Abstract
Background
HIV-associated nephropathy (HIVAN) is a renal parenchymal disease that occurs exclusively in people living with HIV. It is a serious kidney condition that may possibly lead to end-stage kidney disease, particularly in the HIV-1 seropositive patients.
Summary
The African-American population has increased susceptibility to this comorbidity due to a strong association found in the APOL1 gene, specifically two missense mutations in the G1 allele and a frameshift deletion in the G2 allele, although a “second-hit” event is postulated to have a role in the development of HIVAN. HIVAN presents with proteinuria, particularly in the nephrotic range, as with other kidney diseases. The diagnosis requires biopsy and typically presents with collapsing subtype focal segmental glomerulosclerosis and microcyst formation in the tubulointerstitial region. Gaps still exist in the definitive treatment of HIVAN - concurrent use of antiretroviral therapy and adjunctive management with like renal-angiotensin-aldosterone system inhibitors, steroids, or renal replacement therapy showed benefits.
Key Message
This study reviews the current understanding of HIVAN including its epidemiology, mechanism of disease, related genetic factors, clinical profile, and pathophysiologic effects of management options for patients.
Keywords: Chronic kidney disease, Glomerular disease, Glomerulonephritis, HIV-associated nephropathy, Pathology
Introduction
HIV-associated nephropathy (HIVAN) is the first kidney disease classically associated with HIV infection discovered in 1984 during the early AIDS epidemic where a subset of patients developed severe nephrotic syndrome, irreversible renal failure, and rapid progression to end-stage kidney disease (ESKD) [1]. HIVAN, characterized as a rapidly progressive form of collapsing focal segmental glomerulosclerosis (FSGS), has dramatically declined in incidence and mortality [2] since the advent of widespread combination antiretroviral therapy (cART) [3, 4], from 80% in biopsies obtained from 1995 to 1997 to 20% in those obtained from 2000 to 2004 [1]. Despite HIVAN still being the most common disease in patients not receiving cART, other causes of chronic kidney disease (CKD) have emerged in those receiving cART, with immune complex glomerulonephritis (17%) and diabetic nephropathy (16%) both outnumbering HIVAN (14%), followed by tenofovir nephrotoxicity (13%), FSGS (12%), and global sclerosis (9%) [3].
Most histopathologic studies revealed collapsing FSGS, interstitial nephritis, visceral epithelial cell proliferation, and microcystic dilatation of the tubules [5, 6]. It is caused by the direct cytopathic effect of the HIV-1 virus actively replicating inside the renal cells [7, 8]. The clinical hallmarks of HIVAN are moderate-to-heavy proteinuria and enlarged, echogenic kidneys on ultrasound [7, 8]. The diagnosis of HIVAN requires a kidney biopsy (KB) because it is very difficult to distinguish HIVAN from other renal lesions on clinical grounds alone [9]. In individuals with nephrotic range proteinuria without HIVAN, the usual suspects are classic focal sclerosing glomerulosclerosis, membranoproliferative glomerulonephritis, amyloid A amyloidosis, and diabetic nephropathy [9]. It is also remarkable to note that there are recent studies reporting membranous nephropathy associated with HIV despite yielding negative anti-PLA2R, the major target antigen in membranous nephropathy [10]. Current recommendation is to initiate highly active antiretroviral therapy (ART) among patients with HIVAN regardless of CD4 count [9]. ACE inhibitors or ARBs should be initiated if there are no contraindications [8, 11]. The mainstay of treatment for ART-naive patient is the initiation of ART and avoiding all nephrotoxic agents [8]. According to IDSA, ART is underused in this population [11]. For patients who develop ESKD, renal transplant is an option for those with CD4 count of more than 200 cells/mm3 and with undetectable viral load [9].
Epidemiology and Clinical Profile
HIVAN usually presents in the setting of severe immunosuppression characterized by low CD4 count and high viral loftad and the absence of antiretrovirals [9]. A comparison between people living with HIV (PLHIV) presenting with HIVAN or classic FSGS demonstrated that the former had significantly lower median CD4 counts (74/mL vs. 367/mL) and higher viral load (36,532 vs. <40, interquartile range) [12].
From a global standpoint, several countries follow the decreased incidence of HIVAN with some nations such as Italy, Thailand [13], and Australia [14] even reporting a complete absence of HIVAN in kidney biopsies, in contrast to South African patients diagnosed with HIV, where the most common KB finding was HIVAN [15], especially considering that the highest HIV rates (>7%) are of South African nations [16]. Despite the drastic global decline in HIVAN incidence, there are still geographical disparities. Bookholane et al. [15] emphasized the difference in prevalence of HIVAN in South Africa compared to other nations as attributable to a strikingly low availability and accessibility to cART, resulting in challenges in providing adequate cART and achieving virologic suppression in South African patients diagnosed with HIV [17]. Apolipoprotein L1 (APOL1) gene is strongly associated with FSGS (odds ratio [OR] 17) and HIVAN (OR 29) in African Americans [18] and with HIVAN in South Africans (OR 89) [19].
In the USA, the disease affects individuals of African ancestry almost exclusively with a prevalence ranging from 3% to 12% [4, 20]. This may be due to the increased prevalence of the APOL1 risk alleles in this population [8, 9]. In a cross-sectional study done on HIV seropositive patients not on cART in Africa, Han et al. [21] suggested that persistent microalbuminuria, which predicted HIVAN findings on biopsy 86% of the time, may be used as an early marker in screening for HIVAN [21]. Based on the US Renal Data System (USRDS), ESKD patients with HIVAN are predominantly male [22].
Using USRDS data from 1989 to 2011, Razzak Chaudhary et al. [22] found that incidence of HIVAN-associated ESKD in the USA remained stable from 1995 to 2006, then decreased from then onward (from 893 in 2006 to 525 in 2011), concurrent with the increasing availability of ART. However, while the number and mortality rate of individuals with HIVAN ESKD declined, the mortality rate still remains >5-fold higher than all other causes of ESKDs [22].
HIVAN usually presents with nephrotic range proteinuria associated with a rapid deterioration of renal function [9]. In a study of 57 biopsy-proven HIVAN patients, the median glomerular filtration rate was 20 mL/min, and patients had heavy proteinuria of 4.1 g/day [23]. Interestingly, bipedal edema and hypertension are not typical of HIVAN, and urinalysis among patients typically shows bland urinary sediments, varying casts, and renal epithelial cells [9].
Racial Disparity in HIV-Related CKD
According to the USRDS, the risk of developing ESKD is 50-fold higher in PLHIV African Americans than in PLHIV whites [24]. These data were corroborated by Lucas et al. [11] where they found significant African American-white disparities in HIV-related CKD, wherein African Americans usually have a more aggressive natural history.
The most common histopathologic diagnosis in the study of Lucas et al. [25] at Johns Hopkins Hospital was HIVAN (37%). They found out that compared to white cohorts, African American subjects with CKD were highly likely to have prevalent disease, to be younger, have had a history for injection drug use, and to be seropositive for hepatitis C infection. Moreover, the African American cohort had low CD4 counts at the time of diagnosis and was more likely to be ART-naive. Furthermore, African American subjects had higher serum creatinine and urinary protein excretion and significantly lower eGFR.
