Abstract
Continuous positive airway pressure (CPAP) is a primary non-invasive mode of respiratory support for preterm infants. However, emerging evidence suggests CPAP could be an underlying contributor to the unintended pathophysiology of wheezing and associated airway hyperreactivity (AHR) in former preterm infants. The therapeutic benefits of mesenchymal stem cells (MSCs) have been demonstrated in a variety of animal models and several clinical trials are currently underway to assess their safety profiles in the setting of prematurity and bronchopulmonary dysplasia (BPD). In the present study, using a mouse model of neonatal CPAP, we investigated whether conditioned medium harvested from cultures of human bone-marrow derived mesenchymal stem cells (hMSC) could rescue the CPAP-induced AHR, based upon previous observations of their anti-AHR properties. Newborn mice (male and female) were fitted with a custom-made mask for delivery of daily CPAP 3 h/day for the first 7 postnatal days. At postnatal day 21 (two weeks after CPAP ended), lungs were removed, precision-cut lung slices were sectioned and incubated for 48 h in vitro in conditioned medium collected from cultures of three different hMSC donors. As expected, CPAP resulted in AHR to methacholine compared to untreated control mice. hMSC conditioned medium from the cultures of all three donors completely reversed AHR. These data reveal potential therapeutic benefits of hMSC therapy, which may be capable of rescuing the long-term adverse effects of neonatal CPAP on human airway function.
Keywords: CPAP, Airway hyperreactivity, Bone marrow, Stem cells, Prematurity
1. Introduction
Supplemental O2 and continuous positive airway pressure (CPAP) comprise the primary modalities of respiratory support for preterm infants with respiratory distress. However, airway hyperreactivity (AHR) associated with wheezing is a significant long-term respiratory morbidity of former preterm infants. While O2 therapy is considered a major contributor to the pathogenesis of wheezing (Greenough, 2006; Reyburn et al., 2012), there is emerging evidence from both clinical and animal studies that also implicates similar adverse effects of neonatal CPAP. The Victorian Infant Collaborative Study Group studied associations between respiratory function in 8 year old infants who were born preterm during 1991–2005 (Doyle et al., 2017) and found that increased use of nasal CPAP in 2005 was associated with worse respiratory function compared to earlier periods. Similarly, using a mouse model, we showed that neonatal CPAP delivered daily to unanesthetized mice during the first postnatal week (independently of hyperoxia) caused long-term AHR weeks after recovery (Mayer et al., 2015, 2021; MacFarlane et al., 2021). Thus, there continues to be a growing demand for therapeutic interventions aimed at mitigating the unfortunate, life-long respiratory morbidities of former preterm infants.
Mesenchymal stem cells (MSCs) are nonhematopoeitic cells found in a variety of both neonatal and adult tissue. Several animal studies have shown therapeutic and regenerative properties of hMSCs (Cereta et al., 2021). Notably, hMSC therapy in rodent models of BPD (Hansmann et al., 2012; Pierro et al., 2013) and asthma (Goldstein et al., 2017) have demonstrated anti-inflammatory properties and reversal of airway remodelling (Bonfield et al., 2010), although there are still lingering clinical concerns about cell delivery to patients which provide clinical benefit (Levy et al., 2020; Wu et al., 2020). While the specific mechanisms associated with the beneficial properties of hMSCs are still being investigated, one commonality in all of the current known mechanisms is the role of paracrine factors (van Heeckeren et al., 2021) and the immune-evasive nature that prevents tissue rejection and engraftment following cell delivery (Ee and Thébaud, 2018). Thus, we harvested the conditioned medium from cultured hMSCs from three different donors to test the hypothesis that paracrine hMSC factors could reverse AHR in vitro in precision cut lung sections isolated from CPAP-exposed mice.
2. Experimental procedures
2.1. Ethical approval
All procedures were carried out in accordance with the National Institute of Health (NIH) guidelines for care and use of laboratory animals and were approved by the Animal Care and Use Committee at Case Western Reserve University.
