Abstract
IMPORTANCE
There is a paucity of evidence to guide physicians regarding prevention strategies for cutaneous squamous cell carcinoma (CSCC) in solid organ transplant recipients (SOTRs).
OBJECTIVE
To examine the development and results of a Delphi process initiated to identify consensus-based medical management recommendations for prevention of CSCC in SOTRs.
EVIDENCE REVIEW
Dermatologists with more than 5 years’ experience treating SOTRs were invited to participate. A novel actinic damage and skin cancer index (AD-SCI), consisting of 6 ordinal stages corresponding to an increasing burden of actinic damage and CSCC, was used to guide survey design. Three sequential web-based surveys were administered from January 1, 2019, to December 31, 2020. Pursuant to Delphi principles, respondents thoroughly reviewed all peer responses between rounds. Supplemental questions were also asked to better understand panelists’ rationale for their responses.
FINDINGS
The Delphi panel comprised 48 dermatologists. Respondents represented 13 countries, with 27 (56%) from the US. Twenty-nine respondents (60%) were Mohs surgeons. Consensus was reached with 80% or higher concordance among respondents when presented with a statement, question, or management strategy pertaining to prevention of CSCC in SOTRs. A near-consensus category of 70% to less than 80% concordance was also defined. The AD-SCI stage–based recommendations were established if consensus or near-consensus was achieved. The panel was able to make recommendations for 5 of 6 AD-SCI stages. Key recommendations include the following: cryotherapy for scattered actinic keratosis (AK); field therapy for AK when grouped in 1 anatomical area, unless AKs are thick in which case field therapy and cryotherapy were recommended; combination lesion directed and field therapy with fluorouracil for field cancerized skin; and initiation of acitretin therapy and discussion of immunosuppression reduction or modification for patients who develop multiple skin cancers at a high rate (10 CSCCs per year) or develop high-risk CSCC (defined by a tumor with approximately ≥20% risk of nodal metastasis). No consensus recommendation was achieved for SOTRs with a first low risk CSCC.
CONCLUSIONS AND RELEVANCE
Physicians may consider implementation of panel recommendations for prevention of CSCC in SOTRs while awaiting high-level-of-evidence data. Additional clinical trials are needed in areas where consensus was not reached.
Subject to lifelong immunosuppression, solid organ transplant recipients (SOTRs) (especially those with white skin and a history of significant sun exposure) have a high risk of developing cutaneous squamous cell carcinoma (CSCC), with a risk ranging from approximately 20 to 200 times higher than in background populations, resulting in an increased risk of mortality from skin cancer.1 Physicians seeking to prevent CSCC in SOTRs engage in primary, secondary, or tertiary strategies aimed at reducing skin cancer risk. Primary prevention aims to prevent the onset of disease, whereas secondary and tertiary prevention reduces the morbidity and mortality of a disease that has already occurred.2,3 Primary prevention in SOTRs may range from patient education, photoprotection, and skin surveillance to treatment of premalignant lesions, such as use of topical4–11 medications and photodynamic therapy (PDT).12–17 Oral chemoprevention18–22 and reduction23,24 and conversion25–27 of immunosuppression are options for secondary28 and tertiary prevention.
Despite the advancing literature regarding prevention of CSCC inSOTRs,15,21,25,29 uncertainty exists regarding best practices for various patient scenarios. Limitations in the current literature for prevention of CSCC in SOTRs include the low number of randomized clinical trials (RCTs) specifically performed in the immunocompromised population and use of surrogate end points. For example, the primary prevention end point of interest for physicians is the development of first CSCC; nevertheless, commonly used end points in the literature are the elimination of actinic keratosis (AK) or the development of subsequent CSCC in patients with a history of CSCC.6,8,10,12,16,30 Large, prospective RCTs in this population are challenging because of disease and patient heterogeneity and the latency to onset of CSCC, requiring extended follow-up times. In scenarios where there is high-level evidence regarding benefit,31 such as the case for field therapy for AK, treatments may have low adherence rates, thus limiting clinical applicability. Once evidence for a treatment modality is established in SOTRs, as in the case of acitretin for secondary chemoprevention,18 additional open clinical questions remain: which patients should be treated, when treatment should be initiated, and in what sequence different strategies should be implemented.
Previous guidelines on the management of CSCC in SOTRs were issued by the International Transplant Skin Cancer Collaborative in 2004.32 The emphasis of these guidelines is on management of existing disease rather than prevention, and their development did not make use of a structured consensus-building approach.
First described in 1948,33 the Delphi method is a well-established tool to build consensus among experts when such uncertainties exist. It is a structured, iterative process whereby experts are provided several rounds of surveys of increasing specificity designed to encourage the convergence of opinion regarding a problem or question.34 Transparent review of pooled peer responses by participants and opportunities to revise previous responses are core elements of involvement in the Delphi method.35
Because of the paucity of evidence-based data in the prevention of CSCC in the SOTR population, we used the Delphi method among a panel of expert dermatologists to identify consensus-based preventive and treatment recommendations. In this article, we report the development and results of the Strategies for the Prevention of Skin Cancer in Solid Organ Transplant Recipients Delphi study.
Methods
At the September 2018 International Immunosuppression and Transplant Skin Cancer Collaborative meeting, an international group of transplant dermatologists convened to design a Delphi study that would identify consensus-based medical management decisions in the prevention of skin cancer in CSCC. Topics related to management of CSCC, such as excision, Mohs surgery, radiation, imaging, or patient follow-up were specifically excluded because these topics were deemed related to management of active disease and did not play a role in the primary, secondary, or tertiary prevention of CSCC. Prevention of melanoma or other non-CSCC skin cancer was similarly excluded. At this meeting, a key barrier identified by the working group was lack of widely accepted categories to quantify actinic damage and skin cancer burden, which would serve as the basis of the Delphi study. The working group developed a categorical actinic damage and skin cancer index (AD-SCI) that consisted of 6 stages, with each stage representing a common clinical scenario in which medical management of the patient may be altered to decrease the risk of further CSCC development (Table 1). The AD-SCI was also presented at the Skin Care in Organ Transplant Patients Europe 2019 meeting for input from its members. An expert panel was drawn from the membership of 3 international organizations with a focus on skin cancer in SOTRs: the International Immunosuppression and Transplant Skin Cancer Collaborative, the Keratinocyte Carcinoma Consortium Immunosuppression Working Group, and Skin Care in Organ Transplant Patients Europe. Prospective respondents were eligible based on 3 criteria: board certification in dermatology, at least 5 years of experience after residency in treatment of transplant patients, and evidence of active membership in 1 of the 3 aforementioned organizations as defined by review of meeting rosters. Informed consent was not required for this study because all responses were anonymous. This study was approved by the Partners Human Research Committee Institutional Review Board.
