Abstract
Introduction
IgA nephropathy is the most common primary glomerular disease. Its pathogenesis is still poorly understood. Alterations of the Janus kinase signal transducer and activator of transcription (JAK-STAT) pathway may play an important role in IgA nephropathy.
Methods
We evaluated the clinical features, pathology, and tissue staining for lymphocytes and phosphorylated STAT1 (pSTAT1) in 43 patients with biopsy proven IgA nephropathy. They were followed to determine their disease outcomes. All had biopsy tissue and multiple laboratory measurements to assess their kidney disease progression. Sixteen patients underwent repeat kidney biopsy to further assess their clinical status.
Results
The median eGFR at baseline was 61 mL/min/1.73 m<sup>2</sup> and the median proteinuria was 2,600 mg/d. The median follow-up was 5 years with an average annual decline in eGFR of 2.25 mL/min/1.73 m<sup>2</sup>. There was significant inflammation and atrophy seen in the first biopsy, which progressed among those who undertook a 2nd biopsy. Compared to healthy kidney tissue, glomeruli and tubulointerstitium demonstrated increased lymphocyte (CD3+) infiltrates and increased pSTAT1 staining by immunohistochemistry. Increased CD3 (p = 0.001) staining and increased pSTAT1 (p = 0.03) correlated with reduced eGFR levels. In repeat biopsy samples, increasing pSTAT1 staining correlated with loss of eGFR over time (p = 0.02).
Conclusion
These findings support the hypothesis that pSTAT1 is activated in IgA nephropathy and may play a role in the progression toward kidney failure.
Keywords: IgA nephropathy, Phosphorylated STAT1, Progression, Biopsy, Immunohistochemistry
Introduction
IgA nephropathy (IgAN) is the most common glomerulonephritis worldwide. Up to 40% of patients will progress to end-stage renal disease within 20 years [1]. The underlying pathogenesis of this autoimmune disease centers on binding of anti-glycan antibodies to galactose-deficient IgA1 (Gd-IgA1), forming pathogenic immune complexes which deposit in and injure the glomerulus [2]. Gd-IgA1 and anti-glycan antibodies are considered disease markers, but only variably predict disease progression [3]. The main defect in IgAN may lie in the regulation and function of peripheral blood mononuclear cells (PBMCs), although little research has focused on subsets of PBMCs in IgAN [4, 5]. The accumulation of interstitial inflammatory cells in the kidney has been correlated with decreased renal function in patients with IgAN and may be an important influence on disease outcomes [6]. In glomerular diseases that primarily involve injury to the glomeruli, the degree of interstitial infiltration, especially with T lymphocytes, indeed correlates well with long-term outcomes, such as impairment of renal function, as compared to the extent of glomerular involvement [7, 8, 9, 10]. Janus kinase signal transducer and activator of transcription (JAK/STAT) is a major pathway that responds to and transduces inflammatory signals from extracellular ligands such as cytokines and chemokines [11]. Our recent study indicated that JAK/STAT signaling is activated in the kidney and PBMCs of patients with FSGS, with findings that compared to control, the baseline phosphorylated STAT1 (pSTAT1) in FSGS was significantly higher in CD4+ lymphocytes, CD8+CD45RA + lymphocytes, and monocytes, suggesting that pSTAT1 may play a key role in the progression of interstitial inflammation [12]. One study analyzing the genomic profile of PBMCs from 3 IgAN patients suggested a role of STAT1 in the exacerbation of gross hematuria, a sign of intense disease activity in IgAN [13]. Another small study in 2008 using immunohistochemistry found that the expression of pSTAT3 in the human kidney was increased in the glomerular and tubulointerstitial cells in IgAN but not in minimal change disease or membranous nephropathy [14]. More recently, we have reported increased tubulointerstitial transcription of components of the JAK/STAT system in IgA nephropathy, which is associated with other disease markers in cross-sectional fashion [15]. We aimed to further explore the expression of pSTAT1 in the kidneys of patients with IgAN, as well as its presence in infiltrated lymphocytes in the kidneys of patients with IgAN, with the hypothesis that altered pSTAT (STAT phosphorylation) facilitates disease progression in IgAN, and could be a novel target for treatment to prevent disease progression.
