL, which is referred to as an undetectable antibody response in this report. Ethnicity was self-selected by patients within this study.
Statistical Analysis
The primary outcomes of the study were (1) antibody response and titers, (2) breakthrough infection, and (3) SARS-CoV-2–related hospitalization. Comparisons for antibody responses were made between the cancer cohort and the control population cohort and within the cancer cohort by cancer subtype. Breakthrough infection and hospitalization rates were compared within the cancer cohort by level of antibody response.
Antibody testing results were linked to vaccination records from the National Immunisation Management Service and linked to hospital records from the Secondary Use Statistics data sets. Linkages required exact matching of National Health Service numbers, and all records were matched. Breakthrough infection was defined as a positive SARS-CoV-2 polymerase chain reaction test result following vaccination. SARS-CoV-2–related hospitalization was defined as a hospitalization episode between 1 day before and 14 days after a positive polymerase chain reaction test result.19 Breakthrough infections and hospitalizations were monitored during the same period as the study, from September 1, 2021, to March 4, 2022. Test results with missing data points required for an analysis were excluded from that analysis. Predefined cancer subgroups included a cancer subtype classification according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision using groups specified in previous analyses and by recorded cancer treatments.20
Logistic regression analyses were performed to identify risk of a SARS-CoV-2 breakthrough infection or hospitalization based on antibody responses. Multivariable adjustments were performed for predefined clinically significant risk factors, including age (in deciles), sex, ethnicity, and levels of deprivation.12,21 Ethnicity was classified by participants, defined according to an approved UK government list of ethnic groups,22 and included in the analysis because ethnicity is a significant variable for CoV infection and hospitalization. Sensitivity analyses were performed by vaccination dose and cancer subtype. Frequency and cross-tabulation of variables were performed with the 2-sided Fisher exact test to compare categorical data and a Mann-Whitney test to compare antibody titers using P < .05 to denote statistical significance.
Results
Overall Description
The overall cohort comprised 298 479 antibody tests. The median age of the individuals in the cohort was in the age band of 40 and 49 years and included 182 741 test results (61.22%) from women and 115 737 (38.78%) from men. There were 10 862 tests (3.64%) taken by individuals identifying as Asian or Asian British, 2243 (0.75%) from individuals identifying as Black or Black British, 279 721 (93.72%) from individuals identifying as White or White British, and 5283 (1.77%) from individuals identifying as mixed or other ethnicity, with other including individuals who identified as having Arab, Hispanic, or Middle Eastern ethnicity. The cancer cohort consisted of 4249 antibody test results from 3555 patients with cancer from September 1, 2021, to March 4, 2022. Of these tests, 2313 were performed following a second vaccination dose, and 1936 were performed following a third vaccination dose. In this cohort, no patients had more than 3 vaccination doses. The population control consisted of 294 230 test results, of which 230 417 tests were performed following a second vaccination dose and 63 813 following a third vaccination booster dose. The baseline characteristics of the cancer cohort and population control by test results are given in the Table.
Table. Baseline Characteristics of Cancer Cohort and Population Control.
