Abstract
Background
Vascular surgery may be followed by internal bleeding due to inadequate surgical haemostasis, abnormal clotting, or surgical complications. Bleeding ranges from minor, with no transfusion requirement, to massive, requiring multiple blood product transfusions. There are a number of drugs, given systemically or applied locally, which may reduce the need for blood transfusion.
Objectives
To assess the effectiveness and safety of anti‐fibrinolytic and haemostatic drugs and agents in reducing bleeding and the need for blood transfusion in people undergoing major vascular surgery or vascular procedures with a risk of moderate or severe (> 500 mL) blood loss.
Search methods
We searched: Cochrane Central Register of Controlled Trials; MEDLINE; Embase; CINAHL, and Transfusion Evidence Library. We also searched the WHO ICTRP and ClinicalTrials.gov trial registries for ongoing and unpublished trials. Searches used a combination of MeSH and free text terms from database inception to 31 March 2022, without restriction on language or publication status.
Selection criteria
We included randomised controlled trials (RCTs) in adults of drug treatments to reduce bleeding due to major vascular surgery or vascular procedures with a risk of moderate or severe blood loss, which used placebo, usual care or another drug regimen as control.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were units of red cells transfused and all‐cause mortality. Our secondary outcomes included risk of receiving an allogeneic blood product, risk of reoperation or repeat procedure due to bleeding, risk of a thromboembolic event, risk of a serious adverse event and length of hospital stay. We used GRADE to assess certainty of evidence.
Main results
We included 22 RCTs with 3393 participants analysed, of which one RCT with 69 participants was reported only in abstract form, with no usable data. Seven RCTs evaluated systemic drug treatments (three aprotinin, two desmopressin, two tranexamic acid) and 15 RCTs evaluated topical drug treatments (drug‐containing bioabsorbable dressings or glues), including fibrin, thrombin, collagen, gelatin, synthetic sealants and one investigational new agent. Most trials were conducted in high‐income countries and the majority of the trials only included participants undergoing elective surgery. We also identified two ongoing RCTs.
We were unable to perform the planned network meta‐analysis due to the sparse reporting of outcomes relevant to this review.
Systemic drug treatments
We identified seven trials of three systemic drugs: aprotinin, desmopressin and tranexamic acid, all with placebo controls. The trials of aprotinin and desmopressin were small with very low‐certainty evidence for all of our outcomes. Tranexamic acid versus placebo was the systemic drug comparison with the largest number of participants (2 trials; 1460 participants), both at low risk of bias. The largest of these included a total of 9535 individuals undergoing a number of different higher risk surgeries and reported limited information on the vascular subgroup (1399 participants).
Neither trial reported the number of units of red cells transfused per participant up to 30 days. Three outcomes were associated with very low‐certainty evidence due to the very wide confidence intervals (CIs) resulting from small study sizes and low number of events. These were: all‐cause mortality up to 30 days; number of participants requiring an allogeneic blood transfusion up to 30 days; and risk of requiring a repeat procedure or operation due to bleeding.
Tranexamic acid may have no effect on the risk of thromboembolic events up to 30 days (risk ratio (RR) 1.10, 95% CI 0.88 to 1.36; 1 trial, 1360 participants; low‐certainty evidence due to imprecision).
There is one large ongoing trial (8320 participants) comparing tranexamic acid versus placebo in people undergoing non‐cardiac surgery who are at high risk of requiring a red cell transfusion. This aims to complete recruitment in April 2023. This trial has primary outcomes of proportion of participants transfused with red blood cells and incidence of venous thromboembolism (DVT or PE).
Topical drug treatments
Most trials of topical drug treatments were at high risk of bias due to their open‐label design (compared with usual care, or liquids were compared with sponges). All of the trials were small, most were very small, and few reported clinically relevant outcomes in the postoperative period. Fibrin sealant versus usual care was the topical drug comparison with the largest number of participants (5 trials, 784 participants).
The five trials that compared fibrin sealant with usual care were all at high risk of bias, due to the open‐label trial design with no measures put in place to minimise reporting bias. All of the trials were funded by pharmaceutical companies.
None of the five trials reported the number of red cells transfused per participant up to 30 days or the number of participants requiring an allogeneic blood transfusion up to 30 days.
The other three outcomes were associated with very low‐certainty evidence with wide confidence intervals due to small sample sizes and the low number of events, these were: all‐cause mortality up to 30 days; risk of requiring a repeat procedure due to bleeding; and risk of thromboembolic disease up to 30 days.
We identified one large trial (500 participants) comparing fibrin sealant versus usual care in participants undergoing abdominal aortic aneurysm repair, which has not yet started recruitment. This trial lists death due to arterial disease and reintervention rates as primary outcomes.
Authors' conclusions
Because of a lack of data, we are uncertain whether any systemic or topical treatments used to reduce bleeding due to major vascular surgery have an effect on: all‐cause mortality up to 30 days; risk of requiring a repeat procedure or operation due to bleeding; number of red cells transfused per participant up to 30 days or the number of participants requiring an allogeneic blood transfusion up to 30 days.
There may be no effect of tranexamic acid on the risk of thromboembolic events up to 30 days, this is important as there has been concern that this risk may be increased.
Trials with sample size targets of thousands of participants and clinically relevant outcomes are needed, and we look forward to seeing the results of the ongoing trials in the future.
Keywords: Adult, Humans, Aprotinin, Blood Transfusion, Deamino Arginine Vasopressin, Deamino Arginine Vasopressin/therapeutic use, Fibrin Tissue Adhesive, Hemorrhage, Hemorrhage/etiology, Hemorrhage/prevention & control, Tranexamic Acid, Tranexamic Acid/therapeutic use
Plain language summary
Are there any drugs which help reduce bleeding after surgery on blood vessels?
• Key messages
We do not yet know what the best drugs are to reduce bleeding and blood transfusions during vascular surgery.
• What is vascular surgery?
Vascular surgery is when a surgeon operates on blood vessels, to repair leaks and areas of weakness, or to clear blockages. This review focused on the types of vascular surgery that are more likely to lead to severe bleeding.
• Why is it important to reduce blood transfusions during vascular surgery?
If people bleed a lot during or after this type of surgery they may need blood transfusions to replace the blood they have lost. It is better to avoid receiving a blood transfusion, if possible, because blood transfusions can cause harm. This is especially important when health services have limited blood supplies.
• What did we want to find out?
We wanted to find out which drug treatments help reduce bleeding and the need for blood transfusion. We also wanted to find out if these treatments increase the risk of side effects, such as blood clots.
• What did we do?
We searched electronic libraries for reports of the most reliable studies (called randomised controlled trials) of drugs to prevent bleeding after surgery on blood vessels.
• What did we find?
We found seven trials of drugs injected before surgery to try to reduce the amount of bleeding. We found 15 trials of dressings or glues with drugs in them. These are used to stop bleeding during surgery and are left inside after the operation. We did not find enough information to be sure which drugs are best for reducing bleeding and transfusions during vascular surgery. Often, the people having surgery were not followed up for very long once they left the operating theatre, so we could not find out whether they needed a blood transfusion afterwards.
One trial, of 9535 people having surgery, tested a drug called tranexamic acid injected before surgery. This included 1399 people who had surgery on their blood vessels, but it did not report much information for this group on its own. The one outcome it did report on, specifically for vascular surgery, was whether there was an increased risk of developing a blood clot if tranexamic acid is given. We found that there may be no difference in the risk of developing a blood clot between tranexamic acid and a placebo.
• What are the limitations of the evidence?
Most of the trials we found were small, with fewer than 100 people included. This is not enough to be sure if any of these treatments might help people. We need to have trials with many hundreds or even thousands of people included to find out if these drugs helped them recover from surgery.
Most of the trials we found did not collect information about blood transfusions after surgery. We think this might be for a few different reasons. Surgeons may transfer the care of patients they have operated on to the care of other healthcare professionals if they require prolonged postoperative care, e.g. physicians. It is more complicated to do a study if they need to work with other healthcare professionals to find out what happened afterwards.
Also, most of the trials were run by the companies that make the treatments. It is cheaper and easier for them to only look at what happens during surgery, especially if people will accept their product without any information about what happens after surgery.
• How up to date is this evidence?
We found all the published trials on this topic up to 31 March 2022. We also found all the trials which have started or are going to be starting soon. The good news is that there is one very large trial on drugs to prevent bleeding in surgery that is already in progress. It is testing tranexamic acid injected before surgery. It plans to recruit 8320 people undergoing a range of surgeries, including surgery on blood vessels, and will report the number of people needing blood transfusions. This will be an important result when the trial is completed, and we hope it will inspire other surgeons to do trials of this sort in future.
Summary of findings
Summary of findings 1. Summary of findings: Tranexamic acid versus placebo.
| Drugs to reduce transfusion after major open vascular or endovascular surgery | ||||||
|
Patient or population: people aged 18 and over undergoing vascular surgery Settings: surgical department Intervention: tranexamic acid (TXA) Comparison: control (placebo, usual care or active comparator) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) |
Relative effect (95% CI) |
No. participants (RCTs) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with TXA | |||||
|
Red cell transfusions (units per participant) up to 30 days post surgery |
There were no data for red cell transfusions (units per participant) in this comparison. | |||||
|
All‐cause mortality up to 30 days |
No deaths occurred in either study arm | 100 (1 RCT) | ⊕⊝⊝⊝a VERY LOW |
We are uncertain whether TXA has any effect on all‐cause mortality up to 30 days after surgery. | ||
|
Risk of receiving any allogeneic blood product (data only available for intraoperative blood product use) |
60 per 1000 |
40 per 1000 (53 fewer to 177 more) |
RR 0.66 (0.11, 3.95) | 100 (1 RCT) | ⊕⊝⊝⊝b VERY LOW |
We are uncertain whether TXA has any effect on the risk of receiving any allogeneic blood product within 30 days of surgery. |
|
Risk of reoperation or repeat procedure for bleeding within 7 days |
20 per 1000 |
7 per 1000 (20 fewer to 153 more) |
RR 0.33 (0.01, 7.99) |
100 (1 RCT) | ⊕⊝⊝⊝c VERY LOW |
We are uncertain whether TXA has any effect on the risk of reoperation or repeat procedure for bleeding. |
|
Risk of a thrombotic/thromboembolic event (MI, CVA, DVT, PE) 30‐day follow‐up |
186 per 1000 |
205 per 1000 (22 fewer to 67 more) |
RR 1.10 (0.88, 1.36) |
1360 (1 RCT) | ⊕⊕⊝⊝d LOW |
TXA may have little to no effect on the risk of experiencing a thrombotic or thromboembolic event. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CVA: cerebrovascular attack; DVT: deep vein thrombosis; MI: myocardial infarction; PE: pulmonary embolus; RCT: randomised controlled trial; RR: risk ratio; TXA: tranexamic acid | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
a We downgraded the evidence three times for imprecision because of very wide confidence intervals resulting from small study sizes and low event rate. b We downgraded the evidence once for indirectness because data were only available for the intraoperative time period, and twice for imprecision because of very wide confidence intervals resulting from small study sizes and low event rate. c We downgraded the evidence three times for imprecision because of very wide confidence intervals resulting from small study sizes and low event rate. d We downgraded the evidence twice for imprecision because of the wide confidence intervals resulting from small study sizes and low event rate.
Summary of findings 2. Summary of findings: Fibrin sealant vs usual care.
| Drugs to reduce transfusion after major open vascular or endovascular surgery | ||||||
|
Patient or population: people aged 18 and over undergoing vascular surgery Settings: surgical department Intervention: fibrin sealant Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) |
Relative effect (95% CI) |
No. participants (RCTs) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with fibrin sealant | |||||
|
Red cell transfusions (units per participant) up to 30 days post surgery |
There were no data for red cell transfusions (units per participant) in this comparison | |||||
|
All‐cause mortality up to 30 days |
29 per 1000 |
13 per 1000 (26 fewer to 22 more) |
RR 0.44 (0.11, 1.76) |
585 (3 RCTs) | ⊕⊝⊝⊝a VERY LOW |
We are uncertain whether fibrin sealant has any impact on all‐cause mortality at 30 days. |
|
Risk of receiving any allogeneic blood product up to 30 days |
There were no data for the risk of receiving any allogeneic blood product in this comparison | |||||
|
Risk of reoperation or repeat procedure for bleeding within 7 days |
62 per 1000 |
64 per 1000 (41 fewer to 148 more) |
RR 1.03 (0.31, 3.40) |
160 (1 RCT) | ⊕⊝⊝⊝a VERY LOW |
We are uncertain whether fibrin sealant has any impact on risk of reoperation or repeat procedure for bleeding within 7 days. |
|
Risk of a thrombotic/thromboembolic event (MI, CVA, DVT, PE) 30‐day follow‐up |
56 per 1000 |
7 per 1000 (56 fewer to 269 more) |
RR 0.11 (0.00, 5.84) |
39 (1 RCT) | ⊕⊝⊝⊝b VERY LOW |
We are uncertain whether fibrin sealant has any impact on the risk of experiencing a thrombotic/thromboembolic event within 30 days. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CVA: cerebrovascular attack; DVT: deep vein thrombosis; MI: myocardial infarction; PE: pulmonary embolus; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
a We downgraded the evidence once for risk of bias in the domains of blinding of participants and personnel, and we downgraded twice for imprecision due to very wide confidence intervals resulting from small study sizes and low event rate. b We downgraded the evidence once for risk of bias in the domain of blinding and selective outcome reporting, and we downgraded three times for imprecision due to extremely wide confidence intervals resulting from small study sizes and low event rate.
Background
Description of the condition
Vascular surgery treats diseases of arteries, veins or lymph vessels, except for those in the heart or brain. The major types of arterial disease are arterial aneurysms, arterial dissections and arterial occlusive disease. These conditions may be treated either with open surgery by vascular surgeons, or with endovascular procedures conducted by vascular surgeons or interventional radiologists (Hirsch 2006). The availability of interventional radiology procedures varies regionally and globally, depending on the availability of trained staff and equipment (Beck 2016; Benson 2020; Boyle 2021; Kline 2017).
Aneurysms
Aneurysms are abnormal dilations in an artery which can progressively enlarge and weaken, with risk of rupture and severe internal bleeding. Vascular services manage aneurysms found in the chest (thoracic aortic aneurysm, TAA), chest and abdomen (thoraco‐abdominal aortic aneurysm, TAAA) or abdomen (abdominal aortic aneurysm, AAA) as well as aneurysms found in peripheral arteries, including iliac, popliteal or femoral arteries. Aneurysms can remain asymptomatic, but rupture can be fatal or life‐threatening. Aneurysm repair can be conducted as an emergency in the case of leak or rupture, or electively to prevent rupture and other complications. Elective repair of AAA is associated with significantly reduced mortality of 2% compared to emergency repair mortality of 20% to 30% (Majd 2016). AAA is the most common type of aneurysm to require repair, whether with open surgery or endovascular repair (Sampson 2014). Risk factors for AAA include older age, male gender, European ancestry, smoking and high blood pressure (Altobelli 2018). Ultrasound screening programmes estimate AAA prevalence at 2.7% in 65 to 74‐year‐olds and 7.3% in 75 to 85‐year‐olds (Makrygiannis 2016). The Centers for Disease Control and Prevention (CDC) ranks AAA as one of the top 15 causes of mortality in the USA for those between 85 and 89 years old (CDC 2015).
Arterial dissection
Arterial dissection is a process in which blood tracks between the layers of an artery wall, forcing them apart. This can be an acute or chronic process, initiated by a defect in the vessel due to shear stress, inflammation, trauma or at the site of an aneurysm. Seventy per cent of major vessel dissections occur in the ascending thoracic aorta (Stanford type A), 7% in the thoracic arch, 20% in the descending thoracic or thoraco‐abdominal aorta (Stanford type B) and 2% in the abdominal aorta (Howard 2014; Roberts 1991). Stanford type A requires urgent or emergency open surgical or endovascular repair, whilst Stanford type B may also be managed by reducing blood pressure with medications (Bannazadeh 2016; Cooper 2016; Elsayed 2017; Ulug 2012). Risk factors for aortic dissection include inherited or acquired connective tissue disorders, high blood pressure and aortic aneurysm (Paterick 2013). Acute aortic dissection has an estimated incidence of 52 per 100,000 per year, 60% occur in men, and it has a high risk of death (approximately 73% 30‐day mortality if Stanford type A, and 13% mortality if Stanford Type B) (Howard 2013). If the aortic valve is involved in thoracic aortic aneurysm or dissection, surgical repair may require cardiac surgeons.
Occlusive arterial disease
Occlusive arterial disease (OAD) is caused by atherosclerosis, in which fat and cholesterol deposition cause inflammation, thickening and hardening of vessel walls, with eventual narrowing or blockage of the artery (Rahman 2017). This can cause inadequate blood flow through the vessel, with poor oxygen delivery to tissues beyond it (ischaemia). Increased blood flow through other (collateral) vessels may compensate to some degree (McDermott 2017). Atherosclerotic deposits (plaques) can also rupture, suddenly blocking blood flow with blood clot (thrombus) and debris, causing sudden and severe (critical) ischaemia and resultant tissue, organ or limb death (Gilliland 2017). OAD risk factors include being male, older age, personal or family history, cardiovascular disease, diabetes, stroke, high cholesterol, high blood pressure, smoking, obesity and inactivity. OAD can occur in many locations, but vascular services typically treat blockage or narrowing (stenosis) of arteries in the neck (carotid arteries), abdomen (aorta), pelvis and legs (iliac, femoral or popliteal arteries). Carotid stenoses or occlusions cause 15% to 25% of strokes (Saw 2014). Carotid OAD can be managed with open surgery (endarterectomy) or endovascular stents (Noiphithak 2017). Occlusive disease in the aorta is classified as above (supra‐renal) or below (infra‐renal) the artery to the kidneys and can also be managed with open bypass grafting surgery or endovascular stenting.
OAD most commonly affects the lower limbs, where it is also known as peripheral arterial disease (PAD) (Fowkes 2017). It is defined by ankle to brachial (upper arm) blood pressure index (ABPI) of less than 0.9. The prevalence of asymptomatic PAD in the middle‐aged to elderly population is estimated at 7% to 15%, and affects over eight million Americans (Swaminathan 2014). The PERART study (a Spanish primary care population) found a PAD prevalence of 10.2% in males and 5.3% in females (Alzamora 2010). The National Health and Nutrition Examination Survey (NHANES, 1999 to 2000) reported a symptomatic PAD prevalence of 4.3% in adults aged over 40 years old and 14.5% in adults over 70 years old (Selvin 2004). However, the British Regional Heart Study, using femoral artery ultrasound assessment, found 64% of subjects aged 56 to 77 years had significant femoral atherosclerosis, of which only 10% were symptomatic (Leng 2000). When vascular disease causes ischaemia with resultant tissue death, vascular surgeons may perform an amputation at the lowest unaffected level, for example below‐knee, above‐knee or hind‐quarter amputation. Retrospective studies show that non‐traumatic amputations are nearly all caused by vascular disease, which may or may not be complicated by diabetes, and have a high risk of death (mortality of 30% at 30 days and 54% at one year) (Kristensen 2012).
Open vascular and endovascular procedures
Open aneurysm or bypass surgery, particularly in the chest, abdomen and pelvis, are invasive major operations associated with complications including bleeding, stroke, cardiac and kidney injury and spinal cord ischaemia, with relatively long recovery and length of hospital stay, and high readmission rates (Fry 2018; Hobson 2018). They may also require periods of aortic cross‐clamping, which adds to complication rates (Zammert 2016). Where cost‐effective and feasible, procedures are conducted endovascularly with stents and grafts, guided by contrast dye and radiological imaging. Endovascular procedures avoid large incisions, cause less postoperative pain, and may have lower mortality and complication rates with reduced hospital length of stay and costs. Intraoperative or postoperative bleeding can occur in endovascular surgery from the vascular access site, around the graft (endoleak), or from vessel rupture. Conversion to open surgery or repeat endovascular procedures are sometimes necessary. Endoleaks (Types I to V) are defined as a persistent blood flow outside the lumen of an endoluminal graft but within the aneurysm sac or the adjacent vascular segments (Society for Vascular Surgery). They are caused by incomplete sealing or exclusion of the aneurysm sac. Endovascular procedures are now feasible in most elective and some emergency settings, particularly in high‐income countries, but remain inappropriate for some complex procedures and require expertise and equipment that may not be available in some settings (Buck 2014).
A 2014 Cochrane Review found that for elective AAA repair, endovascular aneurysm repair (EVAR) was associated with lower short‐term mortality than open surgical repair (OSR), particularly with regard to respiratory complications. At intermediate and long‐term follow‐up, however, they performed comparably. Additionally, individuals undergoing EVAR had a higher re‐intervention rate to manage endoleaks, but these were mostly catheter‐based interventions associated with low mortality and were not associated with any difference in terms of 30‐day mortality (Paravastu 2014). Elective AAA repair may also be conducted with laparoscopic (keyhole) surgery (Robertson 2017). A 2017 Cochrane Review found that for urgent or emergency repair of ruptured AAA, EVAR and OSR had similar 30‐day mortality rates, but did not find a difference in complication rates (Badger 2017). A 2016 Cochrane Review found no randomised controlled evidence to support thoracic endovascular aneurysm repair (TEVAR) compared to open surgical thoracic aorta repair; observational studies, however, support the use of TEVAR (Abraha 2016). A 2017 Cochrane Review found that endovascular treatment (percutaneous transluminal angioplasty) for chronic limb ischaemia was associated with fewer early complications and shorter hospital stay compared with bypass grafting surgery. However, open surgical treatment had better flow in vessels one year on. Endovascular treatment of lower limb occlusive arterial disease may therefore be particularly beneficial in people with significant comorbidities which make them high‐risk surgical candidates (Antoniou 2017). In general, surgical or interventional radiologist experience and anatomy of the defect determine whether endovascular or open surgery is preferable.
Bleeding and transfusion in vascular procedures
Internal bleeding may occur before surgery (in the case of arterial dissection or rupture), during an intervention, or after an intervention, due to inadequate surgical haemostasis, abnormal clotting, graft failure, migration or endoleaks. Bleeding ranges from minor, with no transfusion requirement, to massive, requiring multiple blood product transfusions.
Operations or procedures with a risk of moderate or severe blood loss (at least 500 mL) include: open or endovascular emergency repair of AAA, TAAA or TAA; open or endovascular repair of thoracic aortic dissection; complex lower limb bypass surgery; and major lower limb amputation. Studies show transfusion rates of 38% in people undergoing elective open AAA repair, 27% in lower limb bypass surgery, 15% in open thromboendarterectomy (removal of blood clot and atherosclerotic plaque), and vary from 17% to 64% in lower limb amputation (D'Ayala 2010; O'Keeffe 2010; Tan 2015).
Some procedures, for example elective endovascular AAA repair or endovascular lower limb stenting, have a low risk of bleeding (Obi 2015). However, NICE 2020 has recommended that open surgical repair of unruptured AAAs should be performed unless it is contraindicated, due to the increase in medium‐ and long‐term harms of EVAR that outweigh its short‐term benefits. EVAR is associated with fewer perioperative deaths, and less time in hospital in general (and critical care in particular). But it has worse long‐term survival than open surgical repair, and more long‐term complications, leading to further procedures (NICE 2020). Other procedures very rarely cause people to experience bleeding or require transfusion; for example, a transfusion rate of less than 1% in elective carotid endarterectomy has been reported (Rubinstein 2013). Importantly, transfusion rates and transfusion practices vary between centres and care providers (Osborne 2018).
Various surgical factors can increase risk of bleeding, including emergency procedures, for example for aneurysm rupture, dissection or critical limb ischaemia, revision or repeat surgery or complex or branching anatomy (Obi 2015). Perioperative factors include systemic anticoagulation with heparin to prevent graft thrombosis or clot extension, pre‐existing use of anticoagulants or antiplatelet drugs, intraoperative hypothermia, cross‐clamp position, acute coagulopathy in the setting of trauma, and systemic inflammatory response in the setting of an infectious disease, for example aortic mycotic abscess (Obi 2015; Samoila 2017). Procedure‐specific models have been developed to predict bleeding for certain vascular procedures (Kapma 2017; Mahmood 2018). For example, using data from a large multicentre quality improvement database, the transfusion rate within 24 hours of EVAR was predicted at 3.2%, with the following risk factors associated with transfusion: haematocrit less than 36%, increased aortic diameter, functional status and chronic obstructive pulmonary disease (O'Donnell 2018).
Interventions to reduce bleeding and allogeneic transfusion
Cell salvage can be used to collect blood from the surgical field for autologous transfusion, and meta‐analyses of vascular surgery randomised controlled trials suggest cell salvage reduces perioperative transfusions by up to 37% (Ashworth 2010; Takagi 2009). However, 30% to 50% of surgical blood loss is absorbed into swabs, therefore swab washing can increase blood available for autotransfusion (Haynes 2005). Using a range of techniques to reduce bleeding may be more useful than cell salvage alone; moreover, cell salvage is not used during endovascular procedures. The ratio of different blood products transfused also appears to be important to people's outcomes, as well as the overall amount of blood product transfused (using a higher or lower transfusion trigger) (Mesar 2017). Strategies to reduce use of any allogeneic blood products include techniques such as arterial cross‐clamping, medications to reduce blood pressure and thus reduce bleeding, and limiting use of crystalloid or other fluid infusions, which can compound bleeding by diluting clotting factors present in the circulation (Chee 2016). In addition, point‐of‐care viscoelastic testing (rotational thromboelastometry (ROTEM) or thromboelastography (TEG)) quantifies coagulation and fibrinolysis parameters and their use can guide and reduce autologous transfusion, though most evidence is in the context of cardiac surgery (Wikkelsø 2016). Finally, various haemostatic drugs, which alter coagulation and fibrinolysis, are an important part of management to reduce bleeding and transfusion risk.
Description of the intervention
When an injury occurs, the formation of a blood clot (normal haemostasis) stops excessive bleeding. Blood clot formation is initiated by tissue injury, endothelial and collagen exposure and release of factors which cause blood vessel constriction (vasoconstriction) and platelet activation (Blanco 2017). Activated platelets stick together, forming a weak plug (Mackman 2007). Multiple enzyme pathways are also activated and amplified, finally producing thrombin, an enzyme that converts fibrinogen to fibrin. Fibrin rapidly polymerises and cross‐links with platelets to form an insoluble, stable blood clot. The clot is further stabilised and contracted by cross‐linking between the fibrin strands by factor XIII (Chapin 2015).
To prevent harmful, unregulated clot extension beyond the injury, blood clots are subsequently contained and broken down by fibrinolysis (Blanco 2017). The enzyme plasmin, a protease, cuts through the fibrin mesh, releasing soluble fragments that are metabolised in the liver and kidneys (Hudson 2017). Plasmin is activated locally from its precursor, plasminogen, as part of the normal clotting process. Plasmin formation and fibrinolytic processes normally occur more slowly than coagulation, such that clot breakdown occurs well after clot formation and tissue remodelling ‐ that is, after bleeding has stopped (Chapin 2015). To prevent plasmin digesting non‐clot tissue or proteins, plasminogen is predominantly converted to plasmin at the site of and within the blood clot, creating bound, rather than free plasmin. Free plasmin will indiscriminately digest plasma proteins, including clotting factors and is normally kept in check and neutralised by circulating alpha‐2‐plasmin inhibitor (Madurska 2018). This reduces pathological, rather than physiological fibrinolysis (Makar 2010).
Antifibrinolytic drugs inhibit the activity of plasmin and thus reduce the breakdown of fibrin within blood clots, resulting in greater early and persistent clot strength (Okamoto 1997). Haemostatic drugs are a broad class of drugs which each act on distinct parts of the coagulation cascade to replace or enhance missing or poorly functioning pro‐coagulant enzymes, substrates or factors. These could be deficient due to inherited conditions, such as haemophilia, or acquired conditions, such as prolonged bleeding (consumption of clotting factors), liver failure, autoimmune disease or drug therapy.
Antifibrinolytic drugs
Tranexamic acid (TXA)
TXA is a synthetic analogue of the amino acid lysine. It binds reversibly to lysine receptor sites on plasminogen, prevents activation of plasminogen into plasmin, and reduces fibrin breakdown. This improves clot formation, stability and duration. TXA has been well validated for use in perioperative, obstetric and trauma care, as well as in cardiac surgery (Henry 2011; Ker 2015; Shakur 2018). A systematic review and network meta‐analysis of antifibrinolytic adverse drugs effects in the setting of cardiac surgery suggests TXA use reduces mortality compared to placebo or aprotinin. In addition, it does not increase myocardial infarction (MI), cerebrovascular attack (CVA) or renal failure or dysfunction (Hutton 2012). In high doses, however, TXA has been associated with seizures in the cardiac surgery setting (Murkin 2010).
ε‐aminocaproic acid (EACA)
EACA is another synthetic lysine analogue, with a similar mechanism of action to tranexamic acid. Comparative potency of EACA and TXA estimates vary but suggest EACA is 7 to 10 times less potent than tranexamic acid (Thomsen 2006). There is no known association with seizures.
Antifibrinolytic drugs such as EACA and TXA are usually administered intravenously after induction of anaesthesia. Usually, a loading dose is given followed by continuous infusion. High doses appear to be more effective than low doses (Henry 2011). Neither TXA nor EACA has been associated with increased risks of adverse effects (Hutton 2012).
Aprotinin
Aprotinin is an enzyme inhibitor with complex effects on haemostasis. It is a competitive inhibitor of various serine proteases, including plasmin and kallikrein (McCarthy 1994). Plasmin inhibition slows the rate of fibrinolysis. Aprotinin exerts a much greater effect on free plasmin, however, with much less effect on bound plasmin. This improves the haemostatic problems caused by excessive or unregulated free plasmin activity, such as consumption of clotting factors. This reduces pathological rather than physiological fibrinolysis (Royston 2015). Kallikrein inhibition reduces factor XIIa activity, which inhibits intrinsic coagulation pathways leading to the formation of thrombin and fibrin. On balance, aprotinin is frequently classed as antifibrinolytic, as it has a net clot‐stabilising effect which outweighs its kallikrein‐mediated anticoagulant effects.
Aprotinin has been associated with a higher rate of adverse effects than the lysine analogues (Henry 2009). Evidence from three observational studies and from a single randomised study, in adults undergoing cardiac surgery, showed an increased risk of renal dysfunction, cardiovascular events, pulmonary embolism and death with aprotinin (Bremerich 2006; Cooper 2006; Mangano 2007; Royston 2015). This led to its withdrawal from many national markets in 2007 (FDA 2007). These data have, however, been revisited and reanalysed, questioning the validity of the conclusions of the four studies (Howell 2013). Despite this, aprotinin remains unavailable or on a restricted license, for example for myocardial revascularisation only, in some countries (Henry 2011).
Other haemostatic drugs
Desmopressin (DDVAP)
Desmopressin is a synthetic analogue of the human anti‐diuretic hormone, vasopressin. It increases the plasma levels of von Willebrand factor (vWF) two‐ to three‐fold by stimulating vWF release from endothelial cells. vWF plays an important role in platelet adhesion to wound sites, and thus early clot formation, so deficiency of vWF leads to bleeding tendencies. vWF also increases the availability of factor VIII, because factor VIII degrades rapidly if not complexed to vWF. Activated factor VIII is required in the enzyme cascade, which produces thrombin and fibrin. vWF deficiency is the most common clotting disorder and is present in about 1% of the population. Desmopressin is mainly used to treat coagulopathy caused either by deficiency of vWF or factor VIII (haemophilia A), but may also be used before procedures to treat reduced platelet adhesiveness due to drugs like aspirin, or from raised serum urea in the setting of severe renal impairment (Kim 2015).
Desmopressin is typically administered at a dose of 0.3 μg per kg subcutaneously or intravenously and takes approximately 30 minutes to reach peak effectiveness, and this effect lasts up to six to eight hours (Franchini 2007). Increases in vWF, factor VIII levels and in tissue plasminogen activator (tPA) if recurrent dosing is used can potentially increase the risk of arterial or venous thrombotic events; this is an important safety consideration (Franchini 2007; Kaufmann 2003). Desmopressin also results in release of nitric oxide from endothelial cells, which can cause vasodilation with symptoms of facial flushing, tachycardia, and hypotension (Kaufmann 2003). In rare cases, desmopressin administration may be associated with hyponatraemia and seizures, especially in young children (Smith 1989).
Prothrombin complex concentrate (PCC)
There are two main types of PCC. 3‐factor PCC contains blood clotting factors II, IX and X, whereas 4‐factor PCC also contains blood clotting factor VII, protein C, and protein S. PCC is a powder concentrate, extracted from human plasma and reconstituted prior to use, dosed at 25 to 50 units per kg. It is used for perioperative prophylaxis or treatment of severe bleeding in people treated with vitamin K antagonists, like warfarin, or in people with clotting factor deficiencies, whether inherited, for example haemophilia, or acquired, such as in severe liver disease (BNF 2019). Side effects include fever, high blood pressure and thromboembolism (migrating blood clots).
Recombinant factor VIIa (rFVIIa)
rFVIIa, also called NovoSeven, is a serine protease which catalyses conversion of factors IX and thrombin (X) into active forms. This increases the conversion of fibrinogen to fibrin by thrombin and promotes clot formation and propagation. It is currently licensed only for bleeding in people with a diagnosis of haemophilia, or severe uncontrolled haemorrhage, but is also used for prevention of haemorrhage in haemophiliacs undergoing invasive procedures like surgery (Simpson 2012). Studies have suggested an association with rFVIIa and arterial thromboembolic events (Levi 2010; Simpson 2012).
Factor XIII (FXIII)
FXIII, is a transglutaminase enzyme which cross‐links fibrin monomers between adjacent fibrin polymer strands to stabilise and strengthen the clot. It also acts to contract the clot into a more dense and insoluble unit (Ariëns 2002). FXIII treatment is currently indicated for congenital or acquired factor XIII deficiencies, identified with quantitative methods, and has been studied as an agent that can reduce bleeding in cardiac surgery (Muszbek 2008).
Fibrinogen concentrate
Fibrinogen is a plasma glycoprotein synthesised by the liver. Fibrinogen is the precursor to fibrin, but also helps platelets activate and aggregate by binding to the platelet’s GPIIb/IIIa receptor. Fibrinogen substitution is believed to normalise and improve the environment for clot formation by providing sufficient amounts of substrate and by enhancing the strength and speed of clot generation in people with depleted or dysfunctional fibrinogen (Nielsen 2005a; Nielsen 2005b). Within the context of cardiac surgery, systemic fibrinogen replacement is currently indicated for prophylaxis or treatment of bleeding in congenital and acquired deficiencies of fibrinogen that have been identified with quantitative methods (Bracey 2017). It has, however, been associated with small reduction in transfusions in a Cochrane Review of people with bleeding in elective and cardiac surgery, though without survival benefit (Wikkelsø 2013).
Internal topical agents (excludes surface dressings)
Internal topical application of drugs or biomaterials can be used as an adjunct to surgical control of bleeding, particularly where there are many microscopic bleeding vessels or raw tissue which cannot be surgically closed (Gabay 2013). A biomaterial is any substance that has been engineered to physically interact with biological tissue for a specific purpose (Park 2007). Topical agents include active drugs or clotting factors applied directly as a liquid, paste, foam or gel, or impregnated into biomaterials, or application of passive biomaterials which promote clotting through physical means (Vyas 2013). There are many agents available, and these have been classified as active, passive and combined haemostatic agents (Bracey 2017). They can also be classified as flowable, or non‐flowable, or fibrin and non‐fibrin sealant.
Active agents enhance enzyme pathways in clotting and include antifibrinolytic drugs, fibrin sealants or topical thrombin. Passive materials include collagens, porcine gelatins, regenerated oxidised cellulose and polysaccharide spheres. Passive synthetic sealants include cyanoacrylate, polyethylene glycol, and bovine serum albumin with glutaraldehyde. Combination agents include liquid gelatins with thrombin, and fibrin sealants with equine collagens. These diverse groups have the advantage of acting locally at the site of bleeding, potentially avoiding systemic side effects (Seyednejad 2008). The passive biomaterial and sealants may have the advantage of promoting clotting even in hypothermia or with deficits in normal clotting factors, as they operate independently of enzymatic biological clotting processes.
How the intervention might work
Antifibrinolytic drugs
Hyperfibrinolysis can contribute to catastrophic bleeding by preventing new clots forming as well as degrading formed clots. This is because fibrin degradation products interfere with platelet activation, adhesion and normal fibrin polymerisation, inhibiting normal coagulation. Additionally, the high level of free plasmin associated with hyperfibrinolysis also causes degradation of the fibrin precursor fibrinogen, reducing the substrate available for fibrin polymerisation. Prophylactic antifibrinolytic use is recommended for all surgery expected to have moderate or severe blood loss (often defined as at least 500 ml blood loss), unless there are specific contraindications (Chee 2016; Kozek‐Langenecker 2017; NICE 2015; WHO 2021).
Other haemostatic drugs
Other haemostatic drugs are currently only recommended where a pre‐existing clotting factor deficiency has been identified with quantitative testing. There is a lack of well‐conducted studies to assess the impact of haemostatic drugs in people who may acquire perioperative deficits in clotting factors or have platelet function deficits due to perioperative medications. DDVAP may be of particular benefit in people with bleeding stemming from GPIIb/IIIa inhibitors and other antiplatelet medications (Raja 2006). rFVIIa is used off‐label for a variety of major surgeries, occasionally as prophylaxis, or more frequently in catastrophic haemorrhage after other options have failed to arrest bleeding. Its usefulness in reducing bleeding in surgery remains unproven (Simpson 2012). Analysis of rFVIIa usage in intractable bleeding in cardiothoracic surgery demonstrated a reduction in transfusion requirement, at the expense of a higher thrombotic event rate; it has not been determined, however, whether this translates into more favourable clinical outcomes (Omar 2015). Fibrinogen may be used during massive transfusion, or acquired hypofibrinoginaemia during major bleeding, but is not routinely used (Chee 2016; Kozek‐Langenecker 2017). Therefore, FXIII, rFVIIa and fibrinogen concentration may be used as a rescue treatment in severe bleeding rather than as prophylaxis due to their cost and risk profile.
Internal topical agents
Several trials have shown improved local haemostasis and reductions in overall blood use with topical agents, and there are theoretical advantages of localised treatments in terms of avoiding unwanted side effects (Vyas 2013). In people with abnormal clotting, however, local active treatments which rely on coagulation pathways to work may also have limited effect due to systemic coagulation derangement.
Why it is important to do this review
Bleeding and reoperation for bleeding are serious adverse outcomes, which are associated with increased mortality, complications, and risk of transfusion (Shaw 2013). Bleeding and the need for a red blood cell transfusion have also been shown to increase the duration of hospital stay and the costs associated with surgery, after taking into consideration confounding factors (Stokes 2011; Zbrozek 2015). The negative impact on outcomes associated with allogeneic transfusion is observed even when a person only receives a transfusion of one or two units of red blood cells (Paone 2014; Paone 2018). These findings have recently been replicated in studies of major vascular surgery: after adjustment of major covariates, perioperative transfusion was associated with increased 30‐day mortality and morbidity (specifically myocardial infarction and pneumonia) in people undergoing major vascular surgery (Obi 2015). In lower limb bypass surgery, transfusion was associated with increased perioperative wound infection and graft thrombosis in a dose‐dependent fashion (Tan 2015). This has also been demonstrated in amputation surgery (Tan 2013). The particular blood product components transfused (red cells, platelet, fresh frozen plasma) may also impact outcome in AAA rupture surgery (Henriksson 2013). This study showed that the ratio of platelets and fresh frozen plasma to red cells increased from 0.8 to 0.9 during the study (1992 to 1999 versus 2000 to 2008), which was associated with improved survival.
Why even a small transfusion of red cells may be associated with poorer outcomes is not fully understood. It may be due to a mixture of pro‐inflammatory and anti‐inflammatory molecules within the transfusion, called transfusion‐related immunomodulation (TRIM) (Karsten 2018; Muszynski 2017; Youssef 2017). Other transfusion‐related adverse effects include incompatibility reactions, transfusion‐related acute lung injury (TRALI), and transfusion‐associated circulatory overload (TACO) (Harvey 2015; Maxwell 2006). In addition, transmission of infectious diseases (e.g. HIV, Hepatitis C, prion disease) remains a concern (Kiely 2017; Rerambiah 2014). This is particularly a concern in countries with higher prevalence of infectious diseases, or less robust screening capabilities, or both (Seo 2015; WHO 2017). Blood components, particularly platelets, can also have bacterial contamination that may cause sepsis in the recipient (Benjamin 2016; Makuni 2015; Morel 2013).
Adjuncts to reduce bleeding include prophylactic haemostatic drugs which alter coagulation and fibrinolysis. Tranexamic acid is probably the most frequently used at present, though aprotinin is re‐emerging after its withdrawal in the late 2000s (Royston 2015). These drugs may be given as a single dose, multiple doses or infusion, and before, during or after surgery, or by various different routes (e.g. topically onto a bleeding internal tissue, subcutaneously or intravenously). Audits of elective surgery show that there is poor uptake of pharmacological adjuncts to reduce bleeding (NCABTPG 2017). Barriers to optimal use may include not knowing which drug, drug combination, dose or timing is most effective. These factors are also important for establishing minimum effective doses and appropriate duration of exposure, so that other drug side effects are minimised. In order to select the most appropriate drug (or drug combination), dose, timing and route, the many different ways of giving these drugs should be compared; this requires clarification and review of available evidence. Finally, this review will investigate the effect of antiplatelet/anticoagulant drug use and compare drug efficacy and safety in open and endovascular procedures, to establish any different performance of drugs in different circumstances (Berger 2012).
Objectives
To assess the effectiveness and safety of anti‐fibrinolytic and haemostatic drugs and agents in reducing bleeding and the need for blood transfusion in people undergoing major vascular surgery or vascular procedures with a risk of moderate or severe (> 500 mL) blood loss.
Methods
Criteria for considering studies for this review
Types of studies
We prespecified our methods for conducting this review in the review protocol (Beverly 2020). We included randomised controlled trials (RCTs) and cluster‐RCTs if the analyses accounted for clustering, or if we were able to adequately adjust for clustering (McKenzie 2016). We included all studies regardless of their language or publication status. We excluded studies with purely experimental laboratory outcomes (for example blood tests for inflammatory markers).
Types of participants
We included adults (18 years or over) undergoing the following emergency, urgent and elective procedures.
-
Open surgical repair (OSR) of aneurysm of the:
abdominal aorta (AAA);
thoracic aorta (TAA);
thoraco‐abdominal aorta (TAAA);
iliac artery;
femoral artery; or
popliteal artery.
-
OSR or endovascular repair of dissection of the:
abdominal aorta;
thoracic aorta; or
thoraco‐abdominal aorta.
-
Open bypass surgery for peripheral arterial disease of the:
aortic artery;
iliac artery;
femoral artery; or
popliteal artery.
-
Major lower limb amputation for vascular disease:
below knee;
above knee; or
hindquarter.
We included adults (18 years or over) undergoing the following emergency or urgent procedures.
-
Endovascular aneurysm repair (EVAR) of the:
abdominal aorta (AAA);
thoracic aorta (TAA);
thoraco‐abdominal aorta (TAAA);
iliac artery;
femoral artery; or
popliteal artery.
-
Endovascular stenting for peripheral arterial disease of the:
aortic artery;
iliac artery;
femoral artery; or
popliteal artery
We included participants undergoing surgery with or without aortic cross clamping and with or without use of hypothermia. We included participants undergoing open, modifications of open, and minimally invasive, e.g. laparoscopic, surgical approaches.
We excluded procedures typically performed by or in conjunction with cardiac surgeons, such as those on the ascending aorta and aortic root, or those using coronary artery bypass grafting. These are the topic of a separate ongoing Cochrane Review entitled Drugs to reduce bleeding and transfusion in adults undergoing cardiac surgery; a systematic review and network meta‐analysis (Beverly 2019).
