SUMMARY
OBJECTIVE:
This study aimed to evaluate the prevalence of ovarian hyperstimulation syndrome (OHSS) and associated risk factors in patients undergoing fertilization cycles at risk of OHSS (≥15 antral follicles or ≥15 oocytes aspirated) and submitted to cryopreservation of all embryos in the Human Reproduction Service of the Pérola Byington Hospital (Referral Center for Women's Health) in São Paulo, SP, Brazil.
METHODS:
This cross-sectional, institutional, descriptive study of secondary data from patients’ charts enrolled in the Assisted Reproduction Service of the Pérola Byington Hospital at risk of OHSS after controlled ovarian stimulation and submitted to cryopreservation of all embryos was conducted between January 2015 and September 2017.
RESULTS:
OHSS occurred in 47.5% of cycles, all with mild severity, and there were no moderate or severe cases of OHSS.
CONCLUSION:
The cryopreservation of all embryos is associated with a reduction in moderate and severe forms of OHSS. Risk factors for OHSS should be evaluated prior to initiation of treatment, with less intense stimulation protocols accordingly.
KEYWORDS: Ovarian hyperstimulation syndrome, Cryopreservation, Fertilization in vitro
INTRODUCTION
One in six couples experience fertility problems and, for 20% of this group, the only way to achieve a pregnancy is by using assisted reproduction technology (ART) 1,2 . These techniques, such as in vitro fertilization (IVF), seek to attain pregnancy by replacing or facilitating the defective stage in the reproduction process 3 . The ART, however, can lead to side effects, such as ovarian hyperstimulation syndrome (OHSS) 4 .
The syndrome can occur iatrogenically due to the high hormone dose administered to the patient during the oocyte stimulation phase. Human chorionic gonadotropin (hCG) is one of the hormones used in the stimulation process. The greater number of oocytes produced increases the chance of fertilization and success of the technique. However, this boosting of hormone level to increase success is associated with a 2–3% incidence of moderate and severe forms of OHSS in ARTs 5 . By comparison, OHSS incidence in a Referral Hospital for Assisted Reproduction in São Paulo, Brazil, was 1.9% 6 .
The syndrome affects 6020 patients annually in the United States and Europe, with an estimated mortality of 1 in 450,000–500,000 7 . Risk factors for OHSS include younger age, history of polycystic ovary syndrome (PCOS), and personal history of high response during a previous IVF cycle and on evaluation of biomarkers, such as anti-Müllerian hormone (AMH) level and follicles using ultrasound 8 .
The pathophysiology of OHSS is complex and not yet fully understood. However, the syndrome involves increased vascular permeability of the mesothelial layer of ovaries and leakage of protein-rich fluids into the interstitial or “third” space. Clinical symptoms reflect the degree of third spacing and hemoconcentration resulting from the depletion in intravascular volume 9 .
Pro-inflammatory vasoactive mediators, such as vascular endothelial growth factor (VEGF), are believed to be involved in this pathogenesis 10 . When stimulated under supraphysiological conditions, ovaries oversecrete VEGF to above the normal levels, promoting excessive vascular permeability with leakage to the third space, leading to reduced perfusion of organs 11 . Some studies have shown stronger association of VEGF with hCG and higher VEGF in peritoneal fluid of patients who used hCG when compared to the gonadotropin-releasing hormone (GnRH) agonist 12 .
The signs and symptoms vary with the severity of the syndrome. Mild symptoms occur in roughly 30% of patients, such as slight discomfort and distended abdomen due to increased volume of one or both ovaries5,13. More severe forms can present with ascites of varying severity; pleural effusion, oliguria secondary to renal failure, thromboembolism, and death can occur as a result of hemoconcentration and reduced perfusion of other organs such as the kidneys, heart, and brain 14,15 .
The two main types of OHSS are early and late onset. In early OHSS, symptom onset takes place 7 days after the application of hCG (administered for final oocyte maturation), whereas late OHSS manifests 10 days after hCG application and is triggered by endogenous hCG produced by trophoblasts following pregnancy. The late form of OHSS, compared with the early form, has a higher probability of becoming severe (72.2% vs. 42%) 15 .