2.2. General experimental outline
A complete overview of the methodology is provided in Fig. 1, which describes the experimental timeline of CPAP, long-term (2 weeks) recovery period, the precision-cut lung slice (PCLS) method for incubation with bone-marrow derived hMSC conditioned medium and airway responses to bath-applied methacholine. Specific details of each step are provided in subsections below. Time-pregnant mice (C57BL/6 J) were purchased from a commercial vendor (Charles River, Willmington, MA). The day after birth (P1), both male and female mouse pups were randomized to be treated with or without CPAP (in 21% O2) for the first week of postnatal life as described previously (Mayer et al., 2015). Two weeks after CPAP ended, P21 mice were euthanized and the lungs prepared for hMSC conditioned medium incubation and measurements of airway reactivity in PCLS preparation.
Fig. 1.
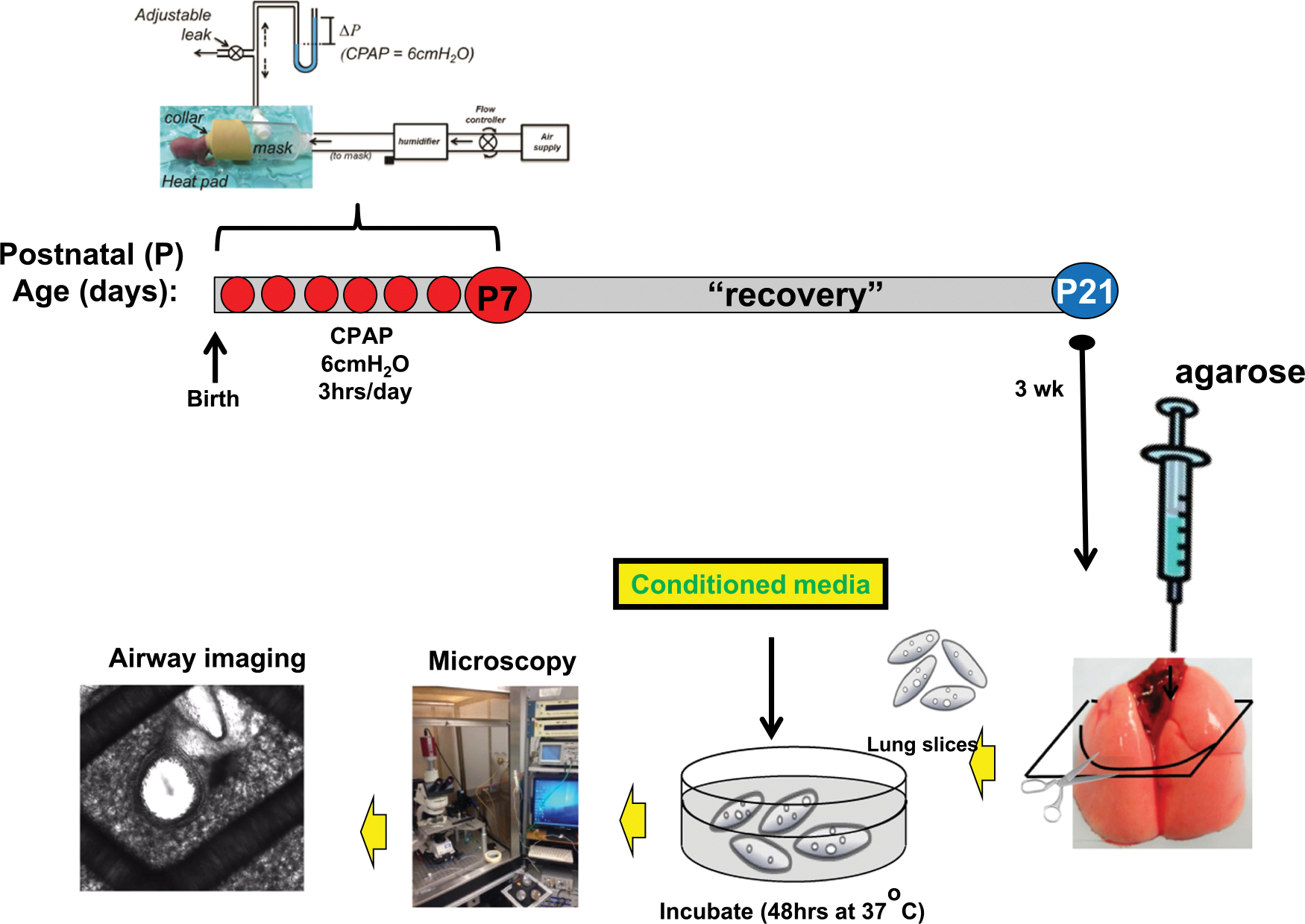
Experimental timeline of CPAP, recovery, preparation of precision lung slices, conditioned medium incubation and imaging of airways for measurements of contractile responses to bath-applied methacholine. CPAP of 6cmH2O, 3 h/day (except day 1, which is limited to 2 h/day) was given for 7 consecutive days (red dots). CPAP was administered via a positive pressure humidified air supply system into a custom-made mask and collar for a neonatal mouse’s head. The level of CPAP was controlled by adjusting a downstream leak and monitored with a manometer. Two weeks after the last bout of CPAP, the mice are sacrificed, lungs inflated with liquid agarose. The lungs are cooled, then sectioned and placed into 6 well plates for incubation with conditioned medium from one of the three different donors. Approximately 48 h after incubation, the lung sections are rinsed with HBSS, placed in a recording chamber and imaged using a microscope to assess changes in airway lumen area in response to increasing doses of bath-applied methacholine.
2.3. Continuous positive airway pressure (CPAP) administration
The method of CPAP administration was performed as described previously (Mayer et al., 2015). Briefly, following the day of birth (P0), the litter (6–8 pups) was divided in half, and both male and female pups were randomly assigned to receive CPAP (6 cmH2O) for 3 h/day for the first 7 postnatal days or control exposure. After each CPAP session, the pups were returned to the dam. To minimize stress from maternal separation following birth, CPAP was administered for 2 h on the first