Abstract
The term mild cognitive impairment (MCI) defines an intermediate state between normal aging and dementia. Vascular cognitive impairment refers to a decline in cognitive function that is caused by or associated with vascular disease and comprises all the spectrum of cognitive impairments, from MCI of vascular origin to vascular dementia. One of the available treatments for cognitive impairment is cytidine diphosphate-choline (CDP-Choline), or citicoline. The objective of the present manuscript is to provide complete evidence about the efficacy of citicoline for MCI, especially of vascular origin, but also due to other neurodegenerative disorders. Citicoline is a pharmaceutical product constituted by the combination of 2 natural molecules (cytidine and choline) and is marketed as a food supplement. It has been proposed to provide neuroprotective effects through diverse mechanisms of action. Taking into account the available literature, citicoline has shown a consistent improvement in cognitive function in patients with MCI, especially of vascular origin. Moreover, it provides beneficial effects on vascular, Alzheimer, and mixed dementias, stroke sequelae, intracerebral hemorrhages, traumatic brain injuries, and neurodegenerative diseases. Long-term treatment with citicoline has also been demonstrated to be well-tolerated and has not been associated with severe adverse events. Citicoline is a safe, well-tolerated, and promising agent with evidenced neuroprotective properties.
Keywords: Alzheimer, cerebrovascular, dementia, Citicoline, cognitive, impairment, neurodegenerative
Introduction
Cytidine diphosphate-choline (CDP-Choline), or citicoline, is a pharmaceutical product constituted by the combination of 2 natural molecules (cytidine and choline).1,2 These endogenous compounds participate in the biosynthesis of phosphatidylcholine, an important phospholipid of the cell membranes.3 Citicoline can be administered orally, intravenously, or both; at doses ranging from 500 to 2000 mg per day.1 Citicoline has an adequate safety profile, with minimum toxicity, and rapid metabolization.4
It has been proposed to provide neuroprotective effects through multiple mechanisms of action. While in vitro studies have shown that anoxia decreases the synthesis of structural phospholipids, studies in animal models have reported a reduction in the incorporation of marked precursors into phospholipids of neuronal subcellular fractions. In addition, cell membrane glycerophospholipids are broken down by the action of different phospholipases, producing free fatty acids and arachidonic acid derivatives.4,5 When ischemia conditions are prolonged, aggression upon membranes becomes more intense, as a loss of function of membranes, accumulation of Na+ and Ca2+ inside the cell, triggering of ischemic cascade, and subsequent cell death are observed. Finally, synthesis of endogenous CDP-choline is compromised under ischemic conditions, as cells lack the high-energy phosphate compounds required for this route. Moreover, since synthesis of structural phospholipids is time-dependent, a higher impact on the metabolism of neuronal phospholipids the longer the time under hypoxia conditions.4,5 Citicoline promotes the biosynthesis of structural phospholipids in neuronal cell membranes (especially phosphatidylcholine), enhances brain metabolism (by increasing class III histone deacetylase sirtuin-1), and raises levels of norepinephrine and dopamine in the central nervous system.4,5 It also increases serotonin levels, while reducing levels of glutamate. Altogether, these effects contribute to neuroprotection in hypoxia. Furthermore, citicoline restores the activity of mitochondrial ATPase and membrane Na+/K+ ATPase, restrains phospholipase A2 activation (and blocking the neuroinflammation caused by ischemia and the production of reactive oxygen species), and accelerates the reabsorption of cerebral edema.4,5
Citicoline has also been shown to modulate neurotransmitter levels, since it acts as a dopaminergic agonist, having a significant effect on both dopamine levels and their metabolites in the corpus striatum. Several studies have reported an increased dopamine synthesis after citicoline administration, which might be triggered by the activation of tyrosine hydroxylase. This higher dopamine synthesis leads to the inhibition of dopamine reuptake, which might be related to the citicoline action upon phospholipid synthesis. In addition, citicoline acts upon other monoamines, serotonin and norepinephrine, muscarinic and nicotinic receptors, and glutamate, opioids, and GABA, having important modulating effects on several intracellular signaling processes (Figure 1).4,5
Figure 1.
