Graphical abstract
Keywords: STimulator of INterferon Genes (STING), Patent landscape, Patent citation network, Agonists, Cancer immunotherapy, Clinical development
Highlights
-
•
R&D efforts and outcomes targeting STING have been rapidly increasing, giving rise to a growing number of drug candidates in clinic and increasing variation in their pharmacologic classes, with a similar trend observed in the patenting activities targeting STING.
-
•
STING patents, as a newly emerging therapeutic field, have exhibited extremely fragmental ownership and an intensively connected citation network compared with other more established fields.
-
•
Over 80% of all STING patents are related to agonist innovation, while less than 10% are focused on STING antagonists.
-
•
Patents for small-molecule STING agonists, including cyclic dinucleotides (CDNs) and non-CDN compounds, account for almost one-half of the nodes in the citation network and constitute the innovative basis of technological developments in this field.
-
•
Improving clinical responses and the potential applicability of STING agonists would be a valuable research focus of next-generation therapies targeting STING.
Abstract
Background
The STimulator of INterferon Genes (STING) plays an essential role in the innate immune system by inducing the expression of type I interferons (IFNs) and inflammatory cytokines upon sensing cytosolic DNA. Although modulating STING has shown promise as a potential treatment for cancers and inflammatory and autoimmune diseases in substantial pre-clinical studies, current preliminary clinical results of STING agonists have demonstrated limited anti-tumor efficacy. Currently, there is ongoing R&D targeting STING and focusing on the delivery of next-generation therapeutics. Whereas no comprehensive analysis on the STING patent landscape has been conducted to fill the gap between basic research progress and drug development and commercialization.
Aim of review
This study summarized the current agents in the clinical stage and global patenting profiles to help identify the current status, development trends, and emerging technologies of the nascent field of STING modulation.
Key scientific concepts of review
Rapidly increasing R&D efforts and outcomes targeting STING were indicated by the recently increasing number and pharmacologic classes of drug candidates in clinic as well as in emergent technological patenting activities. Despite the overall fragmental ownership of patents, several pioneers that have advanced the clinical evaluation of novel STING agonists have established the basis of STING-relevant inventions through their influential patents in the field. These patents also facilitated progress on novel STING modulators, relevant delivery systems, pharmaceutical compositions, and combination strategies with the potential for further enhancing therapeutic outcomes by targeting STING.
Introduction
The STimulator of INterferon Genes (STING) is a critical protein in the innate immune system that acts as a sensor of cytosolic DNA, including exogenous and endogenous DNA [1]. The activation of the STING pathway by recognition of cytosolic DNA can induce the production of type I interferons (IFNs) and other cytokines [1], [2], and modulation of STING activity has recently become an attractive therapeutic strategy in the field of immunology for the treatment of cancers, autoimmune diseases, and inflammatory diseases [3], [4].
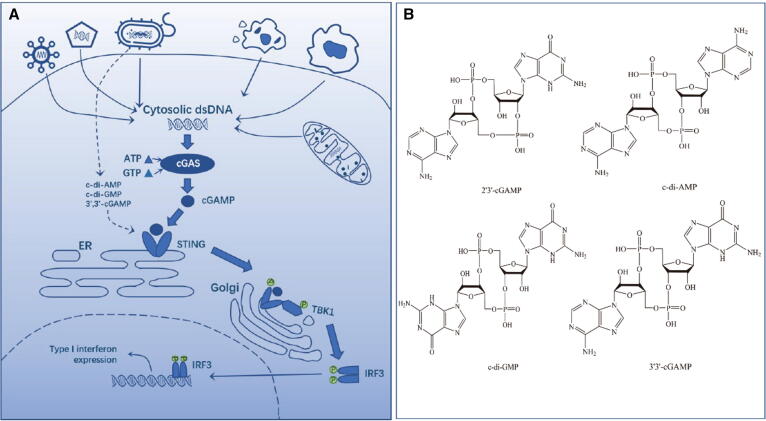
STING proteins are located in the endoplasmic reticulum (ER) and function as a symmetric dimer that can be activated by cytosolic DNA species (Fig. 1A). Double-stranded DNA (dsDNA) in the cytoplasm, which can be derived from pathogens (e.g., bacteria or viruses) and “self” cells (e.g., cancers, dead cells, or damaged mitochondria), binds to and activates cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS). The activated DNA-cGAS then catalyzes the synthesis of 2’,3’-cyclic GMP-AMP (2’,3’-cGAMP, Fig. 1B) using cytosolic GTP and ATP as substrates [5]. After the binding of 2’,3’-cGAMP to STING, STING is activated and the complex translocates from the ER to the Golgi apparatus, where STING recruits and activates TANK-binding kinase 1 (TBK1). The activated TBK1 in turn phosphorylates STING, which results in the recruitment and phosphorylation of interferon regulatory factor 3 (IRF3). The phosphorylated IRF3 is dimerized and enters the nucleus, resulting in the expression of type I IFNs and inflammatory cytokines and chemokines [6], [7]. The IFNs and cytokines have significant immune-stimulatory functions by promoting the priming and activation of T cells, dendritic cells (DCs), and natural killer (NK) cells [8]. In addition, bacteria-derived cyclic dinucleotides (CDNs), including cyclic dimeric GMP (c-di-GMP or CDG), cyclic dimeric AMP (c-di-AMP or CDA), and 3’,3’-cGAMP, can also directly bind to and activate STING like 2’,3’-cGAMP (Fig. 1B).
Fig. 1.
CDN-driven cGAS-STING signaling pathway for inducing type I interferon expression (A) and representative natural CDNs (B).
On the one hand, activation of STING pathway can stimulate innate and adaptive immune responses, which reflects its potential for cancer immunotherapy and action against infection by pathogens [9]. On the other hand, excessive IFN production caused by abnormal STING pathway activation or the aberrant accumulation of intracellular DNA may lead to the development of autoimmune diseases [10], [11] such that the inhibition of STING pathway may provide an option for the treatment of autoimmune diseases.
Drug development targeting STING modulators focuses on CDN-based agonists, which are designed with the expectation of overcoming the poor penetrating capability and high susceptibility to enzymatic degradation of natural CDNs [12]. More than 10 molecules are currently undergoing clinical evaluation [12]. The first and most advanced CDN-based STING agonist in clinical development, ADU-S100, was developed by Aduro Biotech in collaboration with Novartis for cancer treatment. However, Novartis dropped this asset from its portfolio at the end of 2019 due to preliminary clinical results [13], [14]. This may raise questions about the response performance of current CDN-based drug candidates under development but also encourages research on the underlying mechanism of STING signaling and next-generation STING modulators. Still, many novel agents targeting STING are under development and undergoing pre-clinical studies [15], and collaborations in the drug development industry in this area are still very active. This situation suggests the ongoing interest in STING pathway. Improvements in clinical responses and in the applicability of STING modulators, which are the focus of STING drug developments, should also consider various advances in new molecules, drug delivery systems, clinical strategies, drug interactions, and so on. This could also encourage patenting activities in this new therapeutic field from the perspective of protecting technological improvements and in light of the real potential for commercial development. Patent information often provides a critical view of emerging fields [16], [17], [18]. Despite extensive literature on the progress of the STING pathway and modulators, a global and comprehensive analysis focusing on the emerging patent landscape for the target STING, one which would help understand the innovation trajectory and technological development process, has not been conducted.
This study reviewed the current agents targeting STING under clinical development and investigated global patents related to STING to present their innovation status, developmental trends, and emerging technologies and to identify the most influential inventions in this field.
Data retrieval and analysis methods
The patent applications reviewed in this study were retrieved from the Derwent Innovation Index (DII) database, which covers more than 40 patent-issuing authorities and provides organized and comprehensive patent information based on original information. The search was performed with keywords related to STING (full names, abbreviations, or synonyms of the gene/protein) and Derwent Class (DC), as a code of B, pharmaceuticals. In addition, a keyword search was performed in the scope of the patent topic (TS), which basically combined titles, abstracts, and claims of patent information. The full search strategy was TS = (“Stimulator of Interferon Gene*” OR “transmembrane protein 173” OR “TMEM173” OR “Stimulator Of Interferon Response CGAMP Interactor” OR”Endoplasmic Reticulum Interferon Stimulator” OR “Mitochondrial Mediator of IRF3 Activation” OR “STING” OR “MPYS” OR “MITA” OR “HMITA” OR “ERIS” OR “NET23” OR “HSTING”) AND DC = (B*). Moreover, the search was performed to collect patent records published by the end of 2020. The dataset was obtained and reviewed to ensure the removal of all palladium-related hits, such as patent applications for medical devices, compositions for insect stings, non-human pharmaceuticals, and so on. After data clearance, a dataset that included the widest possible range of STING patents was prepared for further analysis.
The patent applications were grouped into families based upon the Derwent World Patents Index (DWPI) to ensure that patents in the same family referred to the same technological invention. A basic patent represents the first document disclosed in this family. Thus, each record of the dataset refers to a patent family with a basic patent as an identifier. The patent analysis in this research focused on family-based patent information, which generally includes the basic patent number, the application date, the applicants, the abstract, the claims, the International Patent Classification (IPC), the legal status, and the citation information. A fundamental step of the analysis was to normalize some non-standard information and organize a lengthy patent description to generate conclusions concerning the field of interest. The applicants’ names were deduplicated due to misspellings and abbreviations, and organizational applicants were grouped into two categories: public organizations (including universities, academic research institutes, hospitals, and government agencies) and private entities (i.e., privately held companies). To identify the partnering pattern of STING-related patents, co-patenting behaviors were collected among organizational applicants based on their sector category. If a member of a patent family was granted by any authority, this patent family was identified as granted.
Patent applications related to STING agonists were classified into several categories based on their technological subjects and applications:
-
•
Class i, small-molecule agonists targeting STING directly (STING agonists);
-
•
Class ii, other STING agonists, including non-small-molecule STING agonists and pharmaceutical compositions that can stimulate STING indirectly (for instance, these pharmaceutical compositions were described as modulators for a number of pathways and targets, which included STING);
-
•
Class iii, new uses for STING agonists, including combinational methods with other drugs for treating diseases and new applications for treating particular disorders with STING agonists;
-
•
Class iv, delivery system and formulations for STING agonists;
-
•
Class v, pharmaceutical compositions comprising STING agonists as a part of the compositions;
-
•
Class vi, other pharmaceutical compositions that could be used in combination with STING agonists.
