Abstract
Background:
Despite improvements in population health, marked racial/ethnic disparities in longevity and cardiovascular disease(CVD) mortality persist. The purpose of this study was to describe risks for all-cause and CVD mortality by race/ethnicity, before and after accounting for socio-economic status (SES) and other factors, in the Multi-Ethnic Study of Atherosclerosis (MESA).
Methods:
MESA recruited 6814 US adults, age 45–84 years, free of clinical CVD at baseline, including Black, White, Hispanic and Chinese individuals (2000–2002). Using Cox proportional hazards modeling with time-updated covariates, we evaluated the association of self-reported race/ethnicity with all-cause and adjudicated CVD mortality, with progressive adjustments for age and sex, SES (neighborhood SES, income, education and health insurance), lifestyle and psychosocial risk factors, clinical risk factors, and immigration history.
Results:
During a median of 15.8 years of follow-up, 22.8% of participants (n=1552) died, of which 5.3% (n=364) died from CVD. After adjusting for age and sex, Black participants had a 34% higher mortality hazard [hazard ratio (HR) 1.34; 95% CI 1.19–1.51], Chinese participants had a 21% lower mortality hazard (HR 0.79; 0.66–0.95), and there was no mortality difference in Hispanic (HR 0.99; 0.86–1.14) compared to White participants. After adjusting for SES, the mortality hazard ratio for Black relative to White participants was reduced (HR 1.16; 1.01–1.34) but still statistically significant. With adjustment for SES, the mortality hazards for Chinese and Hispanic participants also decreased relative to White participants. After further adjustment for additional risk factors and immigration history, Hispanic participants (HR 0.77; 0.63–0.94) had a lower mortality risk relative to White participants, and hazard ratios for Black (HR 1.08; 0.92–1.26) and Chinese (HR 0.81;0.60–1.08) were not significantly different from those of White participants. Similar trends were seen for CVD mortality, although the age and sex- adjusted hazard ratio for CVD mortality for Black compared to White participants was greater than all-cause mortality (HR 1.72; 1.34–2.21, compared with HR 1.34; 1.19–1.51).
Conclusions:
These results highlight persistent racial/ethnic differences in overall and CVD mortality, largely attributable to social determinants of health, and support the need to identify and act on systemic factors that shape differences in health across racial/ethnic groups.
Public health advances and medical discoveries have led to dramatic reductions in cardiovascular disease (CVD) mortality and improvements in adult life expectancy in the United States over the last few decades. Despite general improvements in population health, marked inequities in longevity and cardiovascular disease (CVD) mortality between racial/ethnic groups persist.1–7 Following Healthy People 2020 we define disparities as a particular type of health difference that is closely linked with economic, social, or environmental disadvantage.8 Thus disparities are not biologically determined, rather they are largely driven by structural and systemic factors related to social organization and racism that operate through the social determinants of health.9, 10
The Multi-Ethnic Study of Atherosclerosis (MESA) is a longitudinal cohort study which includes a diverse, community-based sample of adults with detailed assessments of SES, lifestyle, psychosocial, environmental and clinical risk factors, and immigrant history/acculturation with follow-up ascertainment of adjudicated CVD events and mortality.11 There have been multiple individual analyses in MESA describing racial/ethnic differences in these factors and associations with CVD.12–23 The purpose of this report was to describe racial/ethnic differences in all-cause and CVD mortality in self-identified White, Black, Hispanic and Chinese adults in MESA, before and after accounting for socio-economic status (SES) and other factors.
Methods:
MESA is an observational cohort study, designed to determine risk factors for progression of subclinical CVD and progression to clinically overt CVD among adults of White, Black, Hispanic and Chinese race/ethnicity.11, 24 MESA recruited 6814 adults in 2000–2002, age 45–84 years, who self-identified their race/ethnicity. All participants were free of clinically recognized CVD at baseline, including a history of myocardial infarction, angina, stroke, transient ischemic attack, heart failure, cardiac surgery or percutaneous intervention, current atrial fibrillation, active treatment for cancer or any serious condition which would prevent long-term participation. Participants were recruited from six urban/suburban field centers in the United States (Baltimore, Maryland; Chicago, IL; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and St Paul, Minnesota). Each field center recruited approximately equal numbers of men and women from two or more of the racial/ethnic groups. The baseline exam was completed between 2000 and 2002 with five subsequent examinations: visit 2 (2002–2004), visit 3 (2004–2005), visit 4 (2005–2007), visit 5 (2010–2012) and visit 6 (2016–2018). Details are available at the MESA website at https://www.mesa-nhlbi.org. Participants have been followed for two decades with systematic ascertainment and adjudication of CVD events and mortality (adjudicated through 2017). Institutional review boards approved the study at each site, and all participants provided written informed consent. Access to anonymized MESA data can be made after review and approval by the MESA publications committee.
Research staff were centrally trained. Blood pressure was measured three times in a seated position with an automated oscillometric sphygmomanometer under standardized conditions. The last two readings were averaged for analyses. Height and weight were measured using a stadiometer and balance beam scale with participants wearing no shoes and light clothing. Body mass index was calculated as weight (kg) divided by height squared (m2). Total and HDL cholesterol, triglycerides and glucose were measured at a central laboratory after a 12 hour fast using standard methodology. LDL-cholesterol was calculated using the Friedewald equation. Use of hypertension, diabetes and statin medications were obtained by self-report. Participants brought their pill bottles to the clinic visit for confirmation. Treated diabetes was defined as the use of insulin or oral hypoglycemic medications. Standardized questionnaires were administered to collect information on level of education, current household income, medical insurance status, smoking status (current, former, never), smoking pack-years (including 0 for non-smokers), and alcohol use. Physical activity was assessed using a detailed, semiquantitative survey based on the Typical Week Physical Activity Survey.25 Intentional exercise was calculated in METS-minutes/week of exercise including moderate walking, dance and individual activities, and vigorous team and dual sports. Typical diet during the previous year was assessed using a 120-item food-frequency questionnaire (FFQ) quantifying usual intake over the past year based on the Insulin Resistance Atherosclerosis Study instrument and modified to include foods typically eaten by Chinese individuals.26 Diet was categorized as poor, intermediate and ideal based on Life’s Simple Seven criteria.27 Intermediate and ideal were combined for analyses. The Social Support Index survey, based on the ENRICHD social support inventory, was administered.28 Chronic Stress was assessed using a modified version of the Chronic Burden Scale.29, 30 Perceived discrimination was assessed with a questionnaire modified from the Everyday Discrimination Scale and the Major Experiences of Discrimination Scale.31
Medical insurance was categorized as no health insurance, government insurance only (Medicare/Medicaid/ Military) or private health insurance (with or without government insurance). SES and household income were assessed by self-report. A neighborhood SES index was developed from the U.S. Census and the American Community Survey (ACS) data at the census tract level, based on data from the U.S. Census 2000 (US Census Bureau. Census, 2000). Sixteen tract-level measures of educational attainment, occupation, income, wealth, poverty, employment status, and housing characteristics were considered using principal factor analysis with varimax rotation. Seven SES variables that loaded together were used to calculate a neighborhood SES score by multiplying the factor weights by the standardized variables. These measures include: median home value, % with ≥ high school education, % with ≥ Bachelor’s degree, % with management/professional occupation, median household income, % with household income>$50,000, % households with interest/dividend/rental income. This score has been used in other MESA analyses. 32–34 Higher values for neighborhood SES score indicate lower SES.
