Abstract
The majority of research to combat SARS-CoV-2 infection exploits the adaptive immune system, but innate immunity, the first line of defense against pathogenic microbes, is equally important in understanding and controlling infectious diseases. Various cellular mechanisms provide physiochemical barriers to microbe infection in mucosal membranes and epithelia, with extracellular polysaccharides, particularly sulfated polysaccharides, being among the most widespread and potent extracellular and secreted molecules blocking and deactivating bacteria, fungi, and viruses. New research reveals that a range of polysaccharides effectively inhibits COV-2 infection of mammalian cells in culture. This review provides an overview of sulfated polysaccharides nomenclature, its significance as immunomodulators, antioxidants, antitumors, anticoagulants, antibacterial, and as potent antivirals. It summarizes current research on various interactions of sulfated polysaccharide with a range of viruses, including SARS-CoV-2, and their application for potential treatments for COVID-19. These molecules interact with biochemical signaling in immune cell responses, by actions in oxidative reactions, cytokine signaling, receptor binding, and through antiviral and antibacterial toxicity. These properties provide the potential for the development of novel therapeutic treatments for SARS-CoV-2 and other infectious diseases from modified polysaccharides.
Keywords: Antiviral saccharides, Multi-target drugs, COVID-19 and comorbidities, Heparin, Viral infection, Immunomodulation, Antioxidant, Anti-bacterial, Cancer, Hypertension, Diabetes
1. Introduction
The current coronavirus disease 2019 (COVID-19) pandemic has caused worldwide disease and disruption on a scale that is unprecedented in our lifetime. Methods to limit transmission of the virus, to inhibit infection, treat the potentially life-risking disease, and its comorbidities, are currently inadequate. Immunization, surgical mask-wearing, and other behavioral means of mitigating the spread of the disease are extremely helpful, but each measure has limitations. Notably, immunization and immunity post-infection are undermined by rapid mutation of the virus. Social and behavioral measures to mitigate the spread of the disease require widespread compliance, and this has become problematic in many respects. A multi-pronged approach is needed that draws on diverse and novel approaches to combat the spread of the virus.
Sulfated polysaccharides (SP) research is yielding evidence of the strong antiviral activity of these compounds, and the potential for novel and effective therapeutic applications for COVID-19 and other infectious diseases. SPs are attractive alternatives to develop against COVID-19, because their mechanism of action is far more diverse than immunization, for example, which has highly selective binding to specific epitopes of the virus. Here we provide an academic review of recent advances, and summary of relevant literature written to explore the central question of how SPs can be used against this and other infections. The literature supports the potential for new drugs that could be developed against SARS-CoV-2 infection and its comorbidities, by exploiting the versatile effects of SP on a range of physiological properties and antiviral actions.
COVID-19 is a challenging novel viral infection that can affect multiple tissues and organs. Moreover, diverse symptoms can be displayed in different patients, from asymptomatic individuals to patients who experience severe affliction of multiple organs via cytokine storm that may end in death ([1]. In addition, comorbidities such as hypertension, respiratory system disease, and cardiovascular disease, are risk factors for severe patients of COVID-19 compared with non-severe patients [2].
Even though vaccinations prevent severe disease symptoms, new and robust therapeutical approaches are essential to control and avoid COVID-19 infections and deaths. The variants that attract the most attention are those of public health concern, including B.1.1.7 (UK), P.1 (Brazilian), and B.1.351 (South African). This list is extended by the variants of interest that emerge and are expanding in certain countries but are found sporadically in others, such as B.1.427 and B.1.429 (Californians), B.1.617 (Indian), and B.1.1.529 (Omicron-South African) [3]. Various antiviral molecules have been assayed with relative success. Nevertheless, there is a compelling urgency to develop new treatments for COVID-19 comorbidities and other risk factors associated with the disease. A deeper understanding of sulfated polysaccharides can contribute to the design of novel and more efficient strategies to fight current COV-2 disease and future ones. Table 1 [[4], [5], [6], [7]], provides a summary of benefits of SP against hypertension, cardiovascular disease, type 2 diabetes and obesity which will be covered in this review.
Table 1.
Summary of COVID-19 comorbidities and possible prevention or treatment with SP.
| COVID-19 comorbidity | S-Polysaccharides | Origin | Proposed mechanisms of action | Current state | References |
|---|---|---|---|---|---|
| Hypertension | Fucoidans | Brown seaweeds | Mimicking natural ligands of protein receptors | Research | [4] |
| Cardiovascular disease | Sulfated fucans and galactans | Brown seaweeds and sea urchin | Inhibition of factor Xa and thrombin generations by the tenase and prothrombinase systems. | Research | [5] |
| Type 2 diabetes/obesity | Sulfated rhamnose, fucoidan | Enteromorpha prolifera | Sulfated rhamnose-Cr (III) ligands can significantly improve the bioactivity of chromium and increase its ability to control diabetes. | Proposed as nutraceutical | [6,7] |
2. Nomenclature and description of glycans
An understanding of the chemical structure and nomenclature of SPs and related molecules is necessary prior to presenting the latest research on these compounds in relation to COVID-19 and similar infections. Glycans are complex biological molecules widely distributed in all the natural kingdoms. They are composed of monosaccharide units in various combinations and linkages, which can be linear, cyclic, or with diverse and asymmetric types of branching [8]. The basic units, monosaccharides, are carbon-based molecules having the empirical formula of (CH2O)n where carbon atoms are hydrated; thus, they are named as carbohydrates. Monosaccharides are ketones or aldehydes with multiple hydroxyl groups. Sugar units can exhibit a variety of isomeric forms, since they can share identical molecular formulas, but have different atomic spatial organization. Also, sugars with more than three carbon atoms present multiple asymmetries that generate different stereoisomers whose number equals 2n, where n represents the number of asymmetric carbons in the molecule [9].
Monosaccharide monomers link to form more complex structures called oligosaccharides and polysaccharides. According to IUPAC “polysaccharide (glycan) is the name given to a macromolecule consisting of a large number of monosaccharide (glycose) residues joined to each other by glycosidic linkages.” Polysaccharides are macromolecules formed by variable number of sugar units. (“ [10]. Most polysaccharides bear one reducing monosaccharide residue at one end and thus so-called reducing saccharides, but other polysaccharides are lacking a reducing sugar end (non-reducing ends). When polysaccharides are composed of only one type of sugar (for instance, cellulose and starch), they are named homopolysaccharides. If the polysaccharide is made of two or more different monomers, it can be named as heteropolysaccharide or heteroglycan.
The short chains comprised of between 2 and 10 saccharide units are known as oligosaccharides, where the termination “ose” is replaced by the suffix “an” (galactan, glycan, xylan, mannan, fucan, etc.). If a polysaccharide is composed of more than one type of monomer unit, it can be named by the combination of sugars starting in alphabetic order and ending with the most abundant saccharide forming the principal chain, finishing with the suffix “an”. For instance, a polysaccharide made up of galactose, glucose and mannose will be named galactoglucomannan. If no sugar is predominant in the chain, then they will be named alphabetically, and the name terminated with the suffix to the corresponding glycan, for instance arabinoxylan. A polysaccharide composed entirely of glycuronic acid units will be named by replacing “ic acid” by “an”, thus, glycuronan. If a polysaccharide is entirely composed of amino sugars will take the appellative given to the amino-sugar units, (i.e., manosamine, glucosamine, galactosamine).
2.1. Saccharide's chemical structure
Monosaccharides, both ketones, and aldehydes exist in cyclic forms in nature as furan or pyran-like intramolecular cycles instead of open chains. Aldehyde or ketone groups can react with an alcohol group to form intramolecular hemiacetals or hemiketals. Then, sugars form an additional asymmetric center, namely C1. According to convention, the D and L isomers are determined by the configuration of the asymmetric carbon atom farthest from the aldehyde or keto group.
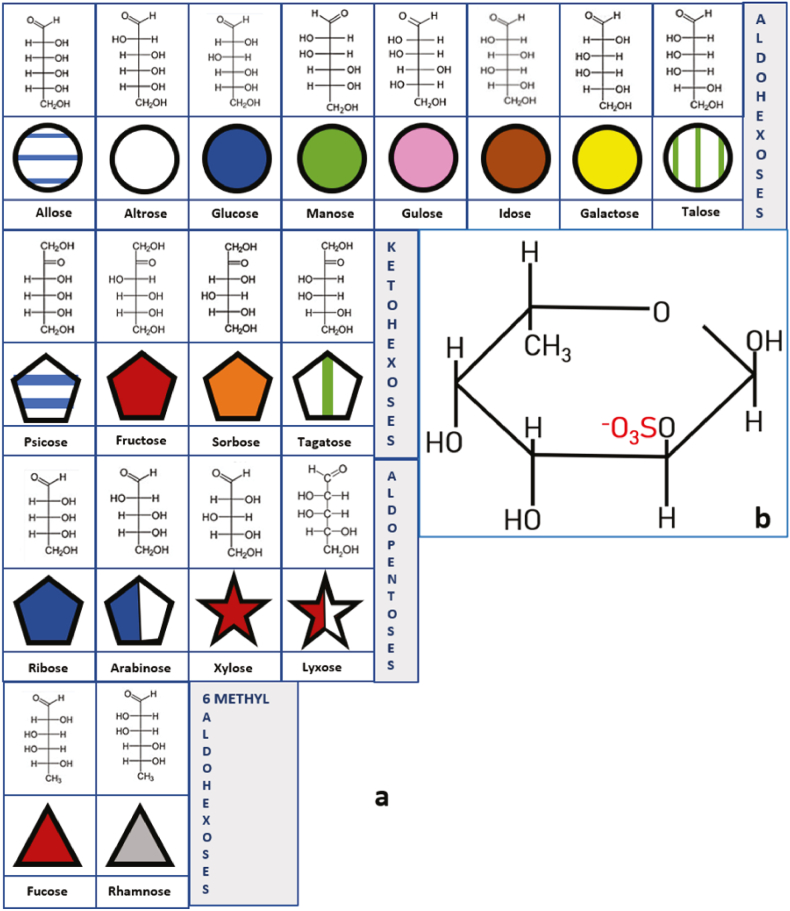
When the farthest alcohol group is located to the right or the left of the open chain, they are assigned as D or L isomers, respectively. Once the additional asymmetric center is formed, as a consequence of intramolecular hemiketals or hemiacetals, the glycoside can be designated as α or β. Designation α indicates that the hydroxyl group attached to C-1 is on the opposite side of the ring as C-6; β indicates that the hydroxyl group is on the same side of the ring as C-6 [9]. The nomenclature for these monosaccharides is determined by all the features mentioned above, as shown in Fig. 1 [8].
