PURPOSE
We present 5-year results from CheckMate 227 Part 1, in which nivolumab plus ipilimumab improved overall survival (OS) versus chemotherapy in patients with metastatic non–small-cell lung cancer, regardless of tumor programmed death ligand 1 (PD-L1) status.
METHODS
Adults with stage IV/recurrent non–small-cell lung cancer without EGFR mutations or ALK alterations and with tumor PD-L1 ≥ 1% or < 1% (n = 1739) were randomly assigned. Patients with tumor PD-L1 ≥ 1% were randomly assigned to first-line nivolumab plus ipilimumab, nivolumab alone, or chemotherapy. Patients with tumor PD-L1 < 1% were randomly assigned to nivolumab plus ipilimumab, nivolumab plus chemotherapy, or chemotherapy. End points included exploratory 5-year results for efficacy, safety, and quality of life.
RESULTS
At a minimum follow-up of 61.3 months, 5-year OS rates were 24% versus 14% for nivolumab plus ipilimumab versus chemotherapy (PD-L1 ≥ 1%) and 19% versus 7% (PD-L1 < 1%). The median duration of response was 24.5 versus 6.7 months (PD-L1 ≥ 1%) and 19.4 versus 4.8 months (PD-L1 < 1%). Among patients surviving 5 years, 66% (PD-L1 ≥ 1%) and 64% (PD-L1 < 1%) were off nivolumab plus ipilimumab without initiating subsequent systemic anticancer treatment by the 5-year time point. Survival benefit continued after nivolumab plus ipilimumab discontinuation because of treatment-related adverse events, with a 5-year OS rate of 39% (combined PD-L1 ≥ 1% and < 1% populations). Quality of life in 5-year survivors treated with nivolumab plus ipilimumab was similar to that in the general US population through the 5-year follow-up. No new safety signals were observed.
CONCLUSION
With all patients off immunotherapy treatment for ≥ 3 years, nivolumab plus ipilimumab increased 5-year survivorship versus chemotherapy, including long-term, durable clinical benefit regardless of tumor PD-L1 expression. These data support nivolumab plus ipilimumab as an effective first-line treatment for patients with metastatic non–small-cell lung cancer.
INTRODUCTION
Patients with metastatic non–small-cell lung cancer (mNSCLC) have historically had a poor prognosis, with a 5-year survival rate of 7%.1 Programmed death (ligand) 1 (PD-[L]1) inhibitors alone or combined with other modalities have recently been recommended as first-line treatment options for mNSCLC without treatable oncogenic driver mutations.2-4 Dual immunotherapy with nivolumab plus ipilimumab, immune check point inhibitors with distinct but complementary mechanisms of action, has shown durable survival benefit in patients with mNSCLC and other advanced tumor types.5-8 However, 5-year survival outcomes are yet to be reported from phase III studies of first-line immunotherapy-based combinations for mNSCLC. These data, including quality-of-life (QoL) outcomes, are key to assess the long-term benefit-risk of such combinations and to foster research that characterizes long-term survivors.
CONTEXT
Key Objective
Patients with metastatic non–small-cell lung cancer (mNSCLC) have historically had a 5-year survival rate of only 7%, but more recently, immune check point inhibitors have improved survival outcomes in mNSCLC without treatable oncogenic driver mutations. To our knowledge, this update from CheckMate 227 is the first report of 5-year clinical outcomes from a phase III study with a first-line immunotherapy combination.
Knowledge Generated
Nivolumab plus ipilimumab conferred long-term clinical benefit versus chemotherapy in patients with mNSCLC, regardless of tumor programmed death ligand 1 status. Dual immunotherapy increased 5-year overall survival rates and preserved quality of life, with the benefits extending beyond discontinuation of immunotherapy; overall, 23% of patients survived at least 5 years.
Relevance
The long-term follow-up of immune check point inhibitor studies provides valuable information about the durability of immunotherapy responses, the outcomes of patients after the completion of therapy, and the proportion of patients alive and without disease progression at later time points. This long-term follow-up of CheckMate 227 revealed that patients who received nivolumab plus ipilimumab with tumor programmed death ligand 1 expression ≥ 1% or < 1% experienced 5-year overall survival rates of 24% and 19%, respectively. Patients who received immunotherapy and experienced a response had superior outcomes, the responses were durable, and many did not require subsequent therapy. With the maturation of immunotherapy studies, landmark analyses will become more important in assessing the long-term benefit of immunotherapy combinations.
CheckMate 227 (ClinicalTrials.gov identifier: NCT02477826), a randomized, open-label, phase III trial, evaluated first-line nivolumab plus ipilimumab or nivolumab-based regimens versus chemotherapy for the treatment of mNSCLC. CheckMate 227 Part 1 met both its independent primary end points: nivolumab plus ipilimumab significantly prolonged progression-free survival (PFS) in patients with high tumor mutational burden (≥ 10 mut/Mb)9 and overall survival (OS) in patients with tumor programmed death ligand 1 (PD-L1) expression ≥ 1% versus chemotherapy.10 In a prespecified descriptive analysis, OS was also prolonged in patients with tumor PD-L1 < 1%.10 Nivolumab plus ipilimumab was consequently approved in the United States and other regions as first-line therapy in adults with mNSCLC with PD-L1 ≥ 1% and no EGFR or ALK aberrations and in some countries regardless of PD-L1 expression.11-13 Nivolumab plus ipilimumab is recommended as a first-line treatment option by the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (National Comprehensive Cancer Network Guidelines) and the European Society for Medical Oncology guidelines for mNSCLC regardless of PD-L1 expression.3,4
Here, we report CheckMate 227 Part 1 efficacy and safety data with a minimum follow-up of 5 years, the longest report from a phase III trial with an immunotherapy combination for NSCLC and an important survival landmark for patients with mNSCLC. In addition, we report post hoc analyses of outcomes in patients alive at 5 years, those who completed 2 years of immunotherapy, and those who discontinued nivolumab plus ipilimumab because of treatment-related adverse events (TRAEs).
METHODS
Patients
Eligibility criteria have been described previously.9,10 Adults with histologically confirmed stage IV/recurrent NSCLC (without sensitizing EGFR mutations or known ALK alterations), Eastern Cooperative Oncology Group performance status ≤ 1, no previous systemic therapy for advanced/metastatic disease, and no untreated CNS metastases were enrolled. Patients provided written informed consent.
Study Design and Treatment
The study design (Data Supplement, online only) has previously been described.9,10 Patients with tumor PD-L1 ≥ 1% or < 1% were randomly assigned to nivolumab 3 mg/kg once every 2 weeks plus ipilimumab 1 mg/kg once every 6 weeks, nivolumab 240 mg once every 2 weeks alone (PD-L1 ≥ 1%) or 360 mg once every 3 weeks with platinum-doublet chemotherapy once every 3 weeks (PD-L1 < 1%), or platinum-doublet chemotherapy once every 3 weeks. Random assignment within each PD-L1 group was stratified by tumor histology (squamous v nonsquamous). Patients were treated until disease progression or unacceptable toxicity or for ≤ 2 years for immunotherapy. Additional details are provided in the Data Supplement.
This trial was conducted per the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice Guidelines. The study Protocol (online only) and amendments were approved by an institutional review board or independent ethics committee at individual sites.
End Points
The independent primary end points, hierarchical secondary end points, and prespecified descriptive analyses have been published previously (Data Supplement).9,10 Exploratory 5-year results for OS and other efficacy measures, including PFS, objective response rate (ORR), and duration of response (DOR) per blinded independent central review, were reported on the basis of RECIST v1.1, where relevant.
Other exploratory end points included PFS after the next line of therapy (PFS2) and QoL in 5-year survivors using the EQ-5D (3-level version) visual analog scale (VAS). Safety and tolerability were also assessed. Post hoc analyses included efficacy in 5-year survivors, patients who completed 2 years of immunotherapy, and patients who discontinued nivolumab plus ipilimumab or chemotherapy because of TRAEs. A post hoc analysis of treatment-free interval (TFI) was measured in patients who discontinued study therapy (for any reason including treatment completion). Additional details are available in the Data Supplement.