Length of time living in the US was reported by each participant at baseline. We derived three categories of immigrant history, including US born participants, foreign-born participants having lived in the US for < 30 years and foreign-born participants having lived in the US for ≥ 30 years.35
Participants were followed for incident cardiovascular events and mortality for a median of 15.8 years from their baseline examinations through 2017. In addition to five follow-up MESA study examinations, a telephone interviewer contacted each participant or their proxy every 9 to 12 months to inquire about all interim hospital admissions, cardiovascular diagnoses, and deaths. To verify self-reported diagnoses, copies were requested of all death certificates and medical records for all hospitalizations and selected outpatient cardiovascular diagnoses. Next-of-kin interviews for out-of-hospital cardiovascular deaths were performed, and autopsy reports reviewed when available. Information about all deaths were collected systematically from the Nation Death Index database, which included underlying cause of death (https://www.cdc.gov/nchs/ndi/index.htm).
Trained personnel abstracted hospital records with possible cardiovascular events and copied materials relevant to the event classification (e.g., ECGs, discharge summaries). Two MESA physicians independently reviewed the abstracted records for end-point classification using pre-specified criteria. If the reviewing physicians disagreed on the event classification, they adjudicated differences. If disagreements persisted, the full events committee made the final classification. Cardiovascular deaths were adjudicated using causes listed on death certificates, medical records and next of kin interviews.
We included all-cause mortality and cardiovascular disease mortality as primary outcomes for analysis. Cardiovascular mortality included death attributed to atherosclerotic coronary heart disease, stroke, primary arrhythmic death, other atherosclerotic disease, other cardiovascular disease, and heart failure. Reviewers classified myocardial infarction as definite, probable, or absent, based primarily on combinations of symptoms (e.g., chest pain), ECG abnormalities, and cardiac biomarker levels. Definite fatal CHD required a myocardial infarction within 28 days of death or chest pain within the 72 h before death or a history of CHD, and the absence of a known nonatherosclerotic or noncardiac cause of death. Stroke required documented focal neurological deficit lasting 24 h or until death, or if <24 hours, a clinically relevant lesion on brain imaging. Patients with focal neurological deficits secondary to brain trauma, tumor, infection, or other nonvascular cause were called no stroke. Stroke included all types, fatal or non-fatal stroke, including hemorrhagic stroke. Non-CVD causes of death were not adjudicated and were classified according to the underlying cause on the death certificate.
Statistical Methods
Baseline measures of demographics, SES, lifestyle and psychosocial variables, clinical risk factors, and immigration history, stratified by the four racial/ethnic groups, were summarized as means (SD) or prevalences. There were 20 (0.3%) participants with unknown mortality status, and were therefore not included in the analyses. The number of events and the rates per 1000 person-years at risk for the outcomes of all-cause mortality, and CVD mortality by race-ethnicity were summarized. Kaplan-Meier curves were used to display events for the outcomes of all-cause mortality and CVD mortality, stratified by race/ethnicity. Cox regression models were adjusted in blocks progressively36 a) demographics, b) SES, c) lifestyle and psychosocial risk factors, d) clinical risk factors and e) immigration history. The following covariates were updated at each exam, if available: Income, neighborhood SES, BMI, drinking status, smoking status (including pack years), statin use, triglycerides, HDL cholesterol, total cholesterol, hypertension medication use, glucose, exercise status, diabetes, and health insurance. The other covariates were from the baseline measurement. We used time-from-baseline as the time scale. Participants were censored at the time of last known contact, or 12/31/2018 if the participant was contacted after 12/31/2018. Most variables were missing in less than 0.05–2.0% of individuals, except for household income (4.0%), diet (4.2%), and neighborhood SES (9.1%). Race-by-sex multiplicative interaction was tested in the demographics adjusted models. Sensitivity analyses were performed to account for birth cohort effects and also adjusting for field center. For all regression models, we computed the hazard ratio with a two-sided 95% confidence interval, indicating when a result was significant at a two-sided 0.05 p-value. We checked the Cox proportional hazards assumption for race/ethnicity and all covariates using scaled Schoenfeld residuals. For covariates with significant results we examined the plot of the time varying coefficient versus time. Significant results were found for statin use, non-private (i.e. government) health insurance, and the emotional social support index. Inspection of the plots revealed that after approximately 15 years of follow-up the associations for statins and government insurance attenuated somewhat. The departure from proportional hazards was not visible to the naked eye for emotional support despite the statistical significance. Inclusion of time varying coefficients for these variables did not alter the race/ethnicity coefficients or conclusions hence for ease of presentation we did not include the time interaction for these variables. As an additional analysis we have provided restricted mean survival estimates over 15 years which do not depend on any proportional hazards assumptions. 37 Analyses were conducted using R (version 4.1.2).
Results:
The baseline characteristics of the study population, stratified by race/ethnicity are provided in Table 1. There were multiple differences in SES, lifestyle, psychosocial, and clinical factors between the groups. For example, White participants had the lowest neighborhood SES score (reflecting highest SES), highest education and household income levels and were least likely to have no private health insurance. White participants were most likely to be former smokers, and currently consume alcohol. Diabetes medication use and fasting glucose were lowest and statin use was highest in White participants.
