This analysis of pooled data from 3 randomized clinical trials evaluates the association between immune-related adverse events and atezolizumab efficacy in patients with advanced non–small cell lung cancer.
Key Points
Question
What is the association between immune-related adverse events (irAEs) and atezolizumab efficacy in advanced nonsquamous non–small cell lung cancer (NSCLC)?
Findings
Post hoc analyses of 3 pooled phase 3 randomized clinical trials of atezolizumab-chemoimmunotherapy combinations including 2503 patients with NSCLC showed that generally, overall survival was longer in patients with low-grade irAEs than without in the atezolizumab-containing and control arms. Grade 1 to 2 irAEs with atezolizumab combinations were generally associated with improved overall survival vs grade 3 to 5 irAEs.
Meaning
These findings suggested an association between low-grade irAEs and improved survival, particularly with atezolizumab, further supporting the use of first-line atezolizumab-containing regimens for advanced NSCLC.
Abstract
Importance
Immune-related adverse events (irAEs) arising from immune checkpoint inhibitor (ICI) cancer therapy may potentially predict improved outcomes.
Objective
To evaluate the association between irAEs and atezolizumab efficacy in patients with advanced non–small cell lung cancer (NSCLC) using pooled data from 3 phase 3 ICI studies.
Design, Setting, and Participants
IMpower130, IMpower132, and IMpower150 were phase 3, multicenter, open-label, randomized clinical trials to evaluate the efficacy and safety of chemoimmunotherapy combinations involving atezolizumab. Participants were chemotherapy-naive adults with stage IV nonsquamous NSCLC. These post hoc analyses were conducted during February 2022.
Interventions
Eligible patients were randomly assigned 2:1 to receive atezolizumab with carboplatin plus nab-paclitaxel, or chemotherapy alone (IMpower130); 1:1 to receive atezolizumab with carboplatin or cisplatin plus pemetrexed, or chemotherapy alone (IMpower132); and 1:1:1 to receive atezolizumab plus bevacizumab plus carboplatin and paclitaxel, atezolizumab plus carboplatin and paclitaxel, or bevacizumab plus carboplatin and paclitaxel (IMpower150).
Main Outcomes and Measures
Pooled data from IMpower130 (cutoff: March 15, 2018), IMpower132 (cutoff: May 22, 2018), and IMpower150 (cutoff: September 13, 2019) were analyzed by treatment (atezolizumab-containing vs control), irAE status (with vs without), and highest irAE grade (1-2 vs 3-5). To account for immortal bias, a time-dependent Cox model and landmark analyses of irAE occurrence at 1, 3, 6, and 12 months from baseline were used to estimate the hazard ratio (HR) of overall survival (OS).
Results
Of 2503 randomized patients, 1577 were in the atezolizumab-containing arm and 926 were in the control arm. The mean (SD) age of patients was 63.1 (9.4) years and 63.0 (9.3) years, and 950 (60.2%) and 569 (61.4%) were male, respectively, in the atezolizumab arm and the control arm. Baseline characteristics were generally balanced between patients with irAEs (atezolizumab, n = 753; control, n = 289) and without (atezolizumab, n = 824; control, n = 637). In the atezolizumab arm, OS HRs (95% CI) in patients with grade 1 to 2 irAEs and grade 3 to 5 irAEs (each vs those without irAEs) in the 1-, 3-, 6-, and 12-month subgroups were 0.78 (0.65-0.94) and 1.25 (0.90-1.72), 0.74 (0.63-0.87) and 1.23 (0.93-1.64), 0.77 (0.65-0.90) and 1.1 (0.81-1.42), and 0.72 (0.59-0.89) and 0.87 (0.61-1.25), respectively.
Conclusions and Relevance
In this pooled analysis of 3 randomized clinical trials, longer OS was observed in patients with vs without mild to moderate irAEs in both arms and across landmarks. These findings further support the use of first-line atezolizumab-containing regimens for advanced nonsquamous NSCLC.
Trial Registration
ClinicalTrials.gov Identifiers: NCT02367781, NCT02657434, and NCT02366143
Introduction
Immune checkpoint inhibitors (ICIs) increase antitumor activity by blocking downregulators of the immune system and have transformed the treatment and prognosis of lung cancer.1,2 However, ICIs are associated with off-target immune and inflammatory adverse effects known as immune-related adverse events (irAEs), which can affect multiple organ systems. The irAE profile, incidence, and time to onset vary among ICIs.3,4 Typically, irAEs tend to be mild to moderate and manageable by steroids, but severe, clinically relevant irAEs can also occur.5,6
Evidence suggests that irAEs could be associated with better ICI efficacy in advanced malignant neoplasms, including non–small cell lung cancer (NSCLC).7,8,9,10,11,12,13,14 Patients who discontinue ICI treatment due to irAEs have continued to experience treatment benefit,15 suggesting a potentially mechanistic association between irAEs and ICI efficacy, even with relatively short exposure.16 Exploratory, retrospective analyses of studies with anti–programmed cell death 1 (PD-1) agents nivolumab and pembrolizumab in NSCLC showed longer progression-free survival (PFS) among patients who experienced irAEs than in those who did not,16,17,18,19,20,21,22,23 and other ICI studies showed that overall survival (OS) was longer in patients with irAEs than in those without.17,18,20 Further evaluation of irAE occurrence as a predictive clinical biomarker for ICI response in NSCLC is warranted.
The PD-1 ligand 1 (PD-L1) inhibitor atezolizumab has demonstrated efficacy and safety alone and in combination with chemotherapy with or without bevacizumab in NSCLC.23,24,25,26,27,28,29,30,31 The approval of chemoimmunotherapy combinations involving atezolizumab for first-line treatment of advanced nonsquamous NSCLC were based on the phase 3 IMpower13028 and IMpower15027 trials, which met their coprimary end points of OS and investigator-assessed PFS. Numerical improvement in median OS was observed in the experimental vs control arm in IMpower132.31
In IMpower130, IMpower132, and IMpower150, the incidence of irAEs ranged from 45% to 60% in the atezolizumab-containing arms.27,28,31 The potential of irAEs to predict atezolizumab efficacy will prove useful for the management of NSCLC and may help improve treatment adherence. In this post hoc exploratory analysis, we evaluated the association between irAEs and atezolizumab efficacy using pooled data from IMpower130, IMpower132, and IMpower150.