Europe, Asia, and South America
In a study done by Post et al. [26] in the UK, 58 of 16,834 patients from 8 HIV centers had HIVAN. All 58 patients were of African descent, and 48 out of 58 patients had HIVAN at the time of HIV diagnosis. Renal survival appears to be poor even for patients on cART, with the study by Post et al. [26] showing no association between early cART initiation, rapid viral suppression, or CD4+ T-cell recovery and renal outcome. In a cross-sectional study done by Al-Sheikh et al. [27] in Saudi Arabia, 16 out of 248 or 6.5% of adult HIV-infected patients had proteinuria without any other possible cause, which the researchers concluded could be due to HIVAN. However, the study was limited by the lack of renal biopsy confirmation for the patients with suspected HIVAN [27].
Africa: West vs East African Ancestry
The presence of 2 APOL1 risk alleles has been strongly associated with HIVAN (OR 29) in African Americans [19] and with HIVAN in South Africans (OR 89) [28]. However, in a more recent cross-sectional study, Hung et al. [29], investigated the relationship between region of African ancestry (East, Central, South, or West Africa) and kidney disease in people of sub-Saharan African ancestry diagnosed with HIV in the UK. They found that West African ancestry as compared to East/South African ancestry was strongly associated with a diagnosis of HIVAN/FSGS. Similarly, Mallipattu et al. [30] investigated the highest prevalence of CKD in Nigeria, the most populous nation in West Africa, and found an association with variability in access to care and local standards of cART initiation. This increased prevalence of HIV-associated CKD and HIVAN in West Africa was in contrast to Ethiopia, the most populous Eastern African nation, where no cases of HIVAN were identified in a cohort of 338 Ethiopian immigrants in Israel, of whom only two individuals carried a single copy of the APOL1 risk alleles [30]. Although HIVAN pathogenesis may be dependent on several factors, the influence of geography has been notable, especially in West African and South African populations.
APOL1 G1 and G2 Alleles as a Risk Factor for HIVAN Requires a “Second Hit”
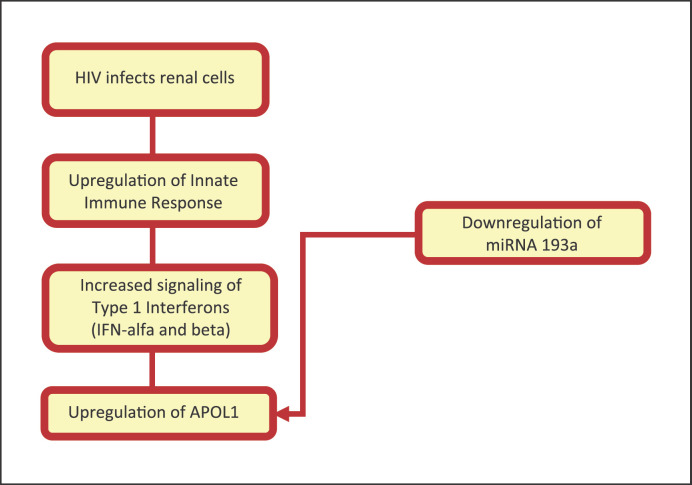
APOL1 is strongly associated with HIVAN with an OR of 29! [4]. It is located on chromosome 22 that codes for APOL1 [31]. APOL1 is a trypanosome lytic factor mostly secreted into the serum by the liver and is found in high-density lipoprotein or complexed to IgM [31, 32]. Two variants of the APOL1 gene, G1 (containing two missense mutations) and G2 (containing a frameshift deletion), have been associated with a significantly increased risk of developing HIVAN in those with HIV infection, and the estimated lifetime risk of carrying 2 APOL1 variants can be as high as 50% for HIVAN [4, 31]. Any combination of the variants is linked with a much greater risk of developing HIVAN [28]. Nevertheless, most people with APOL1 G1 and G2 alleles do not necessarily develop kidney disease. It has been postulated that a second hit may play a role to cause the development of disease [31, 32]. The most well-accepted mechanism in the setting of HIVAN is that the innate immune response to HIV upregulates APOL1 production via the type 1 interferon pathway (shown in Fig. 1) [31].
Fig. 1.
Mechanism of APOL1 upregulation by the human immunodeficiency virus.
The question on testing for high-risk APOL1 alleles remains controversial. Given the known risks of carrying high-risk variants, information on which patients' renal function may be expected to decline faster would allow for closer monitoring and could possibly encourage lifestyle modification on the patient's end [33]. However, the lack of targeted therapy at this time would mitigate the possible benefit [33], which is the rationale for current guidelines. Additionally, as previously mentioned, not all of those with high-risk variants develop ESKD. Identifying exactly which patients with high-risk variants will develop ESKD is difficult, and knowing the APOL1 risk allele status may lead to unnecessary medical interventions and undue stress to both patients and physicians [34].
Mechanism of Disease
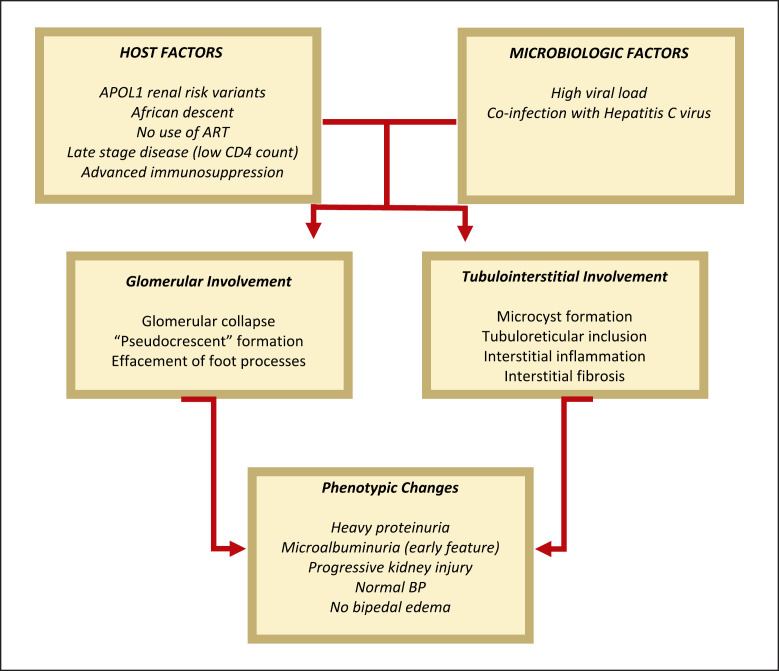
Despite the available evidence showing the direct role of HIV-1 in the pathogenesis of HIVAN, the mechanism of how the virus enters the renal epithelial cells remains elusive [9]. Normal kidney cells lack the receptors CCR5 and CXCR4 which function as co-receptors for HIV-1 entry into CD4+ cells. However, a study done by Li et al. [35] showed that expression of transmembrane TNF-α in cultured renal tubular epithelial cells enabled the infection of these cells, which solidifies the role of inflammation to prime renal cells for infection. Another proposed mechanism for HIV entry into epithelial cells is direct cell-to-cell transmission via infected mononuclear cells. Several studies have shown that HIV-1 utilizes tunneling nanotube networks to move from cell to cell, enabling infection of cells without surface co-receptors for direct HIV-1 entry [36, 37, 38, 39, 40]. The hallmarks of HIVAN glomerular and tubulointerstitial injury are cell cycle dysregulation and dedifferentiation, which can be expressed by an upregulation of the proliferation marker Ki67 in podocytes and reticuloendothelial cells. Ki67 reflects an increased proportion of cells arrested in cell cycle phases other than G0, such as G2/M, which has recently been shown to be a mechanism for tubulointerstitial injury and fibrosis [9, 41]. Aside from Ki67 upregulation, cytoskeletal dysregulation is also evidenced by a reduction of certain podocyte genes such as synaptopodin, which is a key mediator of cytoskeletal integrity, possibly due to HIV-induced basic fibroblast growth factor (bFGF)-mediated dysregulation of MAPK and RhoA. These promote further proliferation and dedifferentiation and decreases synaptopodin expression, jeopardizing the crucial maintenance of the complex and dynamic cytoskeleton [9, 41]. Additionally, HIVAN is characterized by deposition of TGF-β and matrix proteins [7]. Specifically, TGF-β-SMAD3 and