Key Points
Question
How does cognitive functioning evolve after cochlear implantation in older adults at risk for mild cognitive impairment?
Findings
In this longitudinal, prospective cohort study including 21 older adult cochlear implant candidates at risk for mild cognitive impairment preoperatively, cognitive functioning improved 12 months after cochlear implantation.
Meaning
These preliminary findings concur with existing literature on cognitive evolution after cochlear implantation and suggest that cochlear implantation is not contraindicated in older adults with lower-than-expected cognitive scores and should be considered after multidisciplinary evaluation.
Abstract
Importance
Given the rapidly rising dementia incidence, management of modifiable risk factors, such as hearing loss, is vital. Multiple studies have demonstrated an improvement of cognitive functioning in older adults with severe hearing loss after cochlear implantation; however, few of these studies, to the authors’ knowledge, specifically analyzed participants achieving poor cognitive results preoperatively.
Objective
To evaluate the cognitive functioning of older adults with severe hearing loss at risk for mild cognitive impairment (MCI) before and after cochlear implantation.
Design, Setting, and Participants
This prospective, longitudinal cohort study performed at a single center reports data obtained over a 6-year period (April 2015 to September 2021) of an ongoing prospective, longitudinal cohort study on cochlear implant outcomes in older adults. A consecutive sample of older adults with severe hearing loss eligible for cochlear implantation was included. All participants obtained a Repeatable Battery for the Assessment of Neuropsychological Status for hearing-impaired patients (RBANS-H) total score indicative of MCI preoperatively. Participants were assessed before cochlear implant activation and 12 months after cochlear implant activation.
Intervention
The intervention consisted of cochlear implantation.
Main Outcome and Measure
The primary outcome measure was cognition, measured by the RBANS-H.
Results
A total of 21 older adult cochlear implant candidates were included in the analysis (mean [SD] age, 72 [9] years; 13 [62%] men). Cochlear implantation was associated with an improvement of overall cognitive functioning 12 months after activation (median [IQR] percentile, 5 [2-8] vs 12 [7-19]; difference, 7 [95% CI, 2-12]). Eight participants (38%) surpassed the MCI cutoff (16th percentile) postoperatively, while the overall median cognitive score remained under this cutoff. In addition, participants’ speech recognition in noise improved, with a lower score indicating improvement (mean [SD] score, +17.16 [5.45] vs +5.67 [6.3]; difference, −11.49 [95% CI, −14.26 to −8.72]), after cochlear implant activation. Improvement of speech recognition in noise was positively associated with improvement in cognitive functioning (rs, −0.48 [95% CI, −0.69 to −0.19]). Years of education, sex, RBANS-H version, and symptoms of depression and anxiety were not related to the evolution in RBANS-H scores.
Conclusions and Relevance
In this prospective, longitudinal cohort study, cognitive functioning and speech perception in noise showed a clinically meaningful improvement 12 months after cochlear implant activation in older adults with severe hearing loss at risk for MCI, suggesting that cochlear implantation is not contraindicated in cochlear implant candidates with cognitive decline and should be considered after multidisciplinary evaluation.
This cohort study evaluates the cognitive functioning of older adults with severe hearing loss at risk for mild cognitive impairment before and after cochlear implantation.