Statistical Analysis
All randomly assigned patients were evaluated for efficacy; those receiving ≥ 1 dose of study treatment were evaluated for safety. OS, PFS, DOR, and TFI were estimated using the Kaplan-Meier methodology. A Cox proportional hazards model stratified by tumor histology was used to calculate hazard ratios (HRs) between treatment arms with associated two-sided CIs in the overall analysis. An unstratified model was used to estimate HRs between treatment arms in patient subgroups. Two-sided exact 95% CIs were calculated for ORRs using the Clopper-Pearson method. A weighted average of the EQ-5D VAS score and 95% CI were analyzed using all available EQ-5D assessments for randomly assigned patients with the OS ≥ 60 months.
RESULTS
Patients
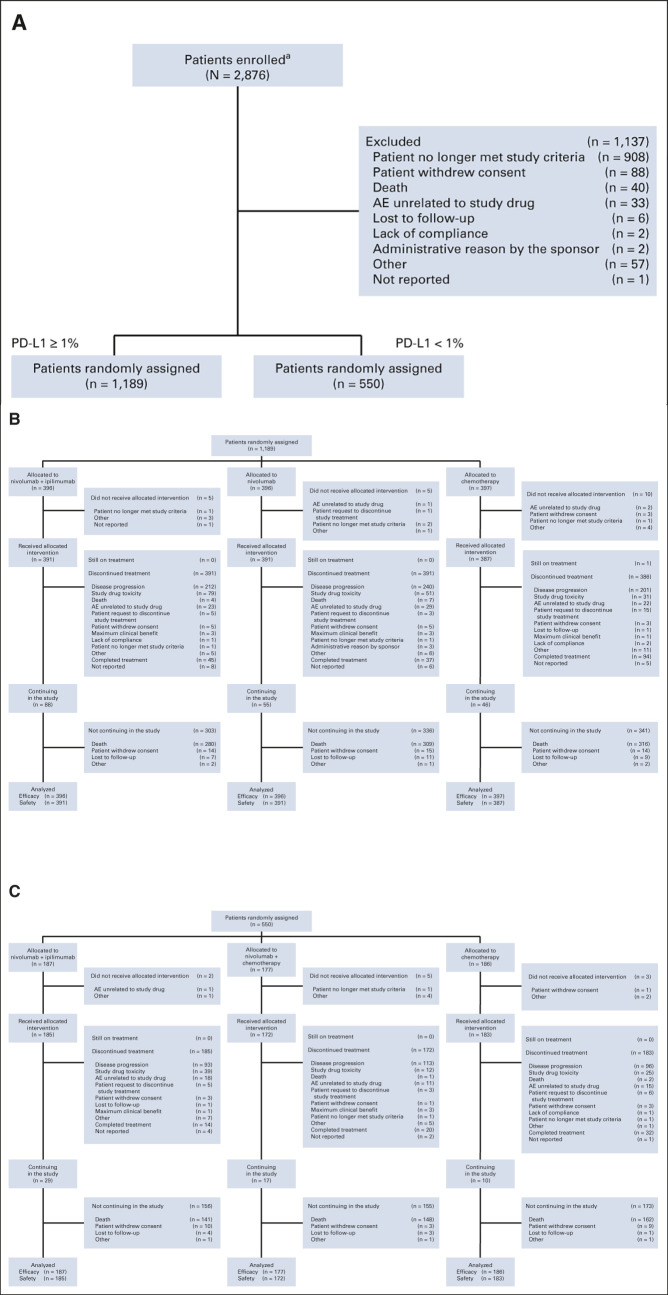
Between August 5, 2015, and November 30, 2016, 2,876 patients were enrolled; 1,739 were randomly assigned (Fig 1).9,10 Baseline characteristics were well-balanced between treatment arms (Data Supplement).9,10 At the current data cutoff (February 15, 2022), the minimum OS follow-up was 61.3 months (median, 66.7 months). All patients had discontinued treatment except one with tumor PD-L1 ≥ 1% treated with chemotherapy who was still receiving maintenance pemetrexed (Fig 1B).
FIG 1.
CheckMate 227 CONSORT diagram. (A) Enrollment and allocation of patients with tumor PD-L1 expression ≥ 1% and < 1%, (B) disposition of patients with tumor PD-L1 expression ≥ 1%, and (C) disposition of patients with tumor PD-L1 expression < 1%. aOne patient was enrolled twice in error but not randomly assigned, not having met study criteria; the number of patients enrolled has been corrected here since the original report.10 AE, adverse event; CONSORT, Consolidated Standards of Reporting Trials; PD-L1, programmed death ligand 1. From the New England Journal of Medicine, Hellmann et al, Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer, 381:2020-2031, 2019 © Massachusetts Medical Society. Reprinted with permission from the Massachusetts Medical Society.10
Efficacy Outcomes
OS, PFS, and response in patients with tumor PD-L1 ≥ 1% (primary end point population) and PD-L1 < 1%.
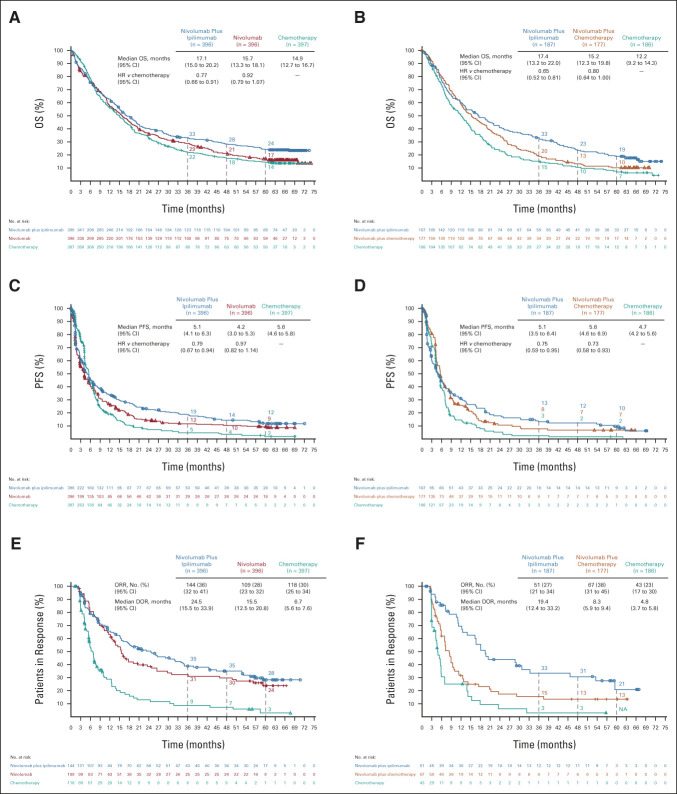
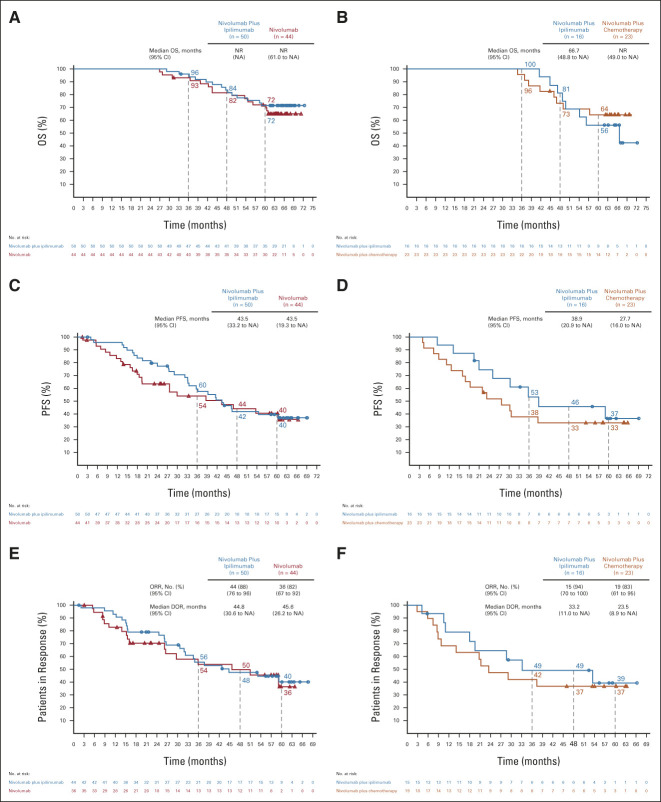
Among patients with tumor PD-L1 ≥ 1% (n = 1,189), nivolumab plus ipilimumab (n = 396) demonstrated continuous long-term, durable OS benefit versus chemotherapy (n = 397; HR, 0.77; 95% CI, 0.66 to 0.91; Fig 2A). Five-year estimated OS rates were 24%, 17%, and 14% with nivolumab plus ipilimumab, nivolumab (n = 396), and chemotherapy, respectively. In patients with tumor PD-L1 < 1% (n = 550), OS benefit also continued with nivolumab plus ipilimumab (n = 187) versus chemotherapy (n = 186; HR, 0.65; 95% CI, 0.52 to 0.81); 5-year estimated OS rates were 19%, 10%, and 7% with nivolumab plus ipilimumab, nivolumab plus chemotherapy (n = 177), and chemotherapy, respectively (Fig 2B). Similar clinical benefit patterns were observed with PFS, ORR, and DOR in both the PD-L1 ≥ 1% and < 1% populations (Figs 2C–2F). Among patients who responded with tumor PD-L1 ≥ 1%, an estimated 28%, 24%, and 3% in the nivolumab plus ipilimumab, nivolumab, and chemotherapy arms, respectively, had responses lasting ≥ 5 years. In patients who responded with tumor PD-L1 < 1%, responses lasting ≥ 5 years occurred in an estimated 21% and 13% in the nivolumab plus ipilimumab and nivolumab plus chemotherapy arms; no patients remained in response in the chemotherapy arm at 5 years.