We excluded studies involving elective endovascular procedures. We excluded procedures associated with minimal bleeding and transfusion, such as carotid procedures, arterio‐venous fistulae formation for dialysis, varicose vein surgery and upper limb or digit amputations. We also excluded procedures typically performed by neurosurgeons, such as repair of aneurysms or dissection of arteries in the head or neck.
We excluded people with known inherited coagulation disorders, such as von Willebrand factor deficiency, haemophilia or hypofibrinogenaemia. This is because the clotting mechanisms that the drugs promote or interact with may be genetically absent, making response atypical.
For trials consisting of mixed populations of participants (e.g. including children, or including procedures other than those specified), we only used data from participants 18 years or over undergoing the specified procedures, without clotting disorders. If the subgroup data required were not provided, we excluded the trial if less than 80% of participants were eligible to be included.
Types of interventions
We included RCTs of the following interventions, compared to usual care, placebo, or each other.
Tranexamic acid (TXA)
ε‐aminocaproic acid (EACA)
Aprotinin
Desmopressin
Prothrombin complex concentrate (PCC)
Recombinant factor VII (rFVII)
Factor XIII (FXIII)
Fibrinogen concentrate
-
Other topical agents, categorised as:
fibrin‐based agents;
thrombin‐based agents;
synthetic sealants;
passive biomaterials; and
combination agents.
We included RCTs that compared one or more of the interventions listed above. We included studies using a combination of the above drugs. We did not exclude trials on the basis of the route, dose, timing, or frequency of drug administration. The comparison groups were as defined by the study, which could be a control group using placebo, standard care, or one of the included drugs, if a second additional drug was being investigated.
Types of outcome measures
We were primarily interested in postsurgical outcomes, and especially the need for blood transfusion. We did not include intraoperative outcomes, such as time to haemostasis, because these outcomes are prone to measurement bias and are of limited clinical relevance or interest to health services.
Primary outcomes
Our primary outcomes were:
Red cell transfusions (units per participant*) at up to 30 days post surgery
All‐cause mortality at up to 30 days; and between 31 and 90 days
*If the red cell transfusion outcome was reported in mL, we converted that into units, according to any local mean unit volume data given in the study, or as per the Guidelines for the Blood Transfusion Services in the UK mean stated volume per unit of red cells of 280 ± 60 mLs (JPAC 2013).
Secondary outcomes
Our secondary outcomes were as follows.
-
Risk of receiving any allogeneic blood product at up to 30 days post surgery
Composite: packed red cells (PRC), fresh frozen plasma (FFP), platelets (PLTs)
Components: PRC, FFP, PLTs
Risk of reoperation or repeat procedure for bleeding within 7 days
-
Risk of a thrombotic/thromboembolic event
Composite: myocardial Infarction (MI), cerebrovascular attack (CVA), deep vein thrombosis (DVT), pulmonary embolus (PE) at up to 30 days and between 31 and 90 days
Components: MI at up to 30 days, CVA at up to 30 days, DVT at up to 90 days, PE at up to 90 days
Risk of a serious adverse event (SAE) at up to 30 days postsurgery
Length of hospital stay (days)
We commented on any cost data, if presented, in a narrative form (Ryan 2016). Cost information was provided as useful additional information, but was not intended to be a formal economic evaluation.
Search methods for identification of studies
We searched bibliographic databases and checked the references of included studies.
Electronic searches
Searches used a combination of MeSH and free text terms and were carried out from database inception to 31 March 2022, without language restriction or publication status.
The Information Specialist (CD) searched the following databases for relevant trials:
Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley, The Cochrane Library, 2022, Issue 3);
MEDLINE (Ovid, 1946 to 31 March 2022);
Embase (Ovid, 1974 to 31 March 2022);
CINAHL (EBSCOhost, 1982 to 31 March 2022);
Transfusion Evidence Library (Evidentia Publishing, 1950 to 31 March 2022).
The Information Specialist (CD) also searched the following trials registries.
World Health Organization International Clinical Trials Registry Platform (ICTRP).
ClinicalTrials.gov.
The Cochrane sensitivity‐ and precision‐maximising RCT filter (Lefebvre 2011) was applied to Ovid MEDLINE, and adaptations of it to Ovid Embase and CINAHL, in combination with a systematic review filter (to include systematic reviews to allow manual screening for additional citations, see Searching other resources), based on the Scottish Intercollegiate Guidelines Network (SIGN) filter (www.sign.ac.uk/methodology/filters.html). Search strategies are displayed in full in Appendix 1.
Searching other resources
We checked the reference lists of all included studies for additional references to trials using SpiderCite. We also examined any relevant retraction statements and errata for included studies.
Data collection and analysis
We conducted and reported the review in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021) and PRISMA.
Selection of studies
Four review authors (AB, PW, CK, JS) used Covidence to screen abstracts of citations identified by the search strategy. Two review authors retrieved and screened the full text of all potentially eligible citations. We translated studies reported in non‐English language journals before assessment. Studies that were ineligible because we could not identify a vascular subgroup are detailed in the Excluded studies table.
Disagreements during screening were resolved by consensus, in consultation with review author LJE where necessary. We recorded the reasons for excluding studies at full text screening.
Data extraction and management
Two of four review authors (AB, GO, CK, JS) independently undertook data extraction from included studies. Data extraction forms were designed by AB and GO, and were piloted and modified before use.
We extracted the following data from each study.
General information: country of study, single or multi‐centre, funding source, publication type (abstract/full text/protocol), trial registration and timing (prospective or retrospective), year of publication.
Trial details: trial design, aims of the trial, funding, location, setting, number of centres, number of treatment arms, intention‐to‐treat analysis, power calculation and whether reached, treatment allocation method, randomisation, blinding, total number recruited, total number randomised, total number analysed in each study group, dropout rate, participant inclusion and exclusion criteria, antiplatelet and anticoagulant cessation protocol, transfusion strategy, comparability of groups according to participants' characteristics, length of follow‐up, stopping rules, thrombotic event definition, SAE definition.
Characteristics of participants: age, sex, weight, preoperative antiplatelet and anticoagulant medication (including washout period).
Characteristics of surgery: type of vascular operation, risk stratification, urgency of surgery (e.g. elective, non‐elective, mixed, not stated), surgical duration, aortic cross‐clamp use, aortic cross‐clamp duration, use of hypothermia, mean minimum temperature, percentage in each arm dropping out (with reasons), percentage in each arm lost to follow‐up.
Characteristics of intervention: number of arms, description of intervention and comparison arms, description of control arms (including placebo, usual care etc.), intervention(s) given, route of administration of intervention, timing of intervention, methods of dosing (e.g. standard, dose/kg, dose categories), dose, dose delivery (single bolus, multiple bolus, infusion).
Grouping interventions into treatment nodes for data synthesis
The included studies used a range of different interventions and control treatments, and we grouped them by type of intervention and comparator. There were not enough network connections, or data, to perform a network meta‐analysis, so we have presented these as pairwise comparisons, grouped by systemic drugs (all of which were compared to placebo) and topical dressings or glues (with a number of different comparators, including some placebo sponges).
Assessment of risk of bias in included studies
Two of four review authors (GO, AB, CK, JS) independently assessed the risk of bias using the Cochrane risk of bias 1 tool (RoB 1) (Higgins 2017). We resolved any disagreements by discussion.
We had planned to use the Confidence in Network Meta Analysis (CiNeMA 2017) tool, but this was not done because no network meta‐analysis was performed. We used the GRADE criteria to summarise the certainty of evidence for pairwise meta‐analysis.
This and other deviations from the published protocol are described in the Differences between protocol and review section.
Measures of treatment effect
We expressed measures of treatment effect using the criteria laid out by Cochrane for dichotomous outcomes and continuous outcomes (Higgins 2022).
For dichotomous outcomes, we recorded the number of events and total number of participants in treatment and control groups. For continuous outcomes, we recorded the mean, standard deviation and total number of participants in both the treatment and control groups, and median, range or interquartile range.
For dichotomous variables, we expressed the results as risk ratio (RR) with 95% confidence intervals (CI). Where the number of observed events was small (less than 5% of sample per group) and the trials had balanced treatment groups, we reported Peto's OR with 95% CI (Deeks 2017).
Where outcomes, for example red cell transfusions, were reported with different units (mLs, mL/kg, units) we converted these to the desired units (e.g. units of packed red cells) where possible.
Where we could not synthesise the data, we provided a descriptive narrative summary and tables with the available information. When we could not report available data in any of the formats described above, we provided a narrative report and, when appropriate, presented the data in tables.
Unit of analysis issues
We considered participants as the unit of analysis (McKenzie 2016).
We did not find any cluster‐randomised trials, or any trials with more than two eligible arms.
Dealing with missing data
We did not contact authors for missing data because most of the missing data was due to the trials not collecting information on the outcomes of relevance to this review.
Assessment of heterogeneity
Where the clinical and methodological characteristics of individual studies were sufficiently homogenous, we combined the data to perform a meta‐analysis (Deeks 2017). In standard pairwise meta‐analyses, we estimated the heterogeneity variances for each pairwise comparison.
Measures and tests for heterogeneity
During initial data extraction, we assessed if clinical and methodological heterogeneity were present by looking at trial and person characteristics across all included trials.
We summarised statistical heterogeneity using Tau2 and I2.
Assessment of reporting biases
We recorded the prespecified outcomes for each trial, where available, and compared them to reported outcomes. There were not enough data to explore small‐study biases; most of the included studies were very small.
Data synthesis
We had planned to perform a network meta‐analysis (NMA) but could not do so due to the large number of treatments with very little data to populate the network. The planned NMA methods were outlined in the protocol (Beverly 2020), and we will undertake this planned analysis should sufficient data be available in future updates.
Methods for direct treatment comparisons
We used RevMan Web to perform pairwise meta‐analysis (RevMan Web 2020), pooling data with a random‐effects model unless there were rare events, in which case we used Peto's OR (if the arms were balanced), which is only available using a fixed‐effect model. We presented the results as the pooled treatment effect with its 95% CI, alongside estimates of Tau2 and I2, and reported all data that could not be included in meta‐analyses in the characteristics of Included studies section.
Subgroup analysis and investigation of heterogeneity
Treatment effect modifiers
We planned to investigate potential effect modifiers by carrying out the following subgroup analyses. However, we were unable to perform any of these analyses due to a lack of data.
Endovascular versus open surgery
Perioperative antiplatelet and anticoagulant therapy
Aortic cross‐clamp use
Hypothermia use
Sensitivity analysis
We planned to do sensitivity analyses based on risk of bias and broad versus narrow treatment groupings, and to explore the impact of missing data, but there were insufficient data for any sensitivity analysis to be informative.
Summary of findings and assessment of the certainty of the evidence
We were unable to do a network meta‐analysis, so we have not used CiNeMA as specified in the protocol. We have detailed this deviation from the protocol in Differences between protocol and review.
We created summary of findings tables using GRADEPro (Schünemann 2021), and used RevMan Web 2020 to present the main findings of this review. We included the following outcomes in the summary of findings tables.
Red cell transfusions (units per participant) up to 30 days post surgery
All‐cause mortality at up to 30 days
Risk of receiving any allogeneic blood product up to 30 days post surgery
Risk of reoperation or repeat procedure for bleeding within 7 days
Risk of a thrombotic/thromboembolic event
We used the five GRADE considerations (risk of bias, inconsistency, imprecision, indirectness, and publication bias) to assess the certainty of the evidence as related to the studies reporting on the prespecified outcome (Atkins 2004). We used the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021; Schünemann 2021). When we downgraded the certainty of evidence we explained our decisions using footnotes, and added comments to aid the reader's understanding of the review when needed.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
See PRISMA flow diagram (Figure 1).
1.
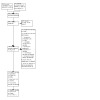
Flow diagram
We searched electronic databases up to 31 March 2022 and identified a total of 8328 records. We also screened a further 397 records that were referenced by the included trials, using SpiderCite. We removed 84 duplicates, leaving 8641 records for further assessment.
On initial assessment of the titles and abstracts of these 8641 records, we excluded 8481 records as irrelevant. Of the remaining 160 records, we excluded 120 studies (135 records) after screening the full text against eligibility criteria (see Excluded studies for further details). Four studies appeared to meet the inclusion criteria but did not report sufficient data to allow a decision on eligibility to be made (jRCTs041180163; NCT00618358; NCT00652314; NCT04083807); details are given in Studies awaiting classification).
We identified 24 potentially eligible trials, two ongoing trials (ChiCTR1900023323; NCT04803747) and 22 completed trials that were eligible for inclusion (Bajardi 2009; Bochicchio 2015; Chetter 2017; Clagett 1995; Czerny 2000; EUCTR2016‐003661‐26‐PL; Giovanacci 2002; Joseph 2004; Leijdekkers 2006; Lethagen 1991; Milne 1996; Minkowitz 2019; Monaco 2020; NCT02094885; Nenezic 2019; O'Donnell 2010 (abstract only); POISE‐3 2022; Qerimi 2013; Ranaboldo 1997; Robinson 2000; Taylor 2003; Weaver 2002).
Included studies
We included 22 RCTs, summarised in Table 3 with more detail for each trial in the Characteristics of included studies. One trial with 69 participants was reported in abstract form only without sufficient data to include in any of our analyses. The remaining 21 trials reported a total of 3324 participants analysed. The total randomised is unknown, as many trials did not explicitly report it.
1. Overview of included studies.
| Study ID | Country (centres); dates | No randomised (analysed) by intervention vs control | Population | % Elective | Intervention | Control | Comparison |
| Bajardi 2009 | Italy (1); June 2007 to June 2008 | 10 (10) vs 10 (10) |
Patients undergoing replacement of infra‐renal AAA.
Indirectness: no 85% male Mean age: 72.7 years (range 63 to 82) |
100% | TachoSil | Usual care | Topical drugs: thrombin + fibrin/collagen sponge vs usual care |
| Bochicchio 2015 | Netherlands (6), UK (10), USA (9); May 2012 to April 2013 | 118 (117) vs 58 (58) |
Patients undergoing arterial bypass 78%, arteriovenous graft formation for haemodialysis access 11%, carotid endarterectomy 9%, other 2%. Indirectness: no 68.8% male Mean age 65.6 years |
NR | Fibrocaps liquid | Gelatin sponge | Topical drugs: fibrin sealant vs gelatin sponge |
| Chetter 2017 | Canada (5), Spain (7), UK (7); March 2013 to December 2015 | 111 (110) vs 57 (57) |
Patients undergoing open arterial surgery Indirectness: "20.9% of fibrin sealant arm and 19.3% of usual care arm underwent carotid endarterectomy with patch angioplasty." 79.8% male Mean age: 65.5 years |
100% | Fibrin sealant (Grifols) | Usual care (manual compression with gauze) | Topical drugs: fibrin sealant vs usual care |
| Clagett 1995 | USA (1); NR | 43 (43) vs 48 (48) |
Patients undergoing infra‐renal aortic aneurysm repair or aortofemoral bypass surgery.
Indirectness: all patients were male 100% male Mean age: 63 years |
100% | Desmopressin | Placebo | Systemic drugs: desmopressin |
| Czerny 2000 | Austria (1), Germany (2); NR | NR (30) vs NR (30) |
Patients undergoing vascular reconstruction surgery with PTFE prostheses. Indirectness: no 73% male Mean age: 65.8 years |
100% | TachoComb H | Usual care (manual compression) | Topical drugs: fibrin/collagen sponge vs usual care |
| EUCTR2016‐003661‐26‐PL | Bosnia and Herzegovina, Croatia, Poland, UK, Serbia (16 centres total); NR |
NR (36) vs NR (18) |
Patients undergoing a planned open liver/soft tissue surgery, vascular surgery or spine surgery. Indirectness: no, vascular subgroup reported 78% male Mean age: 68.1 years |
100% | Peprostat soaked gelatin sponge | Saline soaked gelatin sponge | Topical drugs: novel agent/gelatin sponge vs oxidised cellulose |
| Giovanacci 2002 | Switzerland (3) July 1998 to January 2001 |
NR (79) vs NR (81) |
Patients undergoing femoral artery surgery with inguinal access. Indirectness: no 59% male Mean age: 71 years |
69% | Fibrin glue | Usual care | Topical drugs: fibrin sealant vs usual care |
| Joseph 2004 | UK (3) NR |
12 (11) vs 12 (11) |
Patients undergoing femoral anastomosis and femoral or carotid patch angioplasty with PTFE grafts. Indirectness: no % male NR Mean age: 68.4 years (range 51 to 86) |
NR | Tachocomb H | Usual care | Topical drugs: thrombin + fibrin/collagen sponge vs usual care |
| Leijdekkers 2006 | Netherlands (1); June 1996 to July 2001 | 16 (16) vs 19 (19) |
Patients undergoing repair of an asymptomatic infrarenal aortic aneurysm. Indirectness: no 80% male Median age: 68 years |
100% | Aprotinin | Placebo | Systemic drugs: aprotinin |
| Lethagen 1991 | Sweden (1); NR | 25 (22) vs 25 (22) |
Patients undergoing aorto‐iliac surgery. Indirectness: no 74% male Mean age: NR |
100% | Desmopressin | Placebo | Systemic drugs: desmopressin |
| Milne 1996 | Scotland (1); NR | 21 (21) vs 18 (18) |
Patients undergoing either arterial bypass surgery with a PTFE bypass graft or aortic aneurysm repair with a woven Dacron graft. Indirectness: no 77% male Median age: Fibrin sealant: 73 years; Usual care: 70 years |
NR | Fibrin sealant (fibrinogen and thrombin, which are mixed in the presence of factor XI11 and calcium to produce insoluble fibrin) | Usual care | Topical drugs: fibrin sealant vs usual care |
| Minkowitz 2019 | USA (20); January 2014 to November 2015 | NR (20) vs NR (11) |
Adult and paediatric patients undergoing non‐laparoscopic, non endovascular surgical procedure involving a native artery graft end to side proximal anastomosis. Indirectness: yes 17.1% vascular; vascular subgroup reported. Paediatric patients also included. Numbers NR 38% male Mean age: 55.8 years |
100% | Human thrombin on Gelfoam sponge | Bovine thrombin on Gelfoam sponge | Topical drugs: fibrin sealant vs usual care |
| Monaco 2020 | Italy (1); March 2015 to October 2017 | 50 (50) vs 50 (50) |
Patients undergoing surgical repair for AAA. Indirectness: no 93% male Median age: TXA: 69 years; Placebo: 71 years |
100% | Tranexamic acid | Placebo | Systemic drugs: TXA |
| NCT02094885 | China (9) NR |
125 (125) vs 127 (127) |
Patients undergoing elective vascular procedures. Indirectness: no 73% male Mean age: 56.9 SD 12.6 |
100% | Bioseal (porcine derived fibrin sealant) | Usual care (manual compression) | Topical drugs: fibrin sealant vs usual care |
| Nenezic 2019 | Hungary, Russian Federation, Serbia, USA (35 centres in total) August 2012 to December 2015 |
109 (109) vs 57 (57) |
Patients undergoing peripheral vascular procedures. Indirectness: 12.8% (fibrin sealant) and 17.5% (usual care) of participants underwent upper extremity vascular access procedures (not target population) 64.4% male Median age: Fibrin sealant: 64; Usual care: 61 (range 22 to 84) |
100% | Fibrin sealant (Grifols) | Usual care (manual compression with gauze) | Topical drugs: fibrin sealant vs usual care |
| O'Donnell 2010 | USA (NR); NR | NR (NR) vs NR (NR) [Total 69] |
Patients undergoing vascular surgery with anastomotic suture line bleeding. Indirectness: no % male NR Mean age NR |
NR | Vascular sealant (no further details) | Gelfoam/Thrombin | Topical drugs: unspecified sealant vs usual care |
| POISE‐3 2022 | Australia (17), Austria (1), Belgium (2), Brazil (2), Canada (12), Chile (2), China (4), Denmark (3), France (1), Germany (4), Hong Kong (1), India (14), Italy (4), Malaysia (5), Netherlands (2), New Zealand (3), Pakistan (2), Poland (3), Russian Federation (7), South Africa (3), Spain (7), UK (2), USA (13) (114 centres total); June 2018 to July 2021 | 699 (699; 684 PPA for safety) vs 700 (700; 676 PPA for safety) |
Patients undergoing major vascular surgery, at risk of developing bleeding or cardiovascular complications. (vascular subgroup of a larger trial of non‐cardiac surgery). Indirectness: no % male NR Mean age NR |
NR | Tranexamic acid | Placebo | Systemic drugs: TXA |
| Qerimi 2013 | Germany (1); February 2009 to July 2009 | 8 (7) vs 8 (8) |
Patients undergoing vascular reconstruction due to peripheral vascular disease with suture hole bleeding of peripheral arterial bypass anastomosis using PTFE graft prosthesis. Indirectness: no 69% male Mean age: Lyostypt: men mean 69.7 SD 7.0 women 80.0; Surgicel: men mean 70.5 SD 2.9 women mean 70.5 SD 8.2 |
100% | Lyostypt | Surgicel | Topical drugs: collagen dressing vs oxidised cellulose |
| Ranaboldo 1997 | UK (1); NR | NR (66) vs NR (62) |
Patients undergoing elective aortic reconstruction surgery. Indirectness: no 75.8% male Median age: Aprotinin: 68 years; Placebo: 70 years |
100% | Aprotinin | Placebo | Systemic drugs: aprotinin |
| Robinson 2000 | UK (9); December 1994 to June 1998 | NR (38) vs NR (39) |
Patients undergoing emergency repair for AAA. Indirectness: no 86% male Median age: Aprotinin: 74 years; Placebo: 73 years (range 52 to 88) |
0% | Aprotinin | Placebo | Systemic drugs: aprotinin |
| Taylor 2003 | USA (26); NR | NR (101) vs NR (99) |
Patients undergoing elective PTFE grafting including at least one end‐to‐side anastomosis of a PTFE graft to the common femoral artery. Indirectness: no 62.5% male Mean age: 64.0 years |
100% | Beriplast (fibrin sealant) | Thrombin‐soaked gelatin sponge | Topical drugs: fibrin sealant vs thrombin/gelatin sponge |
| Weaver 2002 | USA (4); NR | 43 (43) vs 46 (46) |
Patients undergoing reconstructive vascular surgery or arteriovenous access procedures. Indirectness: no % male: NR Mean age: 65.4 years |
NR | FloSeal (glutaraldehyde cross linked gelatin with thrombin) | Gelfoam thrombin | Topical drugs: synthetic sealant vs thrombin/gelatin sponge |
AAA ‐ Abdominal aortic aneurysm; NR ‐ not reported; PPA ‐ per protocol analysis; PTFE ‐ polytetrafluoroethylene; TXA ‐ tranexamic acid
Trial design
All of the included studies were two‐arm parallel randomised controlled trials. Six were multinational trials (Bochicchio 2015; Chetter 2017; Czerny 2000; EUCTR2016‐003661‐26‐PL; Nenezic 2019; POISE‐3 2022); seven single‐country multicentre trials (Giovanacci 2002; Joseph 2004; Minkowitz 2019; NCT02094885; Robinson 2000; Taylor 2003; Weaver 2002), and eight of the trials were single centre trials (Bajardi 2009; Clagett 1995; Leijdekkers 2006; Lethagen 1991; Milne 1996; Monaco 2020; Qerimi 2013; Ranaboldo 1997). O'Donnell 2010 is published as an abstract only, and it is unclear if this was a single or multicentre trial.
Trial size
The numbers of participants enrolled in all trials, and relevant for this review, ranged between 16 (Qerimi 2013) and 1399 (POISE‐3 2022); POISE‐3 2022 was a multinational trial comparing tranexamic acid to placebo for higher risk surgical procedures conducted in 22 countries, which recruited 9535 people in total. There were nine trials that included more than 100 participants (Bochicchio 2015; Chetter 2017; Giovanacci 2002; Minkowitz 2019; NCT02094885; Nenezic 2019; POISE‐3 2022; Ranaboldo 1997; Taylor 2003) (Table 3).
Participants
Most of the trials only included people undergoing elective procedures (Table 3). Robinson 2000 was conducted in participants undergoing emergency repair of ruptured abdominal aortic aneurysms, and Giovanacci 2002 included 31% of participants who were undergoing urgent or emergency femoral artery surgery. Six trials did not specify the proportion of elective or urgent surgery (Bochicchio 2015; Joseph 2004; Milne 1996; O'Donnell 2010; POISE‐3 2022; Weaver 2002).
Setting
Most of the trials were only conducted in high income countries according to the World Bank classification (Table 3). POISE‐3 2022 was conducted in middle‐ (India and Pakistan) and upper‐middle‐income countries (Brazil, China, Malaysia, Russian Federation and South Africa) as well as high‐income countries. EUCTR2016‐003661‐26‐PL and Nenezic 2019 were conducted in upper‐middle‐income countries (Serbia and Bosnia EUCTR2016‐003661‐26‐PL; Serbia and Russian Federation Nenezic 2019) as well as high‐income countries. NCT02094885 was conducted in China.
Intervention
Of the 22 included RCTs:
-
seven assessed systemic drug treatments versus placebo:
three aprotinin (Leijdekkers 2006; Ranaboldo 1997; Robinson 2000);
two desmopressin (Clagett 1995; Lethagen 1991);
two tranexamic acid (Monaco 2020; POISE‐3 2022).
-
15 assessed topical drugs, dressings or glues:
nine compared an intervention versus usual care: five assessed fibrin sealant (Chetter 2017; Giovanacci 2002; Milne 1996; NCT02094885; Nenezic 2019); two assessed thrombin plus fibrin/collagen sponge (Bajardi 2009; Joseph 2004); one assessed fibrin/collagen sponge (Czerny 2000); and one assessed an unspecified sealant (O'Donnell 2010);
six compared two different interventions: human thrombin gelatin sponge versus bovine thrombin gelatin sponge (Minkowitz 2019); collagen dressing versus oxidised cellulose (Qerimi 2013); fibrin sealant versus thrombin gelatin sponge (Taylor 2003); fibrin sealant versus gelatin sponge (Bochicchio 2015); synthetic sealant versus thrombin gelatin sponge (Weaver 2002); and a new agent (Prepostat) that polymerises fibrinogen versus gelatin sponge (EUCTR2016‐003661‐26‐PL).
We did not identify any completed or ongoing trials that assessed the following interventions: ε‐aminocaproic acid (EACA); prothrombin complex concentrate (PCC); recombinant factor VII (rFVII); factor XIII (FXIII); fibrinogen concentrate. No systemic drugs were compared against each other.
Outcomes
One trial did not report any of this review's outcomes (O'Donnell 2010). Many of the trials only reported a limited number of this review's outcomes. Two of these trials were vascular subgroups of larger trials with a mixed surgical population, so there was limited outcome data for the vascular subgroup (EUCTR2016‐003661‐26‐PL; POISE‐3 2022). Table 4 summarises the outcomes reported by the trials.
2. Reporting of review endpoints.
| Trial ID | Max FU | RBCu | M30 | M90 | AABT | PRC | FFP | PLT | Redo | TE | MI | CVA | DVT | PE | SAE | LoHS |
| Systemic drugs | ||||||||||||||||
| Aprotinin vs placebo | ||||||||||||||||
| Leijdekkers 2006 | In‐hospital | Y | Ya | ‐ | ‐ | Nb | Nb | Nb | Y | ‐ | ‐ | ‐ | ‐ | ‐ | Y | ‐c |
| Ranaboldo 1997 | 30 days | ≈ | Y | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Y | Y | Y | Y | ≈ | ‐ |
| Robinson 2000 | 30 days | ≈ | Y | ‐ | ‐ | ‐ | Nb | Nb | ‐ | ‐ | Y | Y | ‐ | ‐ | Y | ≈ |
| Desmopressin vs placebo | ||||||||||||||||
| Clagett 1995 | 30 days | Y | Y | ‐ | ‐ | Y | N | N | ‐ | Y | Y | N | Y | Y | ‐ | ‐ |
| Lethagen 1991 | Unclear | Y | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ≈ | ≈ | ‐ | ‐ | ‐ | ≈ | ‐ |
| Tranexamic acid vs placebo | ||||||||||||||||
| Monaco 2020 | 28 days/1 year | ‐ | Y | N | Yd | Y | Yd | ‐ | Y | ≈ | Y | ‐ | Y | Y | ‐ | Y |
| POISE‐3 2022 | 30 days | ‐ | ‐e | ‐ | ‐ | ‐e | ‐ | ‐ | ‐e | Y | ‐e | ‐e | ‐e | ‐e | ‐e | ‐e |
| Trial ID | Max FU | RBCu | M30 | M90 | AABT | PRC | FFP | PLT | Redo | TE | MI | CVA | DVT | PE | SAE | LoHS |
| Topical drugs | ||||||||||||||||
| Fibrin/collagen sponge vs usual care | ||||||||||||||||
| Czerny 2000 | Intraoperative (90 days AEs) |
‐ | ‐ | ‐ | Yd | ‐ | ‐ | ‐ | ‐ | ≈ | ‐ | ‐ | ‐ | ‐ | ≈ | ≈ |
| Thrombin+fibrin/collagen sponge vs usual care | ||||||||||||||||
| Bajardi 2009 | Unclear | Yf | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ≈ | Y | ‐ | ‐ | ‐ | ‐ | ‐ |
| Joseph 2004 | Intraoperative | ‐ | Ya | ‐ | Yd | ‐ | ‐ | ‐ | ‐ | Y | ‐ | ‐ | ‐ | ‐ | Y | ‐ |
| Human thrombin/gelatin sponge vs bovine thrombin/gelatin sponge | ||||||||||||||||
| Minkowitz 2019 | Unclear | ‐ | Ya | Y | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Ne | ‐ |
| Collagen dressing vs oxidised cellulose | ||||||||||||||||
| Qerimi 2013 | 30 days | ‐ | Y | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Y | Y | Y | Y | Y | Y | ‐ |
| Novel agent (Peprostat) vs placebo/gelatin sponge | ||||||||||||||||
| EUCTR2016‐003661‐26‐PL | 30 days | ‐ | Y | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Y | Y | ‐ |
| Trial ID | Max FU | RBCu | M30 | M90 | AABT | PRC | FFP | PLT | Redo | TE | MI | CVA | DVT | PE | SAE | LoHS |
| Fibrin sealant vs usual care | ||||||||||||||||
| Chetter 2017 | 24 hours | ‐ | Y | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ≈ | ‐ |
| Giovanacci 2002 | Unclear | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Y | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Milne 1996 | 26 weeks | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Y | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| NCT02094885 | 30 days | ‐ | Y | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Y | Y | Y | Y | Y | ‐ |
| Nenezic 2019 | 7 months | ‐ | Y | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Yg | ‐ |
| Fibrin sealant vs gelatin sponge | ||||||||||||||||
| Bochicchio 2015 | 29 days | ‐ | Y | ‐ | ‐ | Y | ‐ | ‐ | Y | ‐ | ‐ | ‐ | ‐ | ‐ | Y | ‐ |
| Fibrin sealant vs thrombin/gelatin sponge | ||||||||||||||||
| Taylor 2003 | 30 days | ‐ | Y | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Synthetic sealant vs thrombin/gelatin sponge | ||||||||||||||||
| Weaver 2002 | 6 to 8 weeks | ‐ | Y | ‐ | ‐ | ‐ | ‐ | ‐ | Y | Y | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Unspecified sealant vs usual care | ||||||||||||||||
| O'Donnell 2010 | 30 days | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Key | ||||||||||||||||
| Max FU | maximum follow‐up | |||||||||||||||
| Primary outcomes (review) | ||||||||||||||||
| RBCu | red cell transfusions (units per participant) at up to 30 days post surgery | |||||||||||||||
| M30 | all‐cause mortality at up to 30 days; | |||||||||||||||
| M90 | all‐cause mortality between 31 to 90 days | |||||||||||||||
| Secondary outcomes (review) | ||||||||||||||||
| AABT | risk of receiving any allogeneic blood product at up to 30 days post surgery (PRC, FFP, PLT composite) | |||||||||||||||
| PRC | packed red cells (PRC) | |||||||||||||||
| FFP | fresh frozen plasma (FFP) | |||||||||||||||
| PLT | platelets (PLTs) | |||||||||||||||
| Redo | risk of reoperation or repeat procedure for bleeding within 7 days | |||||||||||||||
| TE | risk of a thrombotic/thromboembolic event (MI, CVA, DVT, PE composite) | |||||||||||||||
| MI | myocardial Infarction at up to 30 days | |||||||||||||||
| CVA | cerebrovascular attack at up to 30 days | |||||||||||||||
| DVT | deep vein thrombosis at up to 90 days | |||||||||||||||
| PE | pulmonary embolus at up to 90 days | |||||||||||||||
| SAE | risk of a serious adverse event at up to 30 days post surgery | |||||||||||||||
| LoHS | length of hospital stay (days) | |||||||||||||||
| Reporting of outcome | ||||||||||||||||
| Y | Reported in a form which can be meta‐analysed | |||||||||||||||
| ≈ | Reported in a form which cannot be meta‐analysed | |||||||||||||||
| I | Measured but not reported | |||||||||||||||
| ‐ | Not measured | |||||||||||||||
a Follow‐up not clearly defined b Units reported c Length of stay in intensive care unit reported d Intraoperative only e Vascular subgroup not reported f “Peri‐operative” (undefined) g At six weeks
Funding source
Funding sources and statements of independence of authors are summarised in Table 5.
3. Industry funding and independence of authors.
| Trial ID | Industry funded? | Statement of independence of authors? | Industry employees or consultants on authorship? |
| Bajardi 2009 | NR | ‐ | ‐ |
| Bochicchio 2015 | Yes | No | Yes |
| Chetter 2017 | Yes | No | Yes |
| Clagett 1995 | NR | ‐ | ‐ |
| Czerny 2000 | Yes | No | Yes |
| EUCTR2016‐003661‐26‐PL | Yes | Trial registration; no publication available | |
| Giovanacci 2002 | Yes | No | No |
| Joseph 2004 | Yes | No | No |
| Leijdekkers 2006 | Yes | No | No |
| Lethagen 1991 | No | NA | NA |
| Milne 1996 | No | NA | NA |
| Minkowitz 2019 | Yes | No | Yes |
| Monaco 2020 | Supported by an unrestricted grant, no direct funding or sponsor control. | ||
| NCT02094885 | Yes | Trial registration; no publication available | |
| Nenezic 2019 | Yes | No | Yes |
| O'Donnell 2010 | NR | ‐ | ‐ |
| POISE‐3 2022 | No | NA | NA |
| Qerimi 2013 | Yes | No | Yes |
| Ranaboldo 1997 | Yes | No | Yes |
| Robinson 2000 | Yes | No | No |
| Taylor 2003 | Yes | No | Yes |
| Weaver 2002 | Yes | No | No |
NR ‐ not reported; NA ‐ not applicable; ‐ Unanswerable
All but three trials were funded by the drug manufacturer (Lethagen 1991; Milne 1996; POISE‐3 2022), or provided no details of funding source (Bajardi 2009; Clagett 1995). One of these trials was funded by an unrestricted grant (Monaco 2020), which implies independence of the authors. Three industry‐sponsored trials did not provide any details of the sponsor's involvement in the trial process (EUCTR2016‐003661‐26‐PL; NCT02094885; O'Donnell 2010).
Thirteen industry‐funded trials did not include a statement of authorial independence (Bochicchio 2015; Chetter 2017; Czerny 2000; Giovanacci 2002; Joseph 2004; Leijdekkers 2006; Minkowitz 2019; Nenezic 2019; Qerimi 2013; Ranaboldo 1997; Robinson 2000; Taylor 2003; Weaver 2002). Eight trials included employees of the manufacturer on the author list (Bochicchio 2015; Chetter 2017; Czerny 2000; Minkowitz 2019; Nenezic 2019; Qerimi 2013; Ranaboldo 1997; Taylor 2003). Six trials received additional material support from the manufacturer such as grants to some of the authors, or the services of medical writers (Bochicchio 2015; Chetter 2017; Minkowitz 2019; Nenezic 2019; Robinson 2000; Taylor 2003).
Ongoing studies
We identified two ongoing trials (Characteristics of ongoing studies). One compares fibrin sealant to an unspecified control and plans to recruit 500 participants (ChiCTR1900023323). The primary outcomes specified are death due to arterial disease and reintervention rates.
The second trial, TRACTION (NCT04803747), compares tranexamic acid against placebo and plans to recruit 8320 participants undergoing a range of different surgeries, including vascular procedures. It was registered in March 2021 and the primary outcomes are the proportion of participants requiring red blood cell transfusion and incidence of venous thromboembolism (DVT or PE). It plans to complete recruitment in April 2023.
Excluded studies
We excluded 135 records of 120 studies at the full‐text screening stage. Of these, 26 studies (26 records) were an ineligible intervention. One study (3 records) used a blood product control, and was excluded as it had an ineligible comparator (Morrison 2019), and 59 studies (71 records) had an ineligible population, including those studies where there was a mixed surgical population with no vascular subgroup available.
Twenty‐eight studies (28 records) were excluded as having an ineligible study design. Where this may be ambiguous, these have been listed in Characteristics of excluded studies (Brunkwall 2007; Koncar 2008; Koncar 2011; Pilon 2010; Sauer 2016; Shimamura 1998). We deemed Clagett 1996 and Thompson 2013 not relevant as they were reviews.
Of the studies we excluded as having an ineligible participant population, nine were excluded at a late stage because they contained mixed populations, with less than 80% of participants meeting our eligibility requirements (Chalmers 2010; Develle 2020; Glickman 2002; Lumsden 2006; NCT00439309; NCT01500135; Saha 2012; Stone 2012; Verhoef 2015). Of these, five studies included more than 20% of the participants receiving arterio‐venous access for haemodialysis, which is not an eligible intervention in this review, and no subgroup of eligible participants was available. The studies were: Chalmers 2010 (33% haemodialysis access); Glickman 2002 (28% haemodialysis); Lumsden 2006 (54% haemodialysis); Saha 2012 (44% haemodialysis) and Stone 2012 (22.7% haemodialysis). We excluded four of the nine studies as we could not establish how many people in each group received arterio‐venous access for dialysis, and subgroup information was not available (Develle 2020; NCT00439309; NCT01500135; Verhoef 2015).
See table of Excluded studies for further details.
Studies awaiting classification
Four studies appeared to meet the inclusion criteria but did not report sufficient data to allow a decision on eligibility to be made (jRCTs041180163; NCT00618358; NCT00652314; NCT04083807); details are given in Studies awaiting classification.
Risk of bias in included studies
The primary risk of bias identified in this group of studies is the lack of blinding in the trials of topical drug treatments, which is unavoidable with most of these interventions. There were also concerns over the independence of the authors for trials funded by the manufacturer of the product.
Overall, the trials were of reasonable quality, with around a third having a low risk of bias and only around 10% having a high risk of bias for reasons other than a lack of blinding or non‐independence from the funder.
Figure 2 is a summary risk of bias chart for the whole review, The risk of bias assessment for each individual trial is described in detail in the trial summaries (Characteristics of included studies) and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Only two trials were at low risk of bias across all domains (Monaco 2020; POISE‐3 2022).
Allocation
Two trials were at high risk of selection bias due to issues with both random sequence generation and allocation concealment (Czerny 2000; Giovanacci 2002).
Twelve trials were at unclear risk of selection bias because the trials gave little or no information about how randomisation was done, or the allocation concealed, or both (Bajardi 2009; Bochicchio 2015; EUCTR2016‐003661‐26‐PL; Joseph 2004; Lethagen 1991; Milne 1996; Minkowitz 2019; NCT02094885; Nenezic 2019; O'Donnell 2010; Ranaboldo 1997; Taylor 2003; Weaver 2002).
Seven trials were at low risk of selection bias for random sequence generation and allocation concealment (Chetter 2017; Clagett 1995; Leijdekkers 2006; Monaco 2020; POISE‐3 2022; Qerimi 2013; Robinson 2000).
Blinding
All seven trials that assessed a systemic intervention were at low risk of performance and detection bias due to the use of a matched placebo (Clagett 1995; Leijdekkers 2006; Lethagen 1991; Monaco 2020; POISE‐3 2022; Ranaboldo 1997; Robinson 2000).
Two trials that compared two different topical interventions were at low risk of performance and detection bias (EUCTR2016‐003661‐26‐PL; Minkowitz 2019).
Around 70% of trials that compared two different topical interventions could not be blinded to healthcare workers performing the procedure because of the distinctive appearance of the topical treatments being compared; for example topical liquids were compared to a sponge. However, in these trials there was no description of how participants or outcomes assessors were blinded to the intervention. We classified 11 trials as at high risk of performance and detection bias because there was no evidence of independent outcome assessors who were blinded to the intervention (Bajardi 2009; Bochicchio 2015; Chetter 2017; Czerny 2000; Giovanacci 2002; Milne 1996; NCT02094885; O'Donnell 2010; Qerimi 2013; Taylor 2003; Weaver 2002).
Joseph 2004 and Nenezic 2019 included independent outcome assessors, but there was no information on blinding of participants; we classified these as high risk for performance bias and unclear risk of bias for detection bias.
Incomplete outcome data
Fourteen trials were at low risk of attrition bias due to little or no loss to follow‐up (Bajardi 2009; Bochicchio 2015; Clagett 1995; Joseph 2004; Leijdekkers 2006; Milne 1996; Monaco 2020; NCT02094885; Nenezic 2019; POISE‐3 2022; Qerimi 2013; Robinson 2000; Taylor 2003; Weaver 2002).
We only considered one trial to be at high risk of attrition bias, due to the proportion who had been randomised but did not receive the allocated intervention (EUCTR2016‐003661‐26‐PL). The other trials were at unclear risk of attrition bias due to some loss to follow‐up, but this appeared to be balanced between arms (Chetter 2017; Czerny 2000; Giovanacci 2002; Lethagen 1991; Minkowitz 2019; O'Donnell 2010; Ranaboldo 1997).
Selective reporting
Around 90% of trials reported all their stated primary and secondary outcomes, although only half had a published protocol or trial registration for verification that these outcomes were prespecified.
Two trials were at high risk of selective reporting bias (Czerny 2000; Milne 1996). In Czerny 2000 the authors stated in their methods section that adverse events were classified as serious or not, but there was no reporting of serious adverse events ‐ only all adverse events. In Milne 1996, the methods stated that they were going to report outcomes, but no numerical data were provided in the results.
Twelve trials were at unclear risk of selective reporting bias (Bajardi 2009; Chetter 2017; Clagett 1995; Giovanacci 2002; Leijdekkers 2006; Lethagen 1991; Minkowitz 2019; O'Donnell 2010; Ranaboldo 1997; Robinson 2000; Taylor 2003; Weaver 2002). In one study this was because serious adverse events were not fully described (Minkowitz 2019). In one study the trial registration referenced is incorrect and we were unable to locate any registration or protocol for the study (Chetter 2017). For one study, the only report available was an abstract with insufficient information to make a judgement about selective outcome reporting (O'Donnell 2010). In the remaining nine studies there was no available trial registration or published protocol (Bajardi 2009; Clagett 1995; Giovanacci 2002; Leijdekkers 2006; Lethagen 1991; Ranaboldo 1997; Robinson 2000; Taylor 2003; Weaver 2002).
Eight studies were at low risk for selective reporting bias (Bochicchio 2015; EUCTR2016‐003661‐26‐PL;Joseph 2004; Monaco 2020; NCT02094885; Nenezic 2019; POISE‐3 2022; Qerimi 2013).
Other potential sources of bias
We identified a high risk of bias for 'other' reasons in three trials (Joseph 2004; Minkowitz 2019; Robinson 2000), of which two were because of early stopping decisions that were not fully explained (Joseph 2004; Robinson 2000), and one was because of concerns about the effectiveness of the masking used (Minkowitz 2019). Risk of bias was unclear in the 'other' domain for four trials (O'Donnell 2010; Qerimi 2013; Ranaboldo 1997; Weaver 2002), of which two were because of insufficient information about baseline characteristics (O'Donnell 2010; Weaver 2002). In one trial there was a change in protocol during the study (Ranaboldo 1997), and in one very small trial (16 participants) there was a large baseline imbalance by sex with an unclear impact on outcomes (Qerimi 2013). The remaining studies were at low risk of other bias (Bajardi 2009; Bochicchio 2015; Chetter 2017; Clagett 1995; Czerny 2000; EUCTR2016‐003661‐26‐PL; Giovanacci 2002; Leijdekkers 2006; Lethagen 1991; Milne 1996; Monaco 2020; NCT02094885; Nenezic 2019; POISE‐3 2022; Taylor 2003).
Effects of interventions
We were able to obtain only very limited data from the trials identified, due to small sample sizes, a lack of reporting of the outcomes we are interested in, and the large number of treatment comparisons (13) relative to the number of trials (22), limiting the amount of information that could be pooled. Network meta‐analysis was not possible and most of the data sought for the pairwise meta‐analyses were unavailable (see Data extraction and management).
One trial did not report any of our review outcomes (O'Donnell 2010), so 21 trials are included in the quantitative analysis.
POISE‐3 2022 was a multinational trial comparing tranexamic acid to placebo for higher risk surgical procedures, which included 1399 people undergoing higher risk vascular surgeries. Of our prespecified review outcomes, only thromboembolic events were reported for the vascular subgroup in this trial.
All the other included trials were small or very small, ranging from 16 to 252 randomised participants, and many did not report our prespecified outcomes. Table 4 summarises the data available for each outcome, grouped by treatment comparison, noting where trials did not measure the outcome or did not report it in a form suitable for meta‐analysis.
The 15 trials of topical dressings or sealants, in particular, were unlikely to report our clinical outcomes of interest. These trials focused on intraoperative outcomes, such as time to haemostasis, with some reporting of adverse events in the weeks following surgery but rarely any postoperative transfusion requirements.
The seven trials of systemic drug treatments were more likely to report transfusion‐related outcomes, although some reported RBC units as medians instead of means and so could not be meta‐analysed. The transfusion‐related outcomes reported by the trials of systemic drug treatments are summarised in Table 6, including information which could not be included in the forest plots.
4. Transfusion outcomes for trials of systemic drugs.
| Trial ID | Red cell transfusions (units per participant) up to 30 days post surgery | Risk of receiving any allogeneic blood product at up to 30 days post surgery | |||
| All | Red blood cells | Fresh frozen plasma (FFP) | Platelets | ||
| Aprotinin vs placebo | |||||
| Leijdekkers 2006 |
Intraoperative Aprotinin Mean (SD) 4.1 (3.1) vs Placebo Mean (SD) 4.1 (2.9) P = 0.95 Postoperative Aprotinin mean (SD) 0.44 (0.7) vs Placebo mean (SD) 2.0 (7.9) P = 0.43 Total NR |
NR |
NR | NR |
Intraoperative “In both groups, only one patient received 1 unit of platelets.” Implied intraoperatively. Postoperative NR Total NR |
| Ranaboldo 1997 |
Intraoperative NR Postoperative < 24 hr: Aprotinin median (IQR) 1 (0 to 2) vs Placebo median 1 (0 to 2) > 24 hr: Aprotinin median (IQR) 0 (0 to 0) vs Placebo median (IQR) 0 (0 to 0) Total Aprotinin median (IQR) 3 (2 to 5) vs Placebo median (IQR) 3 (2 to 5) See footnotea |
NR |
NR |
NR |
NR |
| Robinson 2000 |
Perioperative Aprotinin median (range) 7 (0 to 20) vs Placebo median (range) 10 (2 to 32) Postoperative Aprotinin median (range) 1 (0 to 14) vs Placebo median (range) 3 (0 to 13) P = 0.02 Total Aprotinin median (range) 10 (2 to 29) vs Placebo median (range) 14 (4 to 38) P = 0.053 |
NR |
NR |
Perioperative NR (units only) |
Perioperative NR (units only) |
| Desmopressin vs placebo | |||||
|
Clagett 1995 |
Intraoperative Desmopressin mean (SD) 1.9 (2.2) vs Placebo mean (SD) 1.7 (1.8) Postoperative Desmopressin mean (SD) 1.2 (1.6) vs Placebo mean (SD) 1.0 (2.0) Total Desmopressin mean (SD) 3.1 (3.0) vs Placebo mean (SD) 2.7 (3.0) |
NR |
Intraoperative NR Postoperative NR Total Desmopressin 33/43 vs Placebo 35/48 |
NR |
NR |
| Lethagen 1991 | Converted to units using 280 mL per unit to provide the values for this study in Analysis 1.1 Intraoperative Desmopressin mean (SD) 600 (124) vs Placebo mean (SD) 960 (168) Postoperative Desmopressin mean (SD) 454 (97) vs Placebo mean (SD) 228 (70) Total Desmopressin mean (SD) 1054 (147) vs Placebo mean (SD) 1186 (178) |
NR |
NR |
NR |
NR |
| Tranexamic acid (TXA) vs placebo | |||||
|
Monaco 2020 |
NR |
Intraoperative TXA 2/50 vs Placebo 3/50 Postoperative NR Total NR |
Intraoperative TXA 1/50 vs Placebo 2/50 Postoperative TXA 6/50 vs Placebo 10/50 Total TXA 7/50 vs Placebo 12/50 |
Intraoperative TXA 1/50 vs Placebo 1/50 Postoperative NR Total NR |
NR |
| POISE‐3 2022 | Blood outcomes not reported for vascular subgroup | ||||
IQR ‐ interquartile range; NR ‐ not reported; SD: standard deviation; TXA ‐ tranexamic acid
aNumbers in text inconsistent with table referred to in the text: “Aprotinin‐treated patients received a mean of 2.2 (range 1‐12, median 2) units of blood, compared with 1.9 (range 1‐7, median 2) units for control patients. No differences were observed between the aprotinin‐ and placebo‐treated patients for transfusion requirements after operation or for the total amount of blood transfused (Table 3).”
Given the very limited amount of data available, we have only summarised two of the thirteen treatment comparisons in summary of findings tables; tranexamic acid vs placebo (Table 1) because it includes the only trial with a reasonably large sample size, and fibrin sealant vs usual care (Table 2) because it includes more trials (5) than any other comparison.
The data we were able to obtain for each outcome are summarised below.
Primary outcomes
Red cell transfusions (units per participant) at up to 30 days post surgery
Systemic drug treatments
Three trials, one of aprotinin (Leijdekkers 2006) and two of desmopressin (Clagett 1995; Lethagen 1991), reported this outcome in a form that can be meta‐analysed (Analysis 1.1). Two others (Ranaboldo 1997; Robinson 2000) reported medians with range or interquartile range (Table 6).
1.1. Analysis.