day but was increased to 3 h/session for the following 6 consecutive days (7 days total). Control mice were also separated from the dam, fitted with a CPAP collar but did not receive CPAP (i.e. 0 cmH2O). At the end of the 7 days of CPAP, the mice were allowed an additional 2 weeks of un-interrupted maternal care at which time the pups were prepared for assessment of airway reactivity to methacholine challenge using the in vitro PCLS preparation following 48 h incubation with hMSC conditioned medium.
2.4. Precision-cut lung slice preparation and measurements of AW reactivity
The PCLS method has been described previously (Bergner and Sanderson, 2002; Mayer et al., 2015). Briefly, at P21 (2 weeks after CPAP treatment ended), mice were euthanized via anesthetic overdose (intraperitoneal injection of a ketamine/xylazine mix, 100 mg/kg/10 mg/kg, respectively), and the trachea cannulated (0.58 mm PE tubing, Clay Adams, Sparks, MD) for inflation with liquefied agarose (Invitrogen, Carlsbad, CA; 38 °C). The preparation was then placed en bloc in the refrigerator, allowed to cool and the lungs were then sliced into 300 μm sections using a vibratome (VT1000, Leica Microsystems, Wetzler, Germany). Individual sections were immersed in the same medium that was used to grow the hMSCs, and placed in an incubator for 48 h (5% CO2; 37 °C). Additional sections from the same lungs were also divided into individual wells for incubation with conditioned medium from either of 3 different hMSC donors (see below). After incubation for 48 h, the lung slices were rinsed in HBSS and placed in an in vitro recording chamber for live imaging of airway responses to increasing doses of methacholine. The recording chamber was mounted on a microscope (DMLFS, Leica Microsystems, Wetzler, Germany) and perfused continuously (7 ml/min) with HBSS at room temperature. After locating an airway, images were acquired using a camera (Rolera Fast, QImaging, Surrey, Canada) mounted to the microscope for an initial baseline 3-minute period. A perfusion system connected to increasing doses of methacholine was sequentially perfused into the in vitro system containing the living lung slice. The extent of airway constriction to methacholine (0.25, 0.5, 1, 2, 4, and 8 μM; Sigma Aldrich, St Louis, MO) was determined at the end of a 2 min exposure at each dose. ImageJ analysis software was used to calculate the luminal area (in pixels) and the magnitude of AW constriction was quantified by assessing changes in lumen area from baseline.