Methods
Patients
The study was approved by the Institutional Review Board of Stanford University Medical Center. Using “IgA” as the key word in the final text diagnosis, Stanford's kidney pathology registry was searched from 2003 to June 2016 and linked to Stanford's electronic medical record. 769 native kidney adult biopsy cases (patient aged >18 years old) were found with IgA in the final pathological diagnosis report. 246 cases were excluded due to: insufficient residual sample for immunohistochemical staining, no clinical follow-up available, clinical diagnosis of IgA vasculitis, comorbidity with HBV, DN, MCD, HIV, FSGS, anti-GBM, HCV, IMN, incidental IgA deposits, smoldering myeloma, or polyarthritis nodosum. Among the remaining 523 cases, we identified 43 cases with at least one follow-up with interval duration of more than 1 month at the Stanford clinic. During this same period, we identified 16 adult IgAN patients (9 women and 7 men) who underwent both a baseline and repeat biopsy at Stanford.
Dual Immunohistochemistry Staining for CD3 and pSTAT1
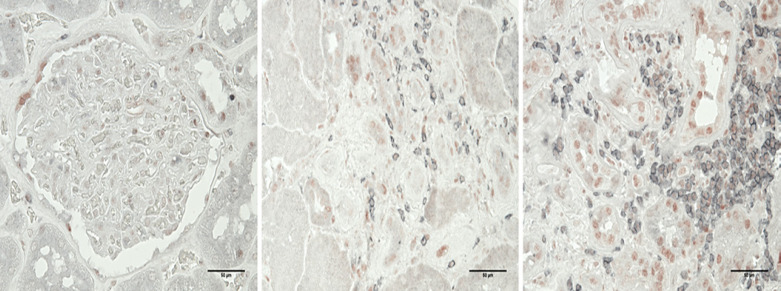
Starting with unstained paraffin-fixed slides, antigen retrieval was done with citrate solution at pH 6.0. Normal serum was used to block the antigens for 30 min at room temperature. Primary antibodies CD3 (DAKO CD3 Clone F7.28) and pSTAT1 (cell signaling #8826) were applied with a dilution of 1:50 and 1:200, respectively, for 60 min at room temperature. Second antibodies to rabbit Ig or mouse Ig (Vector labs) were incubated for 30 min at room temperature. DAB was incubated for 3 min at room temperature to develop the staining. Hematoxylin was counter-stained for 1 min at room temperature. The representative images are shown in Figure 1. Semi-quantification criterion: CD3 density: 0: no lymphoid aggregate (>50 cells/focus); 1: 1 aggregate/bx; 2: 2 aggregates/bx; 3: 3 or more aggregates/biopsy; % cells dual +: 0: none; 1: <20%; 2: 20–50%; 3: >50%; pSTAT1 scoring 0: none, 1: <50% cells weak, 2: 51–100% cells, slightly strong or 20–50% very strong, 3: 51–100% cells strong staining. The semi-quantification of inflammation was done by routine pathology evaluation of cellular infiltrates and in atrophic or non-atrophic areas was set as 0: no inflammation; 1: sparse inflammation with no collections of greater than 10 cells/focus/slide; 2: moderate inflammation with a collection of more than 10 cells/focus/slide; 3: severe inflammation with several large collections of more than 50 cells/focus/slide.
Fig. 1.
Representative images of dual staining in control and 2 IgAN patients (×400). Left: control; middle: IgAN biopsy with mild lymphocytic (CD3 stained as gray) infiltration; right: IgAN biopsy with massive lymphocytic infiltration (CD3 stained as gray) and pSTAT1 (stained as red) expression.
Statistical Analysis
Descriptive statistics, including mean and SD for normally distributed variables, median and interquartile range for skewed variables, and proportions for categorical variables, were used to characterize participant characteristics. Spearman's rank correlation coefficient (rho) was used to evaluate the relationship between semi-quantification of CD3, pSTAT1, or pSTAT1 in CD3 with eGFR, urine protein creatinine ratio, or the decline of eGFR. Analyses were performed using Excel. p < 0.05 was considered statistically significant.