Table 1.
Actinic Damage and Skin Cancer Index
| Stage | Description |
|---|---|
| 1 | No AK; photodamage only (lentigines, poikiloderma, rhytides) |
| 2 | Discrete AK |
| 3 | Diffuse AK with or without SCCis in a given field |
| 4 | First invasive low-risk CSCC |
| 5 | Multiple invasive low-risk CSCCsa |
| 6 | High-risk CSCCb |
Abbreviations: AK, actinic keratosis; CSCC, cutaneous squamous cell carcinoma, SCCis, squamous cell carcinoma in situ.
Development of 2 or more CSCCs.
American Joint Committee on Cancer stage T3 or Brigham and Women’s Hospital stage T2B tumor.
Steering Committee
Several of us (P.R.M., A.J.P., C.A.H., and C.D.S.) formed the steering committee charged with guiding the Delphi process. The steering committee reviewed and interpreted data from each round, promptly disseminated results to the panel, and formulated subsequent surveys based on results from previous rounds.
Enrollment and Data Collection
Prospective respondents were sent an email that contained a link to a web-based Research Electronic Data Capture (REDCap) survey inviting them to participate. If respondents agreed, they proceeded to the first round of the Delphi study. All 3Delphi rounds were conducted over REDCap, and survey data were housed in REDCap for the duration of the study. Limited demographic information, including country of origin, affiliation, and subspecialty were collected as part of enrollment in round 1.
Consensus Building and Information Gathering
In accordance with the Delphi method,36 the first round administered consisted of open-ended questions tailored to each AD-SCI stage of CSCC development. Multiple-choice format answers that comprised plausible management options identified by the steering committee were provided. Responses from round 1 were used to formulate rounds 2 and 3, whereby more directed questions were asked. The steering committee plan for consensus-building subsequent to the open-ended round 1 is shown in the Figure. Supplemental questions, aimed at better understanding respondents’ rationale for their choices (eg, why for some modalities, such as nicotinamide, respondents did not reach consensus despite randomized data supporting their use) were asked at the completion of each round; participants were unable to change their answer choices for that round after review of supplemental questions. Participants were also invited to offer open-ended feedback at the end of each Delphi round. All administered surveys can be found in the eAppendix in the Supplement.
Figure.
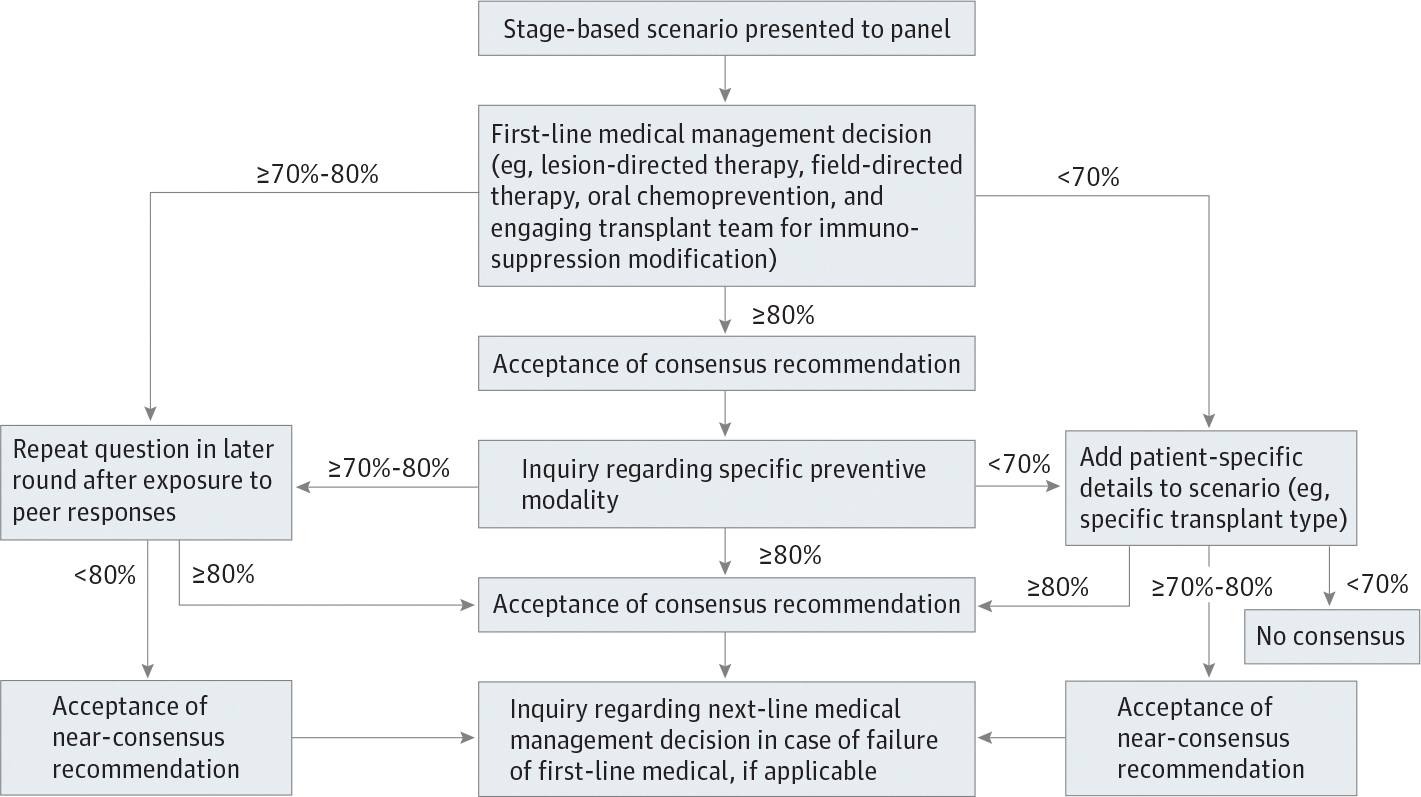
Strategy for Building Consensus in Strategies for the Prevention of Skin Cancer in Solid Organ Transplant Recipients
Definition of Consensus and Threshold for Panel Recommendation
The percentage agreement of a panel of experts in response to a query or statement is a common measure of consensus in Delphi processes.37 A wide range of consensus thresholds are found in the literature, ranging from 51% to 97%; 75% is cited as a median value.37 In this study, a threshold of 80% or higher was used to delineate consensus, evaluate the responses from each round, and develop subsequent rounds (Figure). A near-consensus category of 70% to less than 80% agreement was also defined. The final panel recommendations included any management strategy that achieved consensus or near-consensus (70%–100%). If at any time in the survey a management strategy was selected by fewer than 10% of panelists, it was not included as an option in subsequent rounds.