Table 1:
Baseline characteristics of Multi-Ethnic Study of Atherosclerosis (MESA) participants, 2000–2002
| Race/Ethnicity (n; %) | White (2622; 38.5%) | Chinese (804; 11.8%) | Black (1892; 27.8%) | Hispanic (1496; 22.0%) | Total (n=6814) |
|---|---|---|---|---|---|
| Age (years) | 62.6 (10.2) | 62.3 (10.3) | 62.1 (10.1) | 61.3 (10.3) | 62.2 (10.2) |
| Sex (% male) | 48.1 | 48.5 | 44.5 | 48.2 | 47.2 |
| Neighborhood SES Score* | −0.90 (1.49) | −0.44 (1.13) | 0.09 (1.02) | 0.43 (1.09) | −0.29 (1.36) |
| Education Level (highest degree) % | |||||
| No Degree | 3.4 | 9.8 | 8.3 | 14.8 | 8.1 |
| High School/Associates Degree | 46.8 | 51.2 | 57.3 | 75.3 | 56.7 |
| Bachelor’s Degree | 22.2 | 22.6 | 17.2 | 5.5 | 17.2 |
| Graduate Degree | 27.3 | 16.2 | 16.4 | 4.3 | 18.0 |
| Income Level % | |||||
| < $25,000 | 26.7 | 21.9 | 32.2 | 32.7 | 31.5 |
| $25,000 to $49,000 | 26.7 | 21.9 | 32.2 | 32.7 | 28.9 |
| $50,000 to $99,000 | 32.8 | 18.5 | 28.9 | 15.4 | 26.1 |
| > $100,000 | 24.3 | 10.0 | 8.3 | 2.4 | 13.5 |
| Health Insurance Status % | |||||
| No Health Insurance | 2.7 | 19.2 | 6.2 | 18.0 | 9.0 |
| Government Health Insurance only | 14.4 | 29.5 | 18.7 | 23.7 | 19.4 |
| Private Health Insurance | 80.9 | 48.4 | 73.1 | 55.7 | 69.3 |
| Intentional exercise (MET-min/week) | 1687 (2301) | 1148 (1518) | 1712 (2785) | 1336 (2110) | 1553 (2340) |
| Diet (% poor) | 63.1 | 30.8 | 62.6 | 66.9 | 59.8 |
| Smoking Status % | |||||
| Current | 11.5 | 5.6 | 18.0 | 13.6 | 13.1 |
| Former | 44.2 | 19.0 | 36.8 | 32.5 | 36.6 |
| Pack Years (among smokers) | 27.0 (6.6–38.0) | 20.0 (4.0– 27.0) | 22.0 (6.9 −30.0) | 16.9 (3.0– 23.9) | 15.6 (5.5–32.0) |
| Alcohol (% drinker) | |||||
| Current | 71.7 | 31.2 | 49.2 | 47.2 | 55.3 |
| Former | 19.0 | 15.0 | 33.7 | 26.9 | 24.3 |
| Perceived Discrimination | 0.57 (0.89) | 0.30 (0.69) | 1.22 (1.32) | 0.67 (1.02) | 0.74 (1.08) |
| Social Support Index | 24.1 (5.2) | 23.8 (5.0) | 24.3 (5.2) | 24.2 (5.6) | 24.2 (5.3) |
| Chronic Stress (Burden) | 1.23 (1.18) | 0.79 (1.07) | 1.36 (1.25) | 1.25 (1.20) | 1.22 (1.21) |
| LDL Cholesterol | 117.1 (30.1) | 115.1 (28.9) | 116.5 (33.0) | 119.5 (32.9) | 117.2 (31.5) |
| HDL Cholesterol | 52.2 (15.7) | 49.5 (12.7) | 52.4 (15.3) | 47.6 (13.1) | 51.0 (14.8) |
| Total Cholesterol | 195.7 (35.1) | 192.6 (31.8) | 189.6 (36.3) | 197.9 (37.5) | 194.2 (35.7) |
| Triglycerides | 132.9 (90.2) | 142.7 (84.7) | 104.8 (68.6) | 157.1 (101.1) | 131.6 (88.8) |
| Statin medications (%) | 16.6 | 12.8 | 15.4 | 12.0 | 14.9 |
| Systolic blood pressure (mmHg) | 123.5 (20.4) | 124.6 (21.6) | 131.7 (21.6) | 126.7 (21.9) | 126.6 (21.5) |
| Diastolic blood pressure (mmHg) | 70.2 (10.0) | 72.0 (10.3) | 74.5 (10.2) | 71.6 (10.1) | 71.9 (10.3) |
| Hypertension Medication (%) | 33.1 | 28.7 | 50.3 | 32.6 | 37.2 |
| Diabetes | |||||
| Diabetes Medication (%) | 4.3 | 10.2 | 14.3 | 14.5 | 10.0 |
| Fasting Glucose (mg/dL) | 91.4 (21.6) | 99.0 (28.2) | 100.0 (32.0) | 103.6 (39.1) | 97.4 (30.3) |
| Body mass index (kg/m2) | 28.3 (5.5) | 24.0 (3.3) | 30.2 (5.9) | 29.4 (5.1) | 28.3 (5.5) |
| US Born | 2441 (93.1) | 82 (10.2) | 1699 (89.8) | 578 (38.6) | 4800 (70.4) |
| Immigrant to US ≥30 years ago | 148 (5.6) | 146 (18.2) | 122 (6.4) | 502 (33.6) | 918 (13.5) |
| Immigrant to US< 30 years ago | 33 (1.3) | 576 (71.6) | 71 (3.8) | 416 (27.8) | 1096 (16.1) |
Categorical variables shown as %, and continuous variables as mean (SD) or median (interquartile range);
Higher values for neighborhood SES score indicate lower SES.
Hispanic participants had the highest neighborhood SES score (reflecting lowest SES), lowest education and household income levels, and had a high prevalence of not having health insurance, poor diet, and diabetes. Hispanic participants had the highest LDL-cholesterol and triglyceride levels, lowest HDL cholesterol levels, the lowest use of statin medications and had high mean BMI.
Chinese participants had a relatively low neighborhood SES score (reflecting high SES), and relatively high education and household income (compared to Hispanic and Black participants), the lowest prevalence of poor diet, former and current smoking, and the lowest amount of intentional exercise time (MET-min/week) and BMI. Chinese participants also had a high prevalence of no health insurance and were least likely to have been born in the US.
Black participants had a relatively high neighborhood SES score (reflecting low SES), low household income and education levels relative to White participants and were less likely to have no health insurance than Hispanic and Chinese participants. Black participants were most likely to be current smokers and had the highest level of intentional exercise. Black participants were more likely to use statin medications than Hispanic and Chinese participants. In addition, Black participants had the highest body mass index, systolic and diastolic blood pressures and use of hypertension medications and had a high prevalence of diabetes, similar to that of Hispanic participants.
