Abstract
COVID-19 vaccination campaigns continue in the United States, with the expectation that vaccines will slow transmission of the virus, save lives, and enable a return to normal life in due course. However, the extent to which faster vaccine administration has affected COVID-19-related deaths is unknown. We assessed the association between US state-level vaccination rates and COVID-19 deaths during the first five months of vaccine availability. We estimated that by May 9, 2021, the US vaccination campaign was associated with a reduction of 139,393 COVID-19 deaths. The association varied in different states. In New York, for example, vaccinations led to an estimated 11.7 fewer COVID-19 deaths per 10,000, whereas Hawaii observed the smallest reduction, with an estimated 1.1 fewer deaths per 10,000. Overall, our analysis suggests that the early COVID-19 vaccination campaign was associated with reductions in COVID-19 deaths. As of May 9, 2021, reductions in COVID-19 deaths associated with vaccines had translated to value of statistical life benefit ranging between $625 billion and $1.4 trillion.
The development, approval, and distribution of COVID-19 vaccines have been a welcome relief in a pandemic that has generated enormous suffering throughout the world. Eighteen months into the pandemic, as of July 18, 2021, more than 4 million people had died from COVID-19 worldwide, including more than 600,000 people in the US alone.1 The total death toll from the pandemic may be even higher when additional non-COVID-19 deaths induced by the pandemic are included.2–4 In addition to the death toll, the pandemic has inflicted enormous social and economic costs and created disparate impacts across racial and ethnic subpopulations.5–13 Unemployment rates worldwide spiked in the early spring and have only partially recovered.14 Governments have implemented restrictive policies designed to mitigate transmission, including school and business closures, stay-at-home mandates, restrictions on gathering and travel, and mask mandates.15 And even in the absence of government mandates, people have taken private but less coordinated measures to protect themselves and reduce transmission.16
Just twelve months since the discovery of the novel pathogen SARS-CoV-2, the first COVID-19 vaccine (Pfizer-BioNTech) was approved for use in the US by the Food and Drug Administration, under an emergency use authorization issued on December 11, 2020.17 Vaccines manufactured by Moderna and Johnson & Johnson were approved for emergency use in the US on December 18, 2020, and February 27, 2021, respectively.18,19 These vaccines had high efficacy estimated during their clinical trials.20–22 Early evidence shows that the Pfizer-BioNTech vaccine has some efficacy against the B.1.1.7 (Alpha) and B.1.351 (Beta) virus variants,23 and COVID-19 vaccines are effective against the B.1.617.2 (Delta) virus variant.24 Additional vaccines based on similar technology are under review in the US, and some have been approved in other countries.25
The existence of multiple COVID-19 vaccines offers hope that the ongoing vaccination campaign will slow COVID-19 transmission, save lives, and enable a return to normal activities. In the US access to vaccines varied substantially across states and subpopulations during the early stages of the vaccine campaign.26–28 As of July 18, 2021, about 49 percent of the US population was fully vaccinated and 56 percent had completed at least one dose.29 Some states, such as Alabama and Mississippi, had fully vaccinated less than 34 percent of their populations. In contrast, Vermont had fully vaccinated 67 percent of its population.29
In this study we examined the extent to which variation in the intensity of state vaccination campaigns was associated with changes in the number of COVID-19 deaths. Specifically, we used regressionmodels with state and week fixed effects to estimate the number of COVID-19 deaths averted by vaccine distribution. By providing insight into the initial impact of the COVID-19 vaccination campaign, these estimates can inform public health, clinical, and policy responses going forward.