Introduction
Along with the increasing proportion of older adults in the world population, the incidence of dementia is rising rapidly. Worldwide, more than 55 million people are living with dementia, and if no cure or disease-modifying therapy is found, this number is expected to triple by 2050.1,2 The most common cause of dementia is Alzheimer disease (AD), which accounts for up to 70% of all dementia cases.1 Mild cognitive impairment (MCI) is an intermediate state between age-related forgetfulness and dementia. People with MCI can still autonomously perform their activities of daily life, whereas people with dementia cannot. However, within 5 years, more than half of all patients with MCI progress to a dementia syndrome.3 In particular, the amnestic subtype of MCI, characterized by an episodic memory loss, could be a prodromal stage of AD because of its high risk of conversion to dementia (annual conversion rate of approximately 10%-15% as compared with 3%-5% among cognitively healthy older adults).3 However, not every person with MCI will develop AD dementia.4 A diagnosis of MCI is based on medical history, physical examination, neuropsychological assessment, and assessment of performance of activities of daily living. A neuropsychological assessment objectively evaluates the degree of cognitive impairment. People with MCI present with cognitive test scores of 1 to 1.5 SDs below the mean compared with age-appropriate and education-appropriate normative data.5
Identifying its risk and protective factors is essential to prevent MCI and overall cognitive decline at the early stage.6 In addition to age, sex, and education level, hearing loss is also related to cognitive decline and dementia in older adults and has been defined as a modifiable risk factor for dementia.2,7,8 The large prospective longitudinal cohort study by Lin et al7 demonstrated that older adults with hearing loss had a 24% higher risk for incident cognitive impairment and accelerated cognitive decline, which were linearly associated with hearing loss severity. However, recent studies showed that improving hearing function in older adults with hearing loss by using hearing aids and/or a cochlear implant was associated with substantial improvement in cognitive functioning.8,9,10,11,12,13,14,15,16,17 A recent study evaluated cognition using the Repeatable Battery for the Assessment of Neuropsychological Status for hearing-impaired patients (RBANS-H) in 24 older adult cochlear implant users compared with 24 older adults without cochlear implants 1 month before cochlear implantation and at a follow-up moment of 14 months after baseline testing.10 The control group with severe hearing loss was matched for age, gender, education, cognition, and residual hearing. Overall, cochlear implant users demonstrated improved cognitive functioning, particularly in the subdomain of attention. However, these older adults with 1-year cochlear implant experience achieved worse cognitive results compared to adults with normal hearing and, thus, did not bridge the performance gap.10
This prospective longitudinal cohort study aimed to assess the evolution of cognitive performance in older adults with severe hearing loss at risk for MCI after cochlear implantation.18 To our knowledge, only a few studies evaluating cognition in cochlear implant users specifically analyzed those participants whose baseline cognitive scores were lower than expected.11,12,13,17 Therefore, this study only included cochlear implant candidates with poor baseline cognitive scores and aimed to evaluate their cognitive function and speech perception in noise after a follow-up period of 12 months.
Methods
Ethics
This prospective, longitudinal cohort study was carried out in conformity with the recommendations of the ethics committee of the Antwerp University Hospital (protocol No. 15/17/181). All participants gave their written informed consent per the Declaration of Helsinki before participation. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline was followed. The study protocol was retrospectively registered at ClinicalTrials.gov on June 9, 2016 (NCT02794350), and was described in detail in the study protocol by Claes et al.18
Participants
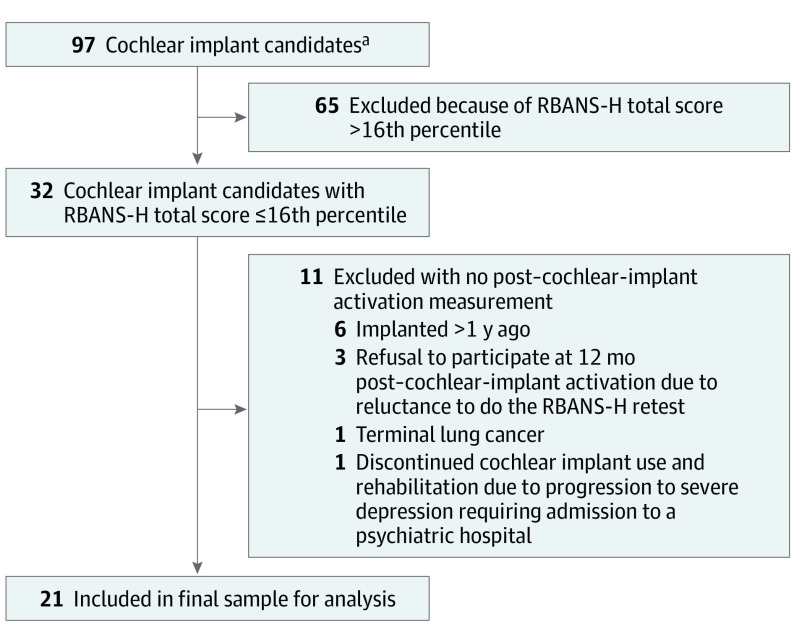
This prospective, longitudinal cohort study highlights part of the cognitive functioning data of a larger study on cochlear implant outcomes in older adults, conducted during a 6-year period (April 2015 to September 2021) at the Antwerp University Hospital in Belgium. A consecutive sample of cochlear implant candidates1 with postlingual, bilateral, severe-to-profound hearing loss2; aged 55 years or older3; receiving a unilateral cochlear implant per the Belgian national reimbursement criteria at the time of enrollment4; and obtaining an RBANS-H total score below or equal to 1 SD below the mean compared with age-appropriate normative data (≤16th percentile) were included in the analysis. All participants underwent routine preoperative brain magnetic resonance imaging, and no abnormalities were detected. The RBANS-H cutoff score was based on the guidelines of Albert et al5 on MCI diagnosis. The age cutoff of 55 years and older was used considering that 55 years was the youngest mean age in which hearing loss presence has been associated with an increased risk of dementia.19 Participants were excluded if they could not complete the study protocol due to additional impairments,1 if they were diagnosed with any neurological disease prior to implantation,2 and if their Dutch language skills were inadequate.3 The activation of the speech processor took place approximately 4 weeks postoperatively and its settings were optimized during regular programming sessions. All participants underwent a thorough multidisciplinary evaluation before implantation, including comprehensive counseling about their expectations toward the cochlear implant outcomes and the rehabilitation process. Participants were assessed 1 month preoperatively and 12 months after the activation of the speech processor.