activation of p53 and dWnt/Notch pathways secondary to increased expression of homeodomain interacting protein kinase 2 (HIPK2) was recently implicated in HIV-induced reticuloendothelial-cell apoptosis and tubulointerstitial fibrosis [41]. On the other hand, dysregulated cell-cell and cell-matrix adhesion also contribute to glomerular injury in HIVAN. HIV expression increases Sidekick-1 (Sdk-1), which mediates cell-cell adhesion, resulting in the characteristic clustering of glomerular epithelial cells (collapsing glomerulopathy) in HIVAN. HIVAN also decreases RAP1, a key regulator of cell-cell and cell-matrix adhesion, by increasing expression of RAP1GAP, causing podocyte detachment in mice, adding to the multiple mechanisms of cellular injury and death in HIVAN [41]. Therefore, HIVAN is a disease marked by increased expression of several proliferation markers. Interestingly, coinfection with hepatitis C virus may influence the pathogenesis of HIVAN [42]. As aforementioned, mutations in the APOL1 gene variants G1 and G2 also increase the risk of FSGS and hypertensive ESKD in the African-American population [43, 44]. These further highlight the role of host genetic factors in the mechanism and the pathogenesis of HIVAN [44]. Figure 2 shows the key summary of the pathophysiology of HIVAN.
Fig. 2.
Pathophysiology of HIVAN.
Diagnosis
A high index of suspicion for HIVAN should be employed in patients with HIV of African descent presenting with persistent heavy proteinuria and progressively declining renal dysfunction. Supportive findings include the presence of advanced HIV infection with high viral load and low CD4 count, nephrotic range proteinuria, and large echogenic kidneys on ultrasound. A study by Atta et al. [45] demonstrated that the sensitivity and specificity for nephrotic range proteinuria in the diagnosis of HIVAN were only 73% and 61%, respectively. In addition, 33% of patients with CD4 counts below 200 had diagnoses other than HIVAN [45]. In another study of 107 PLHIV African-American patients with proteinuria >3 g/day, only 56% had biopsy-proven HIVAN. The remaining patients had classic FSGS, MPGN, amyloidosis, diabetic nephropathy, and other findings. The addition of APOL1 genotype did not significantly improve the prediction rate of HIVAN [46]. HIVAN is not the only cause of renal pathology in patients with HIV infection. The spectrum of the disease varies from FSGS, minimal change disease, thrombotic microangiopathy, and lupus-like HIV-immune complex diseases [47]. Other differentials include glomerular manifestations of other infections such as hepatitis B and C, immune complex-mediated glomerulonephritis, and drug-induced nephrotoxicity from antiretroviral agents and CKD from traditional risk factors such as diabetes. The myriad of alternative diagnoses highlight the importance of KB to confirm the definitive diagnosis of HIVAN [48]. Table 1 summarizes the clinical features and histopathologic findings of other differential diagnoses of patients suspected of HIVAN.
Table 1.
Differential diagnosis: possible histologic patterns in patients suspected of having HIVAN
| Clinical features | Histopathologic findings | |
|---|---|---|
| HIVAN | Nephrotic range proteinuria, renal insufficiency; edema and hypertension are less prominent findings even in the setting of renal failure [49] | Collapsing type of FSGS with segmental or global retraction of the glomerular capillary walls and luminal occlusion, cystic tubular dilatation, interstitial inflammation, the presence of TRI bodies |
|
| ||
| Classic FSGS | Nephrotic syndrome (proteinuria, edema, hypercholesterolemia, hypoalbuminemia) | Idiopathic or secondary to segmental necrosis or nephron loss. Segment of tuft replaced by fibrosis |
|
| ||
| Membranous proliferative glomerulonephritis | Nephrotic syndrome or mixed nephrotic-nephritic syndrome (hematuria, hypertension, oliguria, edema) | Hypercellular, hyperlobular glomeruli with double-contour GBM |
|
| ||
| Renal amyloidosis | Proteinuria often severe enough to produce nephrotic syndrome | Deposition of insoluble fibrillar protein in glomerulus and vessel walls, stains with Congo and Sirius red |
|
| ||
| Diabetic nephropathy | Proteinuria, sometimes with nephrotic syndrome but may progress to features of chronic renal failure | Diffuse and nodular glomerulosclerosis, hyalinized arterioles, capsular drops, fibrin caps, thickened GBM |
|
| ||
| ICGN | Variable depending on etiology. Usually within a spectrum of nephrotic and nephritic symptoms | The site of immune complex deposition is dependent on the size of the complexes which is dependent on the antigen and the type of immunoglobulin produced by the host |
|
| ||
| Minimal-change nephropathy | Nephrotic syndrome without hematuria and usually without impairment of renal function | Normal light microscopy and immunofluorescence, “fused” podocyte foot processes on electron microscopy |
|
| ||
| Thrombotic microangiopathy | Symptoms of acute kidney injury with variable extrarenal manifestations such as thrombocytopenia, microangiopathic hemolytic anemia, and fever [50] | Thrombi in glomerular capillaries, arteries, and arterioles which distend the vascular lumen and entrap red and white blood cells [51]. Bloodless appearance with glomerular endothelial cell swelling. [51] |
|
| ||
| HIVICK | Lupus-like lesions in some PLHIV without serologic evidence of lupus. [47] Commonly presents with proteinuria, hematuria, reduced GFR, and low levels of complements [47] | Variable mesangial and endocapillary hypercellularity [47]. Tubuloreticular aggregates in endothelial cells [47]. “Full-house” staining with mesangial and variable capillary wall granular deposits [47]. Immune complex deposits on electron microscopy [47] |
HIVAN, HIV-associated nephropathy. FSGS, focal segmental glomerulosclerosis; PLHIV, people living with HIV; TRI, tubuloreticular inclusion; ICGN, immune complex glomerulonephritis; HIVICK, HIV-immune complex disease. Adapted from Wheater's Functional Histology E-Book: a Text and Colour Atlas, by Young B et al. [52], p. 214. Copyright 2013 by Elsevier Health Sciences.
