PURPOSE
CARTITUDE-1, a phase Ib/II study evaluating the safety and efficacy of ciltacabtagene autoleucel (cilta-cel) in heavily pretreated patients with relapsed/refractory multiple myeloma, yielded early, deep, and durable responses at 12 months. Here, we present updated results 2 years after last patient in (median follow-up [MFU] approximately 28 months), including analyses of high-risk patient subgroups.
METHODS
Eligible patients had relapsed/refractory multiple myeloma, had received ≥ 3 prior lines of therapy or were double refractory to a proteasome inhibitor and immunomodulatory drug and had received prior proteasome inhibitor, immunomodulatory drug, and anti-CD38 therapy. Patients received a single cilta-cel infusion 5-7 days after lymphodepletion. Responses were assessed by an independent review committee.
RESULTS
At a MFU of 27.7 months (N = 97), the overall response rate was 97.9% (95% CI, 92.7 to 99.7); 82.5% (95% CI, 73.4 to 89.4) of patients achieved a stringent complete response. Median duration of response was not estimable. Median progression-free survival (PFS) and overall survival (OS) were not reached; 27-month PFS and OS rates were 54.9% (95% CI, 44.0 to 64.6) and 70.4% (95% CI, 60.1 to 78.6), respectively. Overall response rates were high across all subgroups (95.1%-100%). Duration of response, PFS, and/or OS were shorter in patients with high-risk cytogenetics, International Staging System stage III, high tumor burden, or plasmacytomas. The safety profile was manageable with no new cilta-cel–related cytokine release syndrome and one new case of parkinsonism (day 914 after cilta-cel) since the last report.
CONCLUSION
At approximately 28 months MFU, patients treated with cilta-cel maintained deep and durable responses, observed in both standard and high-risk subgroups. The risk/benefit profile of cilta-cel remained favorable with longer follow-up.
INTRODUCTION
The standard of care (SOC) for relapsed multiple myeloma (MM) involves a multidrug regimen that may include a proteasome inhibitor (PI), an immunomodulatory drug (IMiD), a monoclonal antibody, and a corticosteroid.1 However, patients may eventually become resistant to these treatments.2,3 Lower depth and durability of response have been reported with each successive line of therapy (LOT),4 and patients who are refractory to multiple drug classes have suboptimal outcomes. The median overall survival (OS) with SOC is 11.2 months for patients who are refractory to < 3 prior LOT and 5.6 months for penta-refractory patients (refractory to anti-CD38 antibody, two PIs, and two IMiDs).2
CONTEXT
Key Objective
To determine if the efficacy and safety profile of ciltacabtagene autoleucel (cilta-cel) previously seen at 12-month median follow-up in patients with heavily pretreated multiple myeloma was maintained at 28 months in the overall and high-risk patient populations.
Knowledge Generated
Deep and durable responses to cilta-cel are demonstrated at 28 months with a positive risk/benefit profile. Median progression-free survival and overall survival have not been reached. All high-risk patient subgroups had high response rates, suggesting that cilta-cel offers greater efficacy than what is observed with other available treatment options in these patients. Long-term monitoring for late-onset toxicities is important, and cilta-cel safety has been shown to be manageable.
Relevance
Cilta-cel is a valuable new treatment option for heavily pretreated patients, including high-risk patients who may be difficult to treat.
There is an unmet medical need to extend survival and delay progression in heavily pretreated patients with refractory MM. Two novel agents with different mechanisms of action, selinexor5 and belantamab mafodotin,6 were recently approved for patients with relapsed or refractory MM (RRMM) who received ≥ 4 prior LOT but had overall response rates (ORRs) of only 21% to 34% in clinical trials.7-9
Personalized immunotherapy using a chimeric antigen receptor (CAR) involves genetically modifying a patient's own T cells so that they can identify and kill malignant plasma cells.10 The first B-cell maturation antigen (BCMA)–directed CAR-T cell immunotherapy, idecabtagene vicleucel (ide-cel), was approved in the United States for patients with RRMM after exposure to ≥ 4 prior LOT11 and was granted conditional approval in 2021 in the European Union for patients with RRMM who received ≥ 3 therapies and progressed on their last therapy.12 Approval was based on the results from the phase II KarMMa trial, which demonstrated an ORR of 73% and a median progression-free survival (PFS) of 8.8 months across all dose cohorts in heavily pretreated patients (median six prior LOT).13
Ciltacabtagene autoleucel (cilta-cel, JNJ-68284528) is a differentiated CAR-T therapy with two BCMA-targeting single-domain antibodies to confer avidity.14 It was recently approved by the US Food and Drug Administration for the treatment of adult patients with RRMM after ≥ 4 prior LOT, including a PI, an IMiD, and an anti-CD38 monoclonal antibody.15 In March 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion of cilta-cel, for an indication in patients with ≥ 3 prior LOT.16 Initial results from the phase Ib/II, single-arm CARTITUDE-1 trial demonstrated that cilta-cel led to early, deep, and durable responses among 97 patients with RRMM exposed to a median of six prior therapies.14 At a median follow-up (MFU) of 12 months, ORR was 97% with 67% of patients reaching stringent complete response (sCR). The median duration of response (DOR) and median PFS were not estimable (NE).14 Here, we present a prespecified analysis of the CARTITUDE-1 study, which was completed in early 2022, with a MFU of 28 months.
METHODS
Study Design and Treatment
CARTITUDE-1 was a single-arm, open-label, multicenter, phase Ib/II study conducted in patients with RRMM to characterize the safety of cilta-cel and confirm the recommended phase II dose (phase Ib) and evaluate clinical efficacy (phase II; ClinicalTrials.gov identifier: NCT03548207). The study design and primary results have been previously reported.14 Patients were required to have received ≥ 3 prior LOT, including a PI, IMiD, and an anti-CD38 antibody, or to be double refractory to PI and IMiD and have received an anti-CD38 antibody, with evidence of progressive disease (PD) within 12 months of the last LOT. Patients received a single cilta-cel infusion (target dose 0.75 × 106 CAR-positive viable T cells/kg; range, 0.5-1.0 × 106) 5-7 days after lymphodepletion (300 mg/m2 cyclophosphamide, 30 mg/m2 fludarabine once daily for 3 days). Retreatment with cilta-cel was permitted within the same dose range in patients with documented PD ≥ 6 months after cilta-cel infusion with best response of at least minimal response who had no ongoing hematologic (grade ≥ 3) or nonhematologic (grade ≥ 2) toxicities.
An independent ethics committee or institutional review board at each study center approved the study protocol, and all patients provided written informed consent. The study Protocol (online only) was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization guidelines for Good Clinical Practice.
End Points and Assessments
The primary efficacy end point of phase II was ORR; secondary end points were rates of sCR, complete response (CR), and very good partial response; minimal residual disease (MRD) negativity; DOR; PFS; and OS.14 Response was assessed by an independent review committee and adjudicated per International Myeloma Working Group (IMWG) criteria.17-19
MRD was assessed at baseline; day 28; and 6, 12, 18, and 24 months using next-generation sequencing (clonoSEQ v2.0; Adaptive Biotechnologies, Seattle, WA) regardless of disease status. An additional sample was collected and assessed at the time of suspected CR and every 12 months until PD for patients who remained on study. MRD negativity was assessed in samples that passed calibration or quality control and included sufficient cells for evaluation at the testing threshold of 10–5.
Extramedullary (EM) plasmacytomas were assessed for patients with a history of plasmacytomas or if clinically indicated at screening, by clinical examination or radiologic imaging, with continuing follow-up using the same method of evaluation at regular assessments post-treatment.
Adverse events (AEs) were graded using National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0. Cytokine release syndrome (CRS) was graded according to Lee criteria20 in phase Ib and American Society for Transplantation and Cellular Therapy (ASTCT) criteria21 in phase II. Neurotoxicity was graded using NCI-CTCAE v5.0 in phase Ib, and immune-effector cell-associated neurotoxicity syndrome (ICANS) was graded by ASTCT criteria21 in phase II.14 Other neurotoxicities (events not reported as ICANS) were graded by NCI-CTCAE version 5.0.