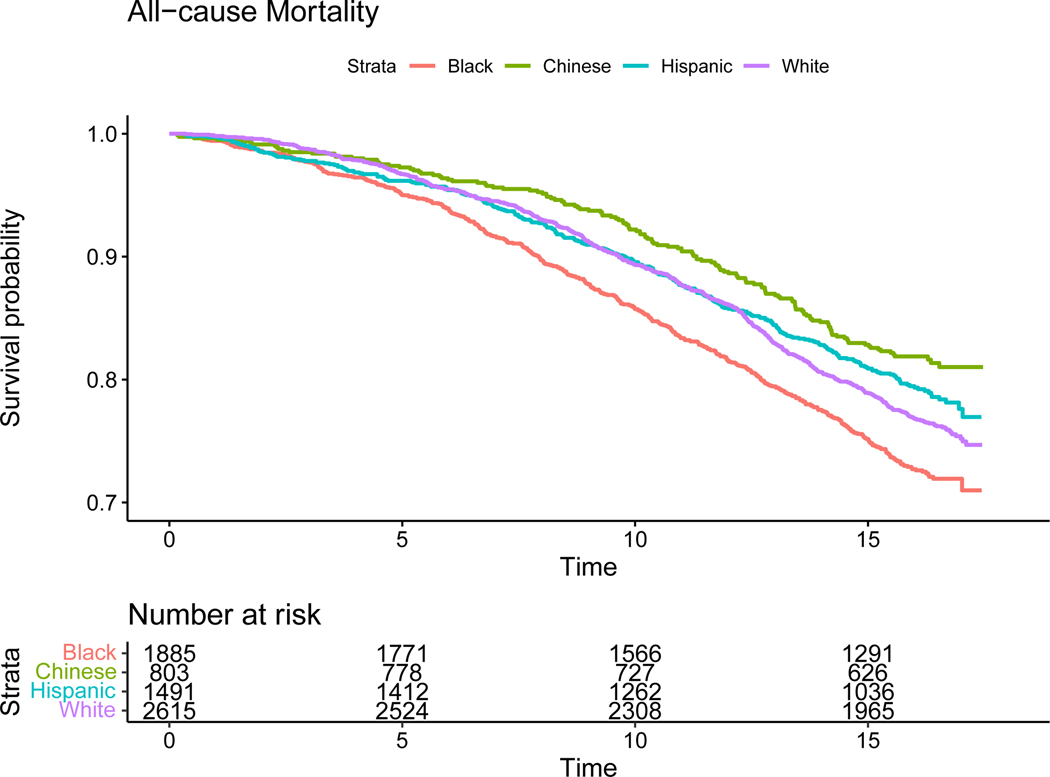
During a median of 15.8 years of follow-up, 22.8% of the participants (n=1552) died. All-cause mortality death rates per 1000 person-years at risk and proportion dying during follow-up were highest in Black ( 19.0; 26.4%), followed by White (15.8; 23.3%), Hispanic (14.0; 19.8%) and Chinese participants (12.1; 18.0%), with similar differences for age at death among participants who died during follow-up. The differences between mean age at death for White compared to Black participants was 3.1 years (Table 2).
Table 2:
All-cause and cardiovascular mortality during follow-up, stratified by race/ethnicity
| Race/Ethnicity | White (2622; 38.5%) | Chinese (804; 11.8%) | Black (1892; 27.8%) | Hispanic (1496; 22.0%) | Total (n=6814) |
|---|---|---|---|---|---|
| All-cause mortality; n (%) | 612 (23.3) | 145 (18.0%) | 499 (26.4) | 296 (19.8) | 1552 (22.8) |
| All-cause death rates* | 15.8 | 12.1 | 19.0 | 14.0 | 15.8 |
| Age at death (mean years) ** | 81.2 | 80.9 | 78.1 | 78.6 | 79.7 |
| Cardiovascular mortality; n (%) | 123 (4.7) | 40 (5.0) | 127 (6.7) | 74 (4.9) | 364 (5.3) |
| Cardiovascular death rates* | 3.2 | 3.4 | 4.8 | 3.5 | 3.7 |
| CVD proportion of mortality | 20.1% | 27.6% | 25.4% | 25.0% | 23.5% |
per 1000 person-years at risk;
Among participants who died during follow-up. CVD= cardiovascular.
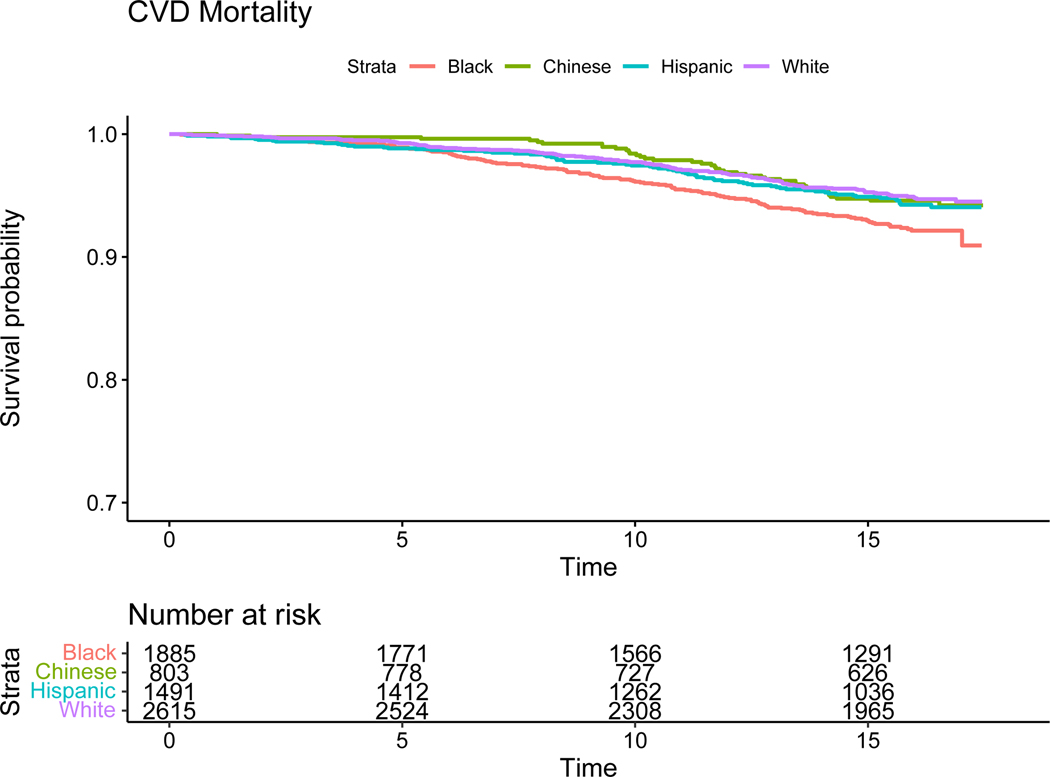
The Kaplan- Meier survival probability curves are displayed in Figure 1. Physician adjudicated cardiovascular mortality occurred in 5.3% of participants and was highest in Black participants (Figure 2). Nearly one quarter (23.5%) of deaths in the entire cohort were attributed to CVD. The proportion of deaths due to CVD was lowest in White (20.1%), highest in Chinese (27.6%) and similar in Black (25.4%) and Hispanic (25.0%) participants.
Figure 1.
Kaplan- Meier Plots for all-cause mortality (unadjusted)
Figure 2.
