Abstract
Cancer-associated fibroblasts (CAFs)—derived exosomes have emerged as a key driver of ovarian cancer (OVCA) tumor progression. The mechanisms behind the specific circular RNA (circRNA) activity encapsulated by CAF-generated exosomes (CAF-exo) requires to be elucidated. Herein, this study selected specific circRNA (hsa_circIFNGR2) molecules and aimed to clarify novel function of CAF-derived exosomal circIFNGR2 on growth, and metastasis of OVCA cells. In this study, we clarified that the exosomes of CAFs originating from human ovarian cancer hindered tumor cell proliferation, metastasis and EMT in vitro. Interestingly, CAFs directly transferred exosomes into OVCA cells to enrich intracellular circIFNGR2 levels. Biologically, activation of exosomal circIFNGR2 blocked cell proliferation, metastasis and EMT. Mechanistically, enhanced circIFNGR2 activated the miR-378/ST5 axis and directly inhibited the malignant evolution of tumor cells. Furthermore, rescue experiments evidenced that circIFNGR2 and ST5 were two essential participants in OVCA, concretely manifested in the co-culture of OVCA cells with exosomes that reversed the effects of intracellular circIFNGR2 and ST5 depletion. Finally, we observed that CAF-exo treatment hindered tumor growth and increased the size and number of metastatic nodules in mice. Our study revealed a previously unknown regulatory pathway whereby CAFs-derived exosomes delivered circIFNGR2 and inhibited the malignant progression of OVCA by circIFNGR2/miR-378/ST5 axis.
Keywords: cancer-associated fibroblasts, circIFNGR2, exosomes, miR-378, OVCA, ST5
INTRODUCTION
The increasing incidence and mortality of ovarian cancer (OVCA) reflects the extreme heterogeneity, high infiltration and metastatic potential of the disease.1 Therefore, it is unavoidable to focus OVCA research on two areas, firstly, finding sensitive early diagnostic markers, and secondly, exploring molecular changes in tumor cell proliferation and metastasis of OVCA.
Tumorigenesis and progression is an extremely complex process in which multiple regulators and transformations are at work.2 The tumor microenvironment (TME) can be likened to the “seed and soil” that provides specific conditions for tumor cells survival and growth.3,4 Cancer-associated fibroblasts (CAFs) within the TME are prone to construct tumor-specific metastatic ecological niches, as well as alter tumor cell growth by secreting one or more soluble factors.5,6 Therefore, CAF-based anticancer therapy has been largely considered. Many studies have focused on the role of biological information carried by CAF-derived exosomes (CAF-exo) in specific biological signaling. For example, after CAF-exo treatment of SKOV-3 and CAOV-3 cells, the cargo TGFβ1 carried by exo-managed the epithelial-mesenchymal transition (EMT) of cancer cells via activating SMAD signaling.7 Exosomes (exo) are extracellular vesicles of 30–200 nm in diameter, generated by various endosomal-lysosomal pathways.8 Cancer-associated exosomes serve as “signal enrichers” for intercellular communication between cancer cells and stroma, through delivering proteins, lipids and genetic material.9,10 The unique vesicle structure of exosomes ensures the integrity of signal population transfer and makes the complex information transmission between cells more precise and comprehensive.11 Not surprisingly, exosomes are strongly connected to the diagnosis, metastasis, and treatment of malignant tumors.
Currently, studies on tumors related to noncoding RNAs have mainly focused on the activation of gene and pathways by circular RNAs (circRNAs) or microRNAs (miRNAs).12 CircRNA is named for its special ring-like closed structure, which is not as quickly degradable as traditional linear RNA.13–15 Prior publications have exhibited that exosomes contained more abundant circRNAs than secretory cells, and that the secretory process could be directed by miRNAs.16 Therefore, the diversity and specificity of circRNAs in exosomes allow them to be used for the ancillary diagnosis of tumors as well as for monitoring tumor progression. And the current research has turned to the fact that exosomes of CAFs (CAF-exo) could translocate circRNAs to adjacent cancer cells for biological roles. It is well-documented that CAF-exo could translocate circ_0088300 to gastric cancer cells, thereby increasing cell motility.17 However, explorations on the mechanisms of CAF-exo regulate OVCA tumorigenesis are still at the beginning, and the working mechanism of circRNAs delivered by CAF-exo in OVCA has not been addressed.
Here, we identified a novel circular RNA (circIFNGR2) derived from CAFs, and illustrated that CAF-exo could transfer circIFNGR2 into OVCA cells to exert antitumor effects. Notably, bioinformatic analysis identified endogenous competitive network between circIFNGR2, miR-378 and ST5, more specifically, the ST5 was implicated in the contribution of circIFNGR2/miR-378 regulatory network to OVCA cell growth, invasion and EMT functions. The present study mirrors the demands to assess the functional contribution of circRNA to OVCA and the interaction of exosome-associated cells with the tumor microenvironment.
RESULTS
Exosomes derived from cancer-associated fibroblasts inhibit the malignant progression of ovarian cancer cells
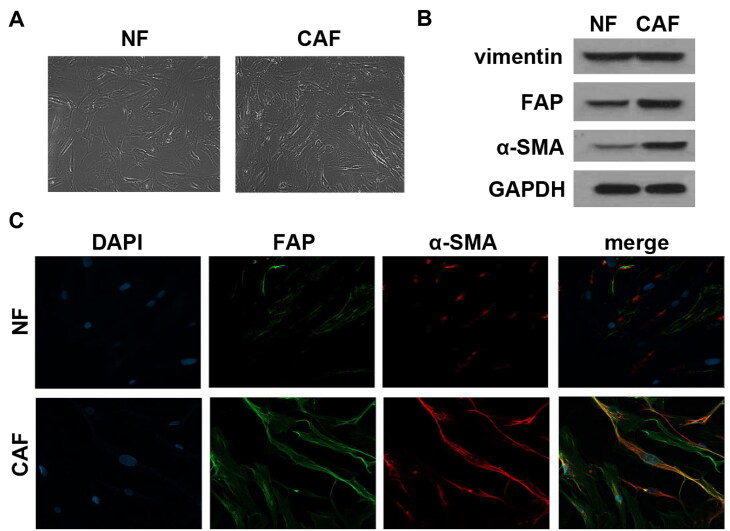
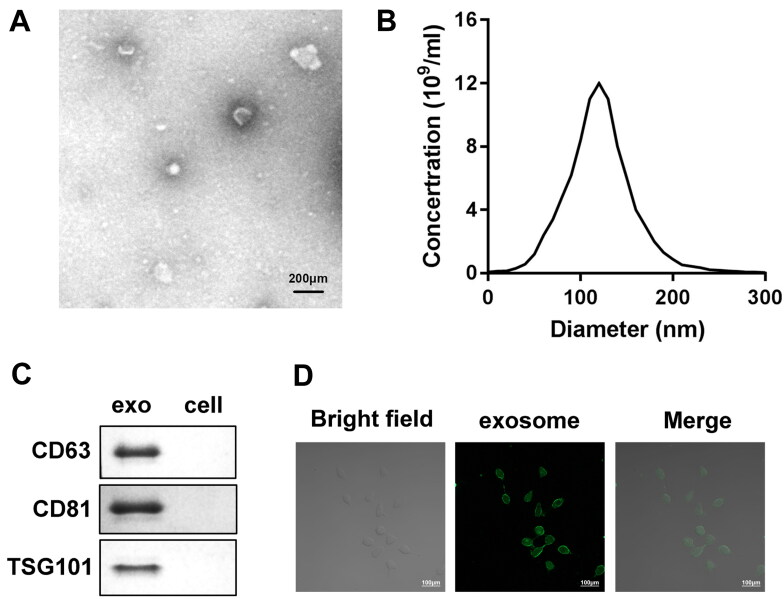
Given that complicated crosstalk between cancer cells and surrounding stromal cells is facilitated by exosomes,18 this reasonably leads to the question of whether exosomes shed by CAFs participated in various OVCA biological functions. To clearly demonstrate this, we first successfully separated CAFs from OVCA tumor tissue. Both CAFs and NFs initially isolated under an inverted microscope exhibited a typical long-fusiform appearance (Fig. 1A). Western blot analysis demonstrated that primary cultures of both CAFs and NFs co-expressed vimentin, while the expression of α-smooth muscle actin (α-SMA) and fibroblast activation protein (FAP) was better in the CAF group than in the NF group (Fig. 1B). Immunofluorescence detection further showed that the obtained CAFs are strongly positive for both α-SMA and FAP (Fig. 1C). Cells separated and identified from OVCA tissues were therefore considered CAFs. Then, the exosomes were isolated and purified from the CAFs supernatant by differential ultracentrifugation. Then, exosomes were isolated and purified from CAFs supernatant by a series of differential ultracentrifugation. As shown in Fig. 2A and B, the exosomes secreted by CAFs all showed a typical round appearance with a uniform size and a diameter of about 100–200 nm, and also seemingly consisted of a planar lipid bilayer. Compared with cell lysates, Western blot analysis suggested that the separated exosomes were positively enriched in exosomal markers CD63, CD81 and TSG101 (Fig. 2C), providing further proof for acquisition identity. These data demonstrated that the exosomes we obtained were pure enough to be used in subsequent experiments. PKH67 lipophilic green fluorescent dye labeled the uptake of exosomes by OV90. Confocal microscopy captured images showed a green fluorescent signal in the surrounding area of OV90 cells, suggesting that OV90 cells internalized exosomes secreted by CAFs (Fig. 2D). Thus, exosomes shed from CAFs could be extended into OVCA cells.
FIG 1.
CAFs were successfully isolated. (A) The morphology of the isolated CAFs and NFs was observed under an inverted microscope. (B) Expression of signature markers α-SMA, FAP and vimentin were determined by Western blot analysis. (C) Immunofluorescence staining was applied to assess the abundance of α-SMA and FAP in CAFs and NFs.
FIG 2.
Characteristic of CAFs-derived exosomes. (A and B) Morphology and particle size variation of exosomes obtained from CAFs conditioned medium were observed and analyzed using Transmission electron microscopy (TEM) and Nanoparticle tracking analysis (NTA). Scale bars: 200μm. (C) Western blot analysis was applied to determine the expression of exosome markers CD63, CD81 and TSG101 in CAFs-derived exosomes and OVCA cell lysates. (D) Confocal laser scanning microscopy was utilized to detect the green fluorescent signal of PKH67-labeled CAFs-exo in OVCA cells. Scale bars: 100 μm.