Statistical Analysis
Statistical analyses and sample size calculations have been described previously.14 The primary efficacy analysis set included all patients who received cilta-cel at the target dose range. Safety was assessed in all cilta-cel–treated patients.
ORR and two-sided 95% CIs were calculated on the basis of the exact binomial distribution. Time-to-event efficacy end points were estimated using the Kaplan-Meier method. Durability of MRD-negative status was characterized by quantifying MRD-negative status rates (10–5) sustained for at least 6 or 12 months.
Subgroup analyses were conducted in the following patient subgroups: age ≥ 65 years, Black/African American, 3 and ≥ 4 prior LOT, triple-class refractory, penta-drug refractory, standard- and high-risk cytogenetic status, International Staging System (ISS) stage III, bone marrow plasma cell percentage at baseline (≤ 30%, > 30% to < 60%, and ≥ 60%), tumor BCMA expression at baseline (< 80%, ≥ 80%), and presence of plasmacytomas (bone-based and EM).
RESULTS
Patients and Treatment
As of the January 11, 2022, data cutoff, 66 of the 97 patients who received cilta-cel infusion remained on study (Fig 1). As previously reported, the median time from receipt of the apheresis material to release of cilta-cel was 29 days (interquartile range 28-33); no patient discontinued the study because of manufacturing failure.14 All patients treated with cilta-cel received a dose within the target range (median, 0.71 × 106 cells/kg; range, 0.51-0.95 × 106). At baseline, patients had received a median of six (range, 3-18) prior LOT. Of 96 patients with evaluable bone marrow biopsy and/or aspirate samples, 21.9% had high disease burden (≥ 60% plasma cells). Plasmacytomas were detected at screening in 19 (19.6%) patients (13 [13.4%] EM, and six [6.2%] bone-based [Appendix Table A1, online only]).
FIG 1.
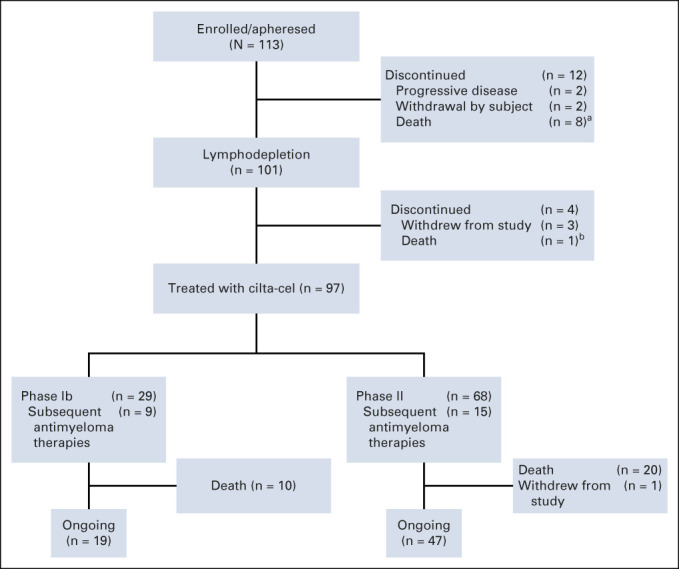
Patient disposition. aBecause of progressive disease (5), acute cardiorespiratory arrest (1), sepsis (1), and subdural hematoma (1). bBecause of acute respiratory failure. cilta-cel, ciltacabtagene autoleucel.
Efficacy
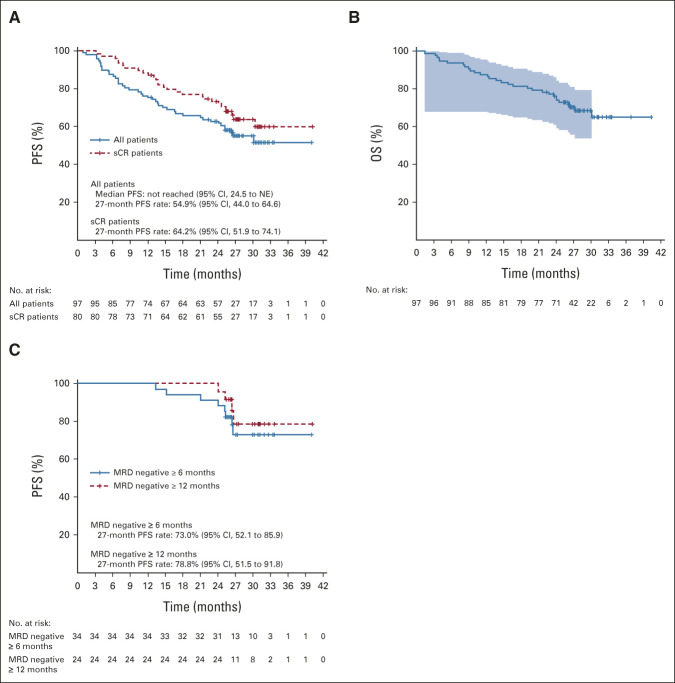
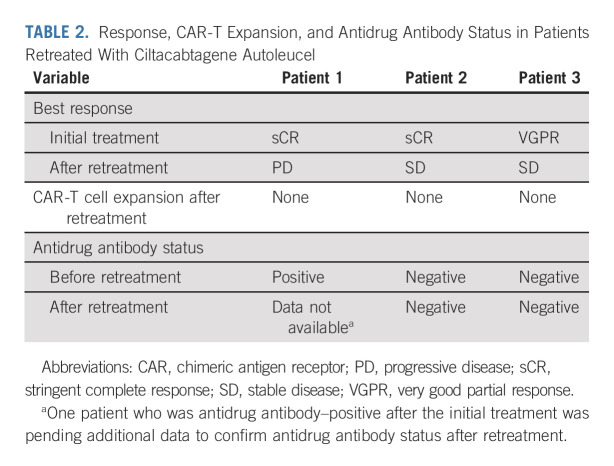
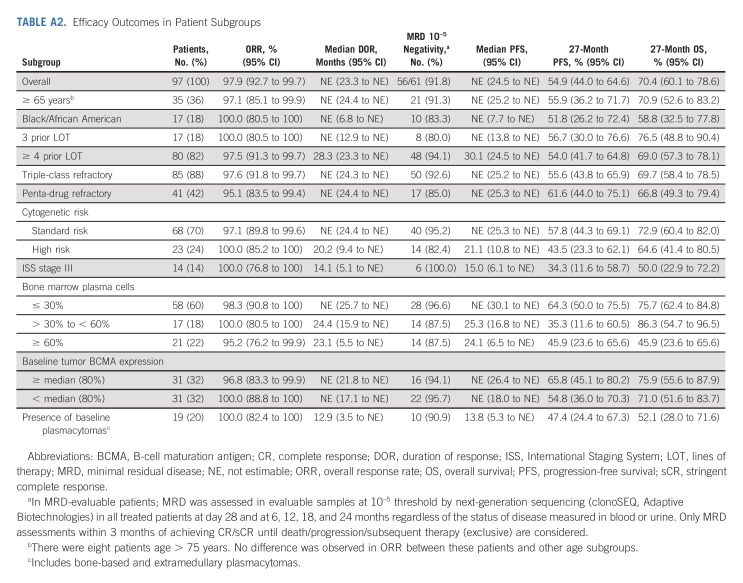
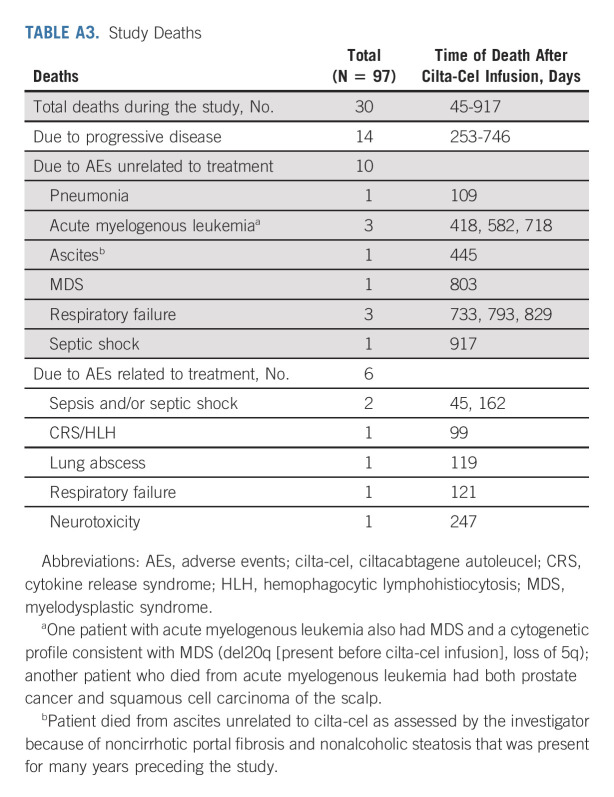
All patients who received cilta-cel were included in the efficacy analyses (N = 97). At a MFU of 27.7 months, ORR was 97.9%, sCR rate was 82.5% (no patient was CR only), very good partial response rate was 12.4%, and PR rate was 3.1% (Table 1). The median time to first response was 1 month, the median time to best response was 2.6 months, and the median time to CR or better was 2.9 months. Median DOR and median PFS were not reached (Fig 2A); the time point at which 75% of patients were progression-free (75th percentile) was 12.9 months (95% CI, 6.97 to 18.04). At the 27-month time point, PFS rates were 54.9% in the overall population and 64.2% in patients with sCR. Median OS was not reached, and the 75th percentile was 24.1 months (95% CI, 14.62 to not estimable). The 27-month OS rate in the overall population was 70.4% (Fig 2B). As of the data cutoff, three patients have been retreated with cilta-cel (same dose as the initial treatment; Table 2).