One method of preventing OHSS is by performing cryopreservation of embryos. The technique entails freezing embryos and implanting them during a later cycle, when ovarian response has normalized after previous hyperstimulation for follicle production. In addition, the use of an antagonist protocol and final follicle maturation with a GnRH agonist trigger, followed by the freeze-all strategy, constitutes an effective option for the prevention of OHSS with high live birth rate 16,17 .
Taken together, this evidence suggests that cryopreservation represents one of the best prevention approaches for patients at high risk of OHSS, given that fresh embryo transfer triggers a further hCG surge following implantation. Therefore, the objective of the present study was to assess cryopreservation as a strategy for the prevention of OHSS and identify the risk factors associated with the syndrome.
METHODS
This retrospective, cross-sectional, institutional, descriptive study of secondary data from patients’ charts enrolled in the Assisted Reproduction Service of the Pérola Byington Hospital at risk of OHSS after controlled ovarian stimulation and submitted to cryopreservation of all embryos was conducted between January 2015 and September 2017.
Those women who had ≥15 antral follicle count (AFC) by ultrasound or ≥15 oocytes aspirated after controlled ovarian stimulation and submitted to cryopreservation of all embryos were included in the study.
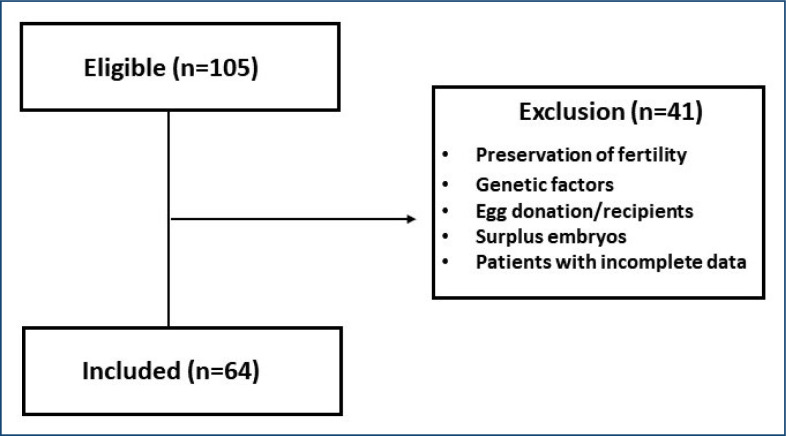
Patients undergoing cryopreservation of embryos for preservation of fertility, genetic factors, egg donation/recipients, and/or those with surplus embryos were excluded. In addition, patients’ charts with incomplete data were excluded.
The variables evaluated were as follows: age, body mass index (BMI) (calculated as weight/height 2 , kg/m 2 ), categorized according to the World Health Organization (WHO) criteria; infertility factors; the levels of AMH, follicle-stimulating hormone (FSH), and luteinizing hormone (LH); AFC by ultrasound; number of aspirated oocytes and number of mature oocytes; fertilization rate; fertilization technique employed, such as intracytoplasmic sperm injection (ICSI) or IVF; embryo transfer; occurrence of OHSS and related complications using the classification of Golan et al. 18 , for mild, moderate, and severe forms; type of OHSS treatment; occurrence of biochemical pregnancy (detection of hCG in maternal plasma 14 days after embryo transfer); and clinical pregnancy (detection of gestational sac on ultrasound from 7 weeks’ gestation).
The protocols for controlled ovarian stimulation for IVF/ICSI employed in the human reproduction service studied were assessed. The protocol used was dictated by AFC as follows:
Protocol A (AFC≤15): 300 IU FSH/hMG (human menopausal gonadotropin) per day; GnRH antagonist (Cetrorelix or Ganirelix) 0.25 mg; trigger with hCG (Choriomon 5000 IU) or GnRH agonist (Gonapeptyl 0.2 mg/day) when at risk of OHSS.
Protocol B (AFC=11–15): long block with GnRH agonist (1.875 mg Triptorelin); stimulation with 300 IU FSH/hMG per day; trigger with hCG (Choriomon 5000 IU).
Protocol C (AFC≥15): stimulation with 150 IU FSH/hMG per day; GnRH antagonist (Cetrorelix or Ganirelix) 0.25 mg; trigger with GnRH agonist (Gonapeptyl 0.2 mg/day).
