PURPOSE
Squamous cell carcinoma of the anus (SCCA) incidence and mortality rates are rising in the United States. Understanding state-level incidence and mortality patterns and associations with smoking and AIDS prevalence (key risk factors) could help unravel disparities and provide etiologic clues.
METHODS
Using the US Cancer Statistics and the National Center for Health Statistics data sets, we estimated state-level SCCA incidence and mortality rates. Rate ratios (RRs) were calculated to compare incidence and mortality in 2014-2018 versus 2001-2005. The correlations between SCCA incidence with current smoking (from the Behavioral Risk Factor Surveillance System) and AIDS (from the HIV Surveillance system) prevalence were evaluated using Spearman's rank correlation coefficient.
RESULTS
Nationally, SCCA incidence and mortality rates (per 100,000) increased among men (incidence, 2.29-3.36, mortality, 0.46-0.74) and women (incidence, 3.88-6.30, mortality, 0.65-1.02) age ≥ 50 years, but decreased among men age < 50 years and were stable among similar-aged women. In state-level analysis, a marked increase in incidence (≥ 1.5-fold for men and ≥ two-fold for women) and mortality (≥ two-fold) for persons age ≥ 50 years was largely concentrated in the Midwestern and Southeastern states. State-level SCCA incidence rates in recent years (2014-2018) among men were correlated (r = 0.47, P < .001) with state-level AIDS prevalence patterns. For women, a correlation was observed between state-level SCCA incidence rates and smoking prevalence (r = 0.49, P < .001).
CONCLUSION
During 2001-2005 to 2014-2018, SCCA incidence and mortality nearly doubled among men and women age ≥ 50 years living in Midwest and Southeast. State variation in AIDS and smoking patterns may explain variation in SCCA incidence. Improved and targeted prevention is needed to combat the rise in SCCA incidence and mitigate magnifying geographic disparities.
INTRODUCTION
The incidence and mortality rates of human papillomavirus (HPV)–associated squamous cell carcinoma of the anus (SCCA) are rising rapidly (nearly 3% per year) in the United States, particularly among adults age 50 years or older (nearly 5% per year).1-4 Notably, SCCA incidence surpassed cervical cancer among non-Hispanic White women age ≥ 65 years and the incidence of these two cancers is approaching parity among women age 50-64 years.3 The population size of US seniors (currently unvaccinated against HPV because of age ineligibility) is projected to rise (from 49 million in 2016 to 81 million in 2040), with women outnumbering men (5:4), implying that the SCCA burden (number of cases) among seniors may continue to rise.5
CONTEXT
Key Objective
What are the recent incidence and mortality patterns in human papillomavirus (HPV)–associated squamous cell carcinoma of the anus (SCCA) in association with state-level prevalence of HIV/AIDS and smoking (key oncogenic cofactors)?
Knowledge Generated
Recently, a more than 1.5-fold rise in anal cancer incidence among persons age ≥ 50 years occurred. A correlation with smoking prevalence patterns was observed for women. For men, SCCA incidence patterns were correlated with HIV/AIDS prevalence patterns. Increases in anal cancer incidence and mortality rates call for improvements in primary (ie, HPV vaccination) and secondary (early detection approaches) prevention and urgent treatment advances.
Relevance (E.M. O'Reilly)
This article describes important epidemiologic trends that have occurred over the past two decades related to the incidence of SCCA in the United States and suggests opportunities for public health interceptions related to smoking cessation, HPV vaccination, and early detection strategies.*
*Relevance section written by JCO Associate Editor Eileen M. O'Reilly, MD.
Risk factors for SCCA include HIV infection, particularly advanced disease (ie, AIDS), and current smoking.6-10 HIV-/AIDS-related immunosuppression enhances the carcinogenicity of HPV, causing an elevated SCCA risk.8 Smoking impairs immune function, prohibiting the ability to clear HPV infection.9 It may also inhibit apoptosis, promoting tumor growth.11 The prevalence of these risk factors varies across US states/territories.12,13 AIDS prevalence is highest (> 450/100,000 in men and > 175/100,000 in women) in the District of Columbia (DC) and coastal US states/territories (eg, in California, New York, and Florida).13 Smoking prevalence is highest (> 20% in men and women) in the Southeastern and Midwestern states (eg, Louisiana, Mississippi, Ohio, and Tennessee).14 Given these differences in the geographic concentration of risk factors, variations in SCCA incidence and mortality patterns by geography may be expected but remain undescribed.