2.5. hMSCs and conditioned media
Human posterior iliac crest bone marrow samples were obtained after written informed consent. This protocol was approved by the Case Western Reserve University and University Hospitals Internal Review Board (IRB, #CASE12Z05). Non-GMP Human MSCs (hMSCs) were expanded in ex vivo culture per previously published methods (Lennon and Caplan, 2006), in medium containing DMEM, 5% lot tested and validated fetal bovine serum, and 10% penicillin/streptomycin (100 U/ml). hMSC were subcultured to passage 2 or 3, washed and the medium replenished in antibiotic free conditions for 3-days (72 h), prior to harvesting the conditioned medium.
2.6. Statistical analysis
Statistical comparisons of responses to methacholine between control, CPAP and the different hMSC donors were made using a two-way repeated measure ANOVA (SigmaPlot Software Inc. San Jose, CA).
3. Results
At P21 days of age, airways from mice exposed to prior CPAP during the first postnatal week were more reactive to methacholine compared to untreated control mice, as indicated by the significant decrease in AW lumen area with increasing doses of bath-applied methacholine (Fig. 2A). Incubation with each of the three conditioned medium samples from bone-marrow derived hMSC for 48 h showed reversal of CPAP-induced airway hyperreactivity compared to unincubated growth medium controls. Airway responses to methacholine from control mice (that did not receive CPAP) were not affected by any of the three different donors. Representative images of airways and lumen area from control (ctrl, n = 4 mice, 9 airways), CPAP (n = 4 mice, 10 airways), ctrl with conditioned medium (Ctrl+M, n = 9 mice, 26 airways, grouped data from the three different donors) and CPAP with each of the three donors (CPAP+2200, 2216, and 2222, n = 8, 10, and 8 airways respectively) are also provided for baseline and maximum dose of methacholine exposure (Fig. 2B).
Fig. 2.
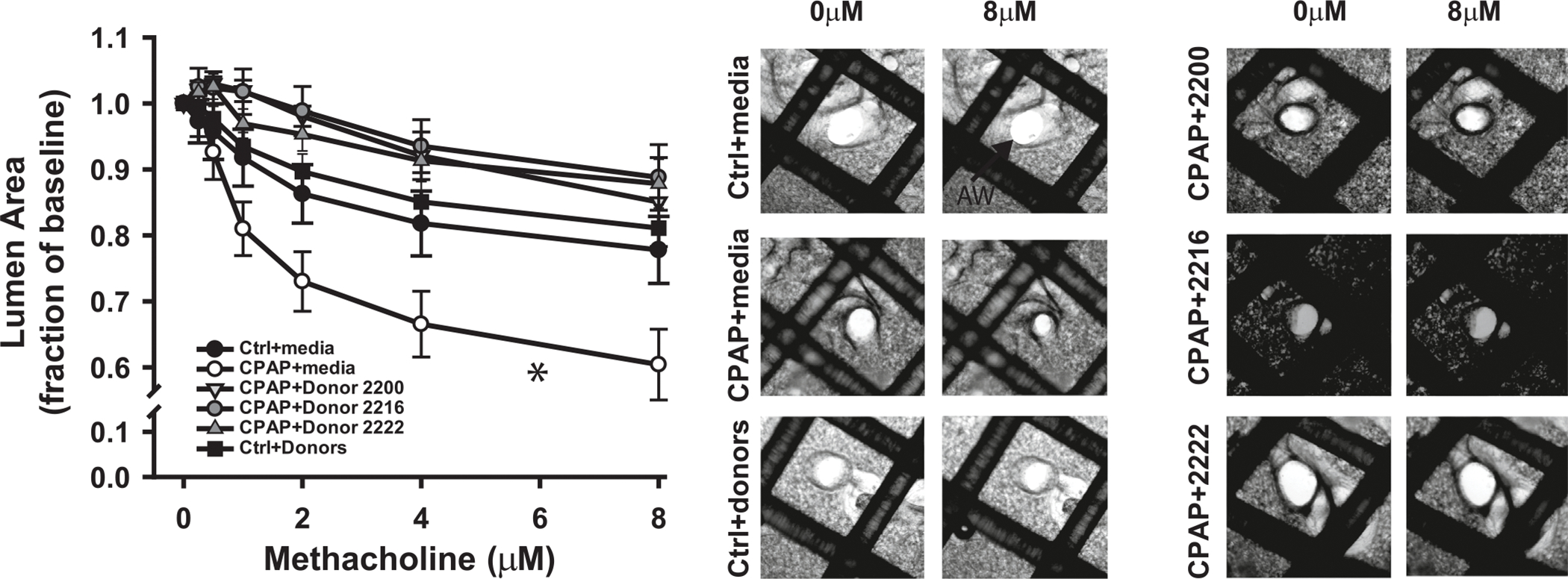