Additionally, patent applications related to inhibiting the STING signaling pathway were grouped into a different class: Class vii, STING antagonists, including compositions of STING antagonists, uses or applications of STING antagonists, and so on. Patents that were excluded from the above classes were grouped into Class viii, others. These subject classifications can help to clarify the technological structure and development of innovations related to STING.
Patent citations can reveal the technological references between STING patents [18]. The patent citation network was generated based on citation information among all of the STING patents, considering patent family members, that could contribute to identifying a significant technological process and trends concerning STING-relevant innovations. If a member of a patent family was cited by any member of another family, then these two families were connected by an edge with directions pointing from the cited patents to the citing patents. The nodes represented patent families with colors based on the technological categories of the patents. The node degree was the number of connections of a patent family in the network, which was also the sum of the in-degree and out-degree of the patent. The in-degree of a node was the number of edges pointed to by other nodes and indicated how often the node was referenced by other patents, whereas the out-degree referred to the number of nodes pointed to by each node, reflecting how often the patent (the node) was referenced by other patent documents. Higher-degree patents represented more active interactions within STING innovations, thereby indicating greater importance in the network. Moreover, the largest component of the network, which included the most interconnected patents, was used to abstract a condensed subnetwork in terms of the importance of patents in the network and application years of basic patents in order to present a main developmental process of a STING innovation.
Before STING patent analysis, a summary on the progress of drug candidates targeting STING is necessary to help understand clinical development situation in this specific field.
Drug candidates targeting STING in clinical development
Given the importance of activating the STING signaling pathway in anti-tumor immunity and its promising anti-tumor activities in substantial pre-clinical studies, more than a dozen STING agonists have been advanced to clinical studies to evaluate their safety and efficacy in various cancer patients (Table 1). The first STING agonist that entered clinical development was ADU-S100, a synthetic CDN that was discovered by Aduro Biotech (acquired by Chinook Therapeutics in 2020). Since 2019, 10 agents have advanced to clinic, including two agents other than small molecules, an engineered bacterial strain, SYN-STING (SYNB1891) [19], [20], and a type of extracellular vesicle loaded with CDN, exoSTING [21]. All of these agonists are in Phase I or Phase II study as monotherapies or in combination with immune-oncology (IO) inhibitors, such as PD-1/PD-L1 (programmed cell death protein 1/programmed cell death protein 1 ligand 1) inhibitors and CTLA-4 (cytotoxic T-lymphocyte antigen-4) inhibitors. It should be noted that only three of these agonists have had preliminary clinical results released (Supplementary Table S1).
Table 1.
STING agonists in clinical development.
| Drug Candidates | Developers | Molecular Type | Administration Route | First Clinical Study Initiation | Clinical Study1 | Ref.2 |
|---|---|---|---|---|---|---|
| ADU-S100 | Aduro Biotech (acquired by Chinook Therapeutics) | CDN analog | IT | Mar-16 | NCT02675439 | [22] |
| NCT03172936 | [13], [14] | |||||
| NCT03937141 | ||||||
| MK-1454 | Merck Sharp & Dohme Corp | CDN analog | IT | Feb-17 | NCT03010176 | [23] |
| NCT04220866 | ||||||
| MK-2118 | Merck Sharp & Dohme | non-CDN molecule | IT/SC | Sep-17 | NCT03249792 | |
| GSK-3745417 | GlaxoSmithKline | non-CDN molecule | IV | Mar-19 | NCT03843359 | |
| BMS-986301 | Bristol-Myers Squibb, IFM Therapeutics | CDN analog | IV/IT/IM | Mar-19 | NCT03956680 | [24] |
| SB-11285 | F-star Therapeutics, Spring Bank Pharmaceuticals | CDN analog | IV | Sep-19 | NCT04096638 | [25] |
| IMSA-101 | Genor Biopharma; ImmuneSensor Therapeutics | CDN analog | IT | Sep-19 | NCT04020185 | |
| SYN-STING (SYNB1891) | Synlogic | Engineered bacteria vectors | IT | Nov-19 | NCT04167137 | [19], [20] |
| BI-1387446 | Boehringer Ingelheim | CDN analog | IT | Mar-20 | NCT04147234 | |
| TAK-676 | Takeda Oncology | CDN analog | IV | Mar-20 | NCT04420884 | |
| NCT04879849 | ||||||
| E-7766 | Eisai | non-CDN molecule | IT | Mar-20 | NCT04144140 | [26] |
| exoSTING | Codiak BioSciences Inc | extracellular vesicle loaded with CDN | IT | Sep-20 | NCT04592484 | [21] |
| SNX281 | Silicon therapeutics | non-CDN molecule | IV | Nov-20 | NCT04609579 | |
| HG-381 | HitGen Ltd | non-CDN molecule | IV | Aug-21 | NCT04998422 | |
| DN-015089 | Shanghai De Novo Pharmatech | undisclosed | IT | Oct-21 | CTR20212462 |
The designs of clinical studies with a NCT code could be identified in https://clinicaltrials.gov/.
References of pre-clinical studies and current clinical study data are listed in the table. Abbreviations: CDN, cyclic dinucleotide; IT, intratumoral; IV, intravenous; IM, intramuscular; SC, subcutaneous.
To improve the biostability of natural CDNs, ADU-S100 was developed to be resistant to digestion by phosphodiesterase and has demonstrated promising anti-tumor activities by upregulating type 1 IFNs and inducing tumor-specific CD8 + T cells in pre-clinical studies [27], [28]. This agent has been evaluated in three trials since 2016, with the preliminary results from trials NCT02675439 and NCT03172936 having been released [13], [14], [22], [29]. In ADU-S100 monotherapy arms (NCT02675439, dose escalation, intratumoral injection), no dose-limiting toxicities were reported, and Grade 3/4 treatment-related adverse events (TRAEs) were reported in 12.2% of 41 pretreated patients with various advanced solid tumors or lymphomas [22]. From the perspective of anti-tumor effectiveness, eleven patients achieved stable disease (26.8%), and two patients achieved partial responses (4.9%) [29]. In the Phase Ib study, which used ADU-S100 in combination with investigational PD-1 inhibitor spartalizumab (developed by Novartis), the preliminary results reported that of eight triple-negative breast cancer (TNBC) patients eligible for evaluation of efficacy, two patients achieved partial responses (25%), and one achieved completed responses (12.5%); of 25 melanoma patients radiologically eligible for evaluation of efficacy, nine patients achieved stable disease (36%), and two achieved partial responses (8%) [14]. Although limited, the efficacy data of the combination therapy seemed better than the results of the ADU-S100 monotherapy. However, the collaboration between Novartis and Aduro Biotech was discontinued because of these weak clinical data, and the enrollment of these clinical trials has also been suspended. A similar situation was seen in MK-1454, which was the second STING agonist assessed in clinic. MK-1454 (CDN, intratumoral injection) has been evaluated in a Phase I/II trial as a monotherapy or in combination with the PD-1 inhibitor, pembrolizumab, in patients with advanced/metastatic solid tumors or lymphomas since 2017 and in a Phase II trial in combination with pembrolizumab versus a pembrolizumab monotherapy in metastatic or unresectable, recurrent head and neck squamous cell carcinoma (HNSCC) since the beginning of 2020. In 2018, preliminary clinical study data were released, which also demonstrated a better efficacy profile in patients treated with MK-1454 in combination with pembrolizumab compared with MK-1454 monotherapy [23]. However, MK-1454 was removed from Merck’s pipeline in the second quarter of 2021 with no further clinical data disclosed, and recruitment for the two trials has been suspended in terms of their status on clinicaltrials.gov.
SYNB1891 was designed with the capability of localized, targeted STING activation by inducing the production of cyclic di-AMP [19]. Its first-in-human study was initiated to evaluate its safety and tolerability as a monotherapy (dose escalation, intratumoral injection) and in combination with the PD-L1 inhibitor, atezolizumab, in patients with refractory advanced solid tumors or lymphoma (Supplementary Table S1). Preliminary data from eleven patients with SYNB1891 monotherapy demonstrated no dose-limiting toxicities or discontinuations due to adverse events, with two patents achieving stable disease [20]. A further dose escalation study for SYNB1891 as a single agent and combination therapy data was expected at the end of 2021.
Besides, dozens of novel STING agonists in multiple pharmacologic classes have undergone pre-clinical study, including second-generation investigation by those who possess assets at the clinical stage as well as by many new participants, especially developers from China. For example, Merck, in collaboration with Vesselon, has used microbubbles and diagnostic ultrasound to enhance the tumor local concentration of a systemically administered CDN-based STING agonist [30]; F-star Therapeutics (under merger with Spring Bank Pharmaceuticals) is developing STING agonist antibody drug conjugates (ADCs) for targeted STING agonist delivery through systemic administration, a strategy also under investigation by Takeda (under in-licensing from Curadev Pharma) [31] and Mersana Therapeutics [32]. These novel agents may have the potential for improved safety and efficacy profiles in future clinical studies and more convenient administration routes.
Moreover, agents inhibiting the STING signaling pathway are also under investigation for disorders associated with aberrant STING activation, like some autoimmune and inflammatory disorders [33], [34]. Several STING antagonist programs are undergoing pre-clinical study and discovery process, although none has yet been advanced to clinical evaluation [35], [36]. Despite the increasing attention on STING antagonist drug development recently, implied by several big collaborations in this field recently, none of them has entered clinical development. The development of drug candidates targeting STING indicates ongoing R&D on the STING pathway, despite the limited and modest clinical data generated thus far. More investigational efforts are needed to illustrate the mechanisms of action of agents and potential issues when being translated to the clinic. In this context, an overall analysis of patents concerning STING, one which would reflect activities from the perspectives of both basic research and industrial applications, could assist in identifying relevant technological innovation trends and important inventions in this field in order to help participants pursue and realize effective developmental strategies.