Fig. 1.
Fisher structure, symbolic representations of most common sugars prone to be sulfated. From upper to lower rows: a. Aldohexoses: six-carbon sugars with an aldehyde group that reacts with the OH group of C6 to form an intramolecular hemiacetal; Ketohexoses: six-carbon sugars with a keto group that can react with the OH group of C6 or C5 to form an intramolecular hemiketal; Aldopentoses: five-carbon sugars with an aldehyde group that reacts to form an intramolecular hemiacetal; and, 6-methyl-aldohexoses: six-carbon sugars with an aldehyde group that reacts to form an intramolecular hemiacetal with the alcohol group of C5. Carbon 6 forms a methyl group. b. One example of sulfation in a Fucose 2 sulfate molecule. OH groups are prone to be esterified with SO3− groups. In the case of fucose there are four potential sulfation sites. Modified from Ref. [8].
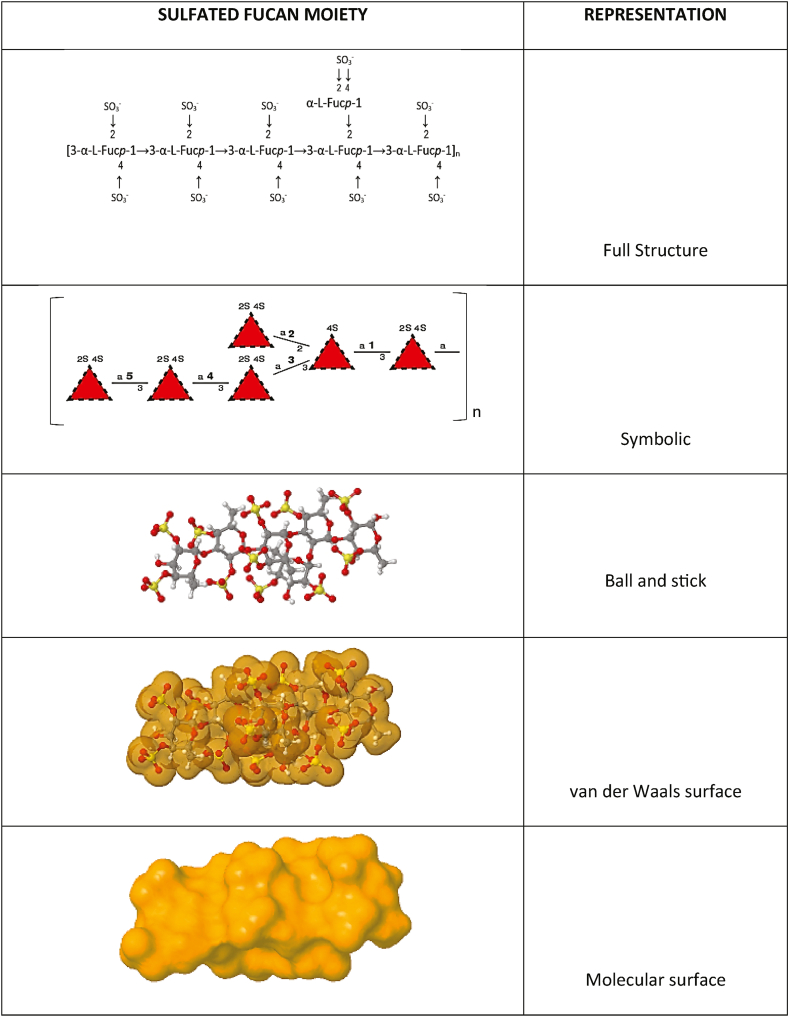
SPs are named in accordance with the composition of the sugar units. For instance, an animal polysaccharide made up of only sulfated fucose units are referred to as fucan sulfate. If the composition is more complex, they are named “fucoidans” [11]. Other examples of nomenclature given to sulfated polysaccharides are galactan sulfate, mannan sulfate, and other variations of the glycan followed by the appellative sulfate. Nevertheless, in the literature it can also be found appellatives such as sulfated galactan or sulfated mannan. In Fig. 2 there is an example of moiety of a sulfated fucan. Sulfate groups are esterified to alcohol groups which provides negative charges and high reactivity.
Fig. 2.
Different representations of a sulfated fucan, a typical polysaccharide found in marine algae. Illustration developed using open-source software from GLYCAM-Web www.glycam.org.
2.2. Sulfation of glycans
Sulfate groups in glycans are esterified to primary or secondary alcohol groups of hexoses such as galactose, fucose, glucose, mannose, arabinose, rhamnose, sialic acid, NAc glucosamine, and glucuronic acid. They can also be found in pentoses such as arabinose and xylose. The sulfation process of sugars in organisms is dependent on sulfotransferase enzymes (SULT) that occur in the lumen of the Golgi apparatus. SULT enzymes catalyze the sulfation of sulfoesters or sulfamates by using a sulfonic group's donor, namely 3' -phosphoadenosyl-5' -phosphosulfate (PAPS).
2.3. Sulfated polysaccharide biology and involvement in viral infection
Phylogenetically distant bacteria genera share old loci, indicating high conservation of SP genes during evolution. Based on genetic studies, it is known that SP appeared early in evolution, probably one billion years ago [12]. Nowadays, sulfate groups in saccharides provide diverse functions across different taxa, from prokaryotes to multicellular species [13].
The highly diverse and widespread SP occurrence and functions in modern organisms are due to the multiple interactions of sulfate groups. Carbohydrate sulfotransferases are crucial in marine macroalgae that use SP for chelation of metals. Sulfate groups bind positively charged molecules that can be harmful to the cell [14]. According to this remarkable property of sulfate groups, some of the most promising therapeutical strategies against COVID-19 could emerge from molecules extracted from organisms producing sulfated fucoidans such as brown, red and green seaweeds, [15,16].
SP are widely distributed in nature. Most of them are obtained from marine algae and terrestrial plants. As an example, the government of India prescribed the use of aqueous extract of the polysaccharide fraction obtained from medicinal plant species such as Allium sativum, Zingiber officinale, Glycyrrhiza glabra, Nigella sativa, Tinospora cordifolia, Cydonia oblonga, and Zizyphus jujube against COVID-19 because of their proven antiviral and immunomodulating activities [17]. Animal sulfated polysaccharides, such as heparans and cheratan sulfates, have been utilized with therapeutical aims for decades [18]. In addition, chemical synthesis would provide new libraries of SPs for further therapeutical applications [19,20].
Among SPs, the group known as Glycosaminoglycans (GAGs) is of special interest with respect to COVID-19 in multiple ways. GAGs are linear-long chain molecules found in virtually all mammalian cell surfaces as components of the extracellular matrix (ECM). When GAGs are attached to proteins, they are called proteoglycans. Proteoglycans (PG) are a group of molecules that take the names of the GAG attached to the protein; for instance, heparan sulfate proteoglycan (HSPG), chondroitin sulfate proteoglycan (CSPG), dermatan sulfate proteoglycan (DSPG) [21]. Bioactivity of these molecules includes interactions with cytokines, cells receptors, and extracellular matrix, which make them paramount in understanding the mechanisms of attachment of SARS-CoV-2 spike protein (S protein) to the cell surface.
The extracellular matrix (ECM) of most cells contains HSPG which is negatively charged, so it serves as a primary anchorage for a range of viruses, including SARS-CoV-2 spike protein [22]. The co-receptor activity of HSPG involves the fusion activation of SARS-CoV-2 and further interaction with angiotensin-converting enzyme 2 (ACE2) [23]. The mechanism by which viruses infect cells is through the HS interaction in ECM, which promotes an increase in viral concentration on the cell surface; the higher the concentration, the more prone a spike protein will be to interact with an ACE2 receptor on cells. Binding of viral S protein to ACE2 receptors is more specific than binding to HS [24].
Sulfated polysaccharides linked to cell surface proteins act as co-receptors for many viruses. As pointed out above, HSPG contributes to SARS-CoV-2 infection by increasing viral concentration on the cell membrane. Potentially, this property could be utilized in novel ways as molecular lures to prevent viruses from accumulating around the cells. In addition to SARS-Cov-2, a large and diverse list of virus species depend on HSPG interaction to infect cells. These include Hepatitis, Adenovirus, Norovirus, Adenovirus, Rabies virus, Chikungunya virus, and many others. For a more complete list see Cagno et al. (2019).