Methods
Trial Designs, Patients, and Oversight
IMpower130 (NCT02367781), IMpower132 (NCT02657434), and IMpower150 (NCT02366143) were phase 3, multicenter, open-label, randomized clinical trials evaluating the safety and efficacy of chemoimmunotherapy combinations involving atezolizumab in advanced nonsquamous NSCLC (trial protocols in Supplements 1, 2, and 3, respectively). Their study designs and methods have been reported previously according to Consolidated Standards of Reporting Trials (CONSORT) guidelines.27,28,31 Briefly, eligible patients were chemotherapy-naive adults with stage IV nonsquamous NSCLC, Eastern Cooperative Oncology Group performance status (ECOG PS) 0 or 1, any PD-L1 status, and no prior treatment for metastatic disease. In IMpower130 and IMpower150, patients with EGFR/ALK genomic alterations must have had disease progression or experienced unacceptable adverse effects from 1 or more approved tyrosine kinase inhibitors. IMpower132 excluded patients with EGFR/ALK alterations.
These trials were conducted in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. The study protocols were approved by independent ethics committees or institutional review boards at each site. All patients provided written informed consent.
Treatment and Assessments
In IMpower130, patients were randomly assigned 2:1 to receive atezolizumab plus chemotherapy (carboplatin plus nab-paclitaxel) or chemotherapy alone, followed by maintenance treatment with atezolizumab (atezolizumab-containing arm) or best supportive care or pemetrexed (chemotherapy arm), until loss of clinical benefit or disease progression, respectively.
Patients in IMpower132 were randomly assigned 1:1 to receive atezolizumab plus chemotherapy (cisplatin or carboplatin plus pemetrexed) or chemotherapy alone, followed by maintenance therapy with atezolizumab plus pemetrexed (atezolizumab-containing arm) or pemetrexed (chemotherapy arm) until disease progression, unacceptable toxic effects, or death.
In IMpower150, patients were randomly assigned 1:1:1 to receive atezolizumab plus bevacizumab plus carboplatin and paclitaxel, atezolizumab plus carboplatin and paclitaxel, or bevacizumab plus carboplatin and paclitaxel, followed by maintenance therapy with atezolizumab plus bevacizumab, atezolizumab, or bevacizumab in the respective arms. Previously described27,28,31 dosages, regimens, stratification factors, and end points are summarized in the eMethods in Supplement 4.
Adverse events were assessed per National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Assessments are described in eMethods in Supplement 4.
Outcomes
The association between irAEs and atezolizumab efficacy was analyzed as follows. Immune-related AEs in each arm were summarized by severity. For ease of comparison, the common term irAE was used, irrespective of the study arm, to describe overlapping toxic effects that have a known immune-based mechanism. The study sponsor defined irAEs as diagnosed immune conditions, or signs and symptoms potentially representative of immune-related events regardless of investigator-assessed causality. The irAEs are medical concepts defined using the Medical Dictionary for Regulatory Activities. This post hoc pooled analysis was based on predefined irAE medical concept “baskets.” Of note, medical events related to disease progression were not reportable as AEs per study protocols and were thus not included in the analysis. Infusion-related reactions are not viewed as true immune-mediated events but are included among the AEs of special interest for atezolizumab and were thus routinely analyzed together with irAEs by the sponsor.
Efficacy, measured by OS, was analyzed by treatment (atezolizumab-containing vs control) in relation to irAE occurrence status, highest irAE grade (grade 1-2 vs grade 3-5), and landmark subgroup (irAE occurrence status 1, 3, 6, and 12 months from treatment initiation). Efficacy by confirmed objective response rates (ORR) and duration of response were analyzed by irAE occurrence status (with vs without irAEs).
Statistical Analysis
For these analyses, data from all 3 studies were pooled (data cutoffs: March 15, 2018 [IMpower130], May 22, 2018 [IMpower132], September 13, 2019 [IMpower150]). Demographic and baseline characteristics were summarized for the intention-to-treat (ITT) population; other analyses included patients who received 1 or more doses of study treatment. For OS, data for patients not reported as having died, including those lost to follow-up, were censored on the date they were last known to be alive. Immortal bias in the OS analyses was adjusted using the following methods: (1) a time-dependent Cox model for estimating the OS hazard ratio (HR [95% CI]), comparing patients with and without irAEs in the atezolizumab-containing and control arms, respectively; the first irAE onset date was used as the time-dependent variable for each patient in the model; and (2) landmark analyses, in which median OS and the corresponding HRs were assessed for patients with vs without irAEs in the atezolizumab-containing and control arms for each landmark subgroup (1, 3, 6, and 12 months), and by severity and irAE status in the atezolizumab-containing arm. The Kaplan-Meier method was used to estimate the median OS, and the Brookmeyer-Crowley method was used to construct the 95% CIs for median OS. In the landmark analyses, OS comparisons were conducted based on a 2-sided unstratified log-rank test at the significance level of .05. SAS, version 9.4 (SAS Institute) was used for the statistical analyses.
Results
Patients and Summary of irAEs
Overall, 2503 patients were included in the analyses of demographic and baseline characteristics: 1577 in the atezolizumab-containing arm and 926 in the control arm (eFigure 1 in Supplement 4). The mean (SD) age of patients was 63.1 (9.4) years and 63.0 (9.3) years, and 950 (60.2%) and 569 (61.4%) were male, respectively, in the atezolizumab arm and the control arm. In both arms, baseline characteristics were generally balanced between patients with irAEs (753 in the atezolizumab-containing arm and 289 in the control arm) and those without irAEs (824 and 637 in the respective arms) (eTable 1 in Supplement 4). Baseline characteristics were generally balanced between arms regardless of irAE status. Treatment disposition by irAE severity is summarized in eTable 2 in Supplement 4, and atezolizumab exposure in patients with grade 3 to 4 irAEs by landmark subgroup is summarized in eTable 3 in Supplement 4.
Any-grade irAEs occurred in 753 (48%) patients in the atezolizumab-containing arm and 289 (32%) in the control arm; these were grade 3 to 5 in 174 (11%) and 45 (5%) patients, respectively; most were grade 1 or 2 (Table 1). The most common irAEs in the atezolizumab-containing vs control arms were rash (in 435 patients [28%] vs 160 [18%]), hepatitis (including laboratory abnormalities and diagnosis; 226 [15%] vs 92 [10%]), and hypothyroidism (192 [12%] vs 33 [4%]).