Sample size calculation and statistical analysis
The sample size was calculated using a confidence interval, adopting an initial prevalence of 3% based on the outcome of OHSS incidence in the study population by Papanikolaou et al. 19 . A 95% level of confidence and 4.5% error margin yielded n=55.
Data collection was carried out based on a review of medical charts using a data collection instrument containing the variables of interest for the study. A database was created using the statistical software SPSS (Statistical Package for Social Sciences) for Windows version 17.0, where data were tabulated and analyzed. The variables were categorized based on the criteria used by the institution's protocol to predict ovarian hyper-response (risk of OHSS). The prevalence of OHSS and respective confidence interval were calculated for a 95% confidence level. Associations between categorical variables were explored with bivariate analysis using chi-square or Fisher's exact test, considering a p-value <0.05 as significant.
RESULTS
A total of 64 fertilization cycles of patients at risk of developing OHSS undergoing cryopreservation of all embryos were assessed (Figure 1). The mean age of patients was 32 years (range 23–40), and 56.1% were nulliparous. The patients’ characteristics are summarized in Table 1.
Figure 1. Patient distribution flowchart as determined by inclusion/exclusion criteria.
Table 1. Characteristics of patients undergoing cryopreservation of all embryos.
| Mean | SD | |
|---|---|---|
| Age | 32.0 | 3.8 |
| BMI | 26.6 | 4.9 |
| AFC | 16.9 | 6.1 |
| Number of aspirated oocytes | 19.0 | 8.6 |
| Number of mature oocytes | 15.6 | 7.9 |
| AMH | 5.1 | 4.1 |
SD: standard deviation; BMI: body mass index; AFC: antral follicle count; AMH: anti-Müllerian hormone.
With regard to BMI, 45.7% of patients were of healthy weight, 34.8% were of overweight, 15.2% belong to Class 1 obesity, and 4.3% belong to Class 3 obesity.
Of the cases evaluated, 43.8% had tubule factor, 28.1% PCOS, 17.2% male factor, 15.6% endometriosis, and 7.8% had unexplained infertility. Of these cases, six had two or more infertility factors.
The level of AMH averaged 5.1 ng/mL, and in 48.4% of cases, it was 3.5 ng/mL. Mean FSH level was 5.9 mIU/mL and LH level was 6.4 mIU/mL.
Protocol C was the most used (50.0%), followed by B (42.2%) and A (7.8%). All patients underwent cryopreservation of embryos to prevent OHSS.
Mean number of antral follicles was 16.9, and 64.1% of cases had ≥15 follicles. Mean number of aspirated follicles was 19, and mean fertilization rate was 72.8%.
ICSI was the most commonly used fertilization technique (78.1% of cases). Embryo transfer was performed in 87.5% of the cycles. Biochemical pregnancy occurred in 50% of these cycles and clinical pregnancy in 39.2%. There were a total of 22 pregnancies, 4 of which were twin pregnancies.
OHSS occurred in 47.5% (95%CI 22.7–68.2) of cycles, all with mild symptoms. There were no cases of moderate or severe OHSS.
The results for risk factors associated with OHSS are given in Table 2.
Table 2. Factors associated with ovarian hyperstimulation syndrome in cycles of patients undergoing cryopreservation of all embryos.
| Mild OHSS | p* | |||
|---|---|---|---|---|
| Yes | No | |||
| Number of aspirated oocytes | ≤15 | 8 (42.1) | 11 (57.9) | 0.567 |
| >15 | 21 (50.0) | 21 (50.0) | ||
| Trigger | Gonapeptyl | 13 (39.4) | 20 (60.6) | 0.212 |
| Choriomon | 15 (55.6) | 12 (44.4) | ||
| AMH | ≤3.4 | 5 (31.3) | 11 (68.8) | 0.213 |
| >3.4 | 8 (53.3) | 7 (46.7) | ||
| BMI | <25 | 10 (45.5) | 12 (54.5) | 0.654 |
| ≥25 | 13(52.0) | 12 (48.0) | ||
| PCOS | No | 21 (46.7) | 24 (53.3) | 0.819 |
| Yes | 8 (50.0) | 8 (50.0) | ||
| Age | >35 | 5 (55.6) | 4 (44.4) | 0.724 |
| ≤35 | 24 (46.2) | 28 (53.8) | ||
OHSS: ovarian hyperstimulation syndrome; AMH: anti-Müllerian hormone; BMI: body mass index; PCOS: polycystic ovarian syndrome.