The above-mentioned properties of SP may have a efficacy in the prevention and treatment of SARS-CoV-2 and other viral diseases [[25], [26], [27]], due to a potential synergistic effect in producing symptoms in COVID-19 patients, including comorbidities. The potential of SPs not only to prevent the comorbidities that worsen the COVID-19 patient's prognosis, but also as a treatment against the viral infection is a promising alternative to the currently existing prevention and healing strategies. In addition, SPs are regarded as low toxicity-molecules that may even be taken as prophylactic administration as in the heparin case [28].
C. Properties directly related to the treatment of COVID-19 - antiviral, immunomodulatory, antioxidant.
Many viral pathogens such as influenza, herpes simplex, HIV, and a range of coronaviruses, including SARS-CoV-2 infect host cells through the interaction of glycans which are attachment factors that constitute the early interaction with the host cells that eventually ends in the entry of the pathogen into the cell [29]. Marine sulfated polysaccharides are being screened for their inhibitory activity against SARS-CoV-2. Polysaccharides obtained from sea cucumber, fucoidan from brown algae, iota-carrageenan from red algae, and chondroitin sulfate C from shark, have been tested and found to have significant antiviral activities at concentrations of 3.90–500 μg/mL. It has been suggested that sulfated polysaccharides can be used medically to bind the virus proteins, consequently blocking cell entry [1].
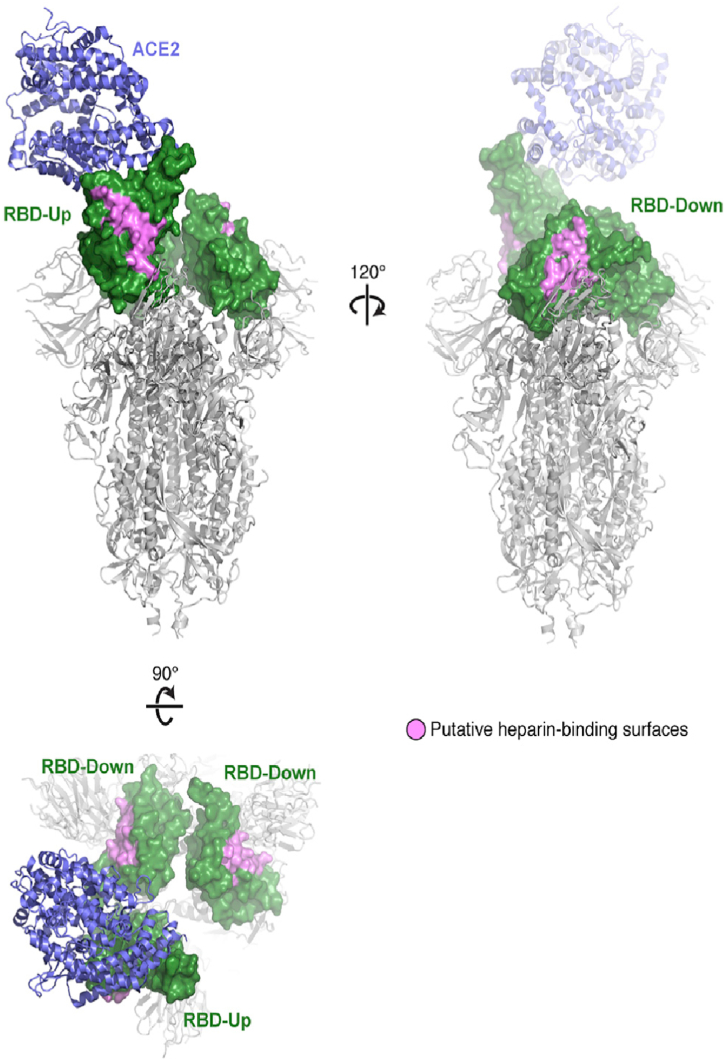
An important member of the glycose amino glycans (GAGs) is heparin, which is regarded as a key binding factor for SARS-CoV-2 and Ebola virus [30]. Most physiological and pathophysiological activities of heparin sulfate (HS) are due to electrostatic interactions with various proteins [31]. Heparin is not a single compound owing to its structural heterogeneity and variability. It is a family of similar sulfated polysaccharides frequently associated with proteins, namely proteoglycans. The physiological activities of heparin and heparan sulfate are largely dependent on their electrostatic nature (Fig. 3 [29]). This makes these sulfated polysaccharides a suitable model for understanding the complexity of these and other sulfated polysaccharides as antiviral agents [31].
Fig. 3.
The residues colored pink on the three Receptor Binding Domain makes up a potential binding site for heparin and heparan sulfate. Reprinted from Ref. [29]; with permission.
Recent work reports promising results using heparin and its derivatives, such as enoxaparin, 6-O-desulfated UFH, and 6-O-desulfated enoxaparin in studies on SARS-CoV-2. This work demonstrates that these polysaccharides can be used effectively in 50% inhibitory concentrations ranging from approximately 1 to 6 mg/L, and they demonstrate the potential of developing prophylactic and therapeutic strategies against SARS-CoV-2 [18]. The ability of sulfated polysaccharides to interact with viruses is exemplified by glycosaminoglycans, which are proteins heavily decorated with sulfated polysaccharides. These proteins are cellular receptors for binding of viruses herpes simplex, HIV dengue virus and many others [32].
In other recent research, Kim et al. found glycosamino glycan binding motifs on SGP of SARS-CoV-2 and SARS-CoV. The study found that heparin binds tightly to monomeric and trimeric SARS-CoV-2 SGP at picomole concentrations [33]. These motifs on the SGP can be exploited for docking the virus proteins. Saccharina japonica is a marine alga utilized as source of sulfated galactofucan and glucuronomannan; both types of molecules display strong inhibition of SARS-CoV-2 spike glycoprotein (SGP). The mechanism suggested is an interaction between algal polysaccharides and host's heparin that is considered key for the interaction of spike glycoprotein and the human ACE2 receptor [34].
As for the toxicity of sulfated polysaccharides [15], reported inhibition of SARS-CoV-2 infection in vitro in Vero cells. They showed the antiviral effect of branched and unbranched sulfated polysaccharides including fucoidans and heparin. They also reported that unbranched fucoidan saccharide was substantially more potent than remdesivir, an approved drug for COVID-19 treatment [15]. Recently, a sulfated fucan from Lytechinus variegatus (sea urchin) and a sulfated galactan from Botryocladia occidentalis (red seaweed) were found to have anti-SARS-CoV-2 activity using pseudotype SARS-CoV-2 particles [35].
2.4. SP to improve the immunomodulation response against SARS-CoV-2
Immunomodulation refers to adjusting the immune response to a certain balance or level of activity by generating or repressing immune responses. Extrinsic SP stimulates a range of responses, chiefly but not exclusively by acting on macrophages. Macrophages are essential in the immune system, and their functions are complex and variated. Upon activation, they can destroy pathogens by endocytosis, but they can also present antigens to lymphocytes while releasing signaling molecules that can regulate the activity of other cells [36]. Also, macrophages are involved in inflammatory processes by the release of pro- or anti-inflammatory cytokines and chemokines [37].
It is becoming clear that molecules modulate response of the immune system, and thus have the potential to ameliorate a vast array of diseases, from fatal infections to autoimmunity and neoplasia [38]. SP are well known as immunomodulators and immunostimulants [36]. Immunomodulators can suppress or stimulate the immune response in both innate and adaptive immune systems. As a consequence of immunomodulation activity, SP could enhance the host defense mechanisms, promoting the cell's antitumor activity and increasing the damage caused by toxic chemicals employed in cancer treatments [39]. Their activity depends upon various factors, including sulfation, monosaccharides composition, degree of polymerization (DP) branched or unbranched chains. Not only the degree of sulfation, but the shape of the polysaccharides can influence their activity [40]. Polysaccharide-stimulated macrophages can synthesize nitric oxide (NO) through the NO synthase (NOS) enzymes that catalyze the reaction of l-arginine and molecular oxygen (O2) to produce NO and l-citrulline. NO plays a fundamental role in macrophages and other cells by reprogramming the tricarboxylic acid cycle. NO is the critical effector molecule to activate macrophages, as seen in murine cells [41]. Studies have shown that NO production in macrophages has a direct relation to the sulfation degree of polysaccharides. These findings suggest that the sulfate groups are necessary for macrophage stimulation through direct interaction with cells [36].
Sulfated polysaccharides extracted from Laminaria japonica have been reported to inhibit macrophage conversion into foam cells [42]. Commonly, foam cells are associated with atherosclerosis and cardiovascular disease, among other disorders [43]. This kind of cell results from a dysregulated lipid metabolism in macrophages, leading to lipid accumulation in the shape of droplet formation (foamy appearance) that impairs the macrophage functions [44]. Another study carried out on murine macrophages shows a marked anti-inflammatory activity of SP extracted from Cyclocaria paliurus [45]. These SP have properties to reduce H2O2 oxidative stress (OS) in murine macrophages [46]. Moreover, SP derived from Pinnus massoniana pollen (Masson pine) promote immunity by induction of cytokines and the proliferation of spleen lymphocytes and macrophages [47].