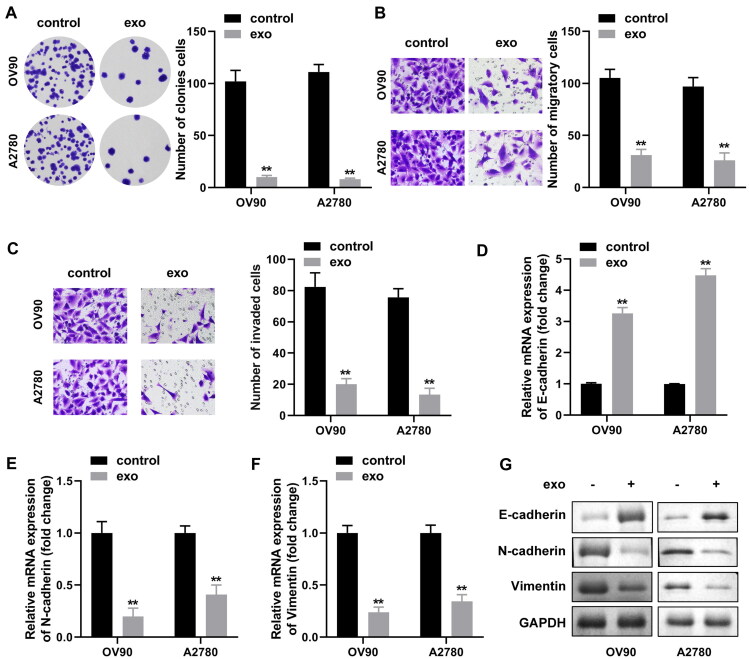
CAF-derived exosomes show more possibilities in regulating OVCA metastasis and invasion19,20 and we conjectured that exosomes released from CAFs may further alter the proliferation and metastatic abilities of OVCA cells, whether they served a promotion or inhibition role needed further discussion. The effect of CAF-exo on tumor cells was assessed by establishing a co-culture system. OVCA cells (OV90, A2780) were co-cultured with exosomes from CAFs or PBS (control), and the following assays were performed. The results of colony formation assay illustrated that exosomes co-culture with OVCA cells silenced the proliferation ability of latter, compared to the controls (Fig. 3A). Consistently, transwell assays demonstrated that complementary addition of exosomes attenuated the migration and invasion abilities of OV90 and A2780 cells, reducing 74%, 71%, 61%, and 62%, respectively (Fig. 3B and C). We also assessed the relevance between CAF-exo co-processing with tumor cells and common epithelial mesenchymal transition (EMT). As expected, after exosome treatment, OV90 and A2780 cells had enriched E-cadherin levels, as well as suppressed N-cadherin and vimentin levels (Fig. 3D to G). The levels of EMT markers in control cells not exposed to CAF-exo were unchanged. In conclusion, our data emphasized that CAF-released exosomes might contribute to the suppression of the OVCA malignant behaviors.
FIG 3.
Ovarian CAFs-derived exosomes partially inhibited ovarian cancer cell proliferation, migration, invasion and EMT in vitro. (A) The number of colonies of OV90 and A2780 cells were examined separately by colony formation assay after co-incubation with or without CAF-derived exosomes. (B and C) Transwell analysis of OV90 and A2780 cells was performed after co-incubation with or without CAF-derived exosomes to examine their migratory and invasive abilities. (D to F) Expression of EMT-related transcription factors (E-cadherin, N-cadherin and vimentin) was detected by qRT-PCR in OV90 and A2780 cells. (G) Expression levels of EMT marker proteins E-cadherin, N-cadherin and vimentin were detected by performing Western blotting in CAFs-exo stimulated OV90 and A2780 cell lines. *P < 0.05, **P < 0.01.
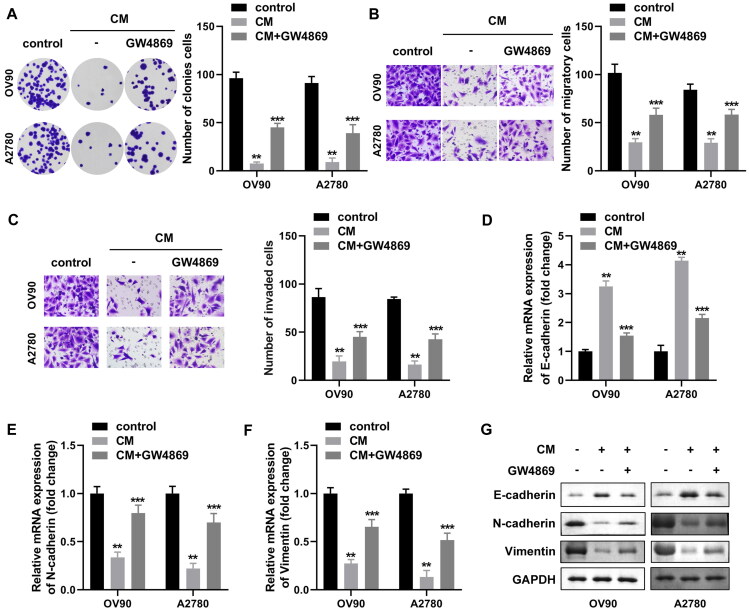
To further validate the effect of exosomes on OVCA tumor cells, CAFs were treated with the exosome inhibitors GW4869 or DMSO prior to co-culture with OV90 and A2780 cells, and further work was produced to assess whether CAFs conditioned medium (CAF-CM) treatment could differ the biological function of OVCA cells. We noticed that the exosome concentration in CAF-CM decreased to one quarter of the original after GW4869 treatment (data not shown). As shown in Fig. 4A, similar to the previous results, CM treatment induced a decrease in colony formation, migration and invasion abilities of OV90 and A2780 cells, and conversely, GW4869 weakened the inhibitory effect of CM on OVCA cells (Fig. 4B and C). In addition, similar results were obtained regarding the expression of mRNA and protein of EMT markers. Specifically, GW4869 rescued N-cadherin and vimentin alleviated by CM treatment at mRNA and protein levels, and attenuated E-cadherin (Fig. 4D to G). The above experimental results confirmed that GW4869 impeded the secretion of exosomes from CAFs, suggesting that CAF-exo inhibited the malignant biological behavior of OVCA depending on its abundance.
FIG 4.
The inhibition of malignant behavior of OVCA cells by CAF-exo depends on its enrichment level. (A to C) The abilities of OVCA cells to form colonies (A), migrate (B) and invade (C) were characterized by colony formation and transwell assays, respectively. (D to F) OV90 and A2780 cells were co-cultured directly with GW4869-treated or untreated CAF-CM, and the expression of E-cadherin (D), N-cadherin (E) and vimentin (F) mRNAs in the cells were assessed by qRT-PCR, respectively. (G) E-cadherin, vimentin and N-cadherin proteins were detected by Western blotting in each experimental group. *P < 0.05, **P < 0.01, ***P < 0.01.
Expression of circIFNGR2 is associated with OVCA
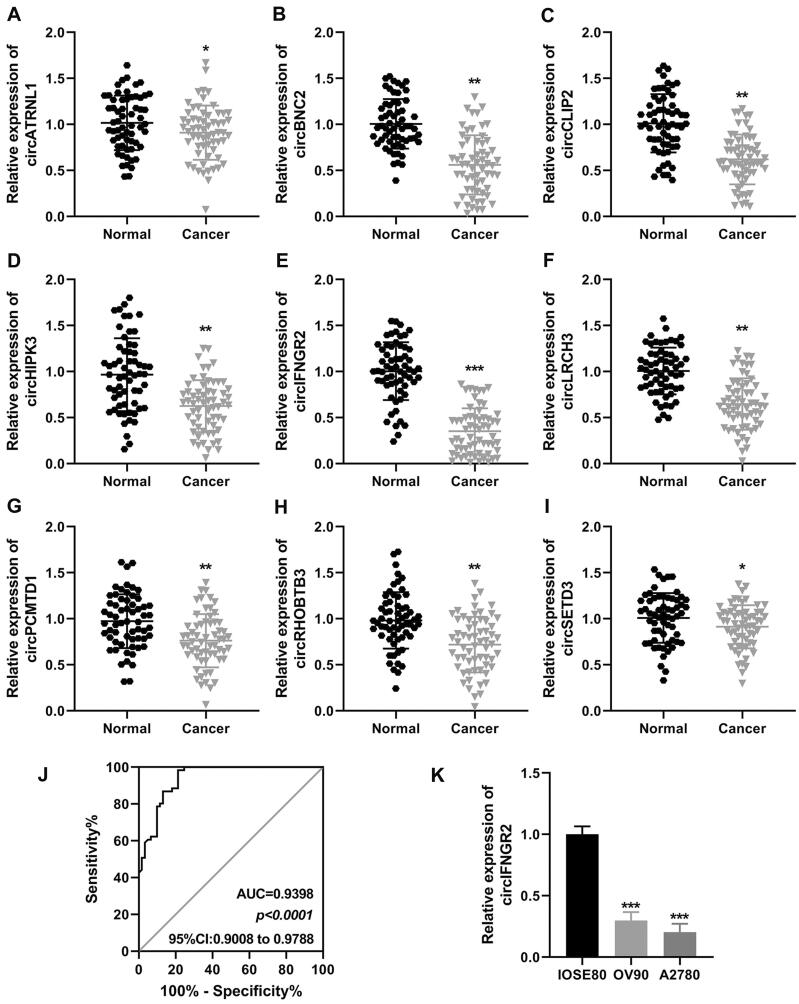
In this study, we pre-screened the literature and identified nine circRNAs that could serve as biomarkers in OVCA, including, circATRNL1, circBNC2, circCLIP2, circCHIPK3, circIFNGR2, circLRCH3, circPCMTD1, circRHOBTB3, circSETD3. Next, the expression of the above genes was detected in the obtained tumor specimens (Fig. 5A to I). We found that circIFNGR2 was significantly reduced in OVCA tumor tissues, and therefore the present study focused on the role of circIFNGR2 in OVCA. Subsequently, we performed receiver operating characteristic (ROC) analysis to explore the diagnostic value of circIFNGR2. The ROC curve of circIFNGR2 showed a significant discriminatory efficiency (AUC = 0.9398, 95%CI: 0.9008–0.9788, P < 0.0001) (Fig. 5J), while showing sufficient sensitivity (100%) in distinguishing ovarian cancer patients from healthy subjects, although the specificity was only 25%. Notably, lower circIFNGR2 was also found in the ovarian cancer cell lines OV90 and A2780 (Fig. 5K). These findings revealed the potential predictive value of circIFNGR2 as an independent risk factor for OVCA.