| Characteristic | Cancer cohort, No. (%) of test results | Population control, No. (%) of test results | ||||
|---|---|---|---|---|---|---|
| Dose 2 (n = 2313) | Dose 3 (n = 1936) | Overall (n = 4249) | Dose 2 (n = 230 417) | Dose 3 (n = 63 813) | Overall (n = 294 230) | |
| Age, median 10-y age band | 50-59 | 60-69 | 60-69 | 40-49 | 50-59 | 40-49 |
| Age group, y | ||||||
| 18-19 | 0 | 0 | 0 | 849 (0.37) | 153 (0.24) | 1002 (0.34) |
| 20-29 | 29 (1.25) | 27 (1.39) | 56 (1.32) | 12 184 (5.29) | 3941 (6.18) | 16 125 (5.48) |
| 30-39 | 99 (4.28) | 105 (5.42) | 204 (4.80) | 35 649 (15.47) | 9703 (15.21) | 45 352 (15.41) |
| 40-49 | 349 (15.09) | 273 (14.10) | 622 (14.64) | 75 411 (32.73) | 15 252 (23.90) | 90 663 (30.81) |
| 50-59 | 704 (30.44) | 493 (25.46) | 1197 (28.17) | 61 095 (26.51) | 16 465 (25.80) | 77 560 (26.36) |
| 60-69 | 720 (31.13) | 586 (30.27) | 1306 (30.74) | 32 976 (14.31) | 11 703 (18.34) | 44 679 (15.19) |
| 70-79 | 378 (16.34) | 387 (19.99) | 765 (18.00) | 11 308 (4.91) | 5796 (9.08) | 17 104 (5.81) |
| 80-89 | 34 (1.47) | 65 (3.36) | 99 (2.33) | 901 (0.39) | 772 (1.21) | 1673 (0.57) |
| ≥90 | 0 | 0 | 0 | 44 (0.02) | 28 (0.04) | 72 (0.02) |
| Sex | ||||||
| Male | 879 (38.00) | 740 (38.22) | 1619 (38.10) | 91 068 (39.52) | 23 050 (36.12) | 114 118 (38.79) |
| Female | 1434 (62.00) | 1196 (61.78) | 2630 (61.90) | 139 348 (60.48) | 40 763 (63.88) | 180 111 (61.21) |
| Ethnicity | ||||||
| Asian/Asian British | 45 (1.95) | 35 (1.81) | 80 (1.88) | 8140 (3.53) | 2642 (4.14) | 10 782 (3.66) |
| Black/Black British | 21 (0.91) | 9 (0.46) | 30 (0.71) | 1617 (0.70) | 596 (0.93) | 2213 (0.75) |
| White/White British | 2225 (96.20) | 1866 (96.38) | 4091 (96.28) | 216 343 (93.89) | 59 287 (92.91) | 275 630 (93.68) |
| Mixed/other ethnic groupa | 21 (0.91) | 25 (1.29) | 46 (1.08) | 4039 (1.75) | 1198 (1.88) | 5237 (1.78) |
| Deprivation, IMD groupb | ||||||
| IMD low (1-3) | 462 (19.97) | 275 (14.20) | 737 (17.35) | 40 501 (17.58) | 12 165 (19.06) | 52 666 (17.90) |
| IMD medium (4-7) | 904 (39.08) | 799 (41.27) | 1703 (40.08) | 94 355 (40.95) | 26 414 (41.39) | 120 769 (41.05) |
| IMD high (8-10) | 947 (40.94) | 862 (44.52) | 1809 (42.57) | 95 505 (41.45) | 25 222 (39.52) | 120 727 (41.03) |
Abbreviation: IMD, index of multiple deprivation.
The other ethnic groups included Arab/North African/Middle Eastern and Hispanic/Latino.
Deprivation represents an English metric of the geographical area in which an individual lives, reflecting 7 domains of deprivation (income, employment, education, health, crime, use of housing services, and living environment).21 Scores range from 1 to 10, where 1 indicates most deprived area and 10 indicates least deprived area.
In patients with at least 2 vaccination doses, undetectable antibody responses were identified in 4.68% of the test results (199 of 4249) from the cancer cohort. In the population control, significantly fewer individuals had undetectable antibody responses (376 of 294 230 test results [0.13%]) (P < .001) (eTable 1 in Supplement 1). For both the cancer cohort and population control, individuals who had received a third dose booster had significantly higher antibody titers than those who had only 2 vaccination doses (11 146.5 vs 8765.0 U/mL, P < .001 for the cancer cohort and 23 667.0 vs 12 126.0 U/mL, P < .001 for the population control; eTable 2 in Supplement 1).