Fucoidans are SP that can be found in marine organisms such as sea cucumbers and brown algae of a range of species, including Fucus vesiculosus, Sargassum stenophyllum, Chorda filum, Ascophyllum nodosum, Dictyota menstrualis, Fucus evanescens, Fucus serratus, Fucus distichus, Caulerpa racemosa, Hizikia fusiforme, Padina gymnospora, Kjellmaniella crassifolia, Analipus japonicus, and Laminaria hyperborea. SP from Fucus evanescens promote the production of specific IgG antibodies against hepatitis B, with significant results increase cytokines (TNF-a, IFN-g and IL-2) in the serum [48]. Fucoidan sulfate are involved in dendritic cell maturation and promotes the migration and adhesion of neutrophils and they can act on monocytes, facilitating antigen presentation. Also, an increase in the cytotoxicity of natural killer cells has been reported [49].
Additionally, these SP have also been tested as vaccine coadjuvants in place of aluminum salts that present several drawbacks. Instead, a natural polysaccharide has several advantages in producing antigen-specific antibodies in a mice model utilizing ovoalbumen as antigen [50]. In another study, fucoidans showed efficiency in preventing autoimmune diabetes in mice [6].
Vast evidence of an ample range of immunomodulatory activities of sulfated polysaccharides demonstrates the high potential of these natural products in the treatment and prevention of diverse diseases, caused by microbial pathogens, autoimmune disorders, immunosuppression or immunodeficiency [51,52]; M [6,53].
2.4.1. Antioxidant effects of SP to prevent COVID-19-related oxidative stress
COVID-19 patients commonly present a cytokine storm and inflammation that ends in cell death and other pathophysiological events linked with a redox imbalance or oxidative stress (OS). Reactive oxygen species (ROS) combined with deprivation of antioxidant mechanisms are key for the replication of SARS-CoV-2 and further associated disease [54]. In addition, several cancers, diabetes mellitus, cardiovascular diseases, chronic kidney disease, and other pathological conditions cause an enhancement of OS that increase the pathogenesis of respiratory viruses [55].
SP from seaweeds are regarded as free radical scavengers, and they prevent oxidative damage in cells. For instance, fucoidans have been tested to scavenge superoxide radicals and hydroxyl radicals. Both activities are closely related to sulfate groups attached to glycans [56]. Evaluation of the antioxidant fucoidans from Caluerpa cupressoides shows high chelating and antioxidant capacity [57].
Chemical modification of polysaccharides enhances their anti-oxidative properties. Due to the well-known attributes that sulfate groups impart to polysaccharides, chemical sulfation has been performed using chlorosulfonic acid-piridine method on Rhodiola sachalinensis, a herb widely used in Chinese medicine, which is acknowledged for its anti-inflammatory and anti-oxidative properties. The resulting SP shows high scavenging activity on ROS [58]. The antioxidant effect of SP may also prevent OS by scavenging ROS and by initiating downstream elements such as catalase and superoxide dismutase, preventing apoptosis [59]. This suggests that the use of SP in COVID-19 treatments, may also prevent apoptosis in respiratory and other tissular damage.
D. Properties related to the management of comorbidities – hypertension and coagulation, hypoglycemic, antibacterial, and cancer growth arrest.
2.5. Antihypertensive effect of SP and coagulation related with SARS-CoV-2
Sulfated polysaccharides have been proposed to treat hypertension because they possess mimetic properties of the natural ligands of protein receptors. They can regulate functions of biological systems via key signaling molecules [4]. Antithrombotic SP are being studied as alternative drugs to prevent thrombosis (cardiovascular disease), which is a prime reason for mortality worldwide [60] and a risk factor regarding SARS-CoV-2 infection. High venous thromboembolism and disseminated intravascular coagulation are reported in COVID-19 patients [61]. The levels of D-dimer, a breakdown product of cross-linked fibrin, correlate with disease severity being an indicative of potential thrombosis, need of ventilatory support, and eventually of mortality. This fact prompted the use of low molecular weight heparin, a SP widely used as anticoagulant [62].
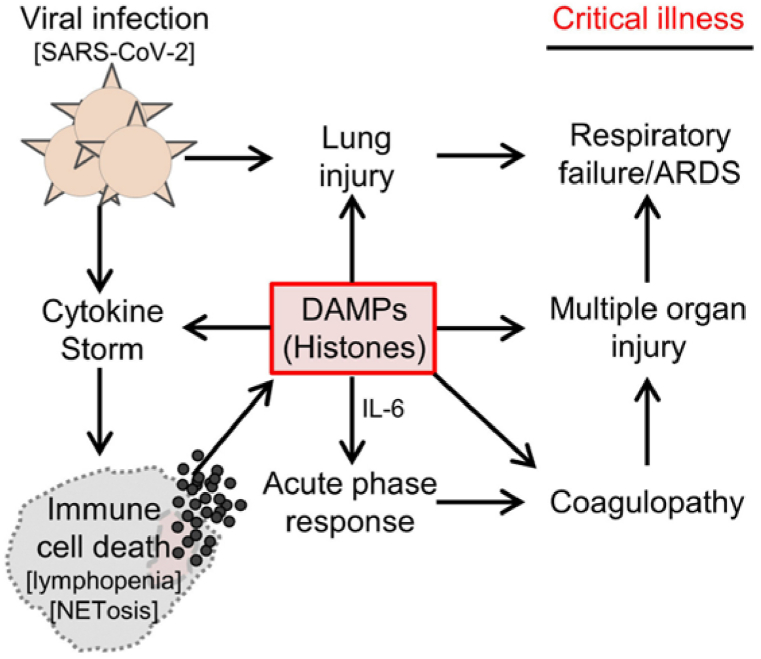
Heparin activates antithrombin by binding, thus, inhibiting proteases that play a role in blood coagulation. Their potential value in terms of COVID-19 treatment include prevention of viral adhesion but also anti-inflammatory activity based on inhibition of neutrophil chemotaxis and leukocyte migration [28]. Heparin/heparan sulfate has affinity with SARS-CoV-2 receptor binding domain whose S1 subunit possesses an ectodomain that interacts with HS. Interactions occur between 2-O or 6-O SO3− groups [63]. It is also known that high negatively charged molecules have a neutralizing effect on histones, which in turn, play a central role in coagulopathy, inflammation, and cardiac troponin. Circulating histones are positively charged small proteins associated to DNA (Fig. 4). These proteins are released from dead immune cells in severe SARS-CoV-2 infections demonstrating a clear association between free histones and deleterious effects of COVID-19 [64]. Heparin and heparinoids not only inhibit the interaction between the virus and cells, but also bind histones, that are associated to thrombosis and inflammation [63].
Fig. 4.
Diagram depicting how circulating histones play a central pathological role in the development of severe COVID-19. Reprinted with permission from Shaw et al.,. (2021).
Other SP with anticoagulant activities have been studied from Caulerpa cupressoides, Holothuria fuscopunctata, Holoturia coluber, and also chemically sulfated guar gum, among other natural sources [57,[65], [66], [67]]. The results indicate the large diversity of SP that can potentially be used in future COVID-19 treatments. Marine sources from seaweeds and invertebrates are being studied to produce antithrombotic SP that can replace low molecular heparin [68]. In addition, brown and red algal SP are also potential sources for the extraction and development of antithrombotic drugs, specially from the Ulva genus [69].
3. Hypoglycemic effect of SP
Type 2 diabetes as a COVID-19 comorbidity might be ameliorated with sulfated rhamnose polysaccharides and fucoidans, which are reported as promising hypoglycemic molecules in type 2 diabetes in mice [6,7]. In addition to the above-mentioned benefits, sulfated polysaccharides are known for their immunomodulating, antitumor, antioxidant, antibacterial, and anticoagulant activities. SP and their anti-infection capacity against pathogenic microorganisms like bacteria, virus, fungus, and protozoan parasites, are reported as beneficial effects on health; [70].
3.1. Antibacterial effect of SP to prevent opportunistic infections associated to COVID-19
Gram-positive or Gram-negative bacteria strains may affect COVID-19 patients as opportunistic infective organisms that may worsen the general prognosis. These kinds of infections are generally associated with intensive care units (ICU). Secondary infections in COVID-19 patients are of great concern given that nearly 58% of them have been reported as nosocomial infections in the ICU. Bacteria species such as Chryseobacterium sp, Pseudomonas aeruginosa, Escherichia coli, Stenotrophomonas maltophilia, Enterococcus faecalis, Staphylococcus aureus, Acinetobacter baumannii, Klebsiella pneumoniae and other microorganisms, including viruses and fungi, have been found in critical COVID-19 patients in hospitals [71]. Antibacterial SP are obtained from various origins. For instance, the smooth hound shark viscera represent a source for these molecules. The degree of sulfation of polysaccharides extracted from shark viscera show a direct relation with the antibacterial effect on Gram positive bacteria such as Staphylococcus aureus, Micrococcus luteus, and Bacillus cereus. Also, Gram-negative bacteria such as Escherichia coli, Klebsiella pneumoniae, Salmonella enterica, Salmonella typhi, and Enterobacter.
The antibacterial mechanism of sulfated polysaccharides resides in disruption of the cell wall and cytoplasmic membranes that eventually results in cell death [25]. Similar antibacterial activity was observed with fractions of SP extracted from Malva aegyptiaca that inhibited cell growth in laboratory conditions [72]. Another source of antibacterial SP is mollusks [73]. Saccharides composed by rhamnose, xylose, mannose, glucuronic acid, and galacturonic acid have been extracted from cuttlefish skin and muscles.