Effects of hMSC conditioned medium treatment on AHR to methacholine challenge in 21 day old mice with or without prior CPAP (7 days) treatment (A). AHR was assessed using the precision-cut lung slice method by determining the changes in airway lumen size in response to increasing doses of methacholine. The smaller lumen size at increasing methacholine concentrations signifies increased AW reactivity. Note, AHR following CPAP (open circles) was reversed by treatment with conditioned medium from three different hMSC donors (Donor 2200, 2216, and 2222). Representative images for each treatment group at baseline (0 μM) and the highest (8 μM) dose of methacholine are also provided (B). *indicates the slope of the response was significantly different from the slope of control (ctrl, no CPAP) mice (*p < 0.05).
4. Discussion
The results of the current study demonstrated that long-term increased airway reactivity following prior neonatal CPAP in mice is reversed by incubation with 72 h conditioned medium derived from three different donors of hMSCs. CPAP therapy in the NICU is an essential life-saving respiratory modality for treatment of premature infants with respiratory distress. As a non-invasive method of respiratory support, its increasing use in the NICU in recent years has been favored over more aggressive invasive forms such as mechanical ventilation. Clinical care has also moved toward limiting supplemental O2 in favor of CPAP, largely because the latter is regarded as relatively benign compared to the damaging effects of hyperoxia. However, in a study by Doyle and colleagues, a 2005 birth cohort of extremely preterm infants (<28 wks of gestation) that had increased use of nasal CPAP had worse pulmonary function outcomes (FEV1/FRC Z-scores) at 8 years of age compared to a 1991–1992 cohort, despite no differences in duration of supplemental O2 (Doyle et al., 2017). In a randomized-controlled trial of weaning off CPAP, slower weans resulting in longer durations of exposure to CPAP were associated with the development of BPD and longer hospitalizations (Todd et al., 2012). Although pulmonary function testing was not assessed in these infants, emerging data in animal studies have begun to implicate CPAP in the pathophysiology of wheezing and airway hyperreactivity disorders (Mayer et al., 2015), which has been corroborated by the results of the present study. Since such modalities are likely to continue as the mainstay of respiratory support for preterm infants, there’s a growing need for development of preventative or even reversible approaches to mitigate the adverse effects that neonatal CPAP may have on lung development.