Analogous to cervical cancer, screening (using high-resolution anoscopy [HRA]) to detect precancers and treatment to prevent progression to SCCA have been used mainly for persons living with HIV (PLWHIV). Mounting data, including the Anal Cancer/HSIL Outcomes Research (ANCHOR) study, demonstrate that anal precancer treatment reduces SCCA risk among PLWHIV.15-17 Given the implementation of screening and treatment practices for PLWHIV in the most populous urban coastal regions for over 15 years (eg, New York state implemented guidelines in 2007, and HIV care providers in California have been screening for SCCA since the 1990s),18-20 SCCA incidence patterns might have been affected by increased diagnostic scrutiny, particularly among men age < 50 years as HIV greatly contributes to SCCA (nearly 60%) in this age group, but 20% among men age 50-79 years.4,21
Understanding the geographic differences in SCCA incidence and mortality patterns could contribute to etiologic hypotheses and have important implications for guiding cancer prevention interventions and clinical practices. Therefore, we evaluated patterns in SCCA incidence, burden, mortality, and associations with smoking and HIV/AIDS prevalence (overall and among persons age < 50 and ≥ 50 years) in all 50 US states, the District of Columbia (DC), and Puerto Rico (PR). In addition, a comprehensive description of screening providers may help guide future infrastructure development. Thus, we described the current screening locations and their distribution across the United States.
METHODS
Data Sources
We used the US Cancer Statistics Data Set from the Centers for Disease Control and Prevention's (CDC) National Program of Cancer Registries (NPCR) and the National Cancer Institute's (NCI) SEER Program data set to analyze SCCA cases diagnosed from 2001 to 2018.22 This data set includes cancer incidence data from central cancer registries reported to NPCR in 46 states, DC, and SEER in four states and covers > 98% of the US population from 2001 through 2018. Anal cancer death data were obtained from information recorded in death certificates ascertained from the National Center for Health Statistics (NCHS). Data for PR were extracted from the PR Central Cancer Registry. Current smoking prevalence by sex, age (< 50 and ≥ 50 years), and state was obtained from The Behavioral Risk Factor Surveillance System surveys. Similarly, stratified HIV/AIDS prevalence was obtained from the CDC's HIV Surveillance system. SCCA screening infrastructure details (ie, location of HRA providers) are compiled and maintained by the International Anal Neoplasia Society (IANS).
Case Definition
We identified anal cancer cases on the basis of the International Classification of Diseases for Oncology, third edition (ICD-O-3; site codes anal, not otherwise specified [C21.0]; anal canal [C21.1]; cloacogenic zone [C21.2]; and overlapping lesion of rectum, anus, and anal canal [C21.8]), and SCCA cases were identified using histologic codes (8050-8076, 8083-8084, and 8123-8124).23 Only malignant and microscopically confirmed cases were included in the analysis. Cases of unknown age and sex and those whose cancer was diagnosed at autopsy or was first documented on the death certificate were excluded (Data Supplement, online only).
Statistical Analysis
SCCA incidence rates and anal cancer mortality rates for 2001-2005 and 2014-2018 were calculated by sex, age (< 50 and ≥ 50 years), and state of residence. The incidence and mortality rates were age-adjusted to the 2000 US standard population and expressed per 100,000 persons. SEER*Stat version 8.3.9. was used for the analysis. US regions were categorized as Southeast, Northeast, Midwest, rocky mountain, Pacific, Southwest, and noncontiguous. We also reported cancer burden (ie, incident cases) as a function of population size and composition. Given the rarity of SCCA incidence and mortality in many states, we calculated the rate ratios (RRs) to examine the change in SCCA incidence rates from earlier (2001-2005) years to recent (2014-2018) years. We also estimated annual percentage changes (APCs) and average APCs (AAPCs) for states with available annual data (ie, minimum number of 16 cases).24 Joinpoint software version 4.7.0 was used to estimate piecewise log-linear trends and derive APCs and AAPCs. P values were estimated using the permutation distribution of the test statistic (statistical significance at P < .05, 2-sided). The relationship between SCCA risk factors (smoking and HIV/AIDS) and incidence was assessed using Spearman's rank correlation coefficient. P values were two-tailed (α = .05) on the basis of the permutation method (incidence) or the Wald χ2 test (prevalence). Screening locations were mapped using latitude and longitude (ie, coordinates) on the basis of the HRA providers' addresses. All maps were created using R (version 3.6.4).
RESULTS
Between 2001 and 2018, 88,159 SCCA cases and 14,483 anal cancer deaths were identified in the United States. Patient characteristics and geographic distribution of SCCA cases and anal cancer deaths in 2001-2005 and 2014-2018 are presented in the Data Supplement.
