Background and Aims:
MAFLD often cooccurs with excessive alcohol consumption, while its prognostic value in this group remains unclear. We aimed to study the mortality risk of MAFLD in relation to excessive alcohol consumption and its potential interactions.
Approach and Results:
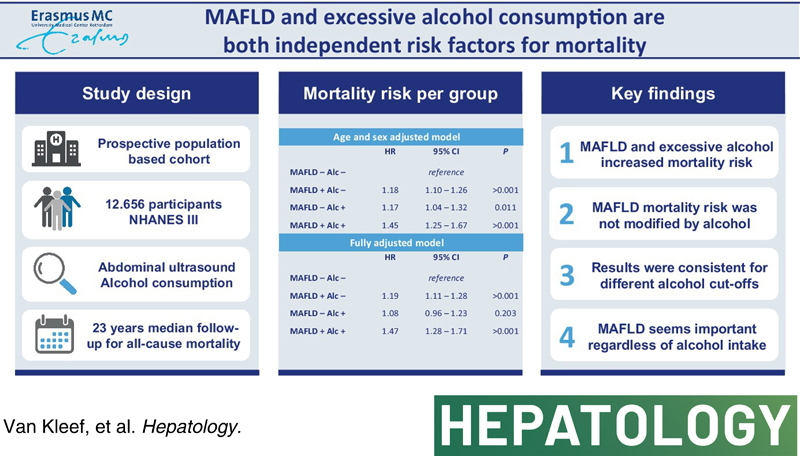
We analyzed persons 25–74 years old enrolled in the National Health and Nutrition Examination Survey III cohort with available steatosis and alcohol data. Participants with viral hepatitis, body mass index < 18.5, and missing data on age or follow‐up were excluded, leaving 12,656 participants for analysis with a median follow‐up of 22.9 [20.9–24.8] years. MAFLD was defined as steatosis on ultrasound in the presence of metabolic dysfunction. Daily alcohol intake of ≥10 g in females and ≥20 g in males was considered excessive alcohol consumption. We quantified mortality risk with multivariate Cox regression for MAFLD and excessive alcohol consumption. Models were adjusted for age, age squared, sex, race, marital status, education, and smoking. MAFLD was present in 31% and excessive alcohol consumption in 13% and were both independently and simultaneously associated with increased mortality risk in fully adjusted models (adjusted HR [aHR], 1.21; 95% CI, 1.13–1.30 and aHR, 1.14; 95% CI, 1.04–1.26, respectively). Similarly, MAFLD was associated with increased mortality risk in participants with and without excessive alcohol consumption. Participants with both MAFLD and excessive alcohol consumption (4.0%) expressed the highest mortality risk (aHR, 1.47; 95% CI, 1.28–1.71). Results were consistent using the initial 10 years of follow‐up, a stringent definition of excessive alcohol, and propensity score weighting.
Conclusions:
MAFLD increases mortality risk independent of excessive alcohol consumption. This underscores the importance of MAFLD, even in patients with excessive alcohol consumption.
INTRODUCTION
Since the recent introduction of the metabolic dysfunction‐associated fatty liver disease (MAFLD) criteria, several research groups have investigated its potential.1–4 The additionally identified group with MAFLD (but not NAFLD) is characterized by metabolic dysfunction with steatosis and also includes the presence of secondary causes for steatosis, such as viral hepatitis (VH) or excessive alcohol consumption. On an important note, the difference between NAFLD and MAFLD is not solely based on the use of alcohol or presence of VH, but also the presence of lean NAFLD without metabolic risk factors. The latter patients do not comply with MAFLD criteria.1
There is now emerging evidence that the prognosis of patients with VH could be negatively affected by MAFLD.5,6 However, in nonendemic regions like Europe and North America, VH accounts for only a rather small proportion of the MAFLD‐only group and additionally identified persons with fatty liver disease have mostly excessive alcohol consumption.2,7,8 It needs to be stressed that, next to alcohol use, these patients have metabolic dysfunction and may likely not be the same group of patients as those with alcohol‐associated liver disease (ALD; without MAFLD). Nevertheless, various research groups have attributed the excess mortality or fibrosis risk of this group predominantly to excessive alcohol consumption and did not therefore support the transition to MAFLD.9,10 Despite the important recent destigmatization steps taken in the field of ALD,11 this point of view yet again presents the risk of stigmatization to a large and growing group of persons. To date, whether the prognosis of MAFLD patients with excessive alcohol consumption is predominantly dependent on alcohol intake or mainly affected by metabolic dysfunction remains a topic for further study. We therefore aimed to study the mortality risk of MAFLD patients in relation to alcohol use.
