Abstract
From late 2019, whole world has been facing COVID-19 pandemic which is caused by SARS-CoV-2 virus. This virus primarily attacks the respiratory tract and enter host cell by binding with angiotensin 2 converting enzyme receptors present on alveoli of the lungs. Despite its binding in the lungs, many patients have reported gastrointestinal symptoms and indeed, RNA of the virus have been found in faecal sample of patients. This observation gave a clue of the involvement of gut-lung axis in this disease development and progression. From several studies reported in past two years, intestinal microbiome has shown to have bidirectional link with lungs i.e., gut dysbiosis increases the tendency of infection with COVID-19 and coronavirus can also cause perturbations in intestinal microbial composition. Thus, in this review we have tried to figure out the mechanisms by which disturbances in the gut composition can increase the susceptibility to COVID-19. Understanding these mechanisms can play a crucial role in decreasing the disease outcomes by manipulating the gut microbiome using prebiotics, probiotics, or combination of two. Even, faecal microbiota transplantation can also show better results, but intensive clinical trials need to be done first.
Keywords: COVID-19, Coronavirus, Gut lung axis, ACE2, Gut microbiome
Abbreviations
- ACE2
Angiotensin converting enzyme 2
- APC
Antigen presenting cells
- AMP
Anti-microbial peptides
- AhR
Aryl hydrocarbon receptor
- CoV
Coronavirus
- COVID-19
Coronavirus disease 2019
- GM
Gut-microbiome
- IM
Intestinal microbiota
- PAMP
Pathogen associated molecular patterns
- PRR
Pathogen recognition receptor
- RAS
Renin Angiotensin system
- Trp
Tryptophan
1. Introduction
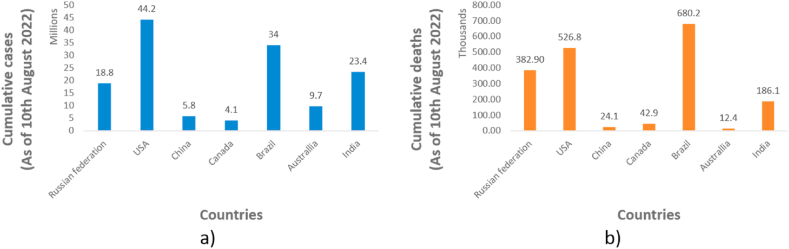
From the past two decades, three serious respiratory tract infections caused by coronaviruses have been emerged in humans. It includes a) Severs acute respiratory syndrome (SARS) caused by SARS-CoV-1, emerged in 2002, b) Middle east respiratory syndrome (MERS) caused by MERS-CoV, emerged in 2012, and c) coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2, emerged in 2019 and has affected millions of life all over the globe and is still affecting [1]. The COVID-19 pandemic spreads all over the globe with its first case identified in Hubei province of China in late 2019 [2]. On March 11, 2020, World Health organisation has declared it as a pandemic [3]. As of August 19, 2022, 591, 683, 619 cases have been confirmed all over the world including 6,443,306 deaths. The confirmed cumulative cases and confirmed deaths as of August 10, 2022 in seven largest countries of the world have been depicted in Fig. 1 [4].
Fig. 1.
a) Cumulative cases and b) cumulative deaths (in the seven largest countries by area) due to COVID-19 as of August 10, 2022.
Interestingly, it has been observed that several cases remained asymptomatic whereas, millions of deaths have also been reported by the same virus. Thus, its aetiology seems to be very complex. It effects mainly the respiratory tract of the human body but several side effects related to gastrointestinal tract like diarrhoea, vomiting etc were also observed in many cases [5,6]. The Gastrointestinal symptoms which appeared in several patients have given a hint regarding the involvement of Gut-lung axis in the disease development and progression. Moreover, increased complications have been observed in either aged patients or the patients with associated co-morbidities like hypertension, diabetes, metabolic, renal, or cardiovascular diseases, or both [[7], [8], [9], [10], [11], [12]]. This observation gave the hint for the role of gut microbiome (GM) in the disease aetiology. This is due to the fact that decrease microbial diversity and abundance have been observed in these patients. Therefore, properly understanding the gut lung axis would help in deciphering the role of GM in the disease development/progression and the role of lung infections in altering the GM towards development of inflammatory cascade [1].
It has been noted that aged patients have decreased abundance of various symbiotic strains of the bacteria (like Lactobacillus and Bifidobacterium) in gut. In addition, these patients also possess lesser number of beneficial bacterial metabolites like butyrate in their gut [13]. Moreover, the patients with chronic inflammation in gastrointestinal tract have higher tendency to develop cytokine storm if exposed to coronavirus [14]. All these evidences clearly linked the involvement of GM in development or the progression of COVID-19. Hence, simultaneous treatment with prebiotics or probiotics can help in correcting gut dysbiosis which would eventually leads to the fast recovery of the patients. Therefore, understanding these mechanisms properly would help in designing the treatment strategy with better results. Therefore, in this review, we have tried to provide evidences linking the GM with COVID-19.