Another study focuses on Sargassum polycystum SP fraction, and the results demonstrate slightly lower antibacterial activity of fucans compared to tetracycline, both in vivo and in vitro. Nevertheless, the antibacterial activity is highly dependent on the fraction of fucans that differentiate alternative forms, chiefly by the degree of sulfation. The antibacterial effect of SP is remarkable also against Vibrio harveyi, Staphylococcus sp., Aeromonas hydrophila, Enterobacter sp., Pseudomonas aeruginosa, Streptococcus sp., Vibrio parahaemolyticus, Vibrio alginolyticus, Vibrio cholerae, Yersinia enterocolitica, and Proteus sp [74].
Not only antibacterial, but metal chelating activities seem to be associated with the degree of sulfation of the alcohol groups of the saccharide units. These and other findings strongly suggest that saccharides associated with a range of marine organisms (from alga to vertebrates) play crucial roles in their innate immunity. Interestingly, most of the pathogenic bacteria species described in the medical literature can be significantly impaired by SP. This represents an attractive therapeutic alternative against opportunistic infections in COVID-19 patients in ICU.
3.1.1. Cancer growth-arrest and SARS-CoV-2 tissue tropism
Cancer patients are reported to be at high risk of SARS-CoV-2 infection due to tumor markers such as CD147 or basigin, that can bind the viral S protein as an additional rout of entry to the ACE2 receptor [75]. Thus, if SP exerts antitumor activities, they could contribute not only to cancer treatments but also potentially to blocking the tumor markers. A group of SP of animal origin include heparan sulfate, chondroitin sulfate, dermatan sulfate, and keratan sulfate, form part of the glycosaminoglycans. These molecules play relevant roles in cancer physiology, being involved in a range of interactions with molecules of varied origins and structures, including enzymes, growth factors, cytokines, and chemokines [76]. Sulfate groups confer negative charges that contribute to the affinity of GAGs for binding different protein targets by electrostatic interactions with positive amino acids ([77]. Heparan sulfate is present in virtually all animal cells, and it has multiple interactions with proteins; for instance, acting as molecular scaffolds and allosteric regulator of thrombin and antithrombin. Heparan sulfate's absence in cells is not compatible with life [78].
In addition to GAGs interacting with growth factors, growth factor receptors, and molecules such as cytokines associated with cancer growth, they provide another approach to develop effective anticancer drugs [77]. Other SPs from various algae origins, such as fucoidans, have shown effectiveness in preventing several different cancer types by interacting with cellular pathways involved in cancer growth; notably the Pi3K/AKT, MAPK, and the caspase pathways [79]. Sulfated fucoidans have shown more antitumor effectiveness when the fucose polymers have high molecular weights. These sulfated molecules cause cancer cell growth-arrest by inducing apoptosis [16]. Recent studies showed a potential relationship between cancer and diabetes since both diseases are somewhat related to glucose metabolism [80].
Fucoidans demonstrate anticancer activities both in vivo and in vitro, and the mechanisms of action are being elucidated. Some natural fucoidan extracts may target specific signaling pathways that impact cancer cells by inhibiting or delaying their growth at various stages. Additionally, fucoidans are specific, exhibit low toxicity and, most importantly, they induce apoptosis of malignant cells [81]. Fucoidans act on four known stages to inhibit metastasis tumors: a. Suppressing normal mitosis in cancer cells [82]; b. Inducing apoptosis of cancer cells [83]; c, vascular endothelial growth factor (VEGF) is inhibited, suppressing angiogenesis and cutting off nutrients and oxygen supply to the tumor [84]; d. By modulating the immune system, enhancing natural killer cells and T cells to destroy tumor cells [85].
Other non-fucoidan polysaccharides can also be used as anticancer drugs. Recently, a sulfated heteropolysaccharide (sulfated galacto-fuco-xylo-glucurono-mannan) has been tested with promising results against lung cancer [34]. Another study focused on cytotoxic effects of sulfated carrageenan and their oligosaccharides resulting from partial degradation. The results show that sulfated disaccharide units can exert apoptotic effects on LM2 tumor cells, impairing their metastatic capacity [86]. Another example of antitumor activity of SP has been described from a 1-4-β-D galactoglucan with 1–6 branches isolated from Antrodia cinnamomea, which was proven effective against lung cancer H1975 cells that underwent apoptosis by means of activation of caspase 3 and PARP. Additionally, this polysaccharide conformation as a “rare boat shape” as a possible attribute of its anticancer activity [87]. The emerging question is whether administering SP to cancer patients infected with COVID-19 would benefit both conditions: cancer growth-arrest and the change of tissue tropism of SARS-CoV-2. Various therapeutic benefits of SP are shown in Table 2 [36,56,70,88], [51,57,59], [70,88], [70,82,86,89], [28,65,66,90,91], [52,72,73,92], [1,15,18,25,32,93].
Table 2.
Potential synergistic effects of treating SARS-CoV-2 infection with SP.
| Activity | S-Polysaccharides | Origin | Proposed mechanisms of action | Current state | References |
|---|---|---|---|---|---|
| Immunomodulation | Acidic sulfated polysaccharides, carrageenans, dextran sulfate, fucoidan, and fucans, Sulfated mannan |
Ulva rigida, Porphyra yezoensis, Laminaria, Gelidum, Ganoderma lucidum Nemalion helmintoides |
Increase the activity and viability of macrophages | In vitro assays and/or regular diet components | [36,56]; J. [88]; S.-Y [70]. |
| Antioxidant | Fucoidans Gluco-manno-xylo-galactans |
Padina boryana Caulerpa cupressoides Gracilaria fisheri |
ROS scavenging, hydrogen-radicals interaction, reduction of lipid peroxidation, catalase activity enhancement. | Research in cell cultures; used in traditional Chinese medicine | [51,57,59]; J [70,88]. |
| Antitumor (cancer growth arrest) | Fucoidan, carrageenans, agarans |
Fucus evanescens Eisenia bicyclis |
Stimulation of phagocytic activities of macrophages and neutrophils; cancer cells' apoptosis | Used as functional foods | [70,82,86,89] |
| Anticoagulant | Sulfated ulvan, glycose amino glycans (heparin sulfate, dermatan sulfate, heparan sulfate), fucans. | Animal cells, Ulva rigida Monostroma angicava, M. oxysperma, Penicillus captatis, Codium divaricatum, Enteromorpha linz, Holoturia fuscopunctata |
Inhibition of antithrombin complexes, Xase inhibition | Routine pharmacologic application (heparin), and experimental approaches | [28,65,66,90,91] |
| Antibacterial | fucoidan | Cuttlefish, Smooth hound, Malva aegyptiaca, Sargassum polycystum | Damage to the bacterial membrane | Research | [52,72,73,92] |
| Anti SARS-CoV-2 | Unfractionated Heparin UFH, Low molecular weight heparins (LMWHs), heparan sulfate proteoglycans, fucoidans, TriS-heparin. Sea cucumber SP, fucoidans, carrageenan and chondroitin sulfate |
Seaweeds, biologically and chemically engineered polysaccharides. Sea cucumber, shark | Molecular docking, leading to a conformational change of S protein that blocks viral attachment and infection. Multivalent interactions | Research | [1,15,18,25,32,93] |
4. Concluding remarks
The development of multi-target drugs with low toxicity extracted from natural sources to fight SARS-CoV-2 and its secondary and concomitant effects is possible. Many sulfated polysaccharide molecules can be directly extracted from living organisms or chemically modified to obtain tailored activities. Sulfated polysaccharides libraries show the great diversity of these compounds and their utility in the prevention, and treatment of comorbidities, but also in fighting SARS-CoV-2 and its collateral deleterious effects. The available data show that fucans, fucoidans, galactans, carrageenans and heparin are promising molecules for drug development, because of their antiviral properties, as well as other beneficial effects to fight COVID-19. Undoubtedly, SP provide promising alternatives as multi-target drugs that may be valuable in treatment of COVID-19 and in future viral pandemics as well as a number of comorbidities. No single SP would possess all the properties mentioned in this review; to achieve all the benefits discussed, one would probably need to use a mixture of several SPs. The present review is limited to the currently available preclinical information, but assembling this previously dispersed information on SP and their wide range of properties should contribute to designing novel therapeutics against SARS-CoV-2 and alleviating the associated comorbidities. Many of the results have been obtained in vitro or through animal experiments, and they must be confirmed in human studies for future treatments. Finally, biological interactions of sulfated polysaccharides in viral infection illuminate puzzling comorbidities and complications associated with CoV-2 infection, including risk factors for developing severe illness and blood clotting that causes strokes in some COVID-19 patients.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Dr. R. Douglas Fields was supported by National Institutes of Child Health and Human Development [ZIAHD000713].