Cognitive Assessment
The primary outcome measure of this study was the change in overall cognitive functioning, measured using the RBANS-H, which compared 2 test moments (preoperatively vs 12 months after cochlear implant activation). The RBANS-H consists of 12 subtests, each contributing to 1 of the following 5 cognitive subdomains: immediate memory, attention, language, visuospatial/constructional, and delayed memory. This cognitive test battery is a modified version of the original RBANS and was developed to assess cognitive functioning in individuals with hearing loss.18,20 In addition to the original RBANS, the RBANS-H includes a slide presentation to visually show written instructions on an external screen along with the standard oral instructions. In 4 of the 12 subtests (list learning, story memory, digit span, and list recognition) simultaneous visual and auditory stimulation is provided in the RBANS-H, while these stimuli are presented only orally in the original RBANS. The subdomains immediate memory, visuospatial/constructional, attention, and language each comprise 2 subtests, of which the raw scores are converted to an index score per cognitive domain. The raw scores of the subtests, list recall, story recall, and figure recall, are summed and combined with the raw score for the list recognition subtest to obtain the total raw score for the delayed memory subdomain, which is also converted to an index score. The index scores of the 5 cognitive domains are combined to derive the RBANS-H total score and total percentile. The subdomain and total scores are age-corrected standard scores, scaled to a normal distribution with a mean of 100 and a standard deviation of 15. The RBANS-H has alternate forms A and B, which were used in this study.
Anxiety and Depression Symptoms Identification
Anxiety and depression symptoms were identified using the Hospital Anxiety and Depression Scale (HADS), a reliable, valid, and commonly used self-assessment questionnaire designed for clinical practice.21,22 The HADS consists of the following 2 subscales, anxiety (HADS-A, 7 items) and depression (HADS-D, 7 items). The HADS scores are categorized as normal (0-7), borderline (8-10), or indicative of a mood disorder (≥11). Participants completed the questionnaire preoperatively and 12 months after the audio processor activation at a routine clinic visit, via email, or via mail.
Speech Recognition in Noise Assessment
Speech recognition in noise was assessed by means of the Leuven Intelligibility Sentences Test (LIST).23 The LIST was performed according to current clinical standards (ISO 8253-1) in a soundproof cabin with a loudspeaker in front of the participant at a 1-m distance in best-aided condition. Participants were instructed to repeat the speech stimuli they heard. The noise level of the LIST was fixed at 65 dB sound pressure level (SPL) and the speech level was adapted depending on the participant’s responses. If participants were able to identify all bold keywords of the current sentence correctly, the speech level of the following sentence was reduced by 2 dB SPL. If not, the speech level of the next sentence was increased by 2 dB SPL. The speech reception threshold (SRT) was calculated by averaging the level of the last 5 sentences and the level of the imaginary 11th sentence on the list. Lower scores on this test indicate better performance.
Statistical Analysis
The statistical analysis was performed using IBM SPSS Statistics software, version 27 (IBM Corporation). A linear mixed-effects model was applied to determine cognitive evolution over time with the RBANS-H total score (primary outcome measure) and each subdomain score (secondary outcome measures) as outcome variable; participant identification as random intercept; and time point (preoperatively/12 months after the activation of the speech processor), sex (male or female), RBANS version (version A/version B preoperatively), HADS-A, HADS-D, and years of education as fixed factors. The final linear mixed-effects model included only time point (preoperatively/12 months after the activation of the speech processor) after backward selection. Post hoc pairwise comparisons between the preoperative and postoperative measurements were performed to identify clinically meaningful effects. To explore the association of hearing restoration with RBANS-H evolution within participants, difference scores for SRT in noise and RBANS-H total and subdomain scores were calculated by subtracting the preoperative score from the postoperative score, and the Spearman rank correlation test was used to study the correlation between these difference scores. The 1-sided significance threshold was assessed at P < .05.
