Abstract
Individual response to immune checkpoint inhibitors (ICIs) is currently unpredictable in patients with melanoma. Recent findings highlight a striking improvement in the clinical outcomes of overweight/obese patients treated with ICIs, which seems driven, at least in part, by programmed cell death protein 1 (PD-1)-mediated T-cell dysfunction. A putative role of butyrophilins (BTNs) is under investigation as a novel mechanism of cancer immune evasion and obesity-associated inflammation. This study investigates the role of baseline plasma levels of soluble PD-1 (sPD-1), soluble programmed cell death ligand 1 (sPD-L1), BTN2A1 (sBTN2A1), BTN3A1 (sBTN3A1), along with body mass index (BMI), as predictive biomarkers of immunotherapy response in metastatic melanoma patients treated with nivolumab or pembrolizumab as first-line treatment. In all, 41 patients were included in the study. The baseline plasma level of sPD-1 was significantly lower, and the sBTN2A1 was significantly higher, in long-responder patients to nivolumab or pembrolizumab (median sPD-1: 10.3 ng/ml versus 16.6 ng/ml, p = 0.001; median sBTN2A1: 4.4 ng/ml versus 3.77 ng/ml, p = 0.004). Lower levels of sPD-1 and higher levels of sBTN2A1 were also significantly associated with better overall response rate. Notably, when we further stratified the study cohort using BMI along with sPD-1, patients with BMI ⩾ 25 and sPD-1 < 11.24 ng/ml had longer time to treatment failure after PD-1 inhibitor than other subgroups of patients (p < 0.001). Circulating sPD-1 and sBTN2A1 detection, along with BMI, could give more insights into the immune-metabolic interactions underlying the benefit observed in overweight/obese patients, improving the use of dynamic, noninvasive, biomarkers for patient selection.
Keywords: BTN2A1, butyrophilins, circulating immune checkpoints, PD-1, predictive biomarker, melanoma, soluble immune checkpoints
Introduction
Melanoma is the most aggressive skin cancer, characterized by high metastatic spread and high mortality rates.1 Melanoma has several unique characteristics that contribute to the renowned high immunogenicity, and make it an attractive tumor for treatment with immune checkpoint inhibitors (ICIs).2
Despite the therapeutic impact of reinvigorating the antitumor immune response by immunotherapy, the clinical benefit of ICIs in advanced melanoma is still limited to selected patients.3 Approximately half of the patients do not derive durable responses.2 Despite elevated tumor programmed cell death ligand 1 (PD-L1) expression in the tumor microenvironment (TME),4 tumor mutational burden, and other markers such as interferon gamma (IFNγ) signature or the presence of T cells in the tumor have been found to correlate with outcomes, these biomarkers cannot accurately predict the response to ICIs in all patients.5 This indicates the clinical need for better, robust, and dynamic predictive, treatment specific, biomarkers.
Recent studies have shown that the programmed cell death protein 1 (PD-1)/PD-L1 axis is responsible for less than half of the dysfunctional antitumor immunity in cancers, suggesting that other mechanisms are involved in tumor immune evasion.6–9 Butyrophilins (BTNs) and butyrophilin-like (BTNL) family of proteins, such as BTN2A1, BTN3A1, and BTNL2, have been shown to play a critical role in modulating γδ T-cell development and differentiation.10–14 γδ T cells are a non-major histocompatibility complex-restricted lymphocyte subset.15 Intriguingly, γδ T cells preferentially infiltrate the epithelial-rich human tissues, such as the skin.16 Concurrent BTN3A1-BTN2A1 interactions seem to be essential for T-cell receptor (TCR)-dependent activation of Vγ9Vδ2+ T cells, the most abundant γδ T cells in peripheral blood.11,13 Recent clinical studies reported the modified expression of molecules belonging to the BTN/BTNL family following immune checkpoint (IC) blockade with anti-PD-1 antibodies,16 suggesting their unexplored role in tumor immune escape.17–19
Furthermore, the new and evolving role of patients’ metabolic state and systemic inflammation is now highlighted in the complex symbiotic and metabolic interactions between tumor cells and dysfunctional/suppressive immune cells in the TME.20,21 Notably, obesity is accompanied by chronic low-grade systemic inflammation as a result of expanded adipose tissue.22 Several clinical data showed that obesity is paradoxically associated with improved outcomes in cancer patients treated with IC blockade.23 Importantly, tumor metabolic dependencies are emerging as key tumor vulnerabilities, and patient-associated features, such as body mass index (BMI), are under investigation as factors to profoundly impact the cancer immune responses.24–28 Although the mechanistic link between metabolic state and immunotherapy benefit was not elucidated, an impact of excess adiposity/obesity on the PD-1/PD-L1 pathway seems to exist. Recent preclinical findings suggested that obesity promotes T-cell exhaustion through leptin-induced upregulation of PD-1.25 This PD-1-mediated immune dysfunction in obesity cancer patients would be reversible by PD-1/PD-L1 inhibitors, making the tumors markedly more responsive to ICIs.29 Furthermore, recent studies indicate a potential ‘gamma-delta T-cell link’ between obesity and cancer.30 Obese adult humans show a decrease in Vγ9δ2 cells in the peripheral blood, which are less capable of IFNγ production, interleukin (IL)2 and IL7 expression, and TCR interactions with BTN2A1/BTN3A1 bound to tumor phosphoantigens (pAgs) expressed on cancer cells.31,32
In human tumors, clinical data linking excess adiposity to IC and BTN expression in patients treated with PD-1 inhibitors are lacking. The soluble forms of immune checkpoints (sICs) and BTNs can be detected in the peripheral blood, and a correlation between sICs/BTNs levels with clinical response was recently described in several tumor types.17–19 Therefore, circulating ICs/BTNs detection, along with BMI, could provide more insights into the immune-metabolic interactions underlying the benefit observed in obese patients, improving the use of dynamic, noninvasive, biomarkers for patient selection. With this goal, we performed a prospective study to investigate the role of BMI and baseline plasma levels of soluble PD-1 (sPD-1), PD-L1 (sPD-L1), BTN2A1 (sBTN2A1), and BTN3A1 (sBTN3A1), as predictive biomarkers of immunotherapy response in metastatic melanoma patients treated with anti-PD-1 nivolumab or pembrolizumab as first-line treatment (Figure 1).
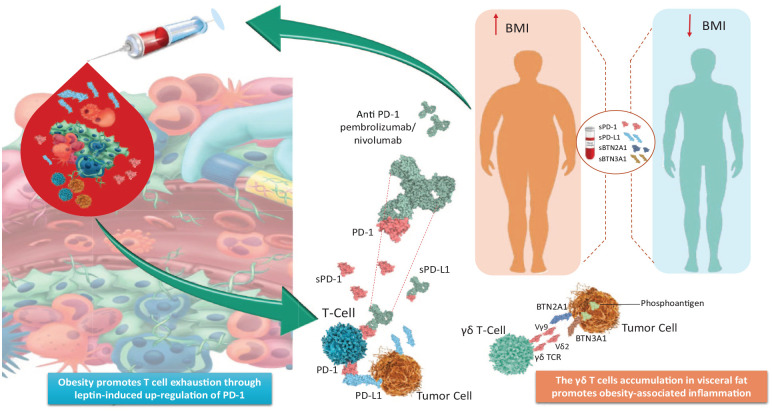
Figure 1.