Study Data And Methods
STATE-LEVEL VACCINE DATA
Data on state vaccine doses administered came from the Bloomberg Covid-19 Vaccine Tracker,30 which collects data from government websites as well as official statements and interviews. The data used in this study were collected May 10, 2021, and are further described in the online appendix.31 We constructed measures of the cumulative number of doses administered in each state by the end of each week, from December 21, 2020 (the first date of Bloomberg data collection), to May 9, 2021.We used 2018 state population counts from the Surveillance, Epidemiology, and End Results (SEER) Program data32 to calculate the number of doses per 100 population ages sixteen and older (henceforth referred to as the adult population) administered each week. The Bloomberg database is a rich source of information on the administration of COVID-19 vaccines at the state-by-time level, but it has some limitations. For example, the data set does not include the age, race/ethnicity, or occupation of the person receiving the vaccine, nor does it separate the number of vaccines by location (for example, at nursing homes versus pharmacies). We were also unable to differentiate whether the vaccine was used as the first or second dose in the regimen.
COVID-19 DEATH DATA
We obtained daily data on COVID-19 deaths for each state from the New York Times database.33 We used the daily data to compute cumulative deaths per week during the study period. Using 2018 state population counts from the SEER data, we calculated the cumulative number of deaths per 100 adults in each state in each week. Data on COVID-19 deaths depend on medical investigation and likely are an undercount.
STATISTICAL METHODS
The aim of our study was to measure the association between time-varying state-level vaccine administration and measures of COVID-19 deaths. Our empirical analysis spanned the period December 21, 2020–May 9, 2021. For each state and week we calculated the cumulative number of vaccine doses per 100 adults that the state had ever administered as of the end of that week. We also calculated the speed with which each of the states administered 5, 10, 20, 40, 80, and 120 vaccine doses per 100 adults. We used a regression-based approach to measure the association between vaccination rates and changes in COVID-19 deaths. Our regression models included fixed effects controls for state, which accounted for underlying differences that exist across states even before vaccinations, and fixed effects controls for week, which accounted flexibly for nationwide trends during our study period. Thus, our multivariate regression models measured the association between within-state changes in the intensity of the vaccination campaign and within-state changes in COVID-19 deaths, after adjusting for systematic differences across states and allowing for flexible nationwide time trends.
Given the natural history of SARS-CoV-2,34,35 we expected that initial vaccinations would affect the number of deaths in a state with a lag, in the sense that current COVID-19 deaths may reflect vaccines administered in earlier weeks. Accordingly, we used a distributed lag model specification to estimate the effects of a state’s vaccination rollout on COVD-19 deaths. In our main specifications we regressed cumulative COVID-19 deaths per 100 adults on state fixed effects, week fixed effects, current-week cumulative vaccinations per 100 adults, and four weeks of lagged cumulative vaccinations per 100 adults. Because the spread of the virus that generates COVID-19 deaths is nonlinear, we used a quasi-Poisson model and estimated standard errors that allowed for heteroskedasticity and clustering at the state level. We used the coefficients on the current and lagged vaccination rates to estimate the number of vaccine-averted deaths up until May 9, 2021, in each state and in the country overall.
A full description of our regression models is in the appendix.31 Analysis was performed in Stata 16.1. This study was deemed Non–Human Subjects research by the Indiana University Institutional Review Board.
SENSITIVITY ANALYSES
We assessed the sensitivity of our results to alternative approaches in several ways. Full details on these models are in the appendix.31
LIMITATIONS
Our study was not without limitations. First, our use of state-level data may have masked important differences across subpopulations and geographic areas within states.27 We also did not consider the effectiveness of individual vaccines but, rather, population-level associations. At the early stage in the rollout spanned by our data, it was challenging to robustly dissect what share of the estimated reduction in COVID-19 deaths was a result of the proportion of the population that was vaccinated or had antibodies and what share was a result of lower population-level risk for COVID-19 transmission. Nor were we able to separately identify the roles of vaccination, natural immunity, and changes in mobility in determining the overall number of COVID-19 deaths. We also used confirmed COVID-19 deaths, acknowledging that asymptomatic infections are rarely detected, and thus our estimated associations may be conservative. In addition, we did not examine whether individual states’ decisions on priority populations affected the number of averted deaths. Finally, we also did not examine whether vaccines received were the first or second dose of the regimen.