Patenting profiles
The STING patent data used in this study were obtained from the DII database. A direct search yielded 1,714 patent families published by the end of 2020, and 588 patent families were further obtained for data analysis by manual reading and noise reduction.
Overview of STING patent applications
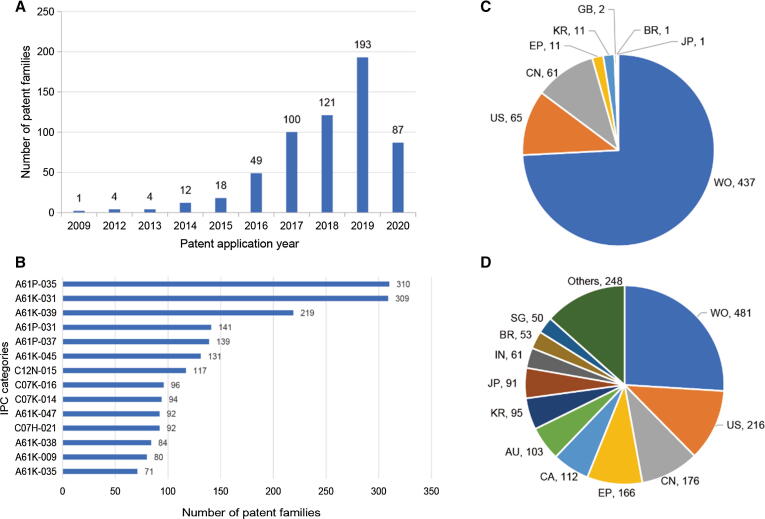
Fig. 2A describes the continuously increasing number of patent applications related to STING over time, except for the patenting activity in 2020. The first STING-related patent, WO2010017248, was applied in 2009 by a team from the University of Miami who had discovered and identified the function of STING in innate immune signaling processes in 2008 [2]. Due to the significant improvement in the understanding and applications of cancer immunotherapy represented by PD-1/PD-L1 inhibitors since 2010, active R&D on and promising pre-clinical performance of STING culminated in a great number of patenting activities from 2016 to 2019, which was much sooner from the first report of STING’s identification in comparison with R&D targeting PD-1/PD-L1 [17]. The significant decline in these activities in 2020 was most likely caused by the COVID-19 pandemic, which restricted R&D efforts and investments from areas other than those devoted to combatting COVID-19 [37].
Fig. 2.
Profiles of global STING patent applications. (A) Annual number of STING patent applications by the year of application of the first member of the patent family (2009–2020). (B) The most frequent international patent classification (IPC) categories of patents targeting STING. The technical areas of these categories can be accessed in the World Intellectual Property Organization (WIPO), https://www.wipo.int/classifications/ipc/en. (C) Country distribution of STING patents by the basic country of patent families. (D) Country distribution of STING patents by application authorities of all of the members in the patent families.
The most frequent IPCs identified by STING patents are listed in Fig. 2B, which emphasizes medicinal preparations with organic active ingredients (A61K-031) as well as their potential as antineoplastic agents (A61P-035) and anti-infectives (A61P-031) and for combatting immunological disorders (A61P-037). More specific technological categories for STING are presented in section of Data retrieval and analysis methods. Of all of the STING patents, 110 families (∼19%) had been granted by at least one authority. This percentage of granted patents was higher than that in the patent landscape analysis of recombinant factor VIII [38] and induced pluripotent stem cell technologies [39].
Almost three-quarters of STING basic patents, referring to the first member of the patent family that was published, were international patent applications filed under the Patent Cooperation Treaty (PCT) (Fig. 2C). This indicates the extent of interest by patent applicants in pursuing patent protection across the world. A similarly high percentage of PCT applications was also observed in patent analysis targeting PD-1/PD-L1 [17]. Taking all members in all patent families into account, the geographic distribution showed that the United States, China, and Europe were the most active countries/regions for STING patent applications, followed by Canada, Australia, South Korea, and Japan (Fig. 2D). The significant position of China and the subtle difference between China and the United States regarding the filings of basic patents and national patent applications is particularly noteworthy. Comparing patents concerning PD-1/PD-L1 [17], China is becoming a much more attractive area for investigators seeking patent protection in such a new field, in turn also indicating that new drug development in China is becoming increasingly active.
Patent applicants and partnerships
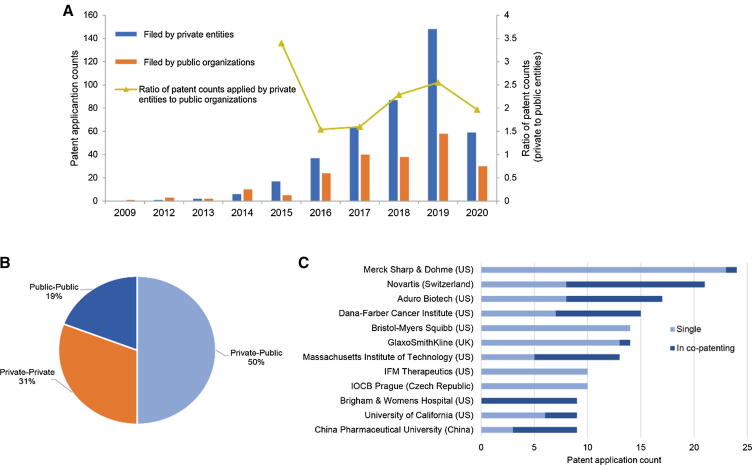
Of all the STING patent families, 421 (72%) were filed by at least one entity in the private sector (i.e., companies from an industry), and 211 (36%) were filed by organizations from the public sector (i.e., universities, hospitals, government/academic institutes). Different patenting activity trends were observed for private entities and public organizations (Fig. 3A). Industrial applicants surpassed those from the public sector and filed more patents since 2015, quickly becoming the main force of STING innovation, especially after the first STING agonist entered clinical development in 2016. This trend is also suggested by the continuously growing gap in patent applications between private and public applicants.
Fig. 3.
Applicant profiles of STING patents. (A) Patenting activity trends for private entities and public organizations. (B) The co-patenting patterns in terms of different sectors of the organizational applicants. (C) The leading applicants that filed the most patents and its relevant percentage of co-patenting activities.
Of all STING patents, 104 (18%) were filed by more than one organizational applicant. Regarding the co-patenting profile in terms of the applicants’ sector, one-half of these patents were the outcome of cooperation between private entities and public organizations, which was much higher than co-patenting activities among those within either the public sector or the private sector alone (Fig. 3B). The leading applicants demonstrated internationally based contributions to STING innovation and were equally split between the public and private sectors (Fig. 3C).
Leading applicants from industry, i.e., Merck Sharp & Dohme, Novartis, Aduro Biotech, Bristol-Myers Squibb, GlaxoSmithKline, and IFM Therapeutics, were the first tier of entrants to develop STING agonists in clinic. Merck Sharp & Dohme is positioned in the first place, with 24 patents filed (4.1%), a slight number of which were in cooperation with other applicants. Novartis and Aduro Biotech, on the other hand, appeared to have similar co-patenting profiles, and more than one-half of their patents were filed in cooperation. These patents were developed mostly based on the established partnership between Novartis and Aduro Biotech. The co-patenting activities of leading applicants from the public sector were more intensive among organizations in the same geographic region, such as cooperation among the Dana-Farber Cancer Institute, the Massachusetts Institute of Technology, and Brigham & Women’s Hospital (Boston, US), and between the China Pharmaceutical University and Nanjing University (Nanjing, China). Moreover, the low percentage of the leading applicants of STING patent filing was much lower than that in the profile in the established field of recombinant factor VIII: 4.1% versus 8.4% for the applicant in the first place [38]. This revealed a highly fragmented patenting landscape in this new field. The difference in terms of maturity in technological and market development could be the main reason for the different concentration profiles between the two fields.
Technological innovation trends
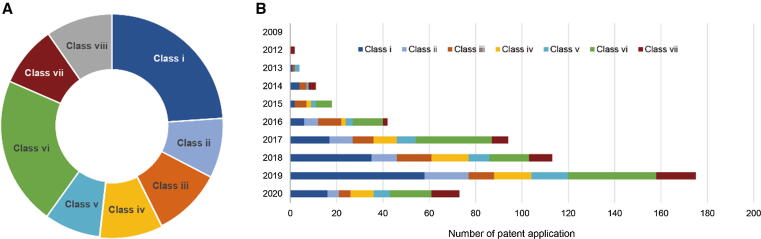
To be able to present technological subjects of STING-specific innovations, all STING patents were grouped into eight classes, Class i ∼ viii, the construction and activity trends of which are shown in Fig. 4. Patents in Class i ∼ vi focused on different subjects related to STING agonists, including (i) small-molecule STING agonists, (ii) other STING agonists, (iii) new uses for STING agonists, (iv) STING agonist delivery systems or formulations, (v) pharmaceutical compositions comprising STING agonists as a part, and (vi) other compositions that could be used in combination with STING agonists. Patents related to STING antagonists were classified as a separate group, Class vii.
Fig. 4.
Technological subject classes of STING patents. (A) Distribution of patent technological subject classes, Class i ∼ viii. (B) Patenting activity trends and construction of technological classes over the years. Class i, small-molecule STING agonists; Class ii, other types of STING agonists; Class iii, new uses for STING agonists; Class iv, STING agonist delivery systems or formulations; Class v, pharmaceutical compositions comprising STING agonists as a part; Class vi, other compositions that could be used in combination with STING agonists; Class vii, patents related to STING antagonists; Class viii, others.