A putative role of PD-1/PD-L1 axis and BTNs is highlighted as mechanism of cancer immune evasion and obesity-associated inflammation.
Obesity promotes T-cell exhaustion through leptin-induced upregulation of PD-1. This PD-1-mediated immune dysfunction in obesity cancer patients would be reversible by PD-1/PD-L1 inhibitors, making the tumors markedly more responsive to ICIs. The γδ T-cell accumulation in visceral fat promotes chronic inflammation. The Vγ9Vδ2+, the major class of γδ T cells, are activated by phosphoantigens produced by tumor cells, and needed the combination of two immunoglobulin superfamily members, BTN2A1 and BTN3A1. The soluble forms of PD-1, PD-L1, BTN2A1, and BTN3A1 can be detected in the peripheral blood. Circulating sICs/BTNs detection at baseline, before starting anti-PD-1 treatment, along with BMI, could give more insights into the symbiotic immune-metabolic interplay, to predict immunotherapy response.
BTNs, butyrophilins; ICIs, immune checkpoint inhibitors; sICs, soluble immune checkpoints; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1.
Patients and methods
Study population
This study included a cohort of 41 patients with a histologically confirmed diagnosis of melanoma, treated at the Section of Medical Oncology of the Department of Surgical, Oncological, and Oral Sciences of the University of Palermo (Italy). The study population included patients with advanced disease, harboring no BRAF, NRAS, or KIT mutations, candidates for first-line treatment based on anti-PD-1 nivolumab or pembrolizumab from January 2016 to March 2018. No patients treated with adjuvant therapy were included in the study.
Peripheral blood samples from melanoma patients were collected at baseline, before starting nivolumab or pembrolizumab treatment (T0).
The clinicopathological information collected included gender, age, histologic subtype, clinical stage according to the TNM system of the American Joint Committee on Cancer, prognostic factors, site of metastases, and pretreatment lactate dehydrogenase (LDH), neutrophil-to-lymphocyte ratio (NLR), and BMI. The NLR was recorded from the routinely performed blood cell count, as the absolute count of neutrophils divided by the absolute count of lymphocytes from peripheral blood samples collected at baseline. BMI was calculated as weight in kilograms divided by height in meters squared. Normal weight (BMI = 18.5–24.9), overweight (BMI = 25–29.9), and obesity (BMI ⩾ 30) were classified based on the World Health Organization recommendations.
The tumor response [progressive disease (PD), stable disease (SD), partial response (PR), complete response (CR)] according to Response Evaluation Criteria in Solid Tumors (RECIST version 1.1.), objective response rate (ORR), time to treatment failure (TTF) to nivolumab or pembrolizumab treatment, and overall survival (OS) were assessed.
The association between clinicopathological variables, sIC, and clinical outcomes was evaluated.
The information was anonymously recorded for all patients who previously provided written informed consent. The ‘G-Land 2017’ study protocol was approved by the ethical committee of the University Hospital AOUP ‘Paolo Giaccone’ of Palermo, Italy (Comitato Etico Palermo 1; approval number: 0103-2017).
Determination of soluble PD-1, PD-L1, BTN2A1, and BTN3A1 concentrations in plasma
The peripheral blood samples from melanoma patients were processed for plasma isolation within 2 h of collection, by centrifugation at 2.200g for 15 min at 4°C in the presence of ethylenediaminetetraacetic acid. The plasma fractions were aliquoted in cryotubes and preserved at −80°C until their use for the determination of soluble ICs concentrations in plasma. The plasma sPD-1, sPD-L1, sBTN2A1, and sBTN3A1 levels were measured using specific homemade enzyme-linked immunosorbent assays (ELISAs) not yet commercially available and designed by DYNABIO S.A. (Parc de Luminy, Marseille, France) according to our indications.17–19 Full steps of the experimental protocol using the homemade ELISA tests developed are reported in Supplemental Text S1, which were previously reported.17–19,31 A key step to underline was the sample compatibility checking regarding serum versus plasma concentrations and interference of the matrix. In fact, our analysis comparing concentrations of the four sICs measured in plasma and serum from the same blood series showed concentrations in serum at least threefold to fivefold lower than in plasma. Therefore, we finally analyzed the concentrations of sPD-1, sPD-L1, sBTN2A1, and sBTN3A1 on plasma samples. Thereby, we observed in all four ELISA assays an interference of the plasma matrix, which became negligible when plasma samples were diluted at least 1/5. For this reason, plasma samples were at least diluted 1/5 before the assay.
Statistical analysis
The primary clinical outcome of the study was TTF. TTF was defined as the time from immunotherapy onset to discontinuation for any reason excluding remission, that is, disease progression, treatment toxicity, patient preference, or death. OS was calculated from the diagnosis of melanoma to death by any cause or last follow-up (censored patients). The analysis of TTF and OS between groups was compared using the Kaplan–Meier method and log-rank test.
To identify independent prognostic factors for TTF and OS, univariate and multivariate Cox proportional hazard regression models were built. All tests were performed with a significance level of p < 0.05. Statistical analyses were conducted using IBM SPSS Statistics for Windows Version 28.0 (IBM Corporation, Armonk, NY, USA). The receiver operating characteristic (ROC) curve analysis was used to determine the optimal cutoff for each marker, to classify short-term versus long-term responders. The optimal cutoff was 11.24 ng/ml for sPD-1 [area under the curve (AUC) = 0.87, p < 0.001], 1.17 ng/ml for sPD-L1 (AUC = 0.93, p < 0.001), 9.7 ng/ml for sBTN3A1 (AUC = 0.93, p < 0.001), and 4.0 ng/ml for sBTN2A1 (AUC = 0.98, p < 0.001).
Results
Patients’ characteristics
In all, 41 melanoma patients were included in the study. Clinical and pathological patient characteristics are summarized in Table 1. In our previous research, the same series of patients were evaluated to investigate the correlation between pretreatment plasma PD-1/PD-L1 expression levels and the presence/absence/class (brisk versus non-brisk) of tumor-infiltrating lymphocytes (TILs) in melanoma primary tumors of the same patients.33 The results showed that lower plasma PD-1 expression levels were statistically correlated with the increased infiltration of brisk-type TILs in primary melanoma, suggesting a more favorable prognosis.33
Table 1.
Clinical features of metastatic melanoma patients.