PARTICIPANTS AND METHODS
Study population
This study was performed within the National Health and Nutrition Examination Survey (NHANES). The NHANES was designed to study participants’ health and nutritional status throughout the USA. In short, from all members of the sample, extensive data on health and nutrition were collected by interview, physical examination, and a battery of clinical measurements and tests. Detailed information regarding the procedures and rationale have been described elsewhere.12 Participants who were part of NHANES III (1988–1994) with available data on steatosis and alcohol were eligible for inclusion. Exclusion criteria were VH, body mass index (BMI) < 18.5, missing data on age at baseline, and lack of follow‐up.
Alcohol
Excessive alcohol consumption was defined as ≥10 g/d for females and ≥20 g/d for males based on interview data in which participants were asked about their drinking habits over the past year, in line with previous studies in the NHANES.9 According to USA standards, alcoholic drinks counted as 14 g of alcohol each. In addition, a more stringent definition of excessive alcohol consumption was used in the additional analysis (≥20 g/d for females and ≥30 g/d for males), which has been suggested to be the limit at which alcohol can induce steatosis.13
Liver assessment
Participants 25–74 years old underwent gallbladder ultrasound (Toshiba Sonolayer SSA‐90A, Tokyo, Japan), for which images were recorded and reassessed in 2009 and 2010 for the presence and grade of hepatic steatosis as described extensively elsewhere.12,14,15
MAFLD was defined as steatosis (irrespective of the gradation) combined with metabolic dysfunction. This comprises either overweight (BMI, ≥25 kg/m2), type 2 diabetes mellitus (defined as antidiabetic drug use, fasting plasma glucose ≥7.0 mmol/L, glycated hemoglobin [HbA1c] > 6.4%, or based on the oral glucose tolerance test [OGTT]), or a combination of at least two of the following metabolic abnormalities: (1) waist circumference > 102 cm for males and >88 cm for females; (2) blood pressure ≥ 130/85 mm Hg or antihypertensive drug use; (3) plasma triglycerides ≥1.70 mmol/L or lipid‐lowering drug treatment; (4) HDL cholesterol (HDL‐C) <1.0 mmol/L for males and <1.3 mmol/L for females or lipid‐lowering drug treatment; (5) prediabetes defined as fasting plasma glucose 5.6–6.9 mmol/L, HbA1c 5.7%–6.4%, or matching OGTT; (6) homeostatic model assessment of insulin resistance ≥2.5; or (7) C‐reactive protein (CRP) level > 2 mg/L.1
Follow‐up and mortality data
Data on vital status were obtained from the national death index and made available in the public use files provided by the National Center for Health Statistics, which contained complete data until December 31, 2015.16
Covariates
Research assistants systematically collected data on age, race, marital status, and smoking. Blood samples were taken, which were analyzed for triglycerides, HDL‐C, and CRP. An OGTT was performed in which, before the test, glucose and insulin were measured, and blood glucose levels were reassessed 2 hours after consuming 75 g of glucose.
Statistical analysis
First, we quantified mortality risk for the presence of MAFLD and excessive alcohol consumption (in one multivariate model) with multivariate Cox proportional hazards analysis and adjusted the results for age, age squared, and sex in model 1 and additionally for race, marital status, education, and smoking status in model 2. Next, we assessed mortality risk for MAFLD stratified for the presence of excessive alcohol consumption. Moreover, mortality risk was quantified for the four mutually exclusive groups based on MAFLD and excessive alcohol status: MAFLD−/Alc−; MAFLD+/Alc−; MAFLD−/Alc−; and MAFLD+/Alc+. In sensitivity analyses, we focused on 10‐year mortality and used stringent definitions of excessive alcohol consumption.
Finally, to address imbalances by an alternative approach, we performed a sensitivity analysis using propensity score weighting to adjust for baseline differences, with regard to age, sex, marital status, and education, to ascertain that these factors did not bias the results. This method is known as inverse probability treatment weighting (IPTW). For this method, a propensity score was constructed based on the probability of being in the MAFLD− or MAFLD+ group using the aforementioned covariates. Next, patients were weighted by the inverse of this propensity, which was stabilized before the analysis by using the estimated marginal means of the calculated propensity. Then, weights were inspected across the groups for comparability and possible extreme outliers. Finally, weights were used in the Cox proportional hazards analysis performing the same analysis as in the primary analysis.