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Song P., Li W., Xie J., Hou Y., You C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta. 2020;509:280–287. doi: 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantón R., De Lucas Ramos P., García-Botella A., García-Lledó A., Gómez-Pavón J., González del Castillo J., Hernández-Sampelayo T., Martín-Delgado M.C., Martín Sánchez F.J., Martínez-Sellés M., Molero García J.M., Moreno Guillén S., Rodríguez-Artalejo F., Ruiz-Galiana J., Bouza E., de Microbiología Servicio. Hospital Ramón y Cajal and Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS). Red Española de Investigación en Patología Infecciosa (REIPI). Madrid. Spain. New variants of SARS-CoV-2. Revista Española de Quimioterapia. 2021;34(5):419–428. doi: 10.37201/req/071.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaporozhets T., Besednova N. Prospects for the therapeutic application of sulfated polysaccharides of brown algae in diseases of the cardiovascular system: review. Pharmaceut. Biol. 2016;54(12):3126–3135. doi: 10.1080/13880209.2016.1185444. [DOI] [PubMed] [Google Scholar]
- 5.Mourão P. Perspective on the use of sulfated polysaccharides from marine organisms as a source of new antithrombotic drugs. Mar. Drugs. 2015;13(5):2770–2784. doi: 10.3390/md13052770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue M., Liang H., Ji X., Liu Y., Ge Y., Hou L., Sun T. Fucoidan prevent murine autoimmune diabetes via suppression TLR4-signaling pathways, regulation DC/Treg induced immune tolerance and improving gut microecology. Nutr. Metabol. 2019;16(1):87. doi: 10.1186/s12986-019-0392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye H., Shen Z., Cui J., Zhu Y., Li Y., Chi Y., Wang J., Wang P. Hypoglycemic activity and mechanism of the sulfated rhamnose polysaccharides chromium(III) complex in type 2 diabetic mice. Bioorg. Chem. 2019;88 doi: 10.1016/j.bioorg.2019.102942. [DOI] [PubMed] [Google Scholar]
- 8.Varki A., Cummings R.D., Aebi M., Packer N.H., Seeberger P.H., Esko J.D., Stanley P., Hart G., Darvill A., Kinoshita T., Prestegard J.J., Schnaar R.L., Freeze H.H., Marth J.D., Bertozzi C.R., Etzler M.E., Frank M., Vliegenthart J.F., Lütteke T.…Kornfeld S. Symbol nomenclature for graphical representations of glycans. Glycobiology. 2015;25(12):1323–1324. doi: 10.1093/glycob/cwv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg J.M., Tymoczko J.L., Gatto G.J., Stryer L. eighth ed. W.H. Freeman & Company, a Macmillan Education Imprint; 2015. Biochemistry. [Google Scholar]
- 10.Polysaccharide Nomenclature Eur. J. Biochem. 1982;126(3):439–441. doi: 10.1111/j.1432-1033.1982.tb06799.x. [DOI] [PubMed] [Google Scholar]
- 11.Usov A.I., Bilan M.I. Fucoidans—sulfated polysaccharides of brown algae. Russ. Chem. Rev. 2009;78(8):785–799. doi: 10.1070/RC2009v078n08ABEH004063. [DOI] [Google Scholar]
- 12.Holt K.E., Lassalle F., Wyres K.L., Wick R., Mostowy R.J. Diversity and evolution of surface polysaccharide synthesis loci in Enterobacteriales. ISME J. 2020;14(7):1713–1730. doi: 10.1038/s41396-020-0628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman E., Best M.D., Hanson S.R., Wong C.-H. Sulfotransferases: structure, mechanism, biological activity, inhibition, and synthetic utility. Angew. Chem. Int. Ed. 2004;43(27):3526–3548. doi: 10.1002/anie.200300631. [DOI] [PubMed] [Google Scholar]
- 14.Ho C.-L. Phylogeny of algal sequences encoding carbohydrate sulfotransferases, formylglycine-dependent sulfatases, and putative sulfatase modifying factors. Front. Plant Sci. 2015;6 doi: 10.3389/fpls.2015.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon P.S., Oh H., Kwon S.-J., Jin W., Zhang F., Fraser K., Hong J.J., Linhardt R.J., Dordick J.S. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discovery. 2020;6 doi: 10.1038/s41421-020-00192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senthilkumar K., Manivasagan P., Venkatesan J., Kim S.-K. Brown seaweed fucoidan: biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013;60:366–374. doi: 10.1016/j.ijbiomac.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Sen I.K., Chakraborty I., Mandal A.K., Bhanja S.K., Patra S., Maity P. A review on antiviral and immunomodulatory polysaccharides from Indian medicinal plants, which may be beneficial to COVID-19 infected patients. Int. J. Biol. Macromol. 2021;181:462–470. doi: 10.1016/j.ijbiomac.2021.03.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tandon R., Sharp J.S., Zhang F., Pomin V.H., Ashpole N.M., Mitra D., Jin W., Liu H., Sharma P., Linhardt R.J. BioRxiv; 2020. Effective Inhibition of SARS-CoV-2 Entry by Heparin and Enoxaparin Derivatives. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee S., Ghosh K., Hahn F., Wangen C., Strojan H., Müller R., Anand N., Ali I., Bera K., Ray B., Hutterer C., Marschall M., Ray S. Chemically sulfated polysaccharides from natural sources: assessment of extraction-sulfation efficiencies, structural features and antiviral activities. Int. J. Biol. Macromol. 2019;136:521–530. doi: 10.1016/j.ijbiomac.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Ray B., Schütz M., Mukherjee S., Jana S., Ray S., Marschall M. Exploiting the amazing diversity of natural source-derived polysaccharides: modern procedures of isolation, engineering, and optimization of antiviral activities. Polymers. 2020;13(1):136. doi: 10.3390/polym13010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Köwitsch A., Zhou G., Groth T. Medical application of glycosaminoglycans: a review: medical application of glycosaminoglycans. Journal of Tissue Engineering and Regenerative Medicine. 2018;12(1):e23–e41. doi: 10.1002/term.2398. [DOI] [PubMed] [Google Scholar]
- 22.Hu Y., Meng X., Zhang F., Xiang Y., Wang J. The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor. Emerg. Microb. Infect. 2021;10(1):317–330. doi: 10.1080/22221751.2021.1888660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan L., Song Y., Xia K., He P., Zhang F., Chen S., Pouliot R., Weiss D.J., Tandon R., Bates J.T., Ederer D.R., Mitra D., Sharma P., Davis A., Linhardt R.J. Heparan sulfates from bat and human lung and their binding to the spike protein of SARS-CoV-2 virus. Carbohydr. Polym. 2021;260 doi: 10.1016/j.carbpol.2021.117797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yue J., Jin W., Yang H., Faulkner J., Song X., Qiu H., Teng M., Azadi P., Zhang F., Linhardt R.J., Wang L. Heparan sulfate facilitates spike protein-mediated SARS-CoV-2 host cell invasion and contributes to increased infection of SARS-CoV-2 G614 mutant and in lung cancer. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.649575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmadi A., Zorofchian Moghadamtousi S., Abubakar S., Zandi K. BioMed Research International, 2015; 2015. Antiviral Potential of Algae Polysaccharides Isolated from Marine Sources: A Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baba M., Nakajima M., Schols D., Pauwels R., Balzarini J., De Clercq E. Pentosan polysulfate, a sulfated oligosaccharide, is a potent and selective anti-HIV agent in vitro. Antivir. Res. 1988;9(6):335–343. doi: 10.1016/0166-3542(88)90035-6. [DOI] [PubMed] [Google Scholar]
- 27.Grice I.D., Mariottini G.L. In: Kloc M., Kubiak J.Z., editors. vol. 65. Springer International Publishing; 2018. Glycans with antiviral activity from marine organisms; pp. 439–475. (Marine Organisms as Model Systems in Biology and Medicine). [DOI] [PubMed] [Google Scholar]
- 28.Lindahl U., Li J. Heparin – an old drug with multiple potential targets in Covid‐19 therapy. J. Thromb. Haemostasis. 2020 doi: 10.1111/jth.14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Perrett H.R., Painter C.D., Narayanan A., Majowicz S.A., Kwong E.M., McVicar R.N., Thacker B.E., Glass C.A., Yang Z., Torres J.L., Golden G.J., Bartels P.L., Porell R.N., Garretson A.F., Laubach L.…Esko J.D. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183(4):1043–1057.e15. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamhankar M., Gerhardt D.M., Bennett R.S., Murphy N., Jahrling P.B., Patterson J.L. Heparan sulfate is an important mediator of Ebola virus infection in polarized epithelial cells. Virol. J. 2018;15 doi: 10.1186/s12985-018-1045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J.-P., Kusche-Gullberg M. International Review of Cell and Molecular Biology. Elsevier; 2016. Heparan sulfate: biosynthesis, structure, and function; pp. 215–273. 325. [DOI] [PubMed] [Google Scholar]