| Characteristic | No. of patients (%) |
|---|---|
| No. of patients | 41 |
| Gender | |
| Male | 23 (56) |
| Female | 18 (44) |
| Age at diagnosis (year) | |
| Median | 49 |
| Mean | 50.6 |
| Range | 23–74 |
| Age groups (years) | |
| ⩽40 | 10 (24.4) |
| 41–50 | 13 (31.7) |
| 51–60 | 6 (14.6) |
| >60 | 12 (29.3) |
| Type of melanoma | |
| Cutaneous | 29 (70.7) |
| Uveal | 5 (12.2) |
| Mucosal | 2 (4.9) |
| Unknown origin | 5 (12.2) |
| Primary cutaneous tumor site | |
| Limbs (acral) | 11 (38) |
| Head and neck | 7 (24.1) |
| Back | 7 (24.1) |
| Chest | 2 (6.9) |
| Abdomen | 2 (6.9) |
| TILs | |
| Brisk | 10 (24.4) |
| Non-brisk | 6 (14.6) |
| Absent | 12 (29.3) |
| NA | 13 (31.7) |
| TILs density | |
| Mild | 6/16 (37.4) |
| Moderate | 1/16 (6.3) |
| Marked | 1/16 (6.3) |
| NA | 8/16 (50) |
| BRAF status | |
| Mutated | 0 (0) |
| Wild type | 41 (100) |
| PS (ECOG) | |
| 0 | 30 (73.2) |
| 1 | 11 (26.8) |
| Site of metastasis, individual | |
| Lymph nodes | 31 (75.6) |
| Lung | 19 (46.3) |
| Liver | 16 (39) |
| Skin | 14 (34.1 |
| Bones | 9 (22) |
| CNS | 8 (19.5) |
| Soft tissues | 8 (19.5) |
| Adrenal gland | 6 (14.6) |
| Others | 18 (43.9) |
| No. of evaluable disease sites, n (%) | |
| ⩽2 | 28 (68.3) |
| ⩾3 | 13 (31.7) |
| LDH* | |
| ⩽300 | 21 (51.2) |
| >300 | 20 (48.8) |
| NLR* | |
| ⩽2.6 | 23 (56.1) |
| >2.6 | 18 (43.9) |
| BMI* | |
| BMI < 25 | 16 (39) |
| BMI ⩾ 25 | 25 (61) |
| Best response to nivolumab/pembrolizumab | |
| CR and PR | 16 (39) |
| SD | 12 (29) |
| PD | 13 (32) |
BMI, body mass index; CNS, central nervous system; CR, complete response; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; PD, progressive disease; PR, partial response; PS, performance status; DS, stable disease; TILs, tumor-infiltrating lymphocytes.
Outcome analysis
First, we focused on the role of the soluble form of ICs (sPD-1, sPD-L1, sBTN3A1, and sBTN2A1) as predictive markers of immunotherapy response.
We classified the plasma levels of each tested biomarker as ‘low’ or ‘high’ concentrations according to the thresholds previously calculated using ROC analysis.
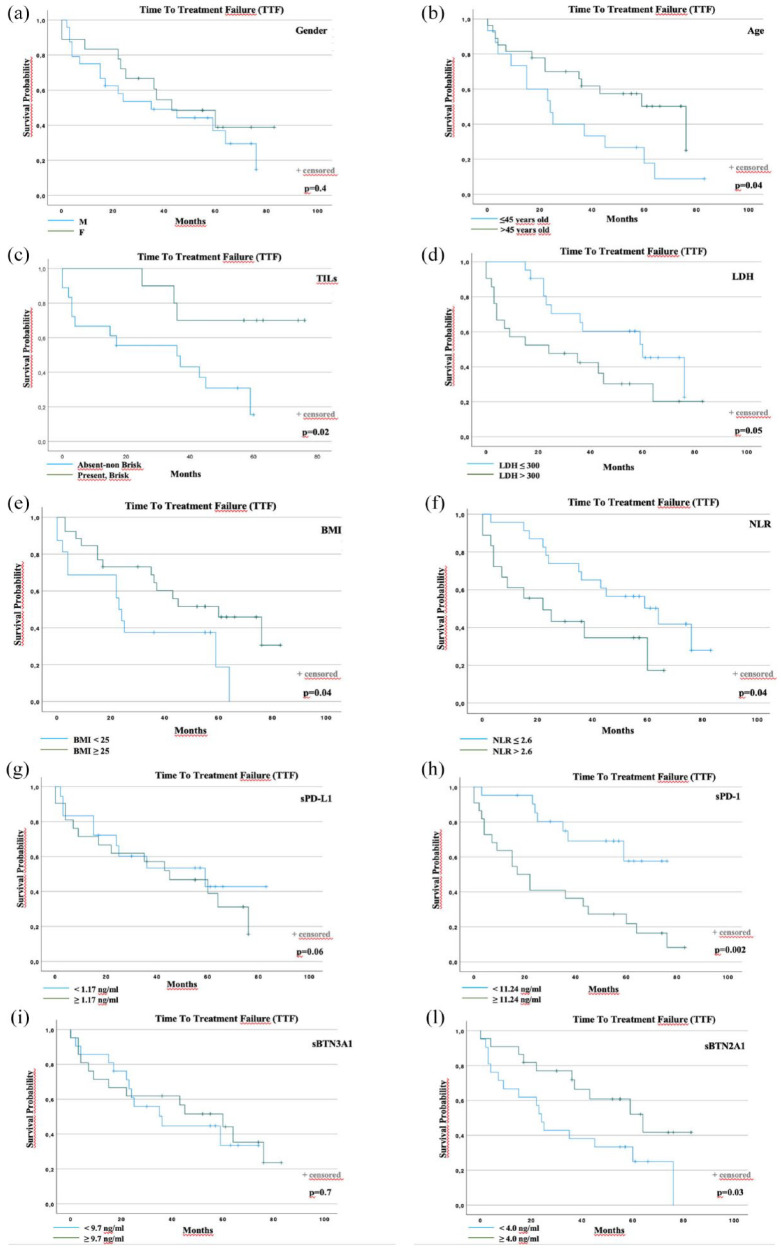
Overall median TTF was 36 months [95% confidence interval (CI): 14.3–57.7]. At the time of data analyses, a total of 26 events (progression or death) occurred (63.4%). Notably, the presence of plasma levels of sPD-1 < 11.24 ng/ml and sBTN2A1 ⩾ 4.0 ng/ml was significantly associated with longer TTF (Figure 2(h) and (l)). Regarding the outcome data according to plasma levels of sPD-1, 7 events were observed in the group of 20 patients with sPD-1 low levels (35%), and 19 events in the group of 21 patients with sPD-1 high levels (90.4%). Regarding the outcome data according to plasma levels of sBTN2A1, 16 events were observed in the group of 20 patients with sBTN2A1 low levels (80%), and 10 events in the group of 21 patients with sBTN2A1 high levels (47.6%). Median TTF was not reached (NR) for the low sPD-1 group, and 17 months (95% CI: 5.0, 28.9) for the high sPD-1 group (p < 0.002).
Figure 2.
TTF according to prognostic factors. TTF according to (a) gender, (b) age at first-line start, (c) TILs on primary melanoma, (d) pretreatment LDH, (e) BMI, (f) NLR, (g) baseline sPD-L1, (h) baseline sPD-1, (i) baseline sBTN2A1, and (l) baseline sBTN3A1.
BMI, body mass index; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; TILs, tumor-infiltrating lymphocytes; sBTN, soluble butyrophilin; sPD-1, soluble programmed cell death protein 1; sPD-L1, soluble programmed cell death ligand 1; TTF, time to treatment failure.
Conversely, pretreatment levels of circulating PD-1 and BTN2A1 were significantly higher and lower, respectively, in patients who failed to respond to the first-line anti-PD-1 treatment with nivolumab or pembrolizumab. The difference was not significant for circulating PD-L1 and BTN3A1 (Figure 2(g) and (i)).