Kaplan- Meier Plots for cardiovascular mortality (unadjusted)
CVD death rates are presented in Table 2. All cause and CVD death rates (unadjusted) stratified by age decade and sex are presented in tables 4 and 5. Cancer deaths, based on death certificate underlying cause of death, were slightly more frequent than adjudicated CVD death (6.9% compared with 5.3%). Cancer mortality was highest in Black (9%), slightly lower in White (7%) and similar in Chinese and Hispanic participants (5%). The proportions of deaths attributed to cancer was higher than CVD (30.2% compared with 23.5%) and was highest in Black (32%) and White (31%) participants, slightly lower in Chinese participants (29%) and lowest in Hispanic participants (25%).
Table 4.
All-cause Death Rates per 1000 Person-years at Risk, stratified by age decade, sex and race/ethnicity
| Age Group at Baseline | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| White | Chinese | Black | Hispanic | White | Chinese | Black | Hispanic | ||
| 45–55 years | 2.8 | 3.4 | 9.0 | 4.6 | 2.1 | 2.2 | 5.3 | 2.2 | |
| 55–65 years | 11.0 | 7.3 | 12.4 | 13.1 | 6.7 | 3.8 | 10.4 | 5.6 | |
| 65–75 years | 25.9 | 17.6 | 30.5 | 26.3 | 17.4 | 13.1 | 16.2 | 14.5 | |
| 75–85 years | 59.7 | 40.5 | 79.2 | 51.7 | 45.9 | 43.8 | 58.7 | 40.3 | |
| Overall | 18.8 | 13.3 | 23.5 | 17.6 | 13.2 | 11.1 | 15.5 | 10.7 | |
Table 5.
Cardiovascular Death Rates per 1000 Person-years at Risk, stratified by age decade, sex and race/ethnicity
| Age Group at Baseline | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| White | Chinese | Black | Hispanic | White | Chinese | Black | Hispanic | ||
| 45–55 years | 0.6 | 0.0 | 1.4 | 1.2 | 0.3 | 0.6 | 0.9 | 0.0 | |
| 55–65 years | 1.7 | 2.4 | 4.3 | 2.7 | 0.7 | 0.6 | 2.7 | 0.6 | |
| 65–75 years | 5.1 | 7.3 | 7.0 | 6.5 | 3.6 | 1.1 | 4.5 | 3.0 | |
| 75–85 years | 13.6 | 8.1 | 25.5 | 15.7 | 10.7 | 18.0 | 12.4 | 14.2 | |
| Overall | 3.8 | 3.8 | 6.3 | 4.5 | 2.6 | 2.9 | 3.7 | 2.6 | |
Adjusted hazard ratios (HR) for all-cause and CVD mortality by race/ethnicity are shown in Table 3. Although male sex was strongly associated with both mortality outcomes, there were no significant race-sex interactions (all-cause mortality p=0.22; CVD mortality p=0.66); therefore, all primary results are presented with men and women combined, except in supplemental tables and figures. After adjusting for age and sex, Black participants had a 34% higher mortality hazard [HR 1.34; 95% CI 1.19–1.51) and Chinese participants had a 21% lower mortality hazard (HR 0.79; 0.66–0.95) than White participants. There was no difference in mortality in Hispanic (HR 0.99; 0.86–1.14) compared to White participants.
Table 3:
Hazard Ratio for all-cause and cardiovascular mortality during follow-up by race/ethnicity
| Hazard Ratio (95% Confidence Interval) | |||||
|---|---|---|---|---|---|
| All-Cause Mortality | |||||
| Race/Ethnicity | + Demographics | + Socio-economic Status (SES) | + Lifestyle and Psychosocial Factors | + Clinical Risk Factors | + Immigrant history |
| White (Reference) | --- | --- | --- | --- | --- |
| Chinese | 0.79 [0.66, 0.95] | 0.62 [0.50; 0.76] | 0.66 [0.53; 0.83] | 0.60 [0.48; 0.75] | 0.81 [0.60; 1.08] |
| Black | 1.34 [1.19, 1.51] | 1.16 [1.01; 1.34] | 1.07 [0.91; 1.24] | 1.08 [0.92; 1.26] | 1.08 [0.92; 1.26] |
| Hispanic | 0.99 [0.86, 1.14] | 0.72 [0.61; 0.85] | 0.74 [0.62; 0.89] | 0.71 [0.59; 0.84] | 0.77 [0.63; 0.94] |
| Cardiovascular Disease Mortality | |||||
| White (Reference) | --- | --- | --- | --- | --- |
| Chinese | 1.09 [0.76; 1.55] | 0.95 [0.63; 1.42] | 1.03 [0.67; 1.58] | 1.06 [0.68; 1.65] | 1.41 [0.80; 2.47] |
| Black | 1.72 [1.34; 2.21] | 1.37 [1.02; 1.84] | 1.06 [0.76; 1.47] | 0.95 [0.68; 1.34] | 0.95 [0.68; 1.34] |
| Hispanic | 1.24 [0.93; 1.66] | 0.97 [0.69; 1.36] | 0.92 [0.64; 1.31] | 0.82 [0.57; 1.18] | 0.84 [0.56; 1.26] |
Cox Proportional Hazards Model with time-varying covariates. p<0.05 in bold.
Demographics: age, sex.
Socio-economic status: neighborhood SES score, education, income, health insurance.
Lifestyle and psychosocial factors: intentional exercise, diet, smoking status, smoking pack years, alcohol, social support index, lifetime perceived discrimination, lifetime chronic stress (burden)
Clinical Risk Factors: Body mass index, total cholesterol, HDL cholesterol, triglycerides, statin medication use, systolic blood pressure, diastolic blood pressure, hypertension medication use, treated diabetes, fasting glucose.
Immigrant history: US Born, Immigrant ≥30 Years, Immigrant <30 Years
Adjustment for SES factors (including neighborhood SES, household income, education and health insurance status) resulted in significant attenuation of the higher risk for mortality in Black compared to White participants. This association decreased from 34% greater mortality in Black compared to White participants down to 16% greater mortality (HR 1.16; 1.01–1.27). SES adjustment resulted in even larger differences between Chinese and White participants than before adjustment (HR 0.62; 0.50–0.76). After adjusting for SES factors, Hispanic participants had a 28% lower mortality hazard compared to White participants (HR 0.72; 0.61–0.85). Additional adjustments were made for lifestyle factors and psychosocial factors (intentional exercise, diet, smoking status, smoking pack years, alcohol, perceived discrimination, chronic stress, social support ), and clinical risk factors (body mass index, total cholesterol, HDL cholesterol, triglycerides, statin medication use, systolic blood pressure, diastolic blood pressure, hypertension medication use, treated diabetes, fasting glucose). These adjustsments led to smaller further attenuation of mortality hazard ratios comparing Black relative to White participants, and resulted in a slightly less inverse association for Chinese and Hispanic participants compared to White participants.