FIG 2.
OS, PFS, and ORR/DOR in randomly assigned patients by tumor PD-L1 expression level. OS in patients with (A) tumor PD-L1 expression ≥ 1% or (B) tumor PD-L1 expression < 1%; PFS in randomly assigned patients with (C) tumor PD-L1 expression ≥ 1% or (D) tumor PD-L1 expression < 1%; ORR and DOR in randomly assigned patients with (E) tumor PD-L1 expression ≥ 1% or (F) tumor PD-L1 expression < 1%. Patients were followed for a minimum of 61.3 months. Ninety-five percent CIs for the nivolumab plus ipilimumab, nivolumab (PD-L1 ≥ 1%) or nivolumab plus chemotherapy (PD-L1 < 1%), and chemotherapy arms at 5-year landmarks, respectively: (A) 20 to 29, 13 to 21, and 11 to 18; (B) 14 to 25, 6 to 15, and 4 to 11; (C) 9 to 16, 6 to 13, and 1 to 5; (D) 5 to 15, 3 to 12, and < 1 to 6; (E) 20 to 37, 15 to 34, and 0 to 11; and (F) 8 to 38, 6 to 24, and NA. DOR, duration of response; HR, hazard ratio; NA, not available; ORR, objective response rate; OS, overall survival; PD-L1, programmed death ligand 1; PFS, progression-free survival.
OS in tumor histology and other subgroups.
OS (HR [95% CI]) was generally improved with nivolumab plus ipilimumab versus chemotherapy across subgroups (Data Supplement), including patients with nonsquamous tumor histology (PD-L1 ≥ 1%: 0.82 [0.67 to 0.99]; PD-L1 < 1%: 0.70 [0.54 to 0.90]), squamous tumor histology (PD-L1 ≥ 1%: 0.69 [0.52 to 0.91]; PD-L1 < 1%: 0.52 [0.34 to 0.82]), PD-L1 ≥ 50% (0.69 [0.54 to 0.86]), and subgroups by most other key demographic/clinical characteristics. There was a lower magnitude of effect in the PD-L1 1%-49% subgroup (0.90 [0.72 to 1.12]), although the study was not powered to assess efficacy in subgroups.
PFS2.
Among randomly assigned patients with a PFS event, smaller proportions of patients received subsequent systemic therapy in the nivolumab plus ipilimumab than the chemotherapy arms (Data Supplement). Greater PFS2 benefit (HR [95% CI]) was observed with nivolumab plus ipilimumab versus chemotherapy (PD-L1 ≥ 1%: 0.73 [0.62 to 0.85]; PD-L1 < 1%: 0.61 [0.49 to 0.76]; Data Supplement).
TFI.
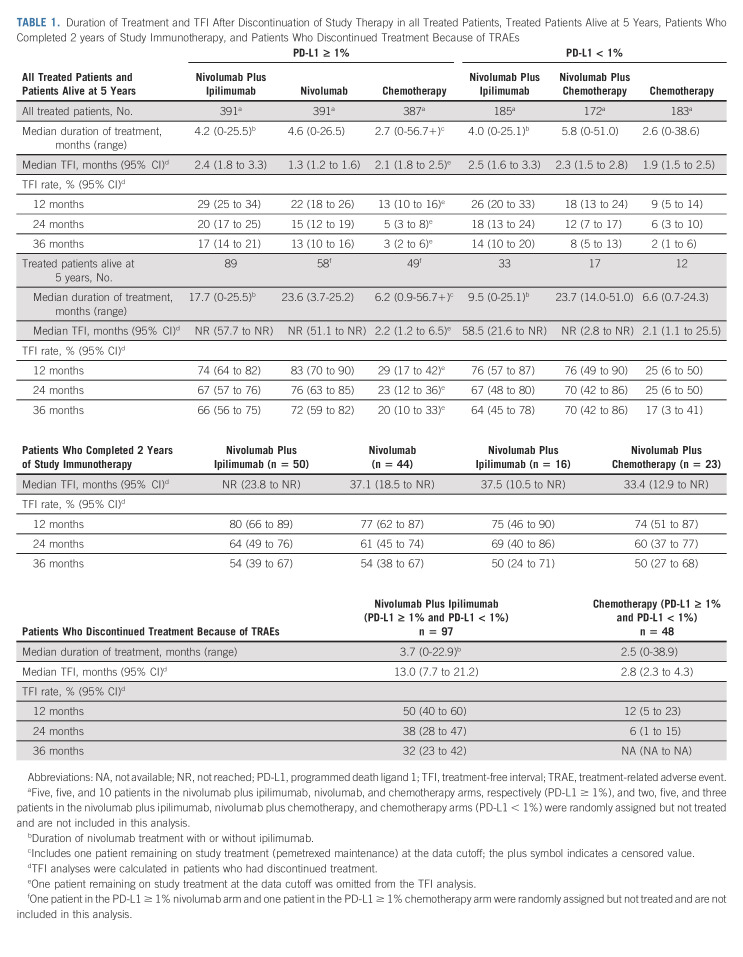
The novel post hoc end point TFI, another measure of study therapy efficacy that may indicate patient experience,14-17 was analyzed in the PD-L1 ≥ 1% and < 1% populations (Table 1). Among patients who discontinued therapy (regardless of reason), 17% of 391 patients (PD-L1 ≥ 1%) and 14% of 185 patients (PD-L1 < 1%) in the nivolumab plus ipilimumab arm were estimated to be alive and treatment-free ≥ 3 years after discontinuing study therapy versus 3% of 386 patients (PD-L1 ≥ 1%) and 2% of 183 patients (PD-L1 < 1%) in the chemotherapy arm. Median therapy duration is shown in Table 1.
TABLE 1.
Duration of Treatment and TFI After Discontinuation of Study Therapy in all Treated Patients, Treated Patients Alive at 5 Years, Patients Who Completed 2 years of Study Immunotherapy, and Patients Who Discontinued Treatment Because of TRAEs
Efficacy and QoL in Patients Alive at 5 Years
OS, PFS, and response.