FIG 5.
circIFNGR2 was identified as a candidate biomarker for OVCA. (A to I) qRT-PCR was used to detect the relative expression of the nine most downregulated circRNAs in OVCA tissues, circATRNL1, circBNC2, circCLIP2, circCHIPK3, circIFNGR2, circLRCH3, circPCMTD1, circRHOBTB3, and circSETD3. (J) ROC curve of circIFNGR2 in OVCA. (K) qRT-PCR was carried out to check the relative expression of circIFNGR2 in two OVCA cell lines. *P < 0.05, **P < 0.01, ***P < 0.001.
Identification of the ring structure and cellular distribution of circIFNGR2 (hsa_circ_0001185)
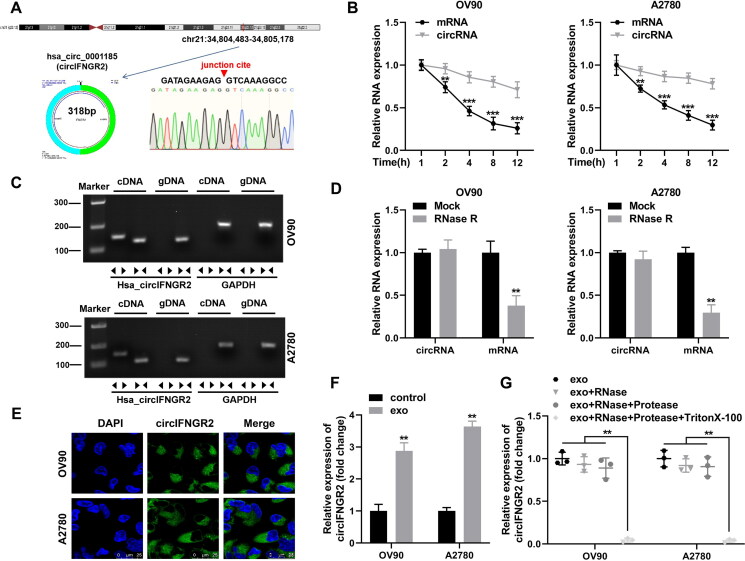
CircIFNGR2 originated from the IFNGR2 gene on chromosome 21q22.12 at chr12: 34,804,483–34,805,178, which was formed by reverse splicing and was 318 bp in spliced length (Fig. 6A). To determine the loop structure of circIFNGR2, we designed four independent experiments. We first amplified the PCR products of circIFNGR2 with different primers, which were subsequently accompanied by Sanger sequencing. As shown in Fig. 6A, the sequencing results confirmed the presence of reverse shear sites for circIFNGR2 and that it was identical to the reverse shear sequence provided by circBank. Next, we further analyzed the stability of circIFNGR2 as a circular RNA molecule after treating OV90 and A2780 cells with actinomycin D, respectively. The data in Fig. 6B presented that circIFNGR2 had a transcriptional half-life of more than 12 h in both cell lines, while the half-life of the related linear molecule was less than 4 h (Fig. 6B). Further, we designed two sets of primers based on the characteristics of circIFNGR2, one for amplifying the circular transcripts (divergent primers) and the other for detecting the linear transcripts (convergent primers). Both sets of primers were amplified via cDNA and genomic DNA (gDNA) from OV90 and A2780 cells as templates, and the resulting products were subsequently accompanied by agarose gel electrophoresis analysis. Conventional convergent primers amplified linear transcripts in both cDNA and gDNA. However, the divergent primers only amplified circular transcripts in cDNA and failed in gDNA (Fig. 6C). Finally, the digestive resistance of circIFNGR2 to RNase R exonuclease proved its closed-loop structure (Fig. 6D). Analysis of the circIFNGR2 distribution in cells using FISH evidenced that circIFNGR2 was expressed in both OV90 and A2780 cell lines, but mainly in the cytoplasm (Fig. 6E). Moreover, we observed the presence of circIFNGR2 in both CAF extracellular medium and CAF exosomes (data not shown), which was subsequently taken up by recipient cells and mediated circIFNGR2 expression. qRT-PCR results verified that circIFNGR2 level was substantially strengthened in CAF-exo treated OVCA cells (Fig. 6F). Combined with the above results, we hypothesized that CAF-exo transferred circIFNGR2 into the cytoplasm of OVCA cells to activate the antitumor effect of circIFNGR2. To verify the above speculation, we performed protease and RNase protection assay. Figure 6G outcomes showed that circIFNGR2 was protected from RNase damage by the exosomes compartment.
FIG 6.
CircIFNGR2 (hsa_circ_0001185) loop structure identification and cellular distribution. (A) Schematic diagram showing that circIFNGR2 originated from the IFNGR2 gene on chromosome 21q22.12, and Sanger sequencing proved the reverse splice junction sites of circIFNGR2. (B) qRT-PCR was applied to examine the transcript levels of linear IFNGR2 and circIFNGR2 in total RNA extracted from OV90 and A2780 cells processed with RNase R or mock, respectively. (C) Agarose gel electrophoresis was used to verify the presence of circIFNGR2 in A2780 and OV90 cells. (D) qRT-PCR was constructed to detect the stability of circIFNGR2 in OV90 and A2780 cells. (E) FISH was used to characterize the localization of circIFNGR2 in cells. (F) Relative expression levels of circIFNGR2 were measured by qRT-PCR in OVCA cells treated with or without exosomes. (G) Expression levels of circIFNGR2 in A2780 and OV90 cells treated with exo, exo + RNase, exo + RNase + Protease, exo + RNase + Protease + Triton X-100, respectively. **P < 0.01, ***P < 0.001.
CircIFNGR2 overexpression contributes to OVCA cell proliferation, migration, invasion and EMT
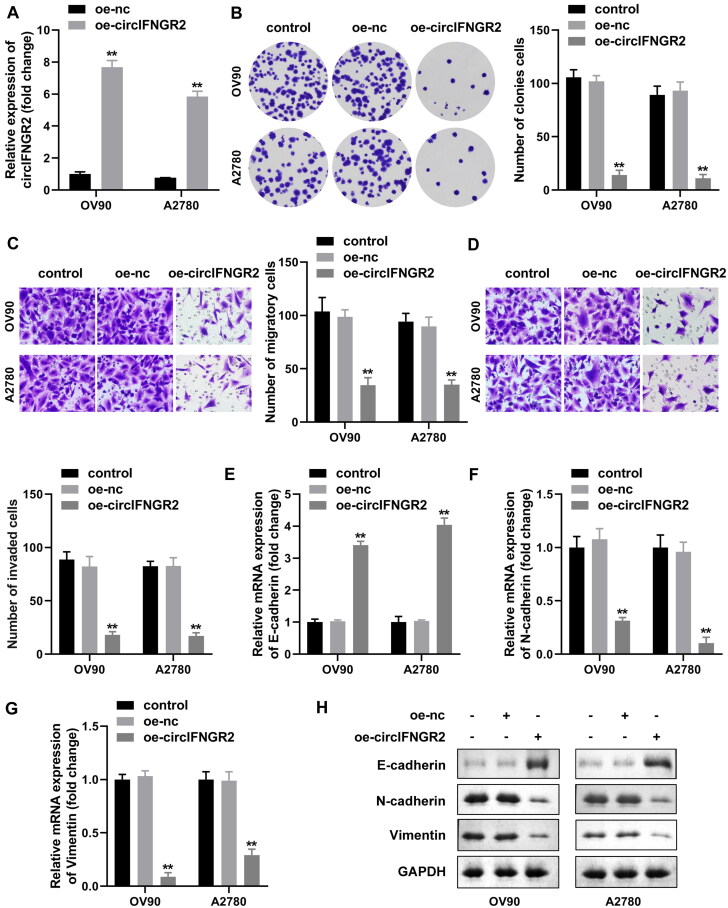
Considering the low levels of circIFNGR2 in OVCA, we attempted to alter the expression of circIFNGR2 in OV90 and A2780 cells to characterize its potential function in the biological behavior of ovarian cancer. As expected, OV90 and A2780 cells exhibited higher expression of circIFNGR2 mRNA after transfection with oe-circIFNGR2 compared with the control, indicating better transfection efficiency (Fig. 7A). Colony formation assays showed that the proliferation capacity of cells was impaired by enhanced circIFNGR2 (Fig. 7B). Furthermore, we explored whether upregulation of circIFNGR2 affected migration and invasion of OV90 and A2780 cells. Transwell results manifested that the athleticism of OV90 and A2780 cells was significantly repressed after introduction of circIFNGR2 (Fig. 7C and D). Higher N-cadherin and vimentin but lower E-cadherin were common indications for EMT. QRT-PCR and Western blotting both presented that cells manifested a substantial decrease in both N-cadherin and vimentin after the introduction of oe-circIFNGR2, while E-cadherin was raised (Fig. 7E to H). According to our results, the enrichment of circIFNGR2 had a resistant effect on the proliferation and metastasis of OVCA cells.
FIG 7.
Excessive circIFNGR2 levels retarded proliferation, migration, invasion and EMT of OVCA cells. (A) qRT-PCR was performed to examine circIFNGR2 levels in OVCA cells transfected with the oe-circIFNGR2 plasmid, compared to oe-NC. (B to D) Colony formation and Transwell assays were applied to calculate the number of colony-forming (B), migrating (C) and invading (D) cells in oe-circIFNGR2-transfected OVCA cells, respectively. (E to G) mRNA expression of E-cadherin (E), N-cadherin (F) and vimentin (G) were assessed by qRT-PCR after transfection with oe-circIFNGR2. (H) E-cadherin, vimentin and N-cadherin proteins were confirmed by Western blotting in each experimental group. **P < 0.01.