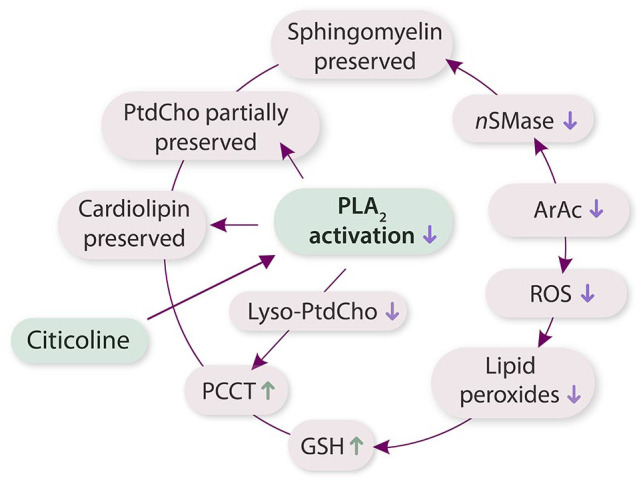
Pathway of citicoline neuroprotection.
Abbreviations: ArAc, arachidonic acid; GSH, glutathione; nSMase, neutral sphingomyelinase; PLA2, phospholipase A2; PtdCho, phosphatidylcholine; ROS, reactive oxygen species.
A worse cardiovascular risk profile has been associated with poorer cognitive function and smaller brain volumes in regions identified as early predictors of cognitive decline6,7. Exogenous citicoline has been suggested to be beneficial for cognitive impairment of diverse causes, stroke, traumatic brain injuries, and brain aging.3,7 The objective of the present review is to provide complete evidence about the efficacy of citicoline for mild cognitive impairment (MCI), especially of vascular origin, but also neurodegenerative disorders.
Mild Cognitive Impairment
The term MCI defines an intermediate state between normal aging and dementia.8 It is characterized by impaired function in one or more of the following domains: learning and memory; language; visuo-spatial; executive; or psychomotor.9 Its prevalence in adults aged 60 or over is estimated to be between 6.7% and 25.2%.10 Clinical studies have determined that 10% to 15% of individuals with a diagnosis of amnestic MCI will progress to dementia each year, compared with 1% to 2% in those without a diagnosis.11
Citicoline on vascular cognitive impairment
Vascular cognitive impairment refers to a decline in cognitive function that is caused by or associated with vascular disease.11 It defines the spectrum of cognitive impairments, from MCI of vascular origin to vascular dementia (VaD).12 Vascular cognitive impairment is characterized by several executive functions, information processing, memory, and personality disorders, usually caused by a disruption of the frontal-subcortical networks due to lesions in the white matter. However, vascular lesions in other subcortical structures such as the thalamus or basal ganglia might contribute to this cognitive impairment.12
A systematic review showed the prevalence of vascular MCI (with no dementia) varying between 24% and 75% in stroke patients, and from 4% to 19% in cohorts with low (or non-reported) stroke prevalence.12,13 Vascular MCI encompasses memory deficit, slowed information processing, mood and personality disorders, and executive dysfunction.12 The disruption of frontal-subcortical networks associated with white matter lesions represents the most important pathological substrate for vascular MCI. Further vascular lesions in the white matter and/or subcortical structures (such as the thalamus and basal ganglia) may also contribute to cognitive decline.12 Cardiovascular factors play a primary role in cognitive impairment, especially arterial hypertension, dyslipidemia, and diabetes. Primary objectives in the management of cognitive impairment include lifestyle modifications and control of vascular risk factors.14 The early detection of cognitive changes is carried out with scales and/or questionnaires. The most frequently used screening instruments are the Mini-Mental Status Examination (MMSE) and the Montreal Cognitive Assessment (MoCA).12 The MMSE has low sensitivity to detect MCI, whereas the MoCa is sensitive to MCI with cerebrovascular disease. The Hachinski Ischemic Score has also been validated as an assessment tool to differentiate major types of dementia (primary degenerative, vascular, and mixed).15 However, the accuracy of the Hachinski score for different dementia subtypes such as vascular dementia, mixed dementia, or Alzheimer’s disease has been considered poor during the last years, with the presence of a significant proportion of misclassifications.16
Diverse studies have revealed the efficacy of citicoline for the treatment of mild cognitive impairment, which is usually administered orally as monotherapy (Table 1).7,17,18 The open-label, multicenter, Italian IDEALE study compared the effectiveness and safety of the administration of oral citicoline (1 g/day) in 265 patients with vascular MCI and 84 patients receiving no treatment (control group), so no placebo was administered to this group, which might represent a limitation of this study.17 The MMSE scores improved slightly after the treatment with citicoline, whereas a significant difference was found between citicoline and control groups at 3 and 9 months (Figure 2). No adverse events were recorded. In this study, citicoline was effective and well tolerated in patients with vascular MCI. The retrospective, observational, multicenter VITA study evaluated the efficacy of citicoline in elderly patients suffering from stupor associated with complex geriatric syndrome.18 Citicoline showed significant improvements in terms of the National Institute of Health Stroke Scale (NIHSS), the Rankin Scale, and the activities of daily living (ADL) Scale. Moreover, a recent observational program aimed to study the effect of citicoline on the state of higher mental functions in patients with MCI. After 2 weeks of treatment, it was observed an improvement in concentration, memory, verbal imagination productivity, counting functions, visual-motor coordination and dynamic praxis, and mental work.19
Table 1.