Table 1. Summary of Immune-Related Adverse Events (irAEs)a.
| irAE | No. (%) | |||
|---|---|---|---|---|
| Atezolizumab-containing arm (n = 1557) | Control arm (n = 900) | |||
| Any grade | Grade 3-5 | Any grade | Grade 3-5 | |
| Any irAE | 753 (48) | 174 (11) | 289 (32) | 45 (5) |
| Rash | 435 (28) | 38 (2) | 160 (18) | 11 (1) |
| Hepatitis | 226 (15) | 73 (5) | 92 (10) | 17 (2) |
| Laboratory abnormalities | 199 (13) | 59 (4) | 90 (10) | 16 (2) |
| Diagnosis | 35 (2) | 16 (1) | 6 (1) | 2 (0.2) |
| Hypothyroidism | 192 (12) | 6 (0.4) | 33 (4) | 0 |
| Pneumonitis | 88 (6) | 25 (2) | 17 (2) | 8 (1) |
| Hyperthyroidism | 59 (4) | 3 (0.2) | 14 (2) | 0 |
| Colitis | 26 (2) | 17 (1) | 3 (0.3) | 2 (0.2) |
| Infusion-related reaction | 17 (1) | 1 (0.1) | 6 (1) | 1 (0.1) |
| Adrenal insufficiency | 19 (1) | 3 (0.2) | 3 (0.3) | 1 (0.1) |
| Pancreatitis | 15 (1) | 6 (0.4) | 4 (0.4) | 2 (0.2) |
Events represent medical concepts and are not single Medical Dictionary for Regulatory Activities preferred terms. Includes events occurring with greater than 1% incidence in any arm. Grade 5 events occurred in 7 patients in the atezolizumab-containing arm (irAEs by medical concepts were hepatitis [n = 2, 0.1%] and pneumonitis [n = 5, 0.3%]) and 2 patients in the control arm (pneumonitis [n = 2, 0.2%]).
Grade 4 irAEs occurred in 22 (1.4%) patients in the atezolizumab arm (vs none in the control arm). These were mostly hepatitis (laboratory abnormalities) in 9 (0.6%), hepatitis (diagnosis) in 5 (0.3%), and pneumonitis in 3 (0.2%). Grade 5 irAEs occurred in 7 (0.4%) and 2 (0.2%) patients in the respective arms (Table 1).
The median (range) time to onset of irAEs was 1.7 (0.0-34.7) months in the atezolizumab-containing arm and 1.4 (0.0-17.2) months in the control arm.
OS and ORR by irAE Status
Median OS was estimated using Kaplan-Meier analyses, unadjusted for the timing of irAE onset. In the atezolizumab-containing arm, median OS (95% CI) was 25.7 (23.9-29.1) months in patients with irAEs vs 13.0 (11.7-13.9) months in those without irAEs (Figure 1A; eFigure 2 in Supplement 4). In the control arm, median OS (95% CI) was 20.2 (18.2-22.8) vs 12.8 (12.0-13.9) months in patients with irAEs vs those without irAEs (Figure 1B; eFigure 2 in Supplement 4). The OS HR (95% CI) from the time-dependent Cox model between patients with and without irAEs was 0.69 (0.60-0.78) in the atezolizumab-containing arm and 0.82 (0.68-0.99) in the control arm. An interaction test of irAE status and treatment arms did not reveal statistical significance.
Figure 1. Overall Survival by Immune-Related Adverse Event (irAE) Status in the Atezolizumab-Containing and Control Arms.
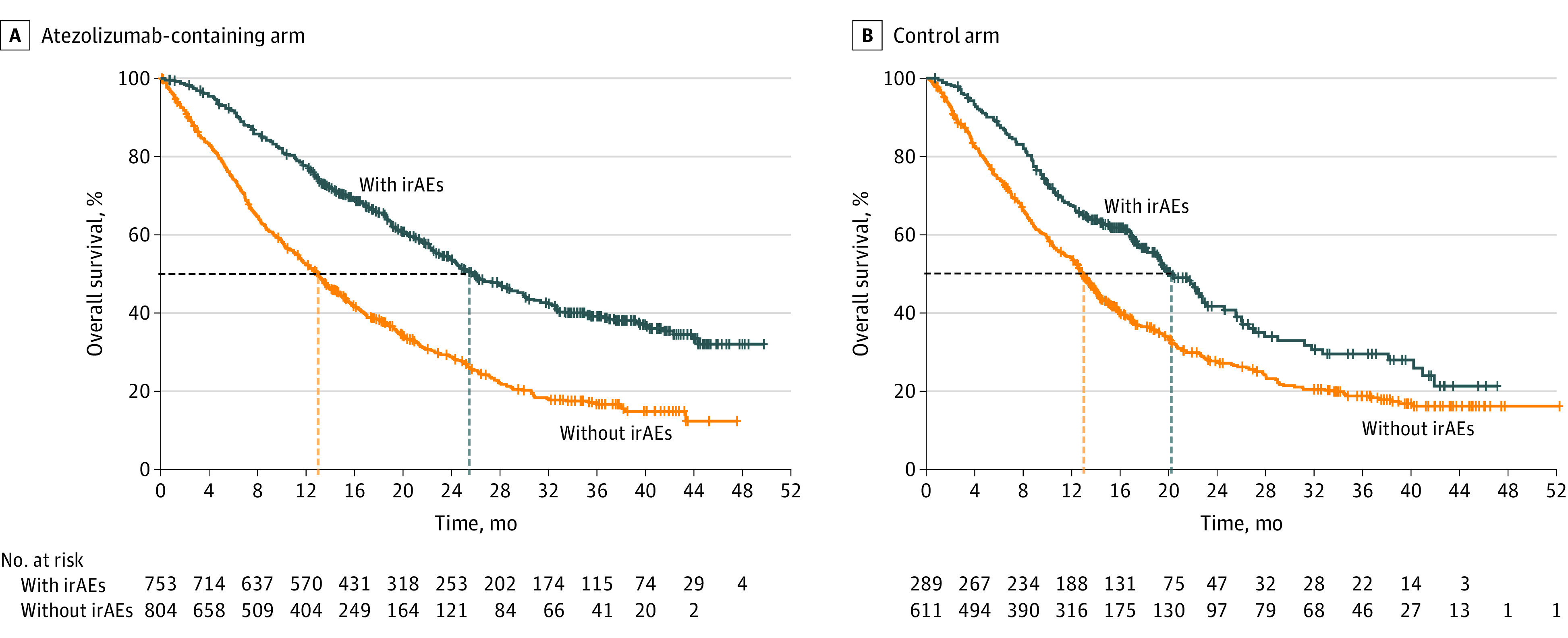
Kaplan-Meier curves are not adjusted for the timing of irAE onset.