Results
This single-center IgAN cohort comprised 43 patients, with a median age at biopsy of 42 years. Their characteristics are depicted in Table 1. There were 17 women. The majority of patients were non-Hispanic. The median eGFR at the biopsy was 47 mL/min/1.73 m2. The median follow-up was 5 years with an annual decline of eGFR of 2.25 mL/min/1.73 m2.
Table 1.
Baseline characteristics of 43 IgAN patients
| Age at biopsy, years | 42 (20–78) |
| Gender (women) | 17 (40.4%) |
| Non-Hispanic/non-Latino | 27 (64.2%) |
| Asian | 7 (16.6%) |
| Unknown | 7 (14.2%) |
| Hispanic | 2 (4.7%) |
| Follow-up, years | 5 (0.1–12.2) |
| eGFR decline during follow-up, mL/min/1.73 m2/years | 2.25 (–31 to 44) |
| eGFR at biopsy, mL/min/1.73 m2 | 47 (20–108) |
| Urine protein creatinine excretion ratio at biopsy, g/g | 1.82 (1.33–15) |
| MEST-C scores in pathology | M0 33/43 M1 10/43 |
| E0 38/43 E1 5/43 | |
| S0 10/43 S1 33/43 | |
| T0 14/43 T1 19/43 T2 10/43 | |
| C0 28/43 C1 15/43 C2 0/43 |
Median (min, max).
In the cohort of 16 IgAN patients who had a second biopsy, there were 4 Asians, 7 non-Hispanic white, 4 with race/ethnicity not available, and 1 Hispanic. Nine were newly diagnosed at the time of the first biopsy; the other 7 had prior disease duration ranging from 1 year to 11 years beforehand. Only 1 patient is still being followed with stable renal function. Six cases required renal replacement or were listed on the transplant wait list (1, 10, 3, 8, 12, and 9 months later). The other 9 cases were ultimately lost to follow up after the second biopsy. One case had been treated with 6 months of prednisone before the first biopsy. Six cases had also been treated with mycophenolic acid or prednisone for several months before the second biopsy. Their clinical profiles are listed in Table 2. Their MEST-C scores are summarized in Table 3, pathological features are listed in Table 4. The average age at biopsy was 40 years old; after an average of 6 years' follow-up, their eGFR significantly decreased from 83 to 48.5 mL/min/1.73 m2. Their systolic blood pressure was significantly increased from 131 to 156 mm Hg, and this was accompanied by increased severity in tubular atrophy and interstitial fibrosis, which was also reflected by the semi-quantification of IFTA and inflammation in non-atrophic areas.
Table 2.
Baseline characteristics of 16 IgAN patients with repeat biopsy
| Age, years | Serum creatinine, mg/dL | GFR, mL/min/ 1.73 m2 | Proteinuria, mg/day | SBP, mm Hg | DBP, mm Hg | |
|---|---|---|---|---|---|---|
| 1st biopsy | ||||||
| Mean | 40.9 | 1.23 | 64.9 | 2,878.1 | 127.8 | 82.3 |
| STDEV | 7.4 | 0.45 | 26.1 | 1,522.8 | 6.9 | 6.1 |
| Median | 42.5 | 1.16 | 61 | 2,800 | 125 | 81 |
| 25 quartile | 34.5 | 1.0 | 48 | 1,625 | 123.3 | 77 |
| 75 quartile | 47.3 | 1.3 | 83 | 4,400 | 131.8 | 87 |
| 2nd biopsy | ||||||
| Mean | 46.1 | 2.98 | 36.1 | 3,772 | 156.1 | 88.3 |
| STDEV | 7.93 | 2.64 | 21.12 | 3,088 | 30.2 | 13.7 |
| Median | 49.5 | 1.8 | 40.5 | 3,100 | 142 | 90 |
| 25 quartile | 38.8 | 1.29 | 17.5 | 1,100 | 137 | 81 |
| 75 quartile | 52 | 3.02 | 48.5 | 4,500 | 183 | 100 |
| p value | 0.032 | 0.005 | 0.361 | 0.032 | 0.32 |
STDEV, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Table 3.