Definition of Low- and High-Risk CSCC and Rate of CSCC Formation
For the purposes of the Delphi study, a low-risk CSCC was defined as an American Joint Committee on Cancer (AJCC)38 or Brigham and Women’s Hospital (BWH)39 T1 tumor. A high-risk CSCC was defined as an AJCC T3 or BWH T2b tumor (because higher stages, AJCC T4 and BWH T3, are rare).With respect to patients diagnosed with multiple CSCCs, a low rate of formation was defined as 1 dermally invasive CSCC diagnosed per year, whereas a high rate of formation was defined as 10 dermally invasive CSCCs diagnosed per year.
Statistical Analysis
Descriptive statistics were performed for each question on completion of each round. Results from preceding rounds were emailed to the panel for review at least 1 week before the subsequent round. All statistical analysis was performed using Excel, version 16.42 (Microsoft Corp).
Results
Three Delphi rounds were completed as planned. Seventy-four prospective expert dermatologists met the predetermined inclusion criteria and were invited participate. Of these, 50 (68%) responded to round 1. Subsequently, 48 respondents completed round 2 and 46 completed round 3. Forty-eight of the initial 50 respondents (96%) participated fully in 2 of 3 rounds and were considered final members of the panel. Baseline demographic characteristics of the final panelists are given in Table 2. Thirteen countries were represented on the panel, with 27 panelists (56%) located in the US. Twenty-nine (60%) were Mohs surgeons.
Table 2.
Panel DemographicCharacteristicsa
| Characteristic | No. (%) of panelists (N = 48) |
|---|---|
| Length of experience, y | |
| 5–<10 | 18(38) |
| 10–<15 | 11 (23) |
| 15–≤20 | 9(19) |
| >20 | 10(21) |
| Mohs surgeon | 29 (60) |
| Medical dermatologist/cutaneous oncologist | 19(40) |
| Country of practice | |
| US | 27 (56) |
| Europe | 18 (38) |
| UK | 4(8) |
| Spain | 4(8) |
| The Netherlands | 3 (6) |
| Belgium | 1 (2) |
| Turkey | 1 (2) |
| Switzerland | 1 (2) |
| Norway | 1 (2) |
| Italy | 1 (2) |
| France | 1 (2) |
| Austria | 1 (2) |
| Brazil | 1 (2) |
| Australia | 2 (4) |
Reflected are panelists who completed more than 2 of 3 Delphi survey rounds.
The Delphi panel was able to make specific management recommendations across 5 of the 6 AD-SCI stages (Table 3). Consensus was not achieved, and no management recommendation was made in the scenario in which an SOTR develops their first low-risk CSCC.
Table 3.
Consensus-Based Medical Management Recommendations for the Prevention of CSCC in Solid Organ Transplant Recipients by AD-SCI Stagea
| Group | Recommendation |
|---|---|
| Stage 1 | |
| Photodamaged skin only | Preventive measures: education, sun protection strategies, sunscreen, and/or skin surveillance (C)b |
| Stage 2 | |
| Discrete AKs | |
| Scattered AKs (2a) | Thin: cryotherapy should be used as first-line treatment (C); if this fails, cryotherapy should be repeated (NC); oral chemoprevention should not be initiated (C)c Thick: cryotherapy should be used as first-line treatment (C); if this fails, lesion directed therapy should be repeated (C); oral chemoprevention should not be initiated (NC) |
| Grouped AKs (2b) | Thin: field therapy should be used as first-line treatment (NC); oral chemoprevention should not be initiated (C)b Thick: lesion-directed therapy (C) using cryotherapy (NC) followed by field therapy (C); a fluorouracil-based modality (NC) should be used as first-line treatment for field therapy; oral chemoprevention should not be initiated (NC)b |
| Stage 3 | |
| Field cancerization | Lesion-directed therapy (C) followed by field therapy (C) using a fluorouracil-based modality (NC) should be used as first-line treatment; speaking with transplant team regarding immunosuppression should not be initiated (C)b |
| Stage 4 | |
| First invasive low-risk CSCCd | No consensus achieved |
| Stage 5 | |
| Multiple invasive low-risk CSCCsd | |
| Low rate (1 CSCC per year) (5a) | Oralchemoprevention should be initiated (NC) |
| High rate (10 CSCCs per year) (5b) | Oralchemoprevention with acitretin should be initiated (C); speak with transplant team regarding modification of immunosuppression (C) |
| Stage 6 | |
| High-risk CSCCe | |
| As first CSCC (6a) | Oral chemoprevention with acitretin should be initiated (NC); speak with transplant team regarding modification of immunosuppression (NC) |
| After multiple low-risk CSCCs (6b) | Oral chemoprevention with acitretin should be initiated (C); speak with transplant team regarding modification of immunosuppression (C) |
Abbreviations: AD-SCI, Actinic Damage and Skin Cancer Index; AK, actinic keratosis; C, consensus; CSCC, cutaneous squamous cell carcinoma; NC, near consensus.
Recommendations made if near consensus (70 to <80%) or consensus (≥80%) was achieved.
Preventive measures were recommended for all AD-SCI stages but are only listed for stage 1 because of table space constraints.