TABLE 1.
Response to Ciltacabtagene Autoleucela
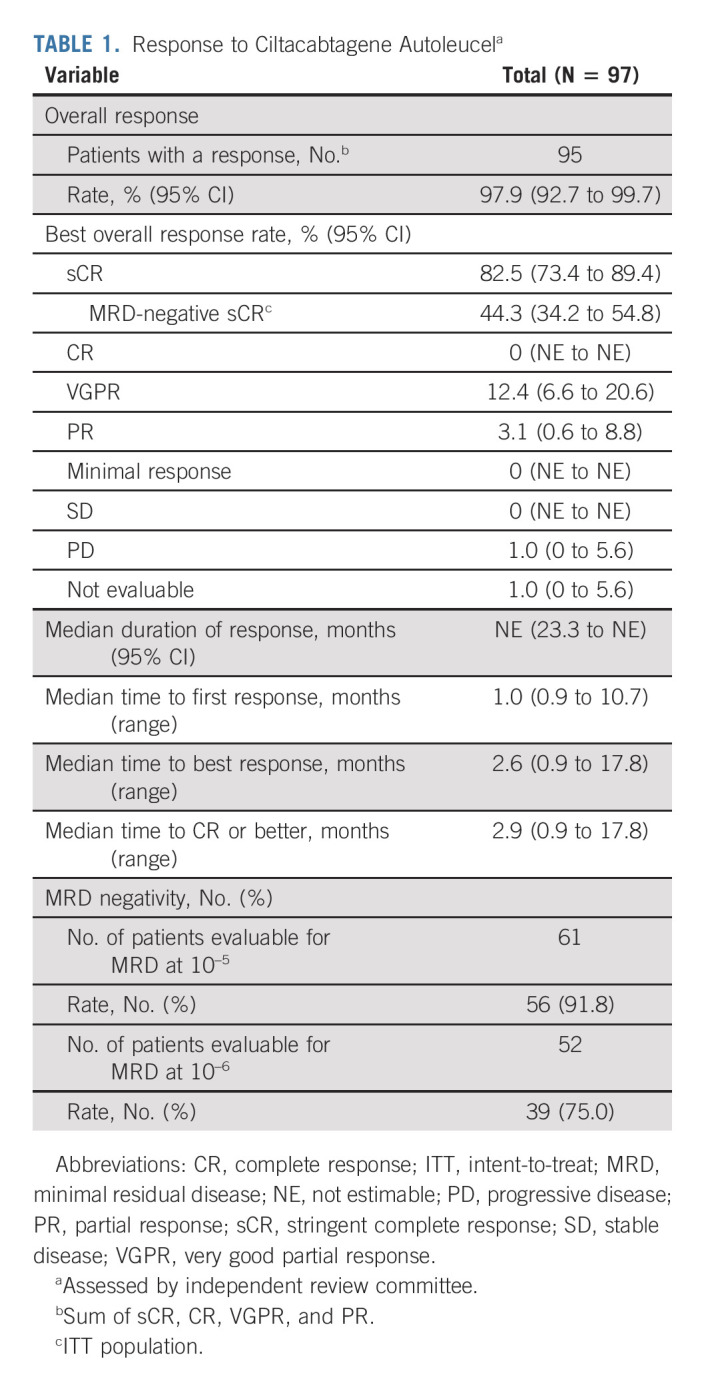
FIG 2.
(A) PFS for the overall population and patients with sCR. (B) OS. Shading shows 95% confidence bands. (C) PFS in patients with sustained MRD negativity (10–5) for ≥ 6 months or ≥ 12 months. MRD, minimal residual disease; NE, not estimable; OS, overall survival; PFS, progression-free survival; sCR, stringent complete response.
TABLE 2.
Response, CAR-T Expansion, and Antidrug Antibody Status in Patients Retreated With Ciltacabtagene Autoleucel
Sixty-one patients had samples evaluable for MRD status, defined as those that passed calibration and quality control and had sufficient cells for evaluation. At the 10–5 threshold, 56 (91.8%) patients achieved MRD negativity, which was sustained for ≥ 6 months in 68% (34 of 50 with sufficient follow-up) and ≥ 12 months in 55% (24 of 44 with sufficient follow-up). PFS rates in patients who achieved sustained MRD negativity for ≥ 6 and ≥ 12 months were 73.0% (95% CI, 52.1 to 85.9) and 78.8% (95% CI, 51.5 to 91.8), respectively (Fig 2C), and OS rates were 93.5% (95% CI, 76.1 to 98.3) and 90.8% (95% CI, 67.7 to 97.6), respectively. Of 52 patients with a sample evaluable for MRD status at the 10–6 threshold, 39 (75.0%) achieved MRD negativity.
Efficacy in Patient Subgroups
Most patients in high-risk subgroups responded to cilta-cel (ORR range, 95.1%-100%), including those treated with three prior LOT (100%), and those with a high-risk cytogenetic profile (100%), high tumor burden (≥ 60% bone marrow plasma cells; 95.2%), or plasmacytomas (100%; Appendix Table A2, online only). MRD negativity rates in evaluable patients (at 10–5) were 80%-100% across all subgroups. Compared with the overall cilta-cel population, patients with ISS stage III disease, high cytogenetic risk, plasmacytomas, or high tumor burden had shorter DOR (Fig 3) and lower PFS and OS rates. Patients with 30%-60% bone marrow plasma cells also had shorter DOR and a lower PFS rate than the overall population, and Black/African American patients had a reduced OS rate.
FIG 3.