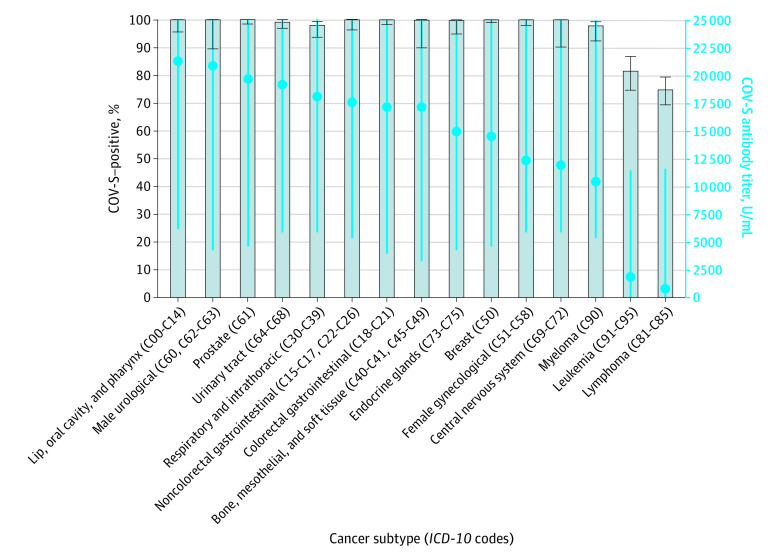
Subgroup analyses identified that undetectable antibody responses were observed in 19.23% of test results (105 of 546) for patients with blood (hematological) cancer and 4.23% of test results (118 of 2791) for patients with solid organ malignant tumors. Patients with blood cancer had significantly lower antibody titers than patients with solid organ malignant tumors (1872.5 U/mL vs 16 165.0 U/mL; P < .001). Individuals with a diagnosis of leukemia or lymphoma had the highest rates of undetectable antibody responses and the lowest antibody titers (Figure 1; eTable 3 in Supplement 1).
Figure 1. Median SARS-CoV-2 Antibody Titers and Responses in Patients With Cancer Based on Cancer Subtype.
Blue dots represent spike protein antibody vaccine response (COV-S) titers. Blue error bars denote IQR; black error bars, 95% CI. ICD-10 indicates International Statistical Classification of Diseases and Related Health Problems, Tenth Revision.
We identified that individuals who were recorded as having had systemic anticancer therapies had lower median antibody titers than individuals who did not receive that therapy (8131.0 U/mL vs 15 443.0 U/mL; P < .001). A difference in antibody titer was not observed for patients who were recorded as having had radiotherapy compared with patients who did not (eFigure 2 in Supplement 1). Stage 4 cancers were associated with a significantly lower antibody titer (eTable 3 and eFigure 2 in Supplement 1).
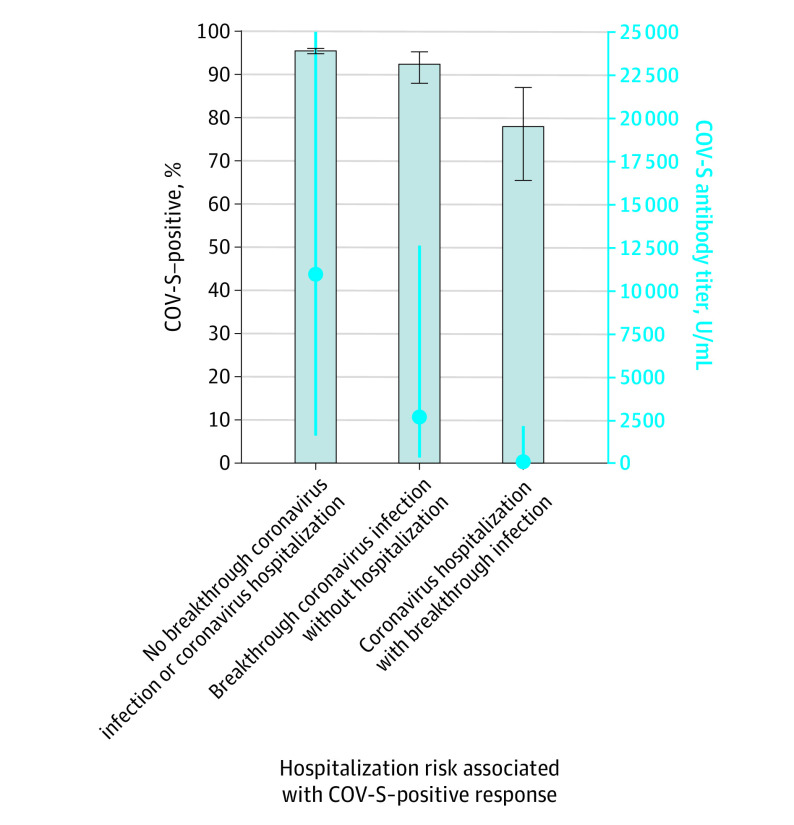
In the cancer cohort, 259 patients had a breakthrough SARS-CoV-2 infection, and 55 patients had a SARS-CoV-2–related hospitalization following their antibody test. No deaths were recorded in the cancer cohort. Patients with cancer who had a SARS-CoV-2–related hospitalization had significantly lower median antibody titers than those who did not (147.0 U/mL; IQR, 6.6-2104.0 U/mL vs 10 961.0 U/mL; IQR, 1611.0-25 000.0 U/mL; P < .001) (Figure 2). Similarly, patients with cancer who had a breakthrough SARS-CoV-2 infection had significantly lower median antibody titers than those who did not (2699.0 U/mL; IQR, 346.9-12 552.0 U/mL vs 10 961.0 U/mL; IQR, 1611.0-25 000.0 U/mL; P < .001) (Figure 3).