Analyses were performed in R (version 4.0.4; R Foundation for Statistical Computing, Vienna, Austria), using the survival package 3.2–10, and SAS software (version 9.4; SAS Institute, Cary, NC). p values < 0.05 were considered statistically significant.
RESULTS
We included 13,225 participants of the NHANES III cohort (1988–1994) with available alcohol and liver ultrasound data. Of these, 283 were excluded for VH, 272 for BMI < 18.5, 3 for missing data on age, and 11 for lack of follow‐up, leaving 12,656 participants for analysis. The median age of the population used for analysis was 41.6 years [30.3–58.4]; 46% were male, and metabolic dysfunction was highly prevalent (e.g., overweight 62%, diabetes 15%, and at least two minor metabolic dysfunction criteria 60%). MAFLD was present in 31% and excessive alcohol consumption in 13%, resulting in the following distribution of mutually exclusive groups: MAFLD−/Alc− (60.1%); MAFLD+/Alc− (26.9%); MAFLD−/Alc+ (9.1%); and MAFLD+/Alc+ (4.0%). Detailed baseline characteristics of these groups are available in Table 1.
TABLE 1. Participants’ characteristics stratified for excessive alcohol and MAFLD status.
| MAFLD−/Alc− n = 7610 | MAFLD+/Alc− n = 3399 | MAFLD−/Alc+ n = 1146 | MAFLD+/Alc+ n = 501 | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 38.7 [28.5, 55.4] | 49.9 [37.4, 63.3] | 36.9 [27.4, 49.8] | 44.8 [34.7, 58.8] |
| Male | 3226 (42.4) | 1553 (45.7) | 706 (61.6) | 360 (71.9) |
| Race | ||||
| Mexican‐American | 2024 (26.6) | 1278 (37.6) | 276 (24.1) | 208 (41.5) |
| Non‐Hispanic Black | 2383 (31.3) | 779 (22.9) | 364 (31.8) | 95 (19.0) |
| Non‐Hispanic White | 2871 (37.7) | 1215 (35.7) | 482 (42.1) | 179 (35.7) |
| Other | 332 (4.4) | 127 (3.7) | 24 (2.1) | 19 (3.8) |
| College | 2538 (33.6) | 792 (23.4) | 367 (32.3) | 121 (24.3) |
| Current smoking | 2100 (27.6) | 699 (20.6) | 654 (57.1) | 221 (44.1) |
| MAFLD criteria | ||||
| BMI ≥ 25 | 3932 (51.7) | 3039 (89.4) | 491 (42.8) | 438 (87.4) |
| Diabetes | 648 (8.8) | 969 (29.1) | 69 (6.2) | 101 (20.4) |
| Metabolic dysfunction | 3624 (47.6) | 3040 (89.4) | 462 (40.3) | 435 (86.8) |
| Biochemistry | ||||
| AST (U/L) | 18 [15, 22] | 20 [17, 26] | 20 [17, 25] | 24 [19, 33] |
| ALT (U/L) | 13 [10, 18] | 17 [12, 26] | 14 [10, 19] | 21 [15, 36] |
| HDL‐C (mmol/L) | 1.35 (0.37) | 1.16 (0.34) | 1.52 (0.49) | 1.31 (0.45) |
| Triglycerides (mmol/L) | 1.13 [0.81, 1.62] | 1.79 [1.23, 2.59] | 1.11 [0.80, 1.65] | 1.80 [1.18, 2.79] |
| HbA1c (%) | 5.4 (0.9) | 6.0 (1.5) | 5.2 (0.6) | 5.5 (1.0) |
Note: Data are presented as mean (SD), median [P25‐P75], or n and percentage.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; P25‐P75, 25th–75th percentile.
In this cohort, 3804 participants died during the median follow‐up of 22.9 [20.9–24.8] years, resulting in a mortality rate of 14.4 per 1000 person‐years. Of these deaths, 31.3% (n = 1193) occurred in the initial 10 years of follow‐up (mortality rate, 9.8 per 1000 person‐years).