Results
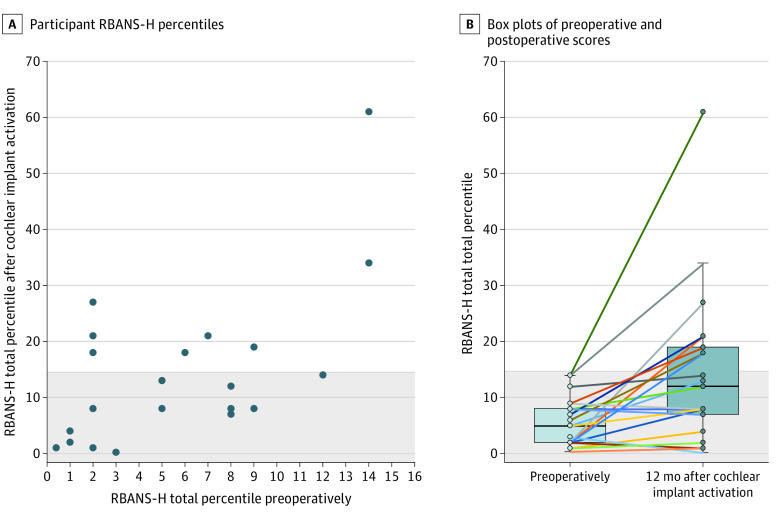
A total of 21 older adult cochlear implant candidates were included in the analysis (mean [SD] age, 72 [9] years; 13 [62%] men) (Figure 1, Table24). The majority of the participants (n = 16) showed improvement in RBANS-H total percentile 12 months after cochlear implant activation. In 1 participant the RBANS-H percentile remained stable, and in 4 participants, the percentile decreased (Figure 25). The linear mixed-effects model revealed that only time points (ie, preoperative vs 12 months after cochlear implant activation) had a clinically meaningful association with the RBANS-H total score (F [1, 20] = 8.58; P = .01; effect size, 0.6 [95% CI, 0.16-1.1]), while HADS-A, HADS-D, sex, years of education, and RBANS-H version did not. Time points also contributed to the linear mixed-effects model for the RBANS-H subdomains, immediate memory (F [1, 20] = 5.13; P = .04; effect size, 0.5 [95% CI, 0.04-0.94]) and delayed memory (F [1, 20] = 8.16; P = .01; effect size, 0.6 [95% CI, 0.15-1.1]). Years of education, sex, RBANS-H version, HADS-D, and HADS-A did not contribute to the model for any RBANS-H subdomain scores. Of all pairwise comparisons between the preoperative and postoperative measurement, the RBANS-H total score (median [IQR] percentile, 5 [2-8] vs 12 [7-19]; difference 7 [95% CI, 2-12]) and the subdomains immediate memory (median [IQR] score, 81 [73-85.5] vs 90 [84.5-100]; difference, 9 [95% CI, 2.29-15.71]) and delayed memory (median [IQR] score, 84 [77.25-94.25] vs 92.5 [84-99.5]; difference, 8.5 [95% CI, 2.59-14.41]) were clinically meaningful. Furthermore, before implantation, all 21 participants were at risk for MCI with RBANS-H total scores of no more than the 16th percentile. At 12 months after cochlear implant activation, 8 out of 21 improved, reaching a score greater than the 16th percentile (difference in proportion, 38% [95% CI, 20%-59%]). The SRT in noise improved after cochlear implantation (mean [SD] score, +17.16 [5.45] vs +5.67 [6.3]; difference, −11.49 [95% CI, −14.26 to −8.72]). Additionally, there was a negative correlation (rs, −0.48 [95% CI, −0.69 to −0.19]) between SRT in noise and RBANS-H difference scores (after cochlear implant activation vs preoperatively). The HADS-D scores improved after implantation (mean [SD] score, 8 [3] vs 5 [4]; difference, 3 [95% CI, 1-5]), whereas HADS-A scores remained stable.
Figure 1. Flowchart of the Study Population.
RBANS-H indicates Repeatable Battery for the Assessment of Neuropsychological Status for hearing-impaired patients.
aThese participants were included in a more extensive prospective study on cognitive evolution in older cochlear implant users at the Antwerp University Hospital (NCT02794350).