Figure 2. Median Antibody Titers and Percentage of Positive Spike Protein Antibody Vaccine Response (COV-S) in Patients With Cancer Who Experienced Breakthrough Infections and Hospitalizations.
Bars represent percentage of positive COV-S responses. Blue dots represent median antibody titers. Blue error bars denote IQR; black error bars, 95% CI.
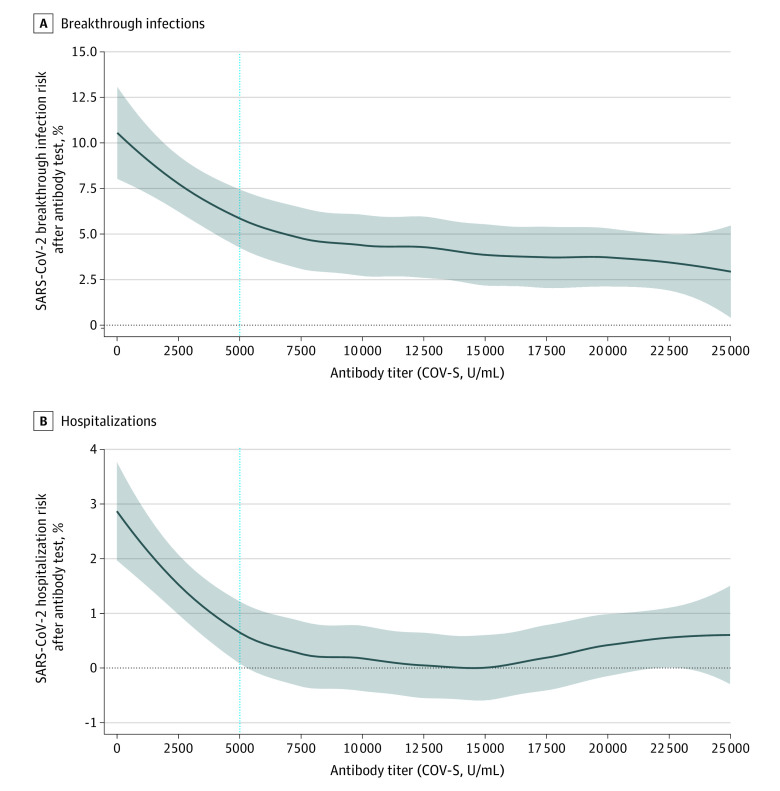
Figure 3. Association Between Antibody Titer and Risk of SARS-CoV-2 Breakthrough Infection and Hospitalization.
Graphs show the association between antibody titer and risk of SARS-CoV-2 breakthrough infection (A) and hospitalization (B). Gray area represents 95% CI. The blue vertical line is the cutoff at 5000 U/mL, at which odds ratios (ORs) were performed.