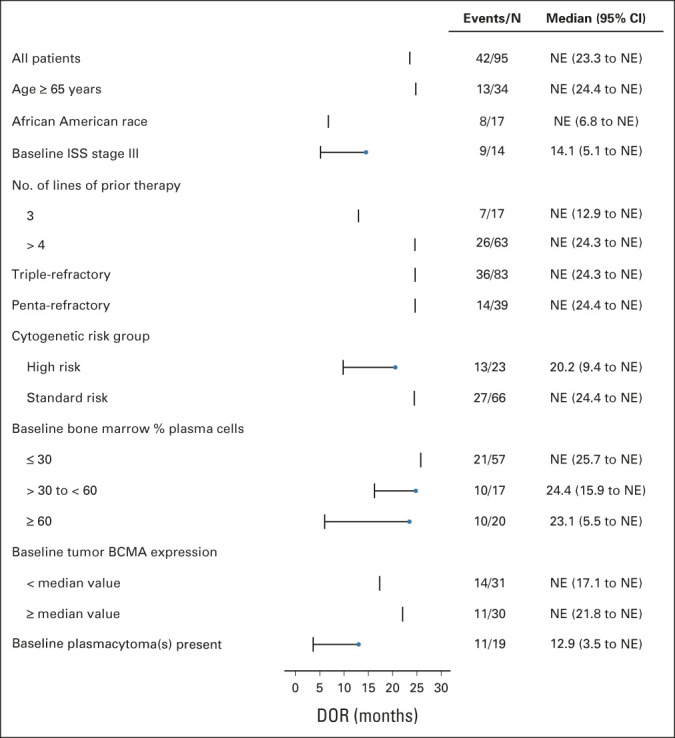
Forest plot of DOR in patient subgroups. BCMA, B-cell maturation antigen; DOR, duration of response; ISS, International Staging System; NE, not estimable.
Safety
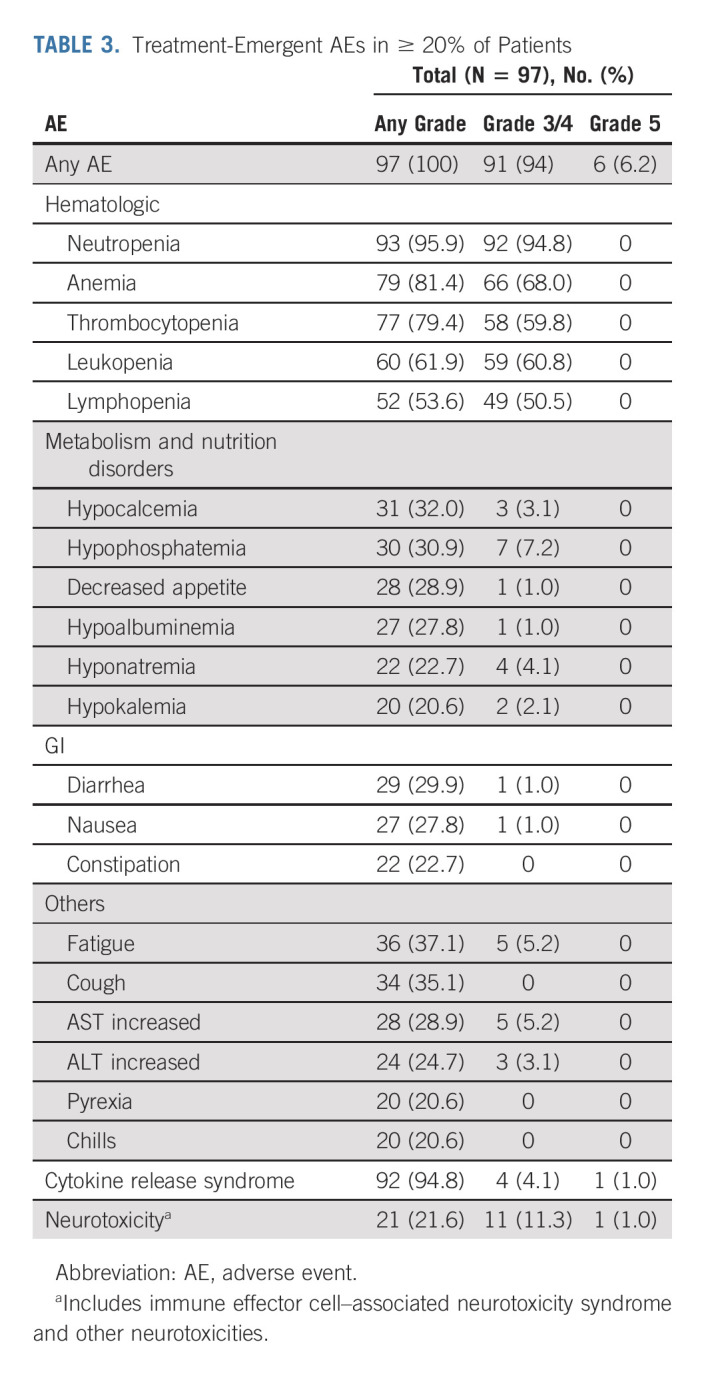
Hematologic adverse events.
The most common (≥ 25%) grade 3/4 treatment-emergent AEs (TEAEs) were hematologic (Table 3). On the basis of laboratory results, grade 3/4 thrombocytopenia occurred in 60 patients; 20 (33.3%) had recovered to grade ≤ 2 by day 30, and 35 (58.3%) recovered by day 60. Grade 3/4 neutropenia was reported in 95 patients; 66 (69.5%) had recovered to grade ≤ 2 by day 30 and 85 (89.5%) by day 60. Grade 3/4 lymphopenia occurred in 96 patients; 84 (87.5%) had recovered to grade ≤ 2 by day 30 and 88 (91.7%) by day 60.
TABLE 3.
Treatment-Emergent AEs in ≥ 20% of Patients
Nonhematologic AEs.
The most common grade 3/4 nonhematologic TEAEs (≥ 5%) were pneumonia (10.3%), hypophosphatemia (7.2%), increased gamma-glutamyl transferase (6.2%), hypertension (6.2%), fatigue (5.2%), and increased AST (5.2%).
Cytokine release syndrome and neurotoxicity.
Since the primary 12-month publication, no new events of CRS (no changes in the incidence, time to onset, or duration) occurred. One new case of signs and symptoms of parkinsonism (previously termed movement and neurocognitive TEAEs) occurred, for a total of six in the CARTITUDE-1 study.14 This patient had seven prior LOT, a history of ongoing peripheral sensory neuropathy at study entry, and grade 2 CRS and grade 3 ICANS after cilta-cel infusion. On day 914, the patient experienced cognitive slowing, gait instability, and neuropathy (all grade 1), and tremor (grade 3). Subsequent lumbar puncture was negative for CAR-T cells. The symptoms did not improve on a short course of levodopa. Without further treatment, the patient is stable and functioning (able to work), with slight improvement in his gait instability, and remains in sCR.14 Three of the six total patients with parkinsonism have died (two from other underlying causes [sepsis and lung abscess] and one related to parkinsonism). Of the other two who are living, one has recovered and one is recovering (ongoing grade 2 symptoms) at the time of the data cut.
Secondary primary malignancies.
In total, 20 secondary primary malignancies (SPMs) were reported in 16 patients; all were unrelated to cilta-cel. Nine patients had hematologic SPM, including one case of low-grade B-cell lymphoma, six cases of myelodysplastic syndrome, and three cases of fatal acute myeloid leukemia (AML; one patient had both myelodysplastic syndrome and fatal AML). Four patients had squamous cell carcinoma; one of these also had basal cell carcinoma. One patient had basal cell carcinoma that was present before cilta-cel infusion. One patient each had malignant melanoma, adenocarcinoma, or myxofibrosarcoma, and one patient had prostate cancer in addition to his squamous cell carcinoma and AML reported above.
Deaths.
A total of 30 deaths occurred during the study after cilta-cel infusion (Fig 1 and Appendix Table A3, online only). No deaths occurred within the first 30 days, two occurred within 100 days, and 28 occurred > 100 days post infusion. Fourteen patients died because of PD. Six deaths were treatment related (investigator assessed) and occurred within the first 12 months; details have been published previously.14 The remaining 10 deaths were due to AEs not related to study treatment (Appendix Table A3).