Table. Overview of the Participants’ Characteristics at Baseline (Preoperatively).
| Variable | No. (%) |
|---|---|
| No. | 21 |
| Age, mean (SD), y | 72 (9) |
| Formal education, mean (SD), ya | 10.0 (2.4) |
| Residual hearing best ear, mean (SD), %b | 16 (10) |
| Unaided PTA best ear, mean (SD), dBc | 94 (16) |
| HADS-A score, mean (SD) | 7 (3) |
| HADS-D score, mean (SD) | 8 (3) |
| Best-aided LIST score, mean (SD), dB SNR | +17.16 (5.45) |
| Sex | |
| Male | 13 (62) |
| Female | 8 (38) |
| Caused | |
| Hereditary | 6 (14) |
| Meningitis | 4 (10) |
| Noise induced | 2 (5) |
| Otosclerosis | 2 (5) |
| Trauma | 3 (7) |
| Unknown | 25 (60) |
| Ear implanted | |
| Right | 12 (57) |
| Left | 9 (43) |
| Retired | |
| Yes | 19 (90) |
| No | 2 (10) |
| Hearing aid use | |
| Yes | 16 (76) |
| No | 5 (24) |
Abbreviations: HADS-A, Hospital Anxiety and Depression Scale–Anxiety; HADS-D, Hospital Anxiety and Depression Scale–Depression; LIST, Leuven Intelligibility Sentences Test; PTA, pure tone average; SNR, signal-to-noise ratio.
Formal education was counted starting from the age of 6 years.
The percentage of residual hearing was calculated based on the Hearing Preservation Classification System of Skarzynski et al.24
The PTA refers to the average of pure tone hearing thresholds at 500 Hz, 1000 Hz, and 2000 Hz.
Cause was determined per ear, so n = 42 for this category.
Figure 2. Overview of the Cognitive Score Percentiles Preoperatively and 12 Months After Cochlear Implant Activation.
A, The cutoff value of the 16th percentile (ie, 1 standard deviation below the mean) was chosen based on the guidelines by Albert et al5 on the diagnosis of mild cognitive impairment. B, The preoperative mean RBANS-H total percentile was 6. The postoperative mean RBANS-H total percentile was 15. The difference between preoperative and postoperative mean RBANS-H total percentile was 9 (Cohen d = 0.6). The horizontal bar inside the box indicates the median; the lower and upper ends of the boxes, the IQR; and the whiskers, values within 1.5-times the IQR above the upper quartile and below the lower quartile. The colored lines in the box plot connect the preoperative score to the postoperative score for each participant.
Discussion
The results of this prospective, longitudinal cohort study suggest an association between hearing rehabilitation using cochlear implantation and improvements in cognitive functioning 12 months after cochlear implant activation in adults aged 55 years and older at risk for MCI. This finding concurs with previous longitudinal studies showing cognitive improvement after cochlear implantation in a general population of older adult cochlear implant users and with the findings of Mosnier et al11 demonstrating an improvement of cognitive abilities as early as 6 months after implantation in older adult cochlear implant users with abnormal cognitive test scores at baseline.9,10,11,12,13,14,15,16,17 In our study, we used the RBANS-H as a cognitive assessment tool, providing audiovisual presentation of the instructions and test items, to avoid individuals not performing well on the test due to their hearing loss and not due to cognitive decline. Still, overall improvement of cognitive scores was associated with better speech perception in noise in this study, supporting the information degradation hypothesis proposed in the review by Wayne and Johnsrude25 in 2015 as a potential explanation for the link between hearing loss and cognition. This hypothesis states that older adults with hearing loss need to rely more on cognitive resources to compensate for impaired auditory input, resulting in more mental fatigue and a higher cognitive load, which leads to a reduction of cognitive resources available for other cognitive tasks.25,26 Hence, by partially restoring hearing function using a cochlear implant, listening effort decreases and more cognitive resources become available, improving cognition even in individuals with low baseline cognitive scores.