MAFLD (adjusted HR [aHR], 1.21; 95% CI, 1.13–1.30) and excessive alcohol consumption (aHR, 1.14; 95% CI, 1.04–1.26) were independently and simultaneously associated with increased mortality in fully adjusted models (Table 2). Furthermore, after stratification for excessive alcohol status, MAFLD increased mortality risk in both participants with and without excessive alcohol consumption (HR, 1.41; 95% CI, 1.17–1.71 and HR, 1.19; 95% CI, 1.10–1.27, respectively). Similarly, by introducing an interaction term between MAFLD and excessive alcohol consumption, we could not demonstrate loss of effect for these components in relation to mortality risk (aHR for effect modification, 1.14; 95% CI, 0.94–1.38).
TABLE 2. Mortality risk for the presence of MAFLD and excessive alcohol consumption.
| HR | 95% CI | p value | |
|---|---|---|---|
| Model 1 | |||
| MAFLD | 1.18 | 1.11–1.26 | >0.001 |
| Excessive alcohol | 1.19 | 1.09–1.31 | >0.001 |
| Model 2 | |||
| MAFLD | 1.21 | 1.13–1.30 | >0.001 |
| Excessive alcohol | 1.14 | 1.04–1.26 | 0.007 |
Note: Results were obtained with Cox proportional hazards and are given as HR with 95% CI for all‐cause mortality as outcome (3804 of 12,656). MAFLD and excessive alcohol consumption were simultaneously added in the multivariate model. Excessive alcohol consumption was defined as ≥10 and ≥20 g/d in females and males. Results were adjusted for age, age squared, and sex (model 1) and in addition for race, marital status, education, and smoking (model 2).
Further investigating the impact of alcohol and MAFLD on mortality using the four mutually exclusive groups, we demonstrated in the age‐ and sex‐adjusted models that the mortality risk for MAFLD+/Alc+ (HR, 1.45; 95% CI, 1.25–1.67) equals the product of MAFLD+/Alc− (HR, 1.18; 95% CI, 1.10–1.26) and MAFLD−/Alc+ (HR, 1.17; 95% CI, 1.04–1.32). In fully adjusted models, similar mortality risk as in the unadjusted models was observed for MAFLD+/Alc− and MAFLD+/Alc+, whereas MAFLD−/Alc+ was no longer associated with increased mortality risk (Table 3 ).
TABLE 3. Mortality risk for the four mutually exclusive groups based on MAFLD and excessive alcohol status.
| HR | 95% CI | p value | |
|---|---|---|---|
| Model 1 | |||
| MAFLD− Alc− | Reference | ||
| MAFLD+ Alc− | 1.18 | 1.10–1.26 | >0.001 |
| MAFLD− Alc+ | 1.17 | 1.04–1.32 | 0.011 |
| MAFLD+ Alc+ | 1.45 | 1.25–1.67 | >0.001 |
| Model 2 | |||
| MAFLD− Alc− | Reference | ||
| MAFLD+ Alc− | 1.19 | 1.11–1.28 | >0.001 |
| MAFLD− Alc+ | 1.08 | 0.96–1.23 | 0.203 |
| MAFLD+ Alc+ | 1.47 | 1.28–1.71 | >0.001 |
Note: Results were obtained with Cox proportional hazards and are given as HR with 95% CI for all‐cause mortality as outcome (3804 of 12,656). Excessive alcohol consumption was defined as ≥10 and ≥20 g/d in females and males. Results were adjusted for age, age squared, and sex (model 1) and in addition for race, marital status, education, and smoking (model 2).
Next, we used a more stringent definition of excessive alcohol consumption (≥20 and ≥30 g/d in females and males). Distribution of the mutually exclusive groups was as follows: MAFLD−/Alc− 64.9%; MAFLD+/Alc− 28.5%; MAFLD−/Alc+ 4.3%; and MAFLD+/Alc+ 2.3%. With this definition, the mortality risk for excessive alcohol consumption was more pronounced (aHR, 1.24; 95% CI, 1.10–1.41), whereas the effect of MAFLD remained stable (aHR, 1.21; 95% CI, 1.13–1.29). Following this trend, the MAFLD−/Alc+ group was at increased mortality risk in contrast to the results obtained with the more lenient definition as mentioned before (Table 4). Moreover, there was again no effect modification of mortality risk for MAFLD and excessive alcohol consumption (aHR, 1.00; 95% CI, 0.78–1.28). Similar to the results with this stringent definition, including alcohol abstinence as a confounder increased the effect size of excessive alcohol consumption (aHR, 1.35; 95% CI, 1.21–1.51). Focusing on participants with exceptionally high alcohol intake (≥60 g/d; n = 212), mortality risk estimates for MAFLD were similar to previous findings, but no longer significant (aHR, 1.56; 95% CI, 0.95–2.59).