- 32.Al-Horani R.A., Kar S., Aliter K.F. Potential anti-COVID-19 therapeutics that block the early stage of the viral life cycle: structures, mechanisms, and clinical trials. Int. J. Mol. Sci. 2020;21(15):5224. doi: 10.3390/ijms21155224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S.Y., Jin W., Sood A., Montgomery D.W., Grant O.C., Fuster M.M., Fu L., Dordick J.S., Woods R.J., Zhang F., Linhardt R.J. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antivir. Res. 2020;181 doi: 10.1016/j.antiviral.2020.104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin W., Tang H., Zhang J., Wei B., Sun J., Zhang W., Zhang F., Wang H., Linhardt R.J., Zhong W. Structural analysis of a novel sulfated galacto-fuco-xylo-glucurono-mannan from Sargassum fusiforme and its anti-lung cancer activity. Int. J. Biol. Macromol. 2020;149:450–458. doi: 10.1016/j.ijbiomac.2020.01.275. [DOI] [PubMed] [Google Scholar]
- 35.Crawford K.H.D., Eguia R., Dingens A.S., Loes A.N., Malone K.D., Wolf C.R., Chu H.Y., Tortorici M.A., Veesler D., Murphy M., Pettie D., King N.P., Balazs A.B., Bloom J.D. Protocol and reagents for pseudotyping lentiviral particles with SARS-CoV-2 spike protein for neutralization assays. Viruses. 2020;12(5):513. doi: 10.3390/v12050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leiro J.M., Castro R., Arranz J.A., Lamas J. Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh. Int. Immunopharm. 2007;7(7):879–888. doi: 10.1016/j.intimp.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Gantzel R.H., Kjær M.B., Laursen T.L., Kazankov K., George J., Møller H.J., Grønbæk H. Macrophage activation markers, soluble CD163 and mannose receptor, in liver fibrosis. Front. Med. 2021;7 doi: 10.3389/fmed.2020.615599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavelle E.C., McLachlan J.B. Editorial overview: immunomodulation: Striking the right balance: using immunomodulators to target infectious diseases, cancer, and autoimmunity. Curr. Opin. Pharmacol. 2018;41 doi: 10.1016/j.coph.2018.07.013. vii–ix. [DOI] [PubMed] [Google Scholar]
- 39.Dobrange E., Peshev D., Loedolff B., Van den Ende W. Fructans as immunomodulatory and antiviral agents: the case of echinacea. Biomolecules. 2019;9(10) doi: 10.3390/biom9100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbosa J.D.S., Costa M.S.S.P., Melo L.F.M.D., Medeiros M.J.C.D., Pontes D.D.L., Scortecci K.C., Rocha H.A.O. Caulerpa cupressoides var. Flabellata. Mar. Drugs. 2019;17(2):105. doi: 10.3390/md17020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmieri E.M., McGinity C., Wink D.A., McVicar D.W. Nitric oxide in macrophage immunometabolism: hiding in plain sight. Metabolites. 2020;10(11):429. doi: 10.3390/metabo10110429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Q.-M., Teng H., Zha X.-Q., Pan L.-H., Luo J.-P. Sulfated Laminaria japonica polysaccharides inhibit macrophage foam cell formation. Int. J. Biol. Macromol. 2018;111:857–861. doi: 10.1016/j.ijbiomac.2018.01.103. [DOI] [PubMed] [Google Scholar]
- 43.Maguire E.M., Pearce S.W.A., Xiao Q. Foam cell formation: a new target for fighting atherosclerosis and cardiovascular disease. Vasc. Pharmacol. 2019;112:54–71. doi: 10.1016/j.vph.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Guerrini V., Gennaro M.L. Foam cells: one size doesn't fit all. Trends Immunol. 2019;40(12):1163–1179. doi: 10.1016/j.it.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z., Xie J., Yang Y., Zhang F., Wang S., Wu T., Shen M., Xie M. Sulfated Cyclocarya paliurus polysaccharides markedly attenuates inflammation and oxidative damage in lipopolysaccharide-treated macrophage cells and mice. Sci. Rep. 2017;7(1) doi: 10.1038/srep40402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z.-J., Xie J.-H., Kan L.-J., Wang J.-Q., Shen M.-Y., Li W.-J., Nie S.-P., Xie M.-Y. Sulfated polysaccharides from Cyclocarya paliurus reduce H 2 O 2 -induced oxidative stress in RAW264.7 cells. Int. J. Biol. Macromol. 2015;80:410–417. doi: 10.1016/j.ijbiomac.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 47.Su F., Sun M., Geng Y. 1H-NMR metabolomics analysis of the effects of sulfated polysaccharides from masson pine pollen in RAW264.7 macrophage cells. Molecules. 2019;24(9):1841. doi: 10.3390/molecules24091841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luthuli S., Wu S., Cheng Y., Zheng X., Wu M., Tong H. Therapeutic effects of fucoidan: a review on recent studies. Mar. Drugs. 2019;17(9):487. doi: 10.3390/md17090487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuznetsova T.A., Smolina T.P., Makarenkova I.D., Ivanushko L.A., Persiyanova E.V., Ermakova S.P., Silchenko A.S., Zaporozhets T.S., Besednova N.N., Fedyanina L.N., Kryzhanovsky S.P. Immunoadjuvant activity of fucoidans from the Brown alga Fucus evanescens. Mar. Drugs. 2020;18(3):155. doi: 10.3390/md18030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Persiyanova E.V., Kuznetsova T.A., Silchenko A.S. Effect of sulfated polysaccharides from marine hydrobionts on humoral immune response to ovalbumin in mice. Bull. Exp. Biol. Med. 2020;169(2):246–248. doi: 10.1007/s10517-020-04860-3. [DOI] [PubMed] [Google Scholar]
- 51.Besednova N.N., Zvyagintseva T.N., Kuznetsova T.A., Makarenkova I.D., Smolina T.P., Fedyanina L.N., Kryzhanovsky S.P., Zaporozhets T.S. Marine algae metabolites as promising therapeutics for the prevention and treatment of HIV/AIDS. Metabolites. 2019;9(5):87. doi: 10.3390/metabo9050087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palanisamy S., Vinosha M., Rajasekar P., Anjali R., Sathiyaraj G., Marudhupandi T., Selvam S., Prabhu N.M., You S. Antibacterial efficacy of a fucoidan fraction (Fu-F2) extracted from Sargassum polycystum. Int. J. Biol. Macromol. 2019;125:485–495. doi: 10.1016/j.ijbiomac.2018.12.070. [DOI] [PubMed] [Google Scholar]
- 53.Wang M., Wang X., Zhang L., Yang R., Fei C., Zhang K., Wang C., Liu Y., Xue F. Effect of sulfated yeast beta-glucan on cyclophosphamide-induced immunosuppression in chickens. Int. Immunopharm. 2019;74 doi: 10.1016/j.intimp.2019.105690. [DOI] [PubMed] [Google Scholar]
- 54.Delgado-Roche L., Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch. Med. Res. 2020;51(5):384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suhail S., Zajac J., Fossum C., Lowater H., McCracken C., Severson N., Laatsch B., Narkiewicz-Jodko A., Johnson B., Liebau J., Bhattacharyya S., Hati S. Role of oxidative stress on SARS-CoV (sars) and SARS-CoV-2 (COVID-19) infection: a review. Protein J. 2020;1 doi: 10.1007/s10930-020-09935-8. –13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abdala Díaz R.T., Casas Arrojo V., Arrojo Agudo M.A., Cárdenas C., Dobretsov S., Figueroa F.L. Immunomodulatory and antioxidant activities of sulfated polysaccharides from Laminaria ochroleuca, porphyra umbilicalis, and gelidium corneum. Mar. Biotechnol. 2019;21(4):577–587. doi: 10.1007/s10126-019-09905-x. [DOI] [PubMed] [Google Scholar]
- 57.Costa M.S.S.P., Costa L.S., Cordeiro S.L., Almeida-Lima J., Dantas-Santos N., Magalhães K.D., Sabry D.A., Albuquerque I.R.L., Pereira M.R., Leite E.L., Rocha H.A.O. Evaluating the possible anticoagulant and antioxidant effects of sulfated polysaccharides from the tropical green alga Caulerpa cupressoides var. Flabellata. J. Appl. Phycol. 2012;24(5):1159–1167. doi: 10.1007/s10811-011-9745-5. [DOI] [Google Scholar]
- 58.Song J., Wu Y., Jiang G., Feng L., Wang Z., Yuan G., Tong H. Sulfated polysaccharides from Rhodiola sachalinensis reduce d-gal-induced oxidative stress in NIH 3T3 cells. Int. J. Biol. Macromol. 2019;140:288–293. doi: 10.1016/j.ijbiomac.2019.08.052. [DOI] [PubMed] [Google Scholar]
- 59.Jayawardena T.U., Wang L., Sanjeewa K.K.A., Kang S.I., Lee J.-S., Jeon Y.-J. Antioxidant potential of sulfated polysaccharides from Padina boryana; protective effect against oxidative stress in in vitro and in vivo zebrafish model. Mar. Drugs. 2020;18(4) doi: 10.3390/md18040212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dwivedi R., Pomin V.H. Marine antithrombotics. Mar. Drugs. 2020;18(10):514. doi: 10.3390/md18100514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al-Samkari H., Karp Leaf R.S., Dzik W.H., Carlson J.C.T., Fogerty A.E., Waheed A., Goodarzi K., Bendapudi P.K., Bornikova L., Gupta S., Leaf D.E., Kuter D.J., Rosovsky R.P. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan N.C., Weitz J.I. COVID-19 coagulopathy, thrombosis, and bleeding. Blood. 2020;136(4):381–383. doi: 10.1182/blood.2020007335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hernández-Huerta M.T., Pérez-Santiago A.D., Pérez-Campos Mayoral L., Sánchez Navarro L.M., Rodal Canales F.J., Majluf-Cruz A., Matias-Cervantes C.A., Pérez-Campos Mayoral E., Romero Díaz C., Mayoral-Andrade G., Martínez Cruz M., Luna Ángel J., Pérez-Campos E. Mechanisms of immunothrombosis by SARS-CoV-2. Biomolecules. 2021;11(11):1550. doi: 10.3390/biom11111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaw R.J., Abrams S.T., Austin J., Taylor J.M., Lane S., Dutt T., Downey C., Du M., Turtle L., Baillie J.K., Openshaw P.J.M., Wang G., Semple M.G., Toh C.-H. Circulating histones play a central role in COVID-19-associated coagulopathy and mortality. Haematologica. 2021;106(9):2493–2498. doi: 10.3324/haematol.2021.278492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Oliveira Barddal H.P., Faria F.A.M., Nogueira A.V., Iacomini M., Cipriani T.R. Anticoagulant and antithrombotic effects of chemically sulfated guar gum. Int. J. Biol. Macromol. 2020;145:604–610. doi: 10.1016/j.ijbiomac.2019.12.210. [DOI] [PubMed] [Google Scholar]