Summary of the studies involving citicoline on vascular cognitive impairment.
| Study design | Sample size | Treatment arms | Outcomes | |
|---|---|---|---|---|
| Controneo et al16 | Open, multicenter | 349 elderly patients | Oral citicoline 500 mg twice a day or placebo | In the treatment group, MMSE scores remained unchanged, while they decreased in the control group (+0.5 and −1.9 respectively over 9 months). Significant differences were observed between the arms at both 3 and 6 months (P < .0001 for both). |
| Putignano et al17 | Retrospective, observational | 197 patients over 65 years | Treatment arm 2000 mg of intravenous in the first phase, 1000 mg of intramuscular citicoline in the second phase. Control group 500 cc saline and 500 cc glucose 5%. | Comparisons between treatment and control groups (after 2 months): NIHS scale: 11.1 ± 4.6 versus 17.7 ± 5.3 Rankin Scale: 3.7 ± 0.7 versus 4.5 ± 0.7 Activities of Daily Living: 2.0 ± 1.4 versus 0.5 ± 0.7 Instrumental Activities of Daily Living: 2.3 ± 2.5 versus 0.2 ± 0.6 |
| Fioravanti and Yanagi18 | Systematic review | 14 studies | – | Standardized mean differences −0.09 (−0.23, 0.05), 0.19 (0.06, 0.32), and −0.60 (−1.05, −0.15) for attention, memory, and behavior |
Figure 2.
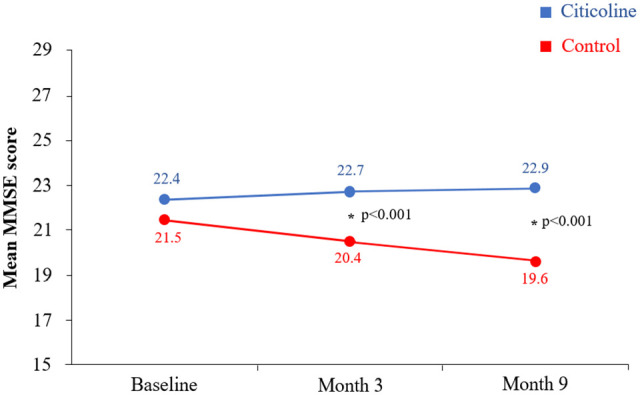
Evolution of the mini-mental status examination scores between citicoline and control groups (modified from Cotroneo et al16).
Abbreviations: MMSE, mini-mental status examination.
Asterisks represent significant differences between groups.
Finally, Cochrane systematic review, with data from 1978 to 2005 of 13 double-blind, placebo-controlled studies using citicoline for cognitive impairment due to chronic cerebral disorders, concluded that citicoline provides an overall positive short- and medium-term effect on memory and behavior.20 When different outcomes were analyzed, a standardized mean difference of −0.09 (−0.23, 0.05), 0.19 (0.06, 0.32), and −0.60 (−1.05, −0.15) were obtained for attention, memory, and behavior. These results highlighted that citicoline had a positive effect on both memory and behavior in at least the medium term, especially in patients suffering from cognitive deficits associated with cerebrovascular disorders. However, there was evidence of little effect on attention. The clinical trials were homogeneous, even though they included patients from different clinical groups, as in the case of attention, or percentages of inconsistent variance of 42.7% among the studies in the case of behavior. Therefore, the authors concluded that further research on this compound is needed, especially regarding the long-term effect of citicoline in patients diagnosed with currently accepted standardized criteria.