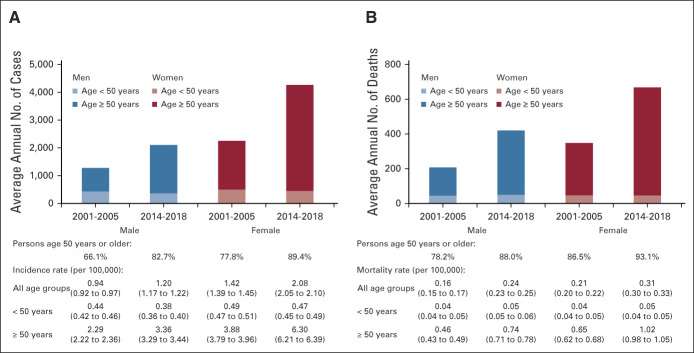
During 2001-2005, annually, on average, 1,266 men (incidence [per 100,000], 0.94) and 2,222 women (incidence, 1.42) were diagnosed with SCCA, which increased during 2014-2018 to 2091 men (incidence, 1.20; RR, 1.27; 95% CI, 1.23 to 1.31) and 4,227 women (incidence, 2.08; RR, 1.46; 95% CI, 1.43 to 1.50; Fig 1A and Data Supplement). Incidence and proportion of cases diagnosed at age ≥ 50 years increased both among men (incidence, from 2.29 to 3.36; RR, 1.47; 95% CI, 1.42 to 1.53; proportion, from 66.1% to 82.7%) and women (incidence, from 3.88 to 6.30; RR, 1.63; 95% CI, 1.58 to 1.67; proportion, from 77.7% to 89.4%; Fig 1A and Data Supplement). By contrast, the SCCA incidence among men and women age < 50 years was < 0.5 per 100,000 and declined among men (RR, 0.87; 95% CI, 0.81 to 0.92) but was stable among women (RR, 0.96; 95% CI, 0.91 to 1.02). Anal cancer mortality followed a similar pattern—increased > 1.5-fold both among men (0.46 to 0.74; RR, 1.62; 95% CI, 1.49 to 1.77) and women (0.65 to 1.02; RR, 1.57; 95% CI, 1.47 to 1.67) age ≥ 50 years (Fig 1B and Data Supplement), with an increased proportion occurring among ≥ 50-year-old men (78.2% to 88.0%) and women (86.5% to 93.1%). Incidence and mortality rates were highest (particularly for women) in the Southeast and Midwest US regions that collectively contributed to more than 50% of the national burden (Figs 2A and 2B).
FIG 1.
Burden and incidence of SCCA and anal cancer deaths among persons age < 50 years and 50 years and older men and women in the United States in 2001-2005 and 2014-2018: National Program of Cancer Registries and SEER Program and National Center for Health Statistics database. In both men and women, (A) the proportion of SCCA cases and (B) the proportion of anal cancer deaths representative of persons age < 50 and ≥ 50 years increased nationally (2001-2005 and 2014-2018). The incidence and mortality ratesa also increased among persons age ≥ 50 years, but not among persons age < 50 years. aRates were calculated as the number of cases per 1,00,000 person-years and age-adjusted to the 2000 US standard population. SCCA, squamous cell carcinoma of the anus.
FIG 2.
Burden and incidence of SCCA and anal cancer deaths according to regions among men and women in the United States in 2001-2005 and 2014-2018: National Program of Cancer Registries and SEER Program and National Center for Health Statistics database. (A) SCCA burden (by region) and (B) anal cancer deaths (by region) among both men and women were highest in the Southeast region, followed by the Midwest region, which collectively contributed to more than 50% of the national burden (2001-2005 and 2014-2018). The incidence ratesa were also high in these regions in recent years, particularly among women. aRates were calculated as the number of cases per 1,00,000 person-years and age-adjusted to the 2000 US standard population. For cases diagnosed from 2003 through 2017, 100% of the population is covered for all 50 states and the District of Columbia. In 2001 and 2002, cases that were diagnosed in Mississippi are not available, and in 2018, cases that were diagnosed in Nevada are not available. SCCA, squamous cell carcinoma of the anus.
In state-specific analysis, SCCA incidence increased in nearly all states, with notable increases in the Midwestern and Southeastern states (Fig 3 and Data Supplement). For men, states with the highest (9 of the top 10) relative increase were concentrated in Midwest and Southeast—Tennessee (RR = 1.92), Iowa (RR = 1.82), Kentucky (RR = 1.66), Louisiana (RR = 1.64), Delaware (RR = 1.55), Wisconsin (RR = 1.54), Alabama (RR = 1.53), Indiana (RR = 1.50), and Michigan (RR = 1.49; all P < .01). For women, marked increases were also concentrated (8 of the top 10) in Midwest and Southeast—Mississippi (RR = 2.12), North Dakota (RR = 2.05), Nebraska (RR = 2.04), West Virginia (RR = 1.92), Minnesota (RR = 1.90), Iowa (RR = 1.85), Tennessee (RR = 1.78), and Wisconsin (RR = 1.78; all P < .01).
FIG 3.
State-specific incidence rates and RRs for SCCA among men and women in 2001-2005 and 2014-2018: National Program of Cancer Registries and SEER Program and National Center for Health Statistics database. The incidence ratesa and RRs for each state are highlighted by subregions among men and women: (A) men incidence rate 2001-2005, (B) men incidence rate 2014-2018, (C) men rate ratios, (D) women incidence rate 2001-2005, (E) women incidence rate 2014-2018, and (F) women rate ratios. Among men and women, incidence increased in nearly all states, with prominent increases largely concentrated in the Midwest and Southeast. aRates were calculated as the number of cases per 1,00,000 person-years and age-adjusted to the 2000 US standard population. For cases diagnosed from 2003 through 2017, 100% of the population is covered for all 50 states and the District of Columbia. In 2001 and 2002, cases that were diagnosed in Mississippi are not available and in 2018, cases that were diagnosed in Nevada are not available. RR, rate ratio; SCCA, squamous cell carcinoma of the anus.