Patents related to STING agonists (Class i ∼ vi) accounted for over 80% of all STING patents, while less than 10% were related to STING antagonist patents (Class vii) (Fig. 4A). The obvious trend toward STING activation in patenting activities is consistent with the drug development profile. The increase in the number of patents filed for new small-molecule STING agonists has principally contributed to the rapid growth of STING patenting activities (Fig. 4B), whereas other types of pharmaceutical compositions that can stimulate STING (Class ii), such as vectors (that can encode STING or cGAS), polypeptides, oncolytic virus, and so on, were in a less significant position. A high proportion of patents for new uses of STING agonists (Class iii) could be observed in the early years (Fig. 4B). Regarding the focus of this class, patenting activities concerning new uses of STING agonists in these early years were mainly concentrated on applications of STING agonists for various disorders rather than on innovations focused on new combinations of STING agonists with other pharmaceuticals for treating diseases. Of all 59 patents filed in Class iii, 36 (∼63%) were focused on combinatorial uses or potential combinatorial uses of STING agonists. These combination strategies could provide important references for the clinical development of STING agonists. Specifically, one of the important categories of other pharmaceuticals in combinations were drugs targeting the immune system in cancers, such as inhibitors of PD-1/PD-L1, CTLA-4, indoleamine 2,3-dioxygenase (IDO) inhibitors, lymphocyte activation gene 3 (LAG3), and CD73.
Patenting for delivery systems or formulations for STING agonists (Class iv) is a significant aspect of STING technological innovations (accounting for 9% of STING patents), with intentions of improving biostability and/or bioavailability as well as of delivering agents to and retaining agents in target tissues, especially for CDN-based STING agonists. Applications in Class iv exhibited a notable increase in 2017, after which they became an important component of STING innovation. Patent filings related to STING antagonists or the inhibition of STING signaling (Class vii) began early. After two years of inactivity, in 2015 ∼ 2016, patenting concerning STING antagonists is now demonstrating a continuous increase in proportion to all patents, mainly focusing on the discovery of new pharmaceuticals as STING antagonists and new uses for treating autoimmune or infectious diseases.
Patent citation network
Citation network profiles
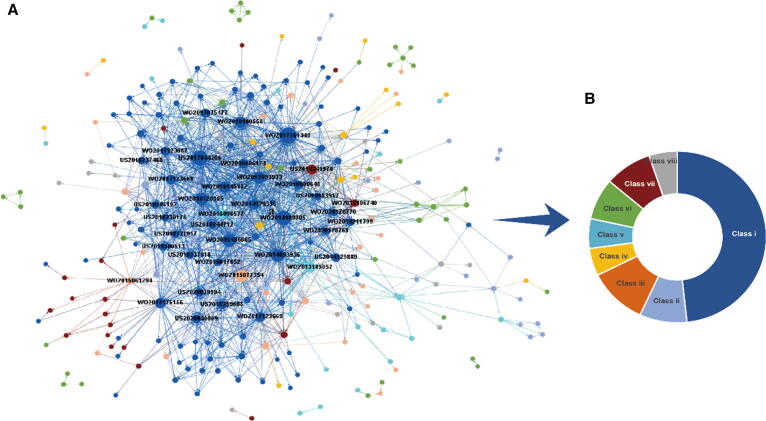
Citation relationships among patents in a specific field can provide a developmental perspective on technological connections and emerging technologies. The citation network of STING patents involved 269 applications and 1,301 citation connections among them, and the patents (nodes) were colored according to their technological classes (Fig. 5A). The node size was set by its degree value, i.e., the number of connections with other patents in the network. In the network, 86% of nodes were connected as the largest component, with many small components around them. This largest component consisted of more than 80% of all patents filed regarding small-molecule STING agonists, much higher than the network involvement of other technological classes. This class also included almost one-half of all patents in the largest component, doubling the percentage of all STING patents (Fig. 4A, 5B). Moreover, the average degree, referring to the average connections per node in the network, increased from 9.7 for the whole network to 14.2 for the network within patents in Class i by removing patents from other classes, which identified intense references among Class i. This broad, intense involvement of patents in Class i in the network implies a high dependence on innovation in the field and high technical coherence within the development of small-molecule STING agonists.
Fig. 5.
Patent citation network and relevant technological distribution. (A) Citation network among STING-related patents. (B) Technological class distribution of patents involved in the largest component of the network. Nodes in the network represent patent applications, and the edges represent citation connections between patent families, pointing from the cited patents toward the citing ones. The node size was set by its degree value, which was also the sum of connections of each node. The colors of nodes in the network and the donut chart reflect the technological subject class of the patents, which are consistent with that in Fig. 4.
Patents for new uses of STING agonists (Class iii) constituted one of the classes that entered the network in the earliest years (2013 ∼ 2015). Especially in 2013, the patent filed by Glen N. Barber was described as uses of modulating immune responses by administrating STING modulators and was cited by two important patent applications by Aduro Biotech (WO2013185052 and WO2014093936, also see Table 2) as indicated by their high degree, which disclosed hundreds of agents of CDN-based STING agonists, including ADU-S100. Then, the network expanded based on these patents and grew to one with intense technological interaction. Patents concerning STING agonist delivery systems or formulations (Class iv) revealed a different scenario, with the lowest percentage of patents in the largest component of the network (∼4.3%, Fig. 5B) and relatively late involvement in the network (from 2017). The most interactive patents regarding STING antagonists (Class vii) in the network, represented by patents with a high degree, were US2014341976 and WO2017106740, also filed by Aduro Biotech, in 2014 and 2016, respectively. In addition, most of the other STING antagonist patents were located in the periphery of the network and were connected with a small number of others. The STING agonist patent WO2017175156, filed by GlaxoSmithKline, and the patent of a new use of STING agonists for treating hepatitis B viral (HBV) infection, WO2015061294, filed by Drexel University, were the two inventions referred to by many STING antagonist patent applications (Fig. 5A).
Table 2.
Detailed information of the influential patent families with high-degree centrality in the patent citation network.
| Basic Patent Number1 | Degree | Out-degree | In-degree | Ratio of Out- to in-degree | Applicant | Technological Subject | Representative Compounds2 |
|---|---|---|---|---|---|---|---|
| WO2017161349 | 92 | 38 | 54 | 0.7 | ImmuneSensorTherapeutics, University of Texas System | CDN-based agonists | Compound 1,2 |
| US2017044206 | 83 | 58 | 25 | 2.32 | Merck Sharp & Dohme | CDN-based agonists | Compound 3 |
| WO2014189805 | 78 | 63 | 15 | 4.2 | University of California, Aduro Biotech | CDN-based agonists |
Compound 4, 5 |
| WO2015185565 | 67 | 54 | 13 | 4.15 | GlaxoSmithKline | CDN-based agonists | Compound 10 |
| WO2014093936 | 64 | 49 | 15 | 3.27 | Aduro Biotech | CDN-based agonists | Compound 6 |
| WO2016120305 | 61 | 48 | 13 | 3.69 | GlaxoSmithKline | CDN-based agonists | Compound 11 |
| WO2017093933 | 59 | 39 | 20 | 1.95 | GlaxoSmithKline | CDN-based agonists | Compound 12,13 |
| WO2015077354 | 56 | 55 | 1 | 55 | University of Chicago, Aduro Biotech | Method of treating cancers with STING agonists | Compound 4 |
| WO2018100558 | 55 | 8 | 47 | 0.17 | Takeda Pharmaceutical | CDN-based agonists | Compound 21 |
| WO2016145102 | 50 | 33 | 17 | 1.94 | Aduro Biotech | CDN-based agonists | Compound 7, 8 |
| WO2014179335 | 48 | 33 | 15 | 2.2 | Sloan Kettering Institute, Rockefeller University, Rutgers University, University of Bonn | CDN-based agonists | Compound 22 |
| WO2017175156 | 47 | 40 | 7 | 5.71 | GlaxoSmithKline | ABZI-based agonists | Compound 14,15 |
| WO2013185052 | 45 | 39 | 6 | 6.5 | Aduro Biotech, Johns Hopkins University | A composition comprising a CDN-based agonist and inactivated tumor cells | N/A |
| WO2017175147 | 40 | 33 | 7 | 4.71 | GlaxoSmithKline | diABZI-based agonists | Compound 16,17 |
| WO2017123669 | 40 | 25 | 15 | 1.67 | Innate Tumor Immunity | CDN-based agonists | Compound 23 |
| US2014341976 | 40 | 37 | 3 | 12.33 | Aduro Biotech | CDN-based antagonists | Compound 9 |
| WO2016096174 | 39 | 35 | 4 | 8.75 | InvivoGen, Kayla Therapeutics | CDN-based agonists | Compound 24 |
| WO2018009648 | 36 | 9 | 27 | 0.33 | Sperovie Biosciences | CDN-based agonists | Compound 25 |
| WO2017123657 | 36 | 33 | 3 | 11 | Innate Tumor Immunity | CDN-based agonists | Compound 26 |
| US2020040009 | 35 | 0 | 35 | 0 | Incyte | Tricyclic heteroaryl compounds as STING agonists | Compound 18 |
| US2020039994 | 35 | 0 | 35 | 0 | Incyte | Tricyclic ABZIs as STING agonists | Compound 19 |
| US2019359608 | 35 | 0 | 35 | 0 | Incyte | Tricyclic ABZIs as STING agonists | Compound 20 |
Abbreviations: CDN, cyclic dinucleotide; ABZI, amidobenzimidazole.
The original patent document can be observed by free access to Espacenet patent search, https://worldwide.espacenet.com/.
Structure of representative compounds of relevant patents can be found in Fig. 6.