In the atezolizumab-containing arm, without adjusting the irAE onset dates, the confirmed ORR (95% CI) was 61% (58%-65%) for patients with irAEs and 37% (34%-41%) for those without irAEs; in the control arm, the confirmed ORR (95% CI) was 42% (36%-48%) and 34% (30%-38%), respectively (eTable 4 in Supplement 4). In the atezolizumab-containing arm, the median (range) time to response was 1.7 (1.1-29.7) and 1.7 (1.0-27.1) months in patients with and without irAEs, respectively; in the control arm, the median (range) time to response was 1.6 (1.1-11.3) and 1.5 (1.1-13.4) months, respectively (eTable 4 in Supplement 4).
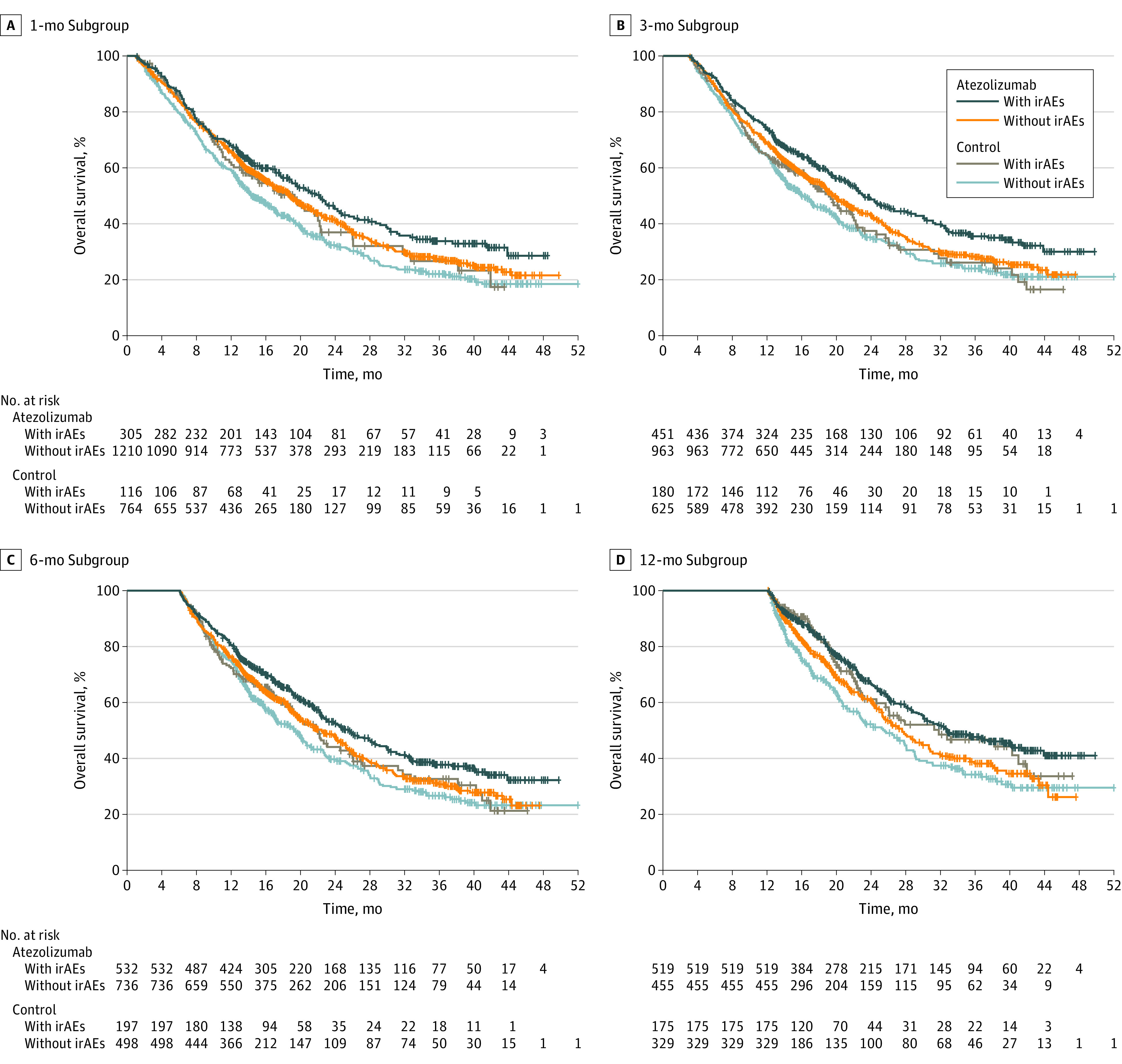
Landmark Analyses
Grade 3 to 5 irAE frequencies in landmark subgroups in the atezolizumab-containing arm are reported in Table 2; treatment withdrawal information for the subgroups is listed in eTable 2 in Supplement 4. The OS data by irAE status in landmark subgroups are shown in Figure 2 and eFigure 3 in Supplement 4. In all landmark subgroups in both arms, median OS for patients with irAEs was longer than for those without irAEs. Across all landmark subgroups, atezolizumab-arm patients who had an irAE had the longest median OS, whereas control-arm patients without an irAE had the shortest median OS.