Comparison 1: Systemic drug treatments, Outcome 1: Number of red blood cells (units) transfused per participant up to 30 days post surgery
There is very limited evidence for this outcome for any of the systemic drugs included in this review.
Topical drug treatments
One trial of topical treatments reported this outcome (Bajardi 2009). This trial compared thrombin + fibrin/collagen sponge versus usual care for the (undefined) "peri‐operative" period only (Analysis 2.1).
2.1. Analysis.

Comparison 2: Topical drug treatments, Outcome 1: Number of red blood cells (units) transfused per participant up to 30 days
There is very limited evidence for this outcome for any of the topical treatments included in this review.
All‐cause mortality at up to 30 days; and between 31 to 90 days
Our other primary outcome, mortality at 30 days and up to 90 days, was reported by a larger proportion of trials than any other outcome, although few had follow‐up beyond 30 days.
Systemic drug treatments
Five trials of systemic drugs reported all‐cause mortality up to 30 days, with Leijdekkers 2006 having only in‐hospital follow‐up (Analysis 1.2). No trials reported all‐cause mortality at 90 days; Monaco 2020 reported mortality at one year. Mortality rates were low and all of the trials reporting this endpoint are small, and so the confidence intervals are very wide.
1.2. Analysis.

Comparison 1: Systemic drug treatments, Outcome 2: All‐cause mortality at up to 30 days
There is very limited evidence for this outcome for any of the systemic drugs included in this review.
Topical drug treatments
Ten trials of topical treatments (Analysis 2.2) reported all‐cause mortality up to 30 days, with Joseph 2004 having only in‐hospital follow‐up. Only one trial reported all‐cause mortality up to 90 days (Minkowitz 2019). Mortality rates were low and all of the trials are small, and so the confidence intervals are very wide.
2.2. Analysis.

Comparison 2: Topical drug treatments, Outcome 2: All‐cause mortality at up to 30 days
There is very limited evidence for this outcome for any of the topical treatments included in this review.
Secondary outcomes
Risk of receiving any allogeneic blood product at up to 30 days post surgery
Systemic drug treatments
One trial of systemic drugs reported this outcome (Monaco 2020) (Analysis 1.3).
1.3. Analysis.

Comparison 1: Systemic drug treatments, Outcome 3: Risk of receiving any allogeneic blood product at up to 30 days post surgery
There is very limited evidence for this outcome for any of the systemic drugs included in this review.
Topical drug treatments
Two trials of topical treatments reported this outcome for the intraoperative period only (Czerny 2000; Joseph 2004) (Analysis 2.3).
2.3. Analysis.

Comparison 2: Topical drug treatments, Outcome 3: Risk of receiving any allogeneic blood product at up to 30 days post surgery
There is no evidence for this outcome for any of the topical treatments included in this review.
Risk of reoperation or repeat procedure for bleeding within 7 days
Systemic drug treatments
Two trials of systemic drugs reported the need for reoperation (Leijdekkers 2006; Monaco 2020) (Analysis 1.4). Reintervention rates were low, and both trials were small, so the confidence intervals are very wide.
1.4. Analysis.

Comparison 1: Systemic drug treatments, Outcome 4: Risk of reoperation or repeat procedure for bleeding within 7 days
There is very limited evidence for this outcome for any of the systemic drugs included in this review.
Topical drug treatments
Three trials of topical treatments reported the need for reoperation (Bochicchio 2015; Giovanacci 2002; Weaver 2002) (Analysis 2.4). Reintervention rates were low and all of the trials were small, so the confidence intervals are very wide.
2.4. Analysis.

Comparison 2: Topical drug treatments, Outcome 4: Risk of reoperation or repeat procedure for bleeding within 7 days
There is very limited evidence for this outcome for any of the topical treatments included in this review.
Risk of a thrombotic/thromboembolic event
Systemic drug treatments
Our secondary composite outcome of thromboembolic events (any of MI, CVA/stroke, DVT, PE; Analysis 1.5) was reported by two trials of systemic drugs (Clagett 1995; POISE‐3 2022), with three others (Monaco 2020; Ranaboldo 1997; Robinson 2000) reporting some or all of these events separately (Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9).
1.5. Analysis.

Comparison 1: Systemic drug treatments, Outcome 5: Risk of a thrombotic/thromboembolic event (MI, CVA, DVT, PE) up to 30‐day follow‐up
1.6. Analysis.

Comparison 1: Systemic drug treatments, Outcome 6: Risk of myocardial infarction up to 30 days
1.7. Analysis.

Comparison 1: Systemic drug treatments, Outcome 7: Risk of CVA or stroke up to 30 days
1.8. Analysis.

Comparison 1: Systemic drug treatments, Outcome 8: Risk of deep vein thrombosis up to 90 days
1.9. Analysis.

Comparison 1: Systemic drug treatments, Outcome 9: Risk of pulmonary embolism up to 90 days
Thromboembolic events were more common in the trials of systemic drugs, all of which included only major vascular procedures; six of them only included aortic aneurysms. There was no evidence that there was a higher risk of thromboembolic events associated with any of these drugs, but the sample sizes were all too small to rule out clinically important differences. This was the only endpoint available for the vascular subgroup of the large POISE‐3 trial, comparing tranexamic acid with placebo (Table 1).
There is very limited evidence for this outcome for any of the systemic drugs included in this review.
Topical drug treatments
Our secondary composite outcome of thromboembolic events (any of MI, CVA/stroke, DVT, PE; (Analysis 2.5) was reported by six trials of topical treatments (Bajardi 2009; Czerny 2000; Joseph 2004; Milne 1996; Qerimi 2013; Weaver 2002), with three other trials reporting some or all of these events separately (EUCTR2016‐003661‐26‐PL; Milne 1996; NCT02094885) (Analysis 2.6; Analysis 2.7; Analysis 2.8; Analysis 2.9).
2.5. Analysis.

Comparison 2: Topical drug treatments, Outcome 5: Risk of a thrombotic/thromboembolic event (MI, CVA, DVT, PE) up to 30‐day follow‐up
2.6. Analysis.

Comparison 2: Topical drug treatments, Outcome 6: Risk of a myocardial infarction up to 30 days
2.7. Analysis.

Comparison 2: Topical drug treatments, Outcome 7: Risk of a CVA or stroke up to 30 days
2.8. Analysis.

Comparison 2: Topical drug treatments, Outcome 8: Risk of a deep vein thrombosis up to 90 days
2.9. Analysis.