Negative consensus or negative near consensus indicates panelists responded negatively when asked about this intervention.
American Joint Committee on Cancer or Brigham andWomen’s Hospital stage T1 tumor.
American Joint Committee on Cancer T3 or Brigham andWomen’s Hospital stage T2B tumor.
Supplemental Information
When asked about the association of the various immunosuppressive medications with skin cancer formation, 42 of 48 respondents (88%) reported that azathioprine was the most associated with skin cancer formation, whereas 33 of 48 respondents (69%) thought that sirolimus was the least associated. A total of 40 of 46 respondents (87%) identified fluorouracil-based therapy as the most effective field agent, but only 10 of 46 (22%) reported that it resulted in the best adherence. Only 2 of 46 respondents (4%) reported that PDT was the most effective field agent, but 34 (74%) reported that its use results in the best adherence. Compliance concerns were identified as the major barrier (78%) to broader use of fluorouracil-based therapy despite RCT data demonstrating its benefit in immunocompetent patients.31 A total of 33 respondents (72%) reported that lack of data specific to SOTRs precluded widespread recommendation of nicotinamide for CSCC prophylaxis, despite RCT data40 demonstrating its benefit in immunocompetent patients and its safety in renal transplant patients.21 A total of 30 respondents (65%) reported that adverse effects limited widespread implementation of early mammalian target of rapamycin (mTOR) conversion in SOTRs despite RCT data25,41 demonstrating its benefit in reducing CSCC risk in this population.
Discussion
The Strategies for the Prevention of Skin Cancer in Solid Organ Transplant Recipients Delphi process was able to provide consensus-based management recommendations regarding optimal prevention strategies of CSCC in SOTRs across 5 of the 6 AD-SCI stages. Areas of consensus may aid physicians in establishing best practices regarding prevention of CSCC in SOTRs in the setting of limited high level of evidence data in this population. The panel recommended routine skin surveillance and sunscreen use for all patients. No studies on the impact of routine skin surveillance in SOTRs have been performed to date, but targeted screening in high-risk populations may be effective in melanoma42 and is in keeping with prior guidelines32 in transplant recipients. The panel’s recommendation for regular sunscreen use in SOTRs is in accordance with literature providing some evidence for a reduction in the incidence of AK and CSCC in this population with regular use.29
Although lesion-directed therapy with cryotherapy was favored for scattered AK, scenarios that involve anatomically grouped AK or field cancerized skin led to a recommendation for the initiation of field therapy with fluorouracil. However, the recommendation for fluorouracil was based on near but not full consensus of the panel. Indeed, no particular field agent reached the 80% or greater consensus threshold at this AD-SCI stage, reflecting a degree of uncertainty in the panel on this point. A prior review by Blomberg et al43 similarly identified uncertainty regarding the optimal field agent for management of AK and prevention of CSCC in SOTRs as a knowledge gap in the literature. This finding is in contrast to recent studies in immunocompetent patients, demonstrating that field therapy with fluorouracil is superior to PDT, ingenol mebutate, and imiquimod in a large, double-blind RCT for treatment of AK,31 reduces the need for lesion-directed therapy,44 and prevents CSCC.45 Although similar large RCTs are lacking for SOTR specifically, small split-patient studies in SOTRs found that PDT outperformed fluorouracil30 and imiquimod16 in the treatment of AK. When the panel was presented with data supporting the use of fluorouracil-based therapy in immunocompetent patients (see Supplemental Information section), 40 of 46 respondents (87%) reported that they believed fluorouracil was the most effective field agent, but adherence concerns limited broader use (reported by 36 respondents [78%]). Acitretin was the sole oral chemoprevention agent recommended by our panel, put forth in scenarios in which SOTRs were developing CSCC at a high rate or had developed a high-risk CSCC. This recommendation is supported by RCTs in renal transplant recipients.18,19 The preferential use of acitretin in SOTRs with advanced CSCC disease as seen in our panel is somewhat discrepant with the existing literature, in which a benefit to acitretin was reported in SOTRs who had a history of, on average, less than 1 nonmelanoma skin cancer per year diagnosed during 5 years and as little as 1 nonmelanoma skin cancer in the prior 5 years.19 Furthermore, even low-rate CSCC formation portends poor outcomes. In a previous analysis,46 development of 10 or more CSCCs during a 10-year period (approximately 1 per year) in a group of mostly immunosuppressed patients was associated with a 26% risk of nodal metastasis, suggesting that early and aggressive preventive intervention is indicated in this population. To date, no RCTs have compared the benefit of acitretin in SOTRs with differential (high vs low) rates of CSCC formation.
Finally, the panel recommended initiating discussions with transplant physicians regarding immunosuppression modification in patients with advanced CSCC (AD-SCI stages 5 and 6) disease but otherwise did not make a recommendation as to the best immunosuppression modification strategy to pursue. Specifically, the panel did not recommend discussing reduction of immunosuppression or conversion to mTOR inhibition, 2 evidence-based24,25,41 mechanisms of secondary and tertiary prevention of CSCC in SOTRs, with transplant physicians. In these scenarios, 58% to 67%, depending on the specific query, of the panel preferred to defer this decision to transplant physicians. Although the absence of such a recommendation runs contrary to previous expert statements32,47,48 and some RCTs have demonstrated that reduction of immunosuppression24 or conversion to mTOR-based immunosuppression25,41 may decrease CSCC formation, this discrepancy is instructive regarding the balance expert physicians must use in management of SOTRs: patients with reduced immunosuppression are at risk for inferior graft survival,49 and conversion to mTOR may affect overall survival.50
The panel did not reach any consensus management recommendation for prevention for an SOTR who develops a first low-risk CSCC. Notably, panel recommendations in this scenario were not affected when asked about the specific organ transplanted (ie, abdominal vs thoracic). As discussed above, the panel did not incorporate the results of the RCT by Euvrard et al41 demonstrating benefit of early conversion to sirolimus in renal transplant recipients (most patients in this study were enrolled after a first CSCC) into its recommendations. Sixty-five percent of respondents reported that widespread use of mTOR conversion in SOTRs is limited by adverse effects. The lack of consensus regarding management for SOTRs who develop a first low-risk CSCC reflects clinical equipoise and should be the subject of further investigation.