Study Results
SPEED OF VACCINE ROLLOUT ACROSS STATES
To summarize variation in the speed of the early vaccination campaigns across states, exhibit 1 shows the amount of time each state took to reach a series of milestones—5 doses per 100 adults, 10 doses per 100 adults, and so on, through 120 doses per 100 adults. The first state to reach 10 doses per 100 adults was West Virginia, achieving that goal on January 16, 2021, and the last state was Idaho, on February 4, 2021. However, states’ positions in vaccination progress varied over time. Alaska was the first to reach 20 doses per 100 adults, on January 29, 2021, and Alabama was the last, on February 21, 2021. On May 6, 2021, California was the first state to reach 120 doses per 100 adults—but many states have still not reached that milestone. The median number of days between the milestones of 10 and 20 doses per 100 adults was 19 days (95% confidence interval: 18, 19), and the median number of days between 20 and 40 doses per 100 adults was 24 days (95% CI: 24, 24).
EXHIBIT 1. Timing of COVID-19 vaccine rollout across US states, 2020–21.
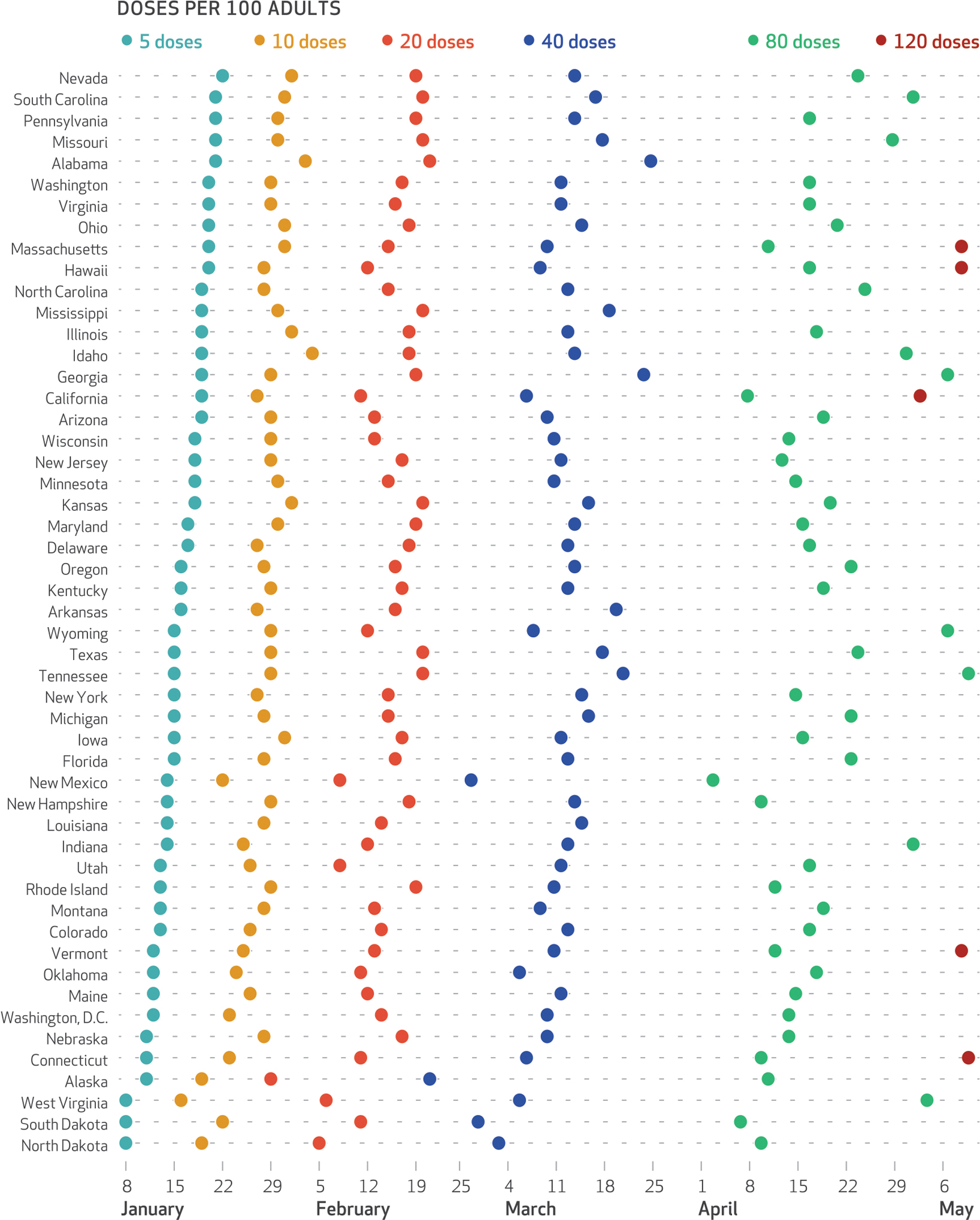
SOURCE Authors’ analysis of data from the Bloomberg Covid-19 Vaccine Tracker, December 21, 2020–May 9, 2021. NOTES Data are shown in order of states’ reaching the benchmark of 5 doses per 100 adults. Adults are those ages sixteen and older.
ASSOCIATION BETWEEN VACCINE ADMINISTRATION AND DEATHS
Appendix exhibit 1 presents a snapshot of the cross-sectional association between the severity of the epidemic and the scale of the early COVID-19 vaccinations across states.31 The vertical axis measures the number of COVID-19 cases (panel a) and deaths (panel b) per 100 adults in