CircIFNGR2 inhibits OVCA cell proliferation, invasion, migration and EMT by targeting the miR-378/ST5 axis
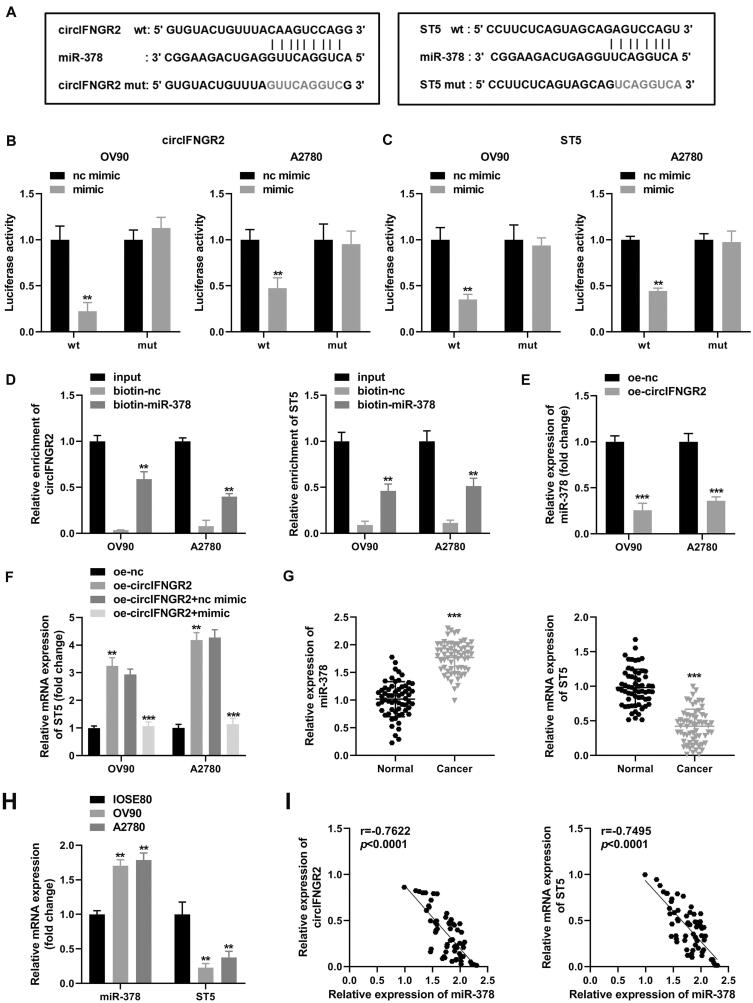
In order to explore the possible molecular mechanism of circIFNGR2 in ovarian cancer, we utilized StarBase (http://starbase.sysu.edu.cn/) database to screen for potential targets of circIFNGR2 and evaluated the possible miRNAs by qRT-PCR. After co-culture with CAF-exo, miR-203, miR-378 and miR-488 presented significant up- or downregulation in OV90 cells compared to the other seven miRNAs (miR-127, miR-188, miR-330, miR-375, miR-574, miR-661, miR-662). Among them, miR-378 was most significantly downregulated. In contrast, only a significant downregulation of miR-378 was observed in A2780 cells (data not shown). Finally, miR-378 with the highest prediction scores was identified as the target of circIFNGR2. Figure 8A displayed the specific binding sites of circIFNGR2 to miR-378. Subsequently, we established a circIFNGR2 fragment (wild-type circIFNGR2 and mutant circIFNGR2) containing the predicted binding sites for miR-378 and introduced it downstream of the luciferase reporter genes. Then, miR-378 mimic and miRNA nc were co-transfected with the reporter genes into OVCA cells. In contrast, miR-378 mimic co-transfected with wild-type circIFNGR2 induced quenching of luciferase activity (Fig. 8B), and the decline rates in the two cells were 80% and 50% of those in the miRNA nc + wild-type circIFNGR2 group. Furthermore, as shown in Fig. 8D, biotin-miR-378 was significantly enriched for circIFNGR2.
FIG 8.
CircIFNGR2 acted as a sponge for miR-378 in OVCA to regulate ST5 expression. (A) Predicted binding sites of miR-378 to wild-type or mutant circIFNGR2 and ST5 sequences. (B and C) Analysis of luciferase activity in OVCA cells co-transfected with miR-378 mimic, miR-NC and wild-type or mutant luciferase reporter vectors. (D) RNA pull-down assays were performed in A2780 and OV90 cells to confirm the direct binding of miR-378 to circIFNGR2 or ST5. (E) Relative expression levels of miR-378 in A2780 and OV90 cells were assessed by qRT-PCR. (F) A2780 and OV90 cells were transfected with oe-NC, oe-circIFNGR2, oe-circIFNGR2+mimic and oe-circIFNGR2+nc mimic, and the expression levels of miR-378 were examined by qRT-PCR. (G) Differential expression of miR-378 and ST5 in ovarian cancer patients and healthy controls was evaluated by qRT-PCR. (H) qRT-PCR was arranged to detect the differential expression of miR-378 and ST5 in OVCA cell lines (A 2780 and OV90) and normal ovarian epithelial cell lines (IOSE80). (I) Correlation between miR-378 and circIFNGR2 or ST5 was analyzed in OVCA tissues by Pearson’s analysis. **P < 0.01, ***P < 0.001 and ***P < 0.01.
The downstream mRNAs of miR-378 were predicted by the TargetScan (http://www.targetscan.org/vert_72/) database. Based on the screening results, ST5 was identified as a preferred target gene for miR-378 (Fig. 8A). The luciferase activity assay revealed that the relative luciferase activity of the reporter gene (wild-type ST5) with special binding sites to miR-378 was diminished (Fig. 8C). RNA pull-down assays also demonstrated that biotin-miR-378 could capture abundant ST5 mRNA (Fig. 8D). These results confirmed that circIFNGR2 could act on the miR-378/ST5 axis via a complementary seed region. More interestingly, qRT-PCR outcomes pointed out that overexpression of endogenous circIFNGR2 directly led to an obvious decrease of miR-378 levels (Fig. 8E) and an indirect upregulation of ST5 mRNA (Fig. 8F). Furthermore, co-transfection of miR-378 mimic reversed the acceleration of oe-circIFNGR2 on ST5 mRNA in OV90 and A2780 cells compared with the corresponding controls (Fig. 8F). Further, CAF-exo treatment alone enriched ST5 levels in OVCA cells (data not shown). Besides, transfection of si-circIFNGR2 into CAFs achieved knockdown of circIFNGR2 in exo (data not shown). By incubation with CAF-exo with low expression of circIFNGR2, the level of circIFNGR2 in recipient cells was remarkably decreased. As circIFNGR2 was inhibited, ST5 expression was reduced in recipient OV90 and A2780 cells, and notably, miR-378 presented an opposite trend (data not shown). These results indicated that CAF-exo treatment not only caused changes in circIFNGR2 expression in ovarian cancer cell lines, but also affected the levels of miR-378 and ST5. Based on the opposite effects of circIFNGR2 and miR-378 on ST5 expression, we proceeded to investigate the expression of miR-378 and ST5 in multiple OVCA tissues and typical cancer cells. Specifically, miR-378 was expressed at higher levels in OVCA tumor tissues and cells (Fig. 8G and H), but ST5 mRNA expression displayed an antithetic trend to miR-378. More interestingly, miR-378 was found to exhibit a negative correlation with circIFNGR2 as well as ST5 in tumor tissues (Fig. 8I). In conclusion, these findings hinted that circIFNGR2 activated transcription of the downstream target gene ST5 via miR-378 in OVCA.
CircIFNGR2 overexpression inhibits the activation of OVCA cells
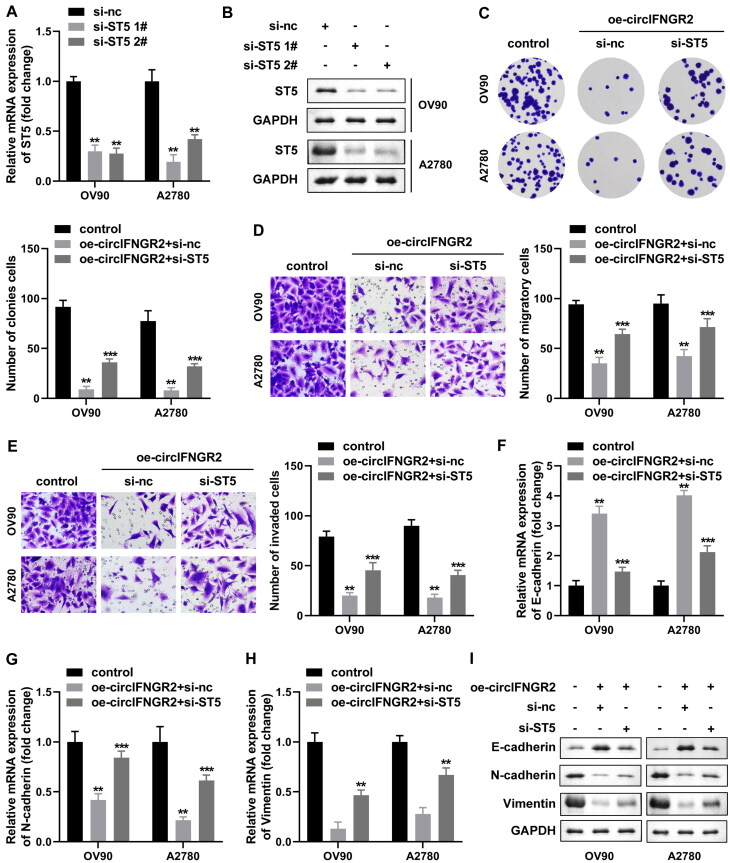
The above findings have shown that circIFNGR2 can indirectly regulate ST5 expression through miR-378. To explore whether circIFNGR2-mediated regulation of ST5 occurs with OVCA cell behaviors change, we designed two small hairpin RNAs targeting ST5 (si-ST5 1# and si-ST5 2#), and then their transfection efficiency was analyzed by qRT-PCR and Western blotting. We observed that two separate sequences successfully reduced ST5 mRNA and protein levels (Fig. 9A and B). To further validate the biological roles of ST5, we designed rescue experiments using oe-circIFNGR2. As indicated by the results of clone formation, transwell assays, si-ST5 partially rescued the inhibitory effect of oe-circIFNGR2 on the proliferation and motility of OV90 and A2780 cells, reactivating the restricted cells (Fig. 9C to E). The expression of key proteins related to the EMT process showed the same trend, si-ST5 transfection followed by N-cadherin and vimentin expression were both substantially elevated, while E-cadherin mRNA and protein levels were sharply reduced (Fig. 9F to I). Above, circIFNGR2 might function in an ST5-dependent manner.
FIG 9.
ST5 low expression impaired the inhibition of OVCA by oe-circIFNGR2 in vitro. (A and B) The levels of ST5 mRNA and protein in OVCA cells transfected with si-ST5 #1 and #2 plasmids were examined by qRT-PCR and Western blotting. (C) Cell colony formation ability was examined by colony formation assay. (D and E) Transwell assay was constructed to assess OVCA cells migration and invasion abilities. (F to H) The mRNA levels of E-cadherin (F), N-cadherin (G) and vimentin (H) were tested by qRT-PCR, respectively. (I) E-cadherin, vimentin and N-cadherin proteins were detected by Western blotting in each experimental group. **P < 0.01 and ***P < 0.01.