Comparison 2: Topical drug treatments, Outcome 9: Risk of a pulmonary embolism up to 90 days
The reported event rates in trials of topical treatments, which tended to include lower risk surgical procedures than the trials of systemic drugs, were generally very low, with many of these small trials reporting that none or very few events were observed.
There is very limited evidence for this outcome for any of the topical treatments included in this review.
Risk of a serious adverse event (SAE) at up to 30 days post surgery
Reporting of adverse events was variable, with some trials not distinguishing between adverse events and serious adverse events, some reporting the number of events rather than the number of people, and some not reporting events split by arm. The data we were able to extract is included in the trial summaries (Characteristics of included studies) and the reporting of this outcome summarised in Table 4. All the reported SAE results are summarised in Table 7.
5. Serious adverse events results.
| Trial ID | SAE |
| Systemic drugs: aprotinin vs placebo | |
| Leijdekkers 2006 | Reports “complications” as 2/16 vs 5/19 OR: 0.4 (95% CI 0.07 to 2.41), P = 0.31 Includes deaths: “In the placebo group, one patient was reoperated for persistent bleeding, who then was resuscitated and developed adult respiratory distress syndrome (ARDS). A second patient was reoperated for persistent bleeding and eventually died due to multiple organ failure. Two patients developed pneumonia, and one developed a wound infection. In the aprotinin group, one patient was reoperated for persistent bleeding due to back‐bleeding from lumbar arteries. He developed colonic ischemia, necessitating a left‐sided hemicolectomy. One patient developed respiratory insufficiency and a fascial dehiscence. One patient died in the aprotinin group due to sepsis after pneumonia.” |
| Ranaboldo 1997 | Reported as “complications”, not all serious. Per event, not per person. (Table 4 in published paper) |
| Robinson 2000 | Reports “complications” not SAEs, most serious. |
| Topical drugs: fibrin/collagen sponge vs usual care | |
| Czerny 2000 | Reports number of events, not number of people. Number of people given in text but for all adverse events: “Throughout the entire study a total of 30 adverse events (31 episodes) were reported in 16 patients in the [TachoComb H] group (including one preoperative adverse event). In the [Control] group 41 adverse events (47 episodes) in 18 patients were reported.” |
| Joseph 2004 | 2/11 vs 2/11 |
| Minkowitz 2019 | NR for vascular subgroup |
| Topical drugs:collagen dressing vs oxidised cellulose | |
| Qerimi 2013 | 1/8 vs 0/8 |
| Topical drugs: novel agent (Peprostat) vs placebo/gelatin sponge | |
| EUCTR2016‐003661‐26‐PL | 6/36 vs 1/18 |
| Topical drugs:fibrin sealant vs usual care | |
| Chetter 2017 | Pooled with a non‐randomised exploratory study: 47/187 vs 9/52 |
| NCT02094885 | 13/125 vs 11/127 |
| Nenezic 2019 | At 6 weeks 21/109 vs 11/57 |
| Topical drugs: fibrin sealant vs gelatin sponge | |
| Bochicchio 2015 | 25/117 vs 12/58 |
CI ‐ confidence interval; NR ‐ not reported; OR ‐ odds ratio: SAE ‐ serious adverse events
There is very limited evidence for this outcome for any of the treatments included in this review.
Length of hospital stay (days)
Only one trial reported means and standard deviation for hospital stay (Monaco 2020). Two other trials reported medians only (Czerny 2000; Robinson 2000), and one reported stay in ICU only (Leijdekkers 2006). The data we were able to extract are included in the trial summaries (Characteristics of included studies) and in Table 8.
6. Length of hospital stay results.
| Study name | Length of hospital stay | Number of participants |
| Systemic drugs | ||
| Aprotinin vs placebo | ||
| Leijdekkers 2006 | Reported length of stay in ICU Aprotinin mean (SD) 44 (61) vs Placebo mean (SD) 120 (228) 95% CI (‐43 to 196) P = 0.20 Aprotinin median (range or IQR unspecified) 22 (19 to 269) vs Placebo median (range or IQR unspecified) 24 (12 to 792) P = 0.55 |
35 (aprotinin 16; placebo 19) |
| Robinson 2000 | Aprotinin median 12 days vs Placebo median 15 days No range or IQR reported. (3 vs 3.5 days in ICU) |
77 (aprotinin 38; placebo 39) |
| TXA vs placebo | ||
| Monaco 2020 | TXA mean (SD) 6 (1.5) vs Placebo mean (SD) 6 (1.2) |
100 (TXA 50; placebo 50) |
| Topical drugs | ||
| Fibrin/collagen sponge vs usual care | ||
| Czerny 2000 | Fibrin/collagen sponge mean 10 vs Usual care mean 10.5 SD not reported |
60 (fibrin/collagen sponge: 30; usual care: 30) |
CI ‐ confidence interval; ICU ‐ intensive care unit; IQR ‐ interquartile range; SD ‐ standard deviation; TXA ‐ tranexamic acid
There is very limited evidence for this outcome for any of the systemic drugs included in this review.
Discussion
We identified 24 eligible trials, two ongoing trials and 22 completed trials with results available.
Summary of main results
We included 22 completed trials within this review with 3393 participants analysed. One trial was not included within the quantitative analysis because it did not report any usable data (O'Donnell 2010), leaving 3324 participants analysed for at least one of our outcomes.
The majority of the trials (15/22) were of bioabsorbable dressings or glues and many reported few or no postoperative outcomes. The seven trials of systemic drugs were somewhat more likely to report outcomes of interest for this review.
Apart from the one outcome for the vascular subgroup reported by the POISE‐3 2022 trial, for tranexamic acid compared to placebo, all the review outcomes were of very low‐certainty evidence due to the small size of the trials and the limited reporting of this review's outcomes (Table 4).
The POISE‐3 2022 trial reported thromboembolic events up to 30 days in its vascular subgroup (1399 participants). There may be no difference in the risk of experiencing a thromboembolic event up to 30 days in those that received TXA compared to placebo, with the reported result for the vascular subgroup being a hazard ratio (HR) of 1.10 (95% CI 0.87 to 1.40), consistent with the whole‐trial result for all 9182 participants having high‐risk surgeries: HR 1.02 (0.92 to 1.14).
POISE‐3 2022 did not report any of our bleeding‐related outcomes for the vascular subgroup but did report subgroups by type of surgery for their primary outcome, a composite of life‐threatening, major, and critical organ bleeding. For the vascular subgroup, the hazard ratio (HR) was 0.86 (95% CI 0.64 to 1.13), consistent with the overall result of 0.76 (95% CI 0.67 to 0.87), with little heterogeneity between subgroups defined by the type of surgery.
There are two ongoing trials planning to recruit 500 and 8320 participants respectively. The smaller of these trials is comparing fibrin sealant versus usual care in participants undergoing abdominal aortic aneurysm repair (ChiCTR1900023323). It lists death due to arterial disease and reintervention rates as primary outcomes. The larger trial, TRACTION NCT04803747), is comparing tranexamic acid to placebo in participants undergoing major non‐cardiac surgery that has at least a 5% risk of requiring a red cell transfusion, and has primary outcomes of proportion transfused with red blood cells and incidence of venous thromboembolism (DVT or PE).
Trials with target sample sizes in the thousands and outcomes related to blood transfusion and thromboembolic events are required, and we look forward to seeing the results of these trials, particularly TRACTION (NCT04803747), in the near future. TRACTION is expected to complete recruitment in April 2023.
Overall completeness and applicability of evidence
This review provides the most up‐to‐date assessment of the effectiveness and safety of drugs to reduce the need for blood transfusion in major open vascular or endovascular surgery.
However, very little data were reported for our primary and secondary outcomes (Table 4). When units of red blood cells were reported, this was not always in a form suitable for meta‐analysis, with around half of these results being reported as medians rather than means.
The majority of trials (15/22) were of bioabsorbable dressings or glues and their primary focus was on intraoperative endpoints, such as time to haemostasis. We rejected this outcome during protocol development as it is prone to bias and of limited clinical relevance. The seven trials of systemic drug treatment, aprotinin (3), desmopressin (2) and tranexamic acid (2) were somewhat more likely to report our outcomes of interest, but there are still substantial gaps and the trials were small.
The lack of clinically relevant outcomes in these trials is likely due to three factors.
1. Some of the included procedures may be considered relatively low risk for postoperative bleeding and so these outcomes were not considered by the trialists. The seven trials involving aortic aneurysms, which includes six of the trials of systemic drugs, were somewhat more likely to report outcomes related to postoperative transfusion requirements.
2. Postoperative follow‐up is logistically challenging and costly, particularly in cases where surgeons may have limited postoperative contact which may be the case after lower‐risk procedures.
3. Most of the included trials were funded by the manufacturers, often with an explicit regulatory or marketing purpose. Clinically relevant outcomes are less likely to be included in trials of this sort because they are of little value to the funder (Svensson 2013).
Even when trials did report clinically relevant outcomes, in all cases their sample sizes were too small to reliably estimate clinically relevant treatment differences. Even for the most widely reported outcome, 30‐day mortality, the confidence intervals were very wide.
Quality of the evidence
Overall, we rated the certainty of the evidence as very low, according to GRADE methodology, for all but one result across all 13 treatment comparisons identified for this review. This was due to trials being at high or unclear risk of bias, and wide confidence intervals due to small sample sizes. We have formally summarised the GRADE outcome for one systemic drug (Table 1) and one topical sealant (Table 2).
Potential biases in the review process
We conducted a comprehensive search; searching data sources (including multiple databases, and clinical trial registries) to ensure that all relevant trials would be captured. There were no restrictions for the language in which the paper was originally published. The relevance of each paper was carefully assessed and all screening and data extractions were performed in duplicate. We prespecified all outcomes and subgroups prior to analysis. There were insufficient numbers of included trials within the meta‐analyses for us to use a funnel plot to examine the risk of publication bias.
However, a large proportion of the potentially eligible trials we identified from the literature search included vascular surgery as one of a mixture of surgical procedures, or a mix of eligible and ineligible vascular surgeries. As specified in the protocol, we only included these trials if they reported the eligible vascular subgroup(s) separately (sample size and at least one review outcome), or if at least 80% of surgeries were eligible vascular procedures.
The usefulness of our review was limited by lack of clinically relevant outcome reporting. Once available, we aim to update this review with data from the currently ongoing studies in order to provide better certainty evidence (ChiCTR1900023323; NCT04803747). Where currently we have consulted with vascular surgeons during the review process, we also plan to include a vascular surgeon in the author team for any updates.
Agreements and disagreements with other studies or reviews
We are not aware of any previous reviews that cover this broad topic of reducing the need for blood transfusion in vascular surgery.
This review is one of a group of five reviews, four of them funded by NIHR and NHSBT, looking at drugs for bleeding due to various causes. The other four focus on cardiac surgery (Beverly 2019), hip and knee replacement (Gibbs 2019a), long bone trauma (Gibbs 2019b), and blunt or penetrating trauma (Erasu 2022). We will consider the similarities and differences between the findings of these reviews, in terms of results, sample sizes and quality of the trials, in joint publications when all the reviews are completed and published.
Authors' conclusions
Implications for practice.
We did not identify any evidence which can reliably inform clinical practice. We were able to obtain only very limited data from the trials identified, due to small sample sizes, a lack of reporting of the outcomes we are interested in, and a large number of treatment comparisons (13) relative to the number of trials (22), limiting the amount of information which could be pooled. Network meta‐analysis was not possible and most of the data for the pairwise meta‐analyses were unavailable.
Because of a lack of data, we are uncertain whether any systemic or topical treatments used to reduce bleeding due to major vascular surgery have an effect on all‐cause mortality up to 30 days; risk of requiring a repeat procedure due to bleeding, or the requirement for blood transfusion; number of red cells transfused per participant up to 30 days or the number of participants requiring an allogeneic blood transfusion up to 30 days.
There is no evidence that tranexamic acid increases the risk of thromboembolic events may be no effect of tranexamic acid on the risk of thromboembolic events up to 30 days, an important concern for vascular surgeons. This is important as there has been concern that this risk may be increased.
Trials with sample size targets of thousands of participants and clinically relevant outcomes are needed, and we look forward to seeing the results of the ongoing trials in the future.
Implications for research.
Vascular surgeons need to do much bigger trials, with follow‐up in the postoperative period. Outcomes related to postoperative transfusion requirements are of interest to the individual, clinicians, and blood services. There is an urgent need for bigger trials in this area with sufficient follow‐up to report clinically relevant endpoints, which are: the need for a red cell transfusion; all‐cause mortality; thromboembolism (venous and arterial); risk of graft thrombosis or occlusion; serious adverse events; length of hospital stay; and need for reoperation. There is one ongoing trial (TRACTION, NCT04803747) that meets both of these requirements, and we look forward to seeing the results. Future trials should also report on specific subgroups that have a higher risk of bleeding, such as patients on antiplatelet agents and anticoagulations; patients who are on cardiopulmonary bypass and are made hypothermic during the procedure; and duration of aortic cross‐clamping.
History
Protocol first published: Issue 6, 2020
Acknowledgements
The authors and the Cochrane Vascular Editorial base are grateful to the following peer reviewers for their time and comments: Robert MacLaren, PharmD, MPH, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, USA; Marc Schermerhorn, MD, Chief, Division of Vascular and Endovascular Surgery Beth Israel Deaconess Medical Center George H. A. Clowes Jr. Professor of Surgery Harvard Medical School Boston, MA, USA; Rahul Velineni, Consultant Vascular Surgeon, Royal Infirmary of Edinburgh, UK; Dr Shailesh B Kolekar, Denmark.
The authors are grateful to the National Institute for Healthcare Research (NIHR) for their grant to support the work of the National Health Service (NHS) Blood and Transplant Systematic Review Initiative.
The authors are grateful to Dr Marialena Trivella, Centre for Statistics in Medicine, University of Oxford, UK, for reviewing the protocol and advising on statistical methodology.
The authors are grateful to Professor Linda Hands, Emeritus Professor of Surgery, University of Oxford and Mr Jeremy Perkins, Oxford University Hospital Trust for their advice on the development of the protocol.
The authors are grateful for the support of the NIHR Complex Review Support Unit for their comments and expertise.
The authors are grateful to Dr Colin Batchelor, Royal Society of Chemistry, Cambridge, UK for his help with translation.
The authors are grateful to NHS Blood and Transplant for their support, systematic review expertise and administrative assistance during the review process.
Appendices
Appendix 1. Search strategies
CENTRAL (The Cochrane Library)
#1 MeSH descriptor: [Vascular Surgical Procedures] explode all trees #2 MeSH descriptor: [Amputation] explode all trees #3 MeSH descriptor: [Blood Vessel Prosthesis Implantation] this term only #4 MeSH descriptor: [Vascular Diseases] explode all trees and with qualifier(s): [surgery ‐ SU] #5 MeSH descriptor: [Iliac Artery] this term only and with qualifier(s): [surgery ‐ SU] #6 MeSH descriptor: [Femoral Artery] this term only and with qualifier(s): [surgery ‐ SU] #7 MeSH descriptor: [Popliteal Artery] this term only and with qualifier(s): [surgery ‐ SU] #8 MeSH descriptor: [Aneurysm] explode all trees and with qualifier(s): [surgery ‐ SU] #9 MeSH descriptor: [Aortic Diseases] explode all trees and with qualifier(s): [surgery ‐ SU] #10 MeSH descriptor: [Arterial Occlusive Diseases] explode all trees and with qualifier(s): [surgery ‐ SU] #11 MeSH descriptor: [Arteriovenous Malformations] explode all trees and with qualifier(s): [surgery ‐ SU] #12 MeSH descriptor: [Diabetic Angiopathies] explode all trees and with qualifier(s): [surgery ‐ SU] #13 MeSH descriptor: [Peripheral Vascular Diseases] explode all trees and with qualifier(s): [surgery ‐ SU] #14 MeSH descriptor: [Venous Insufficiency] explode all trees and with qualifier(s): [surgery ‐ SU] #15 MeSH descriptor: [Radiology, Interventional] this term only #16 (interventional radiolog* or surgical radiolog*) #17 ((vascular or vessel* or aneurysm or aortic or aorta or AAA or TAA or TAAA or aortofemoral or artery or arterial or interarterial or arterioarterial or atheroma or carotid or vein or venous or endovascular* or intravascular*) near/6 (surg* or operat* or reconstruct* or repair* or resection* or repair* or bypass* or graft* or homograft* or transplant* or bypass* or transpos* or implant* or reimplant* or clamp* or ligat* or crossover* or cross‐over*)) #18 ((endovascular or intravascular or laparoscop* or angioscop*) near/3 (surg* or operat* or reconstruct* or repair* or resection* or repair* or bypass* or graft* or homograft* or transplant* or bypass* or transpos* or implant* or reimplant* or procedure*)) #19 (bypass graft* or by‐pass graft* or bypass surgery or by‐pass surgery or enarterectom* or (minimally invasive near/1 surg*) or teflon graft* or dacron graft*) #20 (arterial dilation* or endoluminal repair*) #21 ((venous or arterial) near/3 catheteri?ation) #22 ((aort* or iliac or femoral or popliteal or femoropop* or fempop* or crural) near/3 (surg* or operat* or bypass or graft or reconstruct* or revascular*)) #23 (angiosurg* or aneurysmectom* or aneurysm clipping* or aortopexy or aortoplast* or arteriotom* or arterioplast* or artery plast* or artery stripping or venostom* or portacaval anastomoses or revasculari?ation or devasculari?ation) #24 ((femoral* or iliac* or aorta* or aortic* or infrapopliteal or popliteal or infra‐pop*) near/3 (stent* or angioplast*)) #25 (endovascular near/6 (dissection* or stent*)) #26 (EVAR or FEVAR or TEVAR or embolectom* or thrombectom* or endarterectom* or thromboendarterectom* or thromboembolectom* or atherectom*) #27 (mechanical* near/3 (thrombolysis or clot disruption*)) #28 (axillo bifemoral bypass graft* or axillo femoral bypass graft* or axillobifemoral bypass graft* or axillofemoral bypass graft*) #29 ((ilio femor* or ilio femor* or infrarenal or infra‐renal or infrainguinal or infra‐inguinal) next (bypass* or by‐pass*)) #30 ((pedal or tibial) near/3 (angio* or bypass* or by‐pass*)) #31 ((lower limb* or knee* or leg* or foot or feet or lower extremit* or hindquarter*) near/5 (salvag* or saving or save* or angioplast* or graft* or bypass* or by‐pass* or revascula* or reconstruct* or amputat*)) #32 ((blood vessel* or vascular or endovascular or vein or venous or arterial or artery or arteriovenous or arterio‐venous or brescia cimino or venoarterial or veno‐arterial or arterioportal) near/3 (anastomosis or shunt*)) #33 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 #34 MeSH descriptor: [Antifibrinolytic Agents] this term only #35 MeSH descriptor: [Tranexamic Acid] this term only #36 MeSH descriptor: [Aminocaproic Acid] explode all trees #37 (antifibrinolytic* or anti‐fibrinolytic* or antifibrinolysin* or antiplasmin* or plasmin inhibitor* or tranexamic or tranhexamic or cyclohexanecarboxylic acid* or amcha or t‐amcha or amca or transamin or amchafibrin or anvitoff or spotof or cyklokapron or femstrual or ugurol):ti,ab #38 (AMCHA or amchafibrin or amikapron or amstat or antivoff or caprilon or cl65336 or cyclocapron or cyclokapron or cyklocapron or cyklokapron or exacyl or frenolyse or fibrinon or hemostan or hexacapron):ti,ab #39 (hexakapron or kalnex or lysteda or rikaparin or ronex or theranex or tranexam or tranexanic or tranexic or "trans achma" or transexamic or trenaxin or TXA):ti,ab #40 (fibrinolysis near/2 inhibitor*):ti,ab #41 (Agretax or Bio‐Stat or Capiloc or Capitrax or Clip Inj or Clot‐XL or Clotawin‐T or Coastat or Cuti or Cymin or Dubatran or Espercil or Examic or Existat or Extam or Fibran or Gynae‐Pil or Hemstate or Kapron or Menogia or Monitex or Nestran or Nexamic or Nexi‐500 or Nexmeff or Nicolda or Nixa‐500 or Pause or Rheonex or Sylstep TX or Synostat or T‐nex or T Stat or T Stat or Tanmic or Temsyl‐T or Texakind or Texanis or Texapar or Texid or Thams or Tonopan or Traklot or Tramic or Tramix or Tranarest or Trance Inj or Tranecid or Tranee or Tranemic or Tranex or Tranexa or Tranfib or Tranlok or Transtat or Transys or Transcam or Tranxi or Trapic or Traxage or Traxamic or Traxyl or Trenaxa or Trexamic or Trim Inj or Tx‐1000 or Tx 500 or Wistran or X‐Tran or Xamic):ti,ab #42 (ecapron or ekaprol or epsamon or epsicaprom or epsicapron or epsilcapramin or epsilon amino caproate or epsilon aminocaproate or epsilonaminocaproic or epsilonaminocapronsav or ethaaminocaproic or ethaaminocaproich or emocaprol or hepin or ipsilon or neocaprol or resplamin or tachostyptan):ti,ab #43 (lederle or acikaprin or afibrin or amicar or caprocid or capracid or capramol or caprogel or caprolest or caprolisin* or caprolysin* or capromol or epsikapron or hemocaprol or caproamin or EACA or caprolest or capralense or hexalense or hamostat or hemocid):ti,ab #44 (aminohexanoic or aminocaproic or aminohexanoic or amino caproic or amino‐caproic or amino‐n‐hexanoic):ti,ab #45 #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 #46 MeSH descriptor: [Aprotinin] this term only #47 (antagosan or antilysin* or aprotimbin or apronitin* or aprotinin* or bayer a128 or contrical or contrycal or contrykal or dilmintal or frey inhibitor or kontrycal or Kunitz inhibitor or gordox or haemoprot or kallikrein‐trypsin inactivator or iniprol or kontrikal or kontrykal or kunitz pancreatic trypsin inhibitor or midran or pulmin or tracylol or trascolan or trasilol or tra?ylol or traskolan or zymofren or pancreas antitrypsin or protinin or riker 52g or Rivilina zymofren):ti,ab #48 #46 or #47 #49 MeSH descriptor: [Factor VIIa] this term only #50 (factor viia or factor 7a or rfviia or fviia or novoseven* or novo seven* or aryoseven or acset or eptacog* or proconvertin):ti,ab #51 (activated near/1 (factor seven or factor vii or rfvii or fvii)):ti,ab #52 (factor seven or factor vii or factor 7):ti #53 #49 or #50 or #51 or #52 #54 MeSH descriptor: [Fibrinogen] this term only #55 ("fibrinogen concentrate" or "factor I" or Haemocomplettan* or Riastap* or Fibryga* or Fibryna*):ti,ab #56 #54 or #55 #57 MeSH descriptor: [Deamino Arginine Vasopressin] this term only #58 (desmopressin* or vasopressin deamino or D amino D arginine vasopressin or deamino 8 d arginine vasopressin or vasopressin desamino 8 arginine or desmotabs or DDAVP or desmogalen or adin or adiuretin or concentraid or d void or dav ritter or deamino 8 dextro arginine vasopressin or deamino 8d arginine vasopressin or deamino dextro arginine vasopressin or deaminovasopressin or defirin or defirin melt or desmirin pr desmomelt or desmopresina or desmospray or desmotab* or desurin or emosint or enupresol or minirin or minirinette or minirinmelt or minrin or minurin or miram or nictur or noctisson or nocturin or nocutil or nordurine or novidin or nucotil or octim or octostim or presinex or stimate or wetirin):ti,ab #59 #57 or #58 #60 MeSH descriptor: [Factor XIII] explode all trees #61 (factor xiii or fxiii or fibrin stabili?ing factor* or Tretten* or Catridecacog):ti,ab #62 #60 or #61 #63 MeSH descriptor: [Tissue Adhesives] explode all trees #64 MeSH descriptor: [Collagen] explode all trees and with qualifier(s): [therapeutic use ‐ TU] #65 MeSH descriptor: [Thrombin] explode all trees and with qualifier(s): [therapeutic use ‐ TU] #66 MeSH descriptor: [Gelatin] explode all trees and with qualifier(s): [therapeutic use ‐ TU] #67 MeSH descriptor: [Gelatin Sponge, Absorbable] this term only #68 ((fibrin* or collagen or cellulose or gelatin or gel or thrombin* or albumin or hemostatic* or haemostatic*) next (glu* or seal* or adhesive* or topical* or local* or matrix or matrices or spong* or fleece* or foam* or scaffold* or patch* or sheet* or bandag* or aerosol* or dressing* or paste or powder*)):ti,ab #69 ((nonfibrin* or non‐fibrin* or synthetic* or non‐biological* or nonbiological* or biological*) near/3 (glue* or seal* or adhesive*)):ti,ab #70 (surgical* near/3 (glue* or sealant* or adhesive*)):ti,ab #71 ((fibrin* or collagen or cellulose or gelatin or thrombin) near/3 (hemosta* or haemosta*)):ti,ab #72 (8Y or Aafact or Actif‐VIII or Advate or Artiss or Bioglue or Biocol or Collaseal or Omrixil or Transglutine or Raplixa or Evarrest or Aleviate or Alphanate or Amofil or Beriate or Beriplast or Biostate or Bolheal or Cluvot or Conco‐Eight‐HT or Crosseel or Crosseal or Crosseight or Emoclot or Evarrest or Evicel or Factane or Fanhdi or Fibrogammin P or Green VIII or Green VIII Factor or Greengene or Greenmono or Greenplast or Haemate or Haemate P or Haemate P or Haemate P500 or Haemate‐P or Haemoctin or Haemoctin SDH or Haemoctin‐SDH or Hemaseel or Hemaseal or Hemofil M or Hemoraas or Humaclot or Humafactor‐8 or Humate‐P or Immunate or Innovate or Koate or Koate‐DVI or Kogenate Bayer or Kogenate FS or Monoclate‐P or NovoThirteen or Octafil or Octanate or Octanate or Optivate or Quixil or Talate or Tisseel or Tisseal or Tissel or Tissucol or Tricos or Vivostat or Voncento or Wilate or Wilnativ or Wilstart or Xyntha):ti,ab #73 (Glubran or Gluetiss or Ifabond or Indermil or LiquiBand or TissuGlu or Evithrom or Floseal or Hemopatch or Gel‐Flow or Gelfoam or Gelfilm or Recothrom or Surgifoam or Surgiflo* or "rh Thrombin" or Thrombi‐Gel or Thrombi‐Pad or Thrombin‐JMI or Thrombinar or Thrombogen or Thrombostat):ti,ab #74 (porcine gelatin or bovine collagen or bovine gelatin or nu‐knit or arista or hemostase or vita sure or thrombin‐jmi or thrombinjmi or avicel or vivagel or lyostypt or tabotamp or arterx or omnex or veriset):ti,ab #75 (polysaccharide next (sphere* or hemostatic powder)):ti,ab #76 MeSH descriptor: [Chitosan] this term only #77 MeSH descriptor: [Polyethylene Glycols] this term only and with qualifier(s): [therapeutic use ‐ TU] #78 MeSH descriptor: [Hydrogel, Polyethylene Glycol Dimethacrylate] explode all trees and with qualifier(s): [therapeutic use ‐ TU] #79 MeSH descriptor: [Polyurethanes] explode all trees and with qualifier(s): [pharmacology ‐ PD, adverse effects ‐ AE, toxicity ‐ TO, administration & dosage ‐ AD, therapeutic use ‐ TU] #80 ((polymer‐derived elastic* or polymer tissue adhesive* or elastic hydrogel* or glutaraldehyde or PEG‐based or polyurethane‐based tissue or polyethylene glycol* or polyvinyl alcohol‐based tissue or PVA‐based tissue or natural biopolymer* or polypeptide‐based or protein‐based or polysaccharide‐based or chitosan or poliglusam or cyanoacrylic or cyanoacrylate or cyacrin or dextran‐based or chondroitin sulfate‐based or mussel‐inspired elastic* or glycol hydrogel or polymer‐based) next (glu* or seal* or adhesive* or topical* or local* or matrix or matrices or spong* or fleece* or foam* or scaffold* or patch* or sheet* or bandag* or aerosol* or dressing* or paste* or powder*)):ti,ab #81 MeSH descriptor: [Cellulose, Oxidized] this term only #82 (absorbable cellulose or resorbable cellulose or oxidi?ed cellulose or carboxycellulose or oxycellulose or cellulosic acid or oxycel or oxidi?ed regenerated cellulose):ti,ab #83 (BioGlue or Progel or Duraseal or Coseal or FocalSeal or ADAL‐1 or AdvaSeal or Pleuraseal or Angio‐Seal or Avitene or Instat or Helitene or Helistat or TDM‐621 or Dermabond or Tissueseal or PolyStat or Raplixa or Spongostan or Surgicel or Surgilux or Tachosil or Traumstem):ti,ab #84 (collagen‐thrombin or thrombin‐collagen or gelatin‐fibrinogen or fibrinogen‐gelatin or gelatin‐thrombin or thrombin‐gelatin or fibrinogen‐thrombin or thrombin‐fibrinogen or collagen‐fibrinogen or fibrinogen‐collagen or microfibrillar collagen or CoStasis or "GRF Glue" or GR‐Dial or Algosterile or TraumaStat or HemCon or ChitoFlex or Celox or QuikClot or WoundStat or Vitagel or TachSeal or TachoComb or Cryoseal):ti,ab #85 #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78 or #79 or #80 or #81 or #82 or #83 or #84 #86 MeSH descriptor: [Waxes] explode all trees #87 (bonewax* or bone wax* or bone putty or hemasorb or ostene):ti,ab #88 #86 or #87 #89 MeSH descriptor: [Blood Coagulation Factors] this term only #90 (prothrombin near/5 (complex* or concentrate*)) #91 (PCC* or 3F‐PCC* or 4F‐PCC* or Beriplex* or Feiba* or Autoplex* or Ocplex* or Octaplex* or Kcentra* or Cofact or Prothrombinex* or "Proplex‐T" or Prothroraas* or Haemosolvex* or Prothromplex* or "HT Defix" or Facnyne* or Kaskadil* or Kedcom* or Confidex* or PPSB or Profil?ine* or Pronativ* or Proplex* or Prothar* or ProthoRAAS* or Protromplex* or "Pushu Laishi" or "Uman Complex") #92 #89 or #90 or #91 #93 (((haemosta* or hemosta* or antihaemorrhag* or antihemorrhag* or anti haemorrhag* or anti‐hemorrhag*) next (drug* or agent* or treat* or therap*)) or ((coagulat* or clotting) next factor*)):ti,ab #94 #45 or #48 or #53 or #56 or #59 or #62 or #85 or #88 or #92 or #93 #95 #33 and #94 [In Trials]
MEDLINE (Ovid)
1. Vascular Surgical Procedures/ 2. Endarterectomy/ 3. exp Endovascular Procedures/ 4. Axillofemoral Bypass Grafting/ 5. Embolectomy/ 6. Limb Salvage/ 7. exp Amputation/ 8. exp Thrombectomy/ 9. Vascular Grafting/ 10. Arteriovenous Shunt, Surgical/ 11. Blood Vessel Prosthesis Implantation/ 12. Vascular Diseases/su 13. Femoral Artery/su or Iliac Artery/su or Popliteal Artery/su 14. exp Aneurysm/su 15. exp Aortic Diseases/su 16. exp Arterial Occlusive Diseases/su 17. exp Arteriovenous Malformations/su 18. Diabetic Angiopathies/su 19. exp Peripheral Vascular Diseases/su 20. exp Spinal Cord Vascular Diseases/su 21. Vascular System Injuries/su 22. exp Venous Insufficiency/su 23. or/1‐22 24. Radiology, Interventional/ 25. (interventional radiolog* or surgical radiolog*).tw,kf. 26. ((vascular or vessel* or aneurysm or aortic or aorta or AAA or TAA or TAAA or aortofemoral or artery or arterial or interarterial or arterioarterial or atheroma or carotid or vein or venous or endovascular* or intravascular*) adj6 (surg* or operat* or reconstruct* or repair* or resection* or repair* or bypass* or graft* or homograft* or transplant* or bypass* or transpos* or implant* or reimplant* or clamp* or ligat* or crossover* or cross‐over*)).tw,kf. 27. ((endovascular or intravascular or laparoscop* or angioscop*) adj3 (surg* or operat* or reconstruct* or repair* or resection* or repair* or bypass* or graft* or homograft* or transplant* or bypass* or transpos* or implant* or reimplant* or procedure*)).tw,kf. 28. (bypass graft* or by‐pass graft* or bypass surgery or by‐pass surgery or enarterectom* or (minimally invasive adj1 surg*) or teflon graft* or dacron graft*).tw,kf. 29. (arterial dilation* or endoluminal repair*).tw,kf. 30. ((venous or arterial) adj3 catheteri?ation).tw,kf. 31. ((aort* or iliac or femoral or popliteal or femoropop* or fempop* or crural) adj3 (surg* or operat* or bypass or graft or reconstruct* or revascular*)).tw,kf. 32. (angiosurg* or aneurysmectom* or aneurysm clipping* or aortopexy or aortoplast* or arteriotom* or arterioplast* or artery plast* or artery stripping or venostom* or portacaval anastomoses or revasculari?ation or devasculari?ation).tw,kf. 33. ((femoral* or iliac* or aorta* or aortic* or infrapopliteal or popliteal or infra‐pop*) adj3 (stent* or angioplast*)).tw,kf. 34. (endovascular adj6 (dissection* or stent*)).tw,kf. 35. (EVAR or FEVAR or TEVAR or embolectom* or thrombectom* or endarterectom* or thromboendarterectom* or thromboembolectom* or atherectom*).tw,kf. 36. (mechanical* adj3 (thrombolysis or clot disruption*)).tw,kf. 37. (axillo bifemoral bypass graft* or axillo femoral bypass graft* or axillobifemoral bypass graft* or axillofemoral bypass graft*).tw,kf. 38. ((ilio femor* or ilio femor* or infrarenal or infra‐renal or infrainguinal or infra‐inguinal) adj (bypass* or by‐pass*)).tw,kf. 39. ((pedal or tibial) adj3 (angio* or bypass* or by‐pass*)).tw,kf. 40. ((lower limb* or knee* or leg* or foot or feet or lower extremit* or hindquarter*) adj5 (salvag* or saving or save* or angioplast* or graft* or bypass* or by‐pass* or revascula* or reconstruct* or amputat*)).tw,kf. 41. ((blood vessel* or vascular or endovascular or vein or venous or arterial or artery or arteriovenous or arterio‐venous or brescia cimino or venoarterial or veno‐arterial or arterioportal) adj3 (anastomosis or shunt*)).tw,kf. 42. or/24‐41 43. 23 or 42 44. Antifibrinolytic Agents/ 45. Tranexamic Acid/ 46. Aminocaproic Acid/ 47. (antifibrinolytic* or anti‐fibrinolytic* or antifibrinolysin* or antiplasmin* or plasmin inhibitor* or tranexamic or tranhexamic or cyclohexanecarboxylic acid* or amcha or trans‐4‐aminomethyl‐cyclohexanecarboxylic acid* or t‐amcha or amca or "kabi 2161" or transamin or amchafibrin or anvitoff or spotof or cyklokapron or cyclo‐F or femstrual or ugurol or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or antivoff or caprilon or cl?65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or cyklokapron or exacyl or frenolyse or fibrinon or hemostan or hexacapron or hexakapron or kalnex or lysteda or rikaparin or ronex or theranex or tranexam or tranexanic or tranexic or trans achma or transexamic or trenaxin or TXA or (fibrinolysis adj2 inhibitor*)).tw,kf. 48. (Agretax or Bio‐Stat or Capiloc or Capitrax or Clip Inj or Clot‐XL or Clotawin‐T or Coastat or Cuti or Cymin or Dubatran or Espercil or Examic or Existat or Extam or Fibran or Gynae‐Pil or Hemstate or Kapron or Menogia or Monitex or Nestran or Nexamic or Nexi‐500 or Nexmeff or Nicolda or Nixa‐500 or Pause or Rheonex or Sylstep TX or Synostat or T‐nex or T Stat or T Stat or Tanmic or Temsyl‐T or Texakind or Texanis or Texapar or Texid or Thams or Tonopan or Traklot or Tramic or Tramix or Tranarest or Trance Inj or Tranecid or Tranee or Tranemic or Tranex or Tranexa or Tranfib or Tranlok or Transtat or Transys or Transcam or Tranxi or Trapic or Traxage or Traxamic or Traxyl or Trenaxa or Trexamic or Trim Inj or Tx‐1000 or Tx 500 or Wistran or X‐Tran or Xamic).tw,kf. 49. (6‐aminohexanoic or amino?caproic or amino?hexanoic or amino caproic or amino‐caproic or amino‐n‐hexanoic or cy‐116 or cy116 or lederle or acikaprin or afibrin or amicar or caprocid or capracid or capramol or caprogel or caprolest or caprolisin* or caprolysin* or capromol or epsikapron or hemocaprol or caproamin or EACA or caprolest or capralense or hexalense or hamostat or hemocid or cl 10304 or cl10304 or ecapron or ekaprol or epsamon or epsicaprom or epsicapron or epsilcapramin or epsilon amino caproate or epsilon aminocaproate or epsilonaminocaproic or epsilonaminocapronsav or etha?aminocaproic or ethaaminocaproich or emocaprol or hepin or ipsilon or jd?177or neocaprol or nsc?26154 or resplamin or tachostyptan).tw,kf. 50. or/44‐49 51. Aprotinin/ 52. (antagosan or antilysin* or aprotimbin or apronitin* or aprotinin* or bayer a128 or contrical or contrycal or contrykal or dilmintal or frey inhibitor or kontrycal or Kunitz inhibitor or gordox or haemoprot or kallikrein‐trypsin inactivator).tw,kf. 53. (iniprol or kontrikal or kontrykal or kunitz pancreatic trypsin inhibitor or midran or pulmin or tracylol or trascolan or trasilol or tra?ylol or traskolan or zymofren or pancreas antitrypsin or protinin or riker 52g or Rivilina zymofren).tw,kf. 54. or/51‐53 55. Factor VIIa/ 56. (factor viia or factor 7a or rfviia or fviia or novoseven* or novo seven* or aryoseven or acset or eptacog* or proconvertin).tw,kf. 57. ((activated adj2 factor seven) or (activated adj2 factor vii) or (activated adj3 rfvii) or (activated adj2 fvii)).tw,kf. 58. (factor seven or factor vii or factor 7).ti. 59. 55 or 56 or 57 or 58 60. Fibrinogen/ad, ae, de, sd, tu, th, to 61. *Fibrinogen/ 62. (fibrinogen concentrate* or factor I or Haemocomplettan* or Riastap* or Fibryga* or Fibryna*).tw,kf. 63. 60 or 61 or 62 64. Deamino Arginine Vasopressin/ 65. (desmopressin* or vasopressin deamino or D‐amino D‐arginine vasopressin or deamino‐8‐d‐arginine vasopressin or vasopressin 1‐desamino‐8‐arginine or desmotabs or DDAVP or desmogalen or adin or adiuretin or concentraid or d‐void or dav ritter or deamino 8 dextro arginine vasopressin or deamino 8d arginine vasopressin or deamino dextro arginine vasopressin or deaminovasopressin or defirin or defirin melt or desmirin or desmomelt or desmopresina or desmospray or desmotab* or desurin or emosint or enupresol or minirin or minirinette or minirinmelt or minrin or minurin or miram or nictur or noctisson or nocturin or nocutil or nordurine or novidin or nucotil or octim or octostim or presinex or stimate or wetirin).tw,kf. 66. 64 or 65 67. exp Factor XIII/ 68. (factor XIII or fXIII or fibrin stabili?ing factor* or Tretten* or Catridecacog).tw,kf. 69. 67 or 68 70. exp Tissue Adhesives/ 71. *Adhesives/ 72. Collagen/tu 73. Thrombin/tu 74. Gelatin/tu 75. Gelatin Sponge, Absorbable/ 76. ((fibrin* or collagen or cellulose or gelatin or gel or thrombin* or albumin or hemostatic* or haemostatic*) adj3 (glu* or seal* or adhesive* or topical* or local* or matrix or matrices or spong* or fleece* or foam* or scaffold* or patch* or sheet* or bandag* or aerosol* or dressing* or paste or powder*)).tw,kf. 77. ((nonfibrin* or non‐fibrin* or synthetic* or non‐biological* or nonbiological* or biological*) adj3 (glue* or seal* or adhesive*)).tw,kf. 78. (surgical* adj3 (glue* or sealant* or adhesive*)).tw,kf. 79. ((fibrin* or collagen or cellulose or gelatin or thrombin) adj3 (hemosta* or haemosta*)).tw,kf. 80. (8Y or Aafact or Actif‐VIII or Advate or Artiss or Bioglue or Biocol or Collaseal or Omrixil or Transglutine or Raplixa or Evarrest or Aleviate or Alphanate or Amofil or Beriate or Beriplast or Biostate or Bolheal or Cluvot or Conco‐Eight‐HT or Crosseel or Crosseal or Crosseight or Emoclot or Evarrest or Evicel or Factane or Fanhdi or Fibrogammin P or Green VIII or Green VIII Factor or Greengene or Greenmono or Greenplast or Haemate or Haemate P or Haemate P or Haemate P500 or Haemate‐P or Haemoctin or Haemoctin SDH or Haemoctin‐SDH or Hemaseel or Hemaseal or Hemofil M or Hemoraas or Humaclot or Humafactor‐8 or Humate‐P or Immunate or Innovate or Koate or Koate‐DVI or Kogenate Bayer or Kogenate FS or Monoclate‐P or NovoThirteen or Octafil or Octanate or Octanate or Optivate or Quixil or Talate or Tisseel or Tisseal or Tissel or Tissucol or Tricos or Vivostat or Voncento or Wilate or Wilnativ or Wilstart or Xyntha).tw,kf. 81. (Glubran or Gluetiss or Ifabond or Indermil or LiquiBand or TissuGlu).tw,kf. 82. (Evithrom or Floseal or Hemopatch or Gel‐Flow or Gelfoam or Gelfilm or Recothrom or Surgifoam or Surgiflo* or "rh Thrombin" or Thrombi‐Gel or Thrombi‐Pad or Thrombin‐JMI or Thrombinar or Thrombogen or Thrombostat).tw,kf. 83. (porcine gelatin or bovine collagen or bovine gelatin or nu‐knit or arista or hemostase or vita sure or thrombin‐jmi or thrombinjmi or avicel or vivagel or lyostypt or tabotamp or arterx or omnex or veriset).tw,kf. 84. (polysaccharide adj (sphere* or hemostatic powder)).tw,kf. 85. *Chitosan/ 86. *Polyethylene Glycols/ 87. *Hydrogel, Polyethylene Glycol Dimethacrylate/ 88. Polyurethanes/ad, ae, pd, tu, to 89. ((polymer‐derived elastic* or polymer tissue adhesive* or elastic hydrogel* or glutaraldehyde or PEG‐based or polyurethane‐based tissue or polyethylene glycol* or polyvinyl alcohol‐based tissue or PVA‐based tissue or natural biopolymer* or polypeptide‐based or protein‐based or polysaccharide‐based or chitosan or poliglusam or cyanoacrylic or cyanoacrylate or cyacrin or dextran‐based or chondroitin sulfate‐based or mussel‐inspired elastic* or glycol hydrogel or polymer‐based) adj3 (glu* or seal* or adhesive* or topical* or local* or matrix or matrices or spong* or fleece* or foam* or scaffold* or patch* or sheet* or bandag* or aerosol* or dressing* or paste* or powder*)).tw,kf. 90. Cellulose, Oxidized/ 91. (absorbable cellulose or resorbable cellulose or oxidi?ed cellulose or carboxycellulose or oxycellulose or cellulosic acid or oxycel or oxidi?ed regenerated cellulose).tw,kf. 92. (BioGlue or Progel or Duraseal or Coseal or FocalSeal or ADAL‐1 or AdvaSeal or Pleuraseal or Angio‐Seal or Avitene or Instat or Helitene or Helistat or TDM‐621 or Dermabond or Tissueseal or PolyStat or Raplixa or Spongostan or Surgicel or Surgilux or Tachosil or Traumstem).tw,kf. 93. (collagen‐thrombin or thrombin‐collagen or gelatin‐fibrinogen or fibrinogen‐gelatin or gelatin‐thrombin or thrombin‐gelatin or fibrinogen‐thrombin or thrombin‐fibrinogen or collagen‐fibrinogen or fibrinogen‐collagen or microfibrillar collagen or CoStasis or "GRF Glue" or GR‐Dial or Algosterile or TraumaStat or HemCon or ChitoFlex or Celox or QuikClot or WoundStat or Vitagel or TachSeal or TachoComb or Cryoseal).tw,kf. 94. or/70‐93 95. exp Waxes/ 96. (bonewax* or bone wax* or bone putty or hemasorb or ostene).tw,kf. 97. 95 or 96 98. Blood Coagulation Factors/ 99. (prothrombin adj5 (complex* or concentrate*)).tw,kf. 100. (PCC* or 3F‐PCC* or 4F‐PCC* or Beriplex* or Feiba* or Autoplex* or Ocplex* or Octaplex* or Kcentra* or Cofact or Prothrombinex* or "Proplex‐T" or Prothroraas* or Haemosolvex* or Prothromplex* or "HT Defix" or Facnyne* or Kaskadil* or Kedcom* or Confidex* or PPSB or Profil?ine* or Pronativ* or Proplex* or Prothar* or ProthoRAAS* or Protromplex* or "Pushu Laishi" or "Uman Complex").tw,kf. 101. or/98‐100 102. (((haemosta* or hemosta* or antihaemorrhag* or antihemorrhag* or anti haemorrhag* or anti‐hemorrhag*) adj5 (drug* or agent* or treat* or therap*)) or ((coagulat* or clotting) adj factor*)).tw,kf. 103. 50 or 54 or 59 or 63 or 66 or 69 or 94 or 97 or 101 or 102 104. 43 and 103 105. Meta‐Analysis.pt. 106. Systematic Review.pt. 107. ((meta analy* or metaanaly*) and (trials or studies)).ab. 108. (meta analy* or metaanaly* or evidence‐based).ti. 109. ((systematic* or evidence‐based) adj2 (review* or overview*)).tw,kf. 110. (evidence synthes* or cochrane or medline or pubmed or embase or cinahl or cinhal or lilacs or "web of science" or science citation index or scopus or search terms or literature search or electronic search* or comprehensive search* or systematic search* or published articles or search strateg* or reference list* or bibliograph* or handsearch* or hand search* or manual* search*).ab. 111. Cochrane Database of systematic reviews.jn. 112. (additional adj (papers or articles or sources)).ab. 113. ((electronic* or online) adj (sources or resources or databases)).ab. 114. (relevant adj (journals or articles)).ab. 115. or/105‐114 116. Review.pt. 117. exp Randomized Controlled Trials as Topic/ 118. selection criteria.ab. or critical appraisal.tw. 119. (data adj (abstract* or extract* or analys*)).ab. 120. exp Randomized Controlled Trial/ 121. or/117‐120 122. 116 and 121 123. 115 or 122 124. Randomized Controlled Trial.pt. 125. Controlled Clinical Trial.pt. 126. (placebo or randomly or groups).ab. 127. (randomi* or trial).tw,kf. 128. exp Clinical Trial as Topic/ 129. or/123‐128 130. exp animals/ not humans/ 131. 129 not 130 132. 104 and 131
Embase (Ovid)