Adjustment for immigrant history (US Born, immigrant ≥30 Years, immigrant <30 Years) reduced the protective associations observed for Chinese and Hispanic participants. In the final model, Hispanic participants (HR 0.77; 0.63– 0.94) were at significantly lower adjusted risk for mortality relative to White participants, but mortality risk in Black participants (HR 1.08; 0.92– 1.26) and Chinese participants (HR 0.81; 0.60–1.08] were not statistically different from those of White participants. In secondary analyses, there were no substantial differences when accounting for birth cohort effects or adjusting for field center.
Similar patterns were seen for CVD mortality, although the age and sex adjusted HR for CVD mortality for Black compared to White participants was greater than for all-cause mortality (HR 1.72; 1.34–2.21 compared with HR 1.34; 1.19–1.51), although confidence intervals overlapped, and no inverse association was seen for Chinese participants. Notably SES adjustment reduced the HR observed for Black compared with White participants, although point estimates still suggested higher risk in Black participants (HR 1.37 CI 1.02–1.84) which was still statistically significant (Table 3). In the final model, there were no statistically significant differences in CVD mortality in Black, Hispanic or Chinese participants compared to White participants; however, point estimates suggested lower risks in Black and Hispanic compared to White participants and higher risks in Chinese participants, but confidence intervals were wide.
Based on a restricted mean survival time model controlling for age and sex, over the course of 15 years Chinese participants would survive an estimated 80 days longer on average than White participants, while Black participants would survive 160 days less, and Hispanic participants 51 days less.
Multiple traditional and non-traditional risk factors were independently associated with mortality in the combined cohort, including greater age, male sex, lower household income, less education, no health insurance, lower intentional exercise levels, former smoking, current smoking, greater smoking pack-years, greater chronic stress/burden, and higher systolic BP, triglycerides, and fasting glucose. Current alcohol use, statin medication use, greater total cholesterol levels, greater diastolic BP, higher body mass index and living in the US for < 30 years were each inversely associated with total mortality (Supplemental Table 1). These results were similar with CVD mortality as the outcome, although there were fewer statistically significant associations (Supplemental Table 2).
Discussion:
One of the primary goals of MESA is to ascertain racial/ethnic differences in the incidence of clinical events, including all-cause and CVD mortality. MESA is a unique, ongoing longitudinal US cohort study which includes self-identified Black, Hispanic, White, and Chinese participants with extensive measured information about many mortality risk factors, including detailed SES, lifestyle and psychosocial measures, in addition to traditional clinical risk factors. The current analysis demonstrates marked racial/ethnic disparities in all-cause mortality among MESA participants over ~15 years of follow-up. The highest all-cause mortality rate was in Black participants and the lowest in Chinese participants. SES factors and access to healthcare, including neighborhood SES, income, education, and health insurance status differed among the four groups and were each independent predictors of mortality. We observed a heterogeneous pattern across the three non-White racial/ethnic groups. Black participants had elevated risk of all-cause mortality that was reduced, but remained elevated, after adjustment for SES (HR 1.16; 1.01–1.34], and was further reduced after additional adjustment for risk factors. In contrast, Hispanic and Chinese participants had lower risk of all-cause mortality after adjustment for SES, that persisted after additional adjustment for risk factors. Further adjustment for immigration history reduced the protective associations observed for Chinese and Hispanic participants, and the HR for Chinese was no longer statistically significant.
We also assessed disparities in CVD mortality, which largely mirrored all-cause mortality, except that somewhat stronger associations were observed for Black participants, both before and after adjustment for SES, and generally weaker protective effects were observed for Hispanic and Chinese participants. However, estimates of associations were imprecise and limited by a smaller number of CVD than all-cause mortality events. CVD mortality accounted for nearly one quarter of the total deaths and cancer for 30%. The higher proportion of cancer deaths is likely related to the fact that all participants were free of known clinical CVD at baseline, which was a selection criterion to enroll in MESA.
The highest risk for mortality was clearly seen in Black participants in MESA. Our analyses suggest that this increased risk for early mortality is largely due to socioeconomic factors and a high prevalence of lifestyle, psychosocial and clinical risk factors. These factors are likely the consequences of structural inequities linked to racism and social inequality that operate over the lifecourse and across generations.10 Although other studies have demonstrated higher mortality and CVD mortality in Black compared to White individuals,4, 38, 39 few have incorporated the broad range of factors available in MESA. The proportions of deaths attributed to CVD and to cancer were both higher in Black than White participants in MESA, suggesting that CVD and cancer mortality contribute to this mortality disparity.
We found that Hispanic participants had a high prevalence of clinical risk factors that are, at least partially, influenced by lifestyle, especially diabetes, elevated BMI, and dyslipidemia. However, after adjustment for SES, Hispanic participants were at lower risk for mortality than White participants. This Hispanic “health paradox” has been described previously, but the contributing factors are not well understood.6 The inverse association was reduced after adjustment for immigration history, suggesting that foreign birth and less time living in the US may be linked to lower risks. This may be due to migrants being more healthy at baseline, and/or less acculturation to the American lifestyle.14, 40–45 Hispanic Americans represent a heterogenous group of individuals with significant within group variation due to differing countries of origin, time living in the US and cultural influences.46,5 We were unable to further stratify the Hispanic MESA participants due to limitations in subgroup sample sizes.
Chinese participants had the greatest longevity and lowest prevalence of most risk factors. This pattern persisted after adjustment for SES and other health-related factors. Chinese participants in MESA were least likely to have been born in the US (only 10%). Most had resided in the US for < 30 years at the baseline MESA exam. As in the case of Hispanic participants, adjustment for immigration history reduced the protective associations of Chinese race/ethnicity with all-cause mortality, suggesting that acculturation and or healthy migrant effects may play a significant role. It is important to note that recruitment in MESA included only Asians of Chinese descent. Americans of East Asian descent, such as Chinese Americans, have been shown in other studies to have a low prevalence of adverse clinical risk factors, especially poor diet, obesity, and low risk for adverse health outcomes, including mortality.2 In contrast, South Asian Americans have a high prevalence of metabolic abnormalities and an increased risk for CVD.47
There are some limitations to this study. The MESA sample was not specifically designed to be representative of the entire US population. MESA participants were selected to be free of clinical cardiovascular diseases at baseline, and therefore represent a healthier selected sample. This may be especially important in groups that have earlier or more prevalent clinical cardiovascular disease. Potential differential participation by racial/ethnic groups could have introduced selection factors that could have impacted the associations that we observed. In addition, the sample size for Chinese participants was smaller than the other three racial/ethnic groups. MESA did not adjudicate causes of death for non-CVD underlying causes of death, but death certificate data are likely accurate enough to compare the broad groups examined here. We also did not include measures of health care received, thus the impact of health care access or bias within the health care system was not assessed.