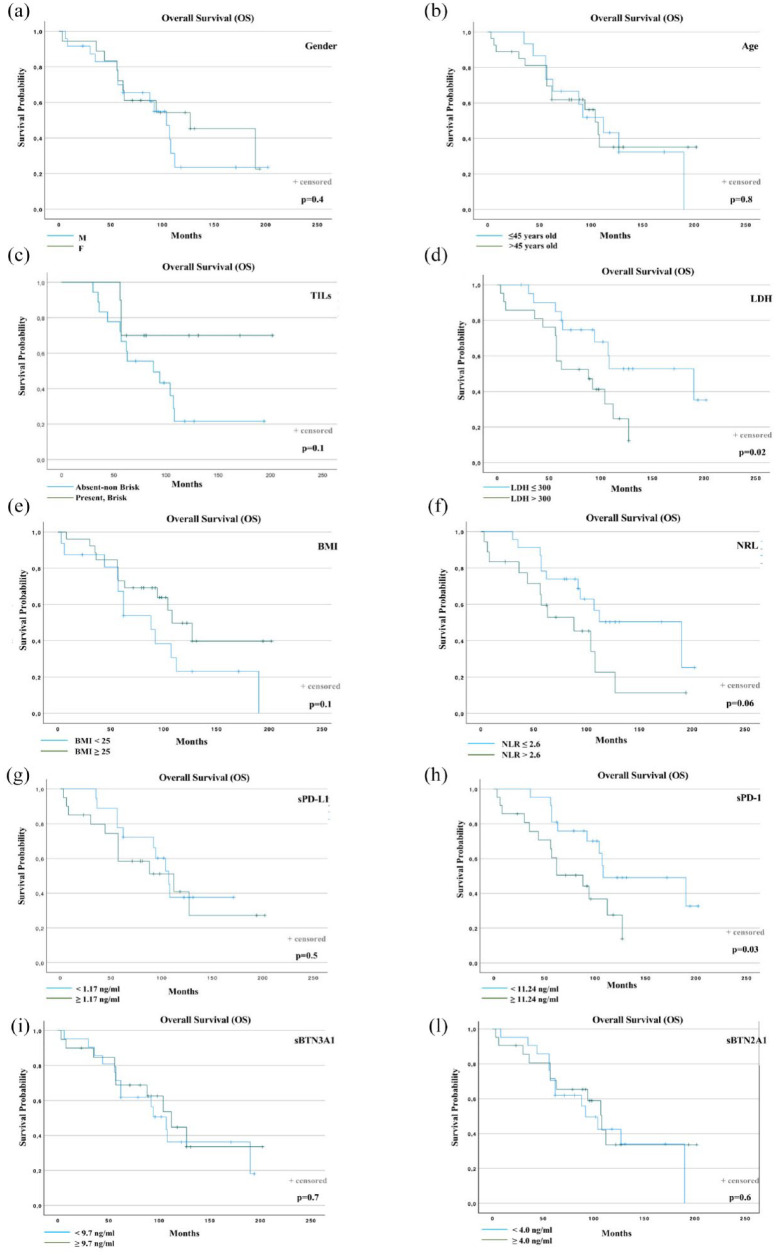
Overall median OS was 107 months (95% CI: 88.7, 125.3). In all, 23 total events (deaths) were observed (56.1%). The distribution of events according to sPD-1 concentrations was: 10 in 20 patients with low sPD-1 levels (50%) and 13 in 21 patients with high sPD-1 levels (61.9%).
Median OS was 108 months (95% CI: 21.1, 194.8) and 88 months (95% CI: 45.6, 130.3) for the low and high sPD-1 groups, respectively (p = 0.03) (Figure 3(h)). The difference was not significant for the other sICs (Figure 3(g), (i), and (l)).
Figure 3.
OS according to prognostic factors. OS according to (a) gender, (b) age at first-line start, (c) TILs on primary melanoma, (d) pretreatment LDH, (e) BMI, (f) NLR, (g) baseline sPD-L1, (h) baseline sPD-1, (i) baseline sBTN2A1, and (l) baseline sBTN3A1.
BMI, body mass index; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; sBTN, soluble butyrophilin; sPD-1, soluble programmed cell death protein 1; sPD-L1, soluble programmed cell death ligand 1; TILs, tumor-infiltrating lymphocytes.
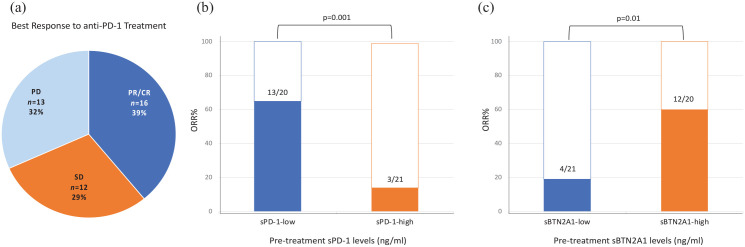
Objective response rate
We then examined the association of sPD-1 and sBTN2A1 pretreatment levels with ORR. Other ICs, because not statistically significant in the previous survival analyses (section ‘Outcome analysis’), were not included in this further investigation.
Following the first-line anti-PD-1 treatment, the patients showed the following best response: 16 patients PR/CR (39%), 12 patients SD (29%), and 13 patients PD (32%) (Figure 4(a)).
Figure 4.
ORR in the groups of patients showing high and low sPD-1 and sBTN2A1 pretreatment plasma levels.
ORR, objective response rate; sBTN, soluble butyrophilin; sPD-1, soluble programmed cell death protein 1.
The patients with low sPD-1 pretreatment levels had a significantly higher ORR (ORR 65%; n = 13/20 patients) compared to the patients with high sPD-1 levels (ORR: 14.3%, 3/21 patients) (p = 0.001) (Figure 4(b)). Conversely, the patients with high sBTN2A1 levels at baseline had a significantly higher ORR (ORR 60%; n = 12/20 patients) compared to the patients with low sBTN2A1 levels (ORR 19%, 4/21 patients) (p = 0.01) (Figure 4(c)).
Next, we examined the level of sPD-L1 and sBTN2A1 in melanoma patients according to TTF to anti-PD-1 therapy. The patients were named ‘long’ and ‘short’ responders using the overall median TTF previously investigated. The pretreatment level of sPD-1 was significantly lower, and the sBTN2A1 was significantly higher, in long-responder patients to nivolumab or pembrolizumab as first-line treatment (p = 0.001 and p = 0.004, respectively) (Supplemental Figure S1A and B). The median sPD-1 values were 10.3 ng/ml (range: 1.7–16.1) for long-responder versus 16.6 ng/ml (range: 8.3−25.0) for short-responder patients. The median sBTN2A1 values were 4.4 ng/ml (range: 3.0–9.4) for long-responder versus 3.77 ng/ml (range: 1.7–5.7) for short-responder melanoma patients.
Therefore, higher levels of sPD-1 and lower levels of sBTN2A1 before the treatment were associated with poorer clinical outcomes and ORR.
Multivariable analysis
Table 2 summarizes the results of the univariable and multivariable prognostic factor analyses for TTF and OS. Variables included in the univariate analysis were as follows: (1) gender (male or female); (2) age at first-line start (⩽45 or >45 years); (3) TILs in primary melanoma (absent/non-brisk or present brisk); (4) BMI at first-line start (<25 or ⩾25); (5) NLR at first-line start (⩽2.6 or >2.6); (6) serum pretreatment level of LDH (⩽300 or >300); (7) baseline plasma levels of sPD-L1 (<1.17 or ⩾1.17 ng/ml); (8) baseline plasma levels of sPD-1 (<11.24 or ⩾11.24 ng/ml); (9) baseline plasma levels of sBTN3A1 (<9.7 or ⩾9.7 ng/ml); and (10) baseline plasma levels of sBTN2A1 (<4.0 or ⩾4.0 ng/ml).