Lower expression of circIFNGR2 reverses the anticancer effects induced by CAFs-derived exosomes
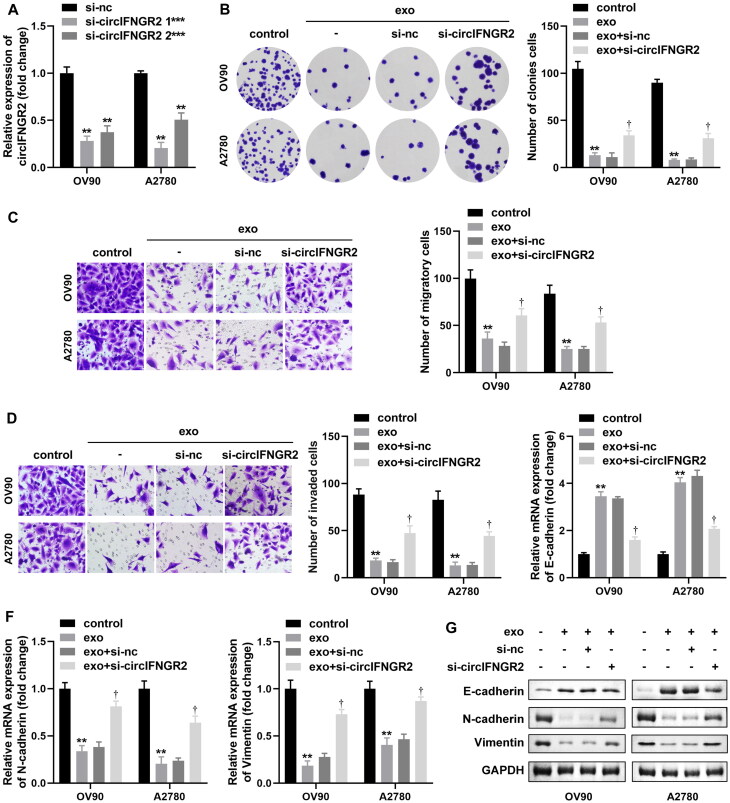
Previous experimental results showed that the level of circIFNGR2 in OVCA cells was closely associated with CAF-exo (Fig. 6F). Therefore, we speculated that circIFNGR2 downregulation could directly rescue the transformations in cell biological behavior caused by CAF-exo treatment. CircIFNGR2 expression was sharply impeded in OV90 and A2780 cells after transfection with si-circIFNGR2 1# or si-circIFNGR2 2# (Fig. 10A). Based on the suppression of OVCA cell viability by CAF-exo, we observed a rescue effect of si-circIFNGR2 transfection on cell proliferation, migration and invasion by implementing clone formation and transwell assays (Fig. 10B to D). After CAF-exo co-incubation with OVCA cells, the EMT process was blocked, while si-circIFNGR2 re-induced the activation of the EMT process. This was manifested by a renewed increase in N-cadherin and vimentin mRNA and protein expression levels and a decrease in E-cadherin mRNA and protein levels (Fig. 10E to G). These results suggested that the effect of CAF-exo on OVCA cells was in connection with the degree of circIFNGR2 enrichment.
FIG 10.
Lower circIFNGR2 partially reversed the inhibition of OVCA by exosomes derived from CAFs in vitro. (A) Relative expression levels of circIFNGR2 in OVCA cells transfected with si-circIFNGR2 #1 and #2 plasmids were examined by qRT-PCR. A2780 and OV90 cells were transfected with si-NC or si-circIFNGR2, and then co-cultured with CAF-exo. (B) Cell colony formation ability was examined by colony formation assay. (C and D) Transwell assay was constructed to assess OVCA cells migration and invasion abilities. (E and F) The mRNAs levels of E-cadherin (E), N-cadherin (F) and vimentin (F) were assessed by qRT-PCR. (G) E-cadherin, vimentin and N-cadherin proteins were verified by Western blotting in each experimental group. Standardized to GAPDH. **P < 0.01, ***P < 0.05 and †P < 0.01.
Lower ST5 affects the anticancer effect induced by exosomes from CAFs
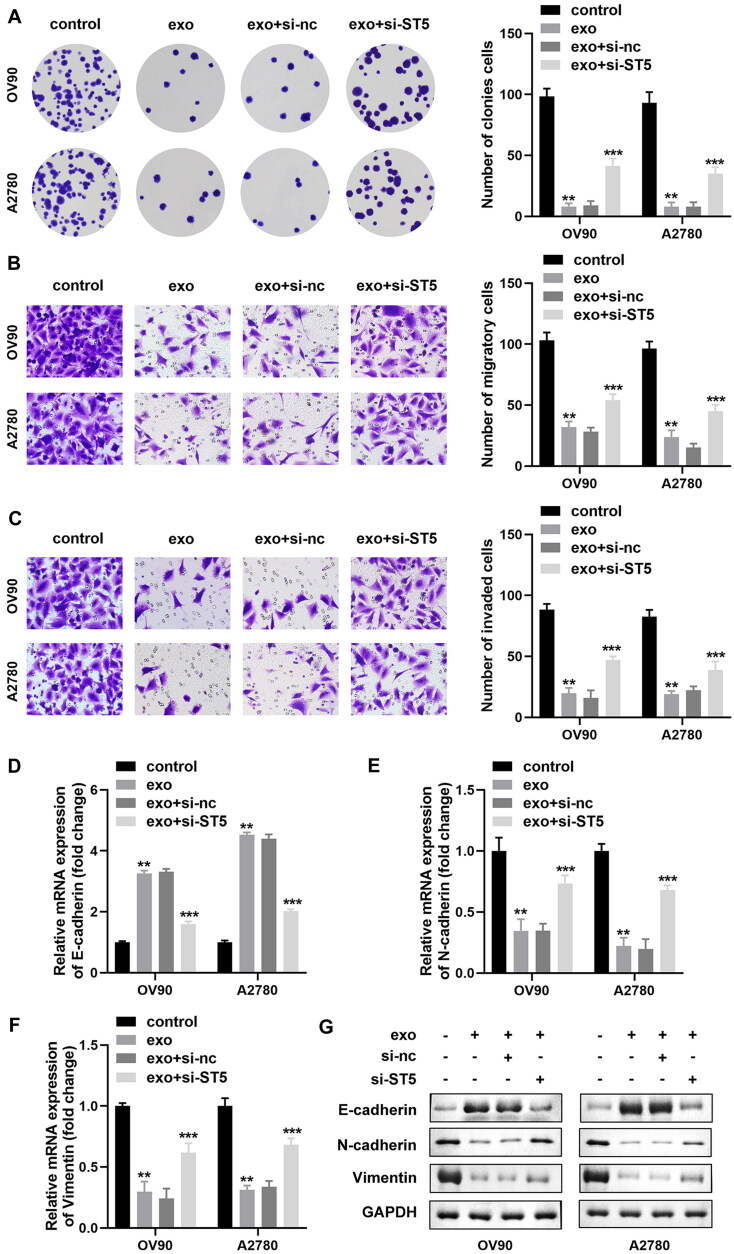
We have shown that circIFNGR2 could be carried into OVCA cells by exosomes from CAFs and that circIFNGR2 acted in an ST5-dependent manner in OVCA cells. Therefore, we predicted that CAF-exo might alter ovarian cancer cell activity in the same manner. Consistent with the previously obtained results, the number of colonies and invasive cells was raised in the exo + si-ST5 group compared to the exo group (Fig. 11A to C). In addition, the mRNA and protein levels of E-cadherin were eliminated in exo-treated OV90 and A2780 cells under si-ST5 induction, while N-cadherin and vimentin showed the opposite trend (Fig. 11D to G). These results implied that exosomes carrying circIFNGR2 inhibited OVCA cell proliferation, migration, invasion and EMT by increasing ST5.
FIG 11.
Low expression of ST5 relieved CAF-derived exosome-mediated inhibition of OVCA cells. (A) The colony formation ability of cells treated with exo was assayed by colony formation assay. (B and C) Transwell assay was managed to evaluate cell migration and invasion abilities. (D to F) E-cadherin (D), N-cadherin € and vimentin (F) mRNA expressions in OVCA cells subjected to the indicated treatments were assessed by qRT-PCR, respectively. (G) E-cadherin, vimentin and N-cadherin protein bands were identified with Western blotting in each experimental group, normalized to GAPDH. **P < 0.01 and ***P < 0.01.
Exosomes of CAFs carrying circIFNGR2 inhibit OVCA tumor growth in vivo
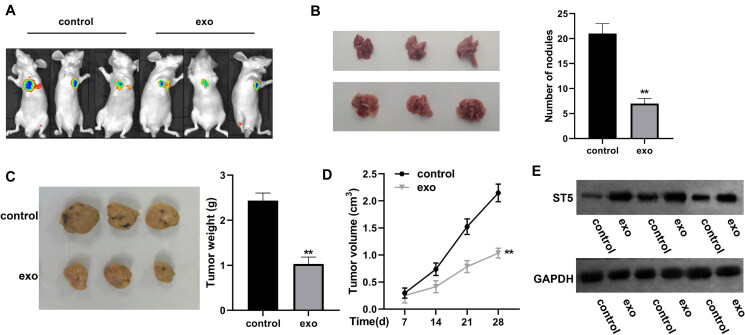
To investigate the protective effect of CAFs-exo circIFNGR2 in vivo, we first injected OV90 cells into the left side of nude mice to construct xenograft tumor model, and later treated with or without CAF-exo (control and exo groups). In combination with bioluminescence imaging to observe the tumor size change, the signal area of the primary tumor of ovarian cancer in the exo treatment group was smaller, as shown in Fig. 12A. Tumor volume and size were measured once per seven days after successful loading. After 28 days, we found that CAFs-exo treatment significantly hindered the increase of the size and number of metastatic tumor nodules, and slowed down the tumor growth (Fig. 12B to D). We also monitored the expression of ST5 in tumor tissues, and the results presented that ST5 level was elevated in CAF-exo treatment group (Fig. 12E). The above experimental results lead us to conclude that CAFs-exo could inhibit tumor progression in vivo. It was further confirmed that CAF-derived exosome-spread circIFNGR2 suppressed OVCA development by sponging miR-378 to upregulate ST5.
FIG 12.
CAF-derived exosomes prolonged OVCA progression in vivo. (A) Representative luminescence images of nude mice subcutaneously injected with OVCA cells in the presence or absence of exosomes. (B) The size (left panel) and number (right panel) of metastatic nodules formed by subcutaneously injected OVCA cells in the presence or absence of exosomes (three per group). (C) Representative images of tumors formed in nude mice (left) and analysis of tumor weights (right) (three per group). (D) Alterations in xenografts tumor volumes of each group (three per group). (E) Evaluation of ST5 level in tumor tissues of mice by Western blot analysis. **P < 0.01.