Histopathologic Findings in Patients with HIVAN
HIVAN is caused by the direct cytopathic effect of HIV that replicates inside the renal cells [7]. The constellation of histopathologic findings that comprise HIVAN include glomerular capillary collapse, glomerular epitheliosis (proliferation of podocyte stem cells which are located in the parietal epithelium), hypertrophy or enlargement of existing podocytes, prominence and hypercellularity of the mesangium, endothelial tubuloreticular inclusions (TRIs), and tubular microcysts [3, 4, 53]. Microcystic dilatation in particular has been suggested as a finding that, together with proteinuria, indicates a worse prognosis [4, 7, 31, 54]. Collapse of the glomeruli has been defined as at least 1 glomerulus with collapsed basement membranes with accompanying hyperplasia and hypertrophy of epithelial cells leading to the so called “pseudocrescents” [4, 9]. It is important to note that at advanced stage of the disease, collapsing FSGS cannot always be distinguished from classic FSGS. Differentials that should be considered in patients with this type of collapsing FSGS include other infectious etiologies (cytomegalovirus, parvovirus B19, Epstein-Barr virus, and SARS-CoV2), drug toxicities, vascular pathologies such as thrombocytic microangiopathy, autoimmune diseases such as SLE, and idiopathic collapsing glomerulopathy [54, 55]. The presence of TRIs on electron microscopy, which are most commonly seen in viral infections and lupus nephropathy, may be a positive indicator of HIVAN. However, TRIs may not always be present in patients who have been treated with cART [54, 55]. By immunofluorescence, the segments of collapse may stain for C3, C1q, and IgM in a nonspecific pattern [4]. In advanced or end-stage disease, there is global glomerulosclerosis composed of retracted sclerotic tufts capped with parietal epithelial cells [4]. The tubulointerstitial component of the lesion can be dramatic causing renal hypertrophy and hyper-echogenicity on renal ultrasound [4].
Treatment
Medical Management
On top of initiating or maintaining ART, current therapeutic modalities for HIVAN include renal-angiotensin-aldosterone system (RAAS) inhibitors, steroids, and renal replacement therapy (RRT). Studies on HIVAN treatment options have been mostly observational, with no clinical trial data available to date [49]. Table 2 shows the current key studies on HIVAN treatment.
Table 2.
Summary of key studies on HIVAN treatment
| Year | Author | Study type | Population | Findings |
|---|---|---|---|---|
| ART 2008 | Post et al. [26] | Retrospective cohort | Biopsy-proven or clinically diagnosed HIVAN | Renal survival not significantly improved by early initiation of ART |
|
| ||||
| 2012 | Bige et al. [23] | Retrospective cohort | Biopsy-proven HIVAN | Median renal survival improved to 40 months compared to prior studies |
|
| ||||
| 2006 | Atta et al. [56] | Retrospective cohort | Biopsy-proven HIVAN | Renal survival better in group treated with ART (HR = 0.3) |
|
| ||||
| 2004 | Szczech et al. [57] | Retrospective cohort | Patients with HIV who underwent renal biopsy | ART slowed progression to ESRD in those with HIVAN (HR = 0.24) |
|
| ||||
| 2012 | Wearne et al. [54] | Retrospective and prospective cohort | Biopsy-proven HIVAN | cART reduced mortality in patients with biopsy features of HIVAN (HR = 0.43) |
|
| ||||
| RAAS inhibitors 1997 | Burns et al. [58] | Prospective cohort | Biopsy-proven and clinically diagnosed HIVAN | ACE inhibitors stabilized serum creatinine and 24-h protein excretion in patients with HIVAN |
|
| ||||
| 2003 | Wei et al. [59] | Prospective cohort | Biopsy-proven HIVAN | ACE inhibitors reduced risk of ESRD (RR = 0.003) and had greater survival |
|
| ||||
| 2004 | Szczech et al. [57] | Retrospective cohort | Patients with HIV who underwent renal biopsy | ACEi/ARB slowed progression to ESRD (HR = 0.41) |
|
| ||||
| Steroids 2000 | Eustace et al. [60] | Retrospective cohort | Biopsy-proven HIVAN | Steroids slowed progression of azotemia on multivariate analysis (OR of improved renal outcome adjusted for serum creatinine = 39.1) |
|
| ||||
| 1998 | Laradi et al. [61] | Retrospective cohort | Biopsy-proven HIVAN | Steroids slowed progression to ESRD (RR = 0.29) |
|
| ||||
| Dialysis 2003 | Ahuja et al. [62] | Retrospective cohort | Patients with HIVAN on dialysis | No significant difference in survival between HD and PD (HR = 1.01) |
|
| ||||
| 2017 | Ndlovu et al. [63] | Prospective cohort | Patients with HIV undergoing PD versus patients without HIV undergoing PD | Increased risk of peritonitis in patients with HIV undergoing PD (HR = 2.38) |
|
| ||||
| Renal transplant 2015 | Waheed et al. [48] | Prospective cohort | Patients with biopsy-proven HIVAN undergoing renal transplant | Graft survival rates: 1 year = 100% 3 year = 81% Acute rejection rates 1 year = 18% 3 year = 27% Delayed graft function in 64% |
|
| ||||
| 2010 | Stock et al. [64] | Prospective cohort | HIV-infected patients on ART undergoing renal transplant | Patient survival rates: 1 year = 94.6±2.0% 3 year = 88.2±3.8% Graft survival rates: 1 year = 90.4% 3 year = 73.7% Rejection rates: 1 year = 31% 3 year = 41% |
HIVAN, HIV-associated nephropathy; RAAS, renal-angiotensin-aldosterone system; ART, antiretroviral therapy; cART, combination antiretroviral therapy; HD, hemodialysis; PD, peritoneal dialysis.
cART is the mainstay of treatment for HIVAN. A 2012 study by Bigé et al. [23] observed an increased median renal survival in patients with biopsy-proven HIVAN on cART. This is in line with previous papers' findings of a delay in progression to ESKD [50, 51]. Additionally, Wearne et al. [54] described a mortality benefit in this population [65]. Despite the benefit of cART, HIVAN still carries a poorer prognosis compared to non-HIV-related kidney disease.
There are no randomized trials of renin-angiotensin-aldosterone system blockade that have been conducted in patients with HIV-related CKD. Most studies using RAAS inhibitors have been extrapolated from studies in other glomerular diseases [52]. The use of RAAS inhibitors is observed to benefit renal survival and delay progression to ESKD [66, 67]. Burns et al. [58] noted stabilization in serum creatinine and 24-h protein excretion in patients treated with fosinopril in both nephrotic and non-nephrotic patients [56]. The benefits of RAAS inhibition in other glomerular diseases have been well-established, and thus, it is reasonable to use these agents in the absence of additional data.
There is a lack of large randomized controlled trials for the use of steroids in HIVAN. It is surmised that steroids may be able to reduce the significant tubulointerstitial inflammation seen in kidney biopsies [52]. Early, smaller studies of steroid in HIVAN observed improvements in protein excretion and serum creatinine and slow progression to ESKD [54, 57]. However, no recent studies have been done to validate these findings. And while some benefit was observed, it must be taken into consideration that current cART regimens are now significantly improved compared to the period of these studies which may impact the actual current clinical benefit of steroids. Additionally, there is a risk of adverse events [57]. Thus, routine use of steroids is not recommended. However, it is reasonable to consider the addition of glucocorticoids in patients with rapidly progressing decline of kidney function despite optimal therapy with cART and angiotensin inhibition.