For men age < 50 years, SCCA incidence decreased in New Jersey (RR = 0.43), California (RR = 0.60), Illinois (RR = 0.71), Florida (RR = 0.72), and Colorado (RR = 0.54; all P < .01; Data Supplement). During the same years, a significant increase was observed in Kentucky and Tennessee (southeastern states), and RRs were > one in seven states for men (all from the southeast and Midwest) and 18 states for women (15 from the Southeast and Midwest; Data Supplement). A significant 34% decline (RR = 0.66, P < .001) also occurred among women age < 50 years in California.
SCCA incidence among individuals age ≥ 50 years increased with a greater magnitude in nearly all states, particularly among women (RR of ≥ 2 for 12 states; 11 of which were from the Midwest and Southeast]; Data Supplement). Notably, the SCCA incidence in 2014-2018 has reached nearly 8 per 100,000 among women age ≥ 50 years in Idaho, Kentucky, Oregon, Tennessee, Vermont, Indiana, West Virginia, and Florida.
Findings were consistent when annual percentage changes were estimated for states with available data (Data Supplement). For instance, the SCCA incidence has decreased by 21.1% per year among men age < 50 years in recent years (2015-2018) in California and 3.2% among similar-aged women (both P < .01). Among men and women age ≥ 50 years, the annual percentage change was > 3% in most states. Notably, the SCCA incidence has increased nearly or above 5% per year among women age ≥ 50 years in Iowa, Minnesota, Ohio, Pennsylvania, South Carolina, Tennessee, and Wisconsin.
In ad hoc analysis, when compared with cervical cancer incidence among women age ≥ 50 years, the gap between cervical cancer and SCCA incidence narrowed between 2001-2005 and 2014-2018 (Data Supplement) and incidence was comparable in 12 states.
Overall, anal cancer mortality rates increased in nearly all US states, with the highest increases concentrated (8 of the top 10 states) in the Midwest and Southeast—Wisconsin (RR = 2.13), Alabama (RR = 2.09), Arkansas (RR = 2.06), Nebraska (RR = 2.00), Maryland (RR = 1.90), Louisiana (RR = 1.86), Georgia (RR = 1.85), and Kentucky (RR = 1.82; all P < .01; Fig 4 and Data Supplement). The relative increase in mortality was greater for persons age ≥ 50 years in all states (Data Supplement).
FIG 4.
State-specific mortality ratesa and RRs for anal cancer in 2001-2005 and 2014-2018: National Center for Health Statistics database; (A) mortality rates 2001-2005, (B) mortality rates 2014-2018, and (C) mortality rate ratios. The mortality rate increased in nearly all states, with a prominent rise concentrated in the Midwest and Southeast. aRates were calculated as the number of cases per 1,00,000 person-years and age-adjusted to the 2000 US standard population. RR, rate ratio.
AIDS prevalence (from 2000 to 2013) increased in nearly all states, particularly among persons age ≥ 50 years (Data Supplement). Current smoking prevalence (from 2001-2005 to 2014-2018) generally decreased in nearly all states, with a greater magnitude of decreases among < 50-year-old persons (Data Supplement).
An association was observed between state-level AIDS prevalence and SCCA incidence in 2014-2018 among men (r = 0.47, P < .01) but not among women (r = –0.08, P = .58; Fig 5). Current smoking prevalence in 2014-2018 was associated with SCCA incidence among women (r = 0.49, P < .001) but not among men (r = 0.22, P = .12). Additional findings comparing AIDS and smoking prevalence with SCCA incidence and AIDS, smoking, and SCCA RRs stratified by age are presented in the Data Supplement. A strong correlation was observed between state-level smoking rates and SCCA incidence among women age < 50 years (r = 0.74, P < .001). AIDS and SCCA trends were correlated (r = 0.58, P < .01) among men age < 50 years.
FIG 5.
Correlation between SCCA incidence and AIDS and smoking prevalence: SCCA incidence and AIDS prevalence among (A) men and (B) women and SCCA incidence and smoking prevalence among (C) men and (D) women. There was an association between AIDS prevalence and anal cancer incidence among men and smoking prevalence and anal cancer incidence among women on the basis of Spearman's rank correlation coefficient. Spearman's rank correlation coefficient with corresponding two-sided P values. SCCA, squamous cell carcinoma of the anus.
Figure 6 provides an illustration of the current screening infrastructure. Nationally, in 2022, there were 181 HRA clinics (a detailed description is available in the Data Supplement). Most (47%) HRA clinics are currently concentrated in California (25), New York (25), Florida (15), Maryland (9), and Massachusetts (9).
FIG 6.
Current SCCA screening infrastructure in each state (as of April 2022). The data markers represent locations of high-resolution anoscopy providers. Location details are available in the Data Supplement. SCCA, squamous cell carcinoma of the anus.
DISCUSSION
Our study provides a recent nationwide view of SCCA incidence and mortality, reveals geographic disparities, and offers etiologic clues for state variation in incidence patterns. Between 2001-2005 and 2014-2018, SCCA incidence nearly doubled among persons age ≥ 50 years living in the Midwest and Southeast. A marked two-fold rise in anal cancer deaths among ≥ 50-year-old persons was also concentrated in the Midwestern and Southeastern states. These regions collectively contributed to more than 50% of the national SCCA burden and deaths. Correlation analysis suggests that state variation in SCCA incidence patterns among men and women may be explained by AIDS and current smoking patterns, respectively.