1. artery surgery/ or arteriotomy/ or exp artery anastomosis/ or exp artery ligation/ or artery reconstruction/ or exp atherectomy/ or endarterectomy/ 2. vascular surgery/ or exp aneurysm surgery/ 3. angioplasty/ or bare metal stenting/ or blunt microdissection/ or exp laser angioplasty/ or patch angioplasty/ or percutaneous transluminal angioplasty/ or transluminal coronary angioplasty/ 4. exp blood vessel shunt/ 5. exp blood vessel transplantation/ 6. devascularization/ or exp embolectomy/ or limb salvage/ or microvascular surgery/ 7. exp endovascular surgery/ or exp thrombectomy/ 8. exp vein surgery/ 9. exp aortic surgery/ 10. exp vascular disease/su 11. exp leg amputation/ 12. foot amputation/ 13. exp thoracic artery/su 14. exp leg artery/su 15. exp artery disease/su 16. or/1‐15 17. interventional radiology/ 18. (interventional radiolog* or surgical radiolog*).tw. 19. ((vascular or vessel* or aneurysm or aortic or aorta or AAA or TAA or TAAA or aortofemoral or artery or arterial or interarterial or arterioarterial or atheroma or carotid or vein or venous or endovascular* or intravascular*) adj6 (surg* or operat* or reconstruct* or repair* or resection* or repair* or bypass* or graft* or homograft* or transplant* or bypass* or transpos* or implant* or reimplant* or clamp* or ligat* or crossover* or cross‐over*)).tw. 20. ((endovascular or intravascular or laparoscop* or angioscop*) adj3 (surg* or operat* or reconstruct* or repair* or resection* or repair* or bypass* or graft* or homograft* or transplant* or bypass* or transpos* or implant* or reimplant* or procedure*)).tw. 21. (bypass graft* or by‐pass graft* or bypass surgery or by‐pass surgery or enarterectom* or (minimally invasive adj1 surg*) or teflon graft* or dacron graft*).tw. 22. (arterial dilation* or endoluminal repair*).tw. 23. ((venous or arterial) adj3 catheteri?ation).tw. 24. ((aort* or iliac or femoral or popliteal or femoropop* or fempop* or crural) adj3 (surg* or operat* or bypass or graft or reconstruct* or revascular*)).tw. 25. (angiosurg* or aneurysmectom* or aneurysm clipping* or aortopexy or aortoplast* or arteriotom* or arterioplast* or artery plast* or artery stripping or venostom* or portacaval anastomoses or revasculari?ation or devasculari?ation).tw. 26. ((femoral* or iliac* or aorta* or aortic* or infrapopliteal or popliteal or infra‐pop*) adj3 (stent* or angioplast*)).tw. 27. (endovascular adj6 (dissection* or stent*)).tw. 28. (EVAR or FEVAR or TEVAR or embolectom* or thrombectom* or endarterectom* or thromboendarterectom* or thromboembolectom* or atherectom*).tw. 29. (mechanical* adj3 (thrombolysis or clot disruption*)).tw. 30. (axillo bifemoral bypass graft* or axillo femoral bypass graft* or axillobifemoral bypass graft* or axillofemoral bypass graft*).tw. 31. ((ilio femor* or ilio femor* or infrarenal or infra‐renal or infrainguinal or infra‐inguinal) adj (bypass* or by‐pass*)).tw. 32. ((pedal or tibial) adj3 (angio* or bypass* or by‐pass*)).tw. 33. ((lower limb* or knee* or leg* or foot or feet or lower extremit* or hindquarter*) adj5 (salvag* or saving or save* or angioplast* or graft* or bypass* or by‐pass* or revascula* or reconstruct* or amputat*)).tw. 34. ((blood vessel* or vascular or endovascular or vein or venous or arterial or artery or arteriovenous or arterio‐venous or brescia cimino or venoarterial or veno‐arterial or arterioportal) adj3 (anastomosis or shunt*)).tw. 35. or/17‐34 36. 16 or 35 37. Antifibrinolytic Agent/ 38. Tranexamic Acid/ 39. Aminocaproic Acid/ 40. (antifibrinolytic* or anti‐fibrinolytic* or antifibrinolysin* or antiplasmin* or plasmin inhibitor* or tranexamic or tranhexamic or cyclohexanecarboxylic acid* or amcha or trans‐4‐aminomethyl‐cyclohexanecarboxylic acid* or t‐amcha or amca or "kabi 2161" or transamin or amchafibrin or anvitoff or spotof or cyklokapron or cyclo‐F or femstrual or ugurol or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or antivoff or caprilon or cl?65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or cyklokapron or exacyl or frenolyse or fibrinon or hemostan or hexacapron or hexakapron or kalnex or lysteda or rikaparin or ronex or theranex or tranexam or tranexanic or tranexic or trans achma or transexamic or trenaxin or TXA or (fibrinolysis adj2 inhibitor*)).tw. 41. (Agretax or Bio‐Stat or Capiloc or Capitrax or Clip Inj or Clot‐XL or Clotawin‐T or Coastat or Cuti or Cymin or Dubatran or Espercil or Examic or Existat or Extam or Fibran or Gynae‐Pil or Hemstate or Kapron or Menogia or Monitex or Nestran or Nexamic or Nexi‐500 or Nexmeff or Nicolda or Nixa‐500 or Pause or Rheonex or Sylstep TX or Synostat or T‐nex or T Stat or T Stat or Tanmic or Temsyl‐T or Texakind or Texanis or Texapar or Texid or Thams or Tonopan or Traklot or Tramic or Tramix or Tranarest or Trance Inj or Tranecid or Tranee or Tranemic or Tranex or Tranexa or Tranfib or Tranlok or Transtat or Transys or Transcam or Tranxi or Trapic or Traxage or Traxamic or Traxyl or Trenaxa or Trexamic or Trim Inj or Tx‐1000 or Tx 500 or Wistran or X‐Tran or Xamic).tw. 42. (6‐aminohexanoic or amino?caproic or amino?hexanoic or amino caproic or amino‐caproic or amino‐n‐hexanoic or cy‐116 or cy116 or lederle or acikaprin or afibrin or amicar or caprocid or capracid or capramol or caprogel or caprolest or caprolisin* or caprolysin* or capromol or epsikapron or hemocaprol or caproamin or EACA or caprolest or capralense or hexalense or hamostat or hemocid or cl 10304 or cl10304 or ecapron or ekaprol or epsamon or epsicaprom or epsicapron or epsilcapramin or epsilon amino caproate or epsilon aminocaproate or epsilonaminocaproic or epsilonaminocapronsav or etha?aminocaproic or ethaaminocaproich or emocaprol or hepin or ipsilon or jd?177or neocaprol or nsc?26154 or resplamin or tachostyptan).tw. 43. or/37‐42 44. Aprotinin/ 45. (antagosan or antilysin* or aprotimbin or apronitin* or aprotinin* or bayer a128 or contrical or contrycal or contrykal or dilmintal or frey inhibitor or kontrycal or Kunitz inhibitor or gordox or haemoprot or kallikrein‐trypsin inactivator).tw. 46. (iniprol or kontrikal or kontrykal or kunitz pancreatic trypsin inhibitor or midran or pulmin or tracylol or trascolan or trasilol or tra?ylol or traskolan or zymofren or pancreas antitrypsin or protinin or riker 52g or Rivilina zymofren).tw. 47. or/44‐46 48. Blood Clotting Factor 7a/ 49. (factor viia or factor 7a or rfviia or fviia or novoseven* or novo seven* or aryoseven or acset or eptacog* or proconvertin).tw. 50. ((activated adj2 factor seven) or (activated adj2 factor vii) or (activated adj3 rfvii) or (activated adj2 fvii)).tw. 51. (factor seven or factor vii or factor 7).ti. 52. 48 or 49 or 50 or 51 53. Fibrinogen Concentrate/ 54. (fibrinogen concentrate* or factor I or Haemocomplettan* or Riastap* or Fibryga* or Fibryna*).tw. 55. 53 or 54 56. Desmopressin/ 57. (desmopressin* or vasopressin deamino or D‐amino D‐arginine vasopressin or deamino‐8‐d‐arginine vasopressin or vasopressin 1‐desamino‐8‐arginine or desmotabs or DDAVP or desmogalen or adin or adiuretin or concentraid or d‐void or dav ritter or deamino 8 dextro arginine vasopressin or deamino 8d arginine vasopressin or deamino dextro arginine vasopressin or deaminovasopressin or defirin or defirin melt or desmirin or desmomelt or desmopresina or desmospray or desmotab* or desurin or emosint or enupresol or minirin or minirinette or minirinmelt or minrin or minurin or miram or nictur or noctisson or nocturin or nocutil or nordurine or novidin or nucotil or octim or octostim or presinex or stimate or wetirin).tw. 58. 56 or 57 59. Blood Clotting Factor 13/ 60. (factor xiii or fxiii or fibrin stabili?ing factor* or Tretten* or Catridecacog).tw. 61. 59 or 60 62. exp Tissue Adhesive/ 63. *Adhesive Agent/ 64. *Hemostatic Agent/ 65. ((fibrin* or collagen or cellulose or gelatin or gel or thrombin* or albumin or hemostatic* or haemostatic*) adj3 (glu* or seal* or adhesive* or topical* or local* or matrix or matrices or spong* or fleece* or foam* or scaffold* or patch* or sheet* or bandag* or aerosol* or dressing* or paste or powder*)).tw. 66. ((nonfibrin* or non‐fibrin* or synthetic* or non‐biological* or nonbiological* or biological*) adj3 (glue* or seal* or adhesive*)).tw. 67. (surgical* adj3 (glue* or sealant* or adhesive*)).tw. 68. ((fibrin* or collagen or cellulose or gelatin or thrombin) adj3 (hemosta* or haemosta*)).tw. 69. (8Y or Aafact or Actif‐VIII or Advate or Artiss or Raplixa or Evarrest or Aleviate or Alphanate or Amofil or Beriate or Beriplast or Biostate or Bolheal or Cluvot or Conco‐Eight‐HT or Crosseel or Crosseal or Crosseight or Emoclot or Evarrest or Evicel or Factane or Fanhdi or Fibrogammin P or Green VIII or Green VIII Factor or Greengene or Greenmono or Greenplast or Haemate or Haemate P or Haemate P or Haemate P500 or Haemate‐P or Haemoctin or Haemoctin SDH or Haemoctin‐SDH or Hemaseel or Hemaseal or Hemofil M or Hemoraas or Humaclot or Humafactor‐8 or Humate‐P or Immunate or Innovate or Koate or Koate‐DVI or Kogenate Bayer or Kogenate FS or Monoclate‐P or NovoThirteen or Octafil or Octanate or Octanate or Optivate or Quixil or Talate or Tisseel or Tisseal or Tissel or Tissucol or Tricos or Vivostat or Voncento or Wilate or Wilnativ or Wilstart or Xyntha).tw. 70. (Glubran or Gluetiss or Ifabond or Indermil or LiquiBand or TissuGlu).tw. 71. Collagen Sponge/ or Collagen Dressing/ 72. Gelatin Sponge/ or Gelfoam/ 73. (Evithrom or Floseal or Hemopatch or Gel‐Flow or Gelfoam or Gelfilm or Recothrom or Surgifoam or Surgiflo* or "rh Thrombin" or Thrombi‐Gel or Thrombi‐Pad or Thrombin‐JMI or Thrombinar or Thrombogen or Thrombostat).tw. 74. *Chitosan/ 75. Hydrogel Dressing/ 76. Fibrinogen plus Thrombin/ 77. Polyvinyl Alcohol Sponge/ 78. (porcine gelatin or bovine collagen or bovine gelatin or nu‐knit or arista or hemostase or vita sure or thrombin‐jmi or thrombinjmi or avicel or vivagel or lyostypt or tabotamp or arterx or omnex or veriset).tw. 79. (polysaccharide adj (sphere* or hemostatic powder)).tw. 80. ((polymer‐derived elastic* or polymer tissue adhesive* or elastic hydrogel* or glutaraldehyde or PEG‐based or polyurethane‐based tissue or polyethylene glycol* or polyvinyl alcohol‐based tissue or PVA‐based tissue or natural biopolymer* or polypeptide‐based or protein‐based or polysaccharide‐based or chitosan or poliglusam or cyanoacrylic or cyanoacrylate or cyacrin or dextran‐based or chondroitin sulfate‐based or mussel‐inspired elastic* or glycol hydrogel or polymer‐based) adj3 (glu* or seal* or adhesive* or topical* or local* or matrix or matrices or spong* or fleece* or foam* or scaffold* or patch* or sheet* or bandag* or aerosol* or dressing* or paste* or powder*)).tw. 81. Oxidized Cellulose/ 82. Oxidized Regenerated Cellulose/ 83. Recombinant Thrombin/ 84. Tachocomb/ 85. (absorbable cellulose or resorbable cellulose or oxidi?ed cellulose or carboxycellulose or oxycellulose or cellulosic acid or oxycel or oxidi?ed regenerated cellulose).tw. 86. (BioGlue or Progel or Duraseal or Coseal or FocalSeal or ADAL‐1 or AdvaSeal or Pleuraseal or Angio‐Seal or Avitene or Instat or Helitene or Helistat or TDM‐621 or Dermabond or Tissueseal or PolyStat or Raplixa or Spongostan or Surgicel).tw. 87. (Tachosil or Traumstem or CoStasis or "GRF Glue" or GR‐Dial or Algosterile or TraumaStat or HemCon or ChitoFlex or Celox or QuikClot or WoundStat or Vitagel or TachSeal or TachoComb or Cryoseal).tw. 88. (collagen‐thrombin or thrombin‐collagen or gelatin‐fibrinogen or fibrinogen‐gelatin or gelatin‐thrombin or thrombin‐gelatin or fibrinogen‐thrombin or thrombin‐fibrinogen or collagen‐fibrinogen or fibrinogen‐collagen or microfibrillar collagen).tw. 89. or/62‐88 90. Bone Wax/ 91. (bonewax* or bone wax* or bone putty or hemasorb or ostene).tw. 92. or/90‐91 93. prothrombin complex/ 94. (prothrombin adj5 (complex* or concentrate*)).tw. 95. (PCC* or 3F‐PCC* or 4F‐PCC* or Beriplex* or Feiba* or Autoplex* or Ocplex* or Octaplex* or Kcentra* or Cofact or Prothrombinex* or "Proplex‐T" or Prothroraas* or Haemosolvex* or Prothromplex* or "HT Defix" or Facnyne* or Kaskadil* or Kedcom* or Confidex* or PPSB or Profil?ine* or Pronativ* or Proplex* or Prothar* or ProthoRAAS* or Protromplex* or "Pushu Laishi" or "Uman Complex").tw. 96. or/93‐95 97. (((haemosta* or hemosta* or antihaemorrhag* or antihemorrhag* or anti haemorrhag* or anti‐hemorrhag*) adj5 (drug* or agent* or treat* or therap*)) or ((coagulat* or clotting) adj factor*)).tw. 98. 43 or 47 or 52 or 55 or 58 or 61 or 89 or 92 or 96 or 97 99. meta analysis/ 100. (meta analy* or metaanaly* or evidence‐based).ti. 101. ((meta analy* or metaanaly*) and (trials or studies)).ab. 102. systematic review/ 103. ((systematic* or evidence‐based) adj2 (review* or overview*)).tw. 104. (evidence synthes* or cochrane or medline or pubmed or embase or cinahl or cinhal or lilacs or "web of science" or science citation index or scopus or search terms or literature search or electronic search* or comprehensive search* or systematic search* or published articles or search strateg* or reference list* or bibliograph* or handsearch* or hand search* or manual* search*).ab. 105. ((electronic* or online) adj (sources or resources or databases)).ab. 106. ((additional adj (papers or articles or sources)) or (relevant adj (journals or articles))).ab. 107. or/99‐106 108. Review.pt. 109. (data extraction or selection criteria).ab. 110. 108 and 109 111. 107 or 110 112. Editorial.pt. 113. 111 not 112 114. crossover‐procedure/ or double‐blind procedure/ or randomized controlled trial/ or single‐blind procedure/ 115. (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or doubl* blind* or singl* blind* or assign* or allocat* or volunteer*).mp. 116. 113 or 114 or 115 117. (exp animal/ or nonhuman/) not exp human/ 118. 116 not 117 119. 36 and 98 and 118
CINAHL (EBSCOhost)
S1 (MH "Vascular Surgery+") S2 (MH "Graft Occlusion, Vascular") S3 (MH "Limb Salvage") S4 (MH "Amputation+") S5 (MH "Vascular Diseases+/SU") S6 (MH "Femoral Artery") OR (MH "Iliac Artery") OR (MH "Popliteal Artery/SU") S7 TX (interventional radiolog* or surgical radiolog*) S8 TI ( ((vascular or vessel* or aneurysm or aortic or aorta or AAA or TAA or TAAA or aortofemoral or artery or arterial or interarterial or arterioarterial or atheroma or carotid or vein or venous or endovascular* or intravascular*) N6 (surg* or operat* or reconstruct* or repair* or resection* or repair* or bypass* or graft* or homograft* or transplant* or bypass* or transpos* or implant* or reimplant* or clamp* or ligat* or crossover* or cross‐over*)) ) OR AB ( ((vascular or vessel* or aneurysm or aortic or aorta or AAA or TAA or TAAA or aortofemoral or artery or arterial or interarterial or arterioarterial or atheroma or carotid or vein or venous or endovascular* or intravascular*) N6 (surg* or operat* or reconstruct* or repair* or resection* or repair* or bypass* or graft* or homograft* or transplant* or bypass* or transpos* or implant* or reimplant* or clamp* or ligat* or crossover* or cross‐over*)) ) S9 TI ( ((endovascular or intravascular or laparoscop* or angioscop*) N3 (surg* or operat* or reconstruct* or repair* or resection* or repair* or bypass* or graft* or homograft* or transplant* or bypass* or transpos* or implant* or reimplant* or procedure*)) ) OR AB ( ((endovascular or intravascular or laparoscop* or angioscop*) N3 (surg* or operat* or reconstruct* or repair* or resection* or repair* or bypass* or graft* or homograft* or transplant* or bypass* or transpos* or implant* or reimplant* or procedure*)) ) S10 TI ( (bypass graft* or by‐pass graft* or bypass surgery or by‐pass surgery or enarterectom* or (minimally invasive N1 surg*) or teflon graft* or dacron graft*) ) OR AB ( (bypass graft* or by‐pass graft* or bypass surgery or by‐pass surgery or enarterectom* or (minimally invasive N1 surg*) or teflon graft* or dacron graft*) ) S11 TI ( (arterial dilation* or endoluminal repair*) ) OR AB ( (arterial dilation* or endoluminal repair*) ) S12 TI ( ((venous or arterial) N3 catheteri?ation) ) OR AB ( ((venous or arterial) N3 catheteri?ation) ) S13 TI ( ((aort* or iliac or femoral or popliteal or femoropop* or fempop* or crural) N3 (surg* or operat* or bypass or graft or reconstruct* or revascular*)) ) AND AB ( ((aort* or iliac or femoral or popliteal or femoropop* or fempop* or crural) N3 (surg* or operat* or bypass or graft or reconstruct* or revascular*)) ) S14 TI ( (angiosurg* or aneurysmectom* or aneurysm clipping* or aortopexy or aortoplast* or arteriotom* or arterioplast* or artery plast* or artery stripping or venostom* or portacaval anastomoses or revasculari?ation or devasculari?ation) ) OR AB ( (angiosurg* or aneurysmectom* or aneurysm clipping* or aortopexy or aortoplast* or arteriotom* or arterioplast* or artery plast* or artery stripping or venostom* or portacaval anastomoses or revasculari?ation or devasculari?ation) ) S15 TI ( ((femoral* or iliac* or aorta* or aortic* or infrapopliteal or popliteal or infra‐pop*) N3 (stent* or angioplast*)) ) OR AB ( ((femoral* or iliac* or aorta* or aortic* or infrapopliteal or popliteal or infra‐pop*) N3 (stent* or angioplast*)) ) S16 TI ( (endovascular N6 (dissection* or stent*)) ) OR AB ( (endovascular N6 (dissection* or stent*)) ) S17 TI ( (EVAR or FEVAR or TEVAR or embolectom* or thrombectom* or endarterectom* or thromboendarterectom* or thromboembolectom* or atherectom*) ) OR AB ( (EVAR or FEVAR or TEVAR or embolectom* or thrombectom* or endarterectom* or thromboendarterectom* or thromboembolectom* or atherectom*) ) S18 TI ( (mechanical* N3 (thrombolysis or clot disruption*)) ) OR AB ( (mechanical* N3 (thrombolysis or clot disruption*)) ) S19 TI ( (axillo bifemoral bypass graft* or axillo femoral bypass graft* or axillobifemoral bypass graft* or axillofemoral bypass graft*) ) OR AB ( (axillo bifemoral bypass graft* or axillo femoral bypass graft* or axillobifemoral bypass graft* or axillofemoral bypass graft*) ) S20 TI ( ((ilio femor* or ilio femor* or infrarenal or infra‐renal or infrainguinal or infra‐inguinal) NEXT (bypass* or by‐pass*)) ) OR AB ( ((ilio femor* or ilio femor* or infrarenal or infra‐renal or infrainguinal or infra‐inguinal) NEXT (bypass* or by‐pass*)) ) S21 TI ( ((ilio femor* or ilio femor* or infrarenal or infra‐renal or infrainguinal or infra‐inguinal) W1 (bypass* or by‐pass*)) ) OR AB ( ((ilio femor* or ilio femor* or infrarenal or infra‐renal or infrainguinal or infra‐inguinal) W1 (bypass* or by‐pass*)) ) S22 TI ( ((pedal or tibial) N3 (angio* or bypass* or by‐pass*)) ) OR AB ( ((pedal or tibial) N3 (angio* or bypass* or by‐pass*)) ) S23 TI ( ((lower limb* or knee* or leg* or foot or feet or lower extremit* or hindquarter*) N5 (salvag* or saving or save* or angioplast* or graft* or bypass* or by‐pass* or revascula* or reconstruct* or amputat*)) ) OR AB ( ((lower limb* or knee* or leg* or foot or feet or lower extremit* or hindquarter*) N5 (salvag* or saving or save* or angioplast* or graft* or bypass* or by‐pass* or revascula* or reconstruct* or amputat*)) ) S24 TI ( ((blood vessel* or vascular or endovascular or vein or venous or arterial or artery or arteriovenous or arterio‐venous or brescia cimino or venoarterial or veno‐arterial or arterioportal) N3 (anastomosis or shunt*)) ) OR AB ( ((blood vessel* or vascular or endovascular or vein or venous or arterial or artery or arteriovenous or arterio‐venous or brescia cimino or venoarterial or veno‐arterial or arterioportal) N3 (anastomosis or shunt*)) ) S25 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 S26 (MH "Antifibrinolytic Agents") OR (MH "Aminocaproic Acids") OR (MH "Tranexamic Acid") S27 TI ( (antifibrinolytic* or anti‐fibrinolytic* or antifibrinolysin* or antiplasmin* or plasmin inhibitor* or tranexamic or tranhexamic or cyclohexanecarboxylic acid* or amcha or trans‐4‐aminomethyl‐cyclohexanecarboxylic acid* or t‐amcha or amca or "kabi 2161" or transamin or amchafibrin or anvitoff or spotof or cyklokapron or cyclo‐F or femstrual or ugurol or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or antivoff or caprilon or cl?65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or cyklokapron or exacyl or frenolyse or fibrinon or hemostan or hexacapron or hexakapron or kalnex or lysteda or rikaparin or ronex or theranex or tranexam or tranexanic or tranexic or trans achma or transexamic or trenaxin or TXA or (fibrinolysis N2 inhibitor*)) ) OR AB ( (antifibrinolytic* or anti‐fibrinolytic* or antifibrinolysin* or antiplasmin* or plasmin inhibitor* or tranexamic or tranhexamic or cyclohexanecarboxylic acid* or amcha or trans‐4‐aminomethyl‐cyclohexanecarboxylic acid* or t‐amcha or amca or "kabi 2161" or transamin or amchafibrin or anvitoff or spotof or cyklokapron or cyclo‐F or femstrual or ugurol or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or antivoff or caprilon or cl?65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or cyklokapron or exacyl or frenolyse or fibrinon or hemostan or hexacapron or hexakapron or kalnex or lysteda or rikaparin or ronex or theranex or tranexam or tranexanic or tranexic or trans achma or transexamic or trenaxin or TXA or (fibrinolysis N2 inhibitor*)) ) S28 TI ( (6‐aminohexanoic or amino?caproic or amino?hexanoic or amino caproic or amino‐caproic or amino‐n‐hexanoic or cy‐116 or cy116 or lederle or acikaprin or afibrin or amicar or caprocid or capracid or capramol or caprogel or caprolest or caprolisin* or caprolysin* or capromol or epsikapron or hemocaprol or caproamin or EACA or caprolest or capralense or hexalense or hamostat or hemocid or cl 10304 or cl10304 or ecapron or ekaprol or epsamon or epsicaprom or epsicapron or epsilcapramin or epsilon amino caproate or epsilon aminocaproate or epsilonaminocaproic or epsilonaminocapronsav or etha?aminocaproic or ethaaminocaproich or emocaprol or hepin or ipsilon or jd?177or neocaprol or nsc?26154 or resplamin or tachostyptan) ) OR AB ( (6‐aminohexanoic or amino?caproic or amino?hexanoic or amino caproic or amino‐caproic or amino‐n‐hexanoic or cy‐116 or cy116 or lederle or acikaprin or afibrin or amicar or caprocid or capracid or capramol or caprogel or caprolest or caprolisin* or caprolysin* or capromol or epsikapron or hemocaprol or caproamin or EACA or caprolest or capralense or hexalense or hamostat or hemocid or cl 10304 or cl10304 or ecapron or ekaprol or epsamon or epsicaprom or epsicapron or epsilcapramin or epsilon amino caproate or epsilon aminocaproate or epsilonaminocaproic or epsilonaminocapronsav or etha?aminocaproic or ethaaminocaproich or emocaprol or hepin or ipsilon or jd?177or neocaprol or nsc?26154 or resplamin or tachostyptan) ) S29 S26 OR S27 OR S28 S30 (MH "Aprotinin") S31 TI ( (antagosan or antilysin* or aprotimbin or apronitin* or aprotinin* or bayer a128 or contrical or contrycal or contrykal or dilmintal or frey inhibitor or kontrycal or Kunitz inhibitor or gordox or haemoprot or kallikrein‐trypsin inactivator) ) OR AB ( (antagosan or antilysin* or aprotimbin or apronitin* or aprotinin* or bayer a128 or contrical or contrycal or contrykal or dilmintal or frey inhibitor or kontrycal or Kunitz inhibitor or gordox or haemoprot or kallikrein‐trypsin inactivator) ) S32 TI ( (iniprol or kontrikal or kontrykal or kunitz pancreatic trypsin inhibitor or midran or pulmin or tracylol or trascolan or trasilol or tra?ylol or traskolan or zymofren or pancreas antitrypsin or protinin or riker 52g or Rivilina zymofren) ) OR AB ( (iniprol or kontrikal or kontrykal or kunitz pancreatic trypsin inhibitor or midran or pulmin or tracylol or trascolan or trasilol or tra?ylol or traskolan or zymofren or pancreas antitrypsin or protinin or riker 52g or Rivilina zymofren) ) S33 S30 OR S31 OR S32 S34 TX ( (factor viia or factor 7a or rfviia or fviia or novoseven* or novo seven* or aryoseven or acset or eptacog* or proconvertin) ) OR TX ( ((activated N2 factor seven) or (activated N2 factor vii) or (activated N3 rfvii) or (activated N2 fvii)) ) S35 TX (factor seven or factor vii or factor 7) S36 S34 OR S35 S37 (MH "Fibrinogen") S38 TX (fibrinogen concentrate* or factor I or Haemocomplettan* or Riastap* or Fibryga* or Fibryna*) S39 S37 OR S38 S40 (MH "Desmopressin") S41 TI ( (desmopressin* or vasopressin deamino or D‐amino D‐arginine vasopressin or deamino‐8‐d‐arginine vasopressin or vasopressin 1‐desamino‐8‐arginine or desmotabs or DDAVP or desmogalen or adin or adiuretin or concentraid or d‐void or dav ritter or deamino 8 dextro arginine vasopressin or deamino 8d arginine vasopressin or deamino dextro arginine vasopressin or deaminovasopressin or defirin or defirin melt or desmirin or desmomelt or desmopresina or desmospray or desmotab* or desurin or emosint or enupresol or minirin or minirinette or minirinmelt or minrin or minurin or miram or nictur or noctisson or nocturin or nocutil or nordurine or novidin or nucotil or octim or octostim or presinex or stimate or wetirin) ) OR AB ( (desmopressin* or vasopressin deamino or D‐amino D‐arginine vasopressin or deamino‐8‐d‐arginine vasopressin or vasopressin 1‐desamino‐8‐arginine or desmotabs or DDAVP or desmogalen or adin or adiuretin or concentraid or d‐void or dav ritter or deamino 8 dextro arginine vasopressin or deamino 8d arginine vasopressin or deamino dextro arginine vasopressin or deaminovasopressin or defirin or defirin melt or desmirin or desmomelt or desmopresina or desmospray or desmotab* or desurin or emosint or enupresol or minirin or minirinette or minirinmelt or minrin or minurin or miram or nictur or noctisson or nocturin or nocutil or nordurine or novidin or nucotil or octim or octostim or presinex or stimate or wetirin) ) S42 S40 OR S41 S43 TX (factor XIII or fXIII or fibrin stabili?ing factor* or Tretten* or Catridecacog) S44 (MH "Tissue Adhesives") S45 (MH "Fibrin Tissue Adhesive") S46 (MH "Collagen/TU") S47 (MH "Thrombin/TU") S48 (MH "Surgical Sponges") S49 TI ( ((fibrin* or collagen or cellulose or gelatin or gel or thrombin* or albumin or hemostatic* or haemostatic*) N3 (glu* or seal* or adhesive* or topical* or local* or matrix or matrices or spong* or fleece* or foam* or scaffold* or patch* or sheet* or bandag* or aerosol* or dressing* or paste or powder*)) ) OR AB ( ((fibrin* or collagen or cellulose or gelatin or gel or thrombin* or albumin or hemostatic* or haemostatic*) N3 (glu* or seal* or adhesive* or topical* or local* or matrix or matrices or spong* or fleece* or foam* or scaffold* or patch* or sheet* or bandag* or aerosol* or dressing* or paste or powder*)) ) S50 TI ( ((nonfibrin* or non‐fibrin* or synthetic* or non‐biological* or nonbiological* or biological*) N3 (glue* or seal* or adhesive*)) ) OR AB ( ((nonfibrin* or non‐fibrin* or synthetic* or non‐biological* or nonbiological* or biological*) N3 (glue* or seal* or adhesive*)) ) S51 TI ( (surgical* N3 (glue* or sealant* or adhesive*)) ) OR AB ( (surgical* N3 (glue* or sealant* or adhesive*)) ) S52 TI ( ((fibrin* or collagen or cellulose or gelatin or thrombin) N3 (hemosta* or haemosta*)) ) OR AB ( ((fibrin* or collagen or cellulose or gelatin or thrombin) N3 (hemosta* or haemosta*)) ) S53 TI ( (8Y or Aafact or Actif‐VIII or Advate or Artiss or Bioglue or Biocol or Collaseal or Omrixil or Transglutine or Raplixa or Evarrest or Aleviate or Alphanate or Amofil or Beriate or Beriplast or Biostate or Bolheal or Cluvot or Conco‐Eight‐HT or Crosseel or Crosseal or Crosseight or Emoclot or Evarrest or Evicel or Factane or Fanhdi or Fibrogammin P or Green VIII or Green VIII Factor or Greengene or Greenmono or Greenplast or Haemate or Haemate P or Haemate P or Haemate P500 or Haemate‐P or Haemoctin or Haemoctin SDH or Haemoctin‐SDH or Hemaseel or Hemaseal or Hemofil M or Hemoraas or Humaclot or Humafactor‐8 or Humate‐P or Immunate or Innovate or Koate or Koate‐DVI or Kogenate Bayer or Kogenate FS or Monoclate‐P or NovoThirteen or Octafil or Octanate or Octanate or Optivate or Quixil or Talate or Tisseel or Tisseal or Tissel or Tissucol or Tricos or Vivostat or Voncento or Wilate or Wilnativ or Wilstart or Xyntha) ) OR AB ( (8Y or Aafact or Actif‐VIII or Advate or Artiss or Bioglue or Biocol or Collaseal or Omrixil or Transglutine or Raplixa or Evarrest or Aleviate or Alphanate or Amofil or Beriate or Beriplast or Biostate or Bolheal or Cluvot or Conco‐Eight‐HT or Crosseel or Crosseal or Crosseight or Emoclot or Evarrest or Evicel or Factane or Fanhdi or Fibrogammin P or Green VIII or Green VIII Factor or Greengene or Greenmono or Greenplast or Haemate or Haemate P or Haemate P or Haemate P500 or Haemate‐P or Haemoctin or Haemoctin SDH or Haemoctin‐SDH or Hemaseel or Hemaseal or Hemofil M or Hemoraas or Humaclot or Humafactor‐8 or Humate‐P or Immunate or Innovate or Koate or Koate‐DVI or Kogenate Bayer or Kogenate FS or Monoclate‐P or NovoThirteen or Octafil or Octanate or Octanate or Optivate or Quixil or Talate or Tisseel or Tisseal or Tissel or Tissucol or Tricos or Vivostat or Voncento or Wilate or Wilnativ or Wilstart or Xyntha) ) S54 TI ( (Glubran or Gluetiss or Ifabond or Indermil or LiquiBand or TissuGlu) ) OR AB ( (Glubran or Gluetiss or Ifabond or Indermil or LiquiBand or TissuGlu) ) S55 TI ( (Evithrom or Floseal or Hemopatch or Gel‐Flow or Gelfoam or Gelfilm or Recothrom or Surgifoam or Surgiflo* or "rh Thrombin" or Thrombi‐Gel or Thrombi‐Pad or Thrombin‐JMI or Thrombinar or Thrombogen or Thrombostat) ) OR AB ( (Evithrom or Floseal or Hemopatch or Gel‐Flow or Gelfoam or Gelfilm or Recothrom or Surgifoam or Surgiflo* or "rh Thrombin" or Thrombi‐Gel or Thrombi‐Pad or Thrombin‐JMI or Thrombinar or Thrombogen or Thrombostat) ) S56 TI ( (porcine gelatin or bovine collagen or bovine gelatin or nu‐knit or arista or hemostase or vita sure or thrombin‐jmi or thrombinjmi or avicel or vivagel or lyostypt or tabotamp or arterx or omnex or veriset) ) OR AB ( (porcine gelatin or bovine collagen or bovine gelatin or nu‐knit or arista or hemostase or vita sure or thrombin‐jmi or thrombinjmi or avicel or vivagel or lyostypt or tabotamp or arterx or omnex or veriset) ) S57 TX (polysaccharide NEXT (sphere* or hemostatic powder)) S58 (MM "Polyethylene Glycols") S59 (MH "Hydrogel Dressings") S60 (MH "Polyurethanes/AD/AE/TU/ST/DE") S61 TI ( ((polymer‐derived elastic* or polymer tissue adhesive* or elastic hydrogel* or glutaraldehyde or PEG‐based or polyurethane‐based tissue or polyethylene glycol* or polyvinyl alcohol‐based tissue or PVA‐based tissue or natural biopolymer* or polypeptide‐based or protein‐based or polysaccharide‐based or chitosan or poliglusam or cyanoacrylic or cyanoacrylate or cyacrin or dextran‐based or chondroitin sulfate‐based or mussel‐inspired elastic* or glycol hydrogel or polymer‐based) N3 (glu* or seal* or adhesive* or topical* or local* or matrix or matrices or spong* or fleece* or foam* or scaffold* or patch* or sheet* or bandag* or aerosol* or dressing* or paste* or powder*)) ) OR AB ( ((polymer‐derived elastic* or polymer tissue adhesive* or elastic hydrogel* or glutaraldehyde or PEG‐based or polyurethane‐based tissue or polyethylene glycol* or polyvinyl alcohol‐based tissue or PVA‐based tissue or natural biopolymer* or polypeptide‐based or protein‐based or polysaccharide‐based or chitosan or poliglusam or cyanoacrylic or cyanoacrylate or cyacrin or dextran‐based or chondroitin sulfate‐based or mussel‐inspired elastic* or glycol hydrogel or polymer‐based) N3 (glu* or seal* or adhesive* or topical* or local* or matrix or matrices or spong* or fleece* or foam* or scaffold* or patch* or sheet* or bandag* or aerosol* or dressing* or paste* or powder*)) ) S62 (MH "Cellulose/TU") S63 TI ( (absorbable cellulose or resorbable cellulose or oxidi?ed cellulose or carboxycellulose or oxycellulose or cellulosic acid or oxycel or oxidi?ed regenerated cellulose) ) OR AB ( (absorbable cellulose or resorbable cellulose or oxidi?ed cellulose or carboxycellulose or oxycellulose or cellulosic acid or oxycel or oxidi?ed regenerated cellulose) ) S64 TI ( (BioGlue or Progel or Duraseal or Coseal or FocalSeal or ADAL‐1 or AdvaSeal or Pleuraseal or Angio‐Seal or Avitene or Instat or Helitene or Helistat or TDM‐621 or Dermabond or Tissueseal or PolyStat or Raplixa or Spongostan or Surgicel or Surgilux or Tachosil or Traumstem) ) OR AB ( (BioGlue or Progel or Duraseal or Coseal or FocalSeal or ADAL‐1 or AdvaSeal or Pleuraseal or Angio‐Seal or Avitene or Instat or Helitene or Helistat or TDM‐621 or Dermabond or Tissueseal or PolyStat or Raplixa or Spongostan or Surgicel or Surgilux or Tachosil or Traumstem) ) S65 TI ( (collagen‐thrombin or thrombin‐collagen or gelatin‐fibrinogen or fibrinogen‐gelatin or gelatin‐thrombin or thrombin‐gelatin or fibrinogen‐thrombin or thrombin‐fibrinogen or collagen‐fibrinogen or fibrinogen‐collagen or microfibrillar collagen or CoStasis or "GRF Glue" or GR‐Dial or Algosterile or TraumaStat or HemCon or ChitoFlex or Celox or QuikClot or WoundStat or Vitagel or TachSeal or TachoComb or Cryoseal) ) OR AB ( (collagen‐thrombin or thrombin‐collagen or gelatin‐fibrinogen or fibrinogen‐gelatin or gelatin‐thrombin or thrombin‐gelatin or fibrinogen‐thrombin or thrombin‐fibrinogen or collagen‐fibrinogen or fibrinogen‐collagen or microfibrillar collagen or CoStasis or "GRF Glue" or GR‐Dial or Algosterile or TraumaStat or HemCon or ChitoFlex or Celox or QuikClot or WoundStat or Vitagel or TachSeal or TachoComb or Cryoseal) ) S66 S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 S67 (MH "Waxes/TU") S68 TI ( (bonewax* or bone wax* or bone putty or hemasorb or ostene) ) OR AB ( (bonewax* or bone wax* or bone putty or hemasorb or ostene) ) S69 S67 OR S68 S70 (MH "Blood Coagulation Factors") S71 TI ( (prothrombin N5 (complex* or concentrate*)) ) OR AB ( (prothrombin N5 (complex* or concentrate*)) ) S72 TI ( (PCC* or 3F‐PCC* or 4F‐PCC* or Beriplex* or Feiba* or Autoplex* or Ocplex* or Octaplex* or Kcentra* or Cofact or Prothrombinex* or "Proplex‐T" or Prothroraas* or Haemosolvex* or Prothromplex* or "HT Defix" or Facnyne* or Kaskadil* or Kedcom* or Confidex* or PPSB or Profil?ine* or Pronativ* or Proplex* or Prothar* or ProthoRAAS* or Protromplex* or "Pushu Laishi" or "Uman Complex") ) OR AB ( (PCC* or 3F‐PCC* or 4F‐PCC* or Beriplex* or Feiba* or Autoplex* or Ocplex* or Octaplex* or Kcentra* or Cofact or Prothrombinex* or "Proplex‐T" or Prothroraas* or Haemosolvex* or Prothromplex* or "HT Defix" or Facnyne* or Kaskadil* or Kedcom* or Confidex* or PPSB or Profil?ine* or Pronativ* or Proplex* or Prothar* or ProthoRAAS* or Protromplex* or "Pushu Laishi" or "Uman Complex") ) S73 S70 OR S71 OR S72 S74 TI ( (((haemosta* or hemosta* or antihaemorrhag* or antihemorrhag* or anti haemorrhag* or anti‐hemorrhag*) N5 (drug* or agent* or treat* or therap*)) or ((coagulat* or clotting) NEXT factor*)) ) OR AB ( (((haemosta* or hemosta* or antihaemorrhag* or antihemorrhag* or anti haemorrhag* or anti‐hemorrhag*) N5 (drug* or agent* or treat* or therap*)) or ((coagulat* or clotting) NEXT factor*)) ) S75 S29 OR S33 OR S36 OR S39 OR S42 OR S66 OR S69 OR S73 OR S74 S76 (MH Clinical Trials+) S77 PT Clinical Trial S78 TI ((controlled trial*) or (clinical trial*)) OR AB ((controlled trial*) or (clinical trial*)) S79 TI ((singl* blind*) OR (doubl* blind*) OR (trebl* blind*) OR (tripl* blind*) OR (singl* mask*) OR (doubl* mask*) OR (tripl* mask*)) OR AB ((singl* blind*) OR (doubl* blind*) OR (trebl* blind*) OR (tripl* blind*) OR (singl* mask*) OR (doubl* mask*) OR (tripl* mask*)) S80 TI randomi* OR AB randomi* S81 MH RANDOM ASSIGNMENT S82 TI ((phase three) or (phase III) or (phase three)) or AB ((phase three) or (phase III) or (phase three)) S83 ( TI (random* N2 (assign* or allocat*)) ) OR ( AB (random* N2 (assign* or allocat*)) ) S84 MH PLACEBOS S85 MH META ANALYSIS S86 MH SYSTEMATIC REVIEW S87 TI ("meta analys*" OR metaanalys* OR "systematic review" OR "systematic overview" OR "systematic search*") OR AB ("meta analys*" OR metaanalys* OR "systematic review" OR "systematic overview" OR "systematic search*") S88 TI ("literature review" OR "literature overview" OR "literature search*") OR AB ("literature review" OR "literature overview" OR "literature search*") S89 TI (cochrane OR embase OR cinahl OR cinhal OR lilacs OR BIDS OR science AND citation AND index OR cancerlit) OR AB (cochrane OR embase OR cinahl OR cinhal OR lilacs OR BIDS OR science AND citation AND index OR cancerlit) S90 TI placebo* OR AB placebo* S91 MH QUANTITATIVE STUDIES S92 S76 or S77 or S78 or S79 or S80 or S81 or S82 or S83 or S84 or S85 or S86 or S87 or S88 or S89 or S90 or S91 S93 S25 AND S75 AND S92
TRANSFUSION EVIDENCE LIBRARY
Clinical Specialty: Cardiovascular Surgery AND Subject Areas: Alternatives to Blood/Antifibrinolytics OR Alternatives to Blood/Fractionated Blood Proucts OR Alternatives to Blood/Recombinant Coagulation Factors
ClinicalTrials.gov
1. Other Terms: (interventional radiology OR vascular surgery OR embolectomy OR interarterial OR intravascular OR arteriovenous OR arterial OR endovascular OR revascularization OR iliac OR femoral OR popliteal OR EVAR or FEVAR or TEVAR OR vascular graft) AND Intervention/Treatment: hemostatic OR antifibrinolytic OR tranexamic OR EACA OR aminocaproic OR aprotinin OR desmopressin OR DDAVP OR factor viia OR novoseven OR aryoseven OR fibrinogen OR haemocomplettan OR Riastap OR Fibryna OR Fibryga OR factor XIII OR Tretten OR Other Terms: (thrombectomy OR venous catheterization OR aortic surgery OR aneurysm surgery OR angiosurgery OR devascularization OR angioplasty OR endarterectomy OR atherectomy OR limb salvage OR amputation OR anastomosis) AND Intervention/Treatment: hemostatic OR antifibrinolytic OR tranexamic OR EACA OR aminocaproic OR aprotinin OR desmopressin OR DDAVP OR factor viia OR novoseven OR aryoseven OR fibrinogen OR haemocomplettan OR Riastap OR Fibryna OR Fibryga OR factor XIII OR Tretten
2. Other Terms: (interventional radiology OR vascular surgery OR embolectomy OR interarterial OR intravascular OR arteriovenous OR arterial OR endovascular OR revascularization OR iliac OR femoral OR popliteal OR EVAR or FEVAR or TEVAR OR vascular graft) AND Intervention/Treatment: sealant OR adhesive OR collagen OR cellulose OR gelatin OR glue OR matrix OR sponge OR fleece OR foam OR scaffold OR patch OR sheet OR gelfoam OR chitosan OR hydrogel OR polyethylene glycol OR tachocomb OR BioGlue OR Surgicel OR Veriset OR Other Terms: (thrombectomy OR venous catheterization OR aortic surgery OR aneurysm surgery OR angiosurgery OR devascularization OR angioplasty OR endarterectomy OR atherectomy OR limb salvage OR amputation OR anastomosis) AND Intervention/Treatment: sealant OR adhesive OR collagen OR cellulose OR gelatin OR glue OR matrix OR sponge OR fleece OR foam OR scaffold OR patch OR sheet OR gelfoam OR chitosan OR hydrogel OR polyethylene glycol OR tachocomb OR BioGlue OR Surgicel OR Veriset
3. Other Terms: (interventional radiology OR vascular surgery OR embolectomy OR interarterial OR intravascular OR arteriovenous OR arterial OR endovascular OR revascularization OR iliac OR femoral OR popliteal OR EVAR or FEVAR or TEVAR OR vascular graft) AND Intervention/Treatment: Evithrom OR Floseal OR Tachosil OR Cryoseal OR Hemopatch OR Progel OR Duraseal OR Coseal OR FocalSeal OR Algosterile OR TraumaStat OR HemCon OR ChitoFlex OR Celox OR QuikClot OR WoundStat OR Vitagel OR TachSeal OR bonewax OR hemasorb OR ostene OR Other Terms: (thrombectomy OR venous catheterization OR aortic surgery OR aneurysm surgery OR angiosurgery OR devascularization OR angioplasty OR endarterectomy OR atherectomy OR limb salvage OR amputation OR anastomosis) AND Intervention/Treatment: Evithrom OR Floseal OR Tachosil OR Cryoseal OR Hemopatch OR Progel OR Duraseal OR Coseal OR FocalSeal OR Algosterile OR TraumaStat OR HemCon OR ChitoFlex OR Celox OR QuikClot OR WoundStat OR Vitagel OR TachSeal OR bonewax OR hemasorb OR ostene
4. Other Terms: (interventional radiology OR vascular surgery OR embolectomy OR interarterial OR intravascular OR arteriovenous OR arterial OR endovascular OR revascularization OR iliac OR femoral OR popliteal OR EVAR or FEVAR or TEVAR OR vascular graft) AND Intervention/Treatment: iniprol or kontrikal OR CloSys OR Glubran OR Gluetiss OR Ifabond OR Indermil OR LiquiBand OR Octafil OR Octanate OR Optivate OR Quixil OR Tisseel OR Tissucol OR TissuGlu OR Thrombi‐Gel OR Vivostat OR Voncento OR Wilate OR Wilnativ OR Wilstart OR Other Terms: (thrombectomy OR venous catheterization OR aortic surgery OR aneurysm surgery OR angiosurgery OR devascularization OR angioplasty OR endarterectomy OR atherectomy OR limb salvage OR amputation OR anastomosis) AND Intervention/Treatment: iniprol or kontrikal OR CloSys OR Glubran OR Gluetiss OR Ifabond OR Indermil OR LiquiBand OR Octafil OR Octanate OR Optivate OR Quixil OR Tisseel OR Tissucol OR TissuGlu OR Thrombi‐Gel OR Vivostat OR Voncento OR Wilate OR Wilnativ OR Wilstart
5. Other Terms: (interventional radiology OR vascular surgery OR embolectomy OR interarterial OR intravascular OR arteriovenous OR arterial OR endovascular OR revascularization OR iliac OR femoral OR popliteal OR EVAR or FEVAR or TEVAR OR vascular graft) AND Intervention/Treatment: "prothrombin complex concentrate" OR Beriplex OR Feiba OR Ocplex OR Kcentra OR Prothrombinex OR Other Terms: (thrombectomy OR venous catheterization OR aortic surgery OR aneurysm surgery OR angiosurgery OR devascularization OR angioplasty OR endarterectomy OR atherectomy OR limb salvage OR amputation OR anastomosis) AND Intervention/Treatment: "prothrombin complex concentrate" OR Beriplex OR Feiba OR Ocplex OR Kcentra OR Prothrombinex
6. Other Terms: (interventional radiology OR vascular surgery OR embolectomy OR interarterial OR intravascular OR arteriovenous OR arterial OR endovascular OR revascularization OR iliac OR femoral OR popliteal OR EVAR or FEVAR or TEVAR OR vascular graft) AND Title: hemostasis OR hemostatic OR antifibrinolytic OR clotting factor OR coagulation factor OR fibrinogen OR thrombin OR collagen OR gelatin OR cellulose OR Other Terms: (thrombectomy OR venous catheterization OR aortic surgery OR aneurysm surgery OR angiosurgery OR devascularization OR angioplasty OR endarterectomy OR atherectomy OR limb salvage OR amputation OR anastomosis) AND Title: hemostasis OR hemostatic OR antifibrinolytic OR clotting factor OR coagulation factor OR fibrinogen OR thrombin OR collagen OR gelatin OR cellulose
7. Other Terms: (interventional radiology OR vascular surgery OR embolectomy OR interarterial OR intravascular OR arteriovenous OR arterial OR endovascular OR revascularization OR iliac OR femoral OR popliteal OR EVAR or FEVAR or TEVAR OR vascular graft) AND Condition: bleeding OR hemorrhage OR blood loss OR bloodloss OR Other Terms: (thrombectomy OR venous catheterization OR aortic surgery OR aneurysm surgery OR angiosurgery OR devascularization OR angioplasty OR endarterectomy OR atherectomy OR limb salvage OR amputation OR anastomosis) AND Condition: bleeding OR hemorrhage OR blood loss OR bloodloss
8. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 [N.B. combined and de‐duplicated in EndNote]
WHO ICTRP
1. Title OR Condition: interventional radiology OR vascular surgery OR embolectomy OR interarterial OR intravascular OR arteriovenous OR arterial OR endovascular OR revascularization OR iliac OR femoral OR popliteal OR EVAR or FEVAR or TEVAR OR vascular graft AND Intervention: hemostatic OR antifibrinolytic OR tranexamic OR EACA OR aminocaproic OR aprotinin OR desmopressin OR DDAVP OR factor viia OR novoseven OR aryoseven OR fibrinogen OR haemocomplettan OR Riastap OR Fibryna OR Fibryga OR factor XIII OR Tretten OR Title OR Condition: thrombectomy OR venous catheterization OR aortic surgery OR aneurysm surgery OR angiosurgery OR devascularization OR angioplasty OR endarterectomy OR atherectomy OR limb salvage OR amputation OR anastomosis AND Intervention: hemostatic OR antifibrinolytic OR tranexamic OR EACA OR aminocaproic OR aprotinin OR desmopressin OR DDAVP OR factor viia OR novoseven OR aryoseven OR fibrinogen OR haemocomplettan OR Riastap OR Fibryna OR Fibryga OR factor XIII OR Tretten
2. Title OR Condition: interventional radiology OR vascular surgery OR embolectomy OR interarterial OR intravascular OR arteriovenous OR arterial OR endovascular OR revascularization OR iliac OR femoral OR popliteal OR EVAR or FEVAR or TEVAR OR vascular graft AND Intervention: sealant OR adhesive OR collagen OR cellulose OR gelatin OR glue OR matrix OR sponge OR fleece OR foam OR scaffold OR patch OR sheet OR gelfoam OR chitosan OR hydrogel OR polyethylene glycol OR tachocomb OR BioGlue OR Surgicel OR Veriset OR Title OR Condition: thrombectomy OR venous catheterization OR aortic surgery OR aneurysm surgery OR angiosurgery OR devascularization OR angioplasty OR endarterectomy OR atherectomy OR limb salvage OR amputation OR anastomosis AND Intervention: sealant OR adhesive OR collagen OR cellulose OR gelatin OR glue OR matrix OR sponge OR fleece OR foam OR scaffold OR patch OR sheet OR gelfoam OR chitosan OR hydrogel OR polyethylene glycol OR tachocomb OR BioGlue OR Surgicel OR Veriset
3. Title OR Condition: interventional radiology OR vascular surgery OR embolectomy OR interarterial OR intravascular OR arteriovenous OR arterial OR endovascular OR revascularization OR iliac OR femoral OR popliteal OR EVAR or FEVAR or TEVAR OR vascular graft AND Intervention: Evithrom OR Floseal OR Tachosil OR Cryoseal OR Hemopatch OR Progel OR Duraseal OR Coseal OR FocalSeal OR Algosterile OR TraumaStat OR HemCon OR ChitoFlex OR Celox OR QuikClot OR WoundStat OR Vitagel OR TachSeal OR bonewax OR hemasorb OR ostene OR Title OR Condition: thrombectomy OR venous catheterization OR aortic surgery OR aneurysm surgery OR angiosurgery OR devascularization OR angioplasty OR endarterectomy OR atherectomy OR limb salvage OR amputation OR anastomosis AND Intervention: Evithrom OR Floseal OR Tachosil OR Cryoseal OR Hemopatch OR Progel OR Duraseal OR Coseal OR FocalSeal OR Algosterile OR TraumaStat OR HemCon OR ChitoFlex OR Celox OR QuikClot OR WoundStat OR Vitagel OR TachSeal OR bonewax OR hemasorb OR ostene
4. Title OR Condition: interventional radiology OR vascular surgery OR embolectomy OR interarterial OR intravascular OR arteriovenous OR arterial OR endovascular OR revascularization OR iliac OR femoral OR popliteal OR EVAR or FEVAR or TEVAR OR vascular graft AND Intervention: iniprol or kontrikal OR CloSys OR Glubran OR Gluetiss OR Ifabond OR Indermil OR LiquiBand OR Octafil OR Octanate OR Optivate OR Quixil OR Tisseel OR Tissucol OR TissuGlu OR Thrombi‐Gel OR Vivostat OR Voncento OR Wilate OR Wilnativ OR Wilstart OR Title OR Condition: thrombectomy OR venous catheterization OR aortic surgery OR aneurysm surgery OR angiosurgery OR devascularization OR angioplasty OR endarterectomy OR atherectomy OR limb salvage OR amputation OR anastomosis AND Intervention: iniprol or kontrikal OR CloSys OR Glubran OR Gluetiss OR Ifabond OR Indermil OR LiquiBand OR Octafil OR Octanate OR Optivate OR Quixil OR Tisseel OR Tissucol OR TissuGlu OR Thrombi‐Gel OR Vivostat OR Voncento OR Wilate OR Wilnativ OR Wilstart ‐ 5. Title OR Condition: interventional radiology OR vascular surgery OR embolectomy OR interarterial OR intravascular OR arteriovenous OR arterial OR endovascular OR revascularization OR iliac OR femoral OR popliteal OR EVAR or FEVAR or TEVAR OR vascular graft AND Intervention: prothrombin complex OR prothrombin concentrate OR Beriplex OR Feiba OR Ocplex OR Kcentra OR Prothrombinex OR Title OR Condition: thrombectomy OR venous catheterization OR aortic surgery OR aneurysm surgery OR angiosurgery OR devascularization OR angioplasty OR endarterectomy OR atherectomy OR limb salvage OR amputation OR anastomosis AND Intervention: prothrombin complex OR prothrombin concentrate OR Beriplex OR Feiba OR Ocplex OR Kcentra OR Prothrombinex
6. Title OR Condition: interventional radiology OR vascular surgery OR embolectomy OR interarterial OR intravascular OR arteriovenous OR arterial OR endovascular OR revascularization OR iliac OR femoral OR popliteal OR EVAR or FEVAR or TEVAR OR vascular graft AND Title OR Intervention: hemostasis OR hemostatic OR antifibrinolytic OR factor OR fibrinogen OR thrombin OR collagen OR gelatin OR cellulose OR Title OR Condition: thrombectomy OR venous catheterization OR aortic surgery OR aneurysm surgery OR angiosurgery OR devascularization OR angioplasty OR endarterectomy OR atherectomy OR limb salvage OR amputation OR anastomosis AND Title OR Intervention: hemostasis OR hemostatic OR antifibrinolytic OR factor OR fibrinogen OR thrombin OR collagen OR gelatin OR cellulose