Significance level <0.05.
DISCUSSION
This study showed that cryopreservation of all embryos prevented moderate and severe forms of OHSS in patients at risk for OHSS, in that there were no moderate or severe cases of the syndrome. This evidence supports embryo freezing as a key strategy for reducing the risk in susceptible patients, given that fresh embryo transfer triggers a further hCG surge following implantation.
These findings are consistent with previous results reported in the literature. A randomized clinical trial describing the use of freeze-all compared elective cryopreservation of all embryos with a new fresh embryo transfer in patients at risk of OHSS. Ferraretti et al. found a reduced risk of moderate/severe OHSS, where 6% of patients in the fresh embryo transfer group developed severe OHSS versus zero cases in the freeze-all group. In addition, there was no significant difference in live birth rates between the two groups 20 .
Several principal clinical parameters have been established, such as the number of follicles on the day of the trigger, to help attenuate the risk of moderate and severe OHSS 21 . Thus, the use of freeze-all strategy after a GnRH agonist trigger is the gold standard strategy for patients at risk of OHSS. With regard to clinical aspects, it is important to note that embryo freezing is a well-established technique with similar pregnancy rates as fresh embryo transfer 4,22 .
In the present study, mild OHSS occurred in 47.5% of the patients assessed. Other studies report mild syndrome in around 30% of IVF cycles 5 . One of the determinants of this rate is the fact that total prevention of OHSS is not possible until the pathogenesis of the syndrome has been fully elucidated. Thus, although it can prevent late OHSS (moderate and severe forms), cryopreservation is not totally effective for the prevention of the syndrome because the strategy cannot prevent early-onset OHSS (mild form) 9 .
Another factor that may influence the outcome of this study for mild cases of OHSS is the protocol employed for the cycle. The choice of protocol was dictated by AFC and risk factors, where women with AFC>15 underwent the protocol with antagonist and GnRH agonist trigger (Protocol C). However, 42.2% of cycles were performed using Protocol B (use of agonist and hCG trigger) because AFC count was between 11 and 15. Nevertheless, this number of follicles subsequently rose following ovarian stimulation to over 15 follicles aspirated, a factor that may have influenced the development of OHSS.
In addition, another possibility for preventing OHSS and its severity reduction is the use of dopamine agonist. But the protocols are not well defined, and more studies are needed to evaluate the potential of dopaminergic agonist in the prevention of OHSS. A recent study showed that bromocriptine did not prevent the moderate or severe cases of early-onset OHSS in high-risk patients subjected to IVF 23 .
Generally, there is a tendency for fewer moderate and severe cases of OHSS. This reduction is due to the greater screening of risk factors, with ovarian marker studies that predict supraphysiological response and allow the use of individualized ovarian stimulation protocols. In addition, wider use of GnRH antagonists for the prevention of premature release of LH and expansion of freeze-all procedures are factors contributing to a reduction in complications related to OHSS 24 .
The specific risk factors (markers of ovarian reserve) are AMH level and AFC 6 . Randomized prospective studies have shown that a basal AMH level to OHSS and allow the use ofresponse with high sensitivity (90.5%) and specificity (81.3%). Women with levels exceeding 5 ng/mL are at 3 times greater risk of developing OHSS 25 . Another study suggests that an AFC>16 has 89% sensitivity and 92% specificity of predicting high ovarian response 26 .
In the present study, risk factors were evaluated and no statistically significant association with OHSS was found, possibly explained by the small sample size, since this was not a primary objective of the study.
The findings of this research are strengthened by the selection of an appropriate sample size and the use of broad eligibility criteria. The prevalence of OHSS in patients undergoing fertilization cycles at risk of OHSS and submitted to cryopreservation of all embryos found in the Service of Human Reproduction of the Pérola Byington Hospital proved to be within the range suggested by the literature. The characterization of patients with OHSS and ARTs employed did not differ from that described in other studies.
This study has some limitations, such as the final pregnancy rate, where some patients had not undergone embryo transfer and were still being treated at the assisted reproduction service during the study period. Other patients also went on to receive a further stimulation cycle. The retrospective design of the study represents another limitation. Further studies involving larger casuistics will allow investigation of other aspects related to this outcome, improving the success of ARTs and preventing complications such as severe forms of OHSS.