TABLE 4. Mortality risk for MAFLD and excessive alcohol consumption using a stringent definition for excessive alcohol in fully adjusted models.
| HR | 95% CI | p value | |
|---|---|---|---|
| MAFLD | 1.21 | 1.13–1.29 | >0.001 |
| Excessive alcohol | 1.24 | 1.10–1.41 | 0.001 |
| Excessive alcohol × MAFLD | |||
| MAFLD− Alc− | Reference | ||
| MAFLD+ Alc− | 1.21 | 1.13–1.29 | >0.001 |
| MAFLD− Alc+ | 1.24 | 1.05–1.47 | 0.010 |
| MAFLD+ Alc+ | 1.50 | 1.24–1.80 | >0.001 |
Note: Results were obtained with Cox proportional hazards and are given as HR with 95% CI for all‐cause mortality as outcome (3804 of 12,656). MAFLD and excessive alcohol consumption were simultaneously added in the multivariate model. Excessive alcohol consumption was defined as ≥20 and ≥30 g/d in females and males. Results were adjusted for age, age squared, sex, race, marital status, education, and smoking.
When only the first 10 years of follow‐up were taken into account, MAFLD was still independent of excessive alcohol consumption associated with increased mortality (aHR, 1.14; 95% CI, 1.01–1.28). Alcohol was only independently associated with 10‐year mortality if the stringent definition of excessive alcohol consumption was used (aHR, 1.37; 95% CI, 1.11–1.71) and not with the lenient definition (aHR, 1.13; 95% CI, 0.95–1.35). As a final sensitivity analysis, we performed a propensity score weighting analysis where patients were matched on age, sex, marital status, and education level. By using the same Cox proportional hazard analysis as stated in Tables 2 and 3, the results were in line with our previous findings.
DISCUSSION
We investigated the role of MAFLD on mortality in relation to excessive alcohol consumption and demonstrated that the simultaneous presence of MAFLD and excessive alcohol consumption cumulatively increased mortality risk.
There is a large proportion of excessive alcohol consumption in persons only selected by MAFLD and not by NAFLD, typically >70%.2,7,8 Some studies indicated that their prognosis is not determined by MAFLD, but rather by their alcohol intake.9,10 Our comprehensive investigation of the potential interactions between alcohol consumption and MAFLD provides evidence that MAFLD, in fact, has prognostic value regardless of excessive alcohol consumption. First, we have shown that MAFLD and excessive alcohol were independent and simultaneous predictors for all‐cause mortality. Second, MAFLD increases mortality risk in patients with and without excessive alcohol. Third, the mortality risk of persons with both excessive alcohol consumption and MAFLD exceeds the risk observed for MAFLD+/Alc− and MAFLD−/Alc+ alone. Finally, we replicated this finding using IPTW, a sophisticated approach to account for imbalances in comparison groups. Altogether, we have shown convincing evidence supporting the clinical relevance of MAFLD independent of excessive alcohol consumption.
There are no extensive data yet available on MAFLD and excessive alcohol consumption, but we previously reported increased liver stiffness in patients captured only by MAFLD independent of alcohol consumption.2 Similarly, Yamamura et al. reported a high prevalence of fibrosis in both MAFLD patients with (19.7%) and without (15.5%) modest alcohol consumption.17 Moreover, Tsutsumi et al. demonstrated an increased risk of cardiovascular disease independent of alcohol consumption in patients with MAFLD.18 Furthermore, our findings are in line with the evidence summarized by Idalsoaga et al. in their review on NAFLD and ALD.19 They concluded that NAFLD and ALD often coexist and that alcohol consumption, even within the arbitrary thresholds allowed for NAFLD, may contribute to disease progression. Within persons with steatosis, Younossi et al. also demonstrated the relevance of alcohol consumption, particularly for higher thresholds of alcohol consumption.20 Although most evidence originated from the NAFLD era, they support the findings of this MAFLD‐oriented article.