As Mosnier et al12 suggested, one could hypothesize that there may be a specific subtype of MCI primarily associated with diminished cognitive reserves due to the impoverished perceptual input caused by severe-to-profound hearing loss. Mosnier et al12 found that cochlear implant users with MCI preoperatively had a low rate of progression to dementia over 7 years as opposed to the high risk of progression to dementia in untreated severe-to-profound hearing loss. Our study confirmed that older adults at risk for MCI before implantation demonstrated improved cognitive function 12 months after the activation of the audio processor rather than a decline and potential further deterioration to dementia. Furthermore, speech perception in noise showed a clinically meaningful improvement as well after cochlear implantation in this study’s cochlear implant users at risk for MCI, indicating that a cochlear implant also benefits individuals with severe hearing loss and poor cognitive scores preoperatively. Additionally, several studies indicated that individuals with poor preoperative cognitive performance showed a greater benefit from cochlear implantation compared with their peers with better cognitive abilities before implantation.11,13,17 These findings highlight that cochlear implantation is not contraindicated in individuals with severe to profound hearing loss and cognitive decline. Moreover, as there is currently no disease-modifying cure available for dementia, management of modifiable risk factors for cognitive decline, such as hearing loss, is vital for the affected or at-risk individuals and their families but also for the economy, considering that dementia yearly costs approximately 1.3 trillion US dollars globally.2,8,27 Proposing a cochlear implant to candidates with cognitive decline (if not diagnosed with dementia) should therefore be considered after multidisciplinary evaluation, and cochlear implantation should not be unnecessarily delayed in these individuals.
Auditory rehabilitation after cochlear implantation is a complex and multidisciplinary process, involving multiple audio processor fitting appointments, speech therapy, psychological counseling, and more, particularly during the first 12 months after implantation. Hence, cochlear implant users receive more cognitive and social stimulation during this period, which might positively influence their cognitive skills, communication abilities, and emotional well-being.13,28 The improvement of depressive symptoms 12 months after cochlear implantation could be attributed to the combination of improved speech perception through cochlear implantation and psychological counseling during auditory rehabilitation.29 Interestingly, depressive symptoms were not associated with cognitive abilities in this study’s cochlear implant users at risk for MCI, although a link between depression and cognitive decline was reported in several extensive studies.2,8,30,31 This inconsistency may be due to the majority of the cochlear implant users in this study sample not showing depressive symptoms at baseline (HADS score, <8), making the influence of depression on cognitive scores loss less likely. Additionally, the HADS is a screening instrument and cannot provide a definite diagnosis of depression or anxiety disorders.
A major strength of this study is the use of the RBANS-H in this population. Given the negative influence of hearing loss on the perception and understanding of auditory-only instructions and stimuli, visual presentation during cognitive testing of individuals with hearing loss is crucial to obtaining reliable results in this population.32,33 Therefore, the RBANS-H, which provides visual support to participants via slide presentation, was used in this study. The RBANS-H allows for differentiation in a wide range from normal to moderately severe cognitive impairment, in contrast to cognitive screening test instruments, such as the Hearing-impaired Montreal Cognitive Assessment,34 which are less sensitive to change. A large study by Duff et al35 showed that practice effects, defined as improvements in cognitive test performance due to repeated evaluation with the same test materials, were largely absent when assessing older adults with the original RBANS across a 1-year retest interval. To further limit practice effects in this study, 2 alternate versions of the RBANS-H were used and the retest interval was approximately 13 months. However, we could not entirely eliminate practice effects due to the absence of a control group, which is the first limitation of this study.
Limitations
Enrolling an untreated matched control group, thereby applying randomization, would have been the most appropriate to rule out risks of bias, but this was not possible due to ethical reasons. This is a common limitation in studies in this field, and so far, to our knowledge, only Mertens et al10 succeeded in enrolling a matched control group; however, they did not focus on cochlear implant users with poor cognitive scores at baseline. A second limitation of our study concerns the fact that extraneous medical conditions, including urinary tract infection, subdural hematoma, metabolic disorders, hypoglycemia, hypothyroidism, medication, infections, dehydration, pain, and depression, could have potentially affected cognitive performance of the study participants. Of these, only depression was systematically evaluated, though all these potential variables take on more importance due to the small sample size and lack of a control group in this study. A third limitation of this study is its small sample size of 21 participants, leading to wide CIs that caused the effect sizes to be imprecise which limits drawing definite conclusions from these data. Further longitudinal research including a larger sample of cochlear implant candidates with cognitive decline is therefore recommended. Lastly, we did not include biomarkers in the diagnostic process regarding cognitive decline, nor did we perform a comprehensive neuropsychological assessment. These assessments would have provided an objective and more specific diagnosis of the degree of cognitive impairment, which would be interesting in further research evaluating cognitive evolution in cochlear implant users with cognitive decline. In addition, the use of biomarkers for AD would allow for research in a more homogeneous MCI population (eg, in prodromal AD) in future studies.