MEST-C classification of 16 IgAN patients with repeat biopsy (classification level, number of the cases)
| 1st biopsya | 2nd biopsyb |
|---|---|
| M M0 11, M1 4 | M M0 10, M1 4 |
| E E0 12, E1 3 | E E0 8, E1 6 |
| S S0 3, S1 12 | S S0 3, S1 11 |
| T T0 9, T1 5, T2 2 | T T0 4, T1 4, T2 6 |
| C C0 7, C1 9 | C C0 8, C1 6 |
All glomeruli were globally sclerotic in 1 case, thus only 15 cases were analyzed for the MEST-C score.
Two cases were not analyzed due to inadequate tissue.
Table 4.
Pathologic characteristics of 16 IgAN patients with repeat biopsy
| G | SG | IFTA | Inflammation intensity | Inflammation in atrophic area, % | Inflammation in non-atrophic area, % | Crescent or necrosis, % | |
|---|---|---|---|---|---|---|---|
| 1st biopsy | |||||||
| Mean | 18.36 | 4.75 | 21.79 | 1.5 | 23.25 | 1.69 | 6.35 |
| SD | 11.35 | 2.98 | 14.72 | 0.52 | 12.61 | 3.50 | 8.7 |
| Median | 16 | 4 | 20 | 1.5 | 21 | 0 | 0 |
| 25 quartile | 11.5 | 2.75 | 10.63 | 1 | 13.75 | 0 | 0 |
| 75 quartile | 23.75 | 7.25 | 30 | 2 | 31.25 | 0.50 | 12.50 |
| 2nd biopsy | |||||||
| Mean | 21.21 | 7.71 | 41.54 | 1.77 | 43.54 | 1.31 | 2.92 |
| SD | 13.99 | 7.08 | 21.89 | 0.80 | 21.33 | 2.87 | 3.51 |
| Median | 15 | 4.5 | 45 | 2 | 45 | 0 | 0 |
| 25 quartile | 10 | 2.25 | 25 | 1 | 23 | 0 | 0 |
| 75 quartile | 28.75 | 12.5 | 50 | 2 | 60 | 0 | 6.15 |
| t test | 0.55 | 0.15 | 0.012 | 0.29 | 0.004 | 0.75 | 0.18 |
G, the number of the glomeruli; SG, the number of globally sclerotic glomeruli; IFTA, interstitial fibrosis tubular atrophy.
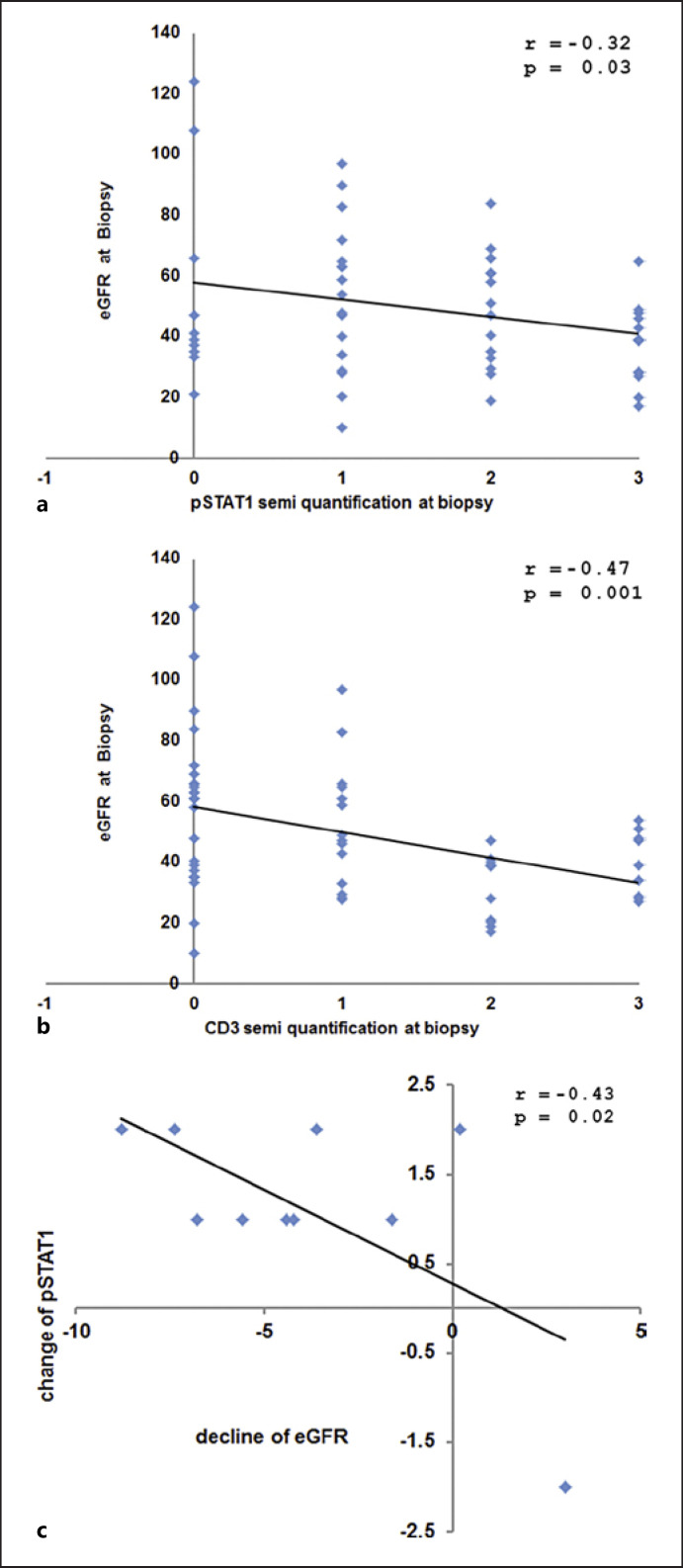
As depicted in Figure 2, correlational analysis showed that the number of resident lymphocytes in the kidney was negatively correlated with eGFR at biopsy (n = 56; p = 0.001, r = 0.47), and pSTAT1 expression in the kidney was negatively correlated with eGFR at biopsy (n = 56; p = 0.03, r = 0.32). In the cohort of repeat biopsy IgAN patients, the change in pSTAT1 scoring was significantly correlated with the decline of