Although the group of participants with both MAFLD and excessive alcohol use had the highest risk of mortality, we found no effect modification between excessive alcohol consumption and MAFLD. Absence of effect modification means that there is a cumulative increase in mortality risk in case both MAFLD and excessive alcohol consumption are present. We therefore have to reject our hypothesis that the simultaneous presence of MAFLD and excessive alcohol consumption might result in synergistically increased mortality risk, as described recently.21
From another point of view, MAFLD might no longer be relevant among persons with exceptionally high alcohol intake attributable to competing risks. Nonetheless, among participants with alcohol intake exceeding 60 g/d, MAFLD seemed equally harmful. However, we note that confidence intervals were widely attributable to the limited number of participants drinking this much (n = 212), hampering us from firm conclusions.
Conflicting results have been obtained in the NHANES III cohort regarding mortality risk of MAFLD.9,22,23 These may be attributed to differences in design, specifically (1) adjusting for several MAFLD criteria, (2) not accounting for age as a nonlinear risk factor, and (3) not considering mild hyperechogenicity as steatosis. For our aim, the interaction between MAFLD and excessive alcohol consumption, we adjusted primarily for demographics and social‐economic status in the mortality risk of MAFLD. However, additional adjusting for BMI again yielded similar mortality risk for patients with and without excessive alcohol consumption, although attenuated.
Several studies have shown that the association between alcohol intake and mortality follows a J‐shaped curve, in which those drinking moderately have the lowest mortality risk. This phenomenon was recently replicated in the NHANES cohort.24 Although this nonlinear association is debated and there is no safe limit of alcohol consumption,25,26 this J‐shaped association might result in underestimating the mortality risk for excessive alcohol consumption. Nonetheless, in our study, a modestly increased mortality risk was observed for excessive alcohol consumption, which increased with a more stringent definition of excessive alcohol consumption. Similarly, by accounting for “abstainer bias” (which may drive the J‐shaped curve) by additional adjusting the final models for alcohol abstinence, we demonstrated relatively larger effect sizes for excessive alcohol consumption (aHR, 1.35; 95% CI, 1.21–1.51) whereas the effect of MAFLD remained stable (aHR, 1.21; 95% CI, 1.13–1.29). This illustrates that the J‐shaped association between alcohol consumption and mortality did not affect our conclusions regarding the clinical relevance of MAFLD.
Interestingly, among the 494 participants with excessive alcohol consumption in the absence of metabolic dysfunction, only 23% (n = 112) had steatosis. Hence, among MAFLD patients with excessive alcohol consumption, the primary driver of steatosis is likely to be metabolic dysfunction and not excessive alcohol consumption. Therefore, MAFLD with excessive alcohol consumption should not be likened to nor seen as ALD.
This study’s findings clearly showed the clinical relevance of MAFLD in patients with excessive alcohol consumption and, in that respect, support the transition from NAFLD toward MAFLD. The cumulative risk of MAFLD and excessive alcohol consumption for mortality illustrates that, within the MAFLD spectrum, these persons are at increased risk. Hence, MAFLD patients should not only be treated for metabolic traits, but their alcohol consumption should also be addressed and vice versa. Given the increased mortality risk of MAFLD patients with excessive alcohol consumption, we support a specific subgroup for these persons—under the same umbrella term of MAFLD—as was proposed recently.9
Although this study decomposed the effects of MAFLD and excessive alcohol comprehensively and had a large sample size with a median follow‐up of 23 years, the following limitations need mentioning. First, coherent to the extensive follow‐up, baseline data from this cohort originated from 1988 to 1994 and the prevalence of MAFLD and excessive alcohol consumption might not reflect its current extent. Second, we did not use the provided weights to modulate the USA general population, because we focused on the concept of interaction between MAFLD and excessive alcohol consumption in relation to mortality rather than estimating exact risks for the USA population. Third, in population studies, alcohol consumption is difficult to assess and often under‐reported. Hence, we applied a rather low cutoff for excessive alcohol data and replicated our findings using other cutoffs. Fourth, although the extended follow‐up is one of the strengths of the NHANES cohort, one can argue the prognostic value of modifiable factors, such as MAFLD and excessive alcohol consumption, beyond a certain time point. Therefore, we confirmed our main results using only the initial 10 years of follow‐up. Fifth, there was an imbalance between the four mutually exclusive groups in terms of age, sex, and socioeconomic status. In addition to taking these factors into account in multivariate models, we performed propensity score weighting, which yielded similar results. Last, although mortality is the ultimate endpoint, we could not differentiate between all‐cause mortality and liver‐related mortality because of the restricted nature of these data, and there were no follow‐up data available on liver‐specific data such as fibrosis stage or liver‐related events. Additional studies should investigate whether, despite the absence of interaction for all‐cause mortality, the risk of liver‐related adverse outcomes is synergistically increased for the simultaneous presence of MAFLD and excessive alcohol consumption.