CONCLUSION
Cryopreservation of all embryos is associated with a reduction in moderate and severe forms of OHSS. Risk factors for OHSS should be evaluated before commencing treatment, with less intense stimulation protocols adopted accordingly. Thus, efforts should be made in OHSS prevention, given that once the syndrome has developed, there is no reliable form of treatment, particularly in severe cases.
Footnotes
Funding: none.
ETHICAL COMMITTEE
The study was approved by the Plataforma Brasil by the Research Ethics Committee under CAAE: 46741721.6.0000.0069 and Permit no. 4.735.050, for report dated May 26, 2021. The study complied with the National Board of Health resolution CNS 196/96 on research involving humans.
REFERENCES
- 1.Lins PGA, Patti EAMR, Peron AC, Barbieri V. O sentido da maternidade e da infertilidade: um discurso singular. Estud Psicol (Campinas) 2014;31(3):387–392. doi: 10.1590/0103-166x2014000300007. [DOI] [Google Scholar]
- 2.Straube KM. In: 1° Consenso de Psicologia em Reprodução Assistida. Borges E Junior, Oliveira CM Filho, Silva AA, editors. São Paulo: Livre Expressão; 2013. 1° consenso de psicologia em reprodução assistida: uma experiência inovadora; pp. 15–20. [Google Scholar]
- 3.Daniluk JC, Koert E. The other side of fertility coin: a comparison of childless men's and women's knowledge of fertility and assisted reproductive technology. Fertil Steril. 2013;99(3):839–846. doi: 10.1016/j.fertnstert.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 4.Thomsen L, Humaidan P. Ovarian hyperstimulation syndrome in the 21st century: the role of gonadotropin-releasing hormone agonist trigger and kisspeptin. Curr Opin Obstet Gynecol. 2015;27(3):210–214. doi: 10.1097/GCO.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 5.Nelson SM. Prevention and management of ovarian hyperstimulation syndrome. Thromb Res. 2017;151(Suppl 1):S61–S64. doi: 10.1016/S0049-3848(17)30070-1. [DOI] [PubMed] [Google Scholar]
- 6.Neves LM, Kurobe FMC, Drezett J, Blake MT, Dzik A, Cavagna M, et al. Ovarian hiperstimulation syndrome: incidence in a public service of assisted reproduction and literature review. Reprodução & Climatério. 2013;28(1):10–17. doi: 10.1016/j.recli.2013.05.001. [DOI] [Google Scholar]
- 7.Alper MM, Smith LP, Sills ES. Ovarian hyperstimulation syndrome: current views on pathophysiology, risk factors, prevention, and management. J Exp Clin Assist Reprod. 2009;6:3–3. [PMC free article] [PubMed] [Google Scholar]
- 8.Humaidan P, Quartarolo J, Papanikolaou EG. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril. 2010;94(2):389–400. doi: 10.1016/j.fertnstert.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Orvieto R, Dratviman-Storobinsky O, Lantsberg D, Haas J, Mashiach R, Cohen Y. Interleukin-2 and SOCS-1 proteins involvement in the pathophysiology of severe ovarian hyperstimulation syndrome--a preliminary proof of concept. J Ovarian Res. 2014;7:106–106. doi: 10.1186/s13048-014-0106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humaidan P, Nelson SM, Devroey P, Coddington CC, Schwartz LB, Gordon K, et al. Ovarian hyperstimulation syndrome: review and new classification criteria for reporting in clinical trials. Hum Reprod. 2016;31(9):1997–2004. doi: 10.1093/humrep/dew149. [DOI] [PubMed] [Google Scholar]
- 11.Bellapu S, Guttman J. Use of point-of-care ultrasound for the diagnosis of ovarian hyperstimulation syndrome. J Emerg Med. 2017;52(4):e101–e104. doi: 10.1016/j.jemermed.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Nastri CO, Teixeira DM, Moroni RM, Leitão VMS, Martins WP. Ovarian hyperstimulation syndrome: physiopathology, staging, prediction and prevention. Ultrasound Obstet Gynecol. 2015;45(4):377–393. doi: 10.1002/uog.14684. [DOI] [PubMed] [Google Scholar]