eGFR per year (r = 0.43, p = 0.02). We evaluated whether we could identify the presence of infiltrating lymphocytes that stained positive for pSTAT1 (dual stain, CD3, and pSTAT1). While these cells could be readily identified, their semi-quantification trended, but was not significantly correlated, with clinical parameters or outcome (r = 0.20, p = 0.19).
Fig. 2.
Correlation analysis between pathology and clinical markers. a eGFR at time of biopsy plotted against pSTAT1 semi-quantification at biopsy. b eGFR at time of biopsy plotted against CD3 semi-quantification at biopsy. c Change of pSTAT1 semi-quantification against change in eGFR per year from Biopsy 1 to Biopsy 2.
Discussion
While IgA nephropathy is the most common primary glomerular disease in the world, its pathogenesis remains poorly understood. Upstream events have indeed been better elucidated within the “multi-hit hypothesis,” but what transduces injury to the kidney remains uncertain. The role of specific subpopulations of lymphocytes in the pathogenesis of IgAN remains an exciting research area, as evidence implicates many specific immune cells, including dendritic cells [16], natural killer cells [17], CD4 and CD8 T lymphocytes, monocytes, neutrophils, mast cells, and T regulatory cells [18] in the pathogenesis of glomerular diseases [19]. Consistent with the findings from D'Amico [8], our study demonstrates that increasing infiltration of lymphocytes (in atrophic and non-atrophic areas) was negatively correlated with kidney structure and function, suggesting that a cause-effect relation may exist. Although we did not find a meaningful correlational relation between the change of the number of lymphocytes with the decline of eGFR in the repeat biopsy cohort, as had been shown in Faria's study [20], we would suggest that this might be due to the small number of cases studied in this cohort (n = 10, repeat biopsy cases). The baseline lymphocyte number did correlate with progression.