Conclusions
In this prospective, longitudinal cohort study, the cognitive abilities of adults aged 55 years and older with severe hearing loss and at risk for MCI showed a clinically meaningful improvement 12 months after cochlear implant activation. Of the 21 patients included in this study, 8 surpassed the MCI cutoff for initial inclusion (16th percentile) 12 months after cochlear implant activation, but, overall, the median postoperative cognitive score remained below this cutoff. In addition, cochlear implantation was positively associated with participants’ speech perception. Therefore, cochlear implantation should be considered in older adults with lower-than-expected cognitive scores and should not be unnecessarily delayed in these at-risk individuals.
Data Sharing Statement
References
- 1.World Health Organization . Risk reduction of cognitive decline and dementia: WHO guidelines. January 2019. Accessed January 10, 2023. https://www.who.int/publications/i/item/9789241550543 [PubMed]
- 2.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673-2734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 3.Bruscoli M, Lovestone S. Is MCI really just early dementia: a systematic review of conversion studies. Int Psychogeriatr. 2004;16(2):129-140. doi: 10.1017/S1041610204000092 [DOI] [PubMed] [Google Scholar]
- 4.Gauthier S, Reisberg B, Zaudig M, et al. ; International Psychogeriatric Association Expert Conference on Mild Cognitive Impairment . Mild cognitive impairment. Lancet. 2006;367(9518):1262-1270. doi: 10.1016/S0140-6736(06)68542-5 [DOI] [PubMed] [Google Scholar]
- 5.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J-H, Lin K-P, Chen Y-C. Risk factors for dementia. J Formos Med Assoc. 2009;108(10):754-764. doi: 10.1016/S0929-6646(09)60402-2 [DOI] [PubMed] [Google Scholar]
- 7.Lin FR, Yaffe K, Xia J, et al. ; Health ABC Study Group . Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293-299. doi: 10.1001/jamainternmed.2013.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claes AJ, Van de Heyning P, Gilles A, Van Rompaey V, Mertens G. Cognitive performance of severely hearing-impaired older adults before and after cochlear implantation: preliminary results of a prospective, longitudinal cohort study using the RBANS-H. Otol Neurotol. 2018;39(9):e765-e773. doi: 10.1097/MAO.0000000000001936 [DOI] [PubMed] [Google Scholar]
- 10.Mertens G, Andries E, Claes AJ, et al. Cognitive improvement after cochlear implantation in older adults with severe or profound hearing impairment: a prospective, longitudinal, controlled, multicenter study. Ear Hear. 2021;42(3):606-614. doi: 10.1097/AUD.0000000000000962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosnier I, Bebear JP, Marx M, et al. Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngol Head Neck Surg. 2015;141(5):442-450. doi: 10.1001/jamaoto.2015.129 [DOI] [PubMed] [Google Scholar]
- 12.Mosnier I, Vanier A, Bonnard D, et al. Long-term cognitive prognosis of profoundly deaf older adults after hearing rehabilitation using cochlear implants. J Am Geriatr Soc. 2018;66(8):1553-1561. doi: 10.1111/jgs.15445 [DOI] [PubMed] [Google Scholar]
- 13.Völter C, Götze L, Bajewski M, Dazert S, Thomas JP. Cognition and cognitive reserve in cochlear implant recipients. Front Aging Neurosci. 2022;14:838214. doi: 10.3389/fnagi.2022.838214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Völter C, Götze L, Dazert S, Falkenstein M, Thomas JP. Can cochlear implantation improve neurocognition in the aging population? Clin Interv Aging. 2018;13:701-712. doi: 10.2147/CIA.S160517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarant J, Harris D, Busby P, et al. The effect of cochlear implants on cognitive function in older adults: initial baseline and 18-month follow up results for a prospective international longitudinal study. Front Neurosci. 2019;13:789. doi: 10.3389/fnins.2019.00789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayakody DMP, Friedland PL, Nel E, Martins RN, Atlas MD, Sohrabi HR. Impact of cochlear implantation on cognitive functions of older adults: pilot test results. Otol Neurotol. 2017;38(8):e289-e295. doi: 10.1097/MAO.0000000000001502 [DOI] [PubMed] [Google Scholar]