Table 2.
Univariable and multivariable analyses of prognostic factors for TTF and OS in melanoma patients treated with first-line nivolumab or pembrolizumab. Gender, age, TILs, BMI, NLR, LDH, sPD-L1, sPD-1, sBTN3A1, and sBTN2A1 were evaluated in the Cox regression model.
| TTF | Univariable Cox regression | Multivariable Cox regression | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Gender (M versus F) |
0.75 (0.34–1.65) | NS | ||
| Age (⩽45 versus >45 years) |
0.46 (0.21–1.00) | 0.04 | 1.13 (0.45–2.82) | NS |
| TILs (Absent/non-brisk versus present brisk) |
0.25 (0.07–0.91) | 0.03 | 0.25 (0.6–1.07) | NS |
| BMI (<25 versus ⩾25) |
0.46 (0.21–1.02) | 0.04 | 0.37 (1.15–0.89) | 0.02 |
| NLR (⩽2.6 versus >2.6) |
2.26 (1.00–5.11) | 0.04 | 1.63 (0.60–4.36) | NS |
| LDH (⩽300 versus >300) |
2.11 (0.96–4.63) | 0.05 | ||
| sPD-L1 (<1.17 versus ⩾1.17) |
1.25 (0.54–2.89) | NS | ||
| sPD-1 (<11.24 versus ⩾11.24) |
3.62 (1.51–8.67) | 0.002 | 4.5 (1.71–12.03) | 0.002 |
| sBTN3A1 (<9.7 versus ⩾ 9.7) |
0.89 (0.34–2.00) | NS | ||
| sBTN2A1 (<4.0 versus ⩾4.0) |
0.44 (0.19–0.97) | 0.03 | 0.57 (0.23–1.37) | NS |
| OS | Univariable Cox regression | Multivariable Cox regression | ||
| HR (95% CI) | P Value | HR (95% CI) | p Value | |
| Gender (M versus F) |
0.74 (0.32–1.70) | NS | ||
| Age (⩽45 versus >45 years) |
0.94 (0.41–2.12) | NS | ||
| TILs (Absent/non-brisk versus present brisk) |
0.35 (0.10–1.25) | NS | ||
| BMI (<25 versus ⩾25) |
0.52 (0.23–1.18) | NS | ||
| NLR (⩽2.6 versus >2.6) |
2.15 (0.94–4.93) | 0.06 | ||
| LDH (⩽300 versus >300) |
2.54 (1.07–6.05) | 0.02 | 1.79 (0.67–4.78) | NS |
| sPD-L1 (<1.17 versus ⩾1.17) |
1.31 (0.55–3.09) | NS | ||
| sPD-1 (<11.24 versus ⩾11.24) |
2.44 (1.05–5.68) | 0.03 | 1.79 (0.67–4.78) | NS |
| sBTN3A1 (<9.7 versus ⩾9.7) |
0.85 (0.37–1.95) | NS | ||
| sBTN2A1 (<4.0 versus ⩾4.0) |
0.85 (0.38–1.91) | NS | ||
BMI, body mass index; CI, confidence interval; HR, hazard ratio; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; sBTN, soluble butyrophilin; sPD-1, soluble programmed cell death protein 1; sPD-L1, soluble programmed cell death ligand 1; TILs, tumor-infiltrating lymphocytes; TTF, time to treatment failure.
Age, BMI, NLR, sPD-1, and sBTN2A1 were found to be statistically significantly associated with TTF in univariable analyses. In the final multivariable Cox regression model, BMI [p = 0.02, hazard ratio (HR): 0.37] and sPD-1 (p = 0.002, HR: 4.5) were significant.
Regarding OS, LDH and sPD-1 were statistically significantly associated with univariable analyses. In the final multivariable model, no prognostic factor considered remains statistically significant.
Therefore, these results showed that, in metastatic melanoma patients treated with first-line PD-1 inhibitors, BMI ⩾ 25 and sPD-1 < 11.24 ng/ml were significant independent prognostic factors for longer TTF.
TTF and OS curves were plotted according to each prognostic factor (Figures 2 and 3).
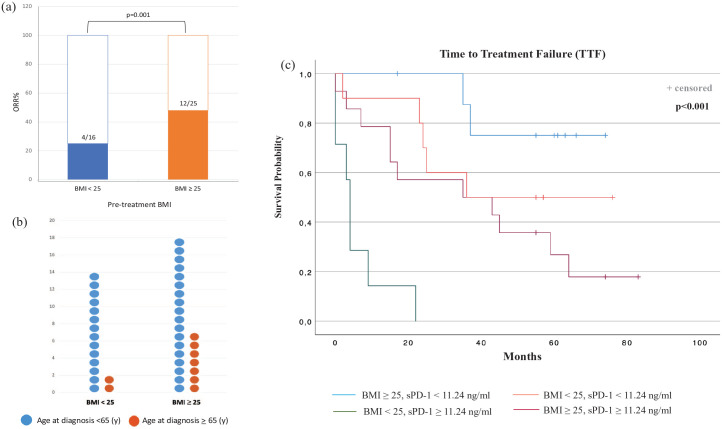
We then assessed the association between BMI and ORR. BMI ⩾ 25 was associated with higher response rates compared with patients having normal weight [48% (12 of 25) versus 25% (4 of 16)] (p = 0.001) (Figure 5(a)). Age did not show a significant impact on BMI; the patients over 65 years old were 9 out 41 (21.9%), and, among these, patients showing BMI < 25 were only 2 (22.2%) (p = 0.4) (Figure 5(b)).
Figure 5.
ORRs according to the BMI (a), BMI and age at diagnosis (b), and TTF analyses of melanoma patients stratified by BMI and sPD-1 (c).
BMI, body mass index; ORRs, objective response rates; sPD-1, soluble programmed cell death protein 1; TTF, time to treatment failure.
We further stratified melanoma patient groups using BMI along with sPD-1, which resulted to be statistically significant and associated with TTF in multivariable analyses.
Notably, patients with a BMI of 25 or higher and sPD-1 < 11.24 ng/ml had improved TTF (median TTF NR) following ICI treatment than patients with a BMI of less than 25 and sPD-1 ⩾ 11.24 ng/ml (median TTF 4 months, 95% CI, 2.8–5.2) (Figure 5(c)). Furthermore, patients with a BMI of 25 or higher and sPD-1 < 11.24 ng/ml showed longer TTF also than patients with a BMI of 25 or higher and sPD-1 ⩾ 11.24 ng/ml, and a BMI of less than 25 and sPD-1 < 11.24 ng/ml (median TTF 35 and 36 months, respectively) (p < 0.001) (Figure 5(c)).
Discussion
Individual response to ICIs is currently unpredictable in patients with advanced melanoma.
Despite the immunotherapy with anti-PD-1, alone or in combination with anti-cytotoxic T lymphocyte antigen 4 (CTLA4), results in remarkable durable responses in selected patients, the ORR remains disappointingly low.16,34–36 The high immunogenicity of melanoma is clear, but immunosuppressive mechanisms are multiple and complex.2 Beyond the historical observations of primary melanoma spontaneous regression,37 and the association with manifestation of an autoimmune reaction against melanocytes, such as vitiligo,38 melanoma has a unique immune microenvironment. Primary tumors can be characterized by dense infiltration of TILs, classified based on their distribution in brisk and non-brisk.39,40 The abundance of peritumoral TILs suggests strongly that a host-versus-tumor immune response is present and is associated with favorable prognosis41,42 and response to therapeutic blockade of the IC proteins PD-1 and CTLA4.43,44
Extensive efforts are underway to discover robust and dynamic biomarkers,34–36 and to elucidate mechanisms of response and resistance to IC blockade.