hMSCs possess reparative, immunological, antimicrobial, antioxidant, anti-inflammatory and angiogenic properties. hMSCs have also been demonstrated to have anti-airway reactivity properties in murine models of asthma (Akkoç and Genç, 2020). However, despite several animal studies showing positive therapeutic potential of MSC therapy, results from clinical studies have not yet been as convincing for neonatal lung diseases such as BPD. Animal MSC studies have focused mostly on oxygen-induced BPD. In hyperoxic rodent models of BPD, MSCs have attenuated lung injury, improved alveolar simplification, and decreased inflammation (Reiter et al., 2017). Systemic administration of mononuclear cells to newborn mice improved hyperoxia-induced changes in respiratory mechanics and prevented AHR (Mills et al., 2017). Using a similar BPD model comprising 95% O2 exposure from P4-P14, Pierro and colleagues (Pierro et al., 2013) showed that intratracheal administration of human umbilical cord blood-derived MSCs after hyperoxia exposure, reversed alveolar simplification and improved respiratory system compliance. The current study showed similar benefits to airway reactivity following incubation with conditioned medium harvested from the MSC cultures, which confirmed a paracrine mechanism of action. The paracrine mode of protection by MSC conditioned medium against neonatal hyperoxia was also confirmed by Hansmann and colleagues (Hansmann et al., 2012), and is likely the primary mechanism given the lack of significant cell engraftment in the lungs. To our knowledge, the results of the current study are the first to show beneficial properties of hMSC conditioned medium in a mouse model of neonatal CPAP, particularly in the context of rescuing long-term airway hyperreactivity weeks after exposure. The specific properties of hMSCs and their secretory agents, however, remain unknown.
4.1. Conclusions
Using a mouse model of neonatal CPAP, we showed that conditioned medium from bone-marrow derived hMSCs rescued the long-term increase in airway reactivity, suggesting a paracrine mechanism of action. These data may be beneficial to our understanding of the therapeutic potential of cell-based therapy for former preterm infants who present with wheezing as a consequence of prior neonatal CPAP treatment. Future studies are needed to explore the effects of CPAP in the clinical setting and test whether allogenic hMSC based treatments may be a viable option as a rescue therapy for infants who develop wheezing and bronchospasm later in life.
Funding
Funded by the National Heart, Lung and Blood Institute (Bethesda, MD) Grants R01HL138402 and R01HL056470, and the Department of Pediatrics, Rainbow Babies and Children’s Hospital, Cleveland, Ohio. This study was also funded in part by generous financial donations from William and Lois Briggs and the David and Virginia Baldwin Fund.
Data Availability
Data will be made available on request.