DISCUSSION
This prespecified analysis of the CARTITUDE-1 trial at 28 months MFU demonstrates the sustained clinical benefit of cilta-cel in patients with RRMM who had received a median of six prior LOT. As median PFS has not yet been reached, clinical benefit continues for many patients. Responses to a single infusion of cilta-cel deepened from 67% with sCR at 12-month MFU to 82.5% at 28 months.14 Response depth and durability were related, as illustrated by the higher rates of PFS in patients with sCR and/or sustained MRD negativity.22
All patient subgroups had high rates of response to cilta-cel, including those with plasmacytomas, high-risk cytogenetics, and ISS stage III. In some high-risk subgroups, DOR, PFS, and/or OS were lower than the overall population, which is expected in these difficult-to-treat groups. Cilta-cel efficacy in these populations was more favorable than other available or recently approved therapies for patients with heavily pretreated RRMM.13,23,24 The subgroup analysis was limited by small sample size of some groups. Furthermore, EM plasmacytomas were not assessed in all patients, but rather evaluated either clinically or radiologically only in those with a history of EM disease. Although the efficacy of cilta-cel in heavily pretreated patients is encouraging, shifting its use to earlier LOTs may improve long-term outcomes for patients. This approach is currently under evaluation in the CARTITUDE-2 (ClinicalTrials.gov identifier: NCT04133636), CARTITUDE-4 (ClinicalTrials.gov identifier: NCT04181827), CARTITUDE-5 (ClinicalTrials.gov identifier: NCT04923893), and Emagine/CARTITUDE-6 (EMN28; ClinicalTrials.gov identifier: NCT05257083) trials.
The depth and durability of responses achieved with cilta-cel highlight the potential of the CAR-T approach to transform the current treatment paradigm for RRMM. Because of the lack of established SOC and clinical equipoise, CARTITUDE-1 was necessarily limited by its design as a single-arm trial. Indirect treatment comparisons between the CARTITUDE-1 results and real-world SOC, in data sets comprising European and/or US patients (MAMMOTH study and the prospective LocoMMotion study), found that cilta-cel significantly improved outcomes versus real-world therapies in triple-class exposed patients with RRMM.3,25 In a matching-adjusted indirect treatment comparison using data from the similarly designed CARTITUDE-114 and KarMMa13 trials, cilta-cel appeared to have higher response rates and longer DOR and PFS than ide-cel.26 After a MFU of 13.3 months, the ORR in the KarMMa trial was 73% across all dose cohorts (33% CR or better) and 81% with the highest dose evaluated (450 × 106 cells; 39% CR or better). The median PFS was 8.8 months across all doses and 12.1 months at the highest dose, with ide-cel demonstrating durable CAR T-cell persistence.13 In contrast, the efficacy results for cilta-cel in CARTITUDE-1 were achieved despite a lack of detectable CAR T-cell persistence in peripheral blood after infusion; most patients did not have detectable cilta-cel CAR transgene levels in peripheral blood at 6-month follow-up.14,27
The unique structural makeup of cilta-cel may contribute to its differentiated efficacy compared with ide-cel. The cilta-cel CAR features two BCMA-targeting, single-domain antibodies comprising two variable regions of heavy chains designed to confer avidity14 while ide-cel comprises an extracellular single-chain variable fragment with one heavy and one light chain targeting a single epitope of BCMA.28-30
Other recently approved agents for treating triple-class refractory RRMM, selinexor5 and belantamab mafodotin,6 have demonstrated limited clinical benefit in heavily pretreated patients with RRMM. ORR with selinexor was 26%, and the median PFS was 3.7 months.7 Similarly, treatment with the recommended dosage of belantamab mafodotin resulted in an ORR of 31% and a median PFS of 2.9 months.8
The safety profile of cilta-cel remained manageable at 28-month MFU, with a risk/benefit profile that remains favorable. TEAEs were consistent with the 1-year MFU.14 Of the SPM observed, none were classified as related to cilta-cel and six were nonmelanoma skin cancers. This patient population was heavily pretreated, including with IMiDs (100%), alkylating agents (melphalan 83%; cyclophosphamide 65%), and/or autologous stem-cell transplantation (90%), all of which are associated with increased risk of SPM.31,32 Furthermore, these heavily treated patients have survived longer with cilta-cel than with other available treatments, and, therefore, continued follow-up is warranted.32
One new case of parkinsonism (day 914 after cilta-cel) was observed since the last report and is manageable as the patient is stable to improving with no CAR-T cell–directed treatments. This patient had grade 2 CRS and grade 3 ICANS, two risk factors that have been associated with parkinsonism after cilta-cel.33,34 Implementation of patient management strategies after CARTITUDE-1 have reduced the incidence of parkinsonism from 6% in CARTITUDE-1 to < 0.5% across the other studies of the cilta-cel program. Parkinsonism events appear to be a class effect of BCMA CAR-T therapies,11 and the delayed onset of this case underscores the need for continued patient monitoring. As cilta-cel is extending the lifespan of heavily pretreated RRMM patients, late-onset side effects may be observed with long-term follow-up.
Patients from the CARTITUDE-1 trial will continue to be followed for up to 15 years after infusion in a separate study (CARTinue, MMY4002) to further evaluate long-term efficacy and safety.
In conclusion, these longer-term data from CARTITUDE-1 in triple-class exposed patients with RRMM demonstrate deep and durable responses to cilta-cel over time, including in high-risk subgroups. The safety profile continues to be manageable. The recent approval of cilta-cel (CARVYKTI; Janssen Biotech, Inc, Horsham, PA) in the US and positive CHMP opinion by the EMA will help fill an unmet medical need in this difficult-to-treat population.
ACKNOWLEDGMENT
We thank the patients who volunteered to participate in the study, their families and caregivers, the physicians and nurses who cared for the patients and supported this clinical trial, staff members at the 16 US study sites who enrolled patients, and staff members involved in data collection and analysis. Medical writing support was provided by Andreja Varjacic, PhD, Karen Pemberton, PhD, and Julie Nowicki, PhD, of Eloquent Scientific Solutions and was funded by Janssen Global Services.
APPENDIX
TABLE A1.
Patient Demographics and Baseline Characteristics
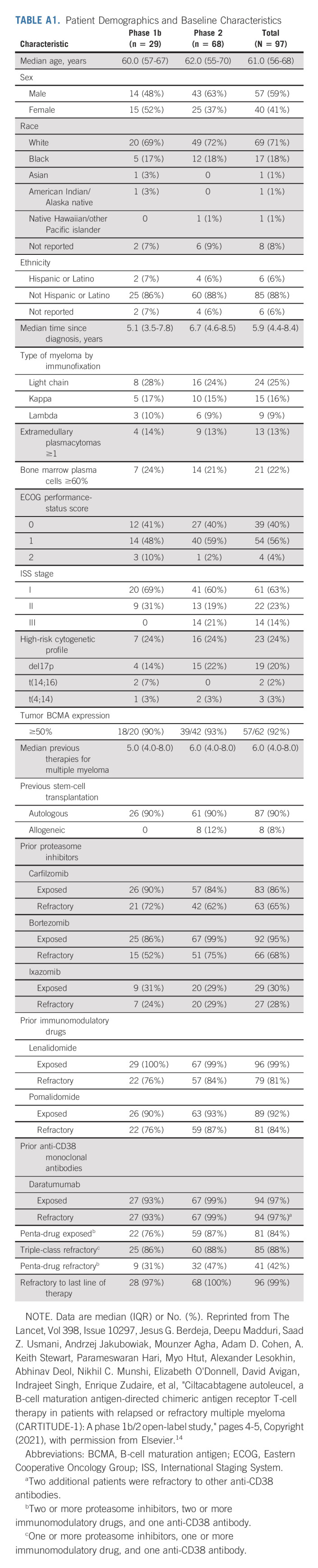
TABLE A2.
Efficacy Outcomes in Patient Subgroups
TABLE A3.