Recent findings highlighted some major concepts. First, tumor metabolic phenotype and patients’ metabolic dysregulation may play critical roles in shaping immune responses.2,25 Second, PD-1 is heavily involved in immune aging and T-cell exhaustion and dysfunction, which affects the antitumor immune response. Third, a striking improvement in the clinical outcomes of obese patients treated with ICIs exists,23 which seems driven, at least in part, by PD-1-mediated T-cell dysfunction.25
In this study, we observed that metastatic melanoma patients with BMI ⩾ 25 along with low plasma levels of PD-1 (sPD-1 < 11.24 ng/ml) assessed at baseline, had TTF and ORR first-line nivolumab or pembrolizumab. The multivariable model confirmed the independent prognostic value of this finding. The biological reason why low plasma sPD-1 is more strongly associated with TTF remains speculative. A possible explanation is that soluble PD-1 might represent a direct target of anti-PD-1 immunotherapy. Thus, high sPD-1 plasma concentration may impair the effectiveness of ICIs by neutralizing the PD-1 inhibitors pembrolizumab and nivolumab, resulting in treatment resistance and consequently a shorter TTF.45,46
Although this hypothesis is intriguing, it does not explain the better clinical outcomes in overweight melanoma patients showing low sPD-1 levels, compared to normal-weight patients with the same low sPD-1. The link between a metabolic factor and the sPD-1 expression in determining the immunotherapy response could be explained by PD-1-mediated T-cell dysfunction induced by excess adiposity.25,47 PD-1 is a key receptor both in early T-cell activation and in later chronic stimulation, where the T-cell exhaustion and anergy phenotype predominate. In fact, in tumor patients, following the chronic antigen stimulation, CD8+ exhausted T cells progressively lose effector functions and overexpress PD-1.48–52,PD-1/PD-L1 blockade can reinvigorate CD8+ exhausted T cells, leading to clinical responses to ICIs.53–55
Chronic inflammation can induce immunosuppression as a result of a protection mechanism against possible autoreactive responses from the immune system.2 In fact, the exhaustion of the immune system by persistent antigenic stimulation can activate homeostatic immunologic feedback, which contributes to the onset of adaptive resistance, ultimately inducing tumor-mediated antitumor immunity suppression.2,22 Obesity promotes a low-grade and chronic systemic inflammation.45 This phenomenon alters the microenvironment of adipose tissue, stimulating the production of pro-inflammatory cytokines, and elevated levels of insulin, glucose, fatty acids, and leptin.56,57 A recent study indicates the role of leptin signaling in increasing PD-1 expression and promoting T-cell exhaustion, resulting in an immune-suppressed phenotype and obesity-related T-cell aging.25 Therefore, our data showing lower sPD-1 concentrations in overweight patients responding to anti-PD-1 antibodies could be indicative of a lower state of CD8+ T-cell exhaustion and dysfunction. An attenuated PD-1-mediated T-cell dysfunction, indirectly expressed by lower sPD-1 levels in plasma patients, could rebalance CD8+ T-cell function through anti-PD-1 treatment more effectively, remarkably leaving the tumors more responsive to ICIs, ultimately explaining the improved outcomes in these subgroups of patients.
Interestingly, our study reveals that baseline soluble concentrations of another IC, BTN2A1, were higher in patients with improved TTF and ORR to anti-PD-1 treatment. sBTN2A1 was found to be statistically significantly associated with TTF in univariable analysis. Although not reaching an independent value in the final multivariable model, this finding could be highly relevant and should be investigated in a larger patient population. BTN2A1 is a butyrophilin (BTN) family member, recently identified as a key molecule in the activation of Vγ9Vδ2+ T cells.12,58 Vγ9Vδ2+ T cells are the most abundant subset of γδ T cells in the blood, with innate-like properties, and are activated by pAgs and phosphorylated antigens produced by many tumors.39 Recent findings indicate that BTN2A1 and BTN3A1 are required for pAg-dependent Vγ9Vδ2+ T-cell activation.12,59,60 Notably, like their adaptive counterparts, human Vγ9Vδ2+ T cells can also express the surface PD-1, leading to an ‘exhausted’ Vγ9Vδ2+ γδ T cells via the PD-1/PD-L1 axis.58 A published case report describes an exceptional prolonged response to pembrolizumab in a patient with epithelioid mesothelioma. Whole-exome sequencing on serial biopsies demonstrated acquired missense mutation in BTN2A1 at tumor relapse.61 These data underline the putative role of BTN2A1 loss of function in the mechanism of cancer immune evasion.53 Of note, our finding shows lower pretreatment levels of sBTN2A1 in patients who failed to respond to the first-line PD-1 inhibitors with nivolumab or pembrolizumab, further providing the biological rationale to better investigate this hypothesis.
A limitation of the study was the small sample size, and the small number of obese patients (BMI ⩾ 30) than overweight patients (BMI ⩾ 25 and <30). Therefore, the results warrant further study with a larger cohort. In addition, BMI is not a perfect assessment of excess adiposity because it cannot distinguish body composition, or rather fat mass, fat-free mass, and muscle quality. Furthermore, adipose tissues are highly plastic and strongly impact inflammatory and immune responses by several, known and unknown, driver mechanisms.
Hence, body fat measurement methods along with baseline and ongoing modifications of circulating ICs and inflammatory biomarkers may have a strong value in future research, linking the symbiotic immune-metabolic interplay to immunotherapy response.
Conclusion
The established link between metabolic and immunologic factors in cancer immune evasion raises important questions regarding the conversion of this knowledge into effective strategies to predict the response to immunotherapy.62 Overall, our data suggest that overweight and plasma sPD-1 low levels assessed at baseline are associated with improved anti-PD-1 treatment outcomes in metastatic melanoma patients. This preliminary report outlines the next potential avenues to select those patients that are most likely to have a long-term response and to drive the therapeutic choice. Circulating ICs detection, along with BMI, could provide more insights into the immune-metabolic interactions underlying the benefit observed in overweight/obese patients, while improving the use of dynamic, noninvasive, biomarkers for patient selection.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359231151845 for Prognostic role of soluble PD-1 and BTN2A1 in overweight melanoma patients treated with nivolumab or pembrolizumab: finding the missing links in the symbiotic immune-metabolic interplay by Lorena Incorvaia, Gaetana Rinaldi, Giuseppe Badalamenti, Alessandra Cucinella, Chiara Brando, Giorgio Madonia, Alessia Fiorino, Angela Pipitone, Alessandro Perez, Federica Li Pomi, Antonio Galvano, Valerio Gristina, Nadia Barraco, Marco Bono, Tancredi Didier Bazan Russo, Francesca Toia, Adriana Cordova, Daniele Fanale, Antonio Russo and Viviana Bazan in Therapeutic Advances in Medical Oncology
Acknowledgments
None
Footnotes
ORCID iDs: Lorena Incorvaia  https://orcid.org/0000-0002-1199-7286
https://orcid.org/0000-0002-1199-7286
Antonio Galvano  https://orcid.org/0000-0003-4365-5683
https://orcid.org/0000-0003-4365-5683
Marco Bono  https://orcid.org/0000-0001-7169-3463
https://orcid.org/0000-0001-7169-3463
Antonio Russo  https://orcid.org/0000-0002-4370-2008
https://orcid.org/0000-0002-4370-2008
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Lorena Incorvaia, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, via del Vespro 129, Palermo 90127, Italy.