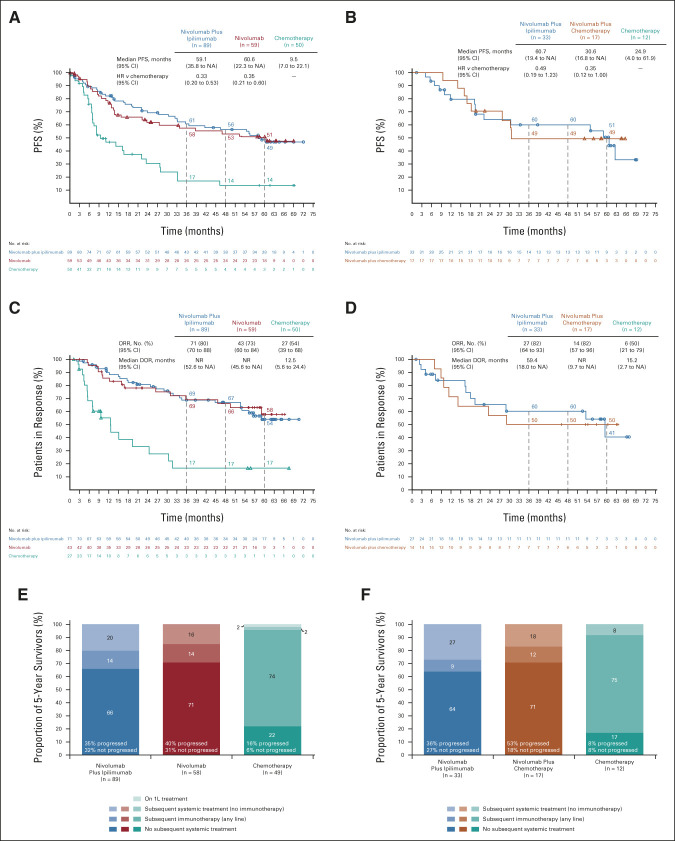
Five years after random assignment, 198 patients were alive in the PD-L1 ≥ 1% group (nivolumab plus ipilimumab, n = 89; nivolumab, n = 59; chemotherapy, n = 50), and 62 in the PD-L1 < 1% group (nivolumab plus ipilimumab, n = 33; nivolumab plus chemotherapy, n = 17; and chemotherapy, n = 12; Fig 3). Baseline characteristics and reasons for treatment discontinuation are shown in the Data Supplement. In the PD-L1 ≥ 1% population, greater PFS benefit was seen with nivolumab plus ipilimumab or nivolumab than chemotherapy (Fig 3A). The ORR was 80%, 73%, and 54% in the nivolumab plus ipilimumab, nivolumab, and chemotherapy arms, respectively; 54%, 58%, and 17% of these patients had ongoing responses for ≥ 5 years (Fig 3C).
FIG 3.
Clinical outcomes and treatment status in patients alive at 5 years by tumor PD-L1 expression level. PFS in randomly assigned patients with (A) tumor PD-L1 expression ≥ 1% or (B) tumor PD-L1 expression < 1%; ORR and DOR in randomly assigned patients with (C) tumor PD-L1 expression ≥ 1% or (D) tumor PD-L1 expression < 1%; treatment status at 5 years in treateda patients with (E) tumor PD-L1 expression ≥ 1% or (F) tumor PD-L1 expression < 1%. Kaplan-Meier curves are not shown for the PD-L1 < 1% chemotherapy arms because of small sample sizes. Ninety-five percent CIs for the nivolumab plus ipilimumab, nivolumab (PD-L1 ≥ 1%) or nivolumab plus chemotherapy (PD-L1 < 1%), and chemotherapy (PD-L1 ≥ 1% only) arms at 5-year landmarks, respectively, (A) 37 to 60, 36 to 64, and 4 to 28; (B) 30 to 68 and 23 to 71; (C) 40 to 66, 39 to 73, and 4 to 36; and (D) 14 to 66 and 23 to 72. aOne patient in the PD-L1 ≥ 1% nivolumab arm and 1 patient in the PD-L1 ≥ 1% chemotherapy arm were randomly assigned but not treated and are not included in the analysis of subsequent treatments. DOR, duration of response; HR, hazard ratio; NA, not available; NR, not reached; ORR, objective response rate; PD-L1, programmed death ligand 1; PFS, progression-free survival.
Treatment status and TFI.
In the PD-L1 ≥ 1% group, the median treatment duration was 17.7, 23.6, and 6.2 months in 5-year survivors treated with nivolumab plus ipilimumab, nivolumab, and chemotherapy, respectively (Table 1), with a median of 36 nivolumab and 10 ipilimumab doses received in the nivolumab plus ipilimumab arm (Data Supplement). Subsequent systemic therapy was administered to 36%, 29%, and 76% of patients in the nivolumab plus ipilimumab, nivolumab, and chemotherapy arms (immunotherapy, 16%, 15%, and 74%; Data Supplement) by data cutoff. At the 5-year landmark, 66%, 71%, and 22% of treated patients, respectively, were off study treatment without having received subsequent systemic treatment (Fig 3E). The median TFI was not reached and 2.2 months in the immunotherapy and chemotherapy arms, respectively (Table 1). Noting the smaller PD-L1 < 1% population, similar clinical outcome patterns were seen in this group (Figs 3B, 3D, and 3F; Table 1; and Data Supplement).
QoL.
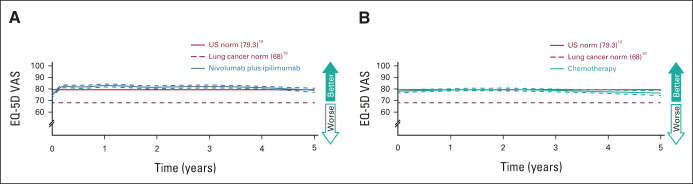
QoL was assessed in 5-year survivors in the combined PD-L1 ≥ 1% and < 1% populations. All 122 treated with nivolumab plus ipilimumab and 60 of 62 treated with chemotherapy completed EQ-5D assessments. EQ-5D VAS scores in the nivolumab plus ipilimumab arm had improvements from baseline and then remained at or above the norm for the general US population18 (Fig 4).
FIG 4.
EQ-5D in 5-year survivors (combined PD-L1 ≥ 1% and < 1% populations). Patients were treated with (A) nivolumab plus ipilimumab (n = 122) or (B) chemotherapy (n = 60). EQ-5D VAS scores range from 0 (worst) to 100 (best), with a minimally important difference cutoff of 7.19 Weighted averages are represented by solid lines and 95% CIs by dashed lines. PD-L1, programmed death ligand 1; VAS, visual analog scale.
Efficacy in Patients Who Completed 2 Years of Immunotherapy
Among patients receiving nivolumab-containing regimens, 133 (12%) completed 2 years of study treatment (PD-L1 ≥ 1%: nivolumab plus ipilimumab, 13% of 391 patients and nivolumab, 11% of 391 patients; PD-L1 < 1%: nivolumab plus ipilimumab, 9% of 185 patients and nivolumab plus chemotherapy, 13% of 172 patients; Fig 5). Baseline characteristics are shown in the Data Supplement. Five-year OS rates were 72% (nivolumab plus ipilimumab) and 72% (nivolumab) for PD-L1 ≥ 1% (Fig 5A) and 56% (nivolumab plus ipilimumab) and 64% (nivolumab plus chemotherapy) for PD-L1 < 1% (Fig 5B); Figures 5C and 5D show PFS. The ORR was 88% and 94% in the PD-L1 ≥ 1% and PD-L1 < 1% nivolumab plus ipilimumab arms, respectively; 40% and 39% of these patients had ongoing responses for ≥ 5 years (Figs 5E and 5F). An estimated 54% and 50% had a TFI ≥ 3 years (Table 1). ORR, DOR, and TFI are also shown for the nivolumab and nivolumab plus chemotherapy arms in Figures 5E and 5F and Table 1.
FIG 5.
OS, PFS, and ORR/DOR in patients who completed 2 years of immunotherapy by the tumor PD-L1 expression level. OS in patients with (A) tumor PD-L1 expression ≥ 1% or (B) tumor PD-L1 expression < 1%; PFS in patients with (C) tumor PD-L1 expression ≥ 1% or (D) tumor PD-L1 expression < 1%; ORR and DOR in patients with (E) tumor PD-L1 expression ≥ 1% or (F) tumor PD-L1 expression < 1%. Ninety-five percent CIs for the nivolumab plus ipilimumab and nivolumab (PD-L1 ≥ 1%) or nivolumab plus chemotherapy (PD-L1 < 1%) arms at 5-year landmarks, respectively: (A) 57 to 82 and 56 to 83, (B) 30 to 76 and 41 to 80, (C) 25 to 53 and 24 to 56, (D) 13 to 61 and 15 to 52, (E) 24 to 56 and 16 to 57, and (F) 14 to 64 and 17 to 57. DOR, duration of response; HR, hazard ratio; NA, not available; NR, not reached; ORR, objective response rate; OS, overall survival; PD-L1, programmed death ligand 1; PFS, progression-free survival.