Citicoline for vascular, Alzheimer, and mixed dementias
Alzheimer’s disease (AD) and cerebrovascular diseases are leading causes of cognitive impairment, accounting for up to 80% of cases.14 AD is a degenerative disorder mainly linked to β-amyloid and tau deposition, which leads to a progressive deterioration in different skills such as memory, language, perceptual proficiency, attention, orientation, and problem-solving. On the other hand, vascular dementia is a heterogeneous disease arising from cerebrovascular lesions, whose number, location, and extent characterize its entity, and in which dementia is only secondary.21,22 In addition, several differences have been reported regarding neurodegeneration of both white and grey matter between both diseases. While AD shows a reduced disruption in white matter and axial diffusivity, both these disturbances are more present in vascular dementia.23 Finally, in older patients, a mixed type of dementia can be observed in AD patients who present a certain degree of cerebrovascular lesions.24
They are frequently presented as a mixture of neuropathologies. Indeed, cognitive differences between vascular MCI preceding AD are not immediately obvious, and cannot be differentiated with simple cognitive assessments like MMSE.13 There is also evidence about the linkage between AD and cerebrovascular diseases such as vascular dementia.25
Sirtuins are NAD-dependent protein deacetylases that can regulate age-related diseases. Out of the 7 different sirtuins described in mammals, SIRT1 plays a key role in regulating the self-renewal of adult hippocampal neural stem cells (aNSCs)/adult neural progenitor cells and acts as a potential mediator of the effect of metabolic changes.26 Therefore, SIRT1 has been widely studied in several diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and cerebral ischemia among others, with its activation representing a therapeutic target regarding these diseases.27 Indeed, CDP-choline has been shown to increase SIRT1 protein expression in rat brain, cultured neurons and circulating blood mononuclear cells, which is strongly linked with the neuroprotection provoked by this drug.28
Diverse studies have evaluated the efficacy of citicoline in VaD, AD, and mixed dementias.29,30 Citicoline is usually administered in combination with cholinesterase inhibitors, compared to monotherapy in patients with mild cognitive impairment, as it has shown improvement in cognition, mood, and behavioral symptoms compared to cholinesterase inhibitors alone.30 Cohen et al,29 in a randomized, double-blind clinical trial, comparing citicoline and placebo, showed no difference in neuropsychological performance at the 12-month follow-up for the 2 groups. The drug was administered for 1 year, with the participants undergoing both MRI imaging and a battery of different tests to assess different neuropsychological functions such as global functioning, attention, memory, visual skills, language, or psychomotor functions. No significant differences were observed in any of the cognitive domains between placebo and citicoline arms (P > .05 in all tests) nor in the MRI (P = .17). Thus, the authors could not demonstrate a beneficial effect of citicoline in patients with VaD.
Regarding AD, citicoline has primarily been evaluated combined with other therapeutic agents. The retrospective case-control CITIRIVAD study demonstrated the superior effectiveness of oral citicoline plus rivastigmine versus rivastigmine alone in terms of slower disease progression.31 Similarly, the Citicholinage study revealed significantly higher scores in MMSE in patients with AD receiving citicoline plus cholinesterase inhibitors (AChEIs) than those with a single AChEI after 3 (17.6 vs 16.0) and 9 months (17.9 vs 15.4).32 A systematic review evaluating the effects of citicoline in combination with other AD drugs analyzed data from 2 retrospective studies involving 563 elderly patients improvement was observed in cognition, mood, and behavioral symptoms with the combination of citicoline and AChEIs versus AChEIs monotherapy. Differences were observed among the different AChEIs. Patients administered with the combination of donepezin + citicoline showed better cognitive function, with MMSE scores of 18.15 ± 4.21 and 18.49 ± 3.98 at the first and second follow-up visits. The trends between baseline and first and second follow-up visits were improved, being more evident between baseline and the second time frame (mean differences of 0.65 and 0.81). In comparison, patients undergoing rivastigmine + citicoline treatment reported values of 16.89 ± 2.53 and 17.07 ± 2.66, respectively (P = .007 and .002 compared to the donepezin + citicoline therapy, respectively).30 Tanaka et al,33 found an association between cognitive performance and the increase in the cerebral blood flow of patients with VaD treated with citicoline. Moreover, Corona et al5,34 suggested that the beneficial effects of citicoline in patients with dementia might derive from its ability to improve the activity of noradrenergic, dopaminergic, and serotonergic systems.