2. Mechanism of action of COVID-19
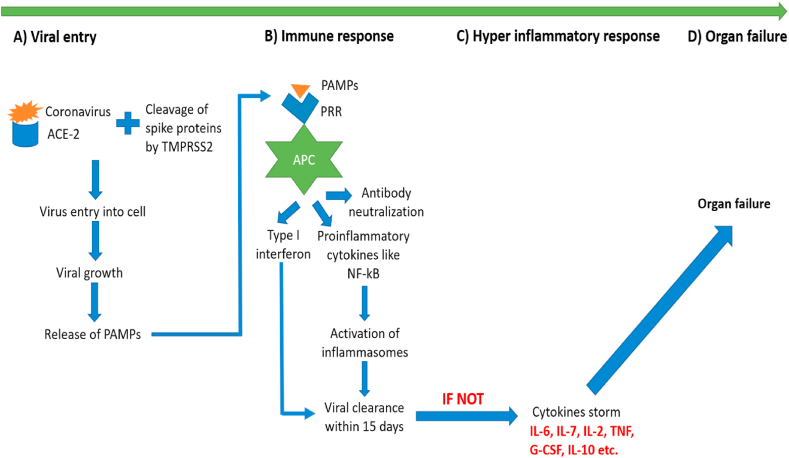
Coronavirus majorly attacks lungs, but in the critically ill patients it attacks many other organs of the body. It attacks in four different phases a) viral entry, b) host immune response, c) hyperinflammatory response, and finally d) organ failure. In most of the patients, the virus got cleared in first two stages, but in immunocompromised, aged or patients with co-morbidities, the attack got exaggerated and all the four stages were seen [[15], [16], [17]].
2.1. Viral entry
Initial entry of the CoV takes place by binding with the angiotensin converting enzyme-2 (ACE2) receptors present in the alveoli of the lungs [[18], [19], [20]]. To understand the role of ACE2, it is very important to first understand the Renin angiotensin system (RAS). The role of RAS has been important in explaining the development and progression of COVID-19 infection. Angiotensinogen is a peptide which is majorly produced in the liver and then distributed in the whole body. The increase in the level of this peptide triggers the release of renin from the kidneys. Renin eventually converts this peptide into angiotensin 1. This decapeptide, angiotensin I is further cleaved by ACE2 to the octapeptide i.e., angiotensin II. This octapeptide finally acts on angiotensin 1 and 2 receptors (AT1R and AT2R) present in different organs and exerts different pharmacological actions. Binding of angiotensin II to AT1R receptor leads to the pro-oxidant, vasoconstrictive, pro-inflammatory, pro-hypertensive and pro-fibrotic actions, whereas its binding on AT2R leads to opposite effects as that of AT1R activation [21].
Coronavirus have shown to bind to ACE2 enzyme and this in turns leads to decreased formation of angiotensin II and increased concentration of angiotensin I in the body [22]. ACE2 enzyme is majorly present in respiratory tract but it is also present in other tissues like gut, liver, heart, kidneys etc [18]. In the alveolar cells of the lungs, coronavirus bind to the ACE2 receptors and the presence of Transmembrane serine protease 2 (TMPRSS2) enzyme causes degradation of spike protein present on the surface of CoV, which in turns help in entry of the virus inside the alveolar cells. This same mechanism is observed for all the three coronaviruses [[23], [24], [25]].
2.2. Host immune response
As soon as the virus enters the cell, it started causing degradation of human alveolar cells. This in turns causes release of pathogen associated molecular patterns (PAMP) which bind to the pattern recognition receptors (PRR), like toll like receptors or nucleotide binding receptors present on the alveolar macrophages that act as antigen presenting cells (APC) [26]. This recognition helps in fighting the infection by stimulating several pathways i.e., a) causes release of type 1 interferons (IFN) [27,28], b) releases various proinflammatory cytokines by stimulating NF-kB signalling and [29], c) production of antibodies against the infection [30]. All these combinations of innate and adaptive immunity help the host to protect itself from the external virus molecule within 15 days.
2.3. Hyperinflammatory response
If the acute inflammatory response is not controlled, it will start harming the body by causing the excess release of pro-inflammatory cytokines which is referred to as a cytokine storm [29,31,32]. Most important inflammatory mediators are IL-2, IL-10, IL-6, IL-17, granulocyte colony stimulating factor, IFN etc. T helper cells (Th1 and Th2) also plays an important role in this cytokine storm [31,33].
2.4. Organ failure
If this inflammatory cascade of cytokine storm is not controlled, it eventually leads to the spread of infection to the whole body and in turns finally causes multiple organ failure [17]. This complete mechanism has been depicted in Fig. 2.