Efficacy in Patients Who Discontinued Study Treatment Because of TRAEs
TRAEs led to discontinuation of all study drugs in 97 (17%) patients treated with nivolumab plus ipilimumab and 48 (8%) treated with chemotherapy (combined PD-L1 ≥ 1% and < 1% populations); the median treatment duration was 3.7 and 2.5 months, respectively (Data Supplement). Five-year OS rates were 39% and 20%, respectively. The 3-year TFI rate was 32% for nivolumab plus ipilimumab and was not applicable for chemotherapy, with no patients estimated to remain treatment-free (Table 1).
Safety
Per protocol, patients discontinued nivolumab-based regimens at 2 years of treatment. Most patients were therefore off study treatment at the primary analysis (minimum follow-up, 29.3 months),10 and all but one patient in the chemotherapy arm had discontinued study therapy by the 4-year analysis.7 Consequently, no new safety signals were observed in the current analysis (Data Supplement). Similarly, there were no new treatment-related deaths.
DISCUSSION
At a minimum follow-up 5 years in CheckMate 227 Part 1, nivolumab plus ipilimumab continued to demonstrate long-term, durable clinical benefit for patients with previously untreated mNSCLC versus chemotherapy, regardless of tumor PD-L1 expression. The 5-year survival rates of 24% and 19% with nivolumab plus ipilimumab in the PD-L1 ≥ 1% and < 1% populations, respectively (v 14% and 7% with chemotherapy), reflect a marked improvement from the preimmunotherapy era: the 5-year relative survival rate for US patients with mNSCLC between 2012 and 2018 was 7%.1 In long-term survivors treated with nivolumab plus ipilimumab, approximately two-thirds had been off study treatment for ≥ 3 years without having started subsequent systemic treatment at the 5-year time point, and QoL was similar to that of the general US population.
Given the recent introduction of immunotherapy for first-line mNSCLC, data regarding long-term impact are limited. Five-year data from KEYNOTE-024 in patients with tumor PD-L1 ≥ 50% indicated that first-line pembrolizumab provides durable OS benefit versus chemotherapy.20 In our study, we observed continued separation of the nivolumab plus ipilimumab and chemotherapy arms in the OS, PFS, and DOR Kaplan-Meier curves at 5 years regardless of PD-L1 expression, consistent with results at 4 years7 and indicative of long-term, durable survival benefit with nivolumab plus ipilimumab treatment. Notably, this benefit continued even after discontinuing therapy, either after completing 2 years of nivolumab plus ipilimumab treatment or because of TRAEs. Among patients completing the maximum 2 years of nivolumab plus ipilimumab, most were alive at 5 years in both the PD-L1 ≥ 1% and < 1% populations. Importantly, among patients who discontinued nivolumab plus ipilimumab before 2 years because of TRAEs, 39% survived ≥ 5 years. In both patients completing 2 years of nivolumab plus ipilimumab and those stopping because of TRAEs, ongoing responses were observed after treatment discontinuation, underscoring the long-term, durable benefits of dual immunotherapy. Although some clinical benefit was seen with chemotherapy, most long-term survivors in the chemotherapy arm received subsequent immunotherapy. Although not statistically powered for comparisons between immunotherapy-containing arms, greater clinical benefit, including improved 5-year OS, was observed with nivolumab plus ipilimumab versus nivolumab monotherapy or nivolumab plus chemotherapy; however, clinical benefit between the nivolumab-based regimens was similar among 5-year survivors.
Immunotherapy combined with chemotherapy has previously shown limited efficacy in PD-L1 < 1% or squamous histology subgroups, which consequently have a high unmet need.21-23 In patients with tumor PD-L1 < 1% in CheckMate 227, durable clinical benefit was observed with nivolumab plus ipilimumab compared with nivolumab plus chemotherapy or chemotherapy. Moreover, although our prespecified subgroup analyses were exploratory and not powered, nivolumab plus ipilimumab appeared to prolong OS in patients with squamous tumors relative to chemotherapy (PD-L1 ≥ 1% and PD-L1 < 1%) or nivolumab plus chemotherapy (PD-L1 < 1%). Considering the limitations of cross-trial comparisons, exploratory subgroup analyses of other studies have shown numerically greater survival benefit with first-line pembrolizumab plus chemotherapy than chemotherapy in the PD-L1 ≥ 1% versus PD-L1 < 1% subgroup in both squamous and nonsquamous tumors.22,23
In CheckMate 227, OS benefit appeared to be generally greater with nivolumab plus ipilimumab than with nivolumab in the PD-L1 ≥ 50% subgroup, consistent with the durability seen with combination immunotherapy in other settings5,6,8; however, in the PD-L1 ≥ 50% squamous histology subgroup, 5-year OS rates were similar with nivolumab plus ipilimumab and nivolumab. The benefit of nivolumab plus ipilimumab versus chemotherapy was less apparent in the PD-L1 1%-49% than ≥ 50% subgroup, consistent with some observations with other anti–PD-(L)1-based regimens in patients with intermediate PD-L1 expression.24,25 DOR was prolonged with nivolumab plus ipilimumab versus other treatments in the PD-L1 ≥ 50% subgroup. By contrast, data from KEYNOTE-598 suggested that ipilimumab combined with pembrolizumab did not improve efficacy versus pembrolizumab in this population at the time of the prespecified interim analysis (12.4-month minimum follow-up) although the analysis was limited by relatively short follow-up.26 A recent pooled analysis suggested that most patient subgroups with PD-L1 ≥ 50% receiving FDA-approved immunotherapy and chemotherapy combinations had comparable survival outcomes versus immunotherapy only although of note, dual nivolumab plus ipilimumab was included in the immunotherapy-only group together with anti–PD-(L)1 monotherapies, confounding direct extrapolations.27 Overall, the optimal regimen for the PD-L1 ≥ 50% subgroup is unclear, highlighting the importance of considering both risks and benefits of different treatments in individual patients. With nivolumab plus ipilimumab, 18% of patients in CheckMate 227 overall discontinued treatment because of TRAEs, which is not substantially higher than the 14% pembrolizumab discontinuation rate for patients with PD-L1 ≥ 50% in KEYNOTE-024.20
As long-term survivorship improves with advancements in cancer therapy, aspects of patient experience such as QoL become increasingly relevant. TFI has recently been adopted as a metric of patient experience in multiple myeloma and other cancers; these intervals are associated with better QoL and typically represent a period of clinically stable disease allowing patients respite from the burden of treatment.14-17 In our study, 5-year survivors experienced an extended treatment-free period after nivolumab plus ipilimumab treatment, with almost two-thirds never receiving subsequent systemic therapy through the 5-year landmark. Furthermore, QoL in 5-year survivors treated with nivolumab plus ipilimumab was similar to that of the general US population. These data reinforce the durable benefit of this regimen, including better patient experience relative to chemotherapy.
Immune-mediated AEs have previously been associated with immunotherapy, particularly ipilimumab.28 However, ipilimumab, approved for cancer treatment in 2011, has well-established algorithms to manage immune-mediated AEs.29 No new safety signals were observed with nivolumab plus ipilimumab in our study. Consistent with previous reports, discontinuing treatment because of TRAEs did not adversely affect long-term efficacy.6 Together with preservation of patient QoL, our study continues to demonstrate the favorable benefit-risk profile of nivolumab plus ipilimumab.