Table 2. Grade 3 to 5 Immune-Related Adverse Events (irAEs) in Landmark Subgroups in the Atezolizumab-Containing Arm.
| Grade 3-5 irAEs | Patients, No. (%)a | |||
|---|---|---|---|---|
| 1-mo Subgroup | 3-mo Subgroup | 6-mo Subgroup | 12-mo Subgroup | |
| No. | 58 | 81 | 101 | 91 |
| Hepatitis (laboratory abnormalities and diagnosis) | 30 (52)b | 37 (46) | 42 (42) | 35 (38) |
| Rash | 20 (35) | 26 (32) | 32 (32) | 29 (32) |
| Pneumonitis | 9 (16)b | 11 (14) | 12 (12)b | 7 (8) |
| Colitis | 3 (5) | 6 (7) | 7 (7) | 6 (7) |
| Severe cutaneous reaction | 3 (5) | 4 (5) | 4 (4) | 3 (3) |
| Pancreatitis | 2 (3) | 2 (3) | 4 (4) | 5 (5) |
| Nephritis | 0 | 0 | 5 (5) | 6 (7) |
| Diabetes | 0 | 3 (4) | 3 (3) | 3 (3) |
| Encephalitis | 2 (3) | 2 (3) | 2 (2) | 2 (2) |
| Hypothyroidism | 0 | 0 | 4 (4) | 4 (4) |
| Meningoencephalitis | 2 (3) | 2 (3) | 2 (2) | 2 (2) |
| Hyperthyroidism | 0 | 1 (1) | 1 (1) | 1 (1) |
| Myositis | 0 | 1 (1) | 1 (1) | 1 (1) |
| Adrenal insufficiency | 0 | 0 | 0 | 2 (2) |
| Hypophysitis | 0 | 0 | 1 (1) | 1 (1) |
| Vasculitis | 0 | 0 | 1 (1) | 1 (1) |
| Infusion-related reaction | 0 | 1 (1) | 1 (1) | 0 |
| Hemolytic anemia | 0 | 1 (1) | 0 | 0 |
| Ocular inflammatory toxic effect | 0 | 0 | 0 | 1 (1) |
Includes events with greater than 1% incidence in any arm.
Grade 5 events observed. Adverse events resulting in death included 1 case of hepatitis and 1 case of pneumonitis at 1 month and 2 cases of pneumonitis at 6 months.
Figure 2. Overall Survival Landmarks by Immune-Related Adverse Event (irAE) Status in the 1-Month, 3-Month, 6-Month, and 12-Month Subgroups.
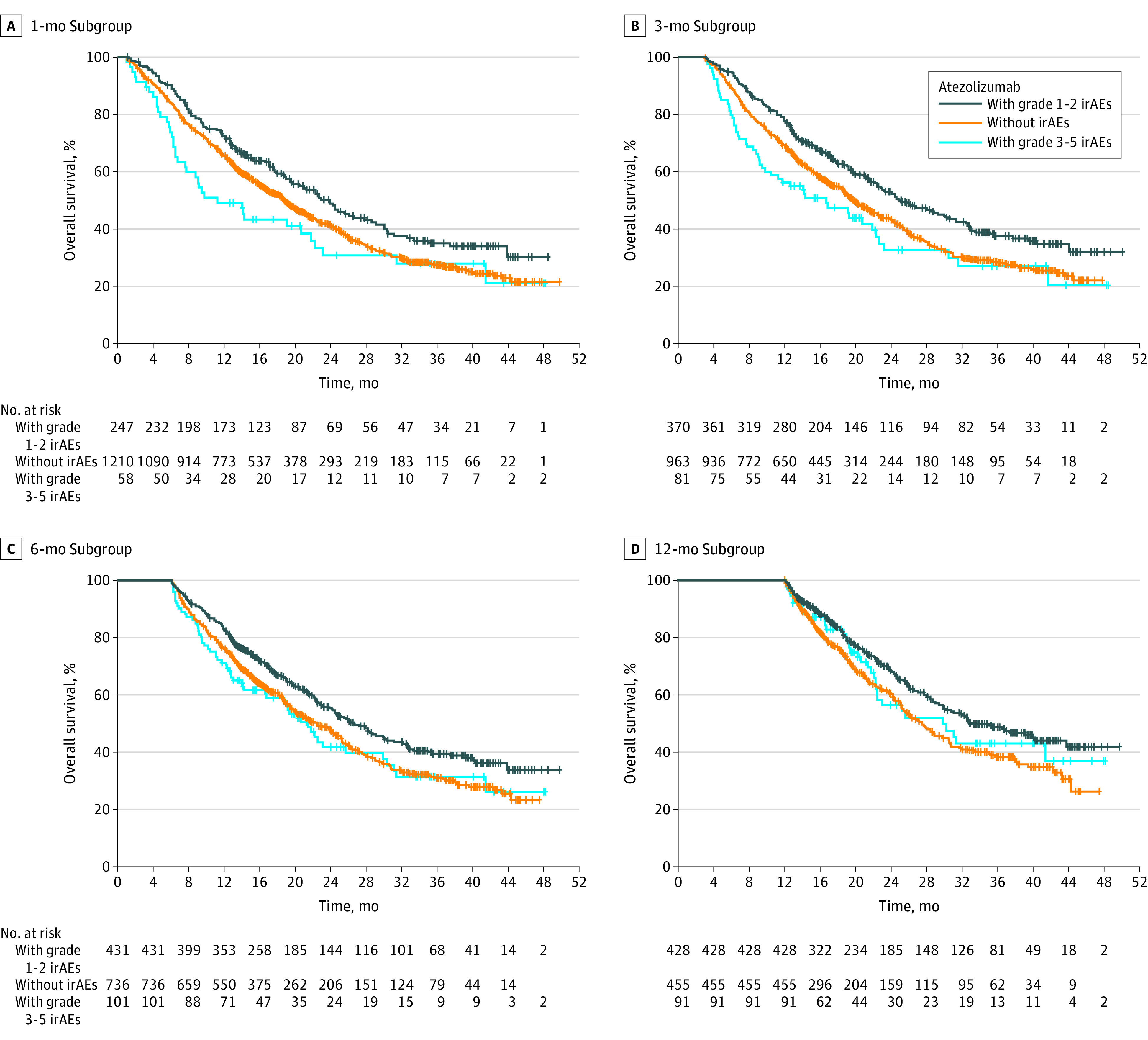
OS by irAE Grade in the Atezolizumab Arm
In all landmark subgroups of the atezolizumab-containing arm, median OS was longer in patients with grade 1 or 2 irAEs than in those with grade 3 to 5 irAEs and those without irAEs (Figure 3; eFigure 4 in Supplement 4). The OS HRs (95% CI) in patients with grade 1 or 2 irAEs and grade 3 to 5 irAEs (each compared with patients without irAEs) were 0.78 (0.65-0.94) and 1.25 (0.90-1.72) in the 1-month landmark subgroup, 0.74 (0.63-0.87) and 1.23 (0.93-1.64) in the 3-month subgroup, 0.77 (0.65-0.90) and 1.1 (0.81-1.42) in the 6-month subgroup, and 0.72 (0.59-0.89) and 0.87 (0.61-1.25) in the 12-month subgroup. The OS was longer in patients without irAEs than in those with grade 3 to 5 irAEs in the 1-, 3-, and 6-month subgroups, but not in the 12-month subgroup. Given that grade 5 events constitute an event in the survival analysis, and these occurred earlier on, landmark analyses including only grade 3 to 4 AEs were performed as sensitivity analyses to assess the impact of grade 5 events. The OS HRs at each landmark remained largely unchanged (eTable 5 in Supplement 4).