Fig. 2.
Mechanism of attack of coronavirus in the human body.
3. Gut microbiota (GM) and gut-lung axis
GM also called as intestinal microbiota (IM), or gut microbiome comprise of trillions of micro-organisms which reside in the gastrointestinal tract [34,35]. It starts developing after the birth and takes almost two years to become stable in composition and abundance. It comprised of more than 1000 different species of the bacteria, of which 90% of the species belongs to four phyla i.e., Bacteroidetes, firmicutes, actinobacteria and proteobacteria [36].
The GM interacts with the lungs through gut-lung axis. Accumulating evidences highlights the importance of gut-lung axis as a bidirectional link between the gut dysbiosis and inflammatory conditions in the lungs [37]. The perturbations in the gut composition not only causes inflammatory cascade in intestine [38] but also in non-intestinal organs like lungs, liver, brain, skin etc [39]. As here we are talking about gut lung axis, the recent studies have shown the involvement of dysbiosis in various lungs related diseases like chronic obstructive pulmonary disease, allergy, cystic fibrosis, asthma and many more [40,41]. These inflammatory cascade in non-intestinal organs were mediated by translocation of microbes or microbial metabolites to the lungs because of the disruption of intestinal membrane which was caused by dys-biotic conditions [36,42,43]. This fact was demonstrated by Prasad et al. that the presence of gut permeability marker i.e., fatty acid binding protein-2, gut microbial antigens i.e., peptidoglycan and lipopolysaccharides were drastically increased in patients suffering from COVID-19 as compared to healthy subjects [44]. Furthermore, the microbes present in the lungs itself got dysbalanced in case of chronic lung cancer despite of gut microbes dys-balance [45,46]. Thus, it has been noted that perturbations in microbial community of intestinal can lead to disturbances in lungs and vice versa [36].
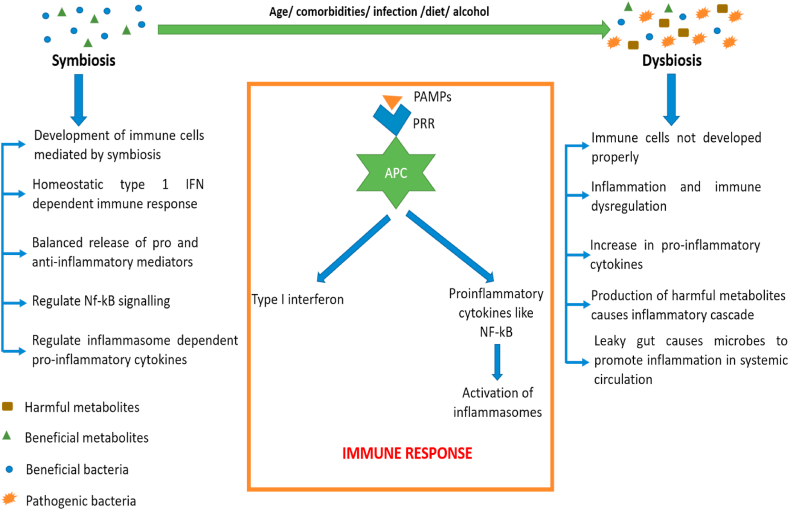
3.1. Role of GM in immune response
The research in past two decades have outlined the importance of GM in modulating the immune response in the human body both locally and systemically. It is not only involved in proper development but also in proper maturation and its functioning of the immune system [47]. Indeed, many researchers have concluded that perturbations in gut microbiome through diet, drugs, alcohol, diseases etc ultimately leads to hampering of the proper functioning of the immune system [48]. This eventually leads to excess release of cytokines both locally and systemically, if not controlled [49]. All these effects on immune system were exerted by the proper balance of bacterial metabolites like short chain fatty acids (SCFAs), Trp, bile acids etc [50]. Furthermore, a decrease in the concentration of SCFAs producing bacteria have been reported in the patients suffering from COVID-19 [[51], [52], [53]].
GM have shown to have impact on cells of both innate and adaptive immune system. Several commensal bacteria like Bifidobacterium infantis, Bacteroides fragilis and Clostridium cluster have demonstrated to play an important role in proper development of T regulatory cells, which is involved in the production of potent anti-inflammatory molecule i.e., IL-10 [54]. The balance between T regulatory and T helper 17 cells is very crucial for preventing the inflammatory response in the body. This balance got disturbed once the level of commensal bacterial species decreases and pathogenic bacterial species increases [54,55]. Furthermore, Lopez P et al., have demonstrated the ability of four Bifidobacterium species in the proper development of dendritic cells which is one of the most important APC. Hence, this study gave a prominent hint of role of GM in proper development of innate immune cells [56]. Moreover, administration of Bifidobacterium lactis to the healthy volunteers have shown drastic increase in the levels of NK cells, mononuclear leukocytes, macrophages etc [57].