JAK/STAT is a critical pathway that responds to and transduces inflammatory signals from extracellular ligands, including many cytokines and chemokines [11]. Strong evidence suggests that it is critical in the differentiation and maturation of the immune system, autoimmune disease processes, cancer development, angiogenesis, and metastasis [11]. Data from kidney cell specific over-expression or knock out transgenic animal models and genetic profile analyses in human samples reveal that the JAK/STAT pathway is extensively activated in diabetic nephropathy, ADPKD, HIVAN, AKI, obstructive uropathy [21], and renal fibrosis [22]. However, its role in the pathogenesis of glomerular disease has rarely been investigated. We have reported a role in focal segmental glomerulosclerosis and in IgA nephropathy based on PBMC activity and transcription in the kidney. The major finding of the present study demonstrates that pSTAT1 expression (judged by histology) was negatively correlated with the eGFR at the time of biopsy, and its change along the disease course was significantly correlated with the decline of eGFR, suggesting that activated STAT1 may indeed play an important role in the clinical and pathological progression of IgAN.
Among seven STATs identified in mammals [23], the biological relevance of STAT1 is to target genes to promote inflammation and antagonize proliferation, which contrasts with the pro-proliferative and anti-inflammatory activities associated with STAT3 [24]. STAT1 has been found to be activated in diffuse proliferative lupus nephritis [25]. STAT1 is implicated in the mediation of Ang II-induced human glomerular mesangial cell senescence through the p53/p21(Cip1) pathway, and losartan could attenuate this senescence by regulating STAT1 [26]. Based on those findings and our own, we propose that STAT1 is active in IgAN, possibly through participating in the mesangial proliferation/cellularity and/or interstitial inflammation or fibrosis that occurs in this disease.
Another effort of this study was to evaluate whether pSTAT1 expression in infiltrated lymphocytes in the kidney at biopsy was correlated with eGFR or proteinuria, or with the change of either marker. We successfully developed a dual stain method to observe the co-localization of those two markers and semi-quantified the positive area. While we find that pSTAT does indeed colocalize with CD3 cells, we did not find any significant correlations with biopsy scores or patient outcomes. We further assessed the repeat biopsy cohort to investigate if the change in pSTAT1 would correlate with any change in proteinuria along the disease course, and we found no significant correlations. The explanations for these negative findings could be due to selection bias, which is hard to avoid in any retrospective clinical study, small number of patients, or accuracy limited by the semi-quantification method itself. Perhaps further development of a digital, fully quantitative method, such as that with image J capture, to estimate the expression of pSTAT1 (shown in red in dual staining in Fig. 1) in lymphocytes (shown in gray in dual staining) would be more accurate in associating with clinical features.
In summary, this study assessed cross sectional and longitudinal data from kidney biopsy tissue in an effort to address contributions made by lymphocyte aggregation and STAT1 activation. It supports the hypothesis that STAT1 is activated during the progression of IgAN. There also remains a possibility that this activation may play a role in the progression of kidney disease in IgA nephropathy. STAT1's activation in lymphocytes and its role in the pathogenesis of IgAN deserve further investigation.
Statement of Ethics
This study protocol was reviewed and approved by the Stanford University Human Subjects Committee (the Institutional Review Board) with a waiver of consent, IRB 39710. Thus, no patient consent was necessary.
Conflict of Interest Statement
Drs. Kambham and Tao and Ms. Kwok report no conflicts. Dr. Lafayette receives research support from the NIH, Travere, Calliditas, Roche, Omeros, Pfizer, Vera, Chinook, and Alexion. He has received consulting fees from Travere, Chemocentryx, Calliditas, Roche, Omeros, Pfizer, Vera, Chinook, Alexion, Reata, and Novartis.