CheckMate 227 is the first phase III study to report 5-year clinical outcomes with a first-line immunotherapy combination for mNSCLC. Nivolumab plus ipilimumab conferred long-term clinical benefit extending beyond treatment discontinuation, regardless of PD-L1 expression. This durable clinical benefit is consistent with the efficacy seen with nivolumab plus ipilimumab across multiple tumor types, including melanoma, renal cell carcinoma, and mesothelioma.5,6,8 Overall, 23% of patients with mNSCLC, regardless of tumor PD-L1 expression, survived for ≥ 5 years after treatment with this dual immunotherapy regimen, signifying a substantial therapeutic advancement. Furthermore, nivolumab plus ipilimumab preserved QoL for these long-term survivors. Future studies are needed to identify reliable biomarkers predicting benefit with immunotherapy-based treatment and to evaluate novel therapeutic combinations for patients with primary or secondary resistance to immunotherapy.
ACKNOWLEDGMENT
We thank the patients and their families for making this trial possible; the clinical study teams who participated; Dako, an Agilent Technologies Inc. company (Santa Clara, CA), for collaborative development of the PD-L1 IHC 28-8 pharmDx assay; Yong Yuan, PhD, for support with data analysis; and Bristol Myers Squibb (Princeton, NJ) and Ono Pharmaceutical Company, Ltd (Osaka, Japan). All the authors contributed to and approved the manuscript. Professional writing and editorial assistance were provided by Sabrina Hom, PhD, and Michele Salernitano of Ashfield MedComms, an Inizio company, funded by Bristol Myers Squibb.
Julie R. Brahmer
Honoraria: Janssen
Consulting or Advisory Role: Bristol Myers Squibb, Lilly, Merck, Amgen, Genentech, GlaxoSmithKline, AstraZeneca, Regeneron, Sanofi, Eisai, Turning Point Therapeutics
Research Funding: Bristol Myers Squibb (Inst), AstraZeneca (Inst), Spectrum Pharmaceuticals (Inst), Revolution (Inst), RAPT Therapeutics (Inst)
Other Relationship: Bristol Myers Squibb
Reyes Bernabe Caro
Consulting or Advisory Role: Roche, AstraZeneca, Bristol Myers Squibb/Celgene
Travel, Accommodations, Expenses: AstraZeneca
Makoto Nishio
Honoraria: Pfizer, Bristol Myers Squibb Japan, Ono Pharmaceutical, Chugai Pharma, Taiho Pharmaceutical, AstraZeneca, Boehringer Ingelheim, MSD, Novartis, Lilly, Nippon Kayaku, Takeda, Merck, Janssen, Amgen
Research Funding: Bristol Myers Squibb (Inst), Taiho Pharmaceutical (Inst), Pfizer (Inst), AstraZeneca (Inst), Lilly (Inst), MSD (Inst), Merck (Inst), Takeda (Inst), Amgen (Inst), Janssen (Inst)
Randeep Sangha
Honoraria: Pfizer, AstraZeneca, Roche/Genentech, Bristol Myers Squibb, Merck, AbbVie, Takeda, Teva, Sanofi, Bayer, Lilly
Consulting or Advisory Role: AstraZeneca, Roche/Genentech, Bristol Myers Squibb, Merck, Novartis, AbbVie, Takeda, Teva, Lilly, Sanofi, Bayer
Research Funding: Bristol Myers Squibb (Inst), AbbVie (Inst), Takeda (Inst), Pharmacyclics (Inst), MorphoSys (Inst), Roche (Inst), Merck Serono (Inst), Novartis (Inst), Celgene (Inst)
Adam Pluzanski
Honoraria: Roche, BMS, AstraZeneca, MSD, Pfizer, Takeda, Boehringer Ingelheim
Consulting or Advisory Role: Takeda, Bristol Myers Squibb/Pfizer, Janssen
Research Funding: BMS, Pfizer
Travel, Accommodations, Expenses: Takeda, MSD Oncology
Jacobus Burgers
Consulting or Advisory Role: Roche (Inst)
Research Funding: Merck (Inst)
Adam J. Schoenfeld
Consulting or Advisory Role: Johnson & Johnson/Janssen, KSQ Therapeutics, Perceptive Advisors, Heat Biologics, Bristol Myers Squibb, Enara Bio, Umoja Biopharma, Oppenheimer, Iovance Biotherapeutics, Lyell Immunopharma
Research Funding: GlaxoSmithKline
Travel, Accommodations, Expenses: Iovance Biotherapeutics, Instil Bio
Other Relationship: Merck, Bristol Myers Squibb, Iovance Biotherapeutics, PACT Pharma, Achilles Therapeutics, GlaxoSmithKline, Harpoon therapeutics, Amgen, Instil Bio
Open Payments Link: https://openpaymentsdata.cms.gov/physician/4222930
Luis G. Paz-Ares
Leadership: Genomica, ALTUM Sequencing
Honoraria: Roche/Genentech, Lilly, Pfizer, Bristol Myers Squibb, MSD, AstraZeneca, Merck Serono, PharmaMar, Novartis, Amgen, Sanofi, Bayer, Takeda, Mirati Therapeutics, Daiichi Sankyo, Hutchmed, BeiGene, GlaxoSmithKline, Janssen, Medscape
Speakers' Bureau: MSD Oncology, BMS, Roche/Genentech, Pfizer, Lilly, AstraZeneca, Merck Serono
Research Funding: BMS (Inst), AstraZeneca (Inst), PharmaMar (Inst), Kura Oncology (Inst), MSD (Inst), Pfizer (Inst)
Other Relationship: Novartis, Ipsen, Pfizer, Servier, Sanofi, Roche, Amgen, Merck, Roche
Martin Reck
Consulting or Advisory Role: Lilly, MSD Oncology, Merck Serono, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Roche/Genentech, AbbVie, Amgen, Mirati Therapeutics, Samsung Bioepis, Sanofi/Regeneron, Daiichi Sankyo Europe GmbH
Speakers' Bureau: Roche/Genentech, Lilly, MSD Oncology, Merck Serono, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen, Mirati Therapeutics, Sanofi/Aventis
Hossein Borghaei
Stock and Other Ownership Interests: Sonnet, Rgenix, Nucleai
Honoraria: Bristol Myers Squibb, Celgene, Axiom Biotechnologies, Pfizer, Amgen
Consulting or Advisory Role: Bristol Myers Squibb, Lilly, Celgene, Genentech, Pfizer, Boehringer Ingelheim, EMD Serono, Novartis, Merck, AstraZeneca, Genmab, Regeneron, Cantargia AB, BioNTech, AbbVie, PharmaMar, Takeda, Amgen, HUYA Bioscience International, Sonnet, Rgenix, BeiGene, Jazz Pharmaceuticals, Mirati Therapeutics, Guardant Health, Janssen Oncology, iTeos Therapeutics, Natera, oncocyte, Da Volterra
Research Funding: Millennium (Inst), Merck (Inst), Celgene (Inst), Bristol Myers Squibb (Inst), Lilly (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Lilly, Clovis Oncology, Celgene, Genentech, Novartis, Merck, Amgen
Other Relationship: University of Pennsylvania, Takeda, Incyte, Novartis
Kenneth J. O'Byrne
Stock and Other Ownership Interests: Carpe Vitae Pharmaceuticals, Replica Pharmaceuticals, DGC diagnostics
Honoraria: Merck Sharp & Dohme, Bristol Myers Squibb, Roche, Boehringer Ingelheim, AstraZeneca, Pfizer/EMD Serono, Novartis, Janssen-Cilag, Yuhan, Merck, TriStar Technology Group, Takeda, Amgen, BeiGene
Consulting or Advisory Role: Merck Sharp & Dohme, Boehringer Ingelheim, Roche/Genentech, Janssen-Cilag, Pfizer, AstraZeneca/MedImmune, Bristol Myers Squibb, Novartis, Yuhan, Sanofi, Amgen, BeiGene
Speakers' Bureau: Merck Sharp & Dohme, Boehringer Ingelheim, Bristol Myers Squibb, Roche, Janssen-Cilag, Pfizer, Merck
Patents, Royalties, Other Intellectual Property: I am named on four active patents, two published and two provisional (Inst)
Ravi G. Gupta
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Judith Bushong
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Li Li
Employment: BMS
Stock and Other Ownership Interests: BMS
Travel, Accommodations, Expenses: BMS
Steven I. Blum
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb, GlaxoSmithKline
Laura J. Eccles
Employment: Bristol Myers Squibb/Celgene
Stock and Other Ownership Interests: Bristol Myers Squibb/Celgene
Suresh S. Ramalingam
Consulting or Advisory Role: Amgen, Genentech/Roche, Lilly/ImClone, Bristol Myers Squibb, AstraZeneca, Merck, Takeda, GlaxoSmithKline, Eisai, Mirati Therapeutics
Research Funding: AbbVie (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Merck (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst), Vertex (Inst), Takeda (Inst), EMD Serono (Inst), Genmab (Inst), Advaxis (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Other Relationship: American Cancer Society
No other potential conflicts of interest were reported.