The interactions of immune system in symbiotic and dys-biotic conditions were illustrated in Fig. 3.
Fig. 3.
Interactions of immune system in symbiotic and dys-biotic conditions.
3.2. Bidirectional link of ACE2 and GM
It has been observed that patients who were hospitalized due to COVID-19 have shown to have reduced diversity and abundance of microbes in the gut. Moreover, it has been observed that the beneficial probiotic strains like Lactobacillus and Bifidobacterium have been reduced whereas, pathogenic strains increased [53,58,59]. These studies have given a clue that there is an involvement of GM in either the disease development/progression or the GM dysbiosis occurs due to COVID-19. Hence, there is a need to understand the causal effect relationship between gut dysbiosis and COVID-19 progression [60].
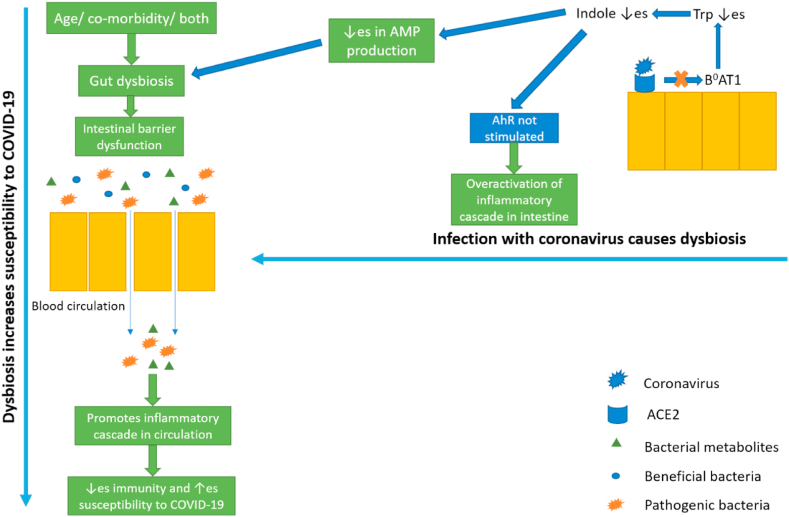
Presence of ACE2 receptors in the gut might seems to be an important mediator between GM and COVID-19. It has been noted that ACE2 receptors are present in the gut which act as chaperone for amino acid transporter i.e., B0AT1 which transports neutral amino acids across the gut mainly glutamine and tryptophan (Trp) [[61], [62], [63]]. Furthermore, a study by Hashimoto et al. have shown that there is a decreased amino acids levels observed in the serum, specifically Trp in ACE2 knock out animals. This decreased level of Trp was accompanied with gut dysbiosis and reduction in the level of antimicrobial peptides. Moreover, the beneficial effects of Trp in these animals were corrected by administering the Trp directly [64]. The role of Trp in modulating the diversity and abundance of GM have been explained in various studies. All the protective effects were exerted due to the production of its metabolite i.e., indole [65,66]. Moreover, experimental studies have shown that gain of function of ACE2 leads to improvement in gut barrier whereas, loss of function of ACE2 leads to further weaking of gut barrier [64,67]. Furthermore, a study by Andring et al. have also shown the role of B0AT1 in COVID-19 [62]. Indeed, a study by Stevens et al. have confirmed the role of B0AT1 in COVID-19 b y exposing the lyophilized enterocyte brush border membrane vesicles to increased doses of high-energy electron radiation. This indicated the interaction of B0AT1 with ACE2 receptors [68]. These results explain that how the decrease in the concentration of ACE2 would leads to perturbations in the gut microbial diversity [63]. Therefore, this helps us in explaining how the COVID-19 infection would leads to the gut dysbiosis.
Now comes the question, does gut dysbiosis leads to increase in susceptibility and progression of COVID-19 infection? The answer to this seems to be yes. As explained in section 3.1, that GM plays a very critical role in the development and functioning of immune system, thus with overacted immune system there is a very high probability that the patients would suffer from COVID-19 and they also develop cytokine storm once infected with the virus [14,69,70]. Thus, there is a bidirectional link between COVID-19 development and gut dysbiosis. This mechanism has been illustrated in Fig. 4.
Fig. 4.
Bidirectional link of COVID-19 infection and gut dysbiosis (AMP: Antimicrobial peptide, AhR: Aryl hydrocarbon receptor, Trp: Tryptophan.