Funding Sources
The authors thank the contribution of the Sobrato Family Foundation and the Perlegos Family fund to this effort. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
Dr. Jianling Tao formulated the project, performed the study, analyzed the data, drafted the manuscript, and approved the final manuscript. Dr. Neeraja Kambham reviewed the pathology, modified the project, and helped drafting and editing the manuscript. She approved the final manuscript. Ms. Shirley Kwok performed the immunohistochemistry, took the histology pictures, reviewed the manuscript, and approved the final manuscript. Dr. Richard Lafayette helped formulate the project, edited the manuscript, and approved the final manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article, as possible. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Dr. Paul Grimm, Professor of Pediatrics at Stanford Lucile Packard Children's Hospital, for his great help with image analysis.
Funding Statement
The authors thank the contribution of the Sobrato Family Foundation and the Perlegos Family fund to this effort. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vecchio M, Bonerba B, Palmer SC, Craig JC, Ruospo M, Samuels JA, et al. Immunosuppressive agents for treating IgA nephropathy. Cochrane Database Syst Rev. 2015;8:CD003965. doi: 10.1002/14651858.CD003965.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Maillard N, Wyatt RJ, Julian BA, Kiryluk K, Gharavi A, Fremeaux-Bacchi V, et al. Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol. 2015;26((7)):1503–1512. doi: 10.1681/ASN.2014101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camilla R, Suzuki H, Daprà V, Loiacono E, Peruzzi L, Amore A, et al. Oxidative stress and galactose-deficient IgA1 as markers of progression in IgA nephropathy. Clin J Am Soc Nephrol. 2011;6((8)):1903–1911. doi: 10.2215/CJN.11571210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox SN, Serino G, Sallustio F, Blasi A, Rossini M, Pesce F, et al. Altered monocyte expression and expansion of non-classical monocyte subset in IgA nephropathy patients. Nephrol Dial Transplant. 2015;30((7)):1122–1232. doi: 10.1093/ndt/gfv017. [DOI] [PubMed] [Google Scholar]
- 5.Stachowski J, Barth C, Michałkiewicz J, Krynicki T, Jarmoliński T, Runowski D, et al. Th1/Th2 balance and CD45-positive T cell subsets in primary nephrotic syndrome. Pediatr Nephrol. 2000;14((8–9)):779–785. doi: 10.1007/PL00013437. [DOI] [PubMed] [Google Scholar]
- 6.Pei G, Zeng R, Han M, Liao P, Zhou X, Li Y, et al. Renal interstitial infiltration and tertiary lymphoid organ neogenesis in IgA nephropathy. Clin J Am Soc Nephrol. 2014;9((2)):255–264. doi: 10.2215/CJN.01150113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noronha IL, Fujihara CK, Zatz R. The inflammatory component in progressive renal disease are interventions possible? Nephrol Dial Transplant. 2002;17((3)):363–368. doi: 10.1093/ndt/17.3.363. [DOI] [PubMed] [Google Scholar]
- 8.D'Amico G. Tubulo-interstitial damage in glomerular diseases its role in the progression of the renal damage. Nephrol Dial Transplant. 1998;13((Suppl 1)):80–85. doi: 10.1093/ndt/13.suppl_1.80. [DOI] [PubMed] [Google Scholar]
- 9.Hooke DH, Gee DC, Atkins RC. Leukocyte analysis using monoclonal antibodies in human glomerulonephritis. Kidney Int. 1987;31((4)):964–972. doi: 10.1038/ki.1987.93. [DOI] [PubMed] [Google Scholar]
- 10.Couser WG, Johnson RJ. The etiology of glomerulonephritis roles of infection and autoimmunity. Kidney Int. 2014;86((5)):905–914. doi: 10.1038/ki.2014.49. [DOI] [PubMed] [Google Scholar]