Figure 3. Overall Survival by Immune-Related Adverse Event (irAE) Grade in the Atezolizumab-Containing Arm in the 1-Month, 3-Month, 6-Month, and 12-Month Subgroups.
Discussion
In this post hoc exploratory pooled analysis of the phase 3 IMpower130, IMpower132, and IMpower150 randomized clinical trials, patients who had irAEs showed longer OS and higher ORR than those without irAEs in the atezolizumab-containing and control arms. The impact of the immortal bias caused by patients having to undergo treatment or living longer to experience irAEs was controlled for by using a time-dependent Cox model and landmark analyses. In both arms, landmark analyses showed longer OS in patients with irAEs than in those without, although patients benefited from atezolizumab vs control irrespective of whether they experienced irAEs. Nevertheless, patients in the atezolizumab-containing arm with grade 3 to 5 irAEs had shorter OS than those with grade 1 or 2 irAEs or no irAEs, except in the 12-month landmark analysis, in which median OS was longer among patients with grade 3 to 5 irAEs than those with none.
In this analysis, the median time to irAE onset (1.7 months) in atezolizumab arms was consistent with the 1.6 months reported for atezolizumab in the OAK trial14 and within the range of weeks to months reported for any-grade irAEs occurring in any organ or system.2,3,31 However, irAEs may occur at any time with ICI therapy, including after stopping treatment.2,3 It has been suggested that the occurrence of irAEs within 8 weeks of starting ICIs indicates that any association with treatment is unlikely to be attributable to longer periods of therapy.17,23
Although the irAE incidence rate in the atezolizumab-containing arm (48%) is consistent with reported rates of less than or equal to 50% for PD-L1/PD-1 inhibitors from various studies,4,32,33,34 it is higher than that observed for atezolizumab monotherapy in the TAIL (10%)35 and OAK (31%)14 NSCLC trials. In IMpower130,28 59% of patients received ICI therapy after disease progression, including atezolizumab at crossover per protocol (41% of the chemotherapy group). In IMpower132,31 46% of the chemotherapy group received ICI therapy in the second line. In IMpower150,27 46.4% and 42% received ICI therapy in the chemotherapy-only arms in the ITT-WT and ITT populations, respectively. The way irAEs were defined for all treatment arms in this analysis may have contributed to the relatively high rate of irAEs observed. It is also possible that concomitant chemotherapy with atezolizumab further exacerbated the off-target immune reactions, increasing the overall occurrence of irAEs. This theory is corroborated by the frequent occurrence of nonspecific irAEs such as rash and hepatitis laboratory abnormalities and the observation of irAEs in the control arms. Chemotherapy-associated lung and kidney injury are difficult to distinguish from pneumonitis and immune-mediated nephritis, posing additional diagnostic challenges and underscoring a greater need for standardized multidisciplinary definitions.36 Control-arm irAE occurrence, while less well delineated, may be related to the documented immunostimulatory effects of chemotherapy in inducing immunogenic cell death and modulating the tumor microenvironment as well as release of cancer antigens and damage-associated molecular patterns,37,38,39,40 although the immunostimulatory effects may be dependent on the chemotherapy class, among other factors.39 The control group included multiple chemotherapies with varied safety profiles and different pharmacokinetics, half-lives, and AE latencies compared with atezolizumab. Nevertheless, we acknowledge that it is not possible to explain this phenomenon completely with the available analysis. Future studies involving associated biopsies to confirm inflammatory mediation of toxicity are warranted.
Immune-related AEs with ICIs are thought to reflect an invigorated immune response that enhances antitumor activity41; therefore, the potential of these events as predictive markers of ICI efficacy in cancer has been explored in several studies. The time-dependent Cox model used suggested an association between irAEs and efficacy, consistent with other findings.7,8,9,10,11,12,17,19,20,21,22,23,33 In the OAK trial,14 median OS and PFS were longer and ORR was higher in patients with irAEs than in those without. The median time to response to atezolizumab coincided with the median time to onset of irAEs in the atezolizumab-containing arm, further indicating a potential association between the occurrence of irAEs and the response to atezolizumab. Although the mechanisms mediating the relationship between irAEs and efficacy have not been fully elucidated, the hypothesis of antigen mimicry suggests that ICIs release shared antigens that prime a secondary immune response to host antigens.42 Whether NSCLC tumor cells share antigens with host tissues affected by irAEs remains to be determined. In support of this theory, the positive association between irAEs and ICI efficacy has been shown to be dictated by the tissue or organ from which the irAEs emanate, with irAEs of dermatologic, endocrinologic, or gastrointestinal origin being associated with improved survival outcomes compared with those of pulmonary or hepatobiliary origin.9,42,43
In our analyses, the most common irAEs overall in the atezolizumab safety population were rash, hepatitis (laboratory abnormalities), and hypothyroidism. The most common grade 3 to 5 irAEs were hepatitis, rash, and pneumonitis in the overall safety population and the landmark subgroups. One case each of hepatic cirrhosis and pneumonitis resulting in death occurred in the 1-month landmark subgroup, and 2 cases of pneumonitis occurred in the 6-month landmark subgroup. Overall, these results are consistent with observations that irAEs commonly involve the gastrointestinal tract, endocrine glands, skin, and liver.2,32 The association of individual irAEs with atezolizumab efficacy we observed requires further investigation.