References
- Akkoç T, Genç D, 2020. Asthma immunotherapy and treatment approaches with mesenchymal stem cells. Immunotherapy 12, 665–674. [DOI] [PubMed] [Google Scholar]
- Bergner A, Sanderson MJ, 2002. Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices. J. Gen. Physiol. 119, 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfield TL, Nolan Koloze MT, Lennon DP, Caplan AI, 2010. Defining human mesenchymal stem cell efficacy in vivo. J. Inflamm. 7, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereta AD, Oliveira VR, Costa IP, Afonso JPR, Fonseca AL, de Souza ART, Silva GAM, Mello D, de Oliveira LVF, da Palma RK, 2021. Emerging cell-based therapies in chronic lung diseases: what about asthma? Front. Pharm. 12, 648506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LW, Carse E, Adams AM, Ranganathan S, Opie G, Cheong JLY, 2017. Ventilation in extremely preterm infants and respiratory function at 8 years. N. Engl. J. Med 377, 329–337. [DOI] [PubMed] [Google Scholar]
- Ee MT, Thébaud B, 2018. The therapeutic potential of stem cells for bronchopulmonary dysplasia: “it’s about time” or “not so fast”? Curr. Pedia Rev. 14, 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BD, Lauer ME, Caplan AI, Bonfield TL, 2017. Chronic asthma and Mesenchymal stem cells: hyaluronan and airway remodeling. J. Inflamm. (Lond. ) 14, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough A, 2006. Bronchopulmonary dysplasia–long term follow up. Paediatr. Respir. Rev. 7 Suppl 1, S189–S191. [DOI] [PubMed] [Google Scholar]
- Hansmann G, Fernandez-Gonzalez A, Aslam M, Vitali SH, Martin T, Mitsialis SA, Kourembanas S, 2012. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm. Circ. 2, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon DP, Caplan AI, 2006. Isolation of human marrow-derived mesenchymal stem cells. Exp. Hematol. 34, 1604–1605. [DOI] [PubMed] [Google Scholar]
- Levy O, Kuai R, Siren EMJ, Bhere D, Milton Y, Nissar N, De Biasio M, Heinelt M, Reeve B, Abdi R, Alturki M, Fallatah M, Almalik A, Alhasan AH, Shah K, Karp JM, 2020. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 6, eaba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Mayer CA, Jafri A, Pabelick CM, Prakash YS, Martin RJ, 2021. CPAP protects against hyperoxia-induced increase in airway reactivity in neonatal mice. Pedia Res. 90, 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer CA, Ganguly A, Mayer A, Pabelick CM, Prakash YS, Hascall VC, Midura RJ, Cali V, Flask CA, Erokwu BO, Martin RJ, MacFarlane PM, 2021. CPAP-induced airway hyper-reactivity in mice is modulated by hyaluronan synthase-3. Pedia Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer CA, Martin RJ, MacFarlane PM, 2015. Increased airway reactivity in a neonatal mouse model of continuous positive airway pressure. Pedia Res. 78, 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills DR, Mao Q, Chu S, Falcon Girard K, Kraus M, Padbury JF, De Paepe ME, 2017. Effects of human umbilical cord blood mononuclear cells on respiratory system mechanics in a murine model of neonatal lung injury. Exp. Lung Res. 43, 66–81. [DOI] [PubMed] [Google Scholar]
- Pierro M, Ionescu L, Montemurro T, Vadivel A, Weissmann G, Oudit G, Emery D, Bodiga S, Eaton F, Péault B, Mosca F, Lazzari L, Thébaud B, 2013. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax 68, 475–484. [DOI] [PubMed] [Google Scholar]
- Reiter J, Drummond S, Sammour I, Huang J, Florea V, Dornas P, Hare JM, Rodrigues CO, Young KC, 2017. Stromal derived factor-1 mediates the lung regenerative effects of mesenchymal stem cells in a rodent model of bronchopulmonary dysplasia. Respir. Res. 18, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyburn B, Martin RJ, Prakash YS, MacFarlane PM, 2012. Mechanisms of injury to the preterm lung and airway: implications for long-term pulmonary outcome. Neonatology 101, 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd DA, Wright A, Broom M, Chauhan M, Meskell S, Cameron C, Perdomi AM, Rochefort M, Jardine L, Stewart A, Shadbolt B, 2012. Methods of weaning preterm babies <30 weeks gestation off CPAP: a multicentre randomised controlled trial. Arch. Dis. Child Fetal Neonatal Ed. 97, F236–F240. [DOI] [PubMed] [Google Scholar]
- van Heeckeren AM, Sutton MT, Fletcher DR, Hodges CA, Caplan AI, Bonfield TL, 2021. Enhancing cystic fibrosis immune regulation. Front. Pharm. 12, 573065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Jiang J, Gu Z, Zhang J, Chen Y, Liu X, 2020. Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress. Stem Cell Res. Ther. 11, 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