7. 1 OR 2 OR 3 OR 4 OR 5 OR 6 [N.B. combined and de‐duplicated in EndNote]
Data and analyses
Comparison 1. Systemic drug treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Number of red blood cells (units) transfused per participant up to 30 days post surgery | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 Aprotinin vs placebo | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐5.65, 2.53] |
| 1.1.2 Desmopressin vs placebo | 2 | 135 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.87, 0.42] |
| 1.2 All‐cause mortality at up to 30 days | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 Aprotinin vs placebo | 3 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.61, 1.52] |
| 1.2.2 Desmopressin vs placebo | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 5.57 [0.27, 112.85] |
| 1.2.3 Tranexamic acid vs placebo | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.3 Risk of receiving any allogeneic blood product at up to 30 days post surgery | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 1.3.1 Tranexamic acid vs placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 1.4 Risk of reoperation or repeat procedure for bleeding within 7 days | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.4.1 Aprotinin vs placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.4.2 Tranexamic acid vs placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5 Risk of a thrombotic/thromboembolic event (MI, CVA, DVT, PE) up to 30‐day follow‐up | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5.1 Desmopressin vs placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5.2 Tranexamic acid vs placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6 Risk of myocardial infarction up to 30 days | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 Aprotinin vs placebo | 2 | 209 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.41 [0.09, 1.82] |
| 1.6.2 Desmopressin vs placebo | 1 | 91 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.11, 2.88] |
| 1.6.3 Tranexamic acid vs placebo | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.14, 7.32] |
| 1.7 Risk of CVA or stroke up to 30 days | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Aprotinin vs placebo | 2 | 209 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.05, 2.62] |
| 1.8 Risk of deep vein thrombosis up to 90 days | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 1.8.1 Aprotinin vs placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 1.8.2 Desmopressin vs placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 1.9 Risk of pulmonary embolism up to 90 days | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 1.9.1 Aprotinin vs placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 1.9.2 Desmopressin vs placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 1.9.3 Tranexamic acid vs placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
Comparison 2. Topical drug treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Number of red blood cells (units) transfused per participant up to 30 days | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 Thrombin+fibrin/collagen sponge vs usual care | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.80, 0.60] |
| 2.2 All‐cause mortality at up to 30 days | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.2.1 Thrombin+fibrin/collagen sponge vs usual care | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.14, 66.53] |
| 2.2.2 Human thrombin/gelatin sponge vs bovine thrombin/gelatin sponge | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.2.3 Collagen dressing vs oxidised cellulose | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.14, 64.26] |
| 2.2.4 Novel agent/gelatin sponge vs placebo/gelatin sponge | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.2.5 Fibrin sealant vs usual care | 3 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.11, 1.76] |
| 2.2.6 Fibrin sealant vs gelatin sponge | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 2.46 [0.29, 20.55] |
| 2.2.7 Fibrin sealant vs thrombin/gelatin sponge | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.09, 2.64] |
| 2.2.8 Synthetic sealant vs thrombin/gelatin sponge | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.71] |
| 2.3 Risk of receiving any allogeneic blood product at up to 30 days post surgery | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.3.1 Fibrin/collagen sponge vs usual care | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.12, 0.92] |
| 2.3.2 Thrombin+fibrin/collagen sponge vs usual care | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 3.74] |
| 2.4 Risk of reoperation or repeat procedure for bleeding within 7 days | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.4.1 Fibrin sealant vs usual care | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.31, 3.40] |
| 2.4.2 Fibrin sealant vs gelatin sponge | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 4.03] |
| 2.4.3 Synthetic sealant vs thrombin/gelatin sponge | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.01, 8.51] |
| 2.5 Risk of a thrombotic/thromboembolic event (MI, CVA, DVT, PE) up to 30‐day follow‐up | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.5.1 Fibrin/collagen sponge vs usual care | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.5.2 Thrombin+fibrin/collagen sponge vs usual care | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.5.3 Collagen dressing vs oxidised cellulose | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.5.4 Fibrin sealant vs usual care | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.5.5 Synthetic sealant vs thrombin/gelatin sponge | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.6 Risk of a myocardial infarction up to 30 days | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.6.1 Thrombin+fibrin/collagen sponge vs usual care | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.6.2 Collagen dressing vs oxidised cellulose | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.6.3 Fibrin sealant vs usual care | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.7 Risk of a CVA or stroke up to 30 days | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.7.1 Collagen dressing vs oxidised cellulose | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.7.2 fibrin sealant vs usual care | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.8 Risk of a deep vein thrombosis up to 90 days | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.8.1 Collagen dressing vs oxidised cellulose | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.8.2 Fibrin sealant vs usual care | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.9 Risk of a pulmonary embolism up to 90 days | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.9.1 Collagen dressing vs oxidised cellulose | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.9.2 Novel agent (Peprostat) vs placebo/gelatin sponge | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.9.3 Fibrin sealant vs usual care | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bajardi 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Between June 2007 and June 2008 a total of 20 patients with intact infrarenal abdominal aortic aneurysm (AAA) were randomized in the same center." Method of sequence generation for randomisation not described. |
| Allocation concealment (selection bias) | Low risk | "Sealed code envelope method was used to randomize patients to either treatment with TachoSil ® use or to standard compression with surgical swabs. The randomization code envelopes were opened just before the application of haemostatic measures." Sealed code method acceptable, though does not specify that envelopes were sealed, opaque and sequentially numbered etc. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The nature of the treatments precluded blinding of the study." Subjective outcomes ‐ high risk of bias due to inadequate personnel blinding because comparator is usual care rather than placebo. Objective outcomes ‐ low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Subjective outcomes ‐ high risk of bias due to absent outcome assessor blinding. Objective outcomes ‐ low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data set reported with denominators and numerators and with no dropouts reported. |
| Selective reporting (reporting bias) | Unclear risk | No available prospective protocol or trial registration. |
| Other bias | Low risk | No other concerns such as early stopping or imbalanced study arms. |
Bochicchio 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The article says "random", but no further details about how it was randomised. Method of sequence generation for randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "this single‐blind trial" Blinding not possible. Subjective outcomes ‐ low risk of bias due to adequate personnel blinding. Objective outcomes ‐ low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Subjective outcomes ‐ low risk of bias due to adequate outcome assessor blinding. Objective outcomes ‐ low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9% of the Fibrocaps group and 3% of the Gelatin sponge group did not complete the study. However because some of these were due to death, overall, less than 5% drop out rate per arm and low risk of bias. Outcome data set reported with denominators and numerators |
| Selective reporting (reporting bias) | Low risk | All outcomes planned in the protocol or prospective trial registration were reported. |
| Other bias | Low risk | No other concerns such as early stopping or imbalanced study arms. |
Chetter 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization function of the statistics software" Adequate method of sequence generation with computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | "and communicated using sealed opaque envelopes." Method of allocation concealment likely to be adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Due to the obvious differences between the 2 treatments, blinding of investigators was not possible following randomization." Subjective outcomes ‐ high risk of bias due to absent personnel blinding. Objective outcomes ‐ low risk of bias regardless. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Subjective outcomes ‐ high risk of bias due to absent outcome assessor blinding. Objective outcomes ‐ low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcome data set reported of both randomised and non‐randomised participants pooled together. |
| Selective reporting (reporting bias) | Unclear risk | The prospective protocol or trial registration mentioned in the article is not correct, and is from another study, so the trial protocol is unknown. |
| Other bias | Low risk | No other concerns such as early stopping or imbalanced study arms. |
Clagett 1995.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was carried out in blocks of 10 and was stratified for repair of abdominal aortic aneurysm or aortofemoral bypass for occlusive disease. Patients were randomized by drawing a sealed envelope that contained a prescription for either 20 txg DDAVI' (Rorer Pharmaceuticals, Fort Washington, Pa.) or placebo." "Patients were randomized by drawing a sealed envelope that contained a prescription for either ...." Method of sequence generation for randomization not described. |
| Allocation concealment (selection bias) | Low risk | "The only person to have knowledge of treatment assignment was the pharmacist who kept records and prepared DDAVP or placebo in identical‐appearing plastic bags of 50 ml normal saline solution. DDAVP or placebo was administered intravenously for 15 minutes immediately after intravenous heparinization with 100 U/kg was performed and just before aortic cross‐clamp application was done." "Patients were randomized by drawing a sealed envelope that contained a prescription for either ...." Adequate method of central allocation concealment by pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The only person to have knowledge of the treatment assignment was the pharmacist who kept records and prepared DDAVP or placebo in identical ‐appearing plastic bags of 50ml normal saline solution." Subjective outcomes ‐ low risk of bias due to adequate personnel blinding. Objective outcomes ‐ low risk of bias. The article says "double blind" and a placebo was used. Subjective outcomes ‐ low risk of bias due to adequate personnel blinding. Objective outcomes ‐ low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The only person to have knowledge of the treatment assignment was the pharmacist who kept records and prepared DDAVP or placebo in identical ‐appearing plastic bags of 50ml normal saline solution." Subjective outcomes ‐ low risk of bias due to adequate outcome assessor blinding. Objective outcomes ‐ low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data set reported with denominators and numerators showing no dropouts or exclusions. |
| Selective reporting (reporting bias) | Unclear risk | No available prospective protocol or trial registration. |
| Other bias | Low risk | No other concerns such as early stopping or imbalanced study arms. |
Czerny 2000.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No meaningful information. "The randomisation was carried out by the sponsor and patients were stratified for the type of reconstruction (anastomosis or patch angioplasty) as the bleeding from patch angioplasty was supposed to be more severe because of longer suture lines." |
| Allocation concealment (selection bias) | High risk | No information. No details of allocation concealment were available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was unblinded. For objective outcomes (e.g. mortality) the RoB is low. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was unblinded. For objective outcomes, the RoB is low. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants randomised was not reported. Unclear if all participants who were randomised were included in the final analysis. |
| Selective reporting (reporting bias) | High risk | The trial was not preregistered, and no protocol is available, so it is unclear if the investigators collected more data that is not reported here. All the outcomes stated in the methods section were reported in the paper. Methods stated that AEs were classified as serious/not, but there was no reporting of SAEs, only all AEs. Eligibility criteria require bleeding (so presumably intraoperative randomisation, but no information reported about randomisation procedures) but a preoperative adverse event was reported. No sample sizes given for any outcome. |
| Other bias | Low risk | No additional concerns noted |
EUCTR2016‐003661‐26‐PL.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only trial registration and results were available to extract. No details of randomisation processes were provided. |
| Allocation concealment (selection bias) | Unclear risk | Only trial registration and results were available to extract. No details of allocation concealment processes were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial is described as "double blind," with subject, investigator, monitor and data analyst all blinded. No details available on how blinding was carried out, as only trial registration and results are provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trial is described as "double blind," with subject, investigator, monitor and data analyst all blinded. No details available on how blinding was carried out, as only trial registration and results are provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Very large number excluded due to not receiving study intervention; randomisation appears to have taken place before surgery, but exclusion from analysis includes not receiving allocated intervention, not just absence of an eligible bleed. "Full Analysis Set represents the planned treatments for patients undergoing all surgeries who received [allocated treatment] and experienced mild bleeding during surgery." |
| Selective reporting (reporting bias) | Low risk | The primary outcomes were reported in the trial registration, but secondary outcomes were not reported, likely because of lack of space. Adverse event reporting was complete. |
| Other bias | Low risk | No additional concerns noted. |
Giovanacci 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No information. Study randomised wounds rather than people. |
| Allocation concealment (selection bias) | High risk | "For randomisation we had two containers with numbered closed envelopes in the operating room, one for unilateral and one for bilateral procedures. For unilateral operation the envelope contained the instruction if fibrin glue had to be utilised or not. For bilateral operations the envelope contained the instruction about which inguinal wound had to be sealed with fibrin glue. The contralateral wound was assigned to the control group." Unclear how "which inguinal wound" was described in the randomisation and whether surgeon would have the freedom to choose (i.e. if randomisation was first wound vs second wound). No mention of whether envelopes were opaque or audit of order in which envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding reported. Surgical team would not be able to be blinded. For objective outcomes, this domain is low RoB. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding reported. Surgical team would not be able to be blinded; no mention of blinded outcome assessors. For objective outcomes, this domain is low RoB. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Six people lost to follow‐up on each arm and one cross‐over on control. 6 vs 7 wounds excluded as a result. Unclear why intrasurgical outcomes not available for these people. Sample sizes not reported but appear to be 136 wounds on control and 130 on intervention. Results keep switching between wounds and people without much clarity. |
| Selective reporting (reporting bias) | Unclear risk | No trial registration. Protocol is not available. It is unclear if more outcome data were recorded and not reported in the paper. |
| Other bias | Low risk | No other concerns noted. |
Joseph 2004.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised by the sealed code envelope method to either treatment with TachoComb w H patches or to standard compression with surgical swabs." Method of sequence generation for randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | "Patients were randomised by the sealed code envelope method to either treatment with TachoComb w H patches or to standard compression with surgical swabs." Method of allocation concealment not described fully enough. Envelopes not described as sealed, opaque and sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The nature of the treatments precluded blinding of the study." Study not blinded. Subjective outcomes ‐ high risk of bias due to unblinded personnel blinding. Objective outcomes ‐ low risk of bias regardless. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The nature of the treatments precluded blinding of the study. Therefore, an independent nurse recorded all the variables assessed." Study was unblinded however, nurse is described as independent so presumable was unaware of treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Whilst two participants were excluded from analysis due to deviation from study protocol it is unlikely to have introduced bias, because the exclusions in both arms were balanced. |
| Selective reporting (reporting bias) | Low risk | All outcomes planned in the protocol or prospective trial registration were reported. |
| Other bias | High risk | "There were no significant differences in any patient or procedural characteristics including the use of anti‐platelet agents." "A total sample size of 60 patients (30 in each treatment group) was originally planned after a power calculation but as the effectiveness of TachoComb w H was also demonstrated in a similar study, conducted simultaneously, the present study was stopped prematurely and only 24 patients were randomised." Study stopped early for ethical reasons (futility) as a different study showed clear efficacy of TachoComb. Although the text in the results suggested that there were no significant differences in the baseline characteristics, there was no table with data backing up this assertion. |
Leijdekkers 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized to receive either placebo or aprotinin, using a standard randomization list stored in the pharmacy department, only to be opened after the study was closed for inclusion." Adequate method of sequence generation with standard randomisation list. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment performed at the pharmacy, though no further details given of how patient's group allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "A double‐blind randomized trial...." "Aprotinin was supplied in bottles of 50ml... the placebo was supplied in the same bottles containing only NaCl..." Subjective outcomes ‐ low risk of bias due to adequate personnel blinding. Objective outcomes ‐ low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomisation list was only opened after study completion. Subjective outcomes ‐ low risk of bias due to adequate outcome assessor blinding. Objective outcomes ‐ low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data set reported with denominators and numerators and with no dropouts reported. |
| Selective reporting (reporting bias) | Unclear risk | No available prospective protocol or trial registration. |
| Other bias | Low risk | No other concerns noted. |
Lethagen 1991.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Fifty patients scheduled for surgery for aorto‐iliac occlusive disease or aneurysms were randomly allocated to receive either desmopressin or a placebo." Method of sequence generation for randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Desmopressin 4 μg/ml (Minirin ®, Ferring, Sweden) was diluted in physiological saline to a volume of 10 ml and injected intravenously over 10min at a dose of 0.3 μg/kg body weight and placebo given as physiological saline." Study stated to be double‐blind, but method not described in detail. However, a need to "break the code" for some exclusions suggests study was properly blinded. Subjective outcomes ‐ low risk of bias due to adequate personnel blinding. Objective outcomes ‐ low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Subjective outcomes ‐ low risk of bias due to adequate outcome assessor blinding. Objective outcomes ‐ low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Six participant in whom surgical complications occurred were excluded, three in the desmopressin group and three in the placebo group. However, the exclusions were balanced in the arms, and thus it is unclear what the effect would have been on the outcome. |
| Selective reporting (reporting bias) | Unclear risk | No available prospective protocol or trial registration. |
| Other bias | Low risk | No other concerns such as early stopping or imbalanced study arms. |
Milne 1996.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Following entry into the trial the patients were randomised in a computer‐generated sequence to treatment or control." Adequate method of sequence generation with computer‐generated code. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The study was a prospective, randomised non‐blinded trial." Unblinded study. Subjective outcomes ‐ high risk of bias due to inadequate or absent personnel blinding. Objective outcomes ‐ low risk of bias regardless |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded study. Subjective outcomes ‐ high risk of bias due to inadequate or absent outcome assessor blinding. Objective outcomes ‐ low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No obvious outcome data missing. |
| Selective reporting (reporting bias) | High risk | Methods state they intend to report a particular outcome (e.g. blood loss, blood product usage) but this is not presented with numerical data in the results. |
| Other bias | Low risk | No other concerns such as early stopping or imbalanced study arms, however limited baseline characteristic data given |
Minkowitz 2019.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Limited information. If blinding was successful unlikely to introduce bias. "Qualified patients were randomized in a 2:1 ratio to the TTH‐Grifols and BT‐JMI treatment groups, stratified by type of operation (vascular, hepatic, soft tissue, and spinal) and subtype of operation in certain cases (vascular, peripheral arterial bypass, and extremity vascular access for hemodialysis) and soft tissue operation (mastopexies and abdominoplasties and non‐mastopexies and non‐abdominoplasties). Randomizations were performed at the study center’s pharmacy using an Interactive Response Technology system, which provided the randomization number and assigned the corresponding thrombin treatment." |
| Allocation concealment (selection bias) | Low risk | "The assigned study drug was then reconstituted by pharmacy staff at each study center and supplied to the investigators in syringes labeled with coded information to mask the treatment identification. Investigator, study nurses, or testing laboratories were blinded to the treatment group and could not identify the agent being assigned." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. "The assigned study drug was then reconstituted by pharmacy staff at each study center and supplied to the investigators in syringes labeled with coded information to mask the treatment identification. Investigator, study nurses, or testing laboratories were blinded to the treatment group and could not identify the agent being assigned." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The assigned study drug was then reconstituted by pharmacy staff at each study center and supplied to the investigators in syringes labeled with coded information to mask the treatment identification. Investigator, study nurses, or testing laboratories were blinded to the treatment group and could not identify the agent being assigned." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intervention group: 17/137 (12%) participants were not dosed and not analysed. 6 discontinued treatment prematurely but were included in mITT population. Control group: 7/68 (10%) participants were not dosed and not analysed. 5 discontinued treatment prematurely but were included in mITT population. Postrandomisation exclusions due to timing of randomisation; few results reported for vascular subgroup. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the protocol were reported. SAEs were counted but not described; the most common treatment‐emergent AEs were listed and fully described. Limited reporting for surgical subgroups, but prespecified outcomes were reported. |
| Other bias | High risk | This suggests that eligibility was determined after randomisation, with potential for bias if placebo not perfectly masked: "A specific bleeding area/site was defined as the target bleeding site (TBS) for each surgical procedure in patients already randomized when it was determined by the surgeon that control of bleeding by conventional surgical techniques (including suture, ligature, and cautery) was ineffective or impractical and required an adjunct treatment to achieve hemostasis". |
Monaco 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation sequence was created by permuted block randomisation with a block size of 20 and a 1:1 allocation generated by a computer." Adequate method of sequence generation with computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | "The allocation sequence was prepared by an independent operator not otherwise involved in the trial and was concealed by opaque sequentially numbered sealed envelopes. Patients were screened for eligibility and were asked to sign an informed consent document to participate in the trial on the day before surgery. Patients were randomised in the trial after they entered the surgical theatre, performed by dedicated study personnel in a separate environment. Patients were randomly allocated to the intervention or the placebo group by assigning them the sequentially numbered envelope with the lowest numeration." Adequate method of central allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients randomised to the intervention group received tranexamic acid (Ugurol; Rottapharm S.p.A., Milan, Italy; 0.5 g 5 ml‐1 vial) according to the following protocol: a loading dose of 500 mg of tranexamic acid diluted in 100 ml saline was intravenously infused slowly 20 min before surgery, and a continuous intravenous infusion of tranexamic acid was then administered at a rate of 250 mg h (2.5 ml h ‐1, using non‐diluted tranexamic acid contained in the vials) from surgical incision until skin closure. Patients randomised to the control group received placebo (saline) with identical volumes and rates of infusion." "We performed a single‐centre, double‐blinded, parallel‐group, randomised clinical trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Subjective outcomes ‐ low risk of bias due to adequate outcome assessor blinding. Objective outcomes ‐ low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All randomised patients received their assigned treatment and were analysed for the primary outcome. All patients were contacted 1 yr after randomisation, and none were lost to follow‐up." Outcome data set reported with denominators and numerators, and with no dropouts reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes planned in the protocol or prospective trial registration are reported. |
| Other bias | Low risk | No other concerns noted. |
NCT02094885.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Extracted from results in trial protocol. No details of randomisation available. The trial registration says that allocation is "randomized", but no further details. Method of sequence generation for randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Extracted from results in trial protocol. No details of allocation concealment available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention and comparison blinding is not possible for personnel. Also, the trial register says that they used "single masking (participant)". Subjective outcomes ‐ high risk of bias due to absent personnel blinding. Objective outcomes ‐ low risk of bias regardless. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Subjective outcomes ‐ high risk of bias due to absent outcome assessor blinding. Objective outcomes ‐ low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data are available for all participants. 1/125 in the intervention group withdrew due to age. 5/127 in the control group did not complete the study due to death. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the trial protocol reported for all participants. Full description of adverse events is available. |
| Other bias | Low risk | No other concerns noted |
Nenezic 2019.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "In the primary part of the study, patients were randomized 2:1 into FS Grifols or MC treatment groups." Method of randomisation not described in sufficient detail. |
| Allocation concealment (selection bias) | Low risk | "All study centers were provided with sealed opaque envelopes with a number printed outside and containing a treatment group. A randomization number was printed on the outside of the envelopes. For each patient undergoing a surgery, the first, sequential, available randomization envelope for the appropriate type of procedure (peripheral arterial bypass or upper extremity vascular access or hemodialysis) was taken to the operating room. The envelope was opened only upon identifying a TBS on the proximal anastomosis with moderate arterial bleeding, according to the investigator’s judgment, and the time of the randomization and the treatment group assignment was recorded." Adequate method of central allocation concealment with sequentially numbered sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Study design. This clinical study was a pivotal confirmatory phase III, controlled, prospective, single‐blinded, randomized, multicenter clinical trial performed at 42 sites in United States, Hungary, Serbia, and Russia (ClinicalTrials.gov Identifier: NCT01662856)." "Data from patients participating in the primary part of the study, including treatment assignment and accumulating efficacy data, were blinded to the sponsor team members, except for personnel from study drug supply groups." Single blinded study ‐ surgeon was aware which patient had received. Subjective outcomes ‐ high risk of bias due to inadequate or absent personnel blinding. Objective outcomes ‐ low risk of bias regardless. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Data from patients participating in the primary part of the study, including treatment assignment and accumulating efficacy data, were blinded to the sponsor team members, except for personnel from study drug supply groups." Hard to establish whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The ITT population consisted of 166 patients recruited in 18 centers. Three of the 109 patients randomized to FS (2.8%) in the primary part discontinued prematurely (two consent withdrawals, one death). One of the 57 patients randomized to MC (1.8%) discontinued (death)." 9% of participants in the fibrin sealant group vs 3% of patients did not complete the study to the end of follow up which could have introduced bias. However, in terms of study completion, only 2.8% and 1.8% did not complete study. Therefore, the risk of bias from incomplete outcomes data is probably low. |
| Selective reporting (reporting bias) | Low risk | All outcomes planned in the protocol or prospective trial registration are reported. |
| Other bias | Low risk | No other concerns such as early stopping or imbalanced study arms. |
O'Donnell 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information (abstract only) |
| Allocation concealment (selection bias) | Unclear risk | No information (abstract only) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label, easily distinguishable treatments. Subjective outcomes ‐ unclear risk of bias due to lack of personnel blinding. Objective outcomes ‐ low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label, easily distinguishable treatments. Primary endpoints assessed intraoperatively. Subjective outcomes ‐ unclear risk of bias due to unclear adequacy of outcome assessor blinding. Objective outcomes ‐ low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only and no available prospective protocol or trial registration. |
| Other bias | Unclear risk | Abstract only; no further information |
POISE‐3 2022.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization was performed by means of a central computerized system with the use of block randomization, with stratification according to center" |
| Allocation concealment (selection bias) | Low risk | "randomization was performed by means of a central computerized system with the use of block randomization, with stratification according to center. Patients were assigned in a 1:1 ratio to receive tranexamic acid (1‐g intravenous bolus) or placebo at the start and end of surgery and, in a 1:1 ratio with the use of a partial factorial design, to a hypotension‐avoidance strategy or a hypertension‐avoidance strategy. Patients, health care providers, data collectors, and outcome adjudicators were unaware of the trial‐group assignments." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients, health care providers, data collectors, and outcome adjudicators were unaware of the trial‐group assignments." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients, health care providers, data collectors, and outcome adjudicators were unaware of the trial‐group assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Unclear for vascular subgroup. ITT analysis for effectiveness, PPA for safety. Limited reporting of vascular subgroup but 1399 (all individuals randomised) for effectiveness, 1360 for safety. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported. |
| Other bias | Low risk | No other concerns |
Qerimi 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sequentially numbered (1–32), opaque, sealed envelopes were used to allocate the patients according to a randomization list prepared by a statistician to receive either microfibrillar collagen (COLL) or oxidized cellulose (ORC)." Adequate method of sequence generation |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered (1–32), opaque, sealed envelopes were used to allocate the patients according to a randomization list prepared by a statistician to receive either microfibrillar collagen (COLL) or oxidized cellulose (ORC)." Adequate method of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "An untreated control group was not included because of the nature of the study and because animal studies have clearly shown that the time to hemostasis increases if no hemostat is used. 8–12 The products make blinding impossible." Subjective outcomes ‐ high risk of bias due to inadequate personnel blinding because comparator is usual care rather than placebo. Objective outcomes ‐ low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Subjective outcomes ‐ high risk of bias due to inadequate or absent personnel blinding. Objective outcomes ‐ low risk of bias regardless |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No obvious outcome data missing. |
| Selective reporting (reporting bias) | Low risk | All outcomes planned in the protocol or prospective trial registration are reported. |
| Other bias | Unclear risk | The percentage male and disease severity seem quite dissimilar. However, only eight participants in each arm were recruited. It is not clear whether these imbalances in study arms would have affected outcomes. |
Ranaboldo 1997.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "prospective, randomized, double‐blind, placebo‐controlled trial." "Some 136 patients were randomized to receive either aprotinin, given as a loading dose of 2 X 106 kallikrein inactivator (KI) units followed by 0.5 X 106 KI units/h or equal volumes of 0.9 per cent saline." Method of sequence generation for randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Randomization, in groups of four, was blinded from the investigators by the use of identical coded bottles containing active drug or placebo (0.9 per cent saline)." It is likely blinding was done adequately, because a placebo was used and the bottles was identical. However, not all details regarding blinding are clear. Subjective outcomes ‐ low risk of bias due to adequate personnel blinding. Objective outcomes ‐ low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It is likely blinding was done adequately, because a placebo was used and the bottles was identical. However, not all details regarding blinding are clear. Subjective outcomes ‐ low risk of bias due to adequate outcome assessor blinding. Objective outcomes ‐ low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Patients surviving to the first week were included in the primary data analysis but clinical follow‐up continued to the 30th day." "Four deaths occurred within 7 days of surgery (two in each group): one following massive haemorrhage, two from myocardial infarction and one from a pulmonary embolus." People who died within 7 days of surgery were excluded, which could have affected outcome. However, the distribution of these 4 excluded people was balanced (2 in each arm), so it is unclear whether or not this would have affected outcomes. |
| Selective reporting (reporting bias) | Unclear risk | No available prospective protocol or trial registration. |
| Other bias | Unclear risk | "After 80 patients had been randomized an independent analysis of the plasma levels achieved, without the investigators receiving code‐breaking information, suggested that the maintenance infusion dose should be increased to 1 X 106 KI units/h in an effort to ensure that plasma levels were similar to those observed in cardiac studies." "Independent assay of the plasma levels of aprotinin achieved dictated a protocol change to reach levels similar to those observed in cardiac studies. The maintenance infusion dose was therefore doubled from 0.5 x 106 KI units/ml/h (standard dose) to 1 X 106 KI units/ml/h (high dose) to achieve this. The median intraoperative aprotinin plasma level was 93 (range 51‐222) KI unit/ml for those treated with the standard regimen and 146 (range 95‐334) KI units/ml for those who received the higher infusion dose (z = 4.15, P < 0.001). Patients treated with the initial standard aprotinin infusion or the higher dose were compared separately with controls; the results were similar to those seen with the whole cohort of patients but there was a dose‐related effect for blood loss on to swabs and for the early postoperative period." There was a change in the intervention to increase the infusion rate, and it is unclear whether this might have introduced bias. |
Robinson 2000.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was in permuted blocks of ten, ensuring approximately equal numbers of each treatment within individual centres. Aprotinin or placebo was administered according to the randomization list drawn up by the pharmacist; the code for determining the contents of each bottle remained with the pharmacist until the end of the trial." Exact method of sequence generation unclear but probably low risk as conducted centrally at pharmaceutical company using code and permuted blocks of 10 |
| Allocation concealment (selection bias) | Low risk | "Bayer PLC (Newbury, UK) supplied the aprotinin and a single pharmacist undertook the preparation of all samples in the sterile production facility at the Royal Hallamshire Hospital (Sheffield, UK). Bottles were prepared containing either 2 3 10 6 units of aprotinin in 200 ml saline or 0´5 3 10 6 units of aprotinin in 50 ml saline. Identical bottles of sterile 0´9 per cent saline were also prepared. Bottles were numbered and sent to the participating centres, where they were kept in the theatre fridge. The randomization was in permuted blocks of ten, ensuring approximately equal numbers of each treatment within individual centres. Aprotinin or placebo was administered according to the randomization list drawn up by the pharmacist; the code for determining the contents of each bottle remained with the pharmacist until the end of the trial." Adequate method of central allocation concealment by pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study report does not state that it was double‐blind, but methodology described suggests that it was adequately blinded. Subjective outcomes ‐ low risk of bias due to adequate personnel blinding. Objective outcomes ‐ low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Subjective outcomes ‐ low risk of bias due to adequate outcome assessor blinding. Objective outcomes ‐ low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No obvious outcome data missing. |
| Selective reporting (reporting bias) | Unclear risk | No available prospective protocol or trial registration. |
| Other bias | High risk | The trial was stopped before the number of participants in each trial was reached, and the article does not really explain why this was done. |
Taylor 2003.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "This was a prospective multicenter randomized (FSBP‐ TSG ratio, 1:1) single‐blinded therapeutic trial conducted at 26 medical centers in the United States." "Because this was a single‐blinded study (study personnel could not be blinded to the nature of the experimental vs control treatment), randomization was delayed until all possible study procedures were completed." Method of sequence generation for randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | "The arterial clamps were then reapplied, and the randomization envelope was opened" Envelopes were used, but no further details supplied. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Because this was a single‐blinded study (study personnel could not be blinded to the nature of the experimental vs control treatment), randomization was delayed until all possible study procedures were completed." Unblinded study. Subjective outcomes ‐ high risk of bias due to inadequate personnel blinding. Objective outcomes ‐ low risk of bias regardless. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Subjective outcomes ‐ high risk of bias due to inadequate outcome assessor blinding. Objective outcomes ‐ low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No obvious outcome data missing, except for one participant randomised to fibrin sealant. |
| Selective reporting (reporting bias) | Unclear risk | No available prospective protocol or trial registration. |
| Other bias | Low risk | No other concerns such as early stopping or imbalanced study arms. |
Weaver 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The article says "randomised", but gives no further details. Method of sequence generation for randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of personnel to the arms not possible. Subjective outcomes ‐ high risk of bias due to inadequate or absent personnel blinding. Objective outcomes ‐ low risk of bias regardless. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Subjective outcomes ‐ unclear risk of bias due to unclear adequacy of outcome assessor blinding. Objective outcomes ‐ low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No obvious outcome data missing. |
| Selective reporting (reporting bias) | Unclear risk | No available prospective protocol or trial registration. |
| Other bias | Unclear risk | Only a few baseline characteristics provided. Insufficient information to assess whether an important risk of bias exists. |