Our study has many strengths. Recruitment was community based with multiple detailed phenotypic assessments covering various domains of potential risk, acquired in a highly standardized fashion. Assessment of CVD mortality was adjudicated by a physician committee, rather than relying on administrative data from billing codes or death certificates. We adjusted for a variety of factors in a sequential fashion, to describe the impact of each set of covariates on the hazard ratio point estimates and included time-updated variables during the follow-up period. We did not do a formal mediation analysis, instead we see the adjustment factors as confounders. The use of mortality as an outcome, with systematic ascertainment, minimizes potential biases that can occur from differential loss to follow-up by race-ethnicity that may occur when studying non-fatal events. Our study is unique in that it includes Chinese and Hispanic American, in addition to Black and White, MESA participants.
We conceptualized race/ethnicity as a social, rather than a biologic, construct. Hence we did not adjust for or attempt to estimate associations with genetic ancestry. Our analyses illustrate the significantly higher risk for all-cause and cardiovascular disease mortality experienced by Black people in the United States. Our results also suggest that this risk is at least partly due to SES and other lifestyle, psychosocial, and clinical risk factors. Given the strong potential for residual confounding by unmeasured or imperfectly measured social factors (including the many manifestations of social inequality and racism within and outside the health care system), the few racial/ethnic associations that remained statistically significant after multivariable adjustment should not be attributed to biologic differences between the groups.9 Our results illustrating the contributions of SES and other risk factors to Black -White mortality differences highlight the need to identify and act on the ways in which systematic factors, including racism and inequality, structure opportunities and exposures in ways that affect risk of all-cause and cardiovascular death, operating through individual experiences, social contexts, and historical processes. Our findings for Hispanic and Chinese participants also highlight the need to better understand the impact of immigration and acculturation on mortality risk, as well as the conditions under which immigrant health can be maximized.
In summary our results highlight heterogeneity across racial/ethnic groups in mortality and the contributions of various sets of factors to these differences. The results of this study lend further support to the need to identify and act on the underlying societal drivers of these persistent differences, including structural factors linked to inequality, racism and history in order to improve population health.48, 49
Supplementary Material
Clinical Perspective.
What is new?
We found marked racial/ethnic disparities in all-cause mortality among MESA participants over ~15 years of follow-up.
The highest all-cause mortality rate was in Black participants and the lowest in Chinese participants.
SES factors and access to healthcare, including neighborhood SES, income, education, and health insurance status differed among the four groups and were each independent predictors of mortality.
What are the clinical implications?
The results of this study lend further support to the need to identify and act on the underlying societal drivers of these persistent differences in order to improve population health.
These differences include structural factors linked to inequality, racism and history.
Sources of Funding
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS) and R01 HL071759 and 2P60MD002249 (to A.V.D.R.). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Disclosures: None of the authors report any disclosures.
References
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang NY and Tsao CW. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 2.Acciai F, Noah AJ and Firebaugh G. Pinpointing the sources of the Asian mortality advantage in the USA. J Epidemiol Community Health. 2015;69:1006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenelon A, Chinn JJ and Anderson RN. A comprehensive analysis of the mortality experience of hispanic subgroups in the United States: Variation by age, country of origin, and nativity. SSM Popul Health. 2017;3:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA Jr., Willis M and Yancy CW. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation. 2017;136:e393–e423. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, Palaniappan L, Piña IL, Ramirez SM, Rodriguez B and Sims M. Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation. 2014;130:593–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorlie PD, Backlund E, Johnson NJ and Rogot E. Mortality by Hispanic status in the United States. Jama. 1993;270:2464–8. [PubMed] [Google Scholar]
- 7.Singh GK, Daus GP, Allender M, Ramey CT, Martin EK, Perry C, Reyes AAL and Vedamuthu IP. Social Determinants of Health in the United States: Addressing Major Health Inequality Trends for the Nation, 1935–2016. Int J MCH AIDS. 2017;6:139–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. http://www.healthypeople.gov/2020/about/disparitiesAbout.aspx.
- 9.Cooper RS, Nadkarni GN and Ogedegbe G. Race, Ancestry, and Reporting in Medical Journals. Jama. 2018;320:1531–1532. [DOI] [PubMed] [Google Scholar]
- 10.Williams DR, Lawrence JA and Davis BA. Racism and Health: Evidence and Needed Research. Annu Rev Public Health. 2019;40:105–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M and Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 12.Ogilvie RP, Everson-Rose SA, Longstreth WT Jr., Rodriguez CJ, Diez-Roux AVand Lutsey PL. Psychosocial Factors and Risk of Incident Heart Failure: The Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2016;9:e002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitaker KM, Everson-Rose SA, Pankow JS, Rodriguez CJ, Lewis TT, Kershaw KN, Diez Roux AV and Lutsey PL. Experiences of Discrimination and Incident Type 2 Diabetes Mellitus: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Epidemiol. 2017;186:445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diez Roux AV, Detrano R, Jackson S, Jacobs DR Jr., Schreiner PJ, Shea S and Szklo M. Acculturation and socioeconomic position as predictors of coronary calcification in a multiethnic sample. Circulation. 2005;112:1557–65. [DOI] [PubMed] [Google Scholar]
- 15.Diez Roux AV, Mujahid MS, Hirsch JA, Moore K and Moore LV. The Impact of Neighborhoods on CV Risk. Glob Heart. 2016;11:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everson-Rose SA, Roetker NS, Lutsey PL, Kershaw KN, Longstreth WT Jr., Sacco RL, Diez Roux AVand Alonso A. Chronic stress, depressive symptoms, anger, hostility, and risk of stroke and transient ischemic attack in the multi-ethnic study of atherosclerosis. Stroke. 2014;45:2318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufman JD, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, Daviglus ML, Diez Roux AV, Gassett AJ, Jacobs DR Jr., Kronmal R, Larson TV, Navas-Acien A, Olives C, Sampson PD, Sheppard L, Siscovick DS, Stein JH, Szpiro AA and Watson KE. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. Lancet. 2016;388:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer H, Han C, Post W, Goff D, Diez-Roux A, Cooper R, Jinagouda S and Shea S. Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA). Am J Hypertens. 2004;17:963–70. [DOI] [PubMed] [Google Scholar]