See accompanying editorial on page 1172
PRIOR PRESENTATION
Presented at the 2022 ASCO annual meeting, Chicago, IL, June 3-7, 2022.
SUPPORT
Supported by Bristol Myers Squibb.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.22.01503.
AUTHOR CONTRIBUTIONS
Conception and design: Julie R. Brahmer, Makoto Nishio, Laszlo Urban, Luis G. Paz-Ares, Martin Reck, Kenneth J. O'Byrne, Suresh S. Ramalingam
Provision of study materials or patients: Tudor-Eliade Ciuleanu, Reyes Bernabe Caro, Makoto Nishio, Laszlo Urban, Lorena Lupinacci, Adam Pluzanski, Jacobus Burgers, Mauricio Mahave, Samreen Ahmed, Adam J. Schoenfeld, Luis G. Paz-Ares, Martin Reck, Hossein Borghaei, Kenneth J. O'Byrne, Suresh S. Ramalingam
Collection and assembly of data: Julie R. Brahmer, Jong-Seok Lee, Tudor-Eliade Ciuleanu, Reyes Bernabe Caro, Makoto Nishio, Laszlo Urban, Clarisse Audigier-Valette, Lorena Lupinacci, Randeep Sangha, Jacobus Burgers, Samreen Ahmed, Luis G. Paz-Ares, Martin Reck, Hossein Borghaei, Ravi G. Gupta, Judith Bushong, Li Li, Suresh S. Ramalingam
Data analysis and interpretation: Julie R. Brahmer, Tudor-Eliade Ciuleanu, Makoto Nishio, Lorena Lupinacci, Randeep Sangha, Adam Pluzanski, Mauricio Mahave, Samreen Ahmed, Adam J. Schoenfeld, Luis G. Paz-Ares, Martin Reck, Hossein Borghaei, Kenneth J. O'Byrne, Ravi G. Gupta, Judith Bushong, Li Li, Steven I. Blum, Laura J. Eccles, Suresh S. Ramalingam
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Five-Year Survival Outcomes With Nivolumab Plus Ipilimumab Versus Chemotherapy as First-Line Treatment for Metastatic Non–Small-Cell Lung Cancer in CheckMate 227
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Julie R. Brahmer
Honoraria: Janssen
Consulting or Advisory Role: Bristol Myers Squibb, Lilly, Merck, Amgen, Genentech, GlaxoSmithKline, AstraZeneca, Regeneron, Sanofi, Eisai, Turning Point Therapeutics
Research Funding: Bristol Myers Squibb (Inst), AstraZeneca (Inst), Spectrum Pharmaceuticals (Inst), Revolution (Inst), RAPT Therapeutics (Inst)
Other Relationship: Bristol Myers Squibb
Reyes Bernabe Caro
Consulting or Advisory Role: Roche, AstraZeneca, Bristol Myers Squibb/Celgene
Travel, Accommodations, Expenses: AstraZeneca
Makoto Nishio
Honoraria: Pfizer, Bristol Myers Squibb Japan, Ono Pharmaceutical, Chugai Pharma, Taiho Pharmaceutical, AstraZeneca, Boehringer Ingelheim, MSD, Novartis, Lilly, Nippon Kayaku, Takeda, Merck, Janssen, Amgen
Research Funding: Bristol Myers Squibb (Inst), Taiho Pharmaceutical (Inst), Pfizer (Inst), AstraZeneca (Inst), Lilly (Inst), MSD (Inst), Merck (Inst), Takeda (Inst), Amgen (Inst), Janssen (Inst)
Randeep Sangha
Honoraria: Pfizer, AstraZeneca, Roche/Genentech, Bristol Myers Squibb, Merck, AbbVie, Takeda, Teva, Sanofi, Bayer, Lilly
Consulting or Advisory Role: AstraZeneca, Roche/Genentech, Bristol Myers Squibb, Merck, Novartis, AbbVie, Takeda, Teva, Lilly, Sanofi, Bayer
Research Funding: Bristol Myers Squibb (Inst), AbbVie (Inst), Takeda (Inst), Pharmacyclics (Inst), MorphoSys (Inst), Roche (Inst), Merck Serono (Inst), Novartis (Inst), Celgene (Inst)
Adam Pluzanski
Honoraria: Roche, BMS, AstraZeneca, MSD, Pfizer, Takeda, Boehringer Ingelheim
Consulting or Advisory Role: Takeda, Bristol Myers Squibb/Pfizer, Janssen
Research Funding: BMS, Pfizer
Travel, Accommodations, Expenses: Takeda, MSD Oncology
Jacobus Burgers
Consulting or Advisory Role: Roche (Inst)
Research Funding: Merck (Inst)
Adam J. Schoenfeld
Consulting or Advisory Role: Johnson & Johnson/Janssen, KSQ Therapeutics, Perceptive Advisors, Heat Biologics, Bristol Myers Squibb, Enara Bio, Umoja Biopharma, Oppenheimer, Iovance Biotherapeutics, Lyell Immunopharma
Research Funding: GlaxoSmithKline
Travel, Accommodations, Expenses: Iovance Biotherapeutics, Instil Bio
Other Relationship: Merck, Bristol Myers Squibb, Iovance Biotherapeutics, PACT Pharma, Achilles Therapeutics, GlaxoSmithKline, Harpoon therapeutics, Amgen, Instil Bio
Open Payments Link: https://openpaymentsdata.cms.gov/physician/4222930
Luis G. Paz-Ares
Leadership: Genomica, ALTUM Sequencing
Honoraria: Roche/Genentech, Lilly, Pfizer, Bristol Myers Squibb, MSD, AstraZeneca, Merck Serono, PharmaMar, Novartis, Amgen, Sanofi, Bayer, Takeda, Mirati Therapeutics, Daiichi Sankyo, Hutchmed, BeiGene, GlaxoSmithKline, Janssen, Medscape
Speakers' Bureau: MSD Oncology, BMS, Roche/Genentech, Pfizer, Lilly, AstraZeneca, Merck Serono
Research Funding: BMS (Inst), AstraZeneca (Inst), PharmaMar (Inst), Kura Oncology (Inst), MSD (Inst), Pfizer (Inst)
Other Relationship: Novartis, Ipsen, Pfizer, Servier, Sanofi, Roche, Amgen, Merck, Roche
Martin Reck
Consulting or Advisory Role: Lilly, MSD Oncology, Merck Serono, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Roche/Genentech, AbbVie, Amgen, Mirati Therapeutics, Samsung Bioepis, Sanofi/Regeneron, Daiichi Sankyo Europe GmbH
Speakers' Bureau: Roche/Genentech, Lilly, MSD Oncology, Merck Serono, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen, Mirati Therapeutics, Sanofi/Aventis
Hossein Borghaei
Stock and Other Ownership Interests: Sonnet, Rgenix, Nucleai
Honoraria: Bristol Myers Squibb, Celgene, Axiom Biotechnologies, Pfizer, Amgen
Consulting or Advisory Role: Bristol Myers Squibb, Lilly, Celgene, Genentech, Pfizer, Boehringer Ingelheim, EMD Serono, Novartis, Merck, AstraZeneca, Genmab, Regeneron, Cantargia AB, BioNTech, AbbVie, PharmaMar, Takeda, Amgen, HUYA Bioscience International, Sonnet, Rgenix, BeiGene, Jazz Pharmaceuticals, Mirati Therapeutics, Guardant Health, Janssen Oncology, iTeos Therapeutics, Natera, oncocyte, Da Volterra
Research Funding: Millennium (Inst), Merck (Inst), Celgene (Inst), Bristol Myers Squibb (Inst), Lilly (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Lilly, Clovis Oncology, Celgene, Genentech, Novartis, Merck, Amgen
Other Relationship: University of Pennsylvania, Takeda, Incyte, Novartis
Kenneth J. O'Byrne
Stock and Other Ownership Interests: Carpe Vitae Pharmaceuticals, Replica Pharmaceuticals, DGC diagnostics
Honoraria: Merck Sharp & Dohme, Bristol Myers Squibb, Roche, Boehringer Ingelheim, AstraZeneca, Pfizer/EMD Serono, Novartis, Janssen-Cilag, Yuhan, Merck, TriStar Technology Group, Takeda, Amgen, BeiGene
Consulting or Advisory Role: Merck Sharp & Dohme, Boehringer Ingelheim, Roche/Genentech, Janssen-Cilag, Pfizer, AstraZeneca/MedImmune, Bristol Myers Squibb, Novartis, Yuhan, Sanofi, Amgen, BeiGene
Speakers' Bureau: Merck Sharp & Dohme, Boehringer Ingelheim, Bristol Myers Squibb, Roche, Janssen-Cilag, Pfizer, Merck
Patents, Royalties, Other Intellectual Property: I am named on four active patents, two published and two provisional (Inst)
Ravi G. Gupta
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Judith Bushong
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Li Li
Employment: BMS
Stock and Other Ownership Interests: BMS
Travel, Accommodations, Expenses: BMS
Steven I. Blum
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb, GlaxoSmithKline
Laura J. Eccles
Employment: Bristol Myers Squibb/Celgene
Stock and Other Ownership Interests: Bristol Myers Squibb/Celgene
Suresh S. Ramalingam
Consulting or Advisory Role: Amgen, Genentech/Roche, Lilly/ImClone, Bristol Myers Squibb, AstraZeneca, Merck, Takeda, GlaxoSmithKline, Eisai, Mirati Therapeutics
Research Funding: AbbVie (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Merck (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst), Vertex (Inst), Takeda (Inst), EMD Serono (Inst), Genmab (Inst), Advaxis (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Other Relationship: American Cancer Society
No other potential conflicts of interest were reported.