Our findings that low-grade irAEs were associated with improved outcomes in the atezolizumab-containing arm vs high-grade irAEs are supported by other studies.10,42,44 High-grade irAEs are potentially life threatening and may require treatment discontinuation or systemic immunosuppression, which may antagonize the effect of ICIs. However, it has also been suggested that because irAEs result from activation of autoreactive T cells, patients who develop severe irAEs theoretically have T cells that are more responsive to ICIs, which may lead to overall improved outcomes in those with high-grade vs low-grade irAEs.45,46 Although this theory is yet to be supported by an NSCLC study, a retrospective melanoma study of nivolumab showed that patients with grade 3 or greater irAEs had longer OS than those with grade 1 or 2 irAEs.47
Strengths and Limitations
The OS landmark analyses over 1 to 12 months, which further suggested an association between irAEs and OS, differentiate our analyses from other studies using landmark time points from 6 to 20 weeks.9,10,17,20,21,22,23 Limitations of this analysis include its post hoc exploratory nature and lack of data on the irAEs that most strongly contributed to the association with atezolizumab efficacy. For irAEs driving the higher rates seen in the atezolizumab-containing arm, the analyses could not truly differentiate between those related to the mechanism of action of atezolizumab vs chemotherapy. Lack of data on the association of high-dose steroid use with efficacy was another limitation.
Conclusions
This pooled analysis of data from 3 atezolizumab randomized clinical trials suggests as association between mild to moderate irAEs and efficacy in NSCLC. Although patients benefited from atezolizumab-containing regimens regardless of whether they experienced irAEs, atezolizumab-treated patients with low-grade irAEs had longer OS than those with high-grade irAEs. These data further support the use of atezolizumab combined with chemotherapy, with or without bevacizumab, for first-line treatment of advanced nonsquamous NSCLC. To the best of our knowledge, this is the first exploratory analysis of large clinical trials to demonstrate an association between irAEs and the efficacy of atezolizumab combined with chemotherapy in patients with advanced nonsquamous NSCLC.
IMpower130 Trial Protocol
IMpower132 Trial Protocol
IMpower150 Trial Protocol
eMethods.
eFigure 1. Patient profile
eFigure 2. OS by irAE status in the atezolizumab-containing (A) and control (B) arms
eFigure 3. OS landmark by irAE status in the 1- (A), 3- (B), 6- (C), and 12-month (D) subgroups
eFigure 4. OS by irAE grade in the atezolizumab-containing arm in the 1- (A), 3- (B), 6- (C), and 12-month (D) subgroups
eTable 1. Key demographics and baseline characteristics
eTable 2. Treatment disposition in patients in the atezolizumab arm with grade 1 or 2 and 3 to 5 irAEs
eTable 3. Atezolizumab exposure based on grade 3 to 5 irAEs in landmark subgroups in the atezolizumab-containing arm
eTable 4. Confirmed ORR and duration of response by irAE status
eTable 5. Sensitivity analyses of OS HR to assess the impact of grade 5 irAEs in landmark analyses
Data Sharing Statement
References
- 1.Jamal S, Hudson M, Fifi-Mah A, Ye C. Immune-related adverse events associated with cancer immunotherapy: a review for the practicing rheumatologist. J Rheumatol. 2020;47(2):166-175. doi: 10.3899/jrheum.190084 [DOI] [PubMed] [Google Scholar]
- 2.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158-168. doi: 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 3.Tang SQ, Tang LL, Mao YP, et al. The pattern of time to onset and resolution of immune-related adverse events caused by immune checkpoint inhibitors in cancer: a pooled analysis of 23 clinical trials and 8,436 patients. Cancer Res Treat. 2021;53(2):339-354. doi: 10.4143/crt.2020.790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu C, Chen YP, Du XJ, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ. 2018;363:k4226. doi: 10.1136/bmj.k4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haanen JBAG, Carbonnel F, Robert C, et al. ; ESMO Guidelines Committee . Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv119-iv142. doi: 10.1093/annonc/mdx225 [DOI] [PubMed] [Google Scholar]
- 6.NCCN Clinical Practice Guidelines . Management of immunotherapy-related toxicities. Version 4.2021. Accessed January 11, 2023. https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf [DOI] [PubMed]
- 7.Akamatsu H, Murakami E, Oyanagi J, et al. Immune-related adverse events by immune checkpoint inhibitors significantly predict durable efficacy even in responders with advanced non-small cell lung cancer. Oncologist. 2020;25(4):e679-e683. doi: 10.1634/theoncologist.2019-0299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortellini A, Buti S, Agostinelli V, Bersanelli M. A systematic review on the emerging association between the occurrence of immune-related adverse events and clinical outcomes with checkpoint inhibitors in advanced cancer patients. Semin Oncol. 2019;46(4-5):362-371. doi: 10.1053/j.seminoncol.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 9.Cortellini A, Chiari R, Ricciuti B, et al. Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin Lung Cancer. 2019;20(4):237-247.e1. doi: 10.1016/j.cllc.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 10.Grangeon M, Tomasini P, Chaleat S, et al. Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-small-cell lung cancer. Clin Lung Cancer. 2019;20(3):201-207. doi: 10.1016/j.cllc.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 11.Hussaini S, Chehade R, Boldt RG, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors—a systematic review and meta-analysis. Cancer Treat Rev. 2021;92:102134. doi: 10.1016/j.ctrv.2020.102134 [DOI] [PubMed] [Google Scholar]
- 12.Khan Z, Di Nucci F, Kwan A, et al. Polygenic risk for skin autoimmunity impacts immune checkpoint blockade in bladder cancer. Proc Natl Acad Sci U S A. 2020;117(22):12288-12294. doi: 10.1073/pnas.1922867117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remon J, Reguart N, Auclin E, Besse B. Immune-related adverse events and outcomes in patients with advanced non-small cell lung cancer: a predictive marker of efficacy? J Thorac Oncol. 2019;14(6):963-967. doi: 10.1016/j.jtho.2019.02.031 [DOI] [PubMed] [Google Scholar]
- 14.Komiya K, Nakamura T, Abe T, et al. Discontinuation due to immune-related adverse events is a possible predictive factor for immune checkpoint inhibitors in patients with non-small cell lung cancer. Thorac Cancer. 2019;10(9):1798-1804. doi: 10.1111/1759-7714.13149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):306. doi: 10.1186/s40425-019-0805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374-378. doi: 10.1001/jamaoncol.2017.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lisberg A, Tucker DA, Goldman JW, et al. Treatment-related adverse events predict improved clinical outcome in NSCLC patients on KEYNOTE-001 at a single center. Cancer Immunol Res. 2018;6(3):288-294. doi: 10.1158/2326-6066.CIR-17-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noguchi S, Suminaga K, Kaki T, et al. Correlation of immune-related adverse events and effects of pembrolizumab monotherapy in patients with non-small cell lung cancer. Lung Cancer (Auckl). 2020;11:53-57. doi: 10.2147/LCTT.S254146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricciuti B, Genova C, De Giglio A, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145(2):479-485. doi: 10.1007/s00432-018-2805-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato K, Akamatsu H, Murakami E, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71-74. doi: 10.1016/j.lungcan.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 21.Teraoka S, Fujimoto D, Morimoto T, et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol. 2017;12(12):1798-1805. doi: 10.1016/j.jtho.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 22.Toi Y, Sugawara S, Kawashima Y, et al. Association of immune-related adverse events with clinical benefit in patients with advanced non-small-cell lung cancer treated with nivolumab. Oncologist. 2018;23(11):1358-1365. doi: 10.1634/theoncologist.2017-0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fehrenbacher L, Spira A, Ballinger M, et al. ; POPLAR Study Group . Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837-1846. doi: 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 24.Felip E, Altorki N, Zhou C, et al. ; IMpower010 Investigators . Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344-1357. doi: 10.1016/S0140-6736(21)02098-5 [DOI] [PubMed] [Google Scholar]
- 25.Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328-1339. doi: 10.1056/NEJMoa1917346 [DOI] [PubMed] [Google Scholar]
- 26.Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group . Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. doi: 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Socinski MA, Jotte RM, Cappuzzo F, et al. ; IMpower150 Study Group . Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301. doi: 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 28.West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924-937. doi: 10.1016/S1470-2045(19)30167-6 [DOI] [PubMed] [Google Scholar]
- 29.Tecentriq (atezolizumab). Summary of product characteristics. Roche GmbH; 2020.