DISCUSSION
In the authenticity of tumor microenvironment cells, tumor-associated fibroblasts are the main cellular components of TME and play a key regulatory role in obtaining cancer metastasis and recurrence by providing cancer cells with protective ecological niches for tumor-promoting factors.21,22 Furthermore, the symbiotic relationship between tumors and CAFs contributes to the availability of key resources for optimal tumor growth and proliferation, such as growth factors and nutrients.23–25 For the above reasons, CAFs have historically been considered to be tumor-promoting components. However, evidence from numerous studies pointed to the fact that CAFs could either promote or suppress tumors depending on the environment.26 Furthermore, the inhibition of cancer progression by CAFs relied on the production of certain tumor suppressor signals that acted as a barrier to tumor cell spread and metastasis.27 It is worth noting that ncRNA-signaling molecules exert an important role in mediating CAF function changes.28 Importantly, the ncRNAs-laden exosomes released by CAFs were taken up by nearby or distant cells, further regulating tumor development. Although the precise physiological roles of ncRNAs remain unknown, previous studies have revealed that exosomes functioned eventful roles in the “crosstalk” procedure between CAFs and tumor cells.29 Most of the studies that have emerged support a tumor-promoting role of CAF-exo, for example, CAFs release exosomes containing nucleic acid information into colorectal or endometrial cancer TME as key contributor to tumor cell growth and migration.30,31 In the present study, we observed that exosomes exhaled by CAFs were enriched in OVCA cells, suggesting that tumorigenesis was associated with the transfer of CAF-exo. It should be noted that the exosomes secreted by CAF do not always lead to the malignant phenotype of cancer cells. For example, CAF-derived exosomes inhibited the growth and metastasis of cholangiocarcinoma tumors.32 The expression of estrogen receptor (Er) in tumor cells was restricted after Er-positive breast cancer cells were treated with CAF conditioned medium, which was related to the release of CAF-exo.33 In accordance with this, our experimental results showed that CAF-derived exosomes transferred between CAFs and OVCA cells and exerted a depleting effect on the malignant process of tumor cells. When the exosomes produced by CAFs were depleted using the exosome secretion inhibitor GW4869, the motility and invasiveness of OVCA cells co-treated with CAF-CM were significantly enhanced, further supporting our results.
Uncontrolled circRNA expression is associated with rapid tumor metastasis and malignant lesions. To date, the complex regulatory network involved in circRNAs in OVCA has also become a major obstacle in the treatment of this aggressive disease and certainly is a popular area of research.34,35 As a part of ncRNAs, circRNAs can be loaded into exosomes and endocytosed into recipient cells in a stable manner to play a post-transcriptional regulatory role. In most cases, the regulation of recipient cells by exosomal circRNAs was beneficial for cancer progression. In gastric cancer (GC), functional circ_0088300 was encapsulated inside exosomes from CAFs and then endocytosed into GC tumor cells, promoting the proliferation, migration and invasive abilities of GC cells.17 In liver cancer, circZFR enriched in CAF-exo induced tumor chemotherapy resistance and boosted tumor growth after being encapsulated by tumor cells.36 In colorectal cancer, the promotion of cancer cell stemness and oxaliplatin resistance by CAF-exo was attributed to the exosomal cricN4BP2L2 it carried.37 Comparatively, current studies on CAFs-exo in OVCA have focused on miRNA. Functional studies have shown that miR-21 could be transferred from CAF-derived exosomes to OVCA cells, inhibit cancer cell apoptosis and increase paclitaxel resistance by targeting APAF1.38 It is noteworthy that the effects of circRNAs from CAFs exosomes on the functional and pathological mechanisms of OVCA cells are largely unaddressed. In the manuscript, we identified a novel circRNA, circIFNGR2, which served as an oncogenic suppressor in OVCA tissue. Importantly, circIFNGR2 also showed sensitivity in the diagnosis of OVCA. A series of procedures confirmed that exosomes derived from ovarian cancer CAFs could transfer circIFNGR2 into the interior of tumor cells and participate in tumor progression.
In addition, ovarian cancer contains multiple bioinformatic molecules, and the sensitivity and specificity of using one substance as biomarker are mostly unsatisfactory. Perhaps better results will be achieved by using multiple molecules as biomarkers. The ceRNA hypothesis proposed that circRNA and mRNA shared a common miRNA response element, and the circRNA-miRNA-mRNA interactive network assembled all co-expressed competition triplets.39 We used bioinformatics and dual luciferase reporter assays to identify miR-378 as a target gene regulated by exosomal circIFNGR2. The decrease of circIFNGR2 level accompanied by the acceleration of miR-378 implied that miR-378 might be one of the important events in circIFNGR2-mediated cancer progression. And the differential expression of miR-378 in stage III ovarian cancer has also been reported.40 In addition, this study also confirmed that ST5 was a downstream target binding gene of miR-378. ST5, also known as DENND2B, is a component of DENN domain protein.41 Current studies on ST5 have focused on its oncogenic properties. For example, ST5 activated the migratory and invasive phenotypes of epithelial cancer cells in the appearance of epidermal growth factor.42 We found that ST5 was regulated by CAF-derived exosomes and acted as a cancer suppressor to inhibit the malignant progression of OVCA cells. An ordinary phenomenon of EMT is the loss expression of epithelial markers (E-calmodulin) accompanied by enrichment of mesenchymal markers (N-Cadherin, vimentin and Snail). In the present study, on the one hand, the enrichment of exosomal circIFNGR2 impaired the ability of E-cadherin downregulation and N-cadherin and vimentin upregulation. On the other hand, inhibition of ST5 expression re-induced downregulation of E-cadherin as well as upregulation of N-cadherin and vimentin. Thus, in the context of OVCA, exosomal circIFNGR2 regulated tumor cells progression in an ST5-dependent manner. It further supported the role of CAFs-derived exosomal circIFNGR2 in regulating the malignant transformation of OVCA by virtue of the 378miR-/ST5 axis.
Overall, we illustrated that exosomes released from CAFs had a significantly debilitating effect on OVCA cell activity. The downregulation of circIFNGR2 was found for the first time in OVCA tissues and cells, and circIFNGR2 levels were closely associated with CAFs-exo. Exosomal circIFNGR2 targeting the miR-378/ST5 axis in tumor cells played a tumor suppressor role in the development of OVCA, which provides good data to support our insight into the mechanism of circRNA as therapeutic agent in OVCA progression.
MATERIALS AND METHODS
Clinical tissue samples
Fresh tumor tissue samples were collected from a total of 61 OVCA patients who underwent surgery at our institution, and all patients signed an informed consent form before surgery. Normal ovarian tissue was obtained from a total of 61 patients who underwent oophorectomy at the same time and had non-oncologic disease. The collection and application of human tissues for the study was licensed and authorized by Yancheng First People's Hospital Ethics Committee (No. 2022-K-026), in accordance with the Declaration of Helsinki.
Cell culture
Two human OVCA cell lines (A2780, OV90) with different histological backgrounds and human normal ovarian epithelial cells (IOSE80) selected for this study were acquired from the American Type Culture Collection (ATCC, USA). Under an atmosphere of 5% CO2 at 37 °C, OV90 cells were cultured in RPMI 1640 medium (Gibco, USA), A2780 and IOSE80 cells were grown in DMEM medium. All media were supplemented with 10% FBS (Gibco, USA) and 1% penicillin/streptomycin (Sigma, USA).
Isolation and culture of primary cells
In this study, tumor samples from five ovarian cancer patients were applied to isolate CAFs, and normal fibroblasts (NFs) were isolated from normal ovary tissues. These patients were at the same tumor stage. Under aseptic conditions, the collected tissues were repeatedly rinsed with saline, and after removing blood clots, peritumor adipose tissue and necrotic tissue, the tumor tissues were evenly cut into uniformly sized pieces of approximately 1 mm3. Processed human tissues were transferred to PBS solution containing antibiotics, washed three times, and added to serum-free DMEM supplemented with 0.1 mg/mL collagenase, 0.1 μL/mL DNAase and 0.1 mg/mL hyaluronidase (all from Sigma-Aldrich, Merck KGaA) followed by digestion at 37 °C. Digestion was terminated after 4 h by adding serum-containing medium. After filtration, the cells were stored in erythrocyte lysate for 8 min, washed in PBS and then transferred to DMEM/DF12 (1:1) (Gibco, USA) complete medium. Anti-fibroblast micro beads and MiniMACS separator were utilized for sorting fibroblasts. To ensure that there was no contamination from other cell types, we used flow cytometry to demonstrate >99% positivity for the fibroblast marker α-SMA, suggesting cancer-associated fibroblasts characteristics. The morphology of sorted CAFs and NFs was observed under an inverted microscope. The expression of CAFs markers α-smooth muscle actin (α-SMA), fibroblast activation protein (FAP) and vimentin were identified by immunofluorescence staining and Western blotting.
Isolation and identification of exosomes from conditioned medium
The exosomes stored in supernatant of CAFs (conditioned medium, CAF-CM) were precipitated by differential ultracentrifugation. Briefly, cells were stored in culture supplemented with 10% exosome-depleted FBS (exo-FBS, SBI) for 24 h, and then cell supernatants were detached and centrifuged at 300 × g for 10 min, 2000 × g for 10 min, and 10,000 × g for 70 min. 0.22 μm PVDF filter (Millipore, USA) was performed to eliminate cells, cell debris, dislodged vesicles, and other larger-sized vesicles. The supernatant was then continued to be collected and centrifuged at 100,000 × g for another 70 min to harvest the exo precipitate. All isolation steps were executed at 4 °C. Finally, the exosomes were resuspended in PBS for immediate use or stored at –80 °C. Exosome morphology was observed using transmission electron microscopy (TEM), and the exosomal markers CD63, CD81, and TSG101 were identified by Western blot analysis.
Characterization and uptake of exosomes
The exosome morphology was traced using TEM. The exosome precipitate harvested from the cell supernatant was resuspended with 100 μL PBS and fixed with 4% paraformaldehyde. Ten microliters of exosome suspension were dropped onto a copper grid, left for 5 min, and excess liquid was attentively aspirated. Subsequently, the specimens were stained for 2–3 min with 2% uranyl acetate and dried at room temperature for 30 min. Finally, images were operated at 80 KV in a JEM-1200 EX microscope (JEOL Ltd, Japan).
The number and size of particles in the measured specimens were directly characterized at 405 nm using ZetaView PMX110 instrument and the matching nanoparticle tracking analysis (NTA) software (ZetaView 8.02.28). The samples were diluted with water and the particle concentration was controlled in the range of 1 × 107/mL and 1 × 109/mL. The images were captured at a rate of 30 pictures/second with an acquisition time of 1 min, and the experimental data were analyzed according to the obtained complete tracks, calculated as: number of exosomes = dilution concentration × measurement volume × dilution times.