Individuals who had an undetectable antibody response were at much higher risk for SARS-CoV-2 breakthrough infection (odds ratio [OR], 2.56; 95% CI, 1.69-4.00; 13.57% [27 of 199 patients] vs 5.73% [232 of 4050 patients]; P < .001) and SARS-CoV-2–related hospitalization (OR, 5.88; 95% CI, 3.13-11.11; 6.03% [12 of 199 patients] vs 1.06% [43 of 4050 patients]; P < .001) than individuals who had a positive antibody response. This increased risk was still observed when a multiple variable adjusted model was fitted (adjusting for age, sex, ethnicity, and levels of deprivation), indicating that antibody responses remained an independent risk factor (breakthrough infection adjusted OR, 3.05; 95% CI, 1.96-4.72; P < .001; SARS-CoV-2–related hospitalization adjusted OR, 6.48; 95% CI, 3.31-12.67; P < .001). Sensitivity analyses confirmed that this risk was observed regardless of whether a third dose had been received and whether the individual had a blood cancer or solid cancer diagnosis (eTable 4 in Supplement 1).
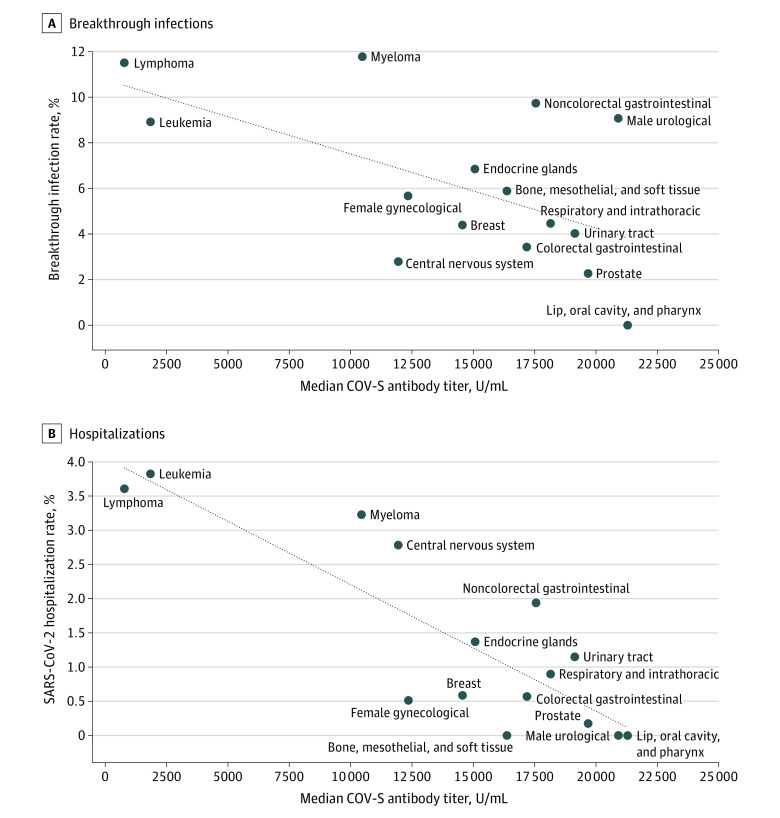
To understand the nature of the association between SARS-CoV-2 antibody titer and risk of breakthrough infection and hospitalization, a logistic regression model was fitted (Figure 3). We observed that the risk of SARS-CoV-2 breakthrough infection and hospitalization increases as the antibody titer decreases to below 5000 U/mL. Comparing those individuals with a titer below 5000 U/mL, the OR was 3.05 (95% CI, 2.33-4.01; 10.08% [167 of 1656 patients] vs 3.55% [92 of 2594 patients]; P < .001) for SARS-CoV-2 breakthrough infection and 7.22 (95% CI, 3.57-16.10; 2.72% [45 of 1656 patients] vs 0.39% [10 of 2594 patients]; P < .001) for SARS-CoV-2–related hospitalization, relative to those with a titer greater than or equal to 5000 U/mL. In the cancer cohort, the association between breakthrough infection and CoV hospitalization and median antibody titers for each cancer subtype is shown in Figure 4.
Figure 4. Association Between Median Antibody Titer and Breakthrough Infections and Hospitalization by Cancer Subtype.