- 30.Tecentriq (atezolizumab). Package insert. Genentech, Inc; 2020.
- 31.Nishio M, Barlesi F, West H, et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol. 2021;16(4):653-664. doi: 10.1016/j.jtho.2020.11.025 [DOI] [PubMed] [Google Scholar]
- 32.Nishio M, Saito H, Goto K, et al. IMpower132: atezolizumab plus platinum-based chemotherapy vs chemotherapy for advanced NSCLC in Japanese patients. Cancer Sci. 2021;112(4):1534-1544. doi: 10.1111/cas.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Pawel J, Syrigos K, Mazieres J, et al. Association between immune-related adverse events (irAEs) and atezolizumab efficacy in advanced NSCLC: analyses from the phase III study OAK. Abstract. Ann Oncol. 2017;28(suppl 5):V469. doi: 10.1093/annonc/mdx380.017 [DOI] [Google Scholar]
- 34.Shankar B, Zhang J, Naqash AR, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 2020;6(12):1952-1956. doi: 10.1001/jamaoncol.2020.5012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793. doi: 10.1136/bmj.k793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan Y, Xie W, Huang H, et al. Association of immune related adverse events with efficacy of immune checkpoint inhibitors and overall survival in cancers: a systemic review and meta-analysis. Front Oncol. 2021;11:633032. doi: 10.3389/fonc.2021.633032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pillai RN, Behera M, Owonikoko TK, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: a systematic analysis of the literature. Cancer. 2018;124(2):271-277. doi: 10.1002/cncr.31043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ardizzoni A, Azevedo S, Rubio-Viqueira B, et al. Primary results from TAIL: a global single-arm safety study of atezolizumab monotherapy in a diverse population of patients with previously treated advanced non-small cell lung cancer. J Immunother Cancer. 2021;9(3):e001865. doi: 10.1136/jitc-2020-001865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conroy M, Naidoo J. Immune-related adverse events and the balancing act of immunotherapy. Nat Commun. 2022;13(1):392. doi: 10.1038/s41467-022-27960-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1-10. doi: 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 41.Ghiringhelli F, Apetoh L. The interplay between the immune system and chemotherapy: emerging methods for optimizing therapy. Expert Rev Clin Immunol. 2014;10(1):19-30. doi: 10.1586/1744666X.2014.865520 [DOI] [PubMed] [Google Scholar]
- 42.Opzoomer JW, Sosnowska D, Anstee JE, Spicer JF, Arnold JN. Cytotoxic chemotherapy as an immune stimulus: a molecular perspective on turning up the immunological heat on cancer. Front Immunol. 2019;10:1654. doi: 10.3389/fimmu.2019.01654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59-73. doi: 10.1038/nri2216 [DOI] [PubMed] [Google Scholar]
- 44.Ho AK, Ho AM, Cooksley T, Nguyen G, Erb J, Mizubuti GB. Immune-related adverse events associated with immune checkpoint inhibitor therapy. Anesth Analg. 2021;132(2):374-383. doi: 10.1213/ANE.0000000000005029 [DOI] [PubMed] [Google Scholar]
- 45.Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? a systematic review and meta-analysis. BMC Med. 2020;18(1):87. doi: 10.1186/s12916-020-01549-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang D, Chen C, Gu Y, et al. Immune-related adverse events predict the efficacy of immune checkpoint inhibitors in lung cancer patients: a meta-analysis. Front Oncol. 2021;11:631949. doi: 10.3389/fonc.2021.631949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujii T, Colen RR, Bilen MA, et al. Incidence of immune-related adverse events and its association with treatment outcomes: the MD Anderson Cancer Center experience. Invest New Drugs. 2018;36(4):638-646. doi: 10.1007/s10637-017-0534-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IMpower130 Trial Protocol
IMpower132 Trial Protocol
IMpower150 Trial Protocol
eMethods.
eFigure 1. Patient profile
eFigure 2. OS by irAE status in the atezolizumab-containing (A) and control (B) arms
eFigure 3. OS landmark by irAE status in the 1- (A), 3- (B), 6- (C), and 12-month (D) subgroups
eFigure 4. OS by irAE grade in the atezolizumab-containing arm in the 1- (A), 3- (B), 6- (C), and 12-month (D) subgroups
eTable 1. Key demographics and baseline characteristics
eTable 2. Treatment disposition in patients in the atezolizumab arm with grade 1 or 2 and 3 to 5 irAEs
eTable 3. Atezolizumab exposure based on grade 3 to 5 irAEs in landmark subgroups in the atezolizumab-containing arm
eTable 4. Confirmed ORR and duration of response by irAE status
eTable 5. Sensitivity analyses of OS HR to assess the impact of grade 5 irAEs in landmark analyses
Data Sharing Statement