3.3. Role of co-morbidities in COVID-19 progression
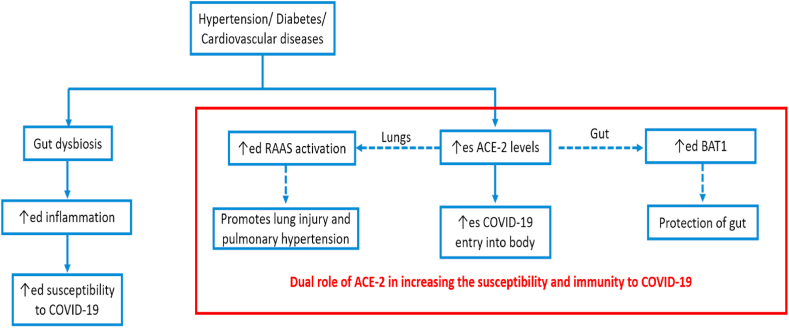
Accumulating data have shown that aged people with pre-existing lung, metabolic, renal, cardiovascular diseases [8] or diabetes [10,12,71,72] are at much higher risk of suffering from severe complications from COVID-19 infection [73,74]. Moreover, the aged persons with co-morbidities have more need for intensive care with high mortality [75,76]. Thus, these studies point out that something is common in between the elder age patients and persons with co-morbidities. Two hypotheses for this have been pointed out in literature, a) dys-biotic gut environment, and b) increased expression of ACE2 in these persons. So, we will explain these two one by one.
Firstly, an altered gut environment has been observed in both elderly patients and the patients suffering for co-morbidities. Due to this dys-biotic condition, the intestine and systemic circulation is more prone towards inflammatory responses, which in turns causes prolonged cytokine storm and finally complicating the conditions of patients suffering from COVID-19. Interestingly, lower abundance of Bacteroides species have been found in persons with co-morbidities and COVID-19 patients [1,[77], [78], [79]].
One more hypothesis have been proposed by Fang et al. related to increased complications in patients suffering from diabetes or hypertension and COVID-19. Mostly, the patients suffering from hypertension were treated with either ACE2 inhibitors or angiotensin II type 1 receptor blockers, which in turns up regulates the ACE2 receptors in the body by the negative feedback mechanism. Therefore, with increased concentration of ACE2, there is increased chances of entry of COVID-19 in this body. Furthermore, the use of thiazolidinediones and ibuprofen in the diabetes also leads to up-regulation of ACE2 receptors [77]. Moreover, a study by Jing Li et al. have shown that increase in expression of ACE2 expression in gut leads to more susceptibility to COVID-19 in hypertensive rats [80]. Hence, increasing the ACE2 concentration in the lungs increase the susceptibility to COVID-19, whereas increase concentration of ACE2 in the gut promotes the symbiotic condition and thus decreases susceptibility to COVID-19 [72].
These two hypotheses are depicting the dual role of ACE2 receptors in both increased and increased susceptibility to the COVID-19 infection. These hypotheses are shown in Fig. 5. Hence, a lot more research is needed to confirm these findings and come to some conclusions.
Fig. 5.
Probable mechanisms of increase in COVID-19 susceptibility due to presence of associated co-morbidities.
4. Conclusions and future directions
From the past two years a lot of researchers have been exploring the role of gut microbiome in the development and progression of COVID-19 in patients. These researches have given strong evidences of the involvement of gut dysbiosis in increased susceptibility to the disease. Moreover, aged patients with associated co-morbidities are most vulnerable to this disease. The most probable reason for this is gut dysbiosis which have been observed in patients suffering from hypertension, diabetes, cardiovascular diseases etc. But, one more hypothesis have also been reported which is stated above. Indeed, these clinical features have given an alternative way to help people in decreasing both the incidence and complications from this disease. It is advised to keep the gut microbiomes in a balance by taking proper diet, doing exercises, administering probiotics, prebiotics and symbiotics. These small lifestyle changes can help in balancing the gut environment which in turns leads to proper development and functioning of host immunity and eventually decreasing the probability of cytokine storm in the patients suffering from COVID-19.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Tanya Ralli was supported by Department of Sciecne and Technology, New Delhi (IF180797).
Data availability statement
No data was used for the research described in the article.
Declaration of interest's statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
No additional information is available for this paper.