Study Deaths
Thomas Martin
Consulting or Advisory Role: GlaxoSmithKline, Legend Biotech
Research Funding: Sanofi (Inst), AMGEN (Inst), Janssen Oncology (Inst)
Saad Z. Usmani
Consulting or Advisory Role: Celgene, Amgen, Janssen Oncology, Seattle Genetics, Takeda, GlaxoSmithKline, Karyopharm Therapeutics, AbbVie, Skyline Diagnostics, Merck, Oncopeptides, Genentech, Gilead Sciences, Bristol Myers Squibb/Celgene
Speakers' Bureau: Takeda, Amgen, Janssen Oncology, Sanofi, Bristol Myers Squibb/Celgene
Research Funding: Celgene, Array BioPharma, Janssen Oncology, Pharmacyclics, Sanofi, Bristol Myers Squibb, Amgen, Seattle Genetics, Merck, Skyline Diagnostics, GlaxoSmithKline
Jesus G. Berdeja
Consulting or Advisory Role: Takeda (Inst), Bristol Myers Squibb (Inst), CRISPR Therapeutics (Inst), Celgene (Inst), Kite, a Gilead company (Inst), Janssen (Inst), Legend Biotech (Inst), Secura Bio (Inst), Bluebird Bio (Inst)
Research Funding: Celgene (Inst), Takeda (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), Janssen (Inst), Novartis (Inst), AbbVie (Inst), Bluebird Bio (Inst), Teva (Inst), Genentech/Roche (Inst), Poseida Therapeutics (Inst), Sanofi (Inst), Acetylon Pharmaceuticals (Inst), Lilly (Inst), Celularity (Inst), CRISPR Therapeutics (Inst), EMD Serono (Inst), Ichnos Sciences (Inst), GlaxoSmithKline (Inst), Incyte (Inst)
Mounzer Agha
Stock and Other Ownership Interests: GenCART, Inc
Adam D. Cohen
Consulting or Advisory Role: Celgene, Janssen Oncology, Oncopeptides, Takeda, Seattle Genetics, GlaxoSmithKline, Novartis, Bristol Myers Squibb, AstraZeneca, Roche/Genentech
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst)
Patents, Royalties, Other Intellectual Property: Patents related to CAR T cells and biomarkers of Cytokine Release Syndrome
Expert Testimony: Janssen Oncology
Travel, Accommodations, Expenses: GlaxoSmithKline, Takeda, Celgene, Janssen Oncology
Parameswaran Hari
Employment: Iovance Biotherapeutics
Honoraria: Sanofi, Amgen, Novartis, BMS, Janssen Oncology, Karyopharm Therapeutics, Takeda, Incyte, Kite, a Gilead company, GlaxoSmithKline, AbbVie/Pharmacyclics
Consulting or Advisory Role: Sanofi, Takeda, Bristol Myers Squibb, Janssen, Amgen, Kite, a Gilead company, Karyopharm Therapeutics, Pharmacyclics
Research Funding: Millennium (Inst), Celgene (Inst), Onyx (Inst), Spectrum Pharmaceuticals (Inst)
David Avigan
Employment: Parexel
Consulting or Advisory Role: Celgene, Kite/Gilead, Chugai Pharma, Karyopharm Therapeutics, Juno Therapeutics, Legend Biotech, Takeda, Bristol Myers Squibb, Aviv MedTech, Partner Therapeutics, Janssen, Sanofi, Kowa Pharmaceutical
Research Funding: Pharmacyclics, Celgene, Kite, a Gilead company
Abhinav Deol
Consulting or Advisory Role: Novartis, Kite/Gilead, Agios, Juno/Celgene, Janssen, Adicet Bio
Myo Htut
Consulting or Advisory Role: Millennium
Research Funding: Judy and Bernard Briskin Center for Multiple Myeloma Research (Inst), BMS (Inst)
Travel, Accommodations, Expenses: Millennium
Alexander Lesokhin
Honoraria: Janssen, Legend Biotech, Trillium Therapeutics, Sanofi, Pfizer, ITeos Therapeutics
Consulting or Advisory Role: Pfizer, Trillium Therapeutics
Research Funding: Bristol Myers Squibb (Inst), Janssen Oncology (Inst), Genentech (Inst), Trillium Therapeutics (Inst), Sanofi (Inst), Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: Serametrix, Inc
Nikhil C. Munshi
Stock and Other Ownership Interests: OncoPep, C4 Therapeutics, Raqia
Consulting or Advisory Role: Takeda, Janssen, OncoPep, AbbVie, Adaptive Biotechnologies, Amgen, BeiGene, Karyopharm Therapeutics, Bristol Myers Squibb, Bristol Myers Squibb/Celgene, Legend Biotech, Novartis, Sebia, Raqia
Patents, Royalties, Other Intellectual Property: OncoPep
Elizabeth O'Donnell
Consulting or Advisory Role: Celgene, BMS, Sanofi, Janssen, Takeda, Karyopharm Therapeutics, Oncopeptides
Research Funding: Takeda (Inst), Amgen (Inst), Janssen (Inst), Sanofi (Inst)
A. Keith Stewart
Leadership: Genomics England, Tempus Inc
Stock and Other Ownership Interests: C4 therapeutics
Consulting or Advisory Role: Janssen, Amgen, Sanofi, GlaxoSmithKline, Skyline Diagnostics, AstraZeneca
Research Funding: Amgen, Celgene, Janssen
Other Relationship: PikSci Inc
Jordan M. Schecter
Employment: Janssen Oncology
Stock and Other Ownership Interests: Janssen Oncology
Jenna D. Goldberg
Employment: Janssen
Stock and Other Ownership Interests: Janssen
Carolyn C. Jackson
Employment: Janssen Research & Development
Consulting or Advisory Role: Memorial Sloan Kettering Cancer Center
Tzu-Min Yeh
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Janssen Research & Development
Arnob Banerjee
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Alicia Allred
Employment: Johnson & Johnson/Janssen
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Enrique Zudaire
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Janssen Research & Development
Patents, Royalties, Other Intellectual Property: Patents pending with Janssen
Travel, Accommodations, Expenses: Janssen Research & Development
William Deraedt
Employment: J&J
Stock and Other Ownership Interests: J&J
Patents, Royalties, Other Intellectual Property: Patent
Changwei Zhou
Employment: Legend Biotech
Lida Pacaud
Employment: Legend Biotech
Stock and Other Ownership Interests: Legend Biotech
Deepu Madduri
Employment: Janssen Oncology
Stock and Other Ownership Interests: Roivant
Consulting or Advisory Role: Takeda, Janssen Oncology, Celgene, Legend Biotech, GlaxoSmithKline, Foundation Medicine
Andrzej Jakubowiak
Honoraria: AbbVie, Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Karyopharm Therapeutics, Sanofi, Gracell
Consulting or Advisory Role: AbbVie, Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Karyopharm Therapeutics, Sanofi, Gracell
Yi Lin
Consulting or Advisory Role: Kite/Gilead (Inst), Novartis (Inst), Bluebird Bio (Inst), Celgene (Inst), Juno Therapeutics (Inst), Bristol Myers Squibb (Inst), Gamida Cell (Inst), Legend Biotech (Inst), Sorrento Therapeutics (Inst), Vineti (Inst), Janssen Oncology (Inst), Pfizer (Inst)
Research Funding: Janssen Oncology (Inst), Merck (Inst), Takeda (Inst), Boston Scientific (Inst), Kite/Gilead (Inst), Bristol Myers Squibb (Inst), Bluebird Bio (Inst)
Sundar Jagannath
Consulting or Advisory Role: Bristol Myers Squibb, Janssen, Karyopharm Therapeutics, Legend Biotech, Takeda, Sanofi
Travel, Accommodations, Expenses: Bristol Myers Squibb, Janssen Biotech
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 3-7, 2022.
SUPPORT
Supported by Janssen Research & Development, LLC, and Legend Biotech, Inc.
CLINICAL TRIAL INFORMATION
Y.L. and S.J. are co-senior authors.