In conclusion, MAFLD increases mortality risk regardless of excessive alcohol consumption, and there was no effect modification regarding mortality between MAFLD and excessive alcohol consumption. Therefore, MAFLD seems an important entity regardless of drinking habits. However, given that mortality risks increase for the simultaneous presence of MAFLD and excessive alcohol consumption, we recommend a specific subgroup for the presence of excessive alcohol consumption, using MAFLD as the umbrella term.
Acknowledgments
AUTHOR CONTRIBUTIONS
Collection of data: Laurens A. van Kleef, Willem Pieter Brouwer. Study design, data analysis, writing of the manuscript: Laurens A. van Kleef, Willem Pieter Brouwer, Robert J. de Knegt. Critical review of the manuscript, approval of final version, and approval of submission: all authors.
ACKNOWLEDGMENT
We gratefully acknowledge the contribution of the participants of the NHANES cohort, research assistants, and facilitating personnel.
FUNDING INFORMATION
Financial support was provided by the Foundation for Liver and Gastrointestinal Research (Rotterdam, The Netherlands). The funding source did not influence study design, data collection, analysis and interpretation of the data, nor the writing of the report and decision to submit for publication.
CONFLICTS OF INTEREST
Robert J. de Knegt advises for and received grants from AbbVie and Gilead. He is on the speakers’ bureau for Echosens. He received grants from GSK.
ETHICS
Participants of the NHANES III provided informed consent. This study was conducted according to the principles as set forth in the Declaration of Helsinki.
DATA AVAILABILITY STATEMENT
Data are publically available from the NHANES database (https://www.cdc.gov/nchs/nhanes/index.htm).
Footnotes
Abbreviations: aHR, adjusted HR; ALD, alcohol‐associated liver disease; BMI, body mass index; CRP, C‐reactive protein; HbA1c, glycated hemoglobin; HDL‐C, HDL cholesterol; MAFLD, metabolic dysfunction‐associated fatty liver disease; NHANES, National Health and Nutrition Examination Survey; OGTT, oral glucose tolerance test; VH, viral hepatitis;
Funding information Stichting voor Lever-en Maag-Darm Onderzoek
REFERENCES
- 1.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero‐Gomez M, et al. A new definition for metabolic associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–9. [DOI] [PubMed] [Google Scholar]
- 2.Van Kleef L, Ayada I, Alferink L, Pan Q, de Knegt RJ. Metabolic dysfunction associated fatty liver disease improves detection of high liver stiffness: the Rotterdam Study. Hepatology. 2021;75:419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40:2082–9. [DOI] [PubMed] [Google Scholar]
- 4.Wong VW, Wong GL, Woo J, Abrigo JM, Chan CK, Shu SS, et al. Impact of the new definition of metabolic associated fatty liver disease on the epidemiology of the disease. Clin Gastroenterol Hepatol. 2021;19:2161–71.e5. [DOI] [PubMed] [Google Scholar]
- 5.van Kleef LA, HSJ C, Brouwer WP, Hansen BE, Patel K, de Man RA, et al. Metabolic dysfunction‐associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B. JHEP Rep. 2021;3:100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Zhou J, Wu L, Zhu X, Deng H. MAFLD is associated with the risk of liver fibrosis and inflammatory activity in HBeAg‐negative CHB patients. Diabetes Metab Syndr Obes. 2022;15:673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciardullo S, Perseghin G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int. 2021;41:1290–3. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen VH, le MH, Cheung RC, Nguyen MH. Differential clinical characteristics and mortality outcomes in persons with NAFLD and/or MAFLD. Clin Gastroenterol Hepatol. 2021;19:2172–81.e6. [DOI] [PubMed] [Google Scholar]
- 9.Younossi ZM, Paik JM, Al Shabeeb R, Golabi P, Younossi I, Henry L. Are there outcome differences between NAFLD and metabolic‐associated fatty liver disease? Hepatology. 2022. Apr 1. 10.1002/hep.32499. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.De A, Ahmad N, Mehta M, Singh P, Duseja A. NAFLD vs. MAFLD—it is not the name but the disease that decides the outcome in fatty liver. J Hepatol. 2022;76:475–7. [DOI] [PubMed] [Google Scholar]