References
- 1.van der Lelie D., Taghavi S.J.M. COVID-19 and the gut microbiome: more than a gut feeling. mSystems. 2020;5(4) doi: 10.1128/mSystems.00453-20. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhar D., Mohanty A. Gut microbiota and Covid-19-possible link and implications. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rishi P., Thakur K., Vij S., Rishi L., Singh A., Kaur I.P., et al. Diet, gut microbiota and COVID-19. Indian J. Microbiol. 2020;60(4):420–429. doi: 10.1007/s12088-020-00908-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh S., Borrós S. Mucoadhesion vs mucus permeability of thiolated chitosan polymers and their resulting nanoparticles using a quartz crystal microbalance with dissipation (QCM-D) Colloids Surf., B. 2016;147:434–441. doi: 10.1016/j.colsurfb.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 5.Din A.U., Mazhar M., Waseem M., Ahmad W., Bibi A., Hassan A., et al. SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han C., Duan C., Zhang S., Spiegel B., Shi H., Wang W., et al. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am. J. Gastroenterol. 2020;115(6):916–923. doi: 10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dey J.K., Dey S.K.J. Computational, Biology S. SARS-CoV-2 pandemic, COVID-19 case fatality rates and deaths per million population in India. J. Bio. Comput. Systems Bio. 2020;2(1):110. JoB. [Google Scholar]
- 8.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du R.-H., Liang L.-R., Yang C.-Q., Wang W., Cao T.-Z., Li M., et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur. Respir. J. 2020;55(5) doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roncon L., Zuin M., Rigatelli G., Zuliani Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104354. oCV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siordia Epidemiology and clinical features of COVID-19: a review of current literature. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104357. JAJJoCV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma R.K., Stevens B.R., Obukhov A.G., Grant M.B., Oudit G.Y., Li Q., et al. ACE2 (Angiotensin-Converting Enzyme 2) in cardiopulmonary diseases: ramifications for the control of SARS-CoV-2. Hypertension. 2020;76(3):651–661. doi: 10.1161/HYPERTENSIONAHA.120.15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangiola F., Nicoletti A., Gasbarrini A., Ponziani F.J.E. Gut microbiota and aging. Science. 2018;22(21):7404–7413. doi: 10.26355/eurrev_201811_16280. RMPS. [DOI] [PubMed] [Google Scholar]
- 14.Luthra-Guptasarma M., Guptasarma P.J.R.G. Inflammation begets hyper-inflammation in: diet-derived chronic inflammation promotes runaway acute inflammation resulting in cytokine storms. Res Gate. 2020;10 [Google Scholar]
- 15.Seyed Hosseini E., Riahi Kashani N., Nikzad H., Azadbakht J., Hassani Bafrani H., Haddad Kashani H. The novel coronavirus Disease-2019 (COVID-19): mechanism of action, detection and recent therapeutic strategies. Virology. 2020;551:1–9. doi: 10.1016/j.virol.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boopathi S., Poma A.B., Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J. Biomol. Struct. Dynam. 2021;39(9):3409–3418. doi: 10.1080/07391102.2020.1758788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohn M.K., Hall A., Sepiashvili L., Jung B., Steele S., Adeli K.J.P. Pathophysiology of COVID-19: mechanisms underlying disease severity and progression. Physiology. 2020;35(5):288–301. doi: 10.1152/physiol.00019.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M.-Y., Li L., Zhang Y., Wang X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infectious Diseases of Poverty. 2020;9(1):45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azushima K., Morisawa N., Tamura K., Nishiyama AJChr. Recent research advances in renin-angiotensin-aldosterone system receptors. Curr. Hypertens. Rep. 2020;22(3):1–10. doi: 10.1007/s11906-020-1028-6. [DOI] [PubMed] [Google Scholar]
- 22.Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S., et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microb. Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271. doi: 10.1016/j.cell.2020.02.052. 80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. USA. 2009;106(14):5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertram S., Glowacka I., Müller M.A., Lavender H., Gnirss K., Nehlmeier I., et al. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J. Virol. 2011;85(24):13363–13372. doi: 10.1128/JVI.05300-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivanov I.I., Honda KJCh, Microbe Intestinal commensal microbes as immune modulators. Cell Host Microbe. 2012;12(4):496–508. doi: 10.1016/j.chom.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viana S.D., Nunes S., Reis FJArr. ACE2 imbalance as a key player for the poor outcomes in COVID-19 patients with age-related comorbidities–role of gut microbiota dysbiosis. Ageing Res. Rev. 2020;62 doi: 10.1016/j.arr.2020.101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy D.E., Marié I.J., Jejcoiv Durbin. Induction and function of type I and III interferon in response to viral infection. Current opinion in virology. 2011;1(6):476–486. doi: 10.1016/j.coviro.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haidar M.K., Demirbolat G.M., Timur S.S., Gürsoy R.N., Nemutlu E., Ulubayram K., et al. Atorvastatin-loaded nanosprayed chitosan nanoparticles for peripheral nerve injury. Bioinspired, Biomimetic Nanobiomaterials. 2020;9:74–84. [Google Scholar]