The panel did not make a recommendation for use of nicotinamide or capecitabine in any of the stages presented. The absence of a recommendation for nicotinamide is notable in the context of a double-blind RCT in immunocompetent patients40 demonstrating benefit in prevention of AKs and CSCCs. A smaller RCT21 in 22 SOTRs did not find a significant benefit from nicotinamide but was underpowered to do so because the study was terminated early because of low enrollment. However, the study demonstrated safety, with no major adverse effects noted. In supplemental questioning, 72% of the panel reported that the lack of efficacy data specifically for SOTR limited their use of nicotinamide. Given the low cost, high safety, and demonstration of CSCC reduction in non-SOTRs, nicotinamide administration may be an area for further consideration and expanded study. Efficacy of capecitabine has been reported in case series in SOTRs,22 but it has not been studied in a randomized manner for chemoprevention. More than half of the panel (52%) reported that they did not have routine access to capecitabine in their practice.
Limitations
Our study has some limitations, including those intrinsic to the Delphi process. Our chosen criteria for consensus were strict (≥80% agreement), and thus more areas of consensus may have been reported with a lower standard. Distinctions were drawn to distinguish patient scenarios, such as the difference between forming low-risk CSCC at a low rate (1 CSCC per year) vs high rate (10 CSCCs per year), which may leave ambiguity, for example, in how an SOTR with multiple low-risk CSCCs per year above the low but below the high threshold should be best managed. Similarly, we explored the association of a first low-risk and high-risk CSCC with prevention strategies but did not inquire as to how an intermediate-risk CSCC (AJCC T2 or BWH T2a) would affect management. The AD-SCI is based on expert opinion and is not a validated instrument. Finally, because the panel comprised an international group of transplant dermatologists, the availability of certain prevention strategies varied, which may have affected survey responses.
Conclusions
This Delphi process resulted in recommendations for management strategies in the prevention of CSCC in SOTRs in 5 of the 6 AD-SCI stages and included cryotherapy for scattered AKs, field therapy for AKs in 1 anatomical area (augmented by cryotherapy if thick), combination lesion directed to hyperkeratotic lesions followed by field therapy with fluorouracil for field cancerized skin, and initiation of acitretin treatment and discussion of immunosuppression reduction or modification for patients developing 10 CSCCs per year or a tumor with 20% or greater risk of nodal metastasis. These recommendations reflect consensus among expert transplant dermatologists and the incorporation of limited and sometimes contradictory evidence into real-world clinical experience across a range of CSCC disease severity. No consensus was reached regarding management of SOTRs who have a first low-risk CSCC; future studies and clinical trials aimed at assessing optimal management in this clinical scenario are much needed, and involvement of transplant medicine colleagues should be considered in such investigations. These recommendations will assist physicians in implementing prevention strategies for management of CSCC in SOTRs while awaiting high level-of-evidence data to guide best practices.
Supplementary Material
Key Points.
Question
What are the recommended medical interventions for skin cancer prevention in solid organ transplant recipients?
Findings
On the basis of the results of the Delphi study, cryotherapy is recommended as first-line therapy for actinic keratosis, with the exception of thin actinic keratoses grouped in 1 area. In that scenario and for field cancerization, field therapy should be performed.
Meaning
Oral chemoprevention and discussion with the transplant team regarding modification of immunosuppression should be initiated when the patient develops multiple low-risk cutaneous squamous cell carcinoma (>10 tumors per year) or a high-risk cutaneous squamous cell carcinoma.
Footnotes
Conflict of Interest Disclosures: Dr Schmults reported serving as a steering committee member for Castle Biosciences, a steering committee member and consultant for Regeneron Pharmaceuticals, and a consultant for Sanofi; receiving research funding from Castle Biosciences, Regeneron Pharmaceuticals, Novartis, Genentech, and Merck; and serving a chair for the National Comprehensive Cancer Network. Dr Arron reported being an employee of Rakuten Medical and serving as a consultant for Castle Biosciences and EnSpectra Health. Dr Asgari reported receiving grants from Pfizer outside the submitted work. Dr Christensen reported receiving grants from PellePharm and serving as a consultant for the Regeneron/Sanofi Advisory Board and LEO Pharma outside the submitted work. Dr DeSimone reported serving as a consultant and receiving speaker fees from Sanofi/Genzyme outside the submitted work. Dr Ferrándiz-Pulido reported receiving advisory fees from Sun Pharma, Sanofi, Almirall, and Galderma outside the submitted work. Dr Shin reported receiving grants from Regeneron outside the submitted work. Dr Zeitouni reported receiving grants from Biofrontera, consulting and speaker fees from Biofrontera, grants and consultant from Sun Pharma, and speaker fees from Genentech and Sanofi/Regeneron outside the submitted work. Dr Harwood reported receiving personal fees from LEO Pharma, Sanofi/Regeneron, Merck, Novartis, and PellePharm and nonfinancial support from Meda and PellePharm trial outside the submitted work and grants from the UK National Institute for Health Research. Dr Jambusaria-Pahlajani reported receiving an unrestricted educational grant from Regeneron outside the submitted work. No other disclosures were reported.
Contributor Information
Paul R. Massey, Department of Dermatology, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts.
Chrysalyne D. Schmults, Department of Dermatology, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts.
Sara J. Li, Department of Dermatology, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts.
Sarah T. Arron, Sarah Arron MD, A Professional Corporation, San Mateo, California.
Maryam M. Asgari, Department of Dermatology, Massachusetts General Hospital, Boston; Department of Population Medicine, Harvard Pilgrim Healthcare Institute, Boston, Massachusetts.
Jan Nico Bouwes Bavinck, Department of Dermatology, Leiden University Medical Center, Leiden, the Netherlands.
Elizabeth Billingsley, Department of Dermatology, Penn State Hershey Medical Center, Hershey, Pennsylvania.
Travis W. Blalock, Department of Dermatology, Emory University School of Medicine, Atlanta, Georgia.
Katie Blasdale, Department of Dermatology, Royal Victoria Infirmary, Newcastle upon Tyne, United Kingdom.
Bryan T. Carroll, Department of Dermatology, University Hospitals Cleveland Medical Center, Cleveland, Ohio; Department of Dermatology, Case Western Reserve University, Cleveland, Ohio.