Among men age < 50 years, SCCA incidence increased in Georgia, Tennessee, Kentucky, and Louisiana. By contrast, a decline occurred in California, Florida, New Jersey, and Illinois. Correlation between AIDS and SCCA trends suggests that immune restoration from antiretroviral therapy might have translated into protective benefits in SCCA prevention in these states with greater linkage to HIV care.25 By contrast, acute barriers to HIV care,26 leading to a greater proportion of undiagnosed HIV and late HIV diagnoses in the southern rural states (consistent with rising AIDS prevalence), might have translated into increased HIV-attributable SCCA incidence, contributing to the overall rise among young men. Recent advancements, including newly approved HIV medications for young individuals (eg, bictegravir/emtricitabine/tenofovir alafenamide) and long-acting injectable pre-exposure prevention, may play a crucial role in reversing HIV-attributable SCCA incidence.27 The greater magnitude of the decline in California may also be due to the combined effect of decreased AIDS prevalence and low smoking rates. The decline in SCCA incidence in urban populous states (ie, Florida, Illinois, and particularly California) may also be partly explained by early adoption and relatively wider implementation of screening and treatment practices. Of note, despite our attempt to provide an explanation for state variation in SCCA incidence trends in men, we caution against overinterpretation of these patterns and correlations because of sparse data in some states.
Among women, SCCA is rising most rapidly in the Midwest and Southeast, where current smoking prevalence is prominent. A stronger correlation between smoking and SCCA that we observed among young women but not among men is consistent with findings from a case-control study from Sweden and Denmark that reported high SCCA risk among premenopausal women who currently smoked (OR, 5.6; 95% CI, 2.4 to 12.7), but no association was observed for postmenopausal women or men.28 The study further reported that anal cancer risk increased linearly by 6.7% (95% CI, 3.0 to 10.7) per pack-year of smoking for premenopausal women (P for trend < .001). Consistent with our finding, a recent study also reported a relatively rapid increase in SCCA incidence, particularly among women (5% per year v 2% per year) living in US counties with high smoking prevalence (27% to 53%) compared with those living in counties where smoking was less prevalent (3% to 18%).29 New data revealed higher prevalence, incidence, and persistence of anal HPV infection among men who were current smokers compared with never smokers.30 Similar evidence for women will be necessary to understand the extent of association between current smoking as a cofactor with HPV in anal cancer carcinogenesis.
The growing number of aging birth cohorts who were never eligible for HPV vaccination and rapidly rising AIDS prevalence among aging persons imply that the SCCA burden among seniors may continue to rise for several more decades. Indeed, the narrowing gap between SCCA and cervical cancer among women age ≥ 50 years implies that age-based screening recommendations for SCCA might also be potentially considered.31 The current screening emphasis is on high-risk PLWHIV given their elevated risk and scarcity of screening resources; however, the contribution of HIV to the SCCA burden among older women is low (< 2%),4,32 which implies that wide implementation of screening for PLWHIV will be unlikely to have any impact on SCCA prevention/early detection among the general population of aging women, indicating the need to identify other risk groups (eg, history of cervical precancer or high-risk cervical HPV and solid organ transplantation) that may contribute to SCCA burden greatly and thus could be potentially targeted for screening evaluation and implementation.6,33-35 Future evaluation and implementation of the approaches for early detection of anal cancer (eg, digital anorectal examination or DARE) may also be an important public health opportunity.36
Unfortunately, the marked increases in anal cancer mortality rates both among men and women were also concentrated in the Midwest and Southeast regions. Notably, a nearly three-fold rise in mortality among men in Wisconsin and South Carolina and a nearly two-fold rise in Tennessee, Maryland, and Georgia (states with high HIV prevalence and limited screening infrastructure) are troubling. With the emergence of evidence-based SCCA screening recommendations, improving screening infrastructure particularly in states with high HIV prevalence should be a priority. Furthermore, continuous improvement in the treatment for SCCA for both early-stage and late-stage disease and the development of appropriate treatment infrastructure are also needed.37 Particularly, unmet needs in terms of the lack of treatment options remain for patients who present with surgically unresectable or metastatic disease. Ongoing clinical trials evaluating novel treatment options (eg, cytotoxic therapy and immunotherapy) represent an important opportunity for treatment advances.37