- 11.Thursz M, Gual A, Lackner C, Mathurin P, Moreno C, Spahr L, et al. EASL Clinical Practice Guidelines: management of alcohol‐related liver disease. J Hepatol. 2018;69:154–81. [DOI] [PubMed] [Google Scholar]
- 12.Marchesini G, Day CP, Dufour JF, Canbay A, Nobili V, Ratziu V, et al. Plan and operation of the third National Health and Nutrition Examination Survey, 1988–94. Washington, D.C.: U.S. Department of Health and Human Services, Public Health Service; 1994. [Google Scholar]
- 13.Marchesini G, Day CP, Dufour JF, Canbay A, Nobili V, Ratziu V, et al. EASL–EASD–EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. [DOI] [PubMed] [Google Scholar]
- 14.National Center for Health Statistics. Third National Health and Nutrition Examination Survey—gallbladder ultrasonography procedure manual. [cited 2022 Jul 22]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes3/manuals/gallblad.pdf
- 15.National Center for Health Statistics. National Health and Nutrition Examination Survey III—hepatic steatosis ultrasound images assessment procedures manual, 2010. [cited 2022 Jul 10]. Available from: https://www.cdc.gov/nchs/data/nhanes/nhanes3/hepatic_steatosis_ultrasound_procedures_manual.pdf
- 16.National Center for Health Statistics. Office of Analysis and Epidemiology, Public‐use Linked Mortality File, 2015. Hyattsville, MD: National Center for Health Statistics. 2020. [cited 2022 Jul 10]. Available from: https://www.cdc.gov/nchs/data‐linkage/mortality‐public.htm [Google Scholar]
- 17.Yamamura S, Eslam M, Kawaguchi T, Tsutsumi T, Nakano D, Yoshinaga S, et al. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int. 2020;40:3018–3030. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsumi T, Eslam M, Kawaguchi T, Yamamura S, Kawaguchi A, Nakano D, et al. MAFLD better predicts the progression of atherosclerotic cardiovascular risk than NAFLD: generalized estimating equation approach. Hepatol Res. 2021;51:1115–28. [DOI] [PubMed] [Google Scholar]
- 19.Idalsoaga F, Kulkarni AV, Mousa OY, Arrese M, Arab JP. Non‐alcoholic fatty liver disease and alcohol‐related liver disease: two intertwined entities. Front Med. 2020;7:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Younossi ZM, Stepanova M, Ong J, Yilmaz Y, Duseja A, Eguchi Y, et al. Effects of alcohol consumption and metabolic syndrome on mortality in patients with nonalcoholic and alcohol‐related fatty liver disease. Clin Gastroenterol Hepatol. 2019;17:1625–33.e1. [DOI] [PubMed] [Google Scholar]
- 21.van Kleef LA, de Knegt RJ. The transition from NAFLD to MAFLD: one size still does not fit all—time for a tailored approach? Hepatology. 2022. May 3. 10.1002/hep.32552. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction‐associated fatty liver disease is associated with increased all‐cause mortality in the United States. J Hepatol. 2021;75:1284–91. [DOI] [PubMed] [Google Scholar]
- 23.Huang Q, Zou X, Wen X, Zhou X, Ji L. NAFLD or MAFLD: Which Has Closer Association With All‐Cause and Cause‐Specific Mortality?—Results From NHANES III. Front Med (Lausanne). 2021;8:693507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricci C, Schutte AE, Schutte R, Smuts CM, Pieters M. Trends in alcohol consumption in relation to cause‐specific and all‐cause mortality in the United States: a report from the NHANES linked to the US mortality registry. Am J Clin Nutr. 2020;111:580–9. [DOI] [PubMed] [Google Scholar]
- 25.Costanzo S, de Gaetano G, di Castelnuovo A, Djoussé L, Poli A, van Velden D. Moderate alcohol consumption and lower total mortality risk: justified doubts or established facts? Nutr Metab Cardiovasc Dis. 2019;29:1003–8. [DOI] [PubMed] [Google Scholar]
- 26.GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are publically available from the NHANES database (https://www.cdc.gov/nchs/nhanes/index.htm).