AAA ‐ abdominal aortic aneurysm; AE ‐ adverse event; AV ‐ arteriovenous; CAD ‐ coronary artery disease; COPD ‐ chronic obstructive pulmonary disease; CVA ‐ cerebrovascular event; DDAVP ‐ Desmopressin acetate 1‐deamino‐8‐D‐arginine vasopressin; DM ‐ diabetes mellitis; DVT ‐ deep vein thrombosis; ITT ‐ intention‐to‐treat; MI ‐ myocardial infarction; mITT ‐ modified intention‐to‐treat; NA ‐ not applicable; NaCL: sodium chloride; NR ‐ not reported; NS EV ‐ normal saline equal volume; OSR ‐ open surgical repair; PE ‐ pulmonary embolism; PPA ‐ per protocol analysis; PTFE ‐ polytetrafluoroethylene; RCT ‐ randomised controlled trial; RoB ‐ risk of bias; SD ‐ standard deviation; TBS ‐ target bleeding site; TTH ‐ time to haemostasis; TXA ‐ tranexamic acid; SAE ‐ serious adverse event;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abiko 2022 | Ineligible participant population |
| Amin 2000 | Ineligible intervention ‐ femoral vascular access closure device or dressing |
| Baum 2002 | Ineligible intervention |
| Bedirhan 2001 | Ineligible participant population ‐ thoracic surgery |
| Brunkwall 2007 | Ineligible study design ‐ single arm study, so participants were not randomised to interventions |
| Casati 2002 | Ineligible participant population ‐ cardiac surgery |
| Cebrian 2017 | Ineligible intervention |
| Chalmers 2010 | Ineligible participant population ‐ mixed population, < 80% receiving included intervention |
| Chapman 2001 | Ineligible participant population |
| Chapman 2006 | Ineligible participant population ‐ mixed surgical population with no vascular subgroup results available |
| Chapman 2007 | Ineligible participant population ‐ mixed surgical population with no vascular subgroup results provided. |
| ChiCTR1900021586 | Ineligible intervention ‐ femoral artery puncture |
| Christenson 2004 | Ineligible participant population ‐ cardiac surgery |
| Clagett 1996 | Not relevant (review) |
| Coselli 2003 | Ineligible participant population ‐ no vascular subgroup available |
| Croxtall 2009 | Ineligible participant population |
| CTRI/2013/04/003580 | Ineligible participant population |
| CTRI/2020/05/025285 | Ineligible intervention ‐ femoral artery puncture |
| CTRI/2021/09/036977 | Ineligible participant population |
| Develle 2020 | Ineligible participant population ‐ < 80% of patients receiving included intervention; no subgroup information provided |
| Devereaux 2008 | Ineligible intervention |
| Donker 2012 | Ineligible intervention |
| Doria 2008 | Ineligible participant population |
| Eder 1986 | Ineligible participant population |
| Ehrlich 1998 | Ineligible participant population ‐ cardiac surgery |
| EUCTR‐2007‐004612‐DE | Ineligible participant population |
| EUCTR‐2013‐003464‐31‐GB | Ineligible participant population ‐ primarily cardiac surgery |
| EUCTR‐2016‐001126‐33‐GB | Ineligible participant population: no vascular subgroup available |
| Fletcher 1995 | Ineligible intervention ‐ "Patients having femoro‐femoral, axillo‐femoral and femoro‐popliteal bypass with prosthetic vascular graft were randomized to receive either PTFE or Gelsoft with or without rifampicin soaking of the vascular graft prior to insertion." |
| Glickman 2002 | Ineligible participant population ‐ mixed population, < 80% receiving included intervention |
| Glineur 2018 | Ineligible participant population ‐ primarily cardiac with no vascular subgroup available |
| Hagberg 2004 | Ineligible participant population ‐ predominantly cardiac surgery |
| Hallak 2007 | Ineligible intervention ‐ vascular access dressing/device |
| Hanks 2003 | Ineligible participant population ‐ no vascular subgroup available |
| Hongo 2021 | Ineligible intervention |
| IRCT2017030432858N1 | Ineligible participant population |
| Khoynezhad 2018 | Ineligible participant population ‐ cardiac surgery |
| Kim 2006 | Ineligible intervention ‐ collagen plug compared to suture mediated vascular access closure |
| Klein‐Wiele 2018 | Ineligible intervention ‐ clip‐based compared to suture mediated vascular closure for femoral access haemostasis |
| Koncar 2008 | Ineligible study design ‐ used historic controls |
| Koncar 2011 | Ineligible study design ‐ used historic controls |
| Lumsden 2006 | Ineligible participant population ‐ mixed population, < 80% receiving included intervention |
| Makhija 2013 | Ineligible participant population ‐ cardiac surgery |
| Minato 2009 | Ineligible participant population ‐ cardiac surgery |
| Morita 2013 | Ineligible participant population ‐ cardiac surgery |
| Morita 2020 | Ineligible participant population ‐ cardiac surgery |
| Morrison 2019 | Ineligible comparator ‐ comparator is FFP, not placebo or usual care |
| Nasso 2009 | Ineligible participant population ‐ cardiac surgery |
| NCT00388284 | Ineligible participant population ‐ peripheral vascular access |
| NCT00428155 | Ineligible intervention ‐ arterial closure device |
| NCT00439309 | Ineligible participant population ‐ mixed population, < 80% receiving included intervention |
| NCT00440401 | Ineligible participant population ‐ cardiac surgery |
| NCT00701142 | Ineligible participant population |
| NCT01500135 | Ineligible participant population ‐ mixed population, < 80% receiving included intervention |
| NCT01669382 | Ineligible intervention ‐ study compares arteriotomy closure devices used in percutaneous coronary intervention |
| NCT01681030 | Ineligible participant population ‐ cardiac surgery remit |
| NCT01879475 | Ineligible participant population ‐ cardiac surgery |
| NCT01959503 | Ineligible participant population ‐ cardiac surgery |
| NCT02540434 | Ineligible participant population ‐ cardiac surgery |
| NCT02640235 | Ineligible participant population ‐ mixed population with no vascular subgroup available |
| NCT03173703 | Ineligible intervention |
| NCT03176225 | Ineligible intervention and participant population ‐ this study compared XenoSure biologic patch with a polyester patch in cardiac surgery |
| NCT03369977 | Ineligible participant population ‐ cardiac surgery |
| NCT03426839 | Ineligible intervention ‐ this study compared haemostasis strategy guided by conventional coagulation tests with transfusion algorithm guided by viscoelastic POC tests and algorithms. |
| NCT03444324 | Ineligible participant population ‐ spinal surgery |
| NCT03558243 | Ineligible participant population ‐ radial access |
| NCT03654560 | Ineligible participant population ‐ no vascular subgroup available |
| NCT03917862 | Ineligible participant population ‐ cardiac surgery |
| Okita 1996 | Ineligible participant population ‐ cardiac surgery |
| Park 2005 | Ineligible intervention ‐ a study of vascular access devices for percutaneous procedures |
| Pathan 2021 | Ineligible participant population |
| Pilon 2010 | Ineligible study design ‐ this was not a randomised study |
| Rahe‐Meyer 2009 | Ineligible study design and participant population ‐ study appears to be cardiac surgery population and is not randomised |
| Rahe‐Meyer 2013 | Ineligible participant population ‐ cardiac surgery remit |
| Rahe‐Meyer 2016 | Ineligible participant population ‐ cardiac surgery remit |
| Rastan 2008 | Ineligible intervention ‐ vascular access closure devices |
| Ratnam 2007 | Ineligible intervention ‐ vascular access closure devices |
| Saha 2012 | Ineligible participant population ‐ mixed population, < 80% receiving included intervention |
| Sanborn 1993 | Ineligible intervention ‐ vascular access closure devices |
| Sauer 2016 | Ineligible study design ‐ study does not appear to be randomised |
| Scheinert 2007 | Ineligible intervention ‐ vascular access closure surface sealant |
| Schenk 2003 | Ineligible participant population ‐ all participants undergoing vascular access surgery |
| Schwarz 2009 | Ineligible intervention ‐ vascular access closure devices |
| Shimamura 1998 | Ineligible study design ‐ participants not randomised |
| Sirlak 2003 | Ineligible participant population ‐ cardiac surgery |
| Stone 2012 | Ineligible participant population ‐ mixed population, < 80% receiving included intervention |
| Takaori 1995 | Ineligible participant population ‐ cardiac surgery |
| Tepe 2008 | Ineligible intervention ‐ vascular access closure device |
| Thompson 2013 | Not relevant (review article) |
| Verhoef 2015 | Ineligible participant population ‐ mixed population, < 80% receiving included intervention |
| Vierhout 2014 | Ineligible intervention ‐ skin sealant |
| Wachol‐Drewek 1996 | Ineligible intervention |
| Weaver 2008 | Ineligible participant population: 46% of participants undergoing excluded vascular procedures at low risk of bleeding |
| Zwischenberger 1999 | Ineligible participant population |
FFP ‐ fresh frozen plasma; POC ‐ point of care; PTFE ‐ polytetrafluoroethylene
Characteristics of studies awaiting classification [ordered by study ID]
jRCTs041180163.
| Methods | Variously described as RCT and historic control |
| Participants |
Inclusion criteria: people who undergo first bifurcated stent graft treatment for abdominal aortic aneurysm and iliac artery aneurysm at Aichi Medical University Hospital Exclusion criteria: people who cannot take internal medicine |
| Interventions | Tranexamic acid group: tranexamic acid 250 mg*3/day given on day 1 to 30 after EVAR Non Tranexamic acid group: do not give any additional drugs |
| Outcomes |
Primary outcome: incidence of type 2 endoleak Secondary outcome: changes in the size of aneurysms after EVAR |
| Notes | Unclear if control participants are randomised, or if historic control is used. Trial registration jRCTs041180163, first posted: 29 March 2019 Actual study start date: 20 May 2017 Estimated primary completion date: NR Estimated study completion date: NR |
NCT00618358.
| Methods | RCT, parallel group |
| Participants |
Inclusion criteria:
Intraoperative inclusion criteria: subjects must meet the following intraoperative inclusion criteria to be eligible for randomisation:
Exclusion criteria: Preoperative exclusion criteria: subjects who meet any of the following criteria are not eligible for participation in the study:
Intraoperative exclusion criteria: subjects who meet any of the following intraoperative exclusion criteria are considered screening failures and are not eligible to be randomised:
|
| Interventions |
Experimental: the Vascular Sealant System is intended for use in vascular reconstructions to achieve adjunctive haemostasis by mechanically sealing areas of leakage Comparator: Gelfoam/Thrombin |
| Outcomes | NR |
| Notes | Trial terminated by sponsor. No results available Trial registration (NCT00618358), first posted: 20 February 2008 Actual study start date: March 2007 Estimated study completion date: terminated, "(Sponsor ‐ Confluent Surgical terminated study re: surgical techniques Letter dated 4/31/2008)" |
NCT00652314.
| Methods | RCT, parallel group |
| Participants |
Inclusion criteria:
Inclusion criteria to be determined during the surgical procedure:
Exclusion criteria:
|
| Interventions |
Experimental: Thrombi‐Gel Control: Gelfoam |
| Outcomes |
Primary outcome: Time to haemostasis ("If hemostasis was not observed within 10 minutes, the treatment site was to be monitored and the research teams were asked to record the specific number of minutes until hemostasis was observed.") Secondary outcomes:
|
| Notes | Awaiting full publication in case a vascular subgroup can be identified. Trial registration NCT00652314, first posted: 3 April 2008 Actual study start date: March 2008 Actual primary completion date: October 2009 Actual completion date: January 2010 |
NCT04083807.
| Methods | RCT, parallel group |
| Participants |
Inclusion criteria:
Intraoperative inclusion criterion:
Exclusion criteria:
Intraoperative exclusion criteria:
|
| Interventions |
Experimental: TISSEEL Lyo, applied once intra‐operatively to the study suture line using the DUPLOJECT Fibrin Sealant Preparation and Application System. Control: manual compression with surgical gauze pads |
| Outcomes |
Primary outcome: number of participants achieving haemostasis at 4 minutes after treatment (time frame: day 0 (4 minutes post‐treatment to closure of surgical wound)) Secondary outcomes:
|
| Notes | Trial completed October 2019 but no results posted Trial registration NCT04083807, first posted: 10 September 2019 Actual study start date: 10 July 2019 Actual primary completion date: 22 October 2019 Actual study completion date: 22 October 2019 |
EVAR ‐ endovascular aneurysm repair; HIV ‐ human immunodeficiency disease; INR ‐ international normalised ratio; NR ‐ not reported; PTFE ‐ polytetrafluoroethylene; RCT ‐ randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1900023323.
| Study name | Fibrin Sealant Used in EVAR as Filling Technique Treating Abdominal Aortic Aneurysm |
| Methods | Single blinded RCT (parallel group) |
| Participants |
|
| Interventions | Intervention: Fibrin sealant Control: usual care |
| Outcomes |
|
| Starting date | 3 June 2019 |
| Contact information | Lu Qing‐sheng, Changhai hospital, 168 Changhai Road, Yangpu District, Shanghai, China; luqs@xueguan.net |
| Notes |
|
NCT04803747.
| Study name | A Trial of a Hospital Policy of Tranexamic Acid Use to Reduce Transfusion in Major Non‐cardiac Surgery (TRACTION) |
| Methods | TRACTION is a pragmatic, multicentre, randomised, registry‐based cluster‐crossover trial. Patients, clinicians and investigators are blinded to treatment allocation |
| Participants |
|
| Interventions | "TXA 1 gram bolus (2 grams for patients over 100 kg) intravenously (IV) administered within 10 minutes of the first surgical incision, followed by 1 additional gram given intravenously at 2 ‐ 4 hours of surgery or prior to skin closure, at the discretion of the anaesthesiologist (e.g. IV bolus at 2 ‐ 4 hours of surgery, at skin closure, or the 1 additional gram given as a continuous infusion throughout the surgical procedure)." "Placebo (0.9% normal saline (1 gram bolus (2 grams for patients over 100 kg) intravenously (IV) administered within 10 minutes of the first surgical incision, followed by 1 additional gram given intravenously at 2‐4 hours of surgery or prior to skin closure, at the discretion of the anaesthesiologist (e.g. IV bolus at 2‐4 hours of surgery, at skin closure, or the 1 additional gram given as a continuous infusion throughout the surgical procedure)." |
| Outcomes | Primary outcome measures :
Secondary outcome measures :
|
| Starting date | 16 February 2022 |
| Contact information | Dayna Solvason, University of Manitoba‐ HSC Campus, 204‐792‐3372, dsolvason@wrha.mb.ca |
| Notes |
|
EVAR ‐ endovascular aneurysm repair; NR ‐ not reported; RBC ‐ red blood cells; RCT ‐ randomised controlled trial; TXA ‐ tranexamic acid
Differences between protocol and review
We had planned to perform a network meta‐analysis (NMA) but were unable to do so due to the large number of treatments with very little data to populate the network. The planned NMA methods are outlined in Beverly 2020.
The protocol states the objective as: "To assess the efficacy and safety of anti‐fibrinolytic and haemostatic drugs and agents in reducing bleeding and transfusion in people undergoing major vascular surgery or vascular procedures with a risk of moderate or severe (> 500 mL) blood loss." This is still our objective, but we have changed "reducing bleeding and transfusion" to "reducing bleeding and the need for blood transfusion" because intra‐surgical bleeding outcomes were rejected at the protocol review stage, and we have not included any direct bleeding outcomes. A large proportion of the trials we identified had 10‐minute follow‐up with intra‐surgical bleeding outcomes only, so we have tightened up the wording to avoid ambiguity over our objectives.
The secondary endpoint of composite thromboembolic events (TE) was reported by very few trials and cannot be reconstructed from reports of specific thromboembolic events (MI, CVA/stroke, DVT, PE) because we cannot tell how many individuals experienced more than one TE. We have reported each of these events separately alongside the composite TE endpoint (where reported).
In the protocol we stated: "We used the International Conference on Harmonisation Good Clinical Practice definition of SAEs (ICH GCP 2018). Where studies reported an SAE outcome using an alternative definition, we recorded what definition of SAE was used and discussed suitability for inclusion in analysis with an expert panel, prior to extracting outcome data." This was overly ambitious. We extracted SAE data as reported by the authors, with very few making any statement about what definition of SAEs they were reporting.
In the protocol we stated: "We will do this after extracting participant, methodology and intervention data but prior to extracting outcome data, to avoid introducing bias. This will therefore be blinded in so far as authors and the panel will not have access to the results of data extraction at this point, though some may have some incidental familiarity with the literature. This two stage approach was devised with experts in the area of complex systematic reviews at the National Institute of Healthcare Research (UK) Complex Review Support Unit. In this way we aim to ensure that any network meta‐analysis of interventions will be meaningful, relevant and feasible." In the event, no network meta‐analysis was possible, and the treatments were grouped after data extraction was completed. This process could not be performed blind to the results, but the subgrouping decisions were based on clinical considerations and the similarity, or otherwise, of the treatments compared, and we do not believe this is an important source of bias.
In the protocol we stated: "For dichotomous variables, we will express the results as odds ratios (OR) with 95% confidence intervals (CI)." The risk ratio (RR) is a more intuitive statistic for reporting the results of RCTs because the meaning of an OR depends on the baseline risk and so is difficult to interpret even for readers who understand what an OR is. We have used RR instead of OR.
In the protocol we stated "If available, we plan to extract and report Hazard Ratios (HRs) for time‐to‐event‐data (mortality or time in hospital). If HRs are not available, we will make every effort to estimate as accurately as possible the HR using the available data and a purpose‐built method based on the Parmar and Tierney approach (Parmar 1998; Tierney 2007). If sufficient studies provide HRs, we plan to use these in favour of other reported treatment effects in the meta‐analysis, otherwise we will perform a separate meta‐analysis for all types of reported treatment effects, for example ORs. If the events are rare and the follow‐up times are similar we will consider the perceived similarity of ORs, risk ratios (RRs) and HRs." Only one trial (POISE‐3 2022) reported any HRs, and we did not attempt to estimate any others because it adds little value compared to RR when there is short‐term follow‐up and little loss to follow‐up. Time‐to‐event analysis for in‐hospital follow‐up also has a number of additional complications which are often not recognised by trial authors (McCaw 2022).
We did not contact authors for missing data because most trials with missing data had primarily intraoperative follow‐up and did not measure the outcomes we are interested in.
We changed the inclusion criteria for type of study to make it clearer that elective endovascular procedures were not included.
Contributions of authors
AB: protocol development, screening, data extraction, and writing up
GO: protocol development, data extraction, and writing up
CK: screening, data extraction, analysis, and writing up
JS: screening, data extraction, analysis, and writing up
CD: search strategy, protocol development, and writing up
NJW: expert advice on network meta‐analysis
PW: content expert, protocol development, and writing up
LJE: content expert, protocol development, and writing up
Sources of support
Internal sources
-
National Institute of Healthcare Research (NIHR), UK
NIHR grant to NHS Blood and Transplant
-
NIHR Biomedical Research Centre at University Hospitals Bristol NHS Foundation Trust and the University of Bristol, UK
Advice on network meta‐analysis
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
AB: NIHR grant to Systematic Review Initiative at NHS Blood and Transplant. NHSBT funded a salary, training costs and provided administrative support during the review process
GO: NIHR grant to Systematic Review Initiative at NHS Blood and Transplant. NHSBT funded a salary, training costs and provided administrative support during the review process
CK: none
JS: none
CD: none
NJW: has received research grants from the NIHR and MRC. Pfizer part‐fund a junior researcher working on a methodology project using historical data in a clinical area unrelated to this project. NJW has received honoraria from ABPI for delivering master classes on evidence synthesis. NJW has delivered a short‐course on network meta‐analysis to ICON plc, the funds from which were paid to her institution.
PW: none
LE: NIHR grant holder. She is Co‐ordinating Editor of Cochrane Haematology, but was not involved in the editorial process for this review.
New
References
References to studies included in this review
Bajardi 2009 {published data only}
- Bajardi G, Pecoraro F, Mirabella D. Efficacy of TachoSil(R) patches in controlling Dacron suture-hole bleeding after abdominal aortic aneurysm open repair. Journal of Cardiothoracic Surgery 2009;4:60. [DOI: 10.1186/1749-8090-4-60] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bochicchio 2015 {published data only}
- Bochicchio GV, Gupta N, Porte RJ, Renkens KL, Pattyn P, Topal B, et al. The FINISH-3 trial: a phase 3, international, randomized, single-blind, controlled trial of topical fibrocaps in intraoperative surgical hemostasis. Journal of the American College of Surgeons 2015;220(1):70-81. [DOI: 10.1016/j.jamcollsurg.2014.09.019] [DOI] [PubMed] [Google Scholar]
Chetter 2017 {published data only}
- Chetter I, Stansby G, Sarralde JA, Riambau V, Gimenez-Gaibar A, MacKenzie K, et al. A prospective, randomized, multicenter clinical trial on the safety and efficacy of a ready-to-use fibrin sealant as an adjunct to hemostasis during vascular surgery. Annals of Vascular Surgery 2017;45:127-37. [DOI: 10.1016/j.avsg.2017.06.043] [DOI] [PubMed] [Google Scholar]
Clagett 1995 {published data only}
- Clagett GP, Valentine RJ, Myers SI, Chervu A, Heller J. Does desmopressin improve hemostasis and reduce blood loss from aortic surgery? A randomized, double-blind study. Journal of Vascular Surgery 1995;22(3):223-9. [DOI] [PubMed] [Google Scholar]
Czerny 2000 {published data only}
- Czerny M, Verrel F, Weber H, Muller N, Kircheis L, Lang W, et al. Collagen patch coated with fibrin glue components. Treatment of suture hole bleedings in vascular reconstruction. Journal of Cardiovascular Surgery 2000;41(4):553-7. [PMID: ] [PubMed] [Google Scholar]
EUCTR2016‐003661‐26‐PL {published data only}
- EUCTR2016-003661-26-PL. A controlled, randomized, multi-centre, double blind, phase II study to evaluate efficacy and safety of topical PeproStat in intraoperative surgical haemostasis. clinicaltrialsregister.eu/ctr-search/trial/2016-003661-26/PL (first received 10 January 2017).
Giovanacci 2002 {published data only}
- Giovannacci L, Eugster T, Stierli P, Hess P, Gurke L. Does fibrin glue reduce complications after femoral artery surgery? A randomised trial. European Journal of Vascular and Endovascular Surgery 2002;24(3):196-201. [DOI: 10.1053/ejvs.2002.1667] [DOI] [PubMed] [Google Scholar]
- Stephens J, Robless P, Jenkins M, Cheshire N. Re: Does fibrin glue reduce complications after femoral artery surgery? A randomised trial. European Journal of Vascular and Endovascular Surgery 2003;25(6):596. [DOI] [PubMed] [Google Scholar]
Joseph 2004 {published data only}
- Joseph T, Adeosun A, Paes T, Bahal V. Randomised controlled trial to evaluate the efficacy of TachoComb H patches in controlling PTFE suture-hole bleeding. European Journal of Vascular and Endovascular Surgery 2004;27(5):549-52. [DOI: 10.1016/j.ejvs.2004.01.018] [DOI] [PubMed] [Google Scholar]
Leijdekkers 2006 {published data only}
- Leijdekkers VJ, Vahl AC, Mackaay AJ, Huijgens PC, Rauwerda JA. Aprotinin does not diminish blood loss in elective operations for infrarenal abdominal aneurysms: a randomized double-blind controlled trial. Annals of Vascular Surgery 2006;20(3):322-9. [DOI: 10.1007/s10016-006-9043-x] [DOI] [PubMed] [Google Scholar]
Lethagen 1991 {published data only}
- Lethagen S, Rugarn P, Bergqvist D. Blood loss and safety with desmopressin or placebo during aorto-iliac graft surgery. European Journal of Vascular Surgery 1991;5(2):173-8. [DOI: 10.1016/s0950-821x(05)80684-x] [DOI] [PubMed] [Google Scholar]
Milne 1996 {published data only}
- Milne AA, Murphy WG, Reading SJ, Ruckley CV. A randomised trial of fibrin sealant in peripheral vascular surgery. Vox Sanguinis 1996;70(4):210-2. [DOI: 10.1111/j.1423-0410.1996.tb01328.x] [DOI] [PubMed] [Google Scholar]
Minkowitz 2019 {published data only}
- Minkowitz H, Navarro-Puerto J, Lakshman S, Singla S, Cousar C, Kim R, et al. Prospective, randomized, phase ii, non-inferiority study to evaluate the safety and efficacy of topical thrombin (human) grifols as adjunct to hemostasis during vascular, hepatic, soft tissue, and spinal open surgery. Journal of the American College of Surgeons 2019;229(5):497-508. [DOI: 10.1016/j.jamcollsurg.2019.07.008] [DOI] [PubMed] [Google Scholar]
Monaco 2020 {published data only}
- Monaco F, Nardelli P, Pasin L, Barucco G, Mattioli C, Di Tomasso N, et al. Tranexamic acid in open aortic aneurysm surgery: a randomised clinical trial. British Journal of Anaesthesia 2020;124(1):35-43. [DOI: 10.1016/j.bja.2019.08.028] [DOI] [PubMed] [Google Scholar]
NCT02094885 {published data only}
- NCT02094885. The Bioseal Vascular Study. clinicaltrials.gov/ct2/show/NCT02094885 (first received 24th March 2014).
Nenezic 2019 {published data only}
- Nenezic D, Ayguasanosa J, Menyhei G, Tamas H, Matyas L, Muluk S, et al. A prospective, single-blind, randomized, phase iii study to evaluate the safety and efficacy of fibrin sealant grifols as an adjunct to hemostasis compared with manual compression in vascular surgery. Journal of Vascular Surgery 2019;70(5):1642-51. [DOI: 10.1016/j.jvs.2018.12.051] [DOI] [PubMed] [Google Scholar]
O'Donnell 2010 {published data only}
- O'Donnell S, Goetchues A, Lins A, Akbari C, Beavers F, Lustgarten J, et al. Effectiveness of vascular sealant compared with GELFOAM/thrombin for control of anastomosis suture line bleeding: a prospective randomized multi-center trial. Journal of Vascular Surgery 2010;51(11S):69S. [DOI: 10.1016/j.jvs.2010.02.182] [DOI] [Google Scholar]
POISE‐3 2022 {published data only}
- Devereaux PJ, Marcucci M, Painter TW, Conen D, Lomivorotov V, Sessler DI, et al. Tranexamic acid in patients undergoing noncardiac surgery. New England Journal of Medicine 2022;386(21):1986-97. [DOI: 10.1056/NEJMoa2201171] [DOI] [PubMed] [Google Scholar]
Qerimi 2013 {published data only}
- Qerimi B, Baumann P, Husing J, Knaebel HP, Schumacher H. Collagen hemostat significantly reduces time to hemostasis compared with cellulose: COBBANA, a single-center, randomized trial. American Journal of Surgery 2013;205(6):636-41. [DOI: 10.1016/j.amjsurg.2012.05.033] [DOI] [PubMed] [Google Scholar]
Ranaboldo 1997 {published data only}
- Ranaboldo CJ, Thompson JF, Davies JN, Shutt AM, Francis JN, Roath OS, et al. Prospective randomized placebo-controlled trial of aprotinin for elective aortic reconstruction. British Journal of Surgery 1997;84(8):1110-3. [PubMed] [Google Scholar]
Robinson 2000 {published data only}
- Robinson J, Nawaz S, Beard JD. Randomized, multicentre, double-blind, placebo-controlled trial of the use of aprotinin in the repair of ruptured abdominal aortic aneurysm. On behalf of the Joint Vascular Research Group. British Journal of Surgery 2000;87(6):754-7. [DOI: 10.1046/j.1365-2168.2000.01475.x] [DOI] [PubMed] [Google Scholar]
Taylor 2003 {published data only}
- Taylor LM, Mueller-Velten G, Koslow A, Hunter G, Naslund T, Kline R, et al. Prospective randomized multicenter trial of fibrin sealant versus thrombin-soaked gelatin sponge for suture- or needle-hole bleeding from polytetrafluoroethylene femoral artery grafts. Journal of Vascular Surgery 2003;38(4):766-71. [DOI: 10.1016/s0741-5214(03)00474-9] [DOI] [PubMed] [Google Scholar]
Weaver 2002 {published data only}
- Weaver FA, Hood DB, Zatina M, Messina L, Badduke B. Gelatin-thrombin-based hemostatic sealant for intraoperative bleeding in vascular surgery. Annals of Vascular Surgery 2002;16(3):286-93. [DOI: 10.1007/s10016-001-0073-0] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abiko 2022 {published data only}
- Abiko S, Yoshikawa A, Harada K, Kawagishi N, Sano I, Oda H, et al. Modified search, coagulation, and clipping with polyglycolic acid sheet and fibrin glue to reduce delayed bleeding risk after endoscopic submucosal dissection near the dentate line. Endoscopy 2022;54(3):E125-6. [DOI: 10.1055/a-1422-2510] [DOI] [PubMed] [Google Scholar]
Amin 2000 {published data only}
- Amin FR, Yousufuddin M, Stables R, Shamim W, Al-Nasser F, Coats AJ, et al. Femoral haemostasis after transcatheter therapeutic intervention: a prospective randomised study of the angio-seal device vs. the femostop device. International Journal of Cardiology 2000;76(2-3):235-40. [DOI: 10.1016/s0167-5273(00)00386-7] [DOI] [PubMed] [Google Scholar]
Baum 2002 {published data only}
- Baum RA, Carpenter JP, Golden MA, Velazquez OC, Clark T, Stavropoulous SW, et al. Treatment of type 2 endoleaks after endovascular repair of abdominal aortic aneurysms: comparison of transarterial and translumbar techniques. Journal of Vascular Surgery 2002;35(1):23-9. [DOI: 10.1067/mva.2002.121068] [DOI] [PubMed] [Google Scholar]
Bedirhan 2001 {published data only}
- Bedirhan MA, Turna A, Yagan N, Tasci O. Aprotinin reduces postoperative bleeding and the need for blood products in thoracic surgery: results of a randomized double-blind study. European Journal of Cardio-Thoracic Surgery 2001;20(6):1122-7. [DOI: 10.1016/s1010-7940(01)01016-8] [DOI] [PubMed] [Google Scholar]
Brunkwall 2007 {published data only}
- Brunkwall J, Ruemenapf G, Florek HJ, Lang W, Schmitz-Rixen T. A single arm, prospective study of an absorbable cyanoacrylate surgical sealant for use in vascular reconstructions as an adjunct to conventional techniques to achieve haemostasis. Journal of Cardiovascular Surgery 2007;48(4):471-6. [PMID: ] [PubMed] [Google Scholar]
Casati 2002 {published data only}
- Casati V, Sandrelli L, Speziali G, Calori G, Grasso MA, Spagnolo S. Hemostatic effects of tranexamic acid in elective thoracic aortic surgery: a prospective, randomized, double-blind, placebo-controlled study. Journal of Thoracic and Cardiovascular Surgery 2002;123(6):1084-91. [DOI: 10.1067/mtc.2002.120717] [DOI] [PubMed] [Google Scholar]
Cebrian 2017 {published data only}
- Cebrian FJ, Mendoza MP, Seco SE, Feria BM, Valdes DS, Hernandex MA, et al. Randomized trial comparing effectiveness and safety of three percutaneous arterial closure devices vs. manual compression in peripheral interventions. Vascular 2017;25(2 Suppl):56. [Google Scholar]
Chalmers 2010 {published data only}
- Chalmers RT, Darling RC, Wingard JT, Chetter I, Cutler B, Kern JA, et al. Randomized clinical trial of tranexamic acid-free fibrin sealant during vascular surgical procedures. British Journal of Surgery 2010;97(12):1784-9. [DOI: 10.1002/bjs.7235] [DOI] [PubMed] [Google Scholar]
- Hickey NC. Randomized clinical trial of tranexamic acid-free fibrin sealant during vascular surgical procedures. British Journal of Surgery 2010;97(12):1790. [DOI] [PubMed] [Google Scholar]
- Zoegall S. Society for the Advancement of Blood Management study shows advantages of fibrin sealant over manual compression. Journal of Vascular Nursing 2008;26(1):5. [DOI] [PubMed] [Google Scholar]
Chapman 2001 {published data only}
- Chapman WC, Sherman R, Boyce S, Malawer M, Hill A, Buncke G, et al. A novel collagen-based composite offers effective hemostasis for multiple surgical indications: results of a randomized controlled trial. Surgery 2001;129:445-50. [DOI: 10.1067/msy.2001.112365] [DOI] [PubMed] [Google Scholar]
Chapman 2006 {published data only}
- Chapman WC, Lockstadt H, Singla N, Kafie FE, Lawson JH. Phase 2, randomized, double-blind, placebo-controlled, multicenter clinical evaluation of recombinant human thrombin in multiple surgical indications. Journal of Thrombosis and Haemostasis 2006;4(9):2083-5. [DOI: 10.1111/j.1538-7836.2006.02067.x] [DOI] [PubMed] [Google Scholar]
- Lawson J, Singla N, Gupta N, Murphy A, Reynolds T. Multicenter, phase 2, randomized evaluation of topical recombinant human thrombin (rhThrombin) for hemostasis in subjects undergoing vascular surgery. In: Abstracts of Peripheral Vascular Surgical Society Annual Vascular Meeting. 2006.
Chapman 2007 {published data only}
- Chapman WC, Singla N, Genyk Y, McNeil JW, Renkens KL Jr, Reynolds TC, et al. A phase 3, randomized, double-blind comparative study of the efficacy and safety of topical recombinant human thrombin and bovine thrombin in surgical hemostasis. Journal of the American College of Surgeons 2007;205(2):256-65. [DOI] [PubMed] [Google Scholar]
- NCT00245336. Study of recombinant human thrombin for bleeding during surgery. clinicaltrials.gov/ct2/show/NCT00245336 (first received 27 October 2005).
ChiCTR1900021586 {published data only}
- ChiCTR1900021586. A prospective, multicenter, randomized controlled clinical trial for evaluation of the safety and effectiveness of MAIHE occlusion hemostasis system and ExoSeal occlusion hemostasis system in femoral artery puncture hemostasis. chictr.org.cn/showprojen.aspx?proj=35950 (first received 28 February 2019).
Christenson 2004 {published data only}
- Christenson JT, Kalangos A. Autologous fibrin glue reinforced by platelets in surgery of ascending aorta. Thoracic and Cardiovascular Surgeon 2004;52(4):225-9. [DOI] [PubMed] [Google Scholar]
Clagett 1996 {published data only}
- Clagett GP. Desmopressin, hemostasis, and vascular surgery. Seminars in Vascular Surgery 1996;9(4):340-6. [PubMed] [Google Scholar]
Coselli 2003 {published data only}
- Coselli JS, Bavaria JE, Fehrenbacher J, Stowe CL, Macheers SK, Gundry SR. Prospective randomized study of a protein-based tissue adhesive used as a hemostatic and structural adjunct in cardiac and vascular anastomotic repair procedures. Journal of the American College of Surgeons 2003;197(2):243-52; discussion 252. [DOI] [PubMed] [Google Scholar]
Croxtall 2009 {published data only}
- Croxtall JD, Scott LJ. Recombinant human thrombin: in surgical hemostasis. Biodrugs 2009;23(5):333-8. [DOI] [PubMed] [Google Scholar]
CTRI/2013/04/003580 {published data only}
- CTRI/2013/04/003580. A clinical trial to study efficacy and safety of Fibrinogen compared to placebo in patients undergoing complex aortic surgeries. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=6290 (first received 9 January 2015).
CTRI/2020/05/025285 {published data only}
- CTRI/2020/05/025285. Ambulate early trial [A prospective trial comparing safety and efficacy of collagen based vs suture based vascular closure device and manual compression in patients who have undergone femoral artery puncture interventional procedure]. ctri.nic.in/Clinicaltrials/showallp.php?mid1=42998&EncHid=&userName=ambulate%20early (first received 21 May 2020).
CTRI/2021/09/036977 {published data only}
- CTRI/2021/09/036977. To evaluate the safety and hemostat efficacy of absorbable hemostat powder. ctri.nic.in/Clinicaltrials/showallp.php?mid1=59949&EncHid=&userName=CTRI/2021/09/036977 (first received 30 September 2021).
Develle 2020 {published data only}
- Develle R, Schaerf R, Najibi S, Conrad J, Abate G. Efficacy and safety of regenerated cellulose topical gauze haemostats in managing secondary haemostasis: a randomised control trial. Journal of Wound Care 2020;29(11):670-7. [DOI: 10.12968/jowc.2020.29.11.670] [DOI] [PubMed] [Google Scholar]
Devereaux 2008 {published data only}
- Devereaux PJ. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008;371(9627):1839-47. [DOI: 10.1016/s0140-6736(08)60601-7] [DOI] [PubMed] [Google Scholar]
Donker 2012 {published data only}
- Donker JM, Tijnagel MJ, Zeeland ML, Veen EJ, Laan L. Anastomoses in the common femoral artery, vascular clips or sutures? A feasibility study. Annals of Vascular Surgery 2012;27(2):194-8. [DOI: 10.1016/j.avsg.2012.02.008] [DOI] [PubMed] [Google Scholar]
Doria 2008 {published data only}
- Doria C, Fischer CP, Wood CG, Li PM, Marra SW, Hart J. Phase 3, randomized, double-blind study of plasma-derived human thrombin versus bovine thrombin in achieving hemostasis in patients undergoing surgery. Current Medical Research and Opinion 2008;24(3):785-94. [DOI: 10.1185/030079908x273426] [DOI] [PubMed] [Google Scholar]
Eder 1986 {published data only}
- Eder G, Neumann M, Cerwenka R, Baumgarten K. Preliminary results of a randomized controlled study on the risk of hepatitis transmission of a two-component fibrin sealant (Tissucol/Tisseel). Fibrin Sealant in Operative Medicine 1986;6:51-9. [DOI: 10.1007/978-3-642-71391-0_5] [DOI] [Google Scholar]
Ehrlich 1998 {published data only}
- Ehrlich M, Grabenwoger M, Cartes-Zumelzu F, Luckner D, Kovarik J, Laufer G, et al. Operations on the thoracic aorta and hypothermic circulatory arrest: is aprotinin safe? Journal of Thoracic and Cardiovascular Surgery 1998;115(1):220-5. [DOI] [PubMed] [Google Scholar]
EUCTR‐2007‐004612‐DE {published data only}
- EUCTR-2007-004612-DE. Efficacy and safety of Haemocomplettan® P in patients experiencing acute bleeding while undergoing aortic replacement surgery. clinicaltrialsregister.eu/ctr-search/trial/2007-004612-31/DE (first received 14 February 2008).
EUCTR‐2013‐003464‐31‐GB {published data only}
- EUCTR2013-003464-31-GB. A study evaluating the effect of EVARREST™ Fibrin Sealant Patch as compared to Tachosil in controlling bleeding during cardivascular surgery. clinicaltrialsregister.eu/ctr-search/trial/2013-003464-31/GB (first received 26 November 2013).
EUCTR‐2016‐001126‐33‐GB {published data only}
- EUCTR-2016-001126-33-GB. A randomised controlled trial to evaluate the feasibility of administering a drug called desmopressin to intensive care patients prior to procedures or radiological interventions, for prophylaxis against bleeding. clinicaltrialsregister.eu/ctr-search/trial/2016-001126-33/GB (first received 19 September 2016).
Fletcher 1995 {published data only}
- Fletcher JP, Robinson B. Prospective randomized trial of PTFE and gelatin-sealed dacron for femoropopliteal, femorofemoral and axillofemoral bypass. Cardiovascular Surgery 1995;3(Suppl):137. [Google Scholar]
Glickman 2002 {published data only}
- Glickman M, Gheissari A, Money S, Martin J, Ballard JL, CoSeal Multicenter Vascular Surgery Study Group. A polymeric sealant inhibits anastomotic suture hole bleeding more rapidly than gelfoam/thrombin: results of a randomized controlled trial. Archives of Surgery 2002;137(3):326-31. [DOI] [PubMed] [Google Scholar]
Glineur 2018 {published data only}
- Glineur D, Hendrikx M, Krievins D, Stradins P, Voss B, Waldow T, et al. A randomized, controlled trial of Veriset TM hemostatic patch in halting cardiovascular bleeding. Medical Devices Evidence and Research 2018;11:65-75. [DOI: 10.2147/MDER.S145651] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hagberg 2004 {published data only}
- Hagberg RC, Safi HJ, Sabik J, Conte J, Block JE. Improved intraoperative management of anastomotic bleeding during aortic reconstruction: results of a randomized controlled trial. American Surgeon 2004;70(4):307-11. [PubMed] [Google Scholar]
Hallak 2007 {published data only}
- Hallak OK, Cubeddu RJ, Griffith RA, Reyes BJ. The use of the D-STAT dry bandage for the control of vascular access site bleeding: a multicenter experience in 376 patients. Cardiovascular and Interventional Radiology 2007;30(4):593-600. [DOI] [PubMed] [Google Scholar]
Hanks 2003 {published data only}
- Hanks JB, Kjaergard HK, Hollingsbee DA. A comparison of the haemostatic effect of Vivostat patient-derived fibrin sealant with oxidised cellulose (Surgicel) in multiple surgical procedures. European Surgical Research 2003;35(5):439-44. [DOI] [PubMed] [Google Scholar]
Hongo 2021 {published data only}
- Hongo N, Kiyosue H, Ota S, Nitta N, Koganemaru M, Inoue M, et al. Vessel occlusion using hydrogel-coated versus nonhydrogel embolization coils in peripheral arterial applications: a prospective, multicenter, randomized trial. Journal of Vascular and Interventional Radiology 2021;32(4):602-9.e1. [DOI: 10.1016/j.jvir.2020.12.001] [DOI] [PubMed] [Google Scholar]
IRCT2017030432858N1 {published data only}
- IRCT2017030432858N1. Comparing ChitoHem powder and sandbags on control of bleeding after femoral angiography. en.irct.ir/trial/25494?revision=25494 (first received 1 June 2017).
Khoynezhad 2018 {published data only}
- Khoynezhad A, DelaRosa J, Moon MR, Brinkman WT, Thompson RB, Desai ND, et al. Facilitating hemostasis after proximal aortic surgery: results of the PROTECT trial. Annals of Thoracic Surgery 2018;105(5):1357-64. [DOI] [PubMed] [Google Scholar]
Kim 2006 {published data only}
- Kim HY, Choo SW, Roh HG, Han H, Kim SS, Lee JY, et al. Efficacy of femoral vascular closure devices in patients treated with anticoagulant, abciximab or thrombolytics during percutaneous endovascular procedures. Catheterization and Cardiovascular Interventions 2006;7(1):35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein‐Wiele 2018 {published data only}
- Klein-Wiele O, Baliota M, Kara K, Kaunicke M, Schafer H, Garbrecht M, et al. Safety and efficacy of clip-based vs. suture mediated vascular closure for femoral access hemostasis: a prospective randomized single center study comparing the StarClose and the ProGlide device. Catheterization and Cardiovascular Interventions 2018;91(3):402-7. [DOI] [PubMed] [Google Scholar]
Koncar 2008 {published data only}
- Koncar IB, Savic N, Davidovic LB, Simic D, Markovic D, Sindjelic RB. Recombinant activated factor VII in the treatment of intractable non-surgical bleeding following major vascular procedures [Aktivirani rekombinantni faktor vii u lečewunekontrolisanog nehirurškog krvarewa poslesloženih operacija u vaskularnoj hirurgiji]. Srpski Arhiv Za Celokupno Lekarstvo 2008;136(7-8):367-72. [DOI] [PubMed] [Google Scholar]
Koncar 2011 {published data only}
- Koncar IB, Davidovic LB, Savic N, Sindelic RB, Ilic N, Dragas M, et al. Role of recombinant factor VIIa in the treatment of intractable bleeding in vascular surgery. Journal of Vascular Surgery 2011;53(4):1032-7. [DOI] [PubMed] [Google Scholar]
Lumsden 2006 {published data only}
- Lumsden AB, Heyman ER, Closure Medical Surgical Sealant Study Group. Prospective randomized study evaluating an absorbable cyanoacrylate for use in vascular reconstructions. Journal of Vascular Surgery 2006;44(5):1002-9. [DOI] [PubMed] [Google Scholar]
Makhija 2013 {published data only}
- Makhija N, Sarupria A, Kumar Choudhary S, Das S, Lakshmy R, Kiran U. Comparison of epsilon aminocaproic acid and tranexamic Acid in thoracic aortic surgery: clinical efficacy and safety. Journal of Cardiothoracic and Vascular Anesthesia 2013;27(6):1201-7. [DOI] [PubMed] [Google Scholar]
Minato 2009 {published data only}
- Minato N, Katayama Y, Yunoki J, Kawasaki H, Satou H. Hemostatic effectiveness of a new application method for fibrin glue, the "rub-and-spray method", in emergency aortic surgery for acute aortic dissection. Annals of Thoracic and Cardiovascular Surgery 2009;15(4):265-71. [PubMed] [Google Scholar]
Morita 2013 {published data only}
- Morita S, Matsuda T, Eto M, Oda S, Tominaga R. Development of hemostatic sealant for arterial anastomosis; clinical application. Kyobu Geka. Japanese Journal of Thoracic Surgery 2013;66(5):395-400. [PubMed] [Google Scholar]
Morita 2020 {published data only}
- Morita S, Matsuda T, Tashiro T, Komiya T, Ogino H, Mukohara N, et al. Randomized clinical trial of an elastomeric sealant for hemostasis in thoracic aortic surgery. General Thoracic and Cardiovascular Surgery 2020;68(2):112-21. [DOI] [PubMed] [Google Scholar]
Morrison 2019 {published data only}
- EUCTR2009-016709-41-GB. Coagulopathy during surgery for the repair of extent 4 thoraco-abdominal aortic aneurysms - feasibility study of the use of fibrinogen concentrate by infusion in place of fresh frozen plasma. - Fibrinogen as an alternative to FFP in aortic surgery. clinicaltrialsregister.eu/ctr-search/trial/2009-016709-41/GB (first received 17 March 2017).
- Morrison GA, Koch J, Royds M, McGee D, Chalmers RT, Anderson J, et al. Fibrinogen concentrate vs. fresh frozen plasma for the management of coagulopathy during thoraco-abdominal aortic aneurysm surgery: a pilot randomised controlled trial. Anaesthesia 2019;74(2):180-9. [DOI] [PubMed] [Google Scholar]
- NCT00994045. Fibrinogen as an alternative to FFP in aortic surgery. clinicaltrials.gov/ct2/show/NCT00994045 (first received 14 October 2009).
Nasso 2009 {published data only}
- Nasso G, Piancone F, Bonifazi R, Romano V, Visicchio G, De Filippo CM, et al. Prospective, randomized clinical trial of the FloSeal matrix sealant in cardiac surgery. Annals of Thoracic Surgery 2009;88(5):1520-6. [DOI] [PubMed] [Google Scholar]
NCT00388284 {published data only}
- NCT00388284. Safety and efficacy study comparing thrombin for arterial sealing. clinicaltrials.gov/ct2/show/NCT00388284 (first received 16 October 2006).
NCT00428155 {published data only}
- NCT00428155. Arterial closure device comparison Trial II – ACDC Trial II. clinicaltrials.gov/ct2/show/NCT00428155 (first received 29 January 2007).
NCT00439309 {published data only}
- NCT00439309. Study to evaluate safety & effectiveness of vascular sealant system. clinicaltrials.gov/ct2/show/NCT00439309 (first received 23 February 2007).
NCT00440401 {published data only}
- EUCTR-2006-000028-15-DK. A randomised, open label, parallel-group, multi-centre trial to compare the efficacy and safety of TachoSil versus standard haemostatic treatment in the cardiovascular surgery. clinicaltrialsregister.eu/ctr-search/trial/2006-000028-15/DK (first received 12 December 2006).
- NCT00440401. TachoSil® Versus Standard Haemostatic Treatment of Haemorrhage in Cardiovascular Surgery (TC-023-IM). clinicaltrials.gov/ct2/show/NCT00440401 (first received 27 February 2007).
NCT00701142 {published data only}
- NCT00701142. Haemocomplettan® P during aortic replacement. clinicaltrials.gov/ct2/show/NCT00701142 (first received 19 June 2008).
NCT01500135 {published data only}