Gaetana Rinaldi, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Giuseppe Badalamenti, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Alessandra Cucinella, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Chiara Brando, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Giorgio Madonia, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Alessia Fiorino, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Angela Pipitone, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Alessandro Perez, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Federica Li Pomi, Department of Clinical and Experimental Medicine, Section of Dermatology, University of Messina, Messina, Italy.
Antonio Galvano, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Valerio Gristina, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Nadia Barraco, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Marco Bono, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Tancredi Didier Bazan Russo, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Francesca Toia, Division of Plastic and Reconstructive Surgery, Department of Surgical, Oncological and Oral Sciences, University of Palermo, Palermo, Italy.
Adriana Cordova, Division of Plastic and Reconstructive Surgery, Department of Surgical, Oncological and Oral Sciences, University of Palermo, Palermo, Italy.
Daniele Fanale, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Antonio Russo, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, via del Vespro 129, Palermo 90127, Italy.
Viviana Bazan, Department of Biomedicine, Neuroscience and Advanced Diagnostics (Bind), Section of Medical Oncology, University of Palermo, Palermo, Italy.
Declarations
Ethics approval and consent to participate: The study protocol was approved by the ethical committee of the University Hospital AOUP ‘Paolo Giaccone’ of Palermo, Italy (Comitato Etico Palermo 1). All patients previously provided written informed consent.
Consent for publication: Not Applicable.
Author contribution(s): Lorena Incorvaia: Conceptualization; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Gaetana Rinaldi: Data curation; Investigation; Writing – review & editing.
Giuseppe Badalamenti: Conceptualization; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Alessandra Cucinella: Data curation; Writing – review & editing.
Chiara Brando: Data curation; Writing – review & editing.
Giorgio Madonia: Data curation; Writing – review & editing.
Alessia Fiorino: Data curation; Writing – review & editing.
Angela Pipitone: Data curation; Writing – review & editing.
Alessandro Perez: Data curation; Writing – review & editing.
Federica Li Pomi: Data curation; Writing – review & editing.
Antonio Galvano: Data curation; Writing – review & editing.
Valerio Gristina: Data curation; Writing – review & editing.
Nadia Barraco: Data curation; Writing – review & editing.
Marco Bono: Data curation; Writing – review & editing.
Tancredi Didier Bazan Russo: Data curation; Writing – review & editing.
Francesca Toia: Data curation; Writing – review & editing.
Adriana Cordova: Data curation; Writing – review & editing.
Daniele Fanale: Conceptualization; Formal analysis; Writing – review & editing.
Antonio Russo: Conceptualization; Formal analysis; Writing – review & editing.
Viviana Bazan: Conceptualization; Formal analysis; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet 2018; 392: 971e984. [DOI] [PubMed] [Google Scholar]
- 2. Huang AC, Zappasodi R. A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat Immunol 2022; 23: 660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol 2021; 40: 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Incorvaia L, Fanale D, Badalamenti G, et al. Programmed death ligand 1 (PD-L1) as a predictive biomarker for pembrolizumab therapy in patients with advanced non-small-cell lung cancer (NSCLC). Adv Ther 2019; 36: 2600–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalaora S, Nagler A, Wargo JA, et al. Mechanisms of immune activation and regulation: lessons from melanoma. Nat Rev Cancer 2022; 22: 195–207. [DOI] [PubMed] [Google Scholar]
- 6. Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4: 127ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Y, Chen L. Classification of advanced human cancers based on tumour immunity in the microenvironment (TIME) for cancer immunotherapy. JAMA Oncol 2016; 2: 1403–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buchan SL, Dou L, Remer M, et al. Antibodies to costimulatory receptor 4-1BB enhance anti- tumour immunity via T regulatory cell depletion and promotion of CD8 T cell effector function. Immunity 2018; 49: 958–970.e7. [DOI] [PubMed] [Google Scholar]
- 9. Wang J, Sanmamed MF, Datar I, et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell 2019; 176: 334–347.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Di Marco Barros R, Roberts NA, Dart RJ, et al. Epithelia use butyrophilin-like molecules to shape organ-specific gamma delta T cell compartments. Cell 2016; 167: 203–218.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rhodes DA, Reith W, Trowsdale J. Regulation of immunity by butyrophilins. Annu Rev Immunol 2016; 34: 151–172. [DOI] [PubMed] [Google Scholar]
- 12. Rigau M, Ostrouska S, Fulford TS, et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 2020; 367: eaay5516. [DOI] [PubMed] [Google Scholar]
- 13. Payne KK, Mine JA, Biswas S, et al. BTN3A1 governs antitumor responses by coordinating αβ and γδ T cells. Science 2020; 369: 942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nguyen T, Liu XK, Zhang Y, et al. BTNL2, a butyrophilin-like molecule that functions to inhibit T cell activation. J Immunol 2006; 176: 7354–7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silva-Santos B, Serre K, Norell H. γδ T cells in cancer. Nat Rev Immunol 2015; 15: 683–691. [DOI] [PubMed] [Google Scholar]
- 16. Du Y, Peng Q, Cheng D, et al. Cancer cell-expressed BTNL2 facilitates tumour immune escape via engagement with IL-17A-producing γδ T cells. Nat Commun 2022; 13: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fanale D, Incorvaia L, Badalamenti G, et al. Prognostic role of plasma PD-1, PD-L1, pan-BTN3As and BTN3A1 in patients affected by metastatic gastrointestinal stromal tumors: can immune checkpoints act as a sentinel for short-term survival? Cancers (Basel) 2021; 13: 2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Incorvaia L, Fanale D, Badalamenti G, et al. Baseline plasma levels of soluble PD-1, PD-L1, and BTN3A1 predict response to nivolumab treatment in patients with metastatic renal cell carcinoma: a step toward a biomarker for therapeutic decisions. Oncoimmunology 2020; 9: 1832348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bian B, Fanale D, Dusetti N, et al. Prognostic significance of circulating PD-1, PD-L1, pan-BTN3As, BTN3A1 and BTLA in patients with pancreatic adenocarcinoma. Oncoimmunology 2019; 8: e1561120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jaiswal AR, Liu AJ, Pudakalakatti S, et al. Melanoma evolves complete immunotherapy resistance through the acquisition of a hypermetabolic phenotype. Cancer Immunol Res 2020; 8: 1365–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Najjar YG, Menk AV, Sander C, et al. Tumor cell oxidative metabolism as a barrier to PD-1 blockade immunotherapy in melanoma. JCI Insight 2019; 4: e124989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park CS, Shastri N. The role of T cells in obesity-associated inflammation and metabolic disease. Immune Netw 2022; 22: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoo SK, Chowell D, Valero C, et al. Outcomes among patients with or without obesity and with cancer following treatment with immune checkpoint blockade. JAMA Netw Open 2022; 5: e220448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lennon H, Sperrin M, Badrick E, et al. The obesity paradox in cancer: a review. Curr Oncol Rep 2016; 18: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med 2019; 25: 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. An Y, Wu Z, Wang N, et al. Association between body mass index and survival outcomes for cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. J Transl Med 2020; 18: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Santoni M, Massari F, Bracarda S, et al. Body mass index in patients treated with cabozantinib for advanced renal cell carcinoma: a new prognostic factor? Diagnostics (Basel) 2021; 11: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vincenzi B, Badalamenti G, Armento G, et al. Body mass index as a risk factor for toxicities in patients with advanced soft-tissue sarcoma treated with trabectedin. Oncology 2018; 95: 1–7. [DOI] [PubMed] [Google Scholar]