To monitor the endocytosis mechanism of exosomes, the interaction between CAF-exo and OVCA cells was observed using fluorescent PKH67 (Sigma-Aldrich, Merck KGaA) labeled exosomes. For cell processing, 2 × 105 recipient cells were maintained with PKH67-labeled exosomes for 48 h, after which cell images were captured using confocal laser scanning microscopy (Nikon Eclipse Ti).
For exosomes secretion inhibition, we pretreated CAFs with or without the exosome production inhibitor GW4869 obtained from APExBIO (Shanghai, China) for 24 h. CAF-CM were harvested and continued to treat OV90 and A2780 cells for 48 h (CM + GW4869 group). Cells treated with DMSO or serum-free medium served as the control group.
Proteinase and RNase treatment
To assess whether circRNA was located inside or outside the exosomes, the obtained exosomes were first processed with proteinase K (1 μg/mL, Promega) at 37 °C for 10 min or RNase A (5 μg/mL, Qiagen) at room temperature for 30 min in the presence or absence of 0.1% Triton X-100 (Sigma, USA). Protease inhibitor was applied to the proteinase K treatment group to inactivate the protease, followed by the immediate addition of RNase A. Then TRIzol reagent was immediately added for RNA extraction and analyzed using qRT-PCR.
Validation of circular structure and head-to-tail splicing of circRNA
To prove the cyclic structure of circIFNGR2, agarose gel electrophoresis was performed to separate the cyclic and linear transcripts of IFNGR2 from the qPCR products after application of convergent and divergent primers together with cDNA and genomic DNA (gDNA) from OVCA cells. Theoretically, the loop transcripts of IFNGR2 can only be amplified by different primers in cDNA, but not in gDNA. In addition, Sanger sequencing was done by Guangzhou Jisai Technology Co., Ltd to verify the reverse splicing sites of circIFNGR2. Two independent experiments were designed to analyze the stability of circIFNGR2. One, total RNA (1 μg) extracted from the OVCA cell line was added with RNase R (3 U/μg, Epicenter, Madison, WI, USA) in a water bath at 37 °C for 30 min, followed by qRT-PCR to quantify the relative expression of circIFNGR2 and IFNGR2 mRNA. Second, OV90 and A2780 cells were grown in six-well plates (5 × 105 cells/well). Cells were exposed to actinomycin D (2μg/mL, Sigma) to degrade linear mRNA, and then collected at the indicated time points (1,4, 8, 12 h). RNA was extracted from the cells and analyzed for circRNA stability using qRT-PCR.
RNA fluorescence in situ hybridization (FISH)
For FISH experiments, firstly, OVCA cell-covered slides were fixed with FISH fixative for 10 min at room temperature. Prior to prehybridization, cells were permeabilized with PBS containing 0.2% Triton X-100 and then maintained in hybridization buffer for 1 h at 37 °C. Then, cells and fluorescently labeled circIFNGR2 (Servicebio Biotechnology, China) specific probes were placed in a wet box and kept overnight at 4 °C. The next day, cells were washed with 2× sodium citrate buffer and re-stained with DAPI solution (Sigma, USA) for 30 min. Finally, images were captured with a fluorescence microscope (Nikon, Japan).
Cell transfection
Cell transfection was performed and done using Lipofectamine 3000 (Invitrogen, USA) and in A2780 and OV90 cells. To alter the expression of circIFNGR2, miR-378, and ST5, small hairpin RNAs targeting the ligation sites of circIFNGR2 (si-circIFNGR2) and its negative control (si-NC), miR-378 mimics/inhibitors and its negative control miR-NC, siRNAs for ST5 and negative control si-NC were obtained from Anhui General Biological Company (China). The circIFNGR2 sequence was cloned into pCD5-ciR used to generate the OE plasmid for circIFNGR2, labeled as oe-circIFNGR2. Twenty-four hours to transfection, cells were implemented into six-well plates referring to the manufacturer’s instructions, and gathered after 24 h of transfection for qRT-PCR and Western blot with the above oligonucleotides or vectors.
Cell proliferation capacity detection
For colony formation assays, the transfected cells in each group were re-inoculated in six-well plates (0.5 × 103 cells/well) and maintained in RPMI 1640 medium containing 10% FBS for 14 days. Whereafter, cells were fixed and stained with anhydrous methanol and 1% crystalline violet solution (Sigma). After rinsing clean, digital images were captured under a microscope (Olympus Inc.), and visible colonies were counted (> 0.05 mm in diameter).
Transwell assay
The abilities of circIFNGR2, miR-378, ST5 on cell migration and invasion were specified by diffusive transwell assays. For migration assay, trypsin-digested OVCA cells were first re-suspended in serum-free medium, and the cell concentration was restricted to 2 × 105 cells/mL. The 200 μL cell suspension was incorporated within the upper chamber of the transwell (Costar, Cambridge, Massachusetts, USA). And 600 μL RPMI1640 medium containing 15% FBS was added back to the bottom counterpart. After 24 h incubation, the non-migrated cells in the top chamber were removed, and the migrated cells were fixed with 4% paraformaldehyde at room temperature for 10 min, stained with 0.1% crystalline violet (Sigma-Aldrich) for 5 min, and washed twice with PBS. Finally, five random fields were acquired to observe and count under a microscope (Olympus). For the quantitative analysis of invasion, OVCA cells were subjected to similar procedures in transwell chamber pretreated with 50 µL Matrigel (BD Biosciences).
Dual luciferase reporter assay
The wild-type (WT) sequences of circIFNGR2 and ST5 3'-UTR containing the putative miR-378 binding sites were cloned into the p-mir-GLO luciferase vector (Promega, Madison, WI, USA) for generating wild-type luciferase reporter vectors (WT- circIFNGR2 and WT-ST5), and the corresponding mutants (MUT- circIFNGR2 and MUT-ST5) were constructed. OVCA cells were transfected with the above luciferase reporter vectors in 96-well plates, together with miR-378 mimics or miR-NC. Forty-eight hours later, the corresponding luciferase activity was determined using a dual luciferase reporter gene assay system (Promega, USA).
Biotin miRNA pull-down assay
The sequences in miR-378 that have binding sites to circIFNGR2, ST5 and the 3'end of miR-NC were severally biotinylated into biotin-miR-378 and biotin-NC (Thermo Fisher, USA), and then transfected into cells. Forty-eight hours after transfection, OV90 and A2780 cells were collected and lysed. Cell extracts were treated with Dynabeads M-280 streptavidin beads (Thermo Scientific) for another 2 h, followed by eluting with biotin elution buffer. Finally, the biotin-coupled RNA complexes were pulled down, and the bound RNA on beads was purified by TRIzol and quantified by qRT-PCR.
Western blot assay
Cells were collected and lysed in lysis buffer (Beyotime) supplemented with 1% protease inhibitor (Roche, USA) and 1% phosphatase inhibitor followed by total protein extraction procedures. Protein concentrations were quantified using the BCA Protein Assay Kit (Pierce, USA). Then, 10–20 µg of denatured protein extracts samples were separated by 10–12% SDS-PAGE and moved to PVDF (Millipore, USA) membranes. The membranes were blocked with 5% fat-free milk for 2 h, followed by mixing with the specific primary antibodies against ST5 (1:500, Abcam), E-cadherin (1:1000, Proteintech), vimentin (1:2000, Cell Signaling Technology), N-cadherin (1:200, Abcam) Abcam), and GADPH (1:2000, Kangchen, China) overnight at 4 °C. Thereafter, incubation of the corresponding secondary antibody was conducted for a further 1.5 h at room temperature. At last, an ECL reagent was employed for proteins visualization (Millipore, USA).
RNA extraction and qRT-PCR
Firstly, total RNA was selectively detached from OVCA tissues and cells with the help of TRIzol (Invitrogen, USA) kits, referring to the instruction. The targeting sequences (circIFNGR2, miR-378, ST5, E-cadherin, N-cadherin, vimentin) were reverse transcribed into cDNA using reverse transcription kits (PrimeScript™ RT kit, Takara; mirVanaTM qRT-PCR miRNA assay kit, Ambion). qRT-PCR was carried out with BeyoFast™ SYBR green qPCR mix (Takara) with specific primers to amplify and detect the expression levels of target mRNA and miRNA on an ABI 7500 PCR system (Applied Biosystems). U6 (for miR-378) and GAPDH (for circIFNGR2 and ST5) acted as internal controls. The relative expression levels of the target genes were calculated by 2–ΔΔCt methods. The primer sequences contained:
circIFNGR2, forward: 5′-CAGGAGCCTGTTTCTTCCTG-3′,
reverse: 5′-AAGCAGTTGTGCCTGGACTT-3′;
miR-378, forward: 5′-TGAGCCCTGTCCTCCCGCAG-3′,
reverse: 5′-CCAGTGCAGGGTCCGAGGT-3′;
ST5, forward: 5′-TCTCCCACTGAGAATGGTACTG-3′,
reverse: 5′-CGGGCTGTTGTACTTCCTGT-3′;
E-cadherin, forward: 5′-CGAGAGCTACACACGTTCACGG-3′,
reverse: 5′-GGGTGTCGAGGGAAAAATAGG-3′;
N-cadherin, forward: 5′-TCAGGCGTCTGTAGAGGCTT-3′,
reverse: 5′-ATGCACATCCTTCGATAAGACTG-3′;
vimentin, forward: 5′-GACGCCATCAACACCGAGTT-3′,
reverse: 5′-CTTTGTCGTTGGTTAGCTGGT-3′;
GAPDH, forward: 5′-GGAGCGAGATCCCTCCAAAAT-3′,
reverse: 5′-GGCTGTTGTCATACTTCTCATGG-3′;
U6, forward: 5′-CTCGCTTCGGCAGCACA-3′,
reverse: 5′-AACGCTTCACGAATTTGCGT-3′.
Animal research
All animal experiments were approved by the Yancheng First People's Hospital Ethics Committee. Female C57Bl/6 nude mice, approximately 6–8 weeks old, weighing 18–20 g, were obtained from Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China). OVCA cells transfected with pLenti-luciferase virus were re-suspended in PBS. Cells in 100 μL PBS were then subcutaneously inoculated into C57Bl/6 mice at a viable density reach to 1 × 106 cells/mL. At day 7 after OVCA cell inoculation, when tumors were palpable, the tumor-bearing mice were randomized into two treatment groups of three mice each for follow-up studies. Treatment groups: control group (PBS treatment) and CAF-exo group (exo treatment). Thirty micrograms of CAF-exo or equivalent amount of PBS were introduced into the mice by intravenous injection every 48 h for 28 days. Mice were monitored once per seven days by measuring two dimensions, width and length using an electronic caliper, estimated as: 0.5 × (length × width2). Tumor site, size and metastasis were characterized by bioluminescence imaging system. After 28 days, the animals were sacrificed, and the tumors were excised, weighed and photographed.