- 19.Mujahid MS, Diez Roux AV, Cooper RC, Shea S and Williams DR. Neighborhood stressors and race/ethnic differences in hypertension prevalence (the Multi-Ethnic Study of Atherosclerosis). Am J Hypertens. 2011;24:187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mujahid MS, Diez Roux AV, Shen M, Gowda D, Sanchez B, Shea S, Jacobs DR, Jr. and Jackson SA. Relation between neighborhood environments and obesity in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008;167:1349–57. [DOI] [PubMed] [Google Scholar]
- 21.Wing JJ, August E, Adar SD, Dannenberg AL, Hajat A, Sanchez BN, Stein JH, Tattersall MC and Diez Roux AV. Change in Neighborhood Characteristics and Change in Coronary Artery Calcium: A Longitudinal Investigation in the MESA (Multi-Ethnic Study of Atherosclerosis) Cohort. Circulation. 2016;134:504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranjit N, Diez-Roux AV, Shea S, Cushman M, Ni H and Seeman T. Socioeconomic position, race/ethnicity, and inflammation in the multi-ethnic study of atherosclerosis. Circulation. 2007;116:2383–90. [DOI] [PubMed] [Google Scholar]
- 23.Shea S, Lima J, Diez-Roux A, Jorgensen NW and McClelland RL. Socioeconomic Status and Poor Health Outcome at 10 Years of Follow-Up in the Multi-Ethnic Study of Atherosclerosis. PLoS One. 2016;11:e0165651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olson JL, Bild DE, Kronmal RA and Burke GL. Legacy of MESA. Glob Heart. 2016;11:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, Jenny NS, Burke GL and Jacobs DR. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169:444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nettleton JA, Polak JF, Tracy R, Burke GL and Jacobs DR Jr. Dietary patterns and incident cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2009;90:647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW and Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 28.Enhancing recovery in coronary heart disease patients (ENRICHD): study design and methods. The ENRICHD investigators. Am Heart J. 2000;139:1–9. [DOI] [PubMed] [Google Scholar]
- 29.Bromberger JT and Matthews KA. A longitudinal study of the effects of pessimism, trait anxiety, and life stress on depressive symptoms in middle-aged women. Psychol Aging. 1996;11:207–13. [DOI] [PubMed] [Google Scholar]
- 30.Garg PK, O’Neal WT, Diez-Roux AV, Alonso A, Soliman EZ and Heckbert S. Negative Affect and Risk of Atrial Fibrillation: MESA. J Am Heart Assoc. 2019;8:e010603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams DR, Yan Y, Jackson JS and Anderson NB. Racial Differences in Physical and Mental Health: Socio-economic Status, Stress and Discrimination. J Health Psychol. 1997;2:335–51. [DOI] [PubMed] [Google Scholar]
- 32.Christine PJ, Auchincloss AH, Bertoni AG, Carnethon MR, Sánchez BN, Moore K, Adar SD, Horwich TB, Watson KE and Diez Roux AV. Longitudinal Associations Between Neighborhood Physical and Social Environments and Incident Type 2 Diabetes Mellitus: The Multi-Ethnic Study of Atherosclerosis (MESA). JAMA Intern Med. 2015;175:1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merkin SS, Karlamangla A, Roux AD, Shrager S, Watson K and Seeman T. Race/ethnicity, neighborhood socioeconomic status and cardio-metabolic risk. SSM Popul Health. 2020;11:100634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore K, Diez Roux AV, Auchincloss A, Evenson KR, Kaufman J, Mujahid M and Williams K. Home and work neighbourhood environments in relation to body mass index: the Multi-Ethnic Study of Atherosclerosis (MESA). J Epidemiol Community Health. 2013;67:846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lê-Scherban F, Albrecht SS, Bertoni A, Kandula N, Mehta N and Diez Roux AV. Immigrant status and cardiovascular risk over time: results from the Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol. 2016;26:429–435.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedhazur EJ. Multiple regression in behavioral research 3rd edition ed. Orlando, Florida: Harcourt Brace; 1997. [Google Scholar]
- 37.Tian L, Zhao L and Wei LJ. Predicting the restricted mean event time with the subject’s baseline covariates in survival analysis. Biostatistics. 2014;15:222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colantonio LD, Gamboa CM, Richman JS, Levitan EB, Soliman EZ, Howard G and Safford MM. Black-White Differences in Incident Fatal, Nonfatal, and Total Coronary Heart Disease. Circulation. 2017;136:152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao D, Post WS, Blasco-Colmenares E, Cheng A, Zhang Y, Deo R, Pastor-Barriuso R, Michos ED, Sotoodehnia N and Guallar E. Racial Differences in Sudden Cardiac Death. Circulation. 2019;139:1688–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osibogun O, Ogunmoroti O, Mathews L, Okunrintemi V, Tibuakuu M and Michos ED. Greater Acculturation is Associated With Poorer Cardiovascular Health in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2021;10:e019828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Day EC, Li Y, Diez-Roux A, Kandula N, Moran A, Rosas S, Shlipak MG and Peralta CA. Associations of acculturation and kidney dysfunction among Hispanics and Chinese from the Multi-Ethnic Study of Atherosclerosis (MESA). Nephrol Dial Transplant. 2011;26:1909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Effoe VS, Chen H, Moran A, Bertoni AG, Bluemke DA, Seeman T, Darwin C, Watson KE and Rodriguez CJ. Acculturation is associated with left ventricular mass in a multiethnic sample: the Multi-Ethnic Study of Atherosclerosis. BMC Cardiovasc Disord. 2015;15:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kandula NR, Diez-Roux AV, Chan C, Daviglus ML, Jackson SA, Ni H and Schreiner PJ. Association of acculturation levels and prevalence of diabetes in the multi-ethnic study of atherosclerosis (MESA). Diabetes Care. 2008;31:1621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lutsey PL, Diez Roux AV, Jacobs DR Jr., Burke GL, Harman J, Shea S and Folsom AR. Associations of acculturation and socioeconomic status with subclinical cardiovascular disease in the multi-ethnic study of atherosclerosis. Am J Public Health. 2008;98:1963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moran A, Diez Roux AV, Jackson SA, Kramer H, Manolio TA, Shrager S and Shea S. Acculturation is associated with hypertension in a multiethnic sample. Am J Hypertens. 2007;20:354–63. [DOI] [PubMed] [Google Scholar]
- 46.Daviglus ML, Talavera GA, Avilés-Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC, LaVange L, Penedo F, Perreira K, Pirzada A, Schneiderman N, Wassertheil-Smoller S, Sorlie PD and Stamler J. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. Jama. 2012;308:1775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volgman AS, Palaniappan LS, Aggarwal NT, Gupta M, Khandelwal A, Krishnan AV, Lichtman JH, Mehta LS, Patel HN, Shah KS, Shah SH and Watson KE. Atherosclerotic Cardiovascular Disease in South Asians in the United States: Epidemiology, Risk Factors, and Treatments: A Scientific Statement From the American Heart Association. Circulation. 2018;138:e1–e34. [DOI] [PubMed] [Google Scholar]
- 48.Skolarus LE, Sharrief A, Gardener H, Jenkins C and Boden-Albala B. Considerations in Addressing Social Determinants of Health to Reduce Racial/Ethnic Disparities in Stroke Outcomes in the United States. Stroke. 2020;51:3433–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mensah GA. Charting the Future for Ethnicity and Health Research: Clinical and Population Science Insights From the MESA. Glob Heart. 2016;11:365–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.