- 17.Zhan KY, Lewis JH, Vasil KJ, et al. Cognitive functions in adults receiving cochlear implants: predictors of speech recognition and changes after implantation. Otol Neurotol. 2020;41(3):e322-e329. doi: 10.1097/MAO.0000000000002544 [DOI] [PubMed] [Google Scholar]
- 18.Claes AJ, Mertens G, Gilles A, et al. The Repeatable Battery for the Assessment of Neuropsychological Status for Hearing Impaired Individuals (RBANS-H) before and after cochlear implantation: a protocol for a prospective, longitudinal cohort study. Front Neurosci. 2016;10:512. doi: 10.3389/fnins.2016.00512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallacher J, Ilubaera V, Ben-Shlomo Y, et al. Auditory threshold, phonologic demand, and incident dementia. Neurology. 2012;79(15):1583-1590. doi: 10.1212/WNL.0b013e31826e263d [DOI] [PubMed] [Google Scholar]
- 20.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310-319. doi: 10.1076/jcen.20.3.310.823 [DOI] [PubMed] [Google Scholar]
- 21.Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. 2003;1:29. doi: 10.1186/1477-7525-1-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27(2):363-370. doi: 10.1017/S0033291796004382 [DOI] [PubMed] [Google Scholar]
- 23.van Wieringen A, Wouters J. LIST and LINT: sentences and numbers for quantifying speech understanding in severely impaired listeners for Flanders and the Netherlands. Int J Audiol. 2008;47(6):348-355. doi: 10.1080/14992020801895144 [DOI] [PubMed] [Google Scholar]
- 24.Skarzynski H, van de Heyning P, Agrawal S, et al. Towards a consensus on a hearing preservation classification system. Acta Otolaryngol Suppl. 2013;(564):3-13. doi: 10.3109/00016489.2013.869059 [DOI] [PubMed] [Google Scholar]
- 25.Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev. 2015;23(Pt B):154-166. doi: 10.1016/j.arr.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 26.Pichora-Fuller MK, Kramer SE, Eckert MA, et al. Hearing impairment and cognitive energy: the Framework for Understanding Effortful Listening (FUEL). Ear Hear. 2016;37(suppl 1):5S-27S. doi: 10.1097/AUD.0000000000000312 [DOI] [PubMed] [Google Scholar]
- 27.Gauthier S, Rosa-Neto P, Morais J, Webster C. World Alzheimer Report 2021: Journey Through the Diagnosis of Dementia. Alzheimer's Disease International; 2021. [Google Scholar]
- 28.Andries E, Gilles A, Topsakal V, et al. Systematic review of quality of life assessments after cochlear implantation in older adults. Audiol Neurootol. 2021;26(2):61-75. doi: 10.1159/000508433 [DOI] [PubMed] [Google Scholar]
- 29.Heinze-Köhler K, Lehmann EK, Hoppe U. Depressive symptoms affect short- and long-term speech recognition outcome in cochlear implant users. Eur Arch Otorhinolaryngol. 2021;278(2):345-351. doi: 10.1007/s00405-020-06096-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly ME, Duff H, Kelly S, et al. The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: a systematic review. Syst Rev. 2017;6(1):259. doi: 10.1186/s13643-017-0632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartels C, Wagner M, Wolfsgruber S, Ehrenreich H, Schneider A; Alzheimer’s Disease Neuroimaging Initiative . Impact of SSRI therapy on risk of conversion from mild cognitive impairment to Alzheimer’s dementia in individuals with previous depression. Am J Psychiatry. 2018;175(3):232-241. doi: 10.1176/appi.ajp.2017.17040404 [DOI] [PubMed] [Google Scholar]
- 32.Claes AJ, Van de Heyning P, Gilles A, Van Rompaey V, Mertens G. Cognitive outcomes after cochlear implantation in older adults: a systematic review. Cochlear Implants Int. 2018;19(5):239-254. doi: 10.1080/14670100.2018.1484328 [DOI] [PubMed] [Google Scholar]
- 33.Andries E, Van Rompaey V, Van de Heyning P, Mertens G. Commentary: assessing cognitive abilities in high-performing cochlear implant users. Front Neurosci. 2019;13:564. doi: 10.3389/fnins.2019.00564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin VY, Chung J, Callahan BL, et al. Development of cognitive screening test for the severely hearing impaired: Hearing-impaired MoCA. Laryngoscope. 2017;127(suppl 1):S4-S11. doi: 10.1002/lary.26590 [DOI] [PubMed] [Google Scholar]
- 35.Duff K, Beglinger LJ, Schoenberg MR, et al. Test-retest stability and practice effects of the RBANS in a community dwelling elderly sample. J Clin Exp Neuropsychol. 2005;27(5):565-575. doi: 10.1080/13803390490918363 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Sharing Statement