Influential patents in the network
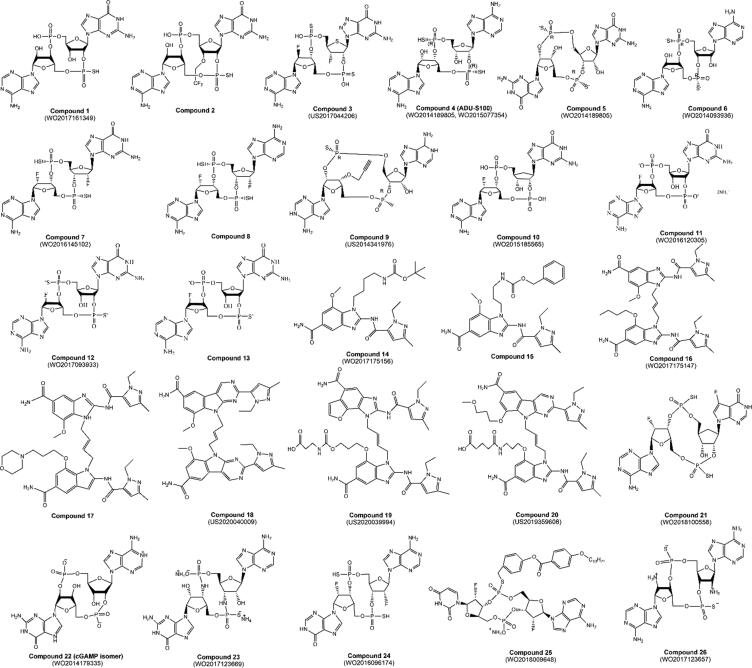
Patents with a high degree of centrality, summarizing out-degree and in-degree, are hubs in the field of interest with many technological connections with others. Table 2 lists these influential inventions, which were identified with the highest degree. All of these patents were filed by companies from the pharmaceutical/biotechnological industry or in collaboration with academic institutes, except for patent WO2014179335, which was co-filed by four academic institutes. The applicants also included the pioneers of STING agonist clinical development, like Aduro Biotech, Merck Sharp & Dohme, GlaxoSmithKline, Immune Sensor Therapeutics, Takeda Pharmaceutical, and IFM Therapeutics. This also indicated the significant contribution from companies in drug development to the intense knowledge flow in innovations related to the exploration of the target STING, implied by basic technologies with a high out-degree that had been cited by subsequent patents and technology summarizers with a high in-degree that had been widely referred to in the prior state of the art. Specifically, the value of the ratio of out- to in-degree was larger than 1, thereby reflecting the incline of a patent as a reference for others comparing its role as a summarizer. More than one-half of these influential patents were positioned as the basic inventions broadly cited by various classes of patents related to STING. Notably, 19 of 22 of these patents focused on small-molecule STING agonists, indicating that inventions concerning STING agonists were the basic foundation of this innovation network.
ImmuneSensor Therapeutics Inc.
Patent WO2017161349, co-filed by ImmuneSensor Therapeutics and the University of Texas System in 2017, had the largest number of total connections to others, with the highest in-degree. This patent disclosed many novel CDNs with modifications on interior phosphor linkages, ribose, and nucleobases. Several of these CDNs (Fig. 6, Compound 1, 2) were reported to have a more promising effectiveness profile (EC50 less than 100 nM or less than 30 μM in THP-1 luciferase reporter cells with or without perfringolysin O to facilitate the compound uptake) as well as the capability of resisting the enzymatic hydrolysis of ENPP1 (less than 10% decrease of the EC50 value after fetal bovine serum incubation) [40]. Since 2019, Immune Sensor Therapeutics has been evaluating the safety and efficacy of IMSA-101 (a CDN-based small molecule) alone or in combination with immune oncology therapies in patients with advanced treatment-refractory solid tumors in a Phase I/IIa clinical trial (NCT04020185, Supplementary Table S1).
Fig. 6.
Representative compounds in the influential patents listed in Table 2.
Merck Sharp & Dohme Corp.
Researchers from Merck Sharp & Dohme generated hundreds of CDNs as STING agonists in patent US2017044206, which had been cited by another 58 patents in the network. These compounds were evaluated in an in vitro STING binding assay (3H-cGAMP filtration binding assay) and IFN-β release in THP1 cells. One of them, Compound 3, exhibited a relatively high binding activity with EC50 of less than 1 nM, and four times the IFN-β production of more than 2ʹ,3ʹ- cGAMP at a concentration of 30 μM [41].
Besides, Merck Sharp & Dohme also filed many other STING patents involved in the network, although the company did not present very comprehensive connections, including patents on CDN-based compounds (US2018244712, WO2019125974, WO2018208667, etc.), benzo[b]thiophen derivatives as STING agonists (US2019337917, WO2019195063, US2018093964, etc.), a new combination use of STING agonists with PD-1 antagonists (WO2019027857, WO2018118664), and a new formulation and compositions for specific drug delivery (WO2020205323, WO2015130584).
Aduro Biotech Inc.
The STING agonist ADU-S100 (dithio-[Rp,Rp]-cyclic-[A(2ʹ-,5ʹ-]pA(3ʹ-,5ʹ-)p), also named ML-RR-CDA (Compound 4, Fig. 6), which first entered clinical research, was disclosed in patent WO2014189805, which was discovered in cooperation between Aduro Biotech and University of California. This patent filing, with the highest out-degree, provided important references for many patents for novel STING agonist identification and applications. In the patent, ADU-S100 and Compound 5 (also named dithio-[Rp,Rp]-cyclic-[G(2ʹ-,5ʹ-]pG(3ʹ-,5ʹ-)p) or ML-RR-CDG) exhibited the strong ability to induce type I IFN production in THP-1 cells and human PBMCs, higher than 2′3′-c-di-AMP and 2′3′-c-di-GMP [42]. ADU-S100 could significantly inhibit tumor growth and metastasis in mice bearing various tumors. Moreover, ADU-S100 exhibited a similar potent resistance to phosphodiesterases (SVPD and NP1) with Compound 6 (dithio-[Rp,Rp]-cyclic-[A(3ʹ-,5ʹ-]pA(3ʹ-,5ʹ-)p), which was discovered by Aduro Biotech earlier in patent WO2014093936.
Aduro Biotech also filed patent WO2015077354 to demonstrate the anti-tumor potential of DMXAA and CDNs by STING pathway activation, leading to a high production of IFN-β, IL-6, TNF-α, and IL-12, enhanced expression of CD86, and profound anti-tumor efficacy in mice models [43]. Other CDN derivatives with modifications on ribose were also reported by Aduro Biotech (represented by Compound 7, 8 in WO2016145102).
Moreover, STING-ADCs where CDN-based STING agonists were conjugated to a specific antibody by a cleavage linker for treating cancers have been reported by Aduro Biotech and Novartis in patents WO2020092617 and WO2020089815 as second-generation compounds.
GlaxoSmithKline PLC
Another prominent patent applicant was GlaxoSmithKline, which also started its investigation of STING stimulators from CDN-based compounds, i.e., WO2015185565, WO2016120305, and WO2017093933, and then further designed a class of amidobenzimidazole (ABZI)-based STING agonists and developed GSK-3745417, the only non-CDN compound as a STING agonist in clinical development. Patent WO2017175156 first disclosed numerous ABZI-based compounds (represented by Compound 14, 15) that exhibited high binding affinities of STING by competition with 3H-cGAMP (pIC50: 3.7 ∼ 6.0) and induced IFN production in human embryonic kidney cells (HEK293T) (pEC50: 6.0 ∼ 6.2) [44]. These activities were significantly improved by further modifications, as diABZIs in patent WO2017175147 (represented by Compound 16, 17), some of which exhibited much higher pIC50 values of larger than 9.0, and a pEC50 of larger than 7.0 [45]. The diABZI, Compound 17, was further reported in patent WO2019069275 with considerable anti-tumor efficacy based on IV administration, which could be rapidly distributed into tumors with a half-life of about 25 h, induce the production of multiple cytokines and MHC-I expression in NK, B, and T cells, and activate CD8 + T cells [46].
IFM Therapeutics Inc.
IFM Therapeutics is another biotech company that focuses on modulating innate immune system including STING pathway. For STING stimulators, IFM Therapeutics filed two important patents (WO2017123669 and WO2017123657) that were assigned to Innate Tumor Immunity under the collaboration of IFM Therapeutics with Bristol-Myers Squibb (Table 2). The patents disclosed dozens of synthetic CDN derivatives and their preparation process, represented by Compound 23 that can stimulate IRF3 with an EC50 of 1 ∼ 10 μM and activate NF-κB signaling with an EC50 of less than 1 μM [47]. Besides STING stimulators, IFM also filed many patents related to STING inhibitors.
Incyte Corp.
Researchers from Incyte synthesized a series of diABZI-based compounds with tricyclic modifications in patents US2020040009, US2020039994, US2019359608, and WO2020146237 that exhibited the activation of IRF3 in a THP-1 dual cell-based assay with an EC50 of less than 100 nM [48], [49], [50], [51]. In contrast, no STING agonist has been released in Incyte’s pipeline so far.
Patents of STING antagonists
Patents of STING antagonists were more likely to be positioned in the periphery of the network with fewer connections with other patents in the field. The most influential STING antagonist patent in the network was filed by Aduro Biotech in 2014, US2014341976. The patent described ML-propargyl-CDA (2ʹ-O-propargyl-cyclic-[A(2ʹ,5ʹ)pA(3ʹ,5ʹ)p] CDA, Compound 9), which can bind to the same binding pocket of STING and thus interfere with the binding of activating CDNs [52]. Following this, patent WO2017106740 and WO2019055750 in the network were also filed by Aduro Biotech, which disclosed other CNDs as STING antagonists.
Like Aduro Biotech, other pioneers in STING pathway also have initiated STING inhibitor development through internal R&D or external partnerships, which include IFM Therapeutics, Novartis (through partnering with IFM Therapeutics), GlaxoSmithKline, Bristol-Myers Squibb (under acquisition of Celgene), and Eli Lilly (through partnering with Aduro Biotech). Moreover, more types of molecules have been disclosed with inhibiting STING function to suppress immune response, and have showed their potential for treatment of autoimmune and inflammatory diseases. For instance, IFM DUE (a subsidiary of IFM Therapeutics) described a bunch of non-CND compounds with potent STING inhibitory activity in several patents, such as WO2020150439 (compounds of indole-based sulfonamide derivatives), WO2020010092 (pyrrolopyridine-based urea derivatives), WO2020010155 (pyrazole Urea-Based derivatives), and so on. These new patent applications targeting STING antagonists extended the citation network of the field of interest, which represents the new drug development opportunities of targeting STING.