- 13.Schirmer DA, 3rd, Kulkarni AD, Zhang Y, Kawwass JF, Boulet SL, Kissin DM. Ovarian hyperstimulation syndrome after assisted reproductive technologies: trends, predictors, and pregnancy outcomes. Fertil Steril. 2020;114(3):567–578. doi: 10.1016/j.fertnstert.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gómez R, Soares SR, Busso C, Garcia-Velasco JA, Simón C, Pellicer A. Physiology and pathology of ovarian hyperstimulation syndrome. Semin Reprod Med. 2010;28(6):448–457. doi: 10.1055/s-0030-1265670. [DOI] [PubMed] [Google Scholar]
- 15.Rizk B, Aboulghar M. In: Classification of ovarian hyperstimulation syndrome. Rizk B, Aboulghar M, editors. New York: Cambridge University Press; 2006. Ovarian hyperstimulation syndrome; pp. 103–129. [Google Scholar]
- 16.Kawwass JF, Kissin DM, Kulkarni AD, Creanga AA, Session DR, Callaghan WM, et al. Safety of assisted reproductive technology in the United States, 2000-2011. JAMA. 2015;313(1):88–90. doi: 10.1001/jama.2014.14488. [DOI] [PubMed] [Google Scholar]
- 17.Blockeal C, Drakopoulos P, Santos-Ribeiro S, Polyzos NP, Tournaye H. A fresh look at the freeze-all protocol: a SWOT analysis. Hum Reprod. 2016;31(3):491–497. doi: 10.1093/humrep/dev339. [DOI] [PubMed] [Google Scholar]
- 18.Golan A, Ron-el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian hyperstimulation syndrome: an update review. Obstet Gynecol Surv. 1989;44(6):430–440. doi: 10.1097/00006254-198906000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Papanikolaou EG, Pozzobon C, Kolibianakis EM, Camus M, Tournaye H, Fatemi HM, et al. Incidence and prediction of ovarian hyperstimulation syndrome in women undergoing gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil Steril. 2006;85(1):112–120. doi: 10.1016/j.fertnstert.2005.07.1292. [DOI] [PubMed] [Google Scholar]
- 20.Ferraretti AP, Gianaroli L, Magli C, Fortini D, Selman HA, Feliciani E. Elective cryopreservation of all pronucleate embryos in women at risk of ovarian hyperstimulation syndrome: efficiency and safety. Hum Reprod. 1999;14(6):1457–1460. doi: 10.1093/humrep/14.6.1457. [DOI] [PubMed] [Google Scholar]
- 21.Griesinger G, Verweij PJM, Gates D, Devroey P, Gordon K, Stegmann BJ, et al. Prediction of ovarian hyperstimulation syndrome in patients treated with corifollitropin alfa or rFSH in a GnRH antagonist protocol. PLoS One. 2016;11(3):e0159615. doi: 10.1371/journal.pone.0149615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dosouto C, Haahr T, Humaidan P. Gonadotropin-releasing hormone agonist (GnRHa) trigger – state of the art. Reprod Biol. 2017;17(1):1–8. doi: 10.1016/j.repbio.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Beltrame AL, Serafini P, Motta ELA, Soares JM, Júnior, Baracat EC. The effects of bromocriptine on VEGF, kidney function and ovarian hyperstimulation syndrome in in vitro fertilization patients: a pilot study. Gynecol Endocrinol. 2013;29(3):201–204. doi: 10.3109/09513590.2012.736554. [DOI] [PubMed] [Google Scholar]
- 24.Vlaisavljević V, Kovačič B, Knez J. Cumulative live birth rate after GnRH agonist trigger and elective cryopreservation of all embryos in high responders. Reprod Biomed Online. 2017;35(1):42–48. doi: 10.1016/j.rbmo.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Practice Committee of the American Society for Reproductive Medicine Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril. 2016;106(7):1634–1647. doi: 10.1016/j.fertnstert.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 26.Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Prediction of high ovarian response to controlled ovarian hyperstimulation: anti-Müllerian hormone versus small antral follicle count (2–6 mm) J Assist Reprod Genet. 2009;26(6):319–325. doi: 10.1007/s10815-009-9319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]