- 30.Bohn M.K., Lippi G., Horvath A., Sethi S., Koch D., Ferrari M., et al. Molecular, serological, and biochemical diagnosis and monitoring of COVID-19: IFCC taskforce evaluation of the latest evidence. Clin. Chem. Lab. Med. 2020;58(7):1037–1052. doi: 10.1515/cclm-2020-0722. [DOI] [PubMed] [Google Scholar]
- 31.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye Q., Wang B., Mao JJJoi. The pathogenesis and treatment of theCytokine Storm'in COVID-19. J. Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ralli T., Neupane Y.R., Saifi Z., Kohli K.J.C.P.D. Gut microbiota as an emerging therapeutic avenue for the treatment of nonalcoholic fatty liver disease. Curr. Pharmaceut. Des. 2021;27(46):4677–4685. doi: 10.2174/1389201022666210625141526. [DOI] [PubMed] [Google Scholar]
- 35.Ralli T., Saifi Z., Tyagi N., Vidyadhari A., Aeri V., Kohli K.J.C. Deciphering the role of gut metabolites in non-alcoholic fatty liver disease. Crit. Rev. Microbiol. 2022:1–19. doi: 10.1080/1040841X.2022.2142091. RiM. [DOI] [PubMed] [Google Scholar]
- 36.Bingula R., Filaire M., Radosevic-Robin N., Bey M., Berthon J.-Y., Bernalier-Donadille A., et al. 2017. Desired Turbulence? Gut-Lung axis, Immunity, and Lung Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keely S., Talley N.J., Hansbro PMJMi. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2012;5(1):7–18. doi: 10.1038/mi.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rapozo D.C., Bernardazzi C., de Souza Hspjwjog Diet and microbiota in inflammatory bowel disease. The gut in disharmony. 2017;23(12):2124. doi: 10.3748/wjg.v23.i12.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carding S., Verbeke K., Vipond D.T., Corfe B.M., Owen L.J.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015;26(1) doi: 10.3402/mehd.v26.26191. Meih, disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rutten E.P., Lenaerts K., Buurman W.A., Wouters E.F.J.C. Disturbed intestinal integrity in patients with COPD: effects of activities of daily living. Chest. 2014;145(2):245–252. doi: 10.1378/chest.13-0584. [DOI] [PubMed] [Google Scholar]
- 41.Anand S., Mande S.S.J. Diet, microbiota and gut-lung connection. Front. Microbiol. 2018;9:2147. doi: 10.3389/fmicb.2018.02147. Fim. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsland B.J., Trompette A., Gollwitzer E.S.J. The gut–lung axis in respiratory disease. Annals of the American Thoracic Society. 2015;12(Supplement 2):S150–S156. doi: 10.1513/AnnalsATS.201503-133AW. AotATS. [DOI] [PubMed] [Google Scholar]
- 43.Schuijt T.J., Lankelma J.M., Scicluna B.P., e Melo FdS., Roelofs J.J., de Boer J.D., et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65(4):575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prasad R., Patton M.J., Floyd J.L., Fortmann S., DuPont M., Harbour A., et al. Plasma microbiome in COVID-19 subjects: an indicator of gut barrier defects and dysbiosis. Int. J. Mol. Sci. 2022;23(16):9141. doi: 10.3390/ijms23169141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H., Liu J.-S., Peng S.-H., Deng X.-Y., Zhu D.-M., Javidiparsijani S., et al. Gut-lung crosstalk in pulmonary involvement with inflammatory bowel diseases. World J. Gastroenterol. 2013;19(40):6794. doi: 10.3748/wjg.v19.i40.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jess T., Horváth-Puhó E., Fallingborg J., Rasmussen H.H., Jacobsen B.A.J. Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: a Danish population-based cohort study. Official J. Am. College of Gastroenterology| ACG. 2013;108(12):1869–1876. doi: 10.1038/ajg.2013.249. OjotACoG, ACG. [DOI] [PubMed] [Google Scholar]
- 47.Samuelson D.R., Welsh D.A., Jejfim Shellito. Regulation of lung immunity and host defense by the intestinal microbiota. Front. Microbiol. 2015;6:1085. doi: 10.3389/fmicb.2015.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen I.-Y., Ichinohe TJTim. Response of host inflammasomes to viral infection. Trends Microbiol. 2015;23 1:55–63. doi: 10.1016/j.tim.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Dang A.T., Marsland BJJMi. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 2019;12(4):843–850. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- 50.Vinolo M.A., Rodrigues H.G., Nachbar R.T., Curi R.J.N. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3(10):858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeoh Y.K., Zuo T., Lui G.C.-Y., Zhang F., Liu Q., Li A.Y.L., et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuo T., Liu Q., Zhang F., Lui G.C.-Y., Tso E.Y., Yeoh Y.K., et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2021;70(2):276–284. doi: 10.1136/gutjnl-2020-322294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zuo T., Zhang F., Lui G.C.Y., Yeoh Y.K., Li A.Y.L., Zhan H., et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944. doi: 10.1053/j.gastro.2020.05.048. 55.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamada N., Seo S.-U., Chen G.Y., Núñez G.J.N.R.I. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013;13(5):321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 55.Chu H., Mazmanian S.K. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat. Immunol. 2013;14(7):668–675. doi: 10.1038/ni.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.López P., Gueimonde M., Margolles A., Suárez AJIjofm. Distinct Bifidobacterium strains drive different immune responses in vitro. Int. J. Food Microbiol. 2010;138(1–2):157–165. doi: 10.1016/j.ijfoodmicro.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 57.Gill H.S., Rutherfurd K.J., Cross M.L., Gopal P.K.J. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am. J. Clin. Nutr. 2001;74(6):833–839. doi: 10.1093/ajcn/74.6.833. TAjocn. [DOI] [PubMed] [Google Scholar]