Renal Replacement Therapy
Patients with HIVAN remain at increased risk of developing ESKD despite medical therapy. Therefore, RRT, either through dialysis or renal transplant, must be considered in these patients.
Both hemodialysis and peritoneal dialysis are acceptable options for dialysis in patients with HIV. Mortality and morbidity are not significantly increased in one modality or another [68]. Old age, low serum albumin, low CD4 count, and lack of cART have been identified as independent risk factors of poor survival [57, 59]. While there may be some concern of an increased risk of peritonitis in these patients undergoing peritoneal dialysis, more data are needed to assess the true impact in this population [57].
Recent studies on renal transplantation in patients with HIVAN have shown favorable results. Graft survival during the 1-year and 3-year mark was 90% and 74%, respectively [48, 58]. However, unique challenges in this population remain. There is a risk of HIVAN recurrence in the graft posttransplantation, particularly in cases wherein the donor is HIV positive [60]. Graft rejection is also a major concern in this population, given the baseline immune dysregulation and the drug interactions between cART and calcineurin inhibitors [48, 58]. Special attention should be paid to managing the risk of rejection and delayed graft function [58]. The Hahnemann University Hospital has shared its best practices in kidney transplantation in patients with HIV, which have greatly improved patient and graft survival rates. Strict criteria for transplantation eligibility included undetectable viral load, CD4 count >200, and cART treatment for at least 6 months. Their addition of IV immunoglobulin to the induction protocol minimized antibody-mediated rejection. The use of belatacept as a maintenance immunosuppressive agent avoided the nephrotoxicity and cART drug interaction associated with tacrolimus [61].
A recent point for consideration in transplant patients is the APOL1 risk allele. Several case reports have noted a quicker decline in renal function posttransplant in both the donor and recipient when the living donor has a high-risk variant of the APOL1 gene [69, 70]. In these cases, the second hit in transplanted kidneys is thought to be from acute ischemic injury such as from thrombotic microangiopathy and cyclosporine nephrotoxicity, as compared to inflammation related to HIV [71]. However, large-scale prospective data on this issue are currently unavailable. Hence, current practices in transplant centers with regard to APOL1 testing in donors are varied [34]. An ongoing clinical trial aims to address this knowledge gap in long-term clinical outcomes of kidney transplants in donors and recipients with APOL1 (NCT03615235).
While APOL1 testing is not currently mandatory for transplant donors, the decision is a significant one. Certain centers may choose to reject donors with high-risk variants, thereby reducing the pool of donors available to the patient. Families who wish to proceed with transplantation may opt out of testing to ensure a suitable donor is found. Ultimately, transplant centers would need to counsel their patients and donors on the risk-benefit analysis of testing should it be available [34].
APOL1 Targeting
Given the link between HIVAN and the APOL1 gene, there is some consideration that therapies targeting APOL1 may be of benefit in this population. While the exact pathogenesis of glomerular injury in those with high-risk APOL1 variants is unclear, studies have linked it with changes in lipid metabolism. It was observed that cellular damage is minimized when APOL1 is localized within lipid droplets [72, 73]. The inhibition of diacylglycerol O-acyltransferase-2 (DGAT-2) in the kidneys promotes translocation of APOL1 to lipid droplets, attenuating damage, and presenting a possible therapeutic mechanism [73]. Early phase 1 and 2 trials are currently underway in developing therapeutics directed toward patients with APOL1 kidney disease (NCT 04340362) [62]. Further investigation may yield more insight on the role of targeted therapy.
Prognosis
HIVAN is usually diagnosed late in the course of HIV infection [74]. Although ART has significant survival benefit in patients with HIV infection, its relationship with renal survival is not clear in patients with HIVAN [50]. HIVAN generally portends a poor prognosis especially without adequate treatment. Many patients with HIVAN progress rapidly to ESKD [63]. In a multivariate model based from 42 patients with collapsing FSGS and 18 patients with HIVAN, the risk of ESKD was greatly increased by interstitial fibrosis of >20%, creatinine level >2.0 mg/dL, proteinuria >8 g/day, glomeruli with collapsing lesions >20%, and HIV infections [64]. In another study, the serum creatinine level at the time of biopsy was highly predictive of progression to ESKD [49]. Studies that compared patients with non-HIVAN ESKD and patients with HIVAN ESKD found that the latter had increased mortality [75, 76]. The earlier literature in 1998 and 2004 reported a median renal survival of 16.6 and 8.5 months, respectively [67, 77]. However, the more recent literature in 2012 and 2008 showed an increased median renal survival of 40 and 42 months, respectively [78, 79]. The increased median renal survival may be attributed to the advancement of and increased access to ART. It is unclear whether the poor renal prognosis is attributed to HIVAN leading to rapidly progressive ESKD or HIVAN presenting in the late stages of HIV infection.
cART has dramatically improved survival in patients with HIVAN in addition to decreasing the incidence of HIVAN in PLHIV and reducing the risk for progression to RRT [67, 75, 79, 80]. Thus, the current recommendation is to initiate cART in patients with HIVAN, regardless of CD4 cell count [49]. It is important to make an early and definitive diagnosis of HIV-related and -unrelated kidney disease in order to provide optimal management as these would lead to better outcomes regardless of etiology [22].
Conclusion
HIVAN is a serious kidney disease affecting those infected with HIV-1, the majority of which are of African descent and harbor 2 APOL1 risk alleles independent of comorbidities and drug use. In susceptible individuals, modifying factors or a second hit such as the activation of the innate immune system from chronic infection may trigger the development of HIVAN.
Nephrotic range proteinuria is characteristic for HIVAN, whereas hypertension and edema are uncommon. Surrogate markers can support the diagnosis of HIVAN but biopsy is needed for confirmation.
HIVAN leads to rapidly progressive ESKD; however, it is not the lone cause of ESKD in those with HIV infection. The possibility of alternative diagnoses highlights the importance of biopsy for definitive diagnosis. Common histopathologic features include collapsing type FSGS, tubulointerstitial microcyst formation, and TRIs.
There is a lack of rigorous trials which investigate the ideal treatment of HIVAN since it would be unethical to withhold cART in these patients. Observational studies have shown benefit for initiation or maintenance of cART in patients with HIVAN. Adjunctive treatment includes RAAS inhibitors, steroids, and RRT. It has increasingly been recognized that APOL1 gene risk alleles play a pivotal role in the development and progression of HIVAN which may be targeted for future therapy. More studies are needed to address gaps in knowledge concerning the treatment of HIVAN.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No specific financial support was obtained for the preparation of this article.
Author Contributions
Credit roles - Frederick B. Rivera, MD, and Pia Gabrielle I. Alfonso, MD: conceptualization, data curation, formal analysis, investigation, methodology, validation, writing - original draft, and writing - review and editing; Marie Francesca Mapua Ansay, MD: conceptualization, validation, and writing - review and editing; Jem Marie Golbin, MD: data curation, formal analysis, investigation, methodology, validation, writing - original draft, and writing - review and editing; Gerard Francis E. Mangubat, MD; Rajiv Hans Solita Menghrajani, MD; Siena Placino; Marianne Taliño, MD; Deogracias de Luna, MD; Nicolo Cabrera, MD; Carlo Nemesio Trinidad, MD; and Amir Kazory, MD: conceptualization, data curation, methodology, validation, and writing - review and editing.