Statistical analysis
The used experiments were performed three times independently and the data obtained are expressed as mean ± SD. Statistical differences between the two or multiple groups were determined using Student's t test or one-way ANOVA. The statistical analyses of gene data were carried out via SPSS 22.0A statistical software (IBM Corporation, Armonk, NY, USA) and graphed in Prism software (GraphPad, Version 8.1.1, CA, USA). P value of less than 0.05 was considered as the criterion for statistical significance.
ETHICS APPROVAL
This study was approved by the Yancheng First People's Hospital Ethics Committee (No.2022-K-026).
CONSENT FORM
All informed consent was obtained from the subjects.
AUTHOR CONTRIBUTIONS
X. C. conceptualization, writing-original draft; X. R. data curation, investigation, validation; J. E. data curation, formal analysis; Y. Z. methodology, data curation, formal analysis; R. B. methodology, supervision, writing-review and editing. All authors read and approved the final version of the manuscript.
DISCLOSURE STATEMENT
The authors declare that they have no conflicts of interest to report regarding the present study.
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F.. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Yamada N, Kuranaga Y, Kumazaki M, Shinohara H, Taniguchi K, Akao Y.. Colorectal cancer cell-derived extracellular vesicles induce phenotypic alteration of T cells into tumor-growth supporting cells with transforming growth factor-β1-mediated suppression. Oncotarget. 2016;7:27033–27043. doi: 10.18632/oncotarget.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bejarano L, Jordāo MJC, Joyce JA.. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021;11:933–959. doi: 10.1158/2159-8290.Cd-20-1808. [DOI] [PubMed] [Google Scholar]
- 4.Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L.. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019;30:36–50. doi: 10.1016/j.cmet.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, Hodi FS, Martín-Algarra S, Mandal R, Sharfman WH, et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell. 2017;171:934–949.e916. doi: 10.1016/j.cell.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klemm F, Joyce JA.. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storti G, Scioli MG, Kim BS, Terriaca S, Fiorelli E, Orlandi A, Cervelli V.. Mesenchymal stem cells in adipose tissue and extracellular vesicles in ovarian cancer patients: a bridge toward metastatic diffusion or a new therapeutic opportunity? Cells. 2021;10:2117. doi: 10.3390/cells10082117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Théry C, Zitvogel L, Amigorena S.. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Deng T, Ge S, Liu Y, Bai M, Zhu K, Fan Q, Li J, Ning T, Tian F, et al. Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7. Oncogene. 2019;38:2844–2859. doi: 10.1038/s41388-018-0619-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Zhang H, Deng T, Liu R, Bai M, Zhou L, Wang X, Li S, Wang X, Yang H, Li J, et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat Commun. 2017;8:15016. doi: 10.1038/ncomms15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrmann IK, Wood MJA, Fuhrmann G.. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 12.Zhong Y, Du Y, Yang X, Mo Y, Fan C, Xiong F, Ren D, Ye X, Li C, et al. Circular RNAs function as ceRNAs to regulate and control human cancer progression. Mol Cancer. 2018;17:79. doi: 10.1186/s12943-018-0827-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holdt LM, Kohlmaier A, Teupser D.. Molecular roles and function of circular RNAs in eukaryotic cells. Cell Mol Life Sci. 2018;75:1071–1098. doi: 10.1007/s00018-017-2688-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J.. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 15.Shang Q, Yang Z, Jia R, Ge S.. The novel roles of circRNAs in human cancer. Mol Cancer. 2019;18:6. doi: 10.1186/s12943-018-0934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Liu J, Ma J, Sun T, Zhou Q, Wang W, Wang G, Wu P, Wang H, Jiang L, et al. Exosomal circRNAs: biogenesis, effect and application in human diseases. Mol Cancer. 2019;18:116. doi: 10.1186/s12943-019-1041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi H, Huang S, Qin M, Xue X, Guo X, Jiang L, Hong H, Fang J, Gao L.. Exosomal circ_0088300 derived from cancer-associated fibroblasts acts as a miR-1305 sponge and promotes gastric carcinoma cell tumorigenesis. Front Cell Dev Biol. 2021;9:676319. doi: 10.3389/fcell.2021.676319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, Zhang B, Meng Q, Yu X, Shi S.. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20:131. doi: 10.1186/s12943-021-01428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong H, Huang Z, Yang Z, Lin Q, Yang B, Fang X, Liu B, Chen H, Kong J.. Recent Progress in Detection and Profiling of Cancer Cell-Derived Exosomes. Small. 2021;17:e2007971. doi: 10.1002/smll.202007971. [DOI] [PubMed] [Google Scholar]
- 20.Pascual-Antón L, Cardeñes B, Sainz de la Cuesta R, González-Cortijo L, López-Cabrera M, Cabañas C, Sandoval P.. Mesothelial-to-mesenchymal transition and exosomes in peritoneal metastasis of ovarian cancer. Int J Mol Sci. 2021;22:11496. doi: 10.3390/ijms222111496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erdogan B, Webb DJ.. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem Soc Trans. 2017;45:229–236. doi: 10.1042/bst20160387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D, Zhang Q, Lin D, Ge S, Bai M, et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer. 2020;19:43. doi: 10.1186/s12943-020-01168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H.. Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers. 2015;7:2443–2458. doi: 10.3390/cancers7040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jena BC, Das CK, Bharadwaj D, Mandal M.. Cancer associated fibroblast mediated chemoresistance: a paradigm shift in understanding the mechanism of tumor progression. Biochim Biophys Acta Rev Cancer. 2020;1874:188416. doi: 10.1016/j.bbcan.2020.188416. [DOI] [PubMed] [Google Scholar]
- 25.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, McAndrews KM, Kalluri R.. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 2021;18:792–804. doi: 10.1038/s41571-021-00546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biffi G, Tuveson DA.. Diversity and biology of cancer-associated fibroblasts. Physiol Rev. 2021;101:147–176. doi: 10.1152/physrev.00048.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang Z, Xu J, Zhang B, Wang W, Liu J, Liang C, Hua J, Meng Q, Yu X, Shi S.. The promising role of noncoding RNAs in cancer-associated fibroblasts: an overview of current status and future perspectives. J Hematol Oncol. 2020;13:154. doi: 10.1186/s13045-020-00988-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pascut D, Pratama MY, Vo NVT, Masadah R, Tiribelli C.. The crosstalk between tumor cells and the microenvironment in hepatocellular carcinoma: the role of exosomal micrornas and their clinical implications. Cancers. 2020;12:823. doi: 10.3390/cancers12040823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS, Yan YR, Song FY, Wang FF, Zhu XH, Liao WJ, et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol Cancer. 2019;18:91. doi: 10.1186/s12943-019-1019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li BL, Lu W, Qu JJ, Ye L, Du GQ, Wan XP.. Loss of exosomal miR-148b from cancer-associated fibroblasts promotes endometrial cancer cell invasion and cancer metastasis. J Cell Physiol. 2019;234:2943–2953. doi: 10.1002/jcp.27111. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Piontek K, Ishida M, Fausther M, Dranoff JA, Fu R, Mezey E, Gould SJ, Fordjour FK, Meltzer SJ, et al. Extracellular vesicles carry microRNA-195 to intrahepatic cholangiocarcinoma and improve survival in a rat model. Hepatology. 2017;65:501–514. doi: 10.1002/hep.28735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah SH, Miller P, Garcia-Contreras M, Ao Z, Machlin L, Issa E, El-Ashry D.. Hierarchical paracrine interaction of breast cancer associated fibroblasts with cancer cells via hMAPK-microRNAs to drive ER-negative breast cancer phenotype. Cancer Biol Ther. 2015;16:1671–81. doi: 10.1080/15384047.2015.1071742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao Y, Xie X, Li M, Gao Y.. CircHIPK2 contributes to DDP resistance and malignant behaviors of DDP-resistant ovarian cancer cells both in vitro and in vivo through circHIPK2/miR-338-3p/CHTOP ceRNA pathway. Onco Targets Ther. 2021;14:3151–3165. doi: 10.2147/ott.S291823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X, Mei J, Wang H, Gu D, Ding J, Liu C.. The emerging roles of circular RNAs in ovarian cancer. Cancer Cell Int. 2020;20:265. doi: 10.1186/s12935-020-01367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y, Tang W, Zhuo H, Zhu D, Rong D, Sun J, Song J.. Cancer-associated fibroblast exosomes promote chemoresistance to cisplatin in hepatocellular carcinoma through circZFR targeting signal transducers and activators of transcription (STAT3)/nuclear factor -kappa B (NF-κB) pathway. Bioengineered. 2022;13:4786–4797. doi: 10.1080/21655979.2022.2032972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qu Z, Yang KD, Luo BH, Zhang F.. CAFs-secreted exosomal cricN4BP2L2 promoted colorectal cancer stemness and chemoresistance by interacting with EIF4A3. Exp Cell Res. 2022;418:113266. doi: 10.1016/j.yexcr.2022.113266. [DOI] [PubMed] [Google Scholar]
- 38.Au Yeung CL, Co NN, Tsuruga T, Yeung TL, Kwan SY, Leung CS, Li Y, Lu ES, Kwan K, Wong KK, et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7:11150. doi: 10.1038/ncomms11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uhan S, Hauptman N.. Metastatic EMT phenotype is governed by microRNA-200-mediated competing endogenous RNA networks. Cells. 2021;11:73. doi: 10.3390/cells11010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Li Y, Zhou JH, Shen FR, Shi X, Chen YG.. CircATRNL1 activates Smad4 signaling to inhibit angiogenesis and ovarian cancer metastasis via miR-378. Mol Oncol. 2021;15:1217–1233. doi: 10.1002/1878-0261.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan D, Qu Y, Zhang L, Ai S, Cheng L.. The lncRNA LINC00691 functions as a ceRNA for miRNA-1256 to suppress osteosarcoma by regulating the expression of ST5. Onco Targets Ther. 2020;13:13171–13181. doi: 10.2147/ott.S266435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ioannou MS, Bell ES, Girard M, Chaineau M, Hamlin JN, Daubaras M, Monast A, Park M, Hodgson L, McPherson PS.. DENND2B activates Rab13 at the leading edge of migrating cells and promotes metastatic behavior. J Cell Biol. 2015;208:629–648. doi: 10.1083/jcb.201407068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.