- 29. Dyck L, Prendeville H, Raverdeau M, et al. Suppressive effects of the obese tumor microenvironment on CD8 T cell infiltration and effector function. J Exp Med 2022; 219: e20210042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Melo AM, Mylod E, Fitzgerald V, et al. Tissue distribution of γδ T cell subsets in oesophageal adenocarcinoma. Clin Immunol 2021; 229: 108797. [DOI] [PubMed] [Google Scholar]
- 31. Costanzo AE, Taylor KR, Dutt S, et al. Obesity impairs γδ T cell homeostasis and antiviral function in humans. PLoS One 2015; 10: e0120918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Silva-Santos B, Mensurado S, Coffelt SB. γδ T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat Rev Cancer 2019; 19: 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Incorvaia L, Badalamenti G, Rinaldi G, et al. Can the plasma PD-1 levels predict the presence and efficiency of tumor-infiltrating lymphocytes in patients with metastatic melanoma? Ther Adv Med Oncol 2019; 11: 1758835919848872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Russo A, Incorvaia L, Malapelle U, et al. The tumor-agnostic treatment for patients with solid tumors: a position paper on behalf of the AIOM- SIAPEC/IAP-SIBioC-SIF Italian Scientific Societies. Crit Rev Oncol Hematol 2021; 165: 103436. [DOI] [PubMed] [Google Scholar]
- 35. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aung P, Nagarajan P, Prieto V. Regression in primary cutaneous melanoma: etiopathogenesis and clinical significance. Lab Invest 2017; 97: 657–668. [DOI] [PubMed] [Google Scholar]
- 38. Cohen BE, Manga P, Lin K, et al. Vitiligo and melanoma-associated vitiligo: understanding their similarities and differences. Am J Clin Dermatol 2020; 21: 669–680. [DOI] [PubMed] [Google Scholar]
- 39. Clemente CG, Mihm MC, Jr, Bufalino R, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996; 77: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 40. Badalamenti G, Fanale D, Incorvaia L, et al. Role of tumor-infiltrating lymphocytes in patients with solid tumors: can a drop dig a stone? Cell Immunol 2019; 343: 103753. [DOI] [PubMed] [Google Scholar]
- 41. Azimi F, Scolyer RA, Rumcheva P, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol 2012; 30: 2678–2683. [DOI] [PubMed] [Google Scholar]
- 42. Thomas NE, Busam KJ, From L, et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J Clin Oncol 2013; 31: 4252–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Majzner RG, Theruvath JL, Nellan A, et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res 2019; 25: 2560–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gide TN, Silva IP, Quek C, et al. Close proximity of immune and tumor cells underlies response to anti-PD-1 based therapies in metastatic melanoma patients. Oncoimmunology 2019; 9: 1659093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Trestini I, Caldart A, Dodi A, et al. Body composition as a modulator of response to immunotherapy in lung cancer: time to deal with it. ESMO Open 2021; 6: 100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Deiuliis J, Shah Z, Shah N, et al. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PLoS One 2011; 6: e16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Russo A, Incorvaia L, Del Re M, et al. The molecular profiling of solid tumors by liquid biopsy: a position paper of the AIOM-SIAPEC-IAP-SIBioC-SIC-SIF Italian Scientific Societies. ESMO Open 2021; 6: 100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Russo A, Incorvaia L, Capoluongo E, et al. The challenge of the molecular tumor board empowerment in clinical oncology practice: a position paper on behalf of the AIOM- SIAPEC/IAP-SIBioC-SIC-SIF-SIGU-SIRM Italian Scientific Societies. Crit Rev Oncol Hematol 2022; 169: 103567. [DOI] [PubMed] [Google Scholar]
- 49. Leto G, Incorvaia L, Flandina C, et al. Clinical impact of cystatin C/cathepsin L and follistatin/activin a systems in breast cancer progression: a preliminary report. Cancer Invest 2016; 34: 415–423. [DOI] [PubMed] [Google Scholar]
- 50. Ugurel S, Schadendorf D, Horny K, et al. Elevated baseline serum PD-1 or PD-L1 predicts poor outcome of PD-1 inhibition therapy in metastatic melanoma. Ann Oncol 2020; 31: 144–152. [DOI] [PubMed] [Google Scholar]
- 51. Incorvaia L, Fanale D, Badalamenti G, et al. A “lymphocyte microRNA signature” as predictive biomarker of immunotherapy response and plasma PD-1/PD-L1 expression levels in patients with metastatic renal cell carcinoma: pointing towards epigenetic reprogramming. Cancers (Basel) 2020; 12: 3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bengsch B, Johnson AL, Kurachi M, et al. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion. Immunity 2016; 45: 358–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huang AC, Orlowski RJ, Xu X, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med 2019; 25: 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017; 545: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444: 860–867. [DOI] [PubMed] [Google Scholar]
- 56. Naylor C, Petri WA., Jr. Leptin regulation of immune responses. Trends Mol Med 2016; 22: 88–98. [DOI] [PubMed] [Google Scholar]
- 57. Saucillo DC, Gerriets VA, Sheng J, et al. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol 2014; 192: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rigau M, Uldrich AP, Behren A. Targeting butyrophilins for cancer immunotherapy. Trends Immunol 2021; 42: 670–680. [DOI] [PubMed] [Google Scholar]
- 59. Harly C, Guillaume Y, Nedellec S, et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood 2012; 120: 2269–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Karunakaran MM, Willcox CR, Salim M, et al. Butyrophilin-2A1 directly binds germline-encoded regions of the Vγ9Vδ2 TCR and is essential for phosphoantigen sensing. Immunity 2020; 52: 487–498.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Minchom A, Yuan W, Crespo M, et al. Molecular and immunological features of a prolonged exceptional responder with malignant pleural mesothelioma treated initially and rechallenged with pembrolizumab. J Immunother Cancer 2020; 8: e000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dimino A, Brando C, Algeri L, et al. Exploring the Dynamic Crosstalk between the Immune System and Genetics in Gastrointestinal Stromal Tumors. Cancers 2023; 15: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359231151845 for Prognostic role of soluble PD-1 and BTN2A1 in overweight melanoma patients treated with nivolumab or pembrolizumab: finding the missing links in the symbiotic immune-metabolic interplay by Lorena Incorvaia, Gaetana Rinaldi, Giuseppe Badalamenti, Alessandra Cucinella, Chiara Brando, Giorgio Madonia, Alessia Fiorino, Angela Pipitone, Alessandro Perez, Federica Li Pomi, Antonio Galvano, Valerio Gristina, Nadia Barraco, Marco Bono, Tancredi Didier Bazan Russo, Francesca Toia, Adriana Cordova, Daniele Fanale, Antonio Russo and Viviana Bazan in Therapeutic Advances in Medical Oncology