Acknowledgments
I would like to thank Dr. Edgar V. Lerma and Dr. Marie Charmaine C. Sy for their support and guidance.
Funding Statement
No specific financial support was obtained for the preparation of this article.
References
- 1.Hou J, Nast CC. Changing concepts of HIV infection and renal disease. Curr Opin Nephrol Hypertens. 2018 May;27((3)):144–152. doi: 10.1097/MNH.0000000000000400. [DOI] [PubMed] [Google Scholar]
- 2.Wearne N, Okpechi IG. HIV-associated renal disease an overview. Clin Nephrol. 2016;86:41–47. doi: 10.5414/CNP86S117. [DOI] [PubMed] [Google Scholar]
- 3.Kudose S, Santoriello D, Bomback AS, Stokes MB, Batal I, Markowitz GS, et al. The spectrum of kidney biopsy findings in HIV-infected patients in the modern era. Kidney Int. 2020 May;97((5)):1006–1016. doi: 10.1016/j.kint.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Swanepoel CR, Atta MG, D'Agati VD, Estrella MM, Fogo AB, Naicker S, et al. Kidney disease in the setting of HIV infection conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2018 Mar 1;93((3)):545–559. doi: 10.1016/j.kint.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banu SG, Banu SS, Saleh FM. HIV-associated nephropathy (HIVAN) a short review of different authors. Mymensingh Med J. 2013 Jul;22((3)):613–617. [PubMed] [Google Scholar]
- 6.Lu TC, Ross M. HIV-associated nephropathy a brief review. Mt Sinai J Med. 2005 May;72((3)):193–199. [PubMed] [Google Scholar]
- 7.Herman ES, Klotman PE. HIV-associated nephropathy epidemiology, pathogenesis, and treatment. Semin Nephrol. 2003 Mar;23((2)):200–208. doi: 10.1053/snep.2003.50018. [DOI] [PubMed] [Google Scholar]
- 8.Alfano G, Cappelli G, Fontana F, Di Lullo L, Di Iorio B, Bellasi A, et al. Kidney disease in HIV infection. J Clin Med. 2019 Aug;8((8)):1254. doi: 10.3390/jcm8081254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atta MG. Diagnosis and natural History of HIV-associated nephropathy. Adv Chronic Kidney Dis. 2010 Jan;17((1)):52–58. doi: 10.1053/j.ackd.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Nikolopoulou A, Teixeira C, Cook HT, Roufosse C, Cairns THD, Levy JB, et al. Membranous nephropathy associated with viral infection. Clin Kidney J. 2021 Mar 1;14((3)):876–883. doi: 10.1093/ckj/sfaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014 Nov 1;59((9)):e96–e138. doi: 10.1093/cid/ciu617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lescure FX, Flateau C, Pacanowski J, Brocheriou I, Rondeau E, Girard PM, et al. HIV-associated kidney glomerular diseases changes with time and HAART. Nephrol Dial Transplant. 2012 Jun 1;27((6)):2349–2355. doi: 10.1093/ndt/gfr676. [DOI] [PubMed] [Google Scholar]
- 13.Verma B, Singh A. Histological spectrum of renal disease in HIV/AIDS patients with significant proteinuria an Indian perspective. J Family Med Prim Care. 2019 Mar;8((3)):860–865. doi: 10.4103/jfmpc.jfmpc_104_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner D, Drak D, Gracey D, Anderson L. Patterns of biopsy-proven renal disease in people living with HIV 10 years experience in Sydney, Australia. BMC Nephrology. 2022 Apr 18;23((1)):148. doi: 10.1186/s12882-022-02695-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bookholane H, Wearne N, Surapaneni A, Ash S, Berghammer-Böhmer R, Omar A, et al. Predictors and prognosis of HIV-associated nephropathy on kidney biopsy in South Africa. Kidney Int Rep. 2020 Oct;5((10)):1799–1804. doi: 10.1016/j.ekir.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.HIV rates by country 2022 [cited 2022 Jun 26] Available from https://worldpopulationreview.com/country-rankings/hiv-rates-by-countrys.
- 17.Johnson LF, Dorrington RE, Moolla H. Progress towards the 2020 targets for HIV diagnosis and antiretroviral treatment in South Africa. South Afr J HIV Med. 2017 Jul 27;18((1)):694. doi: 10.4102/sajhivmed.v18i1.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limou S, Nelson GW, Kopp JB, Winkler CA. APOL1 kidney risk alleles Population Genetics and Disease Associations. Adv Chronic Kidney Dis. 2014 Sep;21((5)):426–433. doi: 10.1053/j.ackd.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011 Nov;22((11)):2129–2137. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atta MG, Lucas GM, Fine DM. HIV-associated nephropathy epidemiology, pathogenesis, diagnosis and management. Expert Rev Anti Infect Ther. 2008 Jun;6((3)):365–371. doi: 10.1586/14787210.6.3.365. [DOI] [PubMed] [Google Scholar]
- 21.Han TM, Naicker S, Ramdial PK, Assounga AG. A cross-sectional study of HIV-seropositive patients with varying degrees of proteinuria in South Africa. Kidney Int. 2006 Jun;69((12)):2243–2250. doi: 10.1038/sj.ki.5000339. [DOI] [PubMed] [Google Scholar]
- 22.Razzak Chaudhary S, Workeneh BT, Montez-Rath ME, Zolopa AR, Klotman PE, Winkelmayer WC. Trends in the outcomes of end-stage renal disease secondary to human immunodeficiency virus-associated nephropathy. Nephrol Dial Transplant. 2015 Oct;30((10)):1734–1740. doi: 10.1093/ndt/gfv207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bigé N, Lanternier F, Viard JP, Kamgang P, Daugas E, Elie C, et al. Presentation of HIV-associated nephropathy and outcome in HAART-treated patients. Nephrol Dial Transplant. 2012 Mar;27((3)):1114–1121. doi: 10.1093/ndt/gfr376. [DOI] [PubMed] [Google Scholar]
- 24.Eggers PW, Kimmel PL. Is there an epidemic of HIV Infection in the US ESRD program? J Am Soc Nephrol. 2004 Sep;15((9)):2477–2485. doi: 10.1097/01.ASN.0000138546.53152.A7. [DOI] [PubMed] [Google Scholar]
- 25.Lucas GM, Lau B, Atta MG, Fine DM, Keruly J, Moore RD. Chronic kidney disease incidence and progression to end-stage renal disease in HIV-infected individuals a tale of two races. J Infect Dis. 2008 Jun 1;197((11)):1548–1557. doi: 10.1086/587994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Post FA, Campbell LJ, Hamzah L, Collins L, Jones R, Siwani R, et al. Predictors of renal outcome in HIV-associated nephropathy. Clin Infect Dis. 2008 Apr 15;46((8)):1282–1289. doi: 10.1086/529385. [DOI] [PubMed] [Google Scholar]