Graphs show rates of breakthrough infections (A) and hospitalization (B) by cancer subtype in association with median antibody titer. In both graphs, the diagonal dotted lines are trend lines to visualize the association between median spike protein antibody vaccine response (COV-S) antibody titer and the outcome rate percentage (infection [A] or hospitalization [B]). These trend lines were calculated from the cancer subtypes (solid circles) for each graph.
Discussion
To our knowledge, our National COVID Cancer Antibody Survey is the first study to demonstrate that COV-S antibody testing is an effective tool to identify individuals with cancer who have the lowest levels of protection from vaccination. The survey was performed at the end of the UK’s Delta variant wave (B.1.617.1) and start of the Omicron variant wave (B.1.1.529), and we observed that antibody titers and responses are negatively correlated with risk of breakthrough SARS-CoV-2 infection and hospitalization. In addition, our results suggest the heterogeneous benefits of vaccination. Low SARS-CoV-2 antibody titers following vaccination are frequently observed in individuals with leukemia and lymphoma. Low levels of antibody titers may also be observed in other tumor types, although at a much lower incidence.
In most countries, SARS-CoV-2 antibody testing is not widely available. There are concerns about measuring humoral immunity alone without a measure of cellular immunity by T cells. Antibody titers are expected to decline over time, even in healthy individuals, and protection against reinfection comes from a combination of circulating antibodies, T cells, and memory B cells, which can rapidly produce antibodies following reexposure. Emerging data show that cellular immunity is well established after vaccination and infection, even in vulnerable groups, and the large and rapid increase in antibody titers following a second vaccine dose strongly supports the presence of a good memory B-cell response.23,24,25
This survey’s results suggest that wider access to antibody testing for individuals with cancer should be evaluated. First, it could inform national guidance for clinicians advising patients and may provide a risk surveillance strategy that can be used to guide vaccination booster programs. This information is important as the risk of severe outcomes of SARS-CoV-2 infection is heterogeneous in different patient groups and changes with SARS-CoV-2 variants, time, availability of effective SARS-CoV-2 treatments, and vaccination status and doses. Second, it may enable individuals to make better-informed choices about personal precautions to reduce the risk of transmission when community SARS-CoV-2 prevalence is high. Last, health care systems could have access to a diagnostic tool to reliably target new interventions, such as early treatment or preexposure prophylactic monoclonal antibodies, to patients at the highest risk of SARS-CoV-2–related hospitalization and breakthrough infections.
Limitations
There were some potential limitations with this study. The most notable was the cross-sectional nature of our study, capturing a range of intervals after vaccination. Cross-sectional periodic testing, however, may offer operational benefits compared with a patient-specific testing schedule. Our study is directly applicable and a useful pilot to a health care periodic testing model. In addition, when a specific cancer subtype is analyzed, numbers of participants are small, and it is difficult to draw firm conclusions. We acknowledge that we have not comprehensively assessed the time between vaccination and antibody tests or controlled for the effect of viral variants, with the study occurring at the end of the Delta wave and start of the Omicron wave. It should be acknowledged that the timing of vaccination relative to immunosuppression is likely to be important in determining the level of antibody response, and this association needs further evaluation. Furthermore, due to their condition or treatments, individuals with cancer often have higher rates of hospitalizations that are unrelated to COVID-19, and some may have been captured in this analysis as the result of a CoV-2 breakthrough infection or as a COVID-19 hospitalization. The effect is to reduce the power of the analysis. Finally, our survey may be influenced by selection bias, with individuals concerned about SARS-CoV-2 more likely to participate. We do not envision that this factor would alter the association between antibody testing and the clinical outcomes of infection or hospitalization because patients were informed of their results. Participants who received a negative test result would, if anything, be expected to reduce their own risk of infection.