- NCT01500135. Comparison of efficacy and safety of TachoSil® versus Surgicel® original for the secondary hemostatic treatment of needle hole bleeding in vascular surgery (VASUS). clinicaltrials.gov/ct2/show/NCT01500135 (first received 26 December 2011).
NCT01669382 {published data only}
- NCT01669382. Angio-Seal® vs. Exo-Seal® for closure of arterial puncture sites. clinicaltrials.gov/ct2/show/NCT01669382 (first received 21 August 2012).
NCT01681030 {published data only}
- NCT01681030. The Fibrin Pad cardiovascular study. clinicaltrials.gov/ct2/show/NCT01681030 (first received 7 September 2012).
NCT01879475 {published data only}
- NCT01879475. Safety and performance of 032-11 haemostat in cardiac and thoracic aortic surgery. clinicaltrials.gov/ct2/show/NCT01879475 (first received 17 June 2013).
NCT01959503 {published data only}
- NCT01959503. Progel vascular sealant. clinicaltrials.gov/ct2/show/NCT01959503 (first received 10 October 2013).
NCT02540434 {published data only}
- NCT02540434. Trial of RiaSTAP versus cryoprecipitate to lower operative transfusions. clinicaltrials.gov/ct2/show/NCT02540434 (first received 4 September 2015).
NCT02640235 {published data only}
- NCT02640235. Effectiveness and safety of CELSTAT for hemostasis in intraoperative tissue bleeding. clinicaltrials.gov/ct2/show/NCT02640235 (first received 28 December 2015).
NCT03173703 {published data only}
- NCT03173703. Evaluate safety and effectiveness of XenoSure® biological patch in the application of peripheral vascular repair. clinicaltrials.gov/ct2/show/results/NCT03173703 (first received 2 June 2017).
NCT03176225 {published data only}
- NCT03176225. Evaluate safety and effectiveness of XenoSure® biological patch in the application of cardiac repair. clinicaltrials.gov/ct2/show/NCT03176225 (first received 5 June 2017).
NCT03369977 {published data only}
- NCT03369977. BioGlue as an adjunct for structural repair and hemostasis in Chinese acute Type A aortic dissections patients. clinicaltrials.gov/ct2/show/NCT03369977 (first received 12 December 2017).
NCT03426839 {published data only}
- NCT03426839. Haemostasis management of abdominal aortic procedures. clinicaltrials.gov/ct2/show/NCT03426839 (first received 8 February 2018).
NCT03444324 {published data only}
- NCT03444324. Adjusted fibrinogen replacement strategy (AdFIrst). clinicaltrials.gov/ct2/show/NCT03444324 (first received 23 February 2018).
NCT03558243 {published data only}
- NCT03558243. Prevention of radial artery occlusion: comparison of three hemostatic methods in transradial intervention. clinicaltrials.gov/ct2/show/NCT03558243 (first received 15 June 2018).
NCT03654560 {published data only}
- NCT03654560. Efficacy and safety of HemoStyp as an adjunct for management of secondary hemostasis in the operative setting. clinicaltrials.gov/ct2/show/study/NCT03654560 (first received 31 August 2018).
NCT03917862 {published data only}
- NCT03917862. Strategy for aortic surgery hemostasis (SASH). clinicaltrials.gov/ct2/show/NCT03917862 (first received 17 April 2019).
Okita 1996 {published data only}
- Okita Y, Takamoto S, Ando M, Morota T, Yamaki F, Kawashima Y. Is use of aprotinin safe with deep hypothermic circulatory arrest in aortic surgery? Investigations on blood coagulation. Circulation 1996;94(9 Suppl):II177‐81. [PubMed] [Google Scholar]
Park 2005 {published data only}
- Park Y, Roh HG, Choo SW, Lee SH, Shin SW, Do YS, et al. Prospective comparison of collagen plug (Angio-Seal) and suture-mediated (the Closer S) closure devices at femoral access sites. Korean Journal of Radiology 2005;6(4):248-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pathan 2021 {published data only}
- Pathan AZ, Aijaz S, Sheikh S, Sattar S. Randomized trial comparing radial hemostasis techniques; catechol conjugated chitosan pad (InnoSEAL) versus pneumatic compression band. Catheterization and Cardiovascular Interventions 2021;98(2):E181-7. [DOI: 10.1002/ccd.29578] [DOI] [PubMed] [Google Scholar]
Pilon 2010 {published data only}
- Pilon F, Tosato F, Danieli D, Campanile F, Zaramella M, Milite D. Intrasac fibrin glue injection after platinum coils placement: the efficacy of a simple intraoperative procedure in preventing type II endoleak after endovascular aneurysm repair. Interactive Cardiovascular and Thoracic Surgery 2010;11(1):78-82. [DOI] [PubMed] [Google Scholar]
Rahe‐Meyer 2009 {published data only}
- Rahe-Meyer N, Tanaka K, Solomon C, Osthaus A, Goerlinger K, Piepenbrock S, et al. The efficacy of fibrinogen concentrate in treating excessive bleeding in aortic surgery. Journal of Thrombosis and Haemostasis 2009;7(S2):1003. [Google Scholar]
Rahe‐Meyer 2013 {published data only}
- Anonymous. Erratum: Effects of fibrinogen concentrate as first-line therapy during major aortic replacement surgery: a randomized, placebocontrolled trial (Anesthesiology (2013) 118 (40-50)). Anesthesiology 2013;118(5):1244. [DOI] [PubMed] [Google Scholar]
- EUCTR2007-004612-31-DE. Efficacy and safety of Haemocomplettan® P in patients experiencing acute bleeding while undergoing aortic replacement surgery - Haemocomplettan® P during aortic replacement. clinicaltrialsregister.eu/ctr-search/trial/2007-004612-31/DE (first received 14 February 2008).
- EUCTR2007-004612-31-DE. Efficacy and safety of Haemocomplettan® P in patients experiencing acute bleeding while undergoing aortic replacement surgery - Haemocomplettan® P during aortic replacement. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2007-004612-31-DE (first received 14 February 2008).
- Rahe-Meyer N, Hanke A, Schmidt DS, Hagl C, Pichlmaier M. Fibrinogen concentrate reduces intraoperative bleeding when used as first-line hemostatic therapy during major aortic replacement surgery: results from a randomized, placebo-controlled trial. Journal of Thoracic and Cardiovascular Surgery 2013;145(3 Suppl):S178-85. [DOI] [PubMed] [Google Scholar]
- Rahe-Meyer N, Solomon C, Hanke A, Schmidt DS, Knoerzer D, Hochleitner G, et al. Effects of fibrinogen concentrate as first-line therapy during major aortic replacement surgery: a randomized, placebo-controlled trial. Anesthesiology 2013;118(1):40-50. [DOI] [PubMed] [Google Scholar]
- Solomon C, Hagl C, Rahe-Meyer N. Time course of haemostatic effects of fibrinogen concentrate administration in aortic surgery. British Journal of Anaesthesia 2013;110(6):947-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon C, Rahe-Meyer N. Fibrinogen concentrate as first-line therapy in aortic surgery reduces transfusion requirements in patients with platelet counts over or under 100x10(9)/L. Blood Transfusion 2015;13(2):248-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahe‐Meyer 2016 {published data only}
- Rahe-Meyer N, Levy JH, Mazer CD, Schramko A, Klein AA, Brat R, et al. Randomized evaluation of fibrinogen versus placebo in complex cardiovascular surgery: post hoc analysis and interpretation of phase III results. Interactive Cardiovascular and Thoracic Surgery 2019;28(4):566-74. [DOI] [PubMed] [Google Scholar]
- Rahe-Meyer N, Levy JH, Mazer CD, Schramko A, Klein AA, Brat R, et al. Randomized evaluation of fibrinogen vs placebo in complex cardiovascular surgery (REPLACE): a double-blind phase III study of haemostatic therapy. British Journal of Anaesthesia 2016;117(1):41-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rastan 2008 {published data only}
- Rastan A, Sixt S, Schwarzwälder U, Schwarz T, Frank U, Burgelin K, et al. VIPER2: a prospective, randomized single-center comparison of 2 different closure devices with a hemostatic wound dressing for closure of femoral artery access sites. Journal of Endovascular Therapy 2008;15(1):83-90. [DOI] [PubMed] [Google Scholar]
Ratnam 2007 {published data only}
- Ratnam LA, Raja J, Munneke GJ, Morgan RA, Belli AM. Prospective nonrandomized trial of manual compression and Angio-Seal and Starclose arterial closure devices in common femoral punctures. Cardiovascular and Interventional Radiology 2007;30(2):182-8. [DOI] [PubMed] [Google Scholar]
Saha 2012 {published data only}
- Saha SP, Muluk S, Schenk W 3rd, Dennis JW, Ploder B, Grigorian A, et al. A prospective randomized study comparing fibrin sealant to manual compression for the treatment of anastomotic suture-hole bleeding in expanded polytetrafluoroethylene grafts. Journal of Vascular Surgery 2012;56(1):134-41. [DOI: 10.1016/j.jvs.2012.01.009] [DOI] [PubMed] [Google Scholar]
Sanborn 1993 {published data only}
- Sanborn TA, Gibbs HH, Brinker JA, Knopf WD, Kosinski EJ, Roubin GS. A multicenter randomized trial comparing a percutaneous collagen hemostasis device with conventional manual compression after diagnostic angiography and angioplasty. Journal of the American College of Cardiology 1993;22(5):1273-9. [DOI] [PubMed] [Google Scholar]
Sauer 2016 {published data only}
- Sauer A, Dierks A, Wolfschmidt F, Hassold N, Bley TA, et al. Hemostatic wound dressing for postinterventional hemostasis in large femoral artery access sites: an initial efficacy and safety study. Journal of Endovascular Therapy 2016;23(5):744-50. [DOI] [PubMed] [Google Scholar]
Scheinert 2007 {published data only}
- Scheinert D, Sievert H, Turco MA, Schmidt A, Hauptmann KE, Mueller R, et al. The safety and efficacy of an extravascular, water-soluble sealant for vascular closure: Initial clinical results for MynxTM. Catheterization and Cardiovascular Interventions 2007;70(5):627-33. [DOI] [PubMed] [Google Scholar]
Schenk 2003 {published data only}
- Schenk WG 3rd, Burks SG, Gagne PJ, Kagan SA, Lawson JH, Spotnitz WD. Fibrin sealant improves hemostasis in peripheral vascular surgery: a randomized prospective trial. Annals of Surgery 2003;237(6):871-6; discussion 876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwarz 2009 {published data only}
- Schwarz T, Rastan A, Pochert V, Sixt S, Schwarzwalder U, Burgelin KH, et al. Mechanical compression versus haemostatic wound dressing after femoral artery sheath removal: a prospective, randomized study. Vasa 2009;38(1):53-9. [DOI] [PubMed] [Google Scholar]
Shimamura 1998 {published data only}
- Shimamura Y, Nakajima M, Hirayama T, Misumi H, Shimokawa T, Uesugi H, et al. The effect of intraoperative high-dose tranexamic acid on blood loss after operation for acute aortic dissection. Japanese Journal of Thoracic and Cardiovascular Surgery 1998;46(7):616-21. [DOI] [PubMed] [Google Scholar]
Sirlak 2003 {published data only}
- Sirlak M, Eryilmaz S, Yazicioglu L, Kiziltepe U, Eyileten Z, Durdu M S, et al. Comparative study of microfibrillar collagen hemostat (Colgel) and oxidized cellulose (Surgicel) in high transfusion-risk cardiac surgery. Journal of Thoracic and Cardiovascular Surgery 2003;126(3):666-70. [DOI] [PubMed] [Google Scholar]
Stone 2012 {published data only}
- Stone WM, Cull DL, Money SR. A randomized prospective multicenter trial of a novel vascular sealant. Annals of Vascular Surgery 2012;26(8):1077-84. [DOI: 10.1016/j.avsg.2012.02.013] [DOI] [PubMed] [Google Scholar]
Takaori 1995 {published data only}
- Takaori M, Fukui A, Kimura K, Mihira M, Endou S, Fujiwara T. Hemostatic mechanism associated with aprotinin during and after the extracorporeal circulation for cardiac and great vessel surgery. Japanese Journal of Anesthesiology 1995;44(12):1661-6. [PubMed] [Google Scholar]
Tepe 2008 {published data only}
- Tepe G, Schmehl J. Safe and effective femoral arteriotomy closure for diseased vessels. Arteriosclerosis, Thrombosis, and Vascular Biology 2008;28(6):e149. [Google Scholar]
Thompson 2013 {published data only}
- Thompson JF. Recombinant activated factor VII and aortic surgery. European Journal of Vascular and Endovascular Surgery 2013;45(6):626. [DOI] [PubMed] [Google Scholar]
Verhoef 2015 {published data only}
- Verhoef C, Singla N, Moneta G, Muir W, Rijken A, Lockstadt H, et al. Fibrocaps for surgical hemostasis: two randomized, controlled phase II trials. Journal of Surgical Research 2015;194:679-87. [DOI: 10.1016/j.jss.2014.12.011] [DOI] [PubMed] [Google Scholar]
Vierhout 2014 {published data only}
- Vierhout BP, Ott A, Reijnen MM, Oskam J, den Dungen JJ, Zeebregts CJ. Cyanoacrylate skin microsealant for preventing surgical site infection after vascular surgery: a discontinued randomized clinical trial. Surgical Infections 2014;15(4):425-30. [DOI] [PubMed] [Google Scholar]
Wachol‐Drewek 1996 {published data only}
- Wachol-Drewek Z, Pfeiffer M, Scholl E. Comparative investigation of drug delivery of collagen implants saturated in antibiotic solutions and a sponge containing gentamicin. Biomaterials 1996;17:1733-8. [DOI: 10.1016/0142-9612(96)87654-x] [DOI] [PubMed] [Google Scholar]
Weaver 2008 {published data only}
- Weaver FA, Lew W, Granke K, Yonehiro L, Delange B, Alexander WA. A comparison of recombinant thrombin to bovine thrombin as a hemostatic ancillary in patients undergoing peripheral arterial bypass and arteriovenous graft procedures. Journal of Vascular Surgery 2008;47(6):1266-73. [DOI: ] [DOI] [PubMed] [Google Scholar]
Zwischenberger 1999 {published data only}
- Zwischenberger JB, Brunston RL, Swann JR, Conti VR. Comparison of two topical collagen-based hemostatic sponges during cardiothoracic procedures. Journal of Investigative Surgery 1999;12:101-6. [DOI: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
jRCTs041180163 {published data only}
- jRCTs 041180163. The effect of tranexamic acid for EL2 after EVAR. rctportal.niph.go.jp/en/detail?trial_id=jRCTs041180163 (first received 29 March 2019).
NCT00618358 {published data only}
- NCT00618358. Vascular Sealant Study (VSS). clinicaltrials.gov/ct2/show/NCT00618358 (first received 20 February 2008).
NCT00652314 {published data only}
- NCT00652314. Study to evaluate safety & effectiveness of vascular sealant system. clinicaltrials.gov/ct2/show/NCT00652314 (first received 3 April 2008).
NCT04083807 {published data only}
- NCT04083807. Efficacy and safety of TISSEEL Lyo compared to manual compression as an adjunct to haemostasis. clinicaltrials.gov/ct2/show/study/NCT04083807 (first received 10 September 2019).
References to ongoing studies
ChiCTR1900023323 {published data only}
- ChiCTR190023323. Fibrin sealant used in EVAR AS filling technique treating abdominal aortic aneurysm. chictr.org.cn/showprojen.aspx?proj=39274 (first received 22 May 2019).
NCT04803747 {published data only}
- NCT04803747. A trial of a hospital policy of tranexamic acid use to reduce transfusion in major non-cardiac surgery (TRACTION). clinicaltrials.gov/ct2/show/NCT04803747 (first received 18 March 2021).
Additional references
Abraha 2016
- Abraha I, Romagnoli C, Montedori A, Cirocchi R. Thoracic stent graft versus surgery for thoracic aneurysm. Cochrane Database of Systematic Reviews 2016, Issue 6. Art. No: CD006796. [DOI: 10.1002/14651858.CD006796.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Altobelli 2018
- Altobelli E, Rapacchietta L, Profeta VF, Fagnano R. Risk factors for abdominal aortic aneurysm in population-based studies: a systematic review and meta-analysis. International Journal of Environmental Research and Public Health 2018;15(12):2805-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alzamora 2010
- Alzamora MT, Fores R, Baena-Diez JM, Pera G, Toran P, Sorribes M, et al. The peripheral arterial disease study (PERART/ARTPER): prevalence and risk factors in the general population. BMC Public Health 2010;10:38. [Google Scholar]
Antoniou 2017
- Antoniou GA, Georgiadis GS, Antoniou SA, Makar RR, Smout JD, Torella F. Bypass surgery for chronic lower limb ischaemia. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD002000. [DOI: 10.1002/14651858.CD002000.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ariëns 2002
- Ariëns RA, Lai T, Weisel JW, Greenberg CS, Grant PJ. Role of factor XIII in fibrin clot formation and effects of genetic polymorphisms. Blood 2002;100(3):743-54. [DOI] [PubMed] [Google Scholar]
Ashworth 2010
- Ashworth A, Klein AA. Cell salvage as part of a blood conservation strategy in anaesthesia. British Journal of Anaesthesia 2010;105(4):401-16. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Badger 2017
- Badger S, Forster R, Blair PH, Ellis P, Kee F, Harkin DW. Endovascular treatment for ruptured abdominal aortic aneurysm. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD005261. [DOI: 10.1002/14651858.CD005261.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bannazadeh 2016
- Bannazadeh M, Tadros RO, McKinsey J, Chander R, Marin ML, Faries PL. Contemporary management of type B aortic dissection in the endovascular era. Surgical Technology International 2016;28:214-21. [PubMed] [Google Scholar]
Beck 2016
- Beck AW, Sedrakyan A, Mao J, Venermo M, Faizer R, Debus S, et al. Variations in abdominal aortic aneurysm care: a report from the International Consortium of Vascular Registries. Circulation 2016;134(24):1948-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benjamin 2016
- Benjamin RJ. Transfusion-related sepsis: a silent epidemic. Blood 2016;127(4):380-1. [DOI] [PubMed] [Google Scholar]
Benson 2020
- Benson RA, Erete L, Stather P, Boyle JR, on behalf of the Rouleaux Club. Patient access to emergency endovascular repair of ruptured infrarenal abdominal aortic aneurysm. Bulletin 2020;102(2):e008. [DOI: 10.1308/rcsbull.2020.e008] [DOI] [Google Scholar]
Berger 2012
- Berger JS, Herout PM, Harshaw Q, Steinhubl SR, Frye CB, Becker RC. Bleeding-associated outcomes with preoperative clopidogrel use in on- and off-pump coronary artery bypass. Journal of Thrombosis and Thrombolysis 2012;34(1):56-64. [DOI] [PubMed] [Google Scholar]
Beverly 2019
- Beverly A, Ong G, Wilkinson KL, Doree C, Welton NJ, Estcourt LJ. Drugs to reduce bleeding and transfusion in adults undergoing cardiac surgery: a systematic review and network meta-analysis. Cochrane Database of Systematic Reviews 2019, Issue 9. Art. No: CD013427. [DOI: 10.1002/14651858.CD013427] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blanco 2017
- Blanco G, Blanco A. Haemostasis. In: Medical Biochemistry. Academic Press, 2017:781-9. [Google Scholar]
BNF 2019
- British National Formulary Online. Dried prothrombin complex. bnf.nice.org.uk/drug/dried-prothrombin-complex.html#indicationsAndDoses (accessed 20 March 2019).
Boyle 2021
- Boyle JR, Mao J, Beck AW, Venermo M, Sedrakyan A, Behrendt CA, et al. Variation in intact abdominal aortic aneurysm repair outcomes by country: analysis of international consortium of vascular registries 2010 - 2016. European Journal of Vascular and Endovascular Surgery 2021;62(1):16-24. [DOI: 10.1016/j.ejvs.2021.03.034] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bracey 2017
- Bracey A, Shander A, Aronson S, Boucher BA, Calcaterra D, Chu MW, et al. The use of topical hemostatic agents in cardiothoracic surgery. Annals of Thoracic Surgery 2017;104(1):353-60. [DOI] [PubMed] [Google Scholar]
Bremerich 2006
- Bremerich DH, Strametz R, Kirchner R, Moritz A, Zwissler B. Aprotinin in cardiac surgery: more risks than usefulness? [Aprotinin in der Kardiochirurgie. Mehr Risiko als Nutzen?]. Der Anaesthesist 2006;55(9):989-92, 994-6. [DOI] [PubMed] [Google Scholar]
Buck 2014
- Buck DB, Herwaarden JA, Schermerhorn ML, Moll FL. Endovascular treatment of abdominal aortic aneurysms. Nature Reviews. Cardiology 2014;11(2):112-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2015
- Centre for Disease Control and Prevention. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race, and sex: United States, 2015. www.cdc.gov/nchs/data/dvs/lcwk1_2015.pdf 2015.
Chapin 2015
- Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood Reviews 2015;29(1):17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chee 2016
- Chee YE, Liu SE, Irwin MG. Management of bleeding in vascular surgery. British Journal of Anaesthesia 2016;117(Suppl 2):ii85-94. [DOI] [PubMed] [Google Scholar]
CiNeMA 2017 [Computer program]
- CINeMA: Confidence in Network Meta-Analysis. Bern: Institute of Social and Preventive Medicine, University of Bern, 2017. Available from cinema.ispm.unibe.ch.
Cooper 2006
- Cooper JR Jr, Abrams J, Frazier OH, Radovancevic R, Radovancevic B, Bracey AW, et al. Fatal pulmonary microthrombi during surgical therapy for end-stage heart failure: possible association with antifibrinolytic therapy. Journal of Thoracic and Cardiovascular Surgery 2006;131(5):963-8. [DOI] [PubMed] [Google Scholar]
Cooper 2016
- Cooper M, Hicks C, Ratchford EV, Salameh MJ, Malas M. Diagnosis and treatment of uncomplicated type B aortic dissection. Vascular Medicine (London, England) 2016;21(6):547-52. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 1 March 2019. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
D'Ayala 2010
- D'Ayala M, Huzar T, Briggs W, Fahoum B, Wong S, Wise L, et al. Blood transfusion and its effect on the clinical outcomes of patients undergoing major lower extremity amputation. Annals of Vascular Surgery 2010;24(4):468-73. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, editor(s), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook/archive/v5.2/.
Elsayed 2017
- Elsayed RS, Cohen RG, Fleischman F, Bowdish ME. Acute type A aortic dissection. Cardiology Clinics 2017;35(3):331-45. [DOI] [PubMed] [Google Scholar]
Erasu 2022
- Erasu V, Novak A, Gibbs VN, Champaneria R, Dorée C, Hafeez A, et al. Pharmacological interventions for the treatment of bleeding in people treated for blunt force or penetrating injury in an emergency department: a systematic review and network meta‐analysis. Cochrane Database of Systematic Reviews 2022, Issue 6. Art. No: CD014600. [DOI: 10.1002/14651858.CD014600] [DOI] [Google Scholar]
FDA 2007
- US Food & Drug Administration. Early communication about an ongoing safety review aprotinin injection (marketed as Trasylol). www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/UCM070281 (accessed 5 September 2016).
Fowkes 2017
- Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nature Reviews. Cardiology 2017;14(3):156-70. [DOI] [PubMed] [Google Scholar]
Franchini 2007
- Franchini M. The use of desmopressin as a hemostatic agent: a concise review. American Journal of Hematology 2007;82(8):731-5. [DOI] [PubMed] [Google Scholar]
Fry 2018
- Fry DE, Nedza SM, Pine M, Reband AM, Huang CJ, Pine G. Medicare risk-adjusted outcomes in elective major vascular surgery. Surgery 2018;164(4):831-8. [DOI] [PubMed] [Google Scholar]
Gabay 2013
- Gabay M, Boucher BA. An essential primer for understanding the role of topical hemostats, surgical sealants, and adhesives for maintaining hemostasis. Pharmacotherapy 2013;33(9):935-55. [DOI] [PubMed] [Google Scholar]
Gibbs 2019a
- Gibbs VN, Champaneria R, Palmer A, Doree C, Estcourt LJ. Pharmacological interventions for the prevention of bleeding in people undergoing elective hip or knee surgery: a systematic review and network meta-analysis. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD013295. [DOI: 10.1002/14651858.CD013295] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibbs 2019b
- Gibbs VN, Champaneria R, Novak A, Doree C, Palmer AJ, Estcourt LJ. Pharmacological interventions for the prevention of bleeding in people undergoing definitive fixation of hip, pelvic and long bone fractures: a systematic review and network meta‐analysis. Cochrane Database of Systematic Reviews 2019, Issue 12. Art. No: CD013499. [DOI: 10.1002/14651858.CD013499] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilliland 2017
- Gilliland C, Shah J, Martin JG, Miller MJ. Acute limb ischemia. Techniques in Vascular and Interventional Radiology 2017;20(4):274-80. [DOI] [PubMed] [Google Scholar]
Harvey 2015
- Harvey AR, Basavaraju SV, Chung KW, Kuehnert MJ. Transfusion-related adverse reactions reported to the National Healthcare Safety Network Hemovigilance Module, United States, 2010 to 2012. Transfusion 2015;55(4):709-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haynes 2005
- Haynes SL, Bennett JR, Torella F, McCollum CN. Does washing swabs increase the efficiency of red cell recovery by cell salvage in aortic surgery? Vox Sanguis 2005;88(4):244-8. [DOI] [PubMed] [Google Scholar]
Henriksson 2013
- Henriksson AE. The impact of blood component transfusion practices on patient survival after abdominal aortic aneurysm surgery. Vascular and Endovascular Surgery 2013;47(1):38-41. [DOI] [PubMed] [Google Scholar]
Henry 2009
- Henry D, Carless P, Fergusson D, Laupacis A. The safety of aprotinin and lysine-derived antifibrinolytic drugs in cardiac surgery: a meta-analysis. Canadian Medical Association Journal 2009;180(2):183-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henry 2011
- Henry DA, Carless PA, Moxey AJ, O'Connell D, Stokes BJ, Fergusson DA, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews 2011, Issue 1. Art. No: CD001886. [DOI: 10.1002/14651858.CD001886.pub4] [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Chruchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook/archive/v5.2/.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Higgins 2022
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook. 2022.
Hirsch 2006
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113(11):e463-654. [DOI] [PubMed] [Google Scholar]
Hobson 2018
- Hobson C, Lysak N, Huber M, Scali S, Bihorac A. Epidemiology, outcomes, and management of acute kidney injury in the vascular surgery patient. Journal of Vascular Surgery 2018;68(3):916-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 2013
- Howard DP, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013;127(20):2031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 2014
- Howard DP, Sideso E, Handa A, Rothwell PM. Incidence, risk factors, outcome and projected future burden of acute aortic dissection. Annals of Cardiothoracic Surgery 2014;3(3):278-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howell 2013
- Howell N, Senanayake E, Freemantle N, Pagano D. Putting the record straight on aprotinin as safe and effective: results from a mixed treatment meta-analysis of trials of aprotinin. Journal of Thoracic and Cardiovascular Surgery 2013;145(1):234-40. [DOI] [PubMed] [Google Scholar]
Hudson 2017
- Hudson NE. Biophysical mechanisms mediating fibrin fiber lysis. Biomed Research International 2017;2017:2748340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2012
- Hutton B, Joseph L, Fergusson D, Mazer CD, Shapiro S, Tinmouth A. Risks of harms using antifibrinolytics in cardiac surgery: systematic review and network meta-analysis of randomised and observational studies. BMJ 2012;345:e5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH GCP 2018
- International Conference on Harmonisation. Good Clinical Practice Glossary. ichgcp.net/1-glossary/2018 (accessed July 2020).
JPAC 2013
- Joint United Kingdom (UK) Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee. Chapter 7: Specifications for blood components (Table 7.2). In: Guidelines for the Blood Transfusion Services in the UK. London: The Stationery Office, 2013. [Google Scholar]
Kapma 2017
- Kapma M, Kahmann O, Stijn I, Zeebregts CJ, Vahl A. Evaluation of risk prediction models, V-POSSUM and GAS, in patients with acute abdominal aortic rupture treated with EVAR or an open procedure. Journal of Cardiovascular Surgery 2017;58(3):439-45. [DOI] [PubMed] [Google Scholar]
Karsten 2018
- Karsten E, Breen E, Herbert BR. Red blood cells are dynamic reservoirs of cytokines. Scientific Reports 2018;8(1):3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaufmann 2003
- Kaufmann JE, Vischer UM. Cellular mechanisms of the hemostatic effects of desmopressin (DDAVP). Journal of Thrombosis and Haemostasis 2003;1(4):682-9. [DOI] [PubMed] [Google Scholar]
Ker 2015
- Ker K, Roberts I, Shakur H, Coats TJ. Antifibrinolytic drugs for acute traumatic injury. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD004896. [DOI: 10.1002/14651858.CD004896.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiely 2017
- Kiely P, Gambhir M, Cheng AC, McQuilten ZK, Seed CR, Wood EM. Emerging infectious diseases and blood safety: modelling the transfusion-transmission risk. Transfusion Medicine Reviews 2017;31(3):154-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2015
- Kim JH, Baek CH, Min JY, Kim JS, Kim SB, Kim H. Desmopressin improves platelet function in uremic patients taking antiplatelet agents who require emergent invasive procedures. Annals of Hematology 2015;94(9):1457-61. [DOI] [PubMed] [Google Scholar]
Kline 2017
- Kline AD, Dixon RG, Brown MK, Culp MP. Interventional Radiology Readiness Assessment Tool for Global Health. Journal of Global Radiology 2017;3(1):Article 2. [Google Scholar]
Kozek‐Langenecker 2017
- Kozek-Langenecker SA, Ahmed AB, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. European Journal of Anaesthesiology 2017;34(6):332-95. [DOI: 10.1097/EJA.0000000000000630] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kristensen 2012
- Kristensen MT, Holm G, Kirketerp-Moller K, Krasheninnikoff M, Gebuhr P. Very low survival rates after non-traumatic lower limb amputation in a consecutive series: what to do? Interactive Cardiovascular and Thoracic Surgery 2012;14(5):543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Leng 2000
- Leng GC, Papacosta O, Whincup P, Wannamethee G, Walker M, Ebrahim S, et al. Femoral atherosclerosis in an older British population: prevalence and risk factors. Atherosclerosis 2000;152(1):167-74. [DOI] [PubMed] [Google Scholar]
Levi 2010
- Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. New England Journal of Medicine 2010;363(19):1791-800. [DOI] [PubMed] [Google Scholar]
Mackman 2007
- Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arteriosclerosis, Thrombosis, and Vascular Biology 2007;27(8):1687-93. [DOI] [PubMed] [Google Scholar]
Madurska 2018
- Madurska MJ, Sachse KA, Jansen JO, Rasmussen TE, Morrison JJ. Fibrinolysis in trauma: a review. European Journal of Trauma and Emergency Surgery 2018;44(1):35-44. [DOI] [PubMed] [Google Scholar]
Mahmood 2018
- Mahmood E, Matyal R, Mueller A, Mahmood F, Tung A, Montealegre-Gallegos M, et al. Multifactorial risk index for prediction of intraoperative blood transfusion in endovascular aneurysm repair. Journal of Vascular Surgery 2018;67(3):778-84. [DOI] [PubMed] [Google Scholar]
Majd 2016
- Majd P, Mylonas S, Gawenda M, Brunkwall J. External validation of risk stratification models predicting the immediate mortality after open repair of ruptured AAA. World Journal of Surgery 2016;40(7):1771-7. [DOI] [PubMed] [Google Scholar]
Makar 2010
- Makar M, Taylor J, Zhao M, Farrohi A, Trimming M, D’Attellis N. Perioperative coagulopathy, bleeding, and hemostasis during cardiac surgery: a comprehensive review. ICU Director 2010;1(1):17-27. [Google Scholar]
Makrygiannis 2016
- Makrygiannis G, Labalue P, Erpicum M, Schlitz M, Seidel L, El Hachemi M, et al. Extending abdominal aortic aneurysm detection to older age groups: preliminary results from the Liege screening programme. Annals of Vascular Surgery 2016;36:55-63. [DOI] [PubMed] [Google Scholar]
Makuni 2015
- Makuni N, Simango C, Mavenyengwa RT. Prevalence of bacterial contamination in blood and blood products at the National Blood Service Zimbabwe. Journal of Infection in Developing Countries 2015;9(4):421-4. [DOI] [PubMed] [Google Scholar]
Mangano 2007
- Mangano DT, Miao Y, Vuylsteke A, Tudor IC, Juneja R, Filipescu D, et al. Mortality associated with aprotinin during 5 years following coronary artery bypass graft surgery. Journal of the American Medical Association 2007;297(5):471-9. [DOI] [PubMed] [Google Scholar]
Maxwell 2006
- Maxwell MJ, Wilson MJ. Complications of blood transfusion. Continuing Education in Anaesthesia, Critical Care & Pain 2006;6(6):225-9. [Google Scholar]
McCarthy 1994
- McCarthy MW, Coley KC. Aprotinin for prophylaxis of blood loss. Annals of Pharmacotherapy 1994;28(11):1246-8. [PubMed] [Google Scholar]
McCaw 2022
- McCaw ZR, Claggett BL, Tian L, Solomon SD, Berwanger O, Pfeffer MA, et al. Practical recommendations on quantifying and interpreting treatment effects in the presence of terminal competing risks: a review. JAMA Cardiology 2022;7(4):450-6. [DOI] [PubMed] [Google Scholar]
McDermott 2017
- McDermott MM, Carroll T, Carr J, Yuan C, Ferrucci L, Guralnik JM, et al. Femoral artery plaque characteristics, lower extremity collaterals, and mobility loss in peripheral artery disease. Vascular Medicine 2017;22(6):473-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKenzie 2016
- McKenzie JE, Beller EM, Forbes AB. Introduction to systematic reviews and meta‐analysis. Respirology 2016;21(4):626-37. [DOI] [PubMed] [Google Scholar]
Mesar 2017
- Mesar T, Larentzakis A, Dzik W, Chang Y, Velmahos G, Yeh DD. Association between ratio of fresh frozen plasma to red blood cells during massive transfusion and survival among patients without traumatic injury. Journal of the American Medical Association (JAMA) Surgery 2017;152(6):574-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morel 2013
- Morel P, Deschaseaux M, Bertrand X, Naegelen C, Leconte des Floris MF, Bardiaux L. Control of the bacterial risk of transfusion in France in 2013. Transfusion Clinique et Biologique 2013;20(2):174-81. [DOI] [PubMed] [Google Scholar]
Murkin 2010
- Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesthesia and Analgesia 2010;110(2):350-3. [DOI] [PubMed] [Google Scholar]
Muszbek 2008
- Muszbek L, Bagoly Z, Bereczky Z, Katona E. The involvement of blood coagulation Factor XIII in fibrinolysis and thrombosis. Cardiovascular & Hematological Agents in Medicinal Chemistry 2008;6(3):190-205. [DOI] [PubMed] [Google Scholar]
Muszynski 2017
- Muszynski JA, Spinella PC, Cholette JM, Acker JP, Hall MW, Juffermans NP, et al. Transfusion-related immunomodulation: review of the literature and implications for pediatric critical illness. Transfusion 2017;57(1):195-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCABTPG 2017
- National Comparative Audit of Blood Transfusion Project Group. 2016 Repeat audit of patient blood management in adults undergoing elective, scheduled surgery. Available from hospital.blood.co.uk/audits/national-comparative-audit/reports-grouped-by-year/patient-blood-management-in-adults-undergoing-elective-scheduled-surgery-2016/ (Accessed 1 July 2020).
NICE 2015
- NICE. Blood transfusion (NG24). Published: 18 November 2015. nice.org.uk/guidance/ng24 (accessed 1 December 2022).
NICE 2020
- NICE. Abdominal aortic aneurysm: diagnosis and management (NG156). Published 19 March 2020. nice.org.uk/guidance/ng156 (accessed 1 December 2022).
Nielsen 2005a
- Nielsen VG, Cohen BM, Cohen E. Effects of coagulation factor deficiency on plasma coagulation kinetics determined via thrombelastography: critical roles of fibrinogen and factors II, VII, X and XII. Acta Anaesthesiologica Scandinavica 2005;49(2):222-31. [DOI] [PubMed] [Google Scholar]
Nielsen 2005b
- Nielsen VG. Colloids decrease clot propagation and strength: role of factor XIII‐fibrin polymer and thrombin‐fibrinogen interactions. Acta Anaesthesiologica Scandinavica 2005;49:1163‐71. [DOI] [PubMed] [Google Scholar]
Noiphithak 2017
- Noiphithak R, Liengudom A. Recent update on carotid endarterectomy versus carotid artery stenting. Cerebrovascular Diseases 2017;43(1-2):68-75. [DOI] [PubMed] [Google Scholar]
O'Donnell 2018
- O'Donnell TF, Shean KE, Deery SE, Bodewes TC, Wyers MC, O'Brien KL, et al. A preoperative risk score for transfusion in infrarenal endovascular aneurysm repair to avoid type and cross. Journal of Vascular Surgery 2018;67(2):442-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Keeffe 2010
- O'Keeffe SD, Davenport DL, Minion DJ, Sorial EE, Endean ED, Xenos ES. Blood transfusion is associated with increased morbidity and mortality after lower extremity revascularization. Journal of Vascular Surgery 2010;51(3):616-21, 621.e1-3. [DOI] [PubMed] [Google Scholar]
Obi 2015
- Obi AT, Park YJ, Bove P, Cuff R, Kazmers A, Gurm HS, et al. The association of perioperative transfusion with 30-day morbidity and mortality in patients undergoing major vascular surgery. Journal of Vascular Surgery 2015;61(4):1000-9.e1. [DOI] [PubMed] [Google Scholar]
Okamoto 1997
- Okamoto S, Hijikata-Okunomiya A, Wanaka K, Okada Y, Okamoto U. Enzyme-controlling medicines: introduction. Seminars in Thrombosis and Hemostasis 1997;23(6):493-501. [DOI] [PubMed] [Google Scholar]
Omar 2015
- Omar HR, Enten G, Karlnoski R, Ching YH, Mangar D, Camporesi EM. Recombinant activated Factor VII significantly reduces transfusion requirements in cardiothoracic surgery. Drugs in R&D 2015;15(2):187-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osborne 2018
- Osborne Z, Hanson K, Brooke BS, Schermerhorn M, Henke P, Faizer R. Variation in transfusion practices and the association with perioperative adverse events in patients undergoing open abdominal aortic aneurysm repair and lower extremity arterial bypass in the Vascular Quality Initiative. Journal of Vascular Surgery 2018;67(2):675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paone 2014
- Paone G, Likosky DS, Brewer R, Theurer PF, Bell GF, Cogan CM, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Annals of Thoracic Surgery 2014;97(1):87-93; discussion 93-4. [DOI] [PubMed] [Google Scholar]
Paone 2018
- Paone G. Anemia, transfusion, and outcome: Both are bad...does it really matter which is worse? Journal of Thoracic and Cardiovascular Surgery 2018;156(1):75-6. [DOI] [PubMed] [Google Scholar]
Paravastu 2014
- Paravastu SC, Jayarajasingam R, Cottam R, Palfreyman SJ, Michaels JA, Thomas SM. Endovascular repair of abdominal aortic aneurysm. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD004178. [DOI: 10.1002/14651858.CD004178.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2007
- Park JB, Lakes RS. Composites as biomaterials. In: Park JB, Lakes RS, editors(s). Biomaterials. New York, NY: Springer New York, 2007:207-24. [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Paterick 2013
- Paterick TE, Humphries JA, Ammar KA, Jan MF, Loberg R, Bush M, et al. Aortopathies: etiologies, genetics, differential diagnosis, prognosis and management. American Journal of Medicine 2013;126(8):670-8. [DOI] [PubMed] [Google Scholar]
PRISMA
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Multrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahman 2017
- Rahman MS, Woollard K. Atherosclerosis. In: Sattler S, Kennedy-Lyndon T, editors(s). The Immunology of Cardiovascular Homeostasis and Pathology (Advances in Experimental Medicine and Biology). Vol. 1003. Springer, Cham, 2017:121-44. [DOI] [PubMed] [Google Scholar]
Raja 2006
- Raja SG, Shahbaz Y. Desmopressin for haemostasis in cardiac surgery: when to use? Annals of Cardiac Anaesthesia 2006;9(2):102. [PubMed] [Google Scholar]
Rerambiah 2014
- Rerambiah LK, Rerambiah LE, Bengone C, Djoba Siawaya JF. The risk of transfusion-transmitted viral infections at the Gabonese National Blood Transfusion Centre. Blood Transfusion 2014;12(3):330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2020 [Computer program]
- Review Manager Web (RevMan Web). Version 1.22.0. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Roberts 1991
- Roberts CS, Roberts WC. Aortic dissection with the entrance tear in abdominal aorta. American Heart Journal 1991;121(6 Pt 1):1834-5. [DOI] [PubMed] [Google Scholar]
Robertson 2017
- Robertson L, Nandhra S. Laparoscopic surgery for elective abdominal aortic aneurysm repair. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD012302. [DOI: 10.1002/14651858.CD012302.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Royston 2015
- Royston D. The current place of aprotinin in the management of bleeding. Anaesthesia 2015;70(Suppl 1):46-9, e17. [DOI] [PubMed] [Google Scholar]
Rubinstein 2013
- Rubinstein C, Davenport DL, Dunnagan R, Saha SP, Ferraris VA, Xenos ES. Intraoperative blood transfusion of one or two units of packed red blood cells is associated with a fivefold risk of stroke in patients undergoing elective carotid endarterectomy. Journal of Vascular Surgery 2013;57(2 Suppl):53s-7s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryan 2016
- Ryan R, Hill S. Cochrane Consumers and Communication Group: meta-analysis. CCCG La Trobe University, 2016. cccrg.cochrane.org/author-resources (accessed 11 June 2020).
Samoila 2017
- Samoila G, Ford RT, Glasbey JC, Lewis MH, Twine CP, Williams IM. The significance of hypothermia in abdominal aortic aneurysm repair. Annals of Vascular Surgery 2017;38:323-31. [DOI] [PubMed] [Google Scholar]
Sampson 2014
- Sampson UK, Norman PE, Fowkes FG, Aboyans V, Song Y, Harrell FE Jr, et al. Estimation of global and regional incidence and prevalence of abdominal aortic aneurysms 1990 to 2010. Global Heart 2014;9(1):159-70. [DOI] [PubMed] [Google Scholar]
Saw 2014
- Saw J. Carotid artery stenting for stroke prevention. Canadian Journal of Cardiology 2014;30(1):22-34. [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Selvin 2004
- Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation 2004;110(6):738-43. [DOI] [PubMed] [Google Scholar]
Seo 2015
- Seo DH, Whang DH, Song EY, Han KS. Occult hepatitis B virus infection and blood transfusion. World Journal of Hepatology 2015;7(3):600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seyednejad 2008
- Seyednejad H, Imani M, Jamieson T, Seifalian AM. Topical haemostatic agents. British Journal of Surgery 2008;95(10):1197-225. [DOI] [PubMed] [Google Scholar]
Shakur 2018
- Shakur H, Beaumont D, Pavord S, Gayet-Ageron A, Ker K, Mousa HA. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database of Systematic Reviews 2018, Issue 2. Art. No: CD012964. [DOI: 10.1002/14651858.CD012964] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaw 2013
- Shaw RE, Johnson CK, Ferrari G, Zapolanski A, Brizzio M, Rioux N, et al. Balancing the benefits and risks of blood transfusions in patients undergoing cardiac surgery: a propensity-matched analysis. Interactive Cardiovascular and Thoracic Surgery 2013;17(1):96-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simpson 2012
- Simpson E, Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database of Systematic Reviews 2012, Issue 3. Art. No: CD005011. [DOI: 10.1002/14651858.CD005011.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1989
- Smith TJ, Gill JC, Ambruso DR, Hathaway WE. Hyponatraemia and seizures in young children given DDAVP. American Journal of Hematology 1989;31(3):199-202. [DOI] [PubMed] [Google Scholar]
Society for Vascular Surgery
- Zhou W. Endoleaks (Type I-V). vascular.org/patient-resources/vascular-conditions/endoleaks-type-i-v (accessed 2 March 2019).
SpiderCite [Computer program]
- SpiderCite. Gold Coast, Australia: Institute for Evidence Based Healthcare, Bond University, 2020.
Stokes 2011
- Stokes ME, Ye X, Shah M, Mercaldi K, Reynolds MW, Rupnow MF, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Services Research 2011;11:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Svensson 2013
- Svensson S, Menkes DB, Lexchin J. Surrogate outcomes in clinical trials: a cautionary tale. JAMA Internal Medicine 2013;173(8):611-2. [DOI: 10.1001/jamainternmed.2013.3037] [DOI] [PubMed] [Google Scholar]
Swaminathan 2014
- Swaminathan A, Vemulapalli S, Patel MR, Jones WS. Lower extremity amputation in peripheral artery disease: improving patient outcomes. Vascular Health and Risk Management 2014;10:417-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takagi 2009
- Takagi H, Manabe H, Kawai N, Goto SN, Umemoto T. Aprotinin increases mortality as compared with tranexamic acid in cardiac surgery: a meta-analysis of randomized head-to-head trials. Interactive Cardiovascular and Thoracic Surgery 2009;9(1):98-101. [DOI] [PubMed] [Google Scholar]
Tan 2013
- Tan TW, Farber A, Hamburg NM, Eberhardt RT, Rybin D, Doros G, et al. Blood transfusion for lower extremity bypass is associated with increased wound infection and graft thrombosis. Journal of the American College of Surgeons 2013;216(5):1005-1014.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2015
- Tan TW, Eslami M, Rybin D, Doros G, Zhang WW, Farber A. Blood transfusion is associated with increased risk of perioperative complications and prolonged hospital duration of stay among patients undergoing amputation. Surgery 2015;158(6):1609-16. [DOI] [PubMed] [Google Scholar]
Thomsen 2006
- Thomsen M, Abel R. Imaging in scoliosis from the orthopaedic surgeon's point of view. European Journal of Radiology 2006;58(1):41-7. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;7(8):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ulug 2012
- Ulug P, McCaslin JE, Stansby G, Powell JT. Endovascular versus conventional medical treatment for uncomplicated chronic type B aortic dissection. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD006512. [DOI: 10.1002/14651858.CD006512.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vyas 2013
- Vyas KS, Saha SP. Comparison of hemostatic agents used in vascular surgery. Expert Opinion on Biological Therapy 2013;13(12):1663-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2017
- World Health Organisation. HIV/AIDS Data and Statistics. www.who.int/hiv/data/en/ (accessed 2 March 2019).
WHO 2021
- Cho BC, Frank SM. Surgery and anaesthesia. In: Educational modules on clinical use of blood. World Health Organization, 2021:161-2. [Google Scholar]
Wikkelsø 2013
- Wikkelsø A, Lunde J, Johansen M, Stensballe J, Wetterslev J, Møller AM, et al. Fibrinogen concentrate in bleeding patients. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD008864. [DOI: 10.1002/14651858.CD008864.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wikkelsø 2016
- Wikkelsø A, Wetterslev J, Møller AM, Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD007871. [DOI: 10.1002/14651858.CD007871.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Youssef 2017
- Youssef LA, Spitalnik SL. Transfusion-related immunomodulation: a reappraisal. Current Opinion in Hematology 2017;24(6):551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zammert 2016
- Zammert M, Gelman S. The pathophysiology of aortic cross-clamping. Best Practice & Research. Clinical Anaesthesiology 2016;30(3):257-69. [DOI] [PubMed] [Google Scholar]
Zbrozek 2015
- Zbrozek A, Magee G. Cost of bleeding in trauma and complex cardiac surgery. Clinical Therapeutics 2015;37(9):1966-74. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Beverly 2020
- Beverly A, Ong G, Doree C, Welton NJ, Estcourt LJ. Drugs to reduce bleeding and transfusion in major open vascular or endovascular surgery: a systematic review and network meta‐analysis. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013649. [DOI: 10.1002/14651858.CD013649] [DOI] [PMC free article] [PubMed] [Google Scholar]