- 11.O'Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36((4)):542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao J, Mariani L, Eddy S, Maecker H, Kambham N, Mehta K, et al. JAK-STAT signaling is activated in the kidney and peripheral blood cells of patients with focal segmental glomerulosclerosis. Kidney Int. 2018;94((4)):795–808. doi: 10.1016/j.kint.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox SN, Sallustio F, Serino G, Loverre A, Pesce F, Gigante M, et al. Activated innate immunity and the involvement of CX3CR1-fractalkine in promoting hematuria in patients with IgA nephropathy. Kidney Int. 2012;82((5)):548–560. doi: 10.1038/ki.2012.147. [DOI] [PubMed] [Google Scholar]
- 14.Arakawa T, Masaki T, Hirai T, Doi S, Kuratsune M, Arihiro K, et al. Activation of signal transducer and activator of transcription 3 correlates with cell proliferation and renal injury in human glomerulonephritis. Nephrol Dial Transplant. 2008;23((11)):3418–3426. doi: 10.1093/ndt/gfn314. [DOI] [PubMed] [Google Scholar]
- 15.Tao J, Mariani L, Eddy S, Maecker H, Kambham N, Mehta K, et al. JAK-STAT Activity in peripheral blood cells and kidney tissue in IgA Nephropathy. Clin J Am Soc Nephrol. 2020;15:973–982. doi: 10.2215/CJN.11010919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickinson BL. Unraveling the immunopathogenesis of glomerular disease. Clin Immunol. 2016;169:89–97. doi: 10.1016/j.clim.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Pereira RL, Reis VO, Semedo P, Buscariollo BN, Donizetti-Oliveira C, Cenedeze MA, et al. Invariant natural killer T cell agonist modulates experimental focal and segmental glomerulosclerosis. PLoS One. 2012;7((3)):e32454. doi: 10.1371/journal.pone.0032454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira F, Brito-Melo GE, Guimarães FT, Carvalho TG, Mateo EC, Simões E, Silva AC. The role of the immune system in idiopathic nephrotic syndrome a review of clinical and experimental studies. Inflamm Res. 2014;63((1)):1–12. doi: 10.1007/s00011-013-0672-6. [DOI] [PubMed] [Google Scholar]
- 19.Kitching AR, Hutton HL. The players cells involved in glomerular disease. Clin J Am Soc Nephrol. 2016;11((9)):1664–1674. doi: 10.2215/CJN.13791215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faria B, Henriques C, Matos AC, Daha MR, Pestana M, Seelen M. Combined C4d and CD3 immunostaining predicts immunoglobulin (Ig)A nephropathy progression. Clin Exp Immunol. 2015;179((2)):354–361. doi: 10.1111/cei.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brosius FC, 3rd, He JC. JAK inhibition and progressive kidney disease. Curr Opin Nephrol Hypertens. 2015;24((1)):88–95. doi: 10.1097/MNH.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang J, Liu CY, Lu MM, Zhang J, Mei WJ, Yang WJ, et al. Fluorofenidone protects against renal fibrosis by inhibiting STAT3 tyrosine phosphorylation. Mol Cell Biochem. 2015;407((1–2)):77–87. doi: 10.1007/s11010-015-2456-5. [DOI] [PubMed] [Google Scholar]
- 23.Stark GR, Darnell JE., Jr The JAK-STAT pathway at twenty. Immunity. 2012;36((4)):503–514. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schindler C, Levy DE, Decker T. JAK-STAT signaling from interferons to cytokines. J Biol Chem. 2007;282((28)):20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Lostao L, Ordi-Ros J, Balada E, Segarra-Medrano A, Majó-Masferrer J, Labrador-Horrillo M, et al. Activation of the signal transducer and activator of transcription-1 in diffuse proliferative lupus nephritis. Lupus. 2007;16((7)):483–488. doi: 10.1177/0961203307079618. [DOI] [PubMed] [Google Scholar]
- 26.Jiao S, Zheng X, Yang X, Zhang J, Wang L. Losartan inhibits STAT1 activation and protects human glomerular mesangial cells from angiotensin II induced premature senescence. Can J Physiol Pharmacol. 2012;90((1)):89–98. doi: 10.1139/y11-105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article, as possible. Further inquiries can be directed to the corresponding author.