REFERENCES
- 1.Surveillance, Epidemiology, and End Results Program (SEER) : Cancer Stat Facts: Lung and Bronchus Cancer. http://seer.cancer.gov/statfacts/html/lungb.html [Google Scholar]
- 2.Hanna NH, Temin S, Masters G: Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update summary. JCO Oncol Pract 16:e844-e848, 2020 [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network Clinical Practice Guidelines: Non-Small Cell Lung Cancer V.3.2022. https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf [Google Scholar]
- 4.Planchard D, Popat S, Kerr K, et al. : Metastatic non-small cell lung cancer: ESMO clinical Practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv192-iv237, 2020. (suppl 4) [DOI] [PubMed] [Google Scholar]
- 5.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. : Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol 40:127-137, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motzer RJ, McDermott DF, Escudier B, et al. : Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer 128:2085-2097, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paz-Ares LG, Ramalingam SS, Ciuleanu TE, et al. : First-line nivolumab plus ipilimumab in advanced NSCLC: 4-year outcomes from the randomized, open-label, phase 3 CheckMate 227 Part 1 trial. J Thorac Oncol 17:289-308, 2022 [DOI] [PubMed] [Google Scholar]
- 8.Peters S, Scherpereel A, Cornelissen R, et al. : First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann Oncol 33:488-499, 2022 [DOI] [PubMed] [Google Scholar]
- 9.Hellmann MD, Ciuleanu TE, Pluzanski A, et al. : Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 378:2093-2104, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. : Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 381:2020-2031, 2019 [DOI] [PubMed] [Google Scholar]
- 11.OPDIVO® (nivolumab) [package insert]. Princeton, NJ, Bristol Myers Squibb. https://packageinserts.bms.com/pi/pi_opdivo.pdf [Google Scholar]
- 12.OPDIVO® (nivolumab) approval. Chuo-ku, Osaka, Japan. Ono Pharmaceutical Co, Ltd, 2020. https://www.ono-pharma.com/sites/default/files/en/news/press/sm_cn201127_1.pdf [Google Scholar]
- 13.OPDIVO® (nivolumab) [prospecto]. Buenos Aires, Argentina, Bristol Myers Squibb Argentina. https://www.bms.com/assets/bms/latam/documents/meds/medicine-prospecto/argentina/ar-es-opdivo-Mar%202020-May%202021-pi-clean.pdf [Google Scholar]
- 14.Richardson P, Roy A, Acharyya S, et al. : Treatment-free interval as a metric of patient experience and a health outcome of value for advanced multiple myeloma: The case for the histone deacetylase inhibitor panobinostat, a next-generation novel agent. Expert Rev Hematol 10:933-939, 2017 [DOI] [PubMed] [Google Scholar]
- 15.McDermott DF, Rini BI, Motzer RJ, et al. : Treatment-free interval (TFI) following discontinuation of first-line nivolumab plus ipilimumab (N+I) or sunitinib (S) in patients (Pts) with advanced renal cell carcinoma (aRCC): CheckMate 214 analysis. Ann Oncol 29, 2018. (suppl 8; abstr VIII309) [Google Scholar]
- 16.Regan MM, Werner L, Rao S, et al. : Treatment-free survival: A novel outcome measure of the effects of immune checkpoint inhibition—A pooled analysis of patients with advanced melanoma. J Clin Oncol 37:3350-3358, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Djebbari F, Sharpley FA, McLain-Smith S, et al. : Treatment-free interval as an additional measure of efficacy in a large UK dataset of transplant ineligible myeloma patients. PLoS One 15:e0229469, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen MF, Szende A, Cabases J, et al. : Population norms for the EQ-5D-3L: A cross-country analysis of population surveys for 20 countries. Eur J Health Econ 20:205-216, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickard AS, Neary MP, Cella D: Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 5:70, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reck M, Rodríguez-Abreu D, Robinson AG, et al. : Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol 39:2339-2349, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Y, Wan B, Chen X, et al. : The association of PD-L1 expression with the efficacy of anti-PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: A meta-analysis of randomized controlled trials. Transl Lung Cancer Res 8:413-428, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paz-Ares L, Vicente D, Tafreshi A, et al. : A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: Protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol 15:1657-1669, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Rodríguez-Abreu D, Powell SF, Hochmair MJ, et al. : Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: Protocol-specified final analysis from KEYNOTE-189. Ann Oncol 32:881-895, 2021 [DOI] [PubMed] [Google Scholar]
- 24.Castro GD, Kudaba I, Wu Y, et al. : 363 KEYNOTE-042 5-year survival update: Pembrolizumab versus chemotherapy in patients with previously untreated, PD-L1–positive, locally advanced or metastatic non–small-cell lung cancer. J Immunother Cancer 9, 2021. (suppl 2; abstr A390) [Google Scholar]
- 25.Jassem J, de Marinis F, Giaccone G, et al. : Updated overall survival analysis from IMpower110: Atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1-selected NSCLC. J Thorac Oncol 16:1872-1882, 2021 [DOI] [PubMed] [Google Scholar]
- 26.Boyer M, Sendur MAN, Rodríguez-Abreu D, et al. : Pembrolizumab plus ipilimumab or placebo for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%: Randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol 39:2327-2338, 2021 [DOI] [PubMed] [Google Scholar]
- 27.Akinboro O, Vallejo JJ, Nakajima EC, et al. : Outcomes of anti–PD-(L)1 therapy with or without chemotherapy for first-line treatment of advanced non–small cell lung cancer with PD-L1 score ≥50%: FDA pooled analysis. J Clin Oncol 40, 2022. (suppl 16; abstr 9000) [Google Scholar]
- 28.Stucci S, Palmirotta R, Passarelli A, et al. : Immune-related adverse events during anticancer immunotherapy: Pathogenesis and management. Oncol Lett 14:5671-5680, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider BJ, Naidoo J, Santomasso BD, et al. : Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol 39:4073-4126, 2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.22.01503.