Disease treatment potential of STING patents
In addition to the treatment exploration on cancers and typical autoimmune and inflammatory diseases, modulating STING also shows a new therapeutic potential for some specific disorders (e.g., liver disease and central nervous system disease) as the improving understanding of the role of STING pathway [53], [54]. However, in the patent applications, cancers still are the most prevalent use for STING monotherapy or in combination with other drugs, which is consistent with the drug development profile in pre- and clinical studies. Moreover, although many indications are always covered by proposed claims of a STING patent application, few have presented an appropriate example to demonstrate the activity of STING modulators in an exact in vitro or in vivo model of a specific disease in patent documents, especially for a specific inflammatory and autoimmune diseases. Specifically, patent WO2015061294 in the citation network first reported that DMXAA, a STING agonist, could result in suppressed HBV DNA replication and reduced the amounts of HBV capsid protein in mouse hepatocytes through inducing type I IFN production in mouse macrophages [55]. This patent application of STING agonists against HBV infection has been approved by the authority of China, the United States, and Australia. Additionally, the patents CN106540260 and CN106539814 described the activities of STING agonists against Alzheimer’s disease and rheumatoid arthritis respectively through in vivo mouse models, while neither of them was approved by the authority.
Patents of STING drug delivery system
Delivering STING modulators to specific tissues is an important direction of developing next-generation STING drugs that overcome the drawbacks of current ones, especially CDN-based STING agonists. In the citation network, patents on delivery system of STING drugs involve compositions of CDNs and carrying systems, such as exosome (WO2019183578), liposomes and other lipid-based carriers (WO2020210317, WO2017186711), hydrogels (WO2018045058), and so on.
More specifically, patent WO2019183578 describes a prostaglandin F2 receptor negative regulator (PTGFRN)-overexpressed exosome comprising CDN-based STING agonists, one which exhibited significantly potent anti-tumor efficacy in a 100-fold lower dose of free STING agonists and highly promoted the selectivity of STING agonist delivery to DCs [56]. Patent WO2020210317 discloses phosphatidylserine (PS)-coated nanoparticles (NP) containing cGAMP (NP-cGAMP), which exhibited significantly more efficient cytosolic uptake of cGAMP than free cGAMP and higher production of type I IFNs in mouse antigen presenting cells [57]. Another example further showed significantly synergic antitumor effect of NP-cGAMP (via inhalation) in combination with radiotherapy in both mouse melanoma and breast cancer lung metastases. Lipid agent complexing with CDNs in order to enable CDNs’ cellular uptake was prepared and demonstrated that the lipid-CDN complex could increase production of various cytokines by several to hundred fold than free CDNs in both in vitro and in vivo models in patent WO2017186711 [58]. This patent further disclosed the anti-tumor activity of lipid-CDN complex at five times lower drug concentration was similar with that of CDN. This obviously indicated the potential of lipid-encapsulated STING agonists in reducing the systemic toxicity of free agonists.
Patent WO2018045058 disclosed a locally slow dissemination system using hydrogels loaded with compounds of interest including STING agonists. The hydrogel drug delivery mode could be implanted in the tumor site. In vivo studies showed significant durable survival benefit with the CDN-payload modes than intratumoral injection of CDNs [59].
Conclusion and perspectives
This study showed rapidly increasing R&D activity targeting STING since its important role in the innate immune response was first reported in 2008. There is an obvious incline toward STING agonists other than STING inhibitors in terms of drug development as well as patenting activities. Several dominant companies in this field, like Audro Biotech, GlaxoSmithKline, IFM Therapeutics, and Merck Sharp & Dohme, have pioneered advancements in the clinical evaluation of novel STING agonists and also established the field of technological innovation of STING with their influential inventions in the network. These innovations were based on generating new small-molecule STING agonists to improve biological activity and overcome pharmaceutical shortages of natural CDNs and were intended for the development of next-generation therapies to improve clinical responses and potential applicability.
Patenting landscape: STING versus PD-1/PD-L1
Targeting the STING pathway is a promising IO strategy as it stimulates the innate immune system and enhances tumor immunogenicity, turning “cold tumors” into “hot tumors” [60]. The rich development experience of checkpoint inhibitors, represented by PD-1/PD-L1 inhibitors, generally promoted the mechanism of understanding and the clinical applications of cancer treatment by targeting the immune system, which helps the rapid translation of STING agonists to clinic.
The profile of STING patents was similar with that of the PD-1/PD-L1 field from some perspectives, such as the significant growth in a short period before the studies, fragmented ownership, and the similar citation network structure (i.e., one main component surrounded by several small ones) [17]. More notably, the patenting activities concerning STING also appear with some particular aspects. First, the patent citation network of STING is much denser than that of PD-1/PD-L1, with a network average degree of 9.6 versus 4.1, respectively. This means that new inventions regarding STING were highly connected with previous patents, especially the discovery of new STING agonist molecules. Second, although most patents filed in the two fields are intended to secure patent protection across the world via the first filing under PCT, the national distribution of patent applications portrayed a significant increase in interest in China as a potential market of STING development. Third, the involvement of companies from the private sector was much more extensive and active in cooperative innovation in the field of STING, including the co-patenting activities between the public and private sectors and among those in the private sector. Whereas, in the field of PD-1/PD-L1 patents, one-half of co-patenting activities were observed among the public sector alone. In this study, some fundamental STING patents were invented based on collaboration between a biotech company and universities, and most of the co-developments concerning therapeutics targeting STING between big pharma and small to medium-sized biotech companies started as early as in the pre-clinical stage, including R&D on next-generation STING agonists. All of these aspects of STING patents are associated with rapid therapeutic developments in this field and with the early and profound involvement of the industry.
What should be noted is that, of all the STING patents which disclosed thousands of compounds, only 15 compounds, by now, entered the clinical evaluation for future commercialization. The extremely low commercialization percentage and the closely connected patent network might raise potential risks of patent infringement in this field, especially for the most typical pharmaceutical class, CDN based compounds. As most patents filed in the past three years have not yet been approved, drug developers need to be aware of legal status and approval claims of important patents in the same category by different authorities, which would impact development and commercialization strategies of compounds in its pipeline.
STING next-generation therapies
Although CDN derivatives showed promising anti-tumor efficacy and potent phosphodiesterase resistance in pre-clinical studies, current preliminary and limited clinical results published from the two STING agonists (ADU-S100 and MK-1454) appeared not so optimistic concerning the modest response rate [13], [14], [22], [23]. However, R&D efforts regarding STING continue to intensify. Many STING patents have been filed, and many new drug candidates have been in pre-clinical development and advanced to clinical evaluation, in the last two years [12].
The development of next-generation therapies targeting STING generally aims to improve clinical responses and potential applicability. First, new classes of STING agonists and related structure modifications are emerging to enhance their bioactivity as well as to promote the potential of systematic administration, including CDN-based molecules and non-CDNs. CDN-based molecules are the first and dominant class in clinical development, and are also the major topics of new patent filings. Multiple modifications on the phosphor linkages (e.g., introducing thiol or boron, changing linkage positions), ribose (e.g., altering hydroxyls), and nucleobases could be demonstrated with refined biological activities and stability against phosphodiepesterases in many fundamental patents. These patents were also closely referred to by the discovery and optimization of non-CDN molecules, like ABZI identification as STING agonists. The promising results of ABZIs in pre-clinical studies provide an important option for STING agonists in clinical development upon systematic administration [61]. Second, the development of delivery systems for STING agonists, especially for CDN-based molecules, are a rapidly increasing topic of innovation concerning STING from the perspective of patent filings, aiming to enhance precise drug delivery and retention in tumor sites and cells. Two therapeutics (SYNB1891 and exoSTING) with specific carriers are under clinical evaluation. Many other drug delivery systems have been employed for CDNs in pre-clinical studies [62], and novel systems are also covered or specifically designed for CDNs, which may improve their efficacy or generate synergistic outcomes in cancer immunotherapy, such as STING ADCs [63], [64], [65]. Third, recent progress on understanding STING signaling activation and relevant tumor microenvironment changes provide theoretical support for the combined development of STING agonists with other therapeutics and further pharmaceutical compositions involving STING agonists [66], [67], [68], [69]. From the limited clinical data of CDN-based STING agonists, combination with PD-1/PD-L1 presented a slight efficacy advantage concerning specific cancer types compared with STING agonist monotherapy [13], [14], [23], and a combination with checkpoint inhibitors is involved in most of all of the current clinical trials of STING agonists. Furthermore, another attempt to improve the clinical outcome of STING agonists may be to differentiate subjects who would not respond to STING agonists or would likely be resistant to STING activation. STING activity may be suppressed as functional mutations or epigenetic silencing of STING genes [69], and, as such, determining STING functional activity or gene status may guide the design of novel biomarkers for STING agonist administration or other anti-cancer drugs [70].
STING antagonist drug development
Drug development regarding STING antagonists for inflammatory and autoimmune diseases is warranting increasing attention but is still in a very early stage. None of the STING antagonists are under clinical development, although some big pharma and biotech companies announced their R&D efforts and compound identifications several years ago, like Eli Lilly and Aduro Biotech/Chinook Therapeutics, IFM Therapeutics, Nimbus Therapeutics, and so on. Recent big collaborations with respect to STING inhibitors (i.e., deals between Bayer and Curadev and between Biogen and ISD Immunotech) have suggested the keen interest of the industry in this field. Patent filings for STING antagonists also entered the network as early as 2014 and expanded to the edge of the network in the last three years. These patents disclosed novel compounds and uses related to inhibiting STING signaling, which were supported by the findings and applications of the STING function and improved understanding of STING signaling in autoimmune diseases.
Conclusion
This study showed a rapidly increasing R&D targeting STING since its important role in the innate immune response was first reported in 2008. There is an obvious incline toward STING agonists other than STING inhibitors in terms of drug development as well as patenting activities. Several dominant companies in this field, like Audro Biotech, GlaxoSmithKline, IFM Therapeutics, and Merck Sharp & Dohme, have pioneered advancements in the clinical evaluation of novel STING agonists and also established the field of technological innovation of STING with their influential inventions in the network. These innovations were based on generating new small-molecule STING agonists to improve biological activity and overcome pharmaceutical shortages of natural CDNs and were intended for the development of next-generation therapies to improve clinical responses and potential applicability. Novel STING modulators (including CDN-based and non-CDN-based molecules), relevant delivery systems, pharmaceutical compositions, and combination strategies are under development with the potential to further promote the therapeutic outcomes of STING agonists. Along with improving understanding of the underlying mechanism of the STING signaling pathway, better performance of STING agonists in clinic is expected in the future but needs to be carefully evaluated in different disease conditions under well-designed trials.