John A. Carucci, The Ronald O. Perelman Department of Dermatology, New York University School of Medicine, New York.
Alvin H. Chong, Skin Health Institute, Victoria, Australia; Department of Medicine (Dermatology), St Vincent’s Hospital Clinical School, The University of Melbourne, Victoria, Australia.
Sean R. Christensen, Department of Dermatology, Yale University School of Medicine, New Haven, Connecticut.
Christina Lee Chung, Montgomery Dermatology, King of Prussia, Pennsylvania; Lankenau Institute for Medical Research, Wynnewood, Pennsylvania.
Jennifer A. DeSimone, Inova Melanoma and Skin Oncology Center, Fairfax, Virginia.
Emilie Ducroux, Dermatology Department, Edouard Herriot Hospital, Hospices Civils de Lyon, Université Claude Bernard Lyon I, Lyon, France.
Begoña Escutia-Muñoz, Department of Dermatology, Hospital Universitario La Fe, Valencia, Spain.
Carla Ferrándiz-Pulido, Department of Dermatology, Hospital Universitari Vall d’Hebron, Barcelona, Spain; Universitat Autònoma de Barcelona, Barcelona, Spain.
Matthew C. Fox, Division of Dermatology, Dell Medical School, University of Texas at Austin, Austin.
Roel E. Genders, Department of Dermatology, Leiden University Medical Center, Leiden, the Netherlands.
Alexandra Geusau, Department of Dermatology, Medical University of Vienna, Vienna, Austria.
Petter Gjersvik, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Department of Dermatology, Oslo University Hospital, Oslo, Norway.
Allison M. Hanlon, Department of Dermatology, Vanderbilt University, Nashville, Tennessee.
Edit B. Olasz Harken, Department of Dermatology, Medical College of Wisconsin, Milwaukee.
Günther F.L. Hofbauer, Department of Dermatology, University Hospital Zurich, Zurich, Switzerland.
R. Samuel Hopkins, Department of Dermatology, Oregon Health and Science University, Portland.
Justin J. Leitenberger, Department of Dermatology, Oregon Health and Science University, Portland.
Manisha J. Loss, Department of Dermatology, Johns Hopkins School of Medicine, Baltimore, Maryland.
Veronique Del Marmol, Department of Dermatology, Hôpital Erasme, Université Libre de Bruxelles, Brussels, Belgium.
José M. Mascaró, Jr, Department of Dermatology, Hospital Clínic, Universitat de Barcelona, Barcelona, Spain.
Sarah A. Myers, Department of Dermatology, Duke University, Durham, North Carolina.
Bichchau T. Nguyen, Department of Dermatology, Tufts Medical Center, Tufts University School of Medicine, Boston, Massachusetts; Department of Dermatology, Boston Medical Center, Boston University School of Medicine, Boston, Massachusetts.
Walmar R. P. Oliveira, Department of Dermatology, Faculdade de Medicina da Universidade de São Paulo, São Paulo, SP, Brazil.
Clark C. Otley, Department of Dermatology, Mayo Clinic, Rochester, Minnesota.
Charlotte M. Proby, Ninewells Hospital and Medical School, University of Dundee, Dundee, United Kingdom.
Emőke Rácz, Department of Dermatology, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands.
Veronica Ruiz-Salas, Department of Dermatology, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
Faramarz H. Samie, Department of Dermatology, Columbia University Irving Medical Center, New York, New York.
Deniz Seçkin, Department of Dermatology, Başkent University Faculty of Medicine, Ankara, Turkey.
Syed N. Shah, Department of Dermatology, Norfolk and Norwich University Hospital, Norwich, United Kingdom.
Thuzar M. Shin, Department of Dermatology, University of Pennsylvania, Philadelphia.
Stephen P. Shumack, Department of Dermatology, University of Sydney, Sydney, Australia.
Seaver L. Soon, Scripps Green Hospital, Private Practice (The Skin Clinic MD), San Diego, California.
Thomas Stasko, Department of Dermatology, University of Oklahoma Health Sciences Center, Oklahoma City.
Elisa Zavattaro, Dermatology Unit, Department of Translational Medicine, University of Piemonte Orientale, Novara, Italy.
Nathalie C. Zeitouni, Medical Dermatology Specialists, Phoenix, Arizona; Division of Dermatology, University of Arizona College of Medicine, Phoenix.
Fiona O’Reilly Zwald, Piedmont Healthcare, Atlanta, Georgia; O’Reilly Comprehensive Dermatology Inc, Atlanta, Georgia; Mount Vernon Medical Center, Atlanta, Georgia.
Catherine A. Harwood, Centre for Cell Biology and Cutaneous Research, Blizard Institute, Barts, United Kingdom; The London School of Medicine and Dentistry, Queen Mary University of London, London, United Kingdom.
Anokhi Jambusaria-Pahlajani, Division of Dermatology, Dell Medical School, University of Texas at Austin, Austin.