the state that occurred between April 9, 2021, and May 9, 2021. The horizontal axis shows the cumulative number of vaccine doses per 100 adults that each state had achieved by March 15, 2021. Measuring vaccines received by March 15 allowed for the inclusion of a four-week lag period to examine how COVID-19 deaths responded to vaccines delivered by March 15. In the point-in-time snapshot, there is a negative association between state-level vaccination rates and COVID-19 deaths.
To move beyond cross-sectional associations, we used the state-level weekly longitudinal data to estimate regression models of cumulative COVID-19 deaths per 100 adults on current and four weeks of lagged vaccination rates. We used the coefficients from the models to estimate the number of COVID-19 deaths that would have prevailed in the absence of vaccinations. The difference between the actual number of deaths and these counterfactual estimates provides a measure of the estimated number of confirmed COVID-19 deaths averted by the vaccination campaign to date.
Exhibit 2 presents the geographic distribution in estimated COVID-19 deaths averted in association with COVID-19 vaccine administrations as of May 9, 2021, expressed here as deaths per 10,000 adults. In population-adjusted terms, New York observed the largest estimated reduction in deaths, with 11.7 fewer COVID-19 deaths per 10,000 adults, and Hawaii observed the smallest reduction, with 1.1 fewer COVID-19 deaths per 10,000 adults. The average state experienced 5 fewer COVID-19 deaths per 10,000 adults (95% CI: 4, 6) in association with COVID-19 vaccination.
EXHIBIT 2. Estimated cumulative COVID-19 deaths averted through vaccination per 10,000 US adults, by state, 2021.