Citicoline on stroke sequelae
Stroke is an important cause of disability.35,36 Up to one-third of stroke survivors may subsequently develop cognitive impairment or dementia.37 Moreover, many patients presenting post-stroke vascular cognitive impairment have a history of multiple strokes.37 Different therapies have been proposed for the recovery and treatment of ischemic stroke, including citicoline.2 The efficacy of citicoline for the treatment of acute stroke has been studied in multiple randomized trials, and most of them have shown positive effects during the acute and subacute phases of ischemic stroke.38 Alvarez-Sabín et al assessed the safety and efficacy of citicoline in the long-term with patients after ischemic stroke.39,40 In an open-label, randomized, parallel study of 1 year of follow-up, patients with first-ever ischemic stroke receiving citicoline showed greater improvement of cognitive function, especially attention-executive functions and temporal orientation (P = .005).41 Among the limitation of this study was the absence of the use of placebo as a control. Therefore, the open-label design of this study could have influenced the results, especially bearing in mind that some cognitive improvement in the citicoline group might be attributable to the expectation bias of patients. Another study published by Bermejo et al40 suggested that citicoline prevented the development of MCI and dementia in patients who suffered a stroke, hemorrhage, or traumatic brain injury. In another study of 2 years of follow-up, citicoline correlated with improvement of cognitive function and better quality of life.41
Citicoline and Parkinson’s disease and glaucoma
Citicoline has demonstrated beneficial effects on neurodegenerative diseases such as Parkinson’s disease (PD) and glaucoma.4 Regarding PD, citicoline might act by increasing dopamine levels and inhibiting dopamine reuptake in the brain.42 Combining citicoline with the main current PD treatment, levodopa, allows for a 50% reduction in levodopa dosage, reducing the adverse effects of this drug.5 Also, it activates biosynthesis of structural phospholipids of neuronal membranes, increases brain metabolism and both norepinephrine and dopamine levels, and restores the activity of mitochondrial ATPase and membrane Na+/K+ ATPase in PD patients.5 These pharmacological characteristics and action mechanisms indicate that citicoline might be used for the treatment of cerebrovascular diseases. A prospective, placebo-controlled, randomized study evaluated the effect of citicoline combination therapy on MCI in 185 patients with PD.43 The MoCA and Scales for Outcomes in Parkinson’s disease-Cognition scores after 12 and 18 months of treatment were significantly higher with citicoline than placebo. Moreover, the decrease in plasma phospholipid concentrations was significantly greater with citicoline after the same period of time. The authors concluded that there was evidence of a positive and consistent effect of the drug on both memory and behavior. However, they also pointed out that the length of the studies was low (3 months or less). A systematic review of data from 2 cross-over studies, 3 randomized, controlled studies, and 2 open-label prospective studies of 355 patients with PD concluded that citicoline provided significant improvements in cognitive status, rigidity, akinesia, tremor, handwriting, and speech.42
Cognitive Impairment, Vascular Disease, and COVID-19
The etiological agent for coronavirus disease (COVID-19), that is, the severe acute respiratory syndrome (SARS)-CoV-2 (SARS-CoV-2), has also been evidenced to cause damage in the brain.44 In fact, numerous case reports and cohort studies have described cognitive impairment and neurodegeneration in COVID-19 patients. One case study described a dysexecutive syndrome (inattention, disorientation, or poorly organized movements in response to a command) in 33% of patients with a severe infection that were discharged from the hospital.45,46 Neurologic syndromes and stroke upon admission predict a significantly greater risk for in-hospital mortality (OR:1.4 and 3.1, respectively), regardless of disease severity.46 Moreover, acute cerebrovascular disease (such as ischemic stroke and intracerebral hemorrhages) is a clinical feature in COVID-19 patients.47 Mechanisms causing the cognitive impairment in COVID-19 (whether infectious, toxic, vascular, and/or metabolic) remain unclear48 although vascular damage seems to be one of the most important factors in this complication.49 For this reason, citicoline has been proposed as a treatment to correct MCI due to COVID-19.49
Recently, Turana et al50 reviewed the potential role of citicoline in combating the pathophysiology and neurological complications of COVID-19. They stated that this therapy could be considered as a treatment option in the management of both short and long-term neurological complications caused by COVID-19-related hyperinflammation and damaging effects on the neurovascular system. The authors based this claim on the maintenance of an adequate phospholipid structure and function in brain cells provoked by citicoline, playing a potential role in coping with both cognitive decline and other neurological complications through its anti-inflammatory, anti-viral, neuroprotective, neurorestorative, and acetylcholine neurotransmitter synthesis actions.