REFERENCES
- 1.Rizvi SMH, Aagnes B, Holdaas H, et al. Long-term change in the risk of skin cancer after organ transplantation: a population-based nationwide cohort study. JAMA Dermatol. 2017;153(12):1270–1277. doi: 10.1001/jamadermatol.2017.2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teutsch SM. A framework for assessing the effectiveness of disease and injury prevention. MMWR Recomm Rep. 1992;41(RR-3):1–12. [PubMed] [Google Scholar]
- 3.Kabir Z, Bennett K, Shelley E, Unal B, Critchley JA, Capewell S. Comparing primary prevention with secondary prevention to explain decreasing coronary heart disease death rates in Ireland, 1985–2000. BMC Public Health. 2007;7:117. doi: 10.1186/1471-2458-7-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown VL, Atkins CL, Ghali L, Cerio R, Harwood CA, Proby CM. Safety and efficacy of 5% imiquimod cream for the treatment of skin dysplasia in high-risk renal transplant recipients: randomized, double-blind, placebo-controlled trial. Arch Dermatol. 2005;141(8):985–993. doi: 10.1001/archderm.141.8.985 [DOI] [PubMed] [Google Scholar]
- 5.Ulrich C, Busch JO, Meyer T, et al. Successful treatment of multiple actinic keratoses in organ transplant patients with topical 5% imiquimod: a report of six cases. Br J Dermatol. 2006;155(2):451–454. doi: 10.1111/j.1365-2133.2006.07233.x [DOI] [PubMed] [Google Scholar]
- 6.Ulrich C, Bichel J, Euvrard S, et al. Topical immunomodulation under systemic immunosuppression: results of a multicentre, randomized, placebo-controlled safety and efficacy study of imiquimod 5% cream for the treatment of actinic keratoses in kidney, heart, and liver transplant patients. Br J Dermatol. 2007;157(suppl 2):25–31. doi: 10.1111/j.1365-2133.2007.08269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith KJ, Germain M, Skelton H. Squamous cell carcinoma in situ (Bowen’s disease) in renal transplant patients treated with 5% imiquimod and 5% 5-fluorouracil therapy. Dermatol Surg. 2001;27(6):561–564. doi: 10.1046/j.1524-4725.2001.00149.x [DOI] [PubMed] [Google Scholar]
- 8.Ulrich C, Hackethal M, Ulrich M, et al. Treatment of multiple actinic keratoses with topical diclofenac 3% gel in organ transplant recipients: a series of six cases. Br J Dermatol. 2007;156(suppl 3):40–42. doi: 10.1111/j.1365-2133.2007.07864.x [DOI] [PubMed] [Google Scholar]
- 9.Mohanna M, Hofbauer G. Pronounced local skin reaction to ingenol mebutate against actinic keratosis in kidney transplant recipient without systemic adverse events. JAAD Case Rep. 2015;1(6):S19–S22. doi: 10.1016/j.jdcr.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garofalo V, Ventura A, Mazzilli S, et al. Treatment of multiple actinic keratosis and field of cancerization with topical piroxicam 0.8% and sunscreen 50+ in organ transplant recipients: a series of 10 cases. Case Rep Dermatol. 2017;9(3):211–216. doi: 10.1159/000481770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Graaf YG, Euvrard S, Bouwes Bavinck JN. Systemic and topical retinoids in the management of skin cancer in organ transplant recipients. Dermatol Surg. 2004;30(4 pt 2):656–661. doi: 10.1111/j.1524-4725.2004.30152.x [DOI] [PubMed] [Google Scholar]
- 12.Dragieva G, Prinz BM, Hafner J, et al. A randomized controlled clinical trial of topical photodynamic therapy with methyl aminolaevulinate in the treatment of actinic keratoses in transplant recipients. Br J Dermatol. 2004;151(1):196–200. doi: 10.1111/j.1365-2133.2004.06054.x [DOI] [PubMed] [Google Scholar]
- 13.Dragieva G, Hafner J, Dummer R, et al. Topical photodynamic therapy in the treatment of actinic keratoses and Bowen’s disease in transplant recipients. Transplantation. 2004;77(1):115–121. doi: 10.1097/01.TP.0000107284.04969.5C [DOI] [PubMed] [Google Scholar]
- 14.Helsing P, Togsverd-Bo K, Veierød MB, Mørk G, Haedersdal M. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169(5):1087–1092. doi: 10.1111/bjd.12507 [DOI] [PubMed] [Google Scholar]
- 15.Togsverd-Bo K, Omland SH, Wulf HC, Sørensen SS, Haedersdal M. Primary prevention of skin dysplasia in renal transplant recipients with photodynamic therapy: a randomized controlled trial. Am J Transplant. 2015;15(11):2986–2990. doi: 10.1111/ajt.13358 [DOI] [PubMed] [Google Scholar]
- 16.Togsverd-Bo K, Halldin C, Sandberg C, et al. Photodynamic therapy is more effective than imiquimod for actinic keratosis in organ transplant recipients: a randomized intraindividual controlled trial. Br J Dermatol. 2018;178(4):903–909. doi: 10.1111/bjd.15884 [DOI] [PubMed] [Google Scholar]
- 17.Willey A, Mehta S, Lee PK. Reduction in the incidence of squamous cell carcinoma in solid organ transplant recipients treated with cyclic photodynamic therapy. Dermatol Surg. 2010;36(5):652–658. doi: 10.1111/j.1524-4725.2009.01384.x [DOI] [PubMed] [Google Scholar]
- 18.Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13(8):1933–1938. doi: 10.1200/JCO.1995.13.8.1933 [DOI] [PubMed] [Google Scholar]
- 19.George R, Weightman W, Russ GR, Bannister KM, Mathew TH. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43(4):269–273. doi: 10.1046/j.1440-0960.2002.00613.x [DOI] [PubMed] [Google Scholar]
- 20.de Sévaux RG, Smit JV, de Jong EM, van de Kerkhof PC, Hoitsma AJ. Acitretin treatment of premalignant and malignant skin disorders in renal transplant recipients: clinical effects of a randomized trial comparing two doses of acitretin. J Am Acad Dermatol. 2003;49(3):407–412. doi: 10.1067/S0190-9622(03)01831-0 [DOI] [PubMed] [Google Scholar]
- 21.Chen AC, Martin AJ, Dalziell RA, et al. A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients. Br J Dermatol. 2016;175(5):1073–1075. doi: 10.1111/bjd.14662 [DOI] [PubMed] [Google Scholar]
- 22.Endrizzi BT, Lee PK. Management of carcinoma of the skin in solid organ transplant recipients with oral capecitabine. Dermatol Surg. 2009;35(10):1567–1572. doi: 10.1111/j.1524-4725.2009.01277.x [DOI] [PubMed] [Google Scholar]
- 23.Otley CC, Maragh SL. Reduction of immunosuppression for transplant-associated skin cancer: rationale and evidence of efficacy. Dermatol Surg. 2005;31(2):163–168. doi: 10.1097/00042728-200502000-00008 [DOI] [PubMed] [Google Scholar]