- 27.Al-Sheikh H, Al-Sunaid M, Alrajhi AA. HIV-associated nephropathy in Saudi Arabia. Ann Saudi Med. 2013;33((4)):347–350. doi: 10.5144/0256-4947.2013.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasembeli AN, Duarte R, Ramsay M, Mosiane P, Dickens C, Dix-Peek T, et al. APOL1 risk variants are strongly associated with HIV-associated nephropathy in Black South Africans. J Am Soc Nephrol. 2015 Nov;26((11)):2882–2890. doi: 10.1681/ASN.2014050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung RKY, Santana-Suarez B, Binns-Roemer E, Campbell L, Bramham K, Hamzah L, et al. The epidemiology of kidney disease in people of African ancestry with HIV in the UK. EClinicalMedicine. 2021 Aug 1;:38. doi: 10.1016/j.eclinm.2021.101006. [cited 2022 Jun 30] https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(21)00286-8/fulltext. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallipattu SK, Salem F, Wyatt CM. The changing epidemiology of HIV-related chronic kidney disease in the era of antiretroviral therapy. Kidney Int. 2014 Aug;86((2)):259–265. doi: 10.1038/ki.2014.44. [DOI] [PubMed] [Google Scholar]
- 31.Goyal R, Singhal PC. APOL1 risk variants and the development of HIV-associated nephropathy. FEBS J. 2021;288((19)):5586–5597. doi: 10.1111/febs.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma L, Divers J, Freedman BI. Mechanisms of injury in APOL1-associated kidney disease. Transplantation. 2019 Mar;103((3)):487–492. doi: 10.1097/TP.0000000000002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopp JB, Winkler CA. Genetic testing for APOL1 genetic variants in clinical practice finally starting to arrive. Clin J Am Soc Nephrol. 2020 Jan 7;15((1)):126–128. doi: 10.2215/CJN.01810219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohan S, Iltis AS, Sawinski D, DuBois JM. APOL1 genetic testing in living kidney transplant donors. Am J Kidney Dis. 2019 Oct;74((4)):538–543. doi: 10.1053/j.ajkd.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Das JR, Tang P, Han Z, Jaiswal JK, Ray PE. Transmembrane TNF-α facilitates HIV-1 infection of podocytes cultured from children with HIV-associated nephropathy. J Am Soc Nephrol. 2017 Mar;28((3)):862–875. doi: 10.1681/ASN.2016050564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross MJ. Advances in the pathogenesis of HIV-associated kidney diseases. Kidney Int. 2014 Aug 1;86((2)):266–274. doi: 10.1038/ki.2014.167. [DOI] [PubMed] [Google Scholar]
- 37.Bracq L, Xie M, Benichou S, Bouchet J. Mechanisms for cell-to-cell transmission of HIV-1. Front Immunol. 2018 Feb 19;9:260. doi: 10.3389/fimmu.2018.00260. [cited 2022 Jun 26] https://www.frontiersin.org/article/10.3389/fimmu.2018.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages a potential mechanism for intercellular HIV trafficking. Cell Immunol. 2009;254((2)):142–148. doi: 10.1016/j.cellimm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Köhler K, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008 Feb;10((2)):211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 40.Kadiu I, Gendelman HE. Human immunodeficiency virus type 1 endocytic trafficking through macrophage bridging conduits facilitates spread of infection. J Neuroimmune Pharmacol. 2011 Dec;6((4)):658–675. doi: 10.1007/s11481-011-9298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rednor S J, MJ Molecular mechanisms of injury in HIV-associated nephropathy. Front Med. 2018 Jun 7;5:177. doi: 10.3389/fmed.2018.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen SD, Kopp JB, Kimmel PL. Kidney diseases associated with human immunodeficiency virus infection. N Engl J Med. 2017 Dec 14;377((24)):2363–2374. doi: 10.1056/NEJMra1508467. [DOI] [PubMed] [Google Scholar]
- 43.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010 Aug 13;329((5993)):841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medapalli RK, He JC, Klotman PE. HIV-associated nephropathy pathogenesis. Curr Opin Nephrol Hypertens. 2011 May;20((3)):306–311. doi: 10.1097/MNH.0b013e328345359a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atta MG, Choi MJ, Longenecker JC, Haymart M, Wu J, Nagajothi N, et al. Nephrotic range proteinuria and CD4 count as noninvasive indicators of HIV-associated nephropathy. Am J Med. 2005 Nov;118((11)):1288. doi: 10.1016/j.amjmed.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 46.Atta MG, Estrella MM, Skorecki KL, Kopp JB, Winkler CA, Wasser WG, et al. Association of APOL1 genotype with renal histology among Black HIV-positive patients undergoing kidney biopsy. Clin J Am Soc Nephrol. 2016 Feb 5;11((2)):262–270. doi: 10.2215/CJN.07490715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fogo AB, Lusco MA, Najafian B, Alpers CE. AJKD atlas of renal pathology HIV-Associated Immune Complex Kidney Disease (HIVICK) Am J Kidney Dis. 2016 Aug 1;68((2)):e9–e10. doi: 10.1053/j.ajkd.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Waheed S, Sakr A, Chheda ND, Lucas GM, Estrella M, Fine DM, et al. Outcomes of renal transplantation in HIV-1 associated nephropathy. PLoS One. 2015 Jun 10;10((6)):e0129702. doi: 10.1371/journal.pone.0129702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross MJ, Klotman PE. HIV-associated nephropathy. AIDS. 2004 May 21;18((8)):1089–1099. doi: 10.1097/00002030-200405210-00002. [DOI] [PubMed] [Google Scholar]
- 50.Brocklebank V, Wood KM, Kavanagh D. Thrombotic microangiopathy and the kidney. Clin J Am Soc Nephrol. 2018 Feb 7;13((2)):300–317. doi: 10.2215/CJN.00620117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gallan AJ, Chang A. A new paradigm for renal thrombotic microangiopathy. Semin Diagn Pathol. 2020 May;37((3)):121–126. doi: 10.1053/j.semdp.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Young B, O'Dowd G, Woodford P. 6th ed. 2013. Wheater's functional histology. [cited 2022 Jul 1]. Available from https://www.elsevier.com/books/wheaters-functional-histology/young/978-0-7020-4747-3. [Google Scholar]
- 53.Rosenberg AZ, Naicker S, Winkler CA, Kopp JB. HIV-associated nephropathies epidemiology, pathology, mechanisms and treatment. Nat Rev Nephrol. 2015 Mar;11((3)):150–160. doi: 10.1038/nrneph.2015.9. [DOI] [PubMed] [Google Scholar]
- 54.Wearne N, Swanepoel CR, Boulle A, Duffield MS, Rayner BL. The spectrum of renal histologies seen in HIV with outcomes prognostic indicators and clinical correlations. Nephrol Dial Transplant. 2012 Nov;27((11)):4109–4118. doi: 10.1093/ndt/gfr702. [DOI] [PubMed] [Google Scholar]