Conclusions
The results of the National COVID Cancer Antibody Study suggest that COV-S antibody testing can identify patients with cancer who have the lowest level of antibody-derived protection and immunity from SARS-CoV-2 and COVID-19. Prevention of SARS-CoV-2 infection in these patients should be prioritized to minimize impact on their cancer treatments. Antibody testing could empower these individuals to take additional preventive measures to reduce their risk of infection. Further expansion of antibody testing to prioritize measures, such as preexposure prophylaxis, vaccination boosters, and early treatment programs, may help mitigate the direct impact on this group as well as the indirect impact arising from delays to effective cancer care. Ongoing caution by patients is warranted regardless of antibody response in view of waning long-term immunity and new variants. Furthermore, antibody testing can only be 1 part of a larger strategy that includes collective efforts such as ventilation, filtration and 2-way masking, which will make life safer for vulnerable and immunocompromised patients. In combination, these measures could maximize prognosis and quality of life for individuals with cancer during the ongoing pandemic.
eTable 1. Coronavirus (COV-S) Antibody Responses in the Cancer Cohort and Population Control
eTable 2. Coronavirus (COV-S) Antibody Titres in the Cancer Cohort and Population Control Following Dose 2, Dose 3, and Following Either Dose 2/3
eTable 3. Cancer Cohort Subgroup Analyses of Median Coronavirus (COV-S) Antibody Titres and Responses
eTable 4. Univariable and Multivariable Adjusted Odds Ratio for the Risk of Coronavirus Breakthrough Infection and Coronavirus Hospitalisation Among Test From Cancer Patients With Undetectable Coronavirus (COV-S) Antibody Responses Relative to Those With Positive Antibody Responses
eFigure 1. Study Eligibility Based on Exclusion Criteria
eFigure 2. Median Coronavirus Antibody Titres and Responses in Cancer Patients Based on Cancer and Treatment
UK COVID Cancer Programme Nonauthor Collaborators
References
- 1.Becerril-Gaitan A, Vaca-Cartagena BF, Ferrigno AS, et al. Immunogenicity and risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after coronavirus disease 2019 (COVID-19) vaccination in patients with cancer: a systematic review and meta-analysis. Eur J Cancer. 2022;160:243-260. doi: 10.1016/j.ejca.2021.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gounant V, Ferré VM, Soussi G, et al. Efficacy of severe acute respiratory syndrome coronavirus-2 vaccine in patients with thoracic cancer: a prospective study supporting a third dose in patients with minimal serologic response after two vaccine doses. J Thorac Oncol. 2022;17(2):239-251. doi: 10.1016/j.jtho.2021.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naranbhai V, Pernat CA, Gavralidis A, et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in patients with cancer: the CANVAX cohort study. J Clin Oncol. 2022;40(1):12-23. doi: 10.1200/JCO.21.01891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fendler A, Au L, Shepherd STC, et al. ; Crick COVID-19 Consortium; CAPTURE Consortium . Functional antibody and T cell immunity following SARS-CoV-2 infection, including by variants of concern, in patients with cancer: the CAPTURE study. Nat Cancer. 2021;2(12):1321-1337. doi: 10.1038/s43018-021-00275-9 [DOI] [PubMed] [Google Scholar]
- 5.Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765-778. doi: 10.1016/S1470-2045(21)00213-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim SH, Campbell N, Johnson M, et al. Antibody responses after SARS-CoV-2 vaccination in patients with lymphoma. Lancet Haematol. 2021;8(8):e542-e544. doi: 10.1016/S2352-3026(21)00199-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ligumsky H, Safadi E, Etan T, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine among actively treated cancer patients. J Natl Cancer Inst. 2022;114(2):203-209. doi: 10.1093/jnci/djab174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakuraba A, Luna A, Micic D. Serologic response following SARS-COV2 vaccination in patients with cancer: a systematic review and meta-analysis. J Hematol Oncol. 2022;15(1):15. doi: 10.1186/s13045-022-01233-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debie Y, Vandamme T, Goossens ME, van Dam PA, Peeters M. Antibody titres before and after a third dose of the SARS-CoV-2 BNT162b2 vaccine in patients with cancer. Eur J Cancer. 2022;163:177-179. doi: 10.1016/j.ejca.2021.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee LYW, Starkey T, Ionescu MC, et al. ; NCRI Consumer Forum . Vaccine effectiveness against COVID-19 breakthrough infections in patients with cancer (UKCCEP): a population-based test-negative case-control study. Lancet Oncol. 2022;23(6):748-757. doi: 10.1016/S1470-2045(22)00202-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Aly Z, Bowe B, Xie Y, et al. Outcomes of SARS-CoV-2 reinfection. Accessed August 11, 2022. 10.21203/rs.3.rs-1749502/v1 [DOI]