- 24.Dantal J, Hourmant M, Cantarovich D, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998;351(9103):623–628. doi: 10.1016/S0140-6736(97)08496-1 [DOI] [PubMed] [Google Scholar]
- 25.Dantal J, Morelon E, Rostaing L, et al. Sirolimus for secondary prevention of skin cancer in kidney transplant recipients: 5-year results. J Clin Oncol. 2018;36(25):2612–2620. doi: 10.1200/JCO.2017.76.6691 [DOI] [PubMed] [Google Scholar]
- 26.Salgo R, Gossmann J, Schöfer H, et al. Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients: reduced rate of (pre-)malignancies and nonmelanoma skin cancer in a prospective, randomized, assessor-blinded, controlled clinical trial. Am J Transplant. 2010;10(6):1385–1393. doi: 10.1111/j.1600-6143.2009.02997.x [DOI] [PubMed] [Google Scholar]
- 27.Vos M, Plasmeijer EI, van Bemmel BC, et al. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant. 2018;37(7):853–859. doi: 10.1016/j.healun.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 28.Lopez AT, Carvajal RD, Geskin L. Secondary prevention strategies for nonmelanoma skin cancer. Oncology. 2018;32(4):195–200. [PubMed] [Google Scholar]
- 29.Ulrich C, Jürgensen JS, Degen A, et al. Prevention of non-melanoma skin cancer in organ transplant patients by regular use of a sunscreen: a 24 months, prospective, case-control study. Br J Dermatol. 2009;161(suppl 3):78–84. doi: 10.1111/j.1365-2133.2009.09453.x [DOI] [PubMed] [Google Scholar]
- 30.Perrett CM, McGregor JM, Warwick J, et al. Treatment of post-transplant premalignant skin disease: a randomized intrapatient comparative study of 5-fluorouracil cream and topical photodynamic therapy. Br J Dermatol. 2007;156(2):320–328. doi: 10.1111/j.1365-2133.2006.07616.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380(10):935–946. doi: 10.1056/NEJMoa1811850 [DOI] [PubMed] [Google Scholar]
- 32.Stasko T, Brown MD, Carucci JA, et al. ; International Transplant-Skin Cancer Collaborative; European Skin Care in Organ Transplant Patients Network. Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Dermatol Surg. 2004;30(4 pt 2):642–650. doi: 10.1111/j.1524-4725.2004.30150.x [DOI] [PubMed] [Google Scholar]
- 33.Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. Am J Public Health. 1984;74(9):979–983. doi: 10.2105/AJPH.74.9.979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powell C The Delphi technique: myths and realities. J Adv Nurs. 2003;41(4):376–382. doi: 10.1046/j.1365-2648.2003.02537.x [DOI] [PubMed] [Google Scholar]
- 35.Mullen PM. Delphi: myths and reality. J Health Organ Manag. 2003;17(1):37–52. doi: 10.1108/14777260310469319 [DOI] [PubMed] [Google Scholar]
- 36.Hsu C-C, Sandford BA. The Delphi technique: making sense of consensus. Res Eval. 2007;12(10):1–9. doi: 10.7275/pdz9-th90 [DOI] [Google Scholar]
- 37.Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67(4):401–409. doi: 10.1016/j.jclinepi.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 38.Amin M, Edge S, Greene F, et al. , eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017. doi: 10.1007/978-3-319-40618-3 [DOI] [Google Scholar]
- 39.Karia PS, Jambusaria-Pahlajani A, Harrington DP, Murphy GF, Qureshi AA, Schmults CD. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol. 2014;32(4):327–334. doi: 10.1200/JCO.2012.48.5326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen AC, Martin AJ, Choy B, et al. A phase 3randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373(17):1618–1626. doi: 10.1056/NEJMoa1506197 [DOI] [PubMed] [Google Scholar]
- 41.Euvrard S, Morelon E, Rostaing L, et al. ; TUMORAPA Study Group. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367(4):329–339. doi: 10.1056/NEJMoa1204166 [DOI] [PubMed] [Google Scholar]
- 42.Schneider JS, Moore DH II, Mendelsohn ML. Screening program reduced melanoma mortality at the Lawrence Livermore National Laboratory, 1984 to 1996. J Am Acad Dermatol. 2008;58(5):741–749. doi: 10.1016/j.jaad.2007.10.648 [DOI] [PubMed] [Google Scholar]
- 43.Blomberg M, He SY, Harwood C, et al. ; NCI Keratinocyte Carcinoma Consortium. Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177(5):1225–1233. doi: 10.1111/bjd.15950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pomerantz H, Hogan D, Eilers D, et al. ; VeteransAffairs Keratinocyte Carcinoma Chemoprevention (VAKCC) Trial Group. Long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol. 2015;151(9):952–960. doi: 10.1001/jamadermatol.2015.0502 [DOI] [PubMed] [Google Scholar]
- 45.Weinstock MA, Thwin SS, Siegel JA, et al. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154(2):167–174. doi: 10.1001/jamadermatol.2017.3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levine DE, Karia PS, Schmults CD. Outcomes of patients with multiple cutaneous squamous cell carcinomas: a 10-year single-institution cohort study. JAMA Dermatol. 2015;151(11):1220–1225. doi: 10.1001/jamadermatol.2015.1702 [DOI] [PubMed] [Google Scholar]
- 47.Otley CC, Berg D, Ulrich C, et al. ; Reduction of Immunosuppression Task Force of the International Transplant Skin Cancer Collaborative and the Skin Care in Organ Transplant Patients Europe. Reduction of immunosuppression for transplant-associated skin cancer: expert consensus survey. Br J Dermatol. 2006;154(3):395–400. doi: 10.1111/j.1365-2133.2005.07087.x [DOI] [PubMed] [Google Scholar]
- 48.Otley CC, Griffin MD, Charlton MR, Edwards BS, Neuburg M, Stasko T; Reduction of Immunosuppression Task Force of the International Transplant Skin Cancer Collaborative. Reduction of immunosuppression for transplant-associated skin cancer: thresholds and risks. Br J Dermatol. 2007;157(6):1183–1188. doi: 10.1111/j.1365-2133.2007.08203.x [DOI] [PubMed] [Google Scholar]
- 49.Yang D, Thamcharoen N, Cardarelli F. Management of immunosuppression in kidney transplant recipients who develop malignancy. J Clin Med. 2019;8(12):E2189. doi: 10.3390/jcm8122189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knoll GA, Kokolo MB, Mallick R, et al. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ. 2014;349:g6679. doi: 10.1136/bmj.g6679 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