- 55.Gaillard F, Ismael S, Sannier A, Tarhini H, Volpe T, Greze C, et al. Tubuloreticular inclusions in COVID-19 − related collapsing glomerulopathy. Kidney Int. 2020 Jul;98((1)):241. doi: 10.1016/j.kint.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atta MG, Gallant JE, Rahman MH, Nagajothi N, Racusen LC, Scheel PJ, et al. Antiretroviral therapy in the treatment of HIV-associated nephropathy. Nephrol Dial Transpl. 2006 Oct;21((10)):2809–2813. doi: 10.1093/ndt/gfl337. [DOI] [PubMed] [Google Scholar]
- 57.Szczech LA, Gupta SK, Habash R, Guasch A, Kalayjian R, Appel R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004 Sep;66((3)):1145–1152. doi: 10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 58.Burns GC, Paul SK, Toth IR, Sivak SL. Effect of angiotensin-converting enzyme inhibition in HIV-associated nephropathy. J Am Soc Nephrol. 1997 Jul;8((7)):1140–1146. doi: 10.1681/ASN.V871140. [DOI] [PubMed] [Google Scholar]
- 59.Wei A, Burns GC, Williams BA, Mohammed NB, Visintainer P, Sivak SL. Long-term renal survival in HIV-associated nephropathy with angiotensin-converting enzyme inhibition. Kidney Int. 2003 Oct;64((4)):1462–1471. doi: 10.1046/j.1523-1755.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- 60.Eustace JA, Nuermberger E, Choi M, Scheel PJ, Moore R, Briggs WA. Cohort study of the treatment of severe HIV-associated nephropathy with corticosteroids. Kidney Int. 2000 Sep;58((3)):1253–1260. doi: 10.1046/j.1523-1755.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- 61.Laradi A, Mallet A, Beaufils H, Allouache M, Martinez F, HIV-associated nephropathy outcome and prognosis factors Groupe d' Etudes Néphrologiques d'Ile de France. J Am Soc Nephrol. 1998 Dec;9((12)):2327–2335. doi: 10.1681/ASN.V9122327. [DOI] [PubMed] [Google Scholar]
- 62.Ahuja TS, Collinge N, Grady J, Khan S. Is dialysis modality a factor in survival of patients with ESRD and HIV-associated nephropathy? Am J Kidney Dis. 2003 May;41((5)):1060–1064. doi: 10.1016/s0272-6386(03)00204-x. [DOI] [PubMed] [Google Scholar]
- 63.Ndlovu KCZ, Sibanda W, Assounga A. Peritonitis outcomes in patients with HIV and end-stage renal failure on peritoneal dialysis a prospective cohort study. BMC Nephrol. 2017 Feb 3;18((1)):48. doi: 10.1186/s12882-017-0466-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stock PG, Barin B, Murphy B, Hanto D, Diego JM, Light J, et al. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med. 2010 Nov 18;363((21)):2004–2014. doi: 10.1056/NEJMoa1001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chertow G, Luyckx V, Marsden P, Skorecki K, Taal M, Yu A. 11th ed. 2019. Brenner and rector's the kidney 2-volume set. [cited 2022 Jul 1]. Available from https://www.elsevier.com/books/brenner-and-rectors-the-kidney-2-volume-set/yu/978-0-323-53265-5. [Google Scholar]
- 66.Ross MJ, Klotman PE, Winston JA. HIV-associated nephropathy case study and review of the literature. AIDS Patient Care STDS. 2000 Dec;14((12)):637–645. doi: 10.1089/10872910050206559. [DOI] [PubMed] [Google Scholar]
- 67.Yahaya I, Uthman OA, Uthman MMB. Interventions for HIV-associated nephropathy. Cochrane Database Syst Rev. 2013 Jan 31;2013((1)):CD007183. doi: 10.1002/14651858.CD007183.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palau L, Menez S, Rodriguez-Sanchez J, Novick T, Delsante M, McMahon BA, et al. HIV-associated nephropathy links, risks and management. HIV AIDS. 2018 May 25;10:73–81. doi: 10.2147/HIV.S141978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kofman T, Audard V, Narjoz C, Gribouval O, Matignon M, Leibler C, et al. APOL1 polymorphisms and development of CKD in an identical twin donor and recipient pair. Am J Kidney Dis. 2014 May;63((5)):816–819. doi: 10.1053/j.ajkd.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 70.Doshi MD, Ortigosa-Goggins M, Garg AX, Li L, Poggio ED, Winkler CA, et al. APOL1 genotype and renal function of black living donors. J Am Soc Nephrol. 2018 Apr;29((4)):1309–1316. doi: 10.1681/ASN.2017060658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Albaqumi M, Soos TJ, Barisoni L, Nelson PJ. Collapsing glomerulopathy. J Am Soc Nephrol. 2006 Oct 1;17((10)):2854–2863. doi: 10.1681/ASN.2006030225. [DOI] [PubMed] [Google Scholar]
- 72.Chun J, Zhang JY, Wilkins MS, Subramanian B, Riella C, Magraner JM, et al. Recruitment of APOL1 kidney disease risk variants to lipid droplets attenuates cell toxicity. Proc Natl Acad Sci U S A. 2019 Feb 26;116((9)):3712–3721. doi: 10.1073/pnas.1820414116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chun J, Riella CV, Chung H, Shah SS, Wang M, Magraner JM, et al. DGAT2 inhibition potentiates lipid droplet formation to reduce cytotoxicity in APOL1 kidney risk variants. J Am Soc Nephrol. 2022 May;33((5)):889–907. doi: 10.1681/ASN.2021050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Atta MG, Fine DM, Kirk GD, Mehta SH, Moore RD, Lucas GM. Survival during renal replacement therapy among African Americans infected with HIV type 1 in Urban Baltimore Maryland. Clin Infect Dis. 2007 Dec 15;45((12)):1625–1632. doi: 10.1086/523728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muller E, Barday Z. HIV-positive kidney donor selection for HIV-positive transplant recipients. J Am Soc Nephrol. 2018 Apr;29((4)):1090–1095. doi: 10.1681/ASN.2017080853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malat GE, Boyle SM, Jindal RM, Guy S, Xiao G, Harhay MN, et al. Kidney transplantation in HIV-positive patients a single-center, 16-year experience. Am J Kidney Dis. 2019 Jan;73((1)):112–118. doi: 10.1053/j.ajkd.2018.02.352. [DOI] [PubMed] [Google Scholar]
- 77.Bruggeman LA, Sedor JR, O'Toole JF. Apolipoprotein L1 and mechanisms of kidney disease susceptibility. Curr Opin Nephrol Hypertens. 2021 May 1;30((3)):317–323. doi: 10.1097/MNH.0000000000000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winston JA, Klotman ME, Klotman PE. HIV-associated nephropathy is a late not early manifestation of HIV-1 infection. Kidney Int. 1999 Mar;55((3)):1036–1040. doi: 10.1046/j.1523-1755.1999.0550031036.x. [DOI] [PubMed] [Google Scholar]
- 79.Carbone L, D'Agati V, Cheng JT, Appel GB. Course and prognosis of human immunodeficiency virus-associated nephropathy. Am J Med. 1989 Oct;87((4)):389–395. doi: 10.1016/s0002-9343(89)80819-8. [DOI] [PubMed] [Google Scholar]
- 80.Laurinavicius A, Hurwitz S, Rennke HG. Collapsing glomerulopathy in HIV and non-HIV patients a clinicopathological and follow-up study. Kidney Int. 1999 Dec;56((6)):2203–2213. doi: 10.1046/j.1523-1755.1999.00769.x. [DOI] [PubMed] [Google Scholar]