Our study also has important implications for primary prevention through HPV vaccination. Unfortunately, regions/states (mostly in the Midwest and Southeast regions) that are currently seeing a marked rise in SCCA incidence have some of the lowest HPV vaccination coverage in the nation and have the highest level of parental HPV vaccine hesitancy, with more than half of parents of unvaccinated adolescents lacking the intention to initiate the HPV vaccination series in each state.38 Unfortunately, in recent years, parental denial driven by unfounded vaccine safety concerns has also increased in nearly all states in these regions.39,40 Coordinated promotional campaigns and aggressive efforts among health care providers, parents, policymakers, and local health agencies should be a public health priority to combat magnifying geographic disparities.41,42
Our study has certain limitations. First, individual-level risk factors, including HIV, sexual behaviors, and smoking status, are not captured in cancer registries. Therefore, their impact on the increasing SCCA incidence cannot be directly measured, limiting our evaluation to measure correlation than causation. Future research is needed to establish reasons for regional disparities. Second, PLWHIV are known to have a high smoking prevalence.43,44 Therefore, state variation in SCCA trends may be influenced by the combined effect of HIV/AIDS and smoking. However, the absence of information on the presence of both risk factors within one database limited us from measuring the combined effect of these risk factors. Furthermore, the unavailability of data limited us from studying the association between the intensity of smoking use and SCCA incidence patterns. Third, although we explore the correlation between SCCA with smoking and HIV/AIDS (ie, established oncogenic cofactors that enhance the carcinogenicity of HPV), state variation in the prevalence of factors that may contribute to greater acquisition (eg, same-sex sexual contact, heterosexual anal intercourse) or persistence (eg, immunosuppression related to organ transplantation) of anal HPV might have also contributed to the observed state variation in the SCCA incidence patterns. Indeed, future research is needed to understand what factors other than HIV/AIDS and smoking may explain the state variation in SCCA incidence and mortality trajectories. Fourth, sample size limitations precluded us from describing AAPC as our primary outcome measure. However, when we compared RRs with AAPCs in states/regions with available data, the RR patterns were consistent with AAPCs, which supports the robustness of our findings. Fifth, because tumor histology information is not collected in death certificates, it was impossible to estimate SCCA mortality. However, most anal cancer deaths (nearly 90%) arise from SCCA cases, and given the similarity to incidence-based mortality trends, anal cancer mortality could serve as a proxy measure for SCCA mortality.1 Finally, although we report an overall decline in SCCA trends among young men, state variation among racial/ethnic groups is likely driven by inequalities in underlying HIV/AIDS prevalence and care.45 Despite these limitations, the principal strength of our study is that the use of nationwide, high-quality, population-based registries and national surveys allowed us to provide a comprehensive and up-to-date view of state-level SCCA incidence and mortality patterns in the United States in association with smoking and AIDS patterns.
In summary, our study reveals geographic disparities and attempts to explain the state variation in SCCA incidence patterns in association with smoking and AIDS patterns. The decline in SCCA incidence among young men in states where AIDS prevalence declined underscores the importance of early HIV diagnosis and care, particularly in the southern US states. Rising SCCA incidence among women living in states with high smoking prevalence highlights the importance of smoking cessation interventions. Our findings imply a greater need to prepare a workforce, particularly in the Midwestern and Southeastern states, to care for the rising SCCA burden among seniors. Finally, our study calls for an urgent need to improve SCCA prevention/early detection and develop a robust screening infrastructure targeted to serve at-risk and potentially screening-eligible risk groups to avert rising mortality and reduce inequity.
ACKNOWLEDGMENT
We thank Eric G. Meissner for his feedback on the manuscript and Carl Heltzel for his help on text editing.
Ana Patricia Ortiz
Consulting or Advisory Role: Merck
Research Funding: Merck (Inst)
Alan G. Nyitray
Research Funding: COPAN (Inst)
Travel, Accommodations, Expenses: EUROGIN
Keith Sigel
Employment: Oscar Health (I)
Stock and Other Ownership Interests: Amarin Corporation, Adial Pharmaceuticals (I), Biohaven Pharmaceuticals (I), Axsome Therapeutics (I)
Naomi Jay
Honoraria: Roche
Vivian Colon Lopez
Consulting or Advisory Role: Merck
Karen J. Ortiz-Ortiz
Research Funding: AbbVie (Inst), Merck (Inst)
Kalyani Sonawane
Consulting or Advisory Role: Value Analytics Labs
Ashish A. Deshmukh
Consulting or Advisory Role: Merck, Value Analytics Labs
No potential conflicts of interest were reported.
See accompanying editorial on page 1180
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under Award Numbers R01CA232888 (A.A.D.), R01CA256660 (A.A.D., K.S., E.C.), 5R01CA232890 (E.Y.C.), U54CA096300 (A.A.D., A.P.O.), U54CA096297 (A.A.D., A.P.O.), and K01MD016440 (K.S.).