Conclusion
Citicoline is a safe, well-tolerated, and promising agent with evidenced neuroprotective properties. Taking into account the available literature, citicoline has shown a consistent improvement in cognitive function in patients with MCI, especially of vascular origin. Moreover, it provides beneficial effects on vascular, AD, and mixed dementias, stroke sequelae, intracerebral hemorrhages, traumatic brain injuries, and neurodegenerative diseases. Long-term treatment with citicoline has also shown to be well-tolerated and has not been associated with severe adverse events.5,51
However, more scientific evidence is needed to support these claims. More causal evidence is needed through the performing of animal studies, in addition to better controlled human studies, since in many of the trials presented here, no placebo was administered to the control groups.
Acknowledgments
Authors express gratitude to Meisys (Madrid, Spain) for writing assistance.
Footnotes
Author Contribution: All authors have contributed to data interpretation and the drafting, critical review, and revision of the manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Pedro E Bermejo  https://orcid.org/0000-0002-2496-4598
https://orcid.org/0000-0002-2496-4598
References
- 1. Grieb P, Jünemann A, Rekas M, Rejdak R. Citicoline: a food beneficial for patients suffering from or threated with glaucoma. Front Aging Neurosci. 2016;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martí-Carvajal AJ, Valli C, Martí-Amarista CE, Solà I, Martí-Fàbregas J, Bonfill Cosp X. Citicoline for treating people with acute ischemic stroke. Cochrane Database Syst Rev. 2020;8:CD013066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fioravanti M, Buckley AE. Citicoline (Cognizin) in the treatment of cognitive impairment. Clin Interv Aging. 2006;1:247-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jasielski P, Piędel F, Piwek M, Rocka A, Petit V, Rejdak K. Application of citicoline in neurological disorders: a systematic review. Nutrients. 2020;12:3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Secades JJ. Citicoline: pharmacological and clinical review, 2016 update. Rev Neurol. 2016;63:S1-S73. [PubMed] [Google Scholar]
- 6. Joosten H, van Eersel ME, Gansevoort RT, Bilo HJ, Slaets JP, Izaks GJ. Cardiovascular risk profile and cognitive function in young, middle-aged, and elderly subjects. Stroke. 2013;44:1543-1549. [DOI] [PubMed] [Google Scholar]
- 7. Srinivasa RN, Rossetti HC, Gupta MK, et al. Cardiovascular risk factors associated with smaller brain volumes in regions identified as early predictors of cognitive decline. Radiology. 2016;278:198-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gareri P, Castagna A, Cotroneo AM, Putignano S, De Sarro G, Bruni AC. The role of citicoline in cognitive impairment: pharmacological characteristics, possible advantages, and doubts for an old drug with new perspectives. Clin Interv Aging. 2015;10:1421-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stephan BC, Matthews FE, Khaw KT, Dufouil C, Brayne C. Beyond mild cognitive impairment: vascular cognitive impairment, no dementia (VCIND). Alzheimers Res Ther. 2009;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knopman DS, Petersen RC. Mild cognitive impairment and mild dementia: a clinical perspective. Mayo Clin Proc. 2014;89(10):1452-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jongsiriyanyong S, Limpawattana P. Mild cognitive impairment in clinical practice: a review article. Am J Alzheimers Dis Other Demen. 2018;33:500-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matthews FE, Stephan BC, McKeith IG, Bond J, Brayne C. Two-year progression from mild cognitive impairment to dementia: to what extent do different definitions agree? J Am Geriatr Soc. 2008;56:1424-1433 [DOI] [PubMed] [Google Scholar]