- 66.Gao N., Chen R., Mou R., Xiang J., Zhou K., Li Z., Zhao J. Purification, structural characterization and anticoagulant activities of four sulfated polysaccharides from sea cucumber Holothuria fuscopunctata. Int. J. Biol. Macromol. 2020;164:3421–3428. doi: 10.1016/j.ijbiomac.2020.08.150. [DOI] [PubMed] [Google Scholar]
- 67.Yang W., Cai Y., Yin R., Lin L., Li Z., Wu M., Zhao J. Structural analysis and anticoagulant activities of two sulfated polysaccharides from the sea cucumber Holothuria coluber. Int. J. Biol. Macromol. 2018;115:1055–1062. doi: 10.1016/j.ijbiomac.2018.04.175. [DOI] [PubMed] [Google Scholar]
- 68.Pomin V.H. Advances in Food and Nutrition Research. Elsevier; 2012. Structure–function relationship of anticoagulant and antithrombotic well-defined sulfated polysaccharides from marine invertebrates; pp. 195–209. 65. [DOI] [PubMed] [Google Scholar]
- 69.Adrien A., Bonnet A., Dufour D., Baudouin S., Maugard T., Bridiau N. Anticoagulant activity of sulfated ulvan isolated from the green macroalga Ulva rigida. Mar. Drugs. 2019;17(5) doi: 10.3390/md17050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu S.-Y., Huang X., Cheong K.-L. Recent advances in marine algae polysaccharides: isolation, structure, and activities. Mar. Drugs. 2017;15(12) doi: 10.3390/md15120388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang H., Zhang Y., Wu J., Li Y., Zhou X., Li X., Chen H., Guo M., Chen S., Sun F., Mao R., Qiu C., Zhu Z., Ai J., Zhang W. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg. Microb. Infect. 2020;9(1):1958–1964. doi: 10.1080/22221751.2020.1812437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fakhfakh N., Abdelhedi O., Jdir H., Nasri M., Zouari N. Isolation of polysaccharides from Malva aegyptiaca and evaluation of their antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2017;105:1519–1525. doi: 10.1016/j.ijbiomac.2017.07.105. [DOI] [PubMed] [Google Scholar]
- 73.Jridi M., Nasri R., Marzougui Z., Abdelhedi O., Hamdi M., Nasri M. Characterization and assessment of antioxidant and antibacterial activities of sulfated polysaccharides extracted from cuttlefish skin and muscle. Int. J. Biol. Macromol. 2019;123:1221–1228. doi: 10.1016/j.ijbiomac.2018.11.170. [DOI] [PubMed] [Google Scholar]
- 74.Marudhupandi T., Kumar T.T.A. Antibacterial effect of fucoidan from Sargassum wightii against the chosen human bacterial pathogens. Int. Curr. Pharmaceut. J. 2013;2(10):156–158. doi: 10.3329/icpj.v2i10.16408. [DOI] [Google Scholar]
- 75.Xia P., Dubrovska A. Tumor markers as an entry for SARS-CoV-2 infection? FEBS J. 2020;287(17):3677–3680. doi: 10.1111/febs.15499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morla S. Glycosaminoglycans and glycosaminoglycan mimetics in cancer and inflammation. Int. J. Mol. Sci. 2019;20(8):1963. doi: 10.3390/ijms20081963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu D., Esko J.D. Demystifying heparan sulfate–protein interactions. Annu. Rev. Biochem. 2014;83(1):129–157. doi: 10.1146/annurev-biochem-060713-035314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stickens D. Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development. 2005;132(22):5055–5068. doi: 10.1242/dev.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Weelden G., Bobiński M., Okła K., Van Weelden W.J., Romano A., Pijnenborg J.M.A. Fucoidan structure and activity in relation to anti-cancer mechanisms. Mar. Drugs. 2019;17(1):32. doi: 10.3390/md17010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mabate B., Daub C.D., Malgas S., Edkins A.L., Pletschke B.I. Fucoidan structure and its impact on glucose metabolism: implications for diabetes and cancer therapy. Mar. Drugs. 2021;19(1):30. doi: 10.3390/md19010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin Y., Qi X., Liu H., Xue K., Xu S., Tian Z. The anti-cancer effects of fucoidan: a review of both in vivo and in vitro investigations. Cancer Cell Int. 2020;20(1):154. doi: 10.1186/s12935-020-01233-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alekseyenko T.V., Zhanayeva S. Ya, Venediktova A.A., Zvyagintseva T.N., Kuznetsova T.A., Besednova N.N., Korolenko T.A. Antitumor and antimetastatic activity of fucoidan, a sulfated polysaccharide isolated from the Okhotsk sea Fucus evanescens brown alga. Bull. Exp. Biol. Med. 2007;143(6):730–732. doi: 10.1007/s10517-007-0226-4. [DOI] [PubMed] [Google Scholar]
- 83.Kim E.J., Park S.Y., Lee J.-Y., Park J.H.Y. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010;10(1):96. doi: 10.1186/1471-230X-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang T.-H., Chiu Y.-H., Chan Y.-L., Chiu Y.-H., Wang H., Huang K.-C., Li T.-L., Hsu K.-H., Wu C.-J. Prophylactic administration of fucoidan represses cancer metastasis by inhibiting vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in lewis tumor-bearing mice. Mar. Drugs. 2015;13(4):1882–1900. doi: 10.3390/md13041882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Atashrazm F., Lowenthal R.M., Woods G.M., Holloway A.F., Karpiniec S.S., Dickinson J.L. Fucoidan suppresses the growth of human acute promyelocytic leukemia cells in vitro and in vivo: fucoidan and acute promyelocytic leukemia. J. Cell. Physiol. 2016;231(3):688–697. doi: 10.1002/jcp.25119. [DOI] [PubMed] [Google Scholar]
- 86.Calvo G.H., Cosenza V.A., Sáenz D.A., Navarro D.A., Stortz C.A., Céspedes M.A., Mamone L.A., Casas A.G., Di Venosa G.M. Disaccharides obtained from carrageenans as potential antitumor agents. Sci. Rep. 2019;9(1):6654. doi: 10.1038/s41598-019-43238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu M.-K., Lin T.-Y., Hu C.-H., Chao C.-H., Chang C.-C., Hsu H.-Y. Characterization of a sulfated galactoglucan from Antrodia cinnamomea and its anticancer mechanism via TGFβ/FAK/Slug axis suppression. Carbohydr. Polym. 2017;167:229–239. doi: 10.1016/j.carbpol.2017.02.104. [DOI] [PubMed] [Google Scholar]
- 88.Lu J., He R., Sun P., Zhang F., Linhardt R.J., Zhang A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 2020;150:765–774. doi: 10.1016/j.ijbiomac.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 89.Qiu W.-L., Tseng A.-J., Hsu H.-Y., Hsu W.-H., Lin Z.-H., Hua W.-J., Lin T.-Y. Fucoidan increased the sensitivity to gefitinib in lung cancer cells correlates with reduction of TGFβ-mediated Slug expression. Int. J. Biol. Macromol. 2020;153:796–805. doi: 10.1016/j.ijbiomac.2020.03.066. [DOI] [PubMed] [Google Scholar]
- 90.Manlusoc J.K.T., Hsieh C.-L., Hsieh C.-Y., Salac E.S.N., Lee Y.-T., Tsai P.-W. Pharmacologic application potentials of sulfated polysaccharide from marine algae. Polymers. 2019;11(7):1163. doi: 10.3390/polym11071163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mulloy B., Hogwood J., Gray E., Lever R., Page C.P. Pharmacology of heparin and related drugs. Pharmacol. Rev. 2016;68(1):76–141. doi: 10.1124/pr.115.011247. [DOI] [PubMed] [Google Scholar]
- 92.Abdelhedi O., Nasri R., Souissi N., Nasri M., Jridi M. Sulfated polysaccharides from common smooth hound: extraction and assessment of anti-ACE, antioxidant and antibacterial activities. Carbohydr. Polym. 2016;152:605–614. doi: 10.1016/j.carbpol.2016.07.048. [DOI] [PubMed] [Google Scholar]
- 93.Mycroft-West C.J., Su D., Pagani I., Rudd T.R., Elli S., Guimond S.E., Miller G., Meneghetti M.C.Z., Nader H.B., Li Y., Nunes Q.M., Procter P., Mancini N., Clementi M., Bisio A., Forsyth N.R., Turnbull J.E., Guerrini M., Fernig D.G.…Skidmore M.A. Heparin inhibits cellular invasion by SARS-CoV-2: Structural dependence of the interaction of the surface protein (spike) S1 receptor binding domain with heparin [Preprint] Biochemistry. 2020 doi: 10.1101/2020.04.28.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.