- 58.Gu S., Chen Y., Wu Z., Chen Y., Gao H., Lv L., et al. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin. Infect. Dis. 2020;71(10):2669–2678. doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu K., Cai H., Shen Y., Ni Q., Chen Y., Hu S., et al. Management of COVID-19: the zhejiang experience. J. Zhejiang Univ. Med. Sci. 2020;49(2):147–157. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He Y., Wang J., Li F., Shi YJFiM. Main clinical features of COVID-19 and potential prognostic and therapeutic value of the microbiota in SARS-CoV-2 infections. Front. Microbiol. 2020;11:1302. doi: 10.3389/fmicb.2020.01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Camargo S.M., Singer D., Makrides V., Huggel K., Pos K.M., Wagner C.A., et al. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology. 2009;136(3):872–882. e3. doi: 10.1053/j.gastro.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andring J.T., McKenna R., Stevens B.R.J.B. 2020. Amino Acid Transporter B0AT1 Influence on ADAM17 Interactions with SARS-CoV-2 Receptor ACE2 Putatively Expressed in Intestine, Kidney, and Cardiomyocytes. [Google Scholar]
- 63.Stevens B.R.J.B. 2020. TMPRSS2 and ADAM17 Interactions with ACE2 Complexed with SARS-CoV-2 and B0AT1 Putatively in Intestine, Cardiomyocytes, and Kidney. [Google Scholar]
- 64.Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M., et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487(7408):477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scott S.A., Fu J., Chang P.V.J. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA. 2020;117(32):19376–19387. doi: 10.1073/pnas.2000047117. PotNAoS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taleb SJFii. Tryptophan dietary impacts gut barrier and metabolic diseases. Front. Immunol. 2019;10:2113. doi: 10.3389/fimmu.2019.02113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duan Y., Prasad R., Feng D., Beli E., Li Calzi S., Longhini A.L.F., et al. Bone marrow-derived cells restore functional integrity of the gut epithelial and vascular barriers in a model of diabetes and ACE2 deficiency. Circ. Res. 2019;125(11):969–988. doi: 10.1161/CIRCRESAHA.119.315743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stevens B.R., Ellory J.C., Preston R.L.J.F. B0AT1 amino acid transporter complexed with SARS-CoV-2 receptor ACE2 forms a heterodimer functional unit: in situ conformation using radiation inactivation analysis. Function. 2021;2(4):zqab027. doi: 10.1093/function/zqab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jose R.J., Manuel A.J. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir. Med. 2020;8(6):e46–e47. doi: 10.1016/S2213-2600(20)30216-2. TLRM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuster G.M., Pfister O., Burkard T., Zhou Q., Twerenbold R., Haaf P., et al. 2020. SARS-CoV2: Should Inhibitors of the Renin–Angiotensin System Be Withdrawn in Patients with COVID-19? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang X., Fang X., Cai Z., Wu X., Gao X., Min J., et al. 2020. Comorbid Chronic Diseases and Acute Organ Injuries Are Strongly Correlated with Disease Severity and Mortality Among COVID-19 Patients: a Systemic Review and Meta-Analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Obukhov A.G., Stevens B.R., Prasad R., Li Calzi S., Boulton M.E., Raizada M.K., et al. SARS-CoV-2 infections and ACE2: clinical outcomes linked with increased morbidity and mortality in individuals with diabetes. Diabetes. 2020;69(9):1875–1886. doi: 10.2337/dbi20-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guan WJC cocd. Ni ZY, Hu Y, Liang Wh, Ou CQ, He JX, et al. 2019:1708-1720.
- 75.Covid C., Team R., Covid C., Team R., Bialek S., Boundy E., et al. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16. Morb. Mortal. Wkly. Rep. 2020;69(12):343. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cai Q., Chen F., Wang T., Luo F., Liu X., Wu Q., et al. Obesity and COVID-19 severity in a designated hospital in shenzhen, China. Diabetes Care. 2020;43(7):1392–1398. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 79.Hill M.A., Mantzoros C., Sowers J.R. Commentary: COVID-19 in patients with diabetes. Metabolism. 2020;107 doi: 10.1016/j.metabol.2020.154217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li J., Stevens B.R., Richards E.M., Raizada M.K.J.H. SARS-CoV-2 receptor ACE2 (Angiotensin-Converting Enzyme 2) is upregulated in colonic organoids from hypertensive rats. Hypertension. 2020;76(3):e26–e28. doi: 10.1161/HYPERTENSIONAHA.120.15725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.