- 13. Harrison SL, Tang EY, Keage HA, et al. A systematic review of the definitions of vascular cognitive impairment, no dementia in cohort studies. Dement Geriatr Cogn Disord. 2016;42:69-79. [DOI] [PubMed] [Google Scholar]
- 14. Farooq MU, Min J, Goshgarian C, Gorelick PB. Pharmacotherapy for vascular cognitive impairment. CNS Drugs. 2017;31:759-776. [DOI] [PubMed] [Google Scholar]
- 15. Kim YH, Kwon OD. Clinical correlates of Hachinski ischemic score and vascular factors in cognitive function of elderly. Biomed Res Int. 2014;2014:852784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Di Nisio M, Prisciandaro M, Rutjes AW, Russi I, Maiorini L, Porreca E. Dementia in patients with atrial fibrillation and the value of the Hachinski ischemic score. Geriatr Gerontol Int. 2015;15:770-777. [DOI] [PubMed] [Google Scholar]
- 17. Cotroneo AM, Castagna A, Putignano S, Lacava R, Fantò F, Monteleone F, et al. Effectiveness and safety of citicoline in mild vascular cognitive impairment: the IDEALE study. Clin Interv Aging. 2013;8:131-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Putignano S, Gareri P, Castagna A, Cerqua G, Cervera P, Cotroneo AM., et al. Retrospective and observational study to assess the efficacy of citicoline in elderly patients suffering from stupor related to complex geriatric syndrome. Clin Interv Aging. 2012;7:113-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nemkova SA, Semenov DV, Petrova EA, Zavadenko NN, Vozvyshaeva MY. Vliyanie preparata Rekognan na sostoyanie vysshikh psikhicheskikh funktsii u patsientov s legkimi kognitivnymi narusheniyami [The effect of the use of the drug recognan (citicoline) on the state of higher mental functions in patients with mild cognitive impairment]. Zh Nevrol Psikhiatr Im S S Korsakova. 2021;121:51-57. [DOI] [PubMed] [Google Scholar]
- 20. Fioravanti M, Yanagi M. Cytidinediphosphocholine (CDP-choline) for cognitive and behavioural disturbances associated with chronic cerebral disorders in the elderly. Cochrane Database Syst Rev. 2005;2:CD000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suri S, Topiwala A, Mackay CE, Ebmeier KP, Filippini N. Using structural and diffusion magnetic resonance imaging to differentiate the dementias. Curr Neurol Neurosci Rep. 2014;14:475. [DOI] [PubMed] [Google Scholar]
- 22. Palesi F, De Rinaldis A, Vitali P, et al. Specific patterns of white matter alterations help distinguishing Alzheimer’s and vascular dementia. Front Neurosci. 2018;12:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jang H, Kwon H, Yang JJ, et al. Correlations between gray matter and white matter degeneration in pure Alzheimer’s disease, pure subcortical vascular dementia, and mixed dementia. Sci Rep. 2017;7:9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer’s disease—lessons from pathology. BMC Med. 2014;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Custodio N, Montesinos R, Lira D, Herrera-Pérez E, Bardales Y, Valeriano-Lorenzo L. Mixed dementia: a review of the evidence. Dement Neuropsychol. 2017;11:364-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gareri P, Castagna A, Cotroneo AM, Putignano S, De Sarro G, Bruni AC. The role of citicoline in cognitive impairment: pharmacological characteristics, possible advantages, and doubts for an old drug with new perspectives. Clin Interv Aging. 2015;10:1421-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang F, Wang S, Gan L, et al. Protective effects and mechanisms of sirtuins in the nervous system. Prog Neurobiol. 2011;95:373-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hurtado O, Hernández-Jiménez M, Zarruk JG, et al. Citicoline (CDP-choline) increases Sirtuin1 expression concomitant to neuroprotection in experimental stroke. J Neurochem. 2013;126:819-826. [DOI] [PubMed] [Google Scholar]
- 29. Cohen RA, Browndyke JN, Moser DJ, Paul RH, Gordon N, Sweet L. Long-term citicoline (cytidine diphosphate choline) use in patients with vascular dementia: neuroimaging and neuropsychological outcomes. Cerebrovasc Dis. 2003;16:199-204. [DOI] [PubMed] [Google Scholar]