AUTHOR CONTRIBUTIONS
Conception and design: Haluk Damgacioglu, Ana Patricia Ortiz, Keith Sigel, Vivian Colon Lopez, Elizabeth Y. Chiao, Kalyani Sonawane, Ashish A. Deshmukh
Financial support: Keith Sigel, Kalyani Sonawane, Ashish A. Deshmukh
Administrative support: Ashish A. Deshmukh
Provision of study materials or patients: Ashish A. Deshmukh
Collection and assembly of data: Haluk Damgacioglu, Gregory M. Barnell, Kalyani Sonawane, Ashish A. Deshmukh
Data analysis and interpretation: Haluk Damgacioglu, Yueh-Yun Lin, Ana Patricia Ortiz, Chi-Fang Wu, Zahed Shahmoradi, Shiang Shiuan Shyu, Ruosha Li, Alan G. Nyitray, Keith Sigel, Gary M. Clifford, Naomi Jay, Vivian Colon Lopez, Elizabeth Y. Chiao, Elizabeth A. Stier, Karen J. Ortiz-Ortiz, Jeslie M. Ramos-Cartagena, Kalyani Sonawane, Ashish A. Deshmukh
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
State Variation in Squamous Cell Carcinoma of the Anus Incidence and Mortality, and Association With HIV/AIDS and Smoking in the United States
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ana Patricia Ortiz
Consulting or Advisory Role: Merck
Research Funding: Merck (Inst)
Alan G. Nyitray
Research Funding: COPAN (Inst)
Travel, Accommodations, Expenses: EUROGIN
Keith Sigel
Employment: Oscar Health (I)
Stock and Other Ownership Interests: Amarin Corporation, Adial Pharmaceuticals (I), Biohaven Pharmaceuticals (I), Axsome Therapeutics (I)
Naomi Jay
Honoraria: Roche
Vivian Colon Lopez
Consulting or Advisory Role: Merck
Karen J. Ortiz-Ortiz
Research Funding: AbbVie (Inst), Merck (Inst)
Kalyani Sonawane
Consulting or Advisory Role: Value Analytics Labs
Ashish A. Deshmukh
Consulting or Advisory Role: Merck, Value Analytics Labs
No potential conflicts of interest were reported.
REFERENCES
- 1.Deshmukh AA, Suk R, Shiels MS, et al. : Recent trends in squamous cell carcinoma of the anus incidence and mortality in the United States, 2001-2015. J Natl Cancer Inst 112:829-838, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortiz-Ortiz KJ, Ramos-Cartagena JM, Deshmukh AA, et al. : Squamous cell carcinoma of the anus incidence, mortality, and survival among the general population and persons living with HIV in Puerto Rico, 2000-2016. JCO Glob Oncol 7:133-143, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deshmukh AA, Suk R, Shiels MS, et al. : Incidence trends and burden of human papillomavirus-associated cancers among women in the United States, 2001-2017. J Natl Cancer Inst 113:792-796, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deshmukh AA, Damgacioglu H, Georges D, et al. : Global burden of HPV-attributable squamous cell carcinoma of the anus in 2020, according to sex and HIV status: A worldwide analysis. Int J Cancer 152:417-428, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vespa J, Armstrong DM, Medina L: Demographic Turning Points for the United States: Population Projections for 2020 to 2060: Population Estimates and Projections. Department of Commerce Economics and Statistics Administration, Washington, DC, US Census Bureau, 2018, pp 25-1144 [Google Scholar]
- 6.Clifford GM, Georges D, Shiels MS, et al. : A meta-analysis of anal cancer incidence by risk group: Toward a unified anal cancer risk scale. Int J Cancer 148:38-47, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colon-Lopez V, Shiels MS, Machin M, et al. : Anal cancer risk among people with HIV infection in the United States. J Clin Oncol 36:68-75, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Martel C, Shiels MS, Franceschi S, et al. : Cancers attributable to infections among adults with HIV in the United States. AIDS 29:2173-2181, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daling JR, Madeleine MM, Johnson LG, et al. : Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer 101:270-280, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Keller K, Ramos-Cartagena JM, Guiot HM, et al. : Association of smoking with anal high-risk HPV infection and histologically confirmed anal high-grade squamous intraepithelial lesions among a clinic-based population in Puerto Rico. Cancer Treat Res Commun 30:100503, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaal C, Chellappan SP: Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol Cancer Res 12:14-23, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grey JA, Bernstein KT, Sullivan PS, et al. : Estimating the population sizes of men who have sex with men in US states and counties using data from the American Community Survey. JMIR Public Health Surveill 2:e14, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention : HIV Surveillance Report, 2019. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html [Google Scholar]
- 14.Centers for Disease Control and Prevention : State Tobacco Activities Tracking and Evaluation System: Map of Current Cigarette Use Among Adults, 2022. https://www.cdc.gov/statesystem/cigaretteuseadult.html [Google Scholar]
- 15.Revollo B, Videla S, Llibre JM, et al. : Routine screening of anal cytology in persons with human immunodeficiency virus and the impact on invasive anal cancer: A prospective cohort study. Clin Infect Dis 71:390-399, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Goldstone SE: ANCHOR trial results are in: So where do we go from here? Dis Colon Rectum 65:1-3, 2022 [DOI] [PubMed] [Google Scholar]
- 17.Palefsky JM, Lee JY, Jay N, et al. : Treatment of anal high-grade squamous intraepithelial lesions to prevent anal cancer. N Engl J Med 386:2273-2282, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Cancer Society : Anal Cancer: Early Detection, Diagnosis, and Staging; Screening in People at High Risk, 2017. https://staging.cancer.org/content/dam/CRC/PDF/Public/8549.00.pdf [Google Scholar]