- 12.Lee LYW, Ionescu MC, Starkey T, et al. ; UK Coronavirus Cancer Programme . COVID-19: third dose booster vaccine effectiveness against breakthrough coronavirus infection, hospitalisations and death in patients with cancer: a population-based study. Eur J Cancer. 2022;175:1-10. doi: 10.1016/j.ejca.2022.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinato DJ, Aguilar-Co J, Ferrante D, et al. ; OnCovid study group . Outcomes of the SARS-CoV-2 Omicron (B.1.1.529) variant outbreak among vaccinated and unvaccinated patients with cancer in Europe: results from the retrospective, multicentre, OnCovid registry study. Lancet Oncol. 2022;23(7):865-875. doi: 10.1016/S1470-2045(22)00273-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinato DJ, Patel M, Scotti L, et al. ; OnCovid Study Group . Time-dependent COVID-19 mortality in patients with cancer: an updated analysis of the OnCovid registry. JAMA Oncol. 2022;8(1):114-122. doi: 10.1001/jamaoncol.2021.6199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbasi J. The flawed science of antibody testing for SARS-CoV-2 immunity. JAMA. 2021;326(18):1781-1782. doi: 10.1001/jama.2021.18919 [DOI] [PubMed] [Google Scholar]
- 16.A United Kingdom pandemic response programme for cancer patients. UK Coronavirus Cancer Programme. Accessed August 11, 2022. http://www.ukcovidcancerprogramme.org
- 17.UK Coronavirus Cancer Programme. Accessed August 11, 2022. https://ukcovidcancerprogramme.org/national-covid-cancer-antibody-survey/
- 18.Department of Health and Social Care . COVID-19 testing data: methodology note. Accessed August 11, 2022. https://www.gov.uk/government/publications/coronavirus-covid-19-testing-data-methodology/covid-19-testing-data-methodology-note
- 19.GOV.UK. Metrics documentation: coronavirus in the UK. Accessed August 11, 2022. https://coronavirus.data.gov.uk/metrics/doc/hospitalCases
- 20.Lee LYW, Cazier JB, Angelis V, et al. ; UK Coronavirus Monitoring Project Team . COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919-1926. doi: 10.1016/S0140-6736(20)31173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GOV.UK. English indices of deprivation 2019. Accessed August 11, 2022. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019
- 22.Ethnicity facts and figures. GOV.UK. Accessed August 11, 2022. https://www.ethnicity-facts-figures.service.gov.uk/style-guide/ethnic-groups
- 23.Röltgen K, Boyd SD. Antibody and B cell responses to SARS-CoV-2 infection and vaccination. Cell Host Microbe. 2021;29(7):1063-1075. doi: 10.1016/j.chom.2021.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner JS, O’Halloran JA, Kalaidina E, et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596(7870):109-113. doi: 10.1038/s41586-021-03738-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swadling L, Diniz MO, Schmidt NM, et al. ; COVIDsortium Investigators . Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature. 2022;601(7891):110-117. doi: 10.1038/s41586-021-04186-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Coronavirus (COV-S) Antibody Responses in the Cancer Cohort and Population Control
eTable 2. Coronavirus (COV-S) Antibody Titres in the Cancer Cohort and Population Control Following Dose 2, Dose 3, and Following Either Dose 2/3
eTable 3. Cancer Cohort Subgroup Analyses of Median Coronavirus (COV-S) Antibody Titres and Responses
eTable 4. Univariable and Multivariable Adjusted Odds Ratio for the Risk of Coronavirus Breakthrough Infection and Coronavirus Hospitalisation Among Test From Cancer Patients With Undetectable Coronavirus (COV-S) Antibody Responses Relative to Those With Positive Antibody Responses
eFigure 1. Study Eligibility Based on Exclusion Criteria
eFigure 2. Median Coronavirus Antibody Titres and Responses in Cancer Patients Based on Cancer and Treatment
UK COVID Cancer Programme Nonauthor Collaborators