CRediT authorship contribution statement
Xiangjun Kong: Conceptualization, Methodology, Visualization, Investigation, Writing – original draft. Huali Zuo: Methodology, Visualization. Hsien-Da Huang: Writing – review & editing. Qianru Zhang: Methodology, Visualization. Jiayu Chen: Writing – review & editing. Chengwei He: Writing – review & editing. Yuanjia Hu: Conceptualization, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by a University of Macau grant [MYRG2019-00011-ICMS]. We gratefully acknowledge Dr. Ying Tan and Dr. Baoyun Zhang from Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, Dr. Da Sun and Ms. Lei Wang from Wenzhou University, and Ms. Jing Yang from University of Macau for their valuable comments and suggestions that have improved this work. We also thank Ms. Yu Chen from University of Macau for the administrative support.
Biographies

Dr. Xiangjun Kong is a researcher at the Institute of Chinese Medical Sciences, University of Macau, where she obtained her Ph.D. in Biomedical Sciences. During her Ph.D., she systematically studied the global collaborative R&D in monoclonal antibody drug development over the past thirty years by social network analysis to understand the network evolution process and the relevant main drivers. Her research interests focus on adapting complex network analysis theory to understand technological connections in biomedical areas.

Dr. Hua-Li Zuo obtained the Ph.D. in Biomedical Sciences from the University of Macau, and now she is a researcher at the Chinese University of Hong Kong, Shenzhen. Her research interest is broad, covering data-based drug discovery and herb-drug interaction. She has abundant experience in natural product-based studies and network pharmacology.

Prof. Hsien-Da Huang is a presidential chair professor at The Chinese University of Hong Kong, Shenzhen. Prof. Huang’s expertise is in biological multi-disciplinary research, including bioinformatics, genomics, metagenomics & microbiome, intelligent biomedical technologies (drug development, genetic test, & precision medicine), AI & machine learning, and biological database design & development.

Prof. Jiayu Chen is an associate professor of at School of Pharmacy, Zunyi Medical University. She works on mechanism research on cancer biology, focusing on the topic of the roles of NFE2L, NDRG and their family members in epithelial-mesenchymal transition of cancer cells.

Prof. Qianru Zhang is an associate professor at School of Pharmacy, Zunyi Medical University, working on innovative pharmaceutical design and development combining network analysis for prediction and biological and pharmaceutical validation. She developed several innovative methods to improve the analysis efficiency and accuracy.

Prof. Chengwei He is an associate professor of at the Institute of Chinese Medical Sciences, University of Macau. He got his Ph.D. at Sun Yat-sen University Zhongshan School of Medicine and completed postdoctoral training at Harvard Medical School and Massachusetts General Hospital. He had a long-term research experience on anticancer and anti-inflammatory pharmacology, molecular basis and therapeutic research for cancer drug resistance and metastasis. He also has research interest in molecular mechanisms on cellular stress responses.

Prof. Yuanjia Hu is an associate professor of at the Institute of Chinese Medical Sciences and Department of Public Health and Medicinal Administration, University of Macau. He conducted interdisciplinary research combining knowledge on data mining and pharmaceutical innovation, as well as integrating theories on complex systems analysis and Chinese medicine discovery. The ultimate goal of his research is to understand the systematic relations in particular areas to improve and accelerate development and innovation.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2022.05.006.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Ishikawa H., Barber G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455(7213):674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishikawa H., Ma Z., Barber G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461(7265):788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao D., Li T., Li X.D., Chen X., Li Q.Z., Wight-Carter M., et al. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc Natl Acad Sci USA. 2015;112(42):E5699–E5705. doi: 10.1073/pnas.1516465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rameshbabu S., Labadie B.W., Argulian A., Patnaik A. Targeting innate immunity in cancer therapy. Vaccines (Basel) 2021;9(2):138. doi: 10.3390/vaccines9020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ablasser A., Goldeck M., Cavlar T., Deimling T., Witte G., Röhl I., et al. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498(7454):380–384. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu S., Cai X., Wu J., Cong Q., Chen X., Li T., et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 2015;347(6227) doi: 10.1126/science.aaa2630. [DOI] [PubMed] [Google Scholar]
- 7.Abe T., Barber G.N., Williams B. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-κB activation through TBK1. J Virol. 2014;88(10):5328–5341. doi: 10.1128/JVI.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vatner R.E., Janssen E.M. STING, DCs and the link between innate and adaptive tumor immunity. Mol Immunol. 2019;110:13–23. doi: 10.1016/j.molimm.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang M., Chen P., Wang L., Li W., Chen B., Liu Y.u., et al. cGAS-STING, an important pathway in cancer immunotherapy. J Hematol Oncol. 2020;13(1) doi: 10.1186/s13045-020-00916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Jesus A.A., Marrero B., Yang D., Ramsey S.E., Montealegre Sanchez G.A., et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371(6):507–518. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gall A., Treuting P., Elkon K., Loo Y.-M., Gale M., Barber G., et al. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity. 2012;36(1):120–131. doi: 10.1016/j.immuni.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amouzegar A., Chelvanambi M., Filderman J.N., Storkus W.J., Luke J.J. STING agonists as cancer therapeutics. Cancers (Basel) 2021;13(11):2695. doi: 10.3390/cancers13112695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meric-Bernstam F., Sandhu S.K., Hamid O., Spreafico A., Kasper S., Dummer R., et al. Phase Ib study of MIW815 (ADU-S100) in combination with spartalizumab (PDR001) in patients (pts) with advanced/metastatic solid tumors or lymphomas. J Clin Oncol. 2019;37(15_suppl):2507. doi: 10.1200/JCO.2019.37.15_suppl.2507. [DOI] [Google Scholar]
- 14.Aduro Biotech, Inc. [Now Chinook Therapeutics, Inc.] Aduro Biotech and Novartis present results from ongoing Phase 1b study of STING agonist ADU-S100 (MIW815) in combination with antI-PD-1 monoclonal antibody spartalizumab (PDR001) in patients with advanced solid tumors or lymphomas, 2019 Jun [cited 2021 June 5]. Available from: https://investors.chinooktx.com/news-releases/news-release-details/aduro-biotech-and-novartis-present-results-ongoing-phase-1b.
- 15.Chen N.N., Zhang H., You Q.D., Xu X.L. Agonist of stimulator of interferon genes as antitumor agents: a patent review (2008–2020) Expert Opin Ther Pat. 2021;31(6):563–584. doi: 10.1080/13543776.2021.1877660. [DOI] [PubMed] [Google Scholar]
- 16.Lyu L., Feng Y., Chen X., Hu Y. The global chimeric antigen receptor T (CAR-T) cell therapy patent landscape. Nat Biotechnol. 2020;38(12):1387–1394. doi: 10.1038/s41467-020-16602-0. [DOI] [PubMed] [Google Scholar]
- 17.Kong X., Zhang Q., Lai Y., Hu H., Chen X., Hu Y. Global patent landscape of programmed cell death 1: implications of the rapid expansion. Expert Opin Ther Pat. 2018;28(1):69–80. doi: 10.1080/13543776.2017.1378349. [DOI] [PubMed] [Google Scholar]
- 18.Érdi P., Makovi K., Somogyvári Z., Strandburg K., Tobochnik J., Volf P., et al. Prediction of emerging technologies based on analysis of the US patent citation network. Scientometrics. 2013;95(1):225–242. doi: 10.1007/s11192-012-0796-4. [DOI] [Google Scholar]
- 19.Leventhal D.S., Sokolovska A., Li N., Plescia C., Kolodziej S.A., Gallant C.W., et al. Immunotherapy with engineered bacteria by targeting the STING pathway for anti-tumor immunity. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-16602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janku F., Luke J.J., Brennan A., Riese R., Varterasian M., Armstrong M.B., et al. Intratumoral injection of SYNB1891, a Synthetic Biotic designed to activate the innate immune system, demonstrates target engagement in humans including intratumoral STING activation. Cancer Res. 2021 doi: 10.1158/1538-7445.AM2021-CT110. [DOI] [Google Scholar]
- 21.Jang S.C., Economides K.D., Moniz R.J., Sia C.L., Lewis N., McCoy C., et al. ExoSTING, an extracellular vesicle loaded with STING agonists, promotes tumor immune surveillance. Commun Biol. 2021;4(1) doi: 10.1038/s42003-021-02004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meric-Bernstam F., Sweis R.F., Hodi F.S., Messersmith W.A., Andtbacka R.H.I., Ingham M., et al. Phase I dose-escalation trial of MIW815 (ADU-S100), an intratumoral STING agonist, in patients with advanced/metastatic solid tumors or lymphomas. Clin Cancer Res. 2022;28(4):677–688. doi: 10.1158/1078-0432.CCR-21-1963. [DOI] [PubMed] [Google Scholar]
- 23.Harrington K.J., Brody J., Ingham M., Strauss J., Cemerski S., Wang M., et al. Preliminary results of the first-in-human (FIH) study of MK-1454, an agonist of stimulator of interferon genes (STING), as monotherapy or in combination with pembrolizumab (pembro) in patients with advanced solid tumors or lymphomas. Ann Oncol. 2018;29:viii712. doi: 10.1093/annonc/mdy424.015. [DOI] [Google Scholar]
- 24.G. Schieven, J. Brown, J. Swanson, C. Stromko, C.P. Ho, R. Zhang, et al. Preclinical characterization of BMS-986301, a differentiated STING agonist with robust antitumor activity as monotherapy or in combination with anti–PD-1, SITC 2018: Proceedings of the 34th Society for Immunotherapy of Cancer (SITC) Annual Meeting, 2018 Nov 7-11; Washington, D.C., USA. P525.