- 19.Aberg JA, Gallant JE, Ghanem KG, et al. : Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis 58:e1-e34, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Stewart DB, Gaertner WB, Glasgow SC, et al. : The American Society of Colon and Rectal Surgeons clinical practice guidelines for anal squamous cell cancers (revised 2018). Dis Colon Rectum 61:755-774, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Zhang ER, Pfeiffer RM, Austin A, et al. : Impact of HIV on anal squamous cell carcinoma rates in the United States, 2001-2015. J Natl Cancer Inst 114:1246-1252, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Program of Cancer Registries and Surveillance Epidemiology and End Results SEER*Stat Database: NPCR and SEER Incidence: US Cancer Statistics Public Use Research Database, 2020 submission (2001-2018), Bethesda, MD, United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. www.cdc.gov/cancer/uscs/public-use [Google Scholar]
- 23.World Health Organization : International Classification of Diseases for Oncology (ed 3). Geneva, Switzerland, World Health Organization, 2000 [Google Scholar]
- 24.Clegg LX, Hankey BF, Tiwari R, et al. : Estimating average annual per cent change in trend analysis. Stat Med 28:3670-3682, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly H, Chikandiwa A, Alemany Vilches L, et al. : Association of antiretroviral therapy with anal high-risk human papillomavirus, anal intraepithelial neoplasia, and anal cancer in people living with HIV: A systematic review and meta-analysis. Lancet HIV 7:e262-e278, 2020 [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention : HIV in the Southern United States. Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, 2019 [Google Scholar]
- 27.US Food and Drug Administration : FDA approves first injectable treatment for HIV pre-exposure prevention. US Department of Health and Human Services, 2001 [Google Scholar]
- 28.Frisch M, Glimelius B, Wohlfahrt J, et al. : Tobacco smoking as a risk factor in anal carcinoma: An antiestrogenic mechanism? J Natl Cancer Inst 91:708-715, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Lin Y-Y, Damgacioglu H, Suk R, et al. : Trends in the incidence of human papillomavirus-associated cancers by county-level income and smoking prevalence in the United States, 2000-2018. JNCI Cancer Spectr 6:pkac004, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umutoni V, Schabath MB, Nyitray AG, et al. : The association between smoking and anal human papillomavirus in the HPV infection in men study. Cancer Epidemiol Biomarkers Prev 31:1546-1553, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tota JE, Isidean SD, Franco EL: Defining benchmarks for tolerable risk thresholds in cancer screening: Impact of HPV vaccination on the future of cervical cancer screening. Int J Cancer 147:3305-3312, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiels MS, Pfeiffer RM, Chaturvedi AK, et al. : Impact of the HIV epidemic on the incidence rates of anal cancer in the United States. J Natl Cancer Inst 104:1591-1598, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin C, Slama J, Gonzalez P, et al. : Cervical determinants of anal HPV infection and high-grade anal lesions in women: A collaborative pooled analysis. Lancet Infect Dis 19:880-891, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suk R, Mahale P, Sonawane K, et al. : Trends in risks for second primary cancers associated with index human papillomavirus-associated cancers. JAMA Netw Open 1:e181999, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarke MA, Deshmukh AA, Suk R, et al. : A systematic review and meta-analysis of cytology and HPV-related biomarkers for anal cancer screening among different risk groups. Int J Cancer 151:1889-1901, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nyitray AG, D'Souza G, Stier EA, et al. : The utility of digital anal rectal examinations in a public health screening Program for anal cancer. J Low Genit Tract Dis 24:192-196, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eng C, Ciombor KK, Cho M, et al. : Anal cancer: Emerging standards in a rare disease. J Clin Oncol 40:2774-2788, 2022 [DOI] [PubMed] [Google Scholar]
- 38.Sonawane K, Zhu Y, Montealegre JR, et al. : Parental intent to initiate and complete the human papillomavirus vaccine series in the USA: A nationwide, cross-sectional survey. Lancet Public Health 5:e484-e492, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonawane K, Lin YY, Damgacioglu H, et al. : Trends in human papillomavirus vaccine safety concerns and adverse event reporting in the United States. JAMA Netw Open 4:e2124502, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonawane K, Zhu Y, Lin YY, et al. : HPV vaccine recommendations and parental intent. Pediatrics 147:e2020026286, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y, Wu CF, Giuliano AR, et al. : Tdap-HPV vaccination bundling in the USA: Trends, predictors, and implications for vaccine series completion. Prev Med 164:107218, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanderpool RC, Stradtman LR, Brandt HM: Policy opportunities to increase HPV vaccination in rural communities. Hum Vaccin Immunother 15:1527-1532, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnston PI, Wright SW, Orr M, et al. : Worldwide relative smoking prevalence among people living with and without HIV. AIDS 35:957-970, 2021 [DOI] [PubMed] [Google Scholar]
- 44.Mdege ND, Shah S, Ayo-Yusuf OA, et al. : Tobacco use among people living with HIV: Analysis of data from Demographic and Health Surveys from 28 low-income and middle-income countries. Lancet Glob Health 5:e578-e592, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Damgacioglu H, Wu CF, Lin YY, et al. : Contemporary patterns in HPV-associated cancer incidence among young US men. J Gen Intern Med, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]