DATA SHARING STATEMENT
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
AUTHOR CONTRIBUTIONS
Conception and design: Thomas Martin, Abhinav Deol, A. Keith Stewart, Jordan M. Schecter, Jenna D. Goldberg, Carolyn C. Jackson, Tzu-Min Yeh, Arnob Banerjee, Enrique Zudaire, William Deraedt, Yunsi Olyslager, Lida Pacaud, Deepu Madduri, Yi Lin, Sundar Jagannath
Administrative support: Sundar Jagannath
Provision of study materials or patients: Thomas Martin, Jesus G. Berdeja, Adam D. Cohen, Parameswaran Hari, David Avigan, Myo Htut, Alexander Lesokhin, Nikhil C. Munshi, A. Keith Stewart, Lida Pacaud, Andrzej Jakubowiak, Sundar Jagannath
Collection and assembly of data: Thomas Martin, Saad Z. Usmani, Mounzer Agha, Adam D. Cohen, Parameswaran Hari, David Avigan, Abhinav Deol, Myo Htut, Alexander Lesokhin, Elizabeth O'Donnell, Jordan M. Schecter, Jenna D. Goldberg, Carolyn C. Jackson, Arnob Banerjee, Alicia Allred, Enrique Zudaire, William Deraedt, Yunsi Olyslager, Lida Pacaud, Deepu Madduri, Andrzej Jakubowiak, Yi Lin, Sundar Jagannath
Data analysis and interpretation: Thomas Martin, Saad Z. Usmani, Jesus G. Berdeja, Mounzer Agha, Adam D. Cohen, Parameswaran Hari, David Avigan, Abhinav Deol, Myo Htut, Nikhil C. Munshi, Elizabeth O'Donnell, A. Keith Stewart, Jordan M. Schecter, Jenna D. Goldberg, Carolyn C. Jackson, Tzu-Min Yeh, Arnob Banerjee, Alicia Allred, Enrique Zudaire, William Deraedt, Yunsi Olyslager, Changwei Zhou, Lida Pacaud, Deepu Madduri, Andrzej Jakubowiak, Yi Lin, Sundar Jagannath
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Ciltacabtagene Autoleucel, an Anti–B-cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Thomas Martin
Consulting or Advisory Role: GlaxoSmithKline, Legend Biotech
Research Funding: Sanofi (Inst), AMGEN (Inst), Janssen Oncology (Inst)
Saad Z. Usmani
Consulting or Advisory Role: Celgene, Amgen, Janssen Oncology, Seattle Genetics, Takeda, GlaxoSmithKline, Karyopharm Therapeutics, AbbVie, Skyline Diagnostics, Merck, Oncopeptides, Genentech, Gilead Sciences, Bristol Myers Squibb/Celgene
Speakers' Bureau: Takeda, Amgen, Janssen Oncology, Sanofi, Bristol Myers Squibb/Celgene
Research Funding: Celgene, Array BioPharma, Janssen Oncology, Pharmacyclics, Sanofi, Bristol Myers Squibb, Amgen, Seattle Genetics, Merck, Skyline Diagnostics, GlaxoSmithKline
Jesus G. Berdeja
Consulting or Advisory Role: Takeda (Inst), Bristol Myers Squibb (Inst), CRISPR Therapeutics (Inst), Celgene (Inst), Kite, a Gilead company (Inst), Janssen (Inst), Legend Biotech (Inst), Secura Bio (Inst), Bluebird Bio (Inst)
Research Funding: Celgene (Inst), Takeda (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), Janssen (Inst), Novartis (Inst), AbbVie (Inst), Bluebird Bio (Inst), Teva (Inst), Genentech/Roche (Inst), Poseida Therapeutics (Inst), Sanofi (Inst), Acetylon Pharmaceuticals (Inst), Lilly (Inst), Celularity (Inst), CRISPR Therapeutics (Inst), EMD Serono (Inst), Ichnos Sciences (Inst), GlaxoSmithKline (Inst), Incyte (Inst)
Mounzer Agha
Stock and Other Ownership Interests: GenCART, Inc
Adam D. Cohen
Consulting or Advisory Role: Celgene, Janssen Oncology, Oncopeptides, Takeda, Seattle Genetics, GlaxoSmithKline, Novartis, Bristol Myers Squibb, AstraZeneca, Roche/Genentech
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst)
Patents, Royalties, Other Intellectual Property: Patents related to CAR T cells and biomarkers of Cytokine Release Syndrome
Expert Testimony: Janssen Oncology
Travel, Accommodations, Expenses: GlaxoSmithKline, Takeda, Celgene, Janssen Oncology
Parameswaran Hari
Employment: Iovance Biotherapeutics
Honoraria: Sanofi, Amgen, Novartis, BMS, Janssen Oncology, Karyopharm Therapeutics, Takeda, Incyte, Kite, a Gilead company, GlaxoSmithKline, AbbVie/Pharmacyclics
Consulting or Advisory Role: Sanofi, Takeda, Bristol Myers Squibb, Janssen, Amgen, Kite, a Gilead company, Karyopharm Therapeutics, Pharmacyclics
Research Funding: Millennium (Inst), Celgene (Inst), Onyx (Inst), Spectrum Pharmaceuticals (Inst)
David Avigan
Employment: Parexel
Consulting or Advisory Role: Celgene, Kite/Gilead, Chugai Pharma, Karyopharm Therapeutics, Juno Therapeutics, Legend Biotech, Takeda, Bristol Myers Squibb, Aviv MedTech, Partner Therapeutics, Janssen, Sanofi, Kowa Pharmaceutical
Research Funding: Pharmacyclics, Celgene, Kite, a Gilead company
Abhinav Deol
Consulting or Advisory Role: Novartis, Kite/Gilead, Agios, Juno/Celgene, Janssen, Adicet Bio
Myo Htut
Consulting or Advisory Role: Millennium
Research Funding: Judy and Bernard Briskin Center for Multiple Myeloma Research (Inst), BMS (Inst)
Travel, Accommodations, Expenses: Millennium
Alexander Lesokhin
Honoraria: Janssen, Legend Biotech, Trillium Therapeutics, Sanofi, Pfizer, ITeos Therapeutics
Consulting or Advisory Role: Pfizer, Trillium Therapeutics
Research Funding: Bristol Myers Squibb (Inst), Janssen Oncology (Inst), Genentech (Inst), Trillium Therapeutics (Inst), Sanofi (Inst), Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: Serametrix, Inc
Nikhil C. Munshi
Stock and Other Ownership Interests: OncoPep, C4 Therapeutics, Raqia
Consulting or Advisory Role: Takeda, Janssen, OncoPep, AbbVie, Adaptive Biotechnologies, Amgen, BeiGene, Karyopharm Therapeutics, Bristol Myers Squibb, Bristol Myers Squibb/Celgene, Legend Biotech, Novartis, Sebia, Raqia
Patents, Royalties, Other Intellectual Property: OncoPep
Elizabeth O'Donnell
Consulting or Advisory Role: Celgene, BMS, Sanofi, Janssen, Takeda, Karyopharm Therapeutics, Oncopeptides
Research Funding: Takeda (Inst), Amgen (Inst), Janssen (Inst), Sanofi (Inst)
A. Keith Stewart
Leadership: Genomics England, Tempus Inc
Stock and Other Ownership Interests: C4 therapeutics
Consulting or Advisory Role: Janssen, Amgen, Sanofi, GlaxoSmithKline, Skyline Diagnostics, AstraZeneca
Research Funding: Amgen, Celgene, Janssen
Other Relationship: PikSci Inc
Jordan M. Schecter
Employment: Janssen Oncology
Stock and Other Ownership Interests: Janssen Oncology
Jenna D. Goldberg
Employment: Janssen
Stock and Other Ownership Interests: Janssen
Carolyn C. Jackson
Employment: Janssen Research & Development
Consulting or Advisory Role: Memorial Sloan Kettering Cancer Center
Tzu-Min Yeh
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Janssen Research & Development
Arnob Banerjee
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Alicia Allred
Employment: Johnson & Johnson/Janssen
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Enrique Zudaire
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Janssen Research & Development
Patents, Royalties, Other Intellectual Property: Patents pending with Janssen
Travel, Accommodations, Expenses: Janssen Research & Development
William Deraedt
Employment: J&J
Stock and Other Ownership Interests: J&J
Patents, Royalties, Other Intellectual Property: Patent
Changwei Zhou
Employment: Legend Biotech
Lida Pacaud
Employment: Legend Biotech
Stock and Other Ownership Interests: Legend Biotech
Deepu Madduri
Employment: Janssen Oncology
Stock and Other Ownership Interests: Roivant
Consulting or Advisory Role: Takeda, Janssen Oncology, Celgene, Legend Biotech, GlaxoSmithKline, Foundation Medicine
Andrzej Jakubowiak
Honoraria: AbbVie, Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Karyopharm Therapeutics, Sanofi, Gracell
Consulting or Advisory Role: AbbVie, Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Karyopharm Therapeutics, Sanofi, Gracell
Yi Lin
Consulting or Advisory Role: Kite/Gilead (Inst), Novartis (Inst), Bluebird Bio (Inst), Celgene (Inst), Juno Therapeutics (Inst), Bristol Myers Squibb (Inst), Gamida Cell (Inst), Legend Biotech (Inst), Sorrento Therapeutics (Inst), Vineti (Inst), Janssen Oncology (Inst), Pfizer (Inst)
Research Funding: Janssen Oncology (Inst), Merck (Inst), Takeda (Inst), Boston Scientific (Inst), Kite/Gilead (Inst), Bristol Myers Squibb (Inst), Bluebird Bio (Inst)
Sundar Jagannath
Consulting or Advisory Role: Bristol Myers Squibb, Janssen, Karyopharm Therapeutics, Legend Biotech, Takeda, Sanofi
Travel, Accommodations, Expenses: Bristol Myers Squibb, Janssen Biotech
No other potential conflicts of interest were reported.