- 25.Challa S.V., Zhou S., Sheri A., Padmanabhan S., Meher G., Gimi R., et al. Preclinical studies of SB 11285, a novel STING agonist for immuno-oncology. J Clin Oncol. 2017;35(15_suppl):e14616. doi: 10.1200/JCO.2017.35.15_suppl.e14616. [DOI] [Google Scholar]
- 26.Kim D.-S., Endo A., Fang F.G., Huang K.-C., Bao X., Choi H.-W., et al. E7766, a macrocycle-bridged stimulator of interferon genes (STING) agonist with potent pan-genotypic activity. ChemMedChem. 2021;16(11):1741–1744. doi: 10.1002/cmdc.202100068. [DOI] [PubMed] [Google Scholar]
- 27.Corrales L., Glickman L., McWhirter S., Kanne D., Sivick K., Katibah G., et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015;11(7):1018–1030. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sivick K.E., Desbien A.L., Glickman L.H., Reiner G.L., Corrales L., Surh N.H., et al. Magnitude of therapeutic STING activation determines CD8(+) T cell-mediated anti-tumor immunity. Cell Rep. 2018;25(11):3074–3085.e5. doi: 10.1016/j.celrep.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 29.Aduro Biotech, Inc. [Now Chinook Therapeutics, Inc.] First patient dosed in Phase 1 study of ADU-S100 (MIW815) in combination with Yervoy (Ipilimumab) for the treatment of relapsed and refractory melanoma, 2019 Feb [cited 2021 June 18]. Available from: https://investors.chinooktx.com/news-releases/news-release-details/aduro-announces-first-patient-dosed-phase-1-study-adu-s100.
- 30.Xu J., Solban N., Wang Y., Ferguson H., Perera S., Lin K., et al. Sonoporation-enhanced delivery of STING agonist induced robust immune modulation and tumor regression. Adv Ther. 2021;4(10):2100154. doi: 10.1002/adtp.202100154. [DOI] [Google Scholar]
- 31.Banerjee M., Basu S., Middya S., Shrivastava R., Ghosh R., Pryde D.C., et al. CRD5500: A versatile small molecule STING agonist amenable to bioconjugation as an ADC. Can Res. 2019 doi: 10.1158/1538-7445.AM2019-LB-061. [DOI] [Google Scholar]
- 32.Cetinbas N.M., Catcott K.C., Avocetien K., Bentley K.W., Bradly S., Carter T., et al. Maryland; USA: 2019. Proceedings of the 34th Society for Immunotherapy of Cancer (SITC) Annual Meeting, Maryland, USA; 2019 Nov 6–10; p. P695. [Google Scholar]
- 33.Jeremiah N., Neven B., Gentili M., Callebaut I., Maschalidi S., Stolzenberg M.-C., et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest. 2014;124(12):5516–5520. doi: 10.1172/JCI79100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma S., Campbell A.M., Chan J., Schattgen S.A., Orlowski G.M., Nayar R., et al. Suppression of systemic autoimmunity by the innate immune adaptor STING. Proc Natl Acad Sci USA. 2015;112(7) doi: 10.1073/pnas.1420217112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding C., Song Z., Shen A., Chen T., Zhang A. Small molecules targeting the innate immune cGAS-STING-TBK1 signaling pathway. Acta Pharm Sin B. 2020;10(12):2272–2298. doi: 10.1016/j.apsb.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Decout A., Katz J.D., Venkatraman S., Ablasser A. The cGAS–STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21(9):538–569. doi: 10.1038/s41577-021-00524-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey C., Black J.R.M., Swanton C. Cancer Research: The lessons to learn from COVID-19. Cancer Discov. 2020;10(9):1263–1266. doi: 10.1158/2159-8290.CD-20-0823. [DOI] [PubMed] [Google Scholar]
- 38.Pereira C.G., Picanco-Castro V., Covas D.T., Porto G.S. Patent mining and landscaping of emerging recombinant factor VIII through network analysis. Nat Biotechnol. 2018;36(7):585–590. doi: 10.1038/nbt.4178. [DOI] [PubMed] [Google Scholar]
- 39.Roberts MacKenna, Wall I.B., Bingham I., Icely D., Reeve B., Bure K., et al. The global intellectual property landscape of induced pluripotent stem cell technologies. Nat Biotechnol. 2014;32(8):742–748. doi: 10.1038/nbt.2975. [DOI] [PubMed] [Google Scholar]
- 40.Zhong B., Sun L., Wei Q., Dai Y., Chen C., Chen Z. Cyclic di-nucleotide compounds and methods of use. Patent WO2017161349A1. 2017 [Google Scholar]
- 41.Altman M.D., Andresen B.M., Chang W., Childers M.L., Cumming J.N., Haidle A.M., et al. Cyclic di-nucleotide compounds as sting agonists. Patent US2017044206A1. 2016 [Google Scholar]
- 42.Dubensky TW Jr, Kanne DB, Leong MLL, Glickman LH, Vance RE, Lemmens EE. Compositions and methods for activating “stimulator of interferon gene”-dependent signalling. Patent WO2014189805A1, 2014.
- 43.Gajewski TF, Woo SR, Corrales L. Use of STING agonist as cancer treatment. Patent WO2015077354A1; 2014.
- 44.Charnley AK, Darcy MG, Dodson JW, Hughes TV, Li Y, Lian Y et al. Heterocyclic amides useful as protein modulators. WO2017175156A1; 2017.
- 45.Charnley AK, Darcy MG, Dodson JW, Dong X, Hughes TV, Kang J et al. Heterocyclic amides useful as protein modulators. Patent WO2017175147A1; 2017.
- 46.Pesiridis GS, Ramanjulu JM, Tran JL, Yang J. Methods for administering sting agonists. Patent WO2019069275A1; 2018.
- 47.Glick G, Ghosh S, Olhava EJ, Roush WR, Jones R. Cyclic dinucleotides for treating conditions associated with sting activity such as cancer. WO2017123669A1; 2017.
- 48.Wu L, Ye Y, Yao W. Tricyclic heteraryl compounds as sting activators. Patent US2020040009A1; 2019.
- 49.Wu L, Lajkiewicz N, Yao W. Heteroaryl amide compounds as sting activators. Patent US2020039994A1; 2019.
- 50.Wu L, Lajkiewicz N, Ye Y, Li Z, Yao W. Tricyclic heterocyclic compounds as sting activators. Patent US2019359608A1; 2019.
- 51.Wu L, Lajkiewicz N, Yao W. Heteroaryl amide compounds as sting activators. Patent WO2020146237A1; 2020.
- 52.Dubensky JTW, Kanne DB. Compositions and methods for inhibiting “stimulator of interferon gene” -dependent signalling. Patent US2014341976A1; 2014.
- 53.Fryer A.L., Abdullah A., Taylor J.M., Crack P.J. The complexity of the cGAS-STING pathway in CNS pathologies. Front Neurosci. 2021;15 doi: 10.3389/fnins.2021.621501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y., He M., Wang Z., Duan Z., Guo Z., Wang Z., et al. STING signaling activation inhibits HBV replication and attenuates the severity of liver injury and HBV-induced fibrosis. Cell Mol Immunol. 2022;19(1):92–107. doi: 10.1038/s41423-021-00801-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang J, Guo F, Block TM, Guo JT. Use of sting agonists to treat chronic hepatitis B virus infection. Patent WO2015061294A2; 2014.
- 56.Jang SC, Sia CL, Lewis ND, Harrison RA, Moniz RJ, Sathyanarayanan S et al. Extracellular vesicles comprising STING-agonist. Patent WO2019183578A1; 2019.
- 57.Zhao D, Liu Y, Crowe W. Immunotherapeutic nanoparticles and methods relating thereto. WO2020210317A1; 2020.
- 58.Vernejoul F, Boularan C, Drocourt D, Lioux T, Qushair G, Romo J et al. Novel complexes of immunostimulatory compounds, and uses thereof. WO2017186711A1; 2017.
- 59.Goldberg MS, Park CG. Drug delivery compositions and uses thereof, WO2018045058A1; 2017.
- 60.Liu Y.T., Sun Z.J. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. 2021;11(11):5365–5386. doi: 10.7150/thno.58390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramanjulu J.M., Pesiridis G.S., Yang J., Concha N., Singhaus R., Zhang S.-Y., et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018;564(7736):439–443. doi: 10.1038/s41586-018-0705-y. [DOI] [PubMed] [Google Scholar]
- 62.Su T., Zhang Y., Valerie K., Wang X.Y., Lin S., Zhu G. STING activation in cancer immunotherapy. Theranostics. 2019;9(25):7759–7771. doi: 10.7150/thno.37574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bukhalid R.A., Duvall J.R., Cetinbas N.M., Catcott K.C., Avocetien K., Bentley K.W., et al. Systemic administration of STING agonist antibody-drug conjugates elicit potent anti-tumor immune responses with minimal induction of circulating cytokines. Can Res. 2020 doi: 10.1158/1538-7445.AM2020-6706. [DOI] [Google Scholar]
- 64.Barnett L, Bender S, Cho C, Cox S, Deane J, Glaser S et al. DC-sign antibody conjugates comprising sting agonists. Patent WO2020092617A1; 2019.
- 65.Hao X, Va P, Wan Y. Antibody conjugates comprising sting agonist. Patent WO2020089815A1; 2019.
- 66.Lemos H., Mohamed E., Huang L., Ou R., Pacholczyk G., Arbab A.S., et al. STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Can Res. 2016;76(8):2076–2081. doi: 10.1158/0008-5472.CAN-15-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lemos H., Ou R., McCardle C., Lin Y., Calver J., Minett J., et al. Overcoming resistance to STING agonist therapy to incite durable protective antitumor immunity. J Immunother Can. 2020;8(2):e001182. doi: 10.1136/jitc-2020-001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang H., Lee W.S., Kong S.J., Kim C.G., Kim J.H., Chang S.K., et al. STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J Clin Invest. 2019;129(10):4350–4364. doi: 10.1172/JCI125413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Della Corte C.M., Byers L.A. Evading the STING: LKB1 loss leads to STING silencing and immune escape in KRAS-mutant lung cancers. Cancer Discov. 2019;9(1):16–18. doi: 10.1158/2159-8290.CD-18-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barber GN. Cancer treatment and diagnosis. Patent WO2016201450A2; 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.