- 30. Piamonte BLC, Espiritu AI, Anlacan VMM. Effects of citicoline as an adjunct treatment for Alzheimer’s disease: a systematic review. J Alzheimers Dis. 2020;76:725-732. [DOI] [PubMed] [Google Scholar]
- 31. Castagna A, Cotroneo AM, Ruotolo G, Gareri P. The CITIRIVAD study: citicoline plus rivastigmine in elderly patients affected with dementia study. Clin Drug Investig. 2016;36:1059-1065. [DOI] [PubMed] [Google Scholar]
- 32. Gareri P, Castagna A, Cotroneo AM, et al. The citicholinage study: citicoline plus cholinesterase inhibitors in aged patients affected with Alzheimer’s disease study. J Alzheimers Dis. 2017;56:557-565. [DOI] [PubMed] [Google Scholar]
- 33. Tanaka Y, Minematsu K, Hirano T, Hayashida K, Yamaguchi T. Effects of CDP-choline on dynamic changes in LCBF and cognitive function in demented subjects – an H2 15O-PET study. Rinsho Shinkeigaku. 1994;34:877-881. [PubMed] [Google Scholar]
- 34. Corona GI, Santagostino G, Frattini P, et al. Preliminary data on monoamine metabolite levels in cerebrospinal fluid and in urine during therapy in dementia. IRCS Med Sci. 1983;11:923-924. [Google Scholar]
- 35. Mijajlović MD, Pavlović A, Brainin M, et al. Post-stroke dementia – a comprehensive review. BMC Med. 2017;15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wolters FJ, Ikram MA. Epidemiology of vascular dementia: nosology in a time of epiomics. Arterioscler Thromb Vasc Biol. 2019;39:1542-1549. [DOI] [PubMed] [Google Scholar]
- 37. Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci. 2017;131:1059-1068. [DOI] [PubMed] [Google Scholar]
- 38. Alvarez-Sabín J, Román GC. The role of citicoline in neuroprotection and neurorepair in ischemic stroke. Brain Sci. 2013;3:1395-13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alvarez-Sabín J, Ortega G, Jacas C, et al. Long-term treatment with citicoline may improve poststroke vascular cognitive impairment. Cerebrovasc Dis. 2013;35:146-154. [DOI] [PubMed] [Google Scholar]
- 40. Bermejo PE, Dorado R. Citicolina en el tratamiento del deterioro cognitivo leve secundario a daño cerebral adquirido. Poster presented at: Sociedad Española de Neurología, 23 November-3 December 2020. [Google Scholar]
- 41. Alvarez-Sabín J, Santamarina E, Maisterra O, Jacas C, Molina C, Quintana M. Long-term treatment with citicoline prevents cognitive decline and predicts a better quality of life after a first ischemic stroke. Int J Mol Sci. 2016;17:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Que DS, Jamora RDG. Citicoline as adjuvant therapy in Parkinson’s disease: a systematic review. Clin Ther. 2021;43:e19-e31. [DOI] [PubMed] [Google Scholar]
- 43. Zhenguang L, Pengfei W, Zhancai Y, et al. Effect of citicoline adjuvant therapy on mild cognitive impairment in Parkinson’s disease. Int J Clin Exp Med. 2016;9:4593-4598. [Google Scholar]
- 44. Miners S, Kehoe PG, Love S. Cognitive impact of COVID-19: looking beyond the short term. Alzheimers Res Ther. 2020;12:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eskandar EN, Altschul DJ, de la Garza Ramos R, et al. Neurologic syndromes predict higher in-hospital mortality in COVID-19. Neurology. 2021;96:e1527-e1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sieracka J, Sieracki P, Kozera G, et al. COVID-19 - neuropathological point of view, pathobiology, and dilemmas after the first year of the pandemic struggle. Folia Neuropathol. 2021;59:1-16. [DOI] [PubMed] [Google Scholar]
- 49. Ostroumova TM, Chernousov PA, Kuznetsov IV. Cognitive impairment in COVID-19 survivors. Neurol Neuropsychiat Psychosom. 2021;13:126-130. [Google Scholar]
- 50. Turana Y, Nathaniel M, Shen R, Ali S, Aparasu RR. Citicoline and COVID-19-related cognitive and other neurologic complications. Brain Sci. 2021;12:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Agencia Española de Medicamentos y Productos Sanitarios. Somazina. Accessed November 26, 2021. http://cima.aemps.es/cima/pdfs/es/ft/53168/53168_ft.pdf