REFERENCES
- 1.Kumar SK, Callander NS, Adekola K, et al. : Multiple myeloma, version 7.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 19:484-493, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Gandhi UH, Cornell RF, Lakshman A, et al. : Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 33:2266-2275, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mateos MV, Weisel K, De Stefano V, et al. : LocoMMotion: A prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma. Leukemia 36:1371-1376, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yong K, Delforge M, Driessen C, et al. : Multiple myeloma: Patient outcomes in real-world practice. Br J Haematol 175:252-264, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karyopharm Therapeutics, Inc: XPOVIO® (selinexor) prescribing information. https://www.karyopharm.com/wp-content/uploads/2019/07/NDA-212306-SN-0071-Prescribing-Information-01July2019.pdf [Google Scholar]
- 6.GlaxoSmithKline: BLENREP® (belantamab mafodotin-blmf) prescribing information. https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Blenrep/pdf/BLENREP-PI-MG.PDF [Google Scholar]
- 7.Chari A, Vogl DT, Gavriatopoulou M, et al. : Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med 381:727-738, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Lonial S, Lee HC, Badros A, et al. : Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol 21:207-221, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Vogl DT, Dingli D, Cornell RF, et al. : Selective inhibition of nuclear export with oral selinexor for treatment of relapsed or refractory multiple myeloma. J Clin Oncol 36:859-866, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feins S, Kong W, Williams EF, et al. : An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol 94:S3-S9, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Celgene Corporation, a Bristol-Myers Squibb Company: ABECMA® (idecabtagene vicleucel) prescribing information. https://packageinserts.bms.com/pi/pi_abecma.pdf [Google Scholar]
- 12.Bristol Myers Squibb : Bristol Myers Squibb receives European Commission approval for Abecma (idecabtagene vicleucel), the first anti-BCMA CAR T Cell therapy for relapsed and refractory multiple myeloma. 2021. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Receives-European-Commission-Approval-for-Abecma-Idecabtagene-Vicleucel-the-First-Anti-BCMA-CAR-T-Cell-Therapy-for-Relapsed-and-Refractory-Multiple-Myeloma/default.aspx [Google Scholar]
- 13.Munshi NC, Anderson LD, Jr, Shah N, et al. : Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med 384:705-716, 2021 [DOI] [PubMed] [Google Scholar]
- 14.Berdeja JG, Madduri D, Usmani SZ, et al. : Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 398:314-324, 2021 [DOI] [PubMed] [Google Scholar]
- 15.Janssen Biotech, Inc and Legend Biotech: CARVYKTI® (ciltacabtagene autoleucel) prescribing information. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/CARVYKTI-pi.pdf [Google Scholar]
- 16.Legend Biotech : CARVYKTI® (ciltacabtagene autoleucel) receives positive CHMP opinion for the treatment of patients with relapsed and refractory multiple myeloma. 2022. https://investors.legendbiotech.com/news-releases/news-release-details/carvyktir-ciltacabtagene-autoleucel-receives-positive-chmp [Google Scholar]
- 17.Durie BG, Miguel JF, Blade J, et al. : Clarification of the definition of complete response in multiple myeloma. Leukemia 29:2416-2417, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Kumar S, Paiva B, Anderson KC, et al. : International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 17:e328-e346, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Rajkumar SV, Harousseau JL, Durie B, et al. : Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood 117:4691-4695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee DW, Gardner R, Porter DL, et al. : Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124:188-195, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DW, Santomasso BD, Locke FL, et al. : ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transpl 25:625-638, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munshi NC, Avet-Loiseau H, Anderson KC, et al. : A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma. Blood Adv 4:5988-5999, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson PG, Lee HC, Abdallah AO, et al. : Single-agent belantamab mafodotin for relapsed/refractory multiple myeloma: Analysis of the lyophilised presentation cohort from the pivotal DREAMM-2 study. Blood Cancer J 10:106, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richter J, Madduri D, Richard S, et al. : Selinexor in relapsed/refractory multiple myeloma. Ther Adv Hematol 11:2040620720930629, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa LJ, Lin Y, Cornell RF, et al. : Comparison of cilta-cel, an anti-BCMA CAR-T cell therapy, versus conventional treatment in patients with relapsed/refractory multiple myeloma. Clin Lymphoma Myeloma Leuk 22:326-335, 2022 [DOI] [PubMed] [Google Scholar]
- 26.Martin T, Usmani SZ, Schecter JM, et al. : Matching-adjusted indirect comparison of efficacy outcomes for ciltacabtagene autoleucel in CARTITUDE-1 versus idecabtagene vicleucel in KarMMa for the treatment of patients with relapsed or refractory multiple myeloma. Curr Med Res Opin 37:1779-1788, 2021 [DOI] [PubMed] [Google Scholar]
- 27.Zudaire E, Madduri D, Usmani SZ, et al. : Translational analysis from CARTITUDE-1, an ongoing phase 1b/2 study of JNJ-4528 BCMA-targeted CAR-T cell therapy in relapsed and/or refractory multiple myeloma (R/R MM), indicates preferential expansion of CD8+ T cell central memory cell subset. Blood 134, 2019. (abstr 928) [Google Scholar]
- 28.Roex G, Timmers M, Wouters K, et al. : Safety and clinical efficacy of BCMA CAR-T-cell therapy in multiple myeloma. J Hematol Oncol 13:164, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruno B, Wäsch R, Engelhardt M, et al. : European Myeloma Network perspective on CAR T-Cell therapies for multiple myeloma. Haematologica 106:2054-2065, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oriol A, Abril L, Torrent A, et al. : The role of idecabtagene vicleucel in patients with heavily pretreated refractory multiple myeloma. Ther Adv Hematol 12:20406207211019622, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palumbo A, Bringhen S, Kumar SK, et al. : Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: A meta-analysis of individual patient data. Lancet Oncol 15:333-342, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Poh C, Keegan T, Rosenberg AS: Second primary malignancies in multiple myeloma: A review. Blood Rev 46:100757, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Einsele H, Parekh S, Madduri D, et al. : Incidence, mitigation, and management of neurologic adverse events in patients with multiple myeloma (MM) treated with ciltacabtagene autoleucel (cilta-cel) in CARTITUDE-2. J Clin Oncol 39, 2021. (suppl 15; abstr 8028) [Google Scholar]
- 34.Cohen AD, Parekh S, Santomasso BD, et al. : Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J 12:32, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.