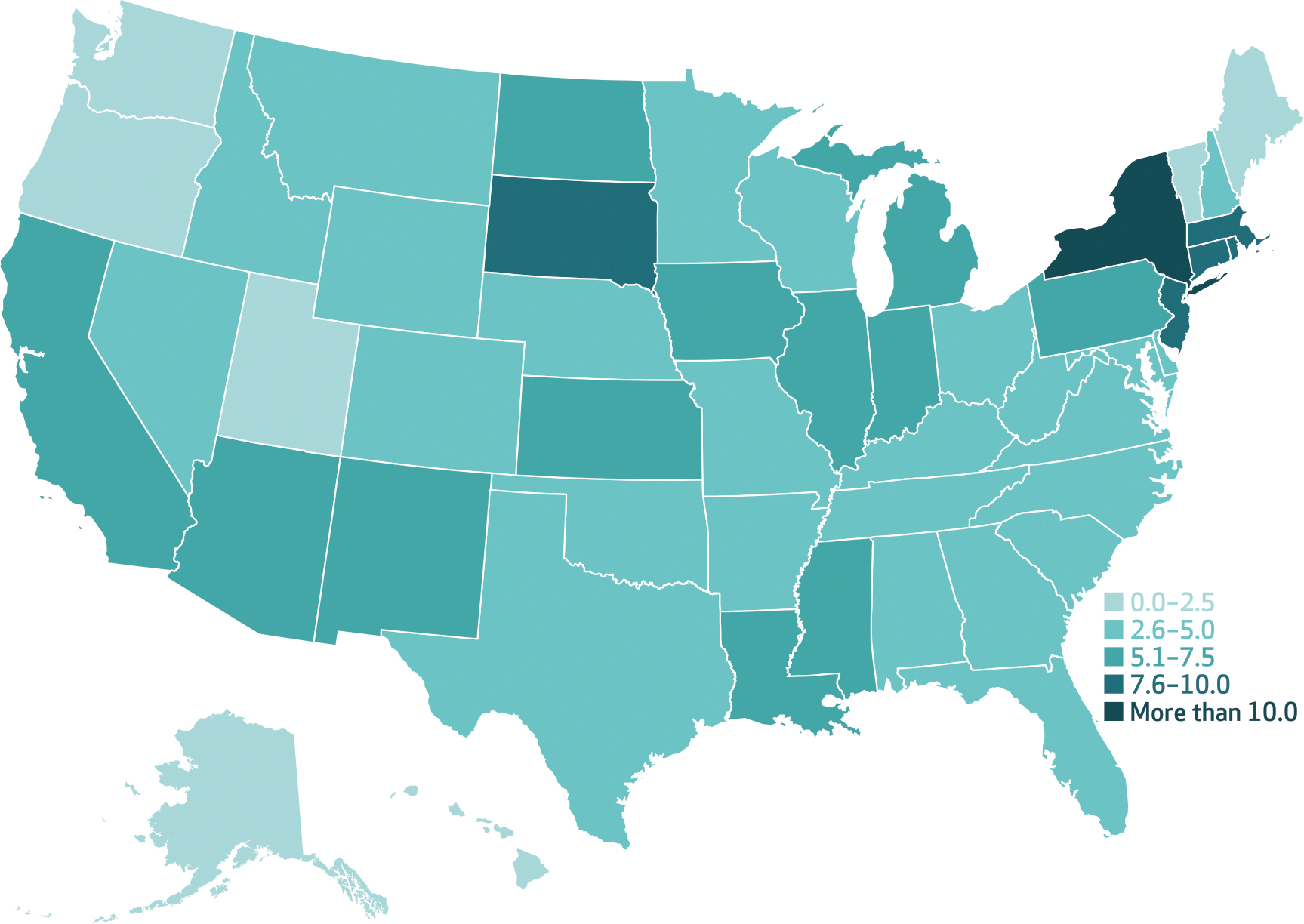
SOURCE Authors’ analysis of state-level vaccine data from the Bloomberg Covid-19 Vaccine Tracker and COVID-19 mortality data from the New York Times Coronavirus (Covid-19) Data in the United States database, December 21, 2020–May 9, 2021. NOTES Averted deaths are the difference between predicted values of deaths at prevailing levels of vaccinations and predicted values of deaths if no vaccinations had occurred during the study period. As described in the Methods section, regression models use state fixed effects, week fixed effects, and four-week lags of the vaccination doses per 100 adults (population ages sixteen and older). A similar map showing confirmed cases averted is in appendix exhibit 3 (see note 30 in text).
Exhibit 3 shows a nationwide weekly time series of the actual number of cumulative COVID-19 deaths among adults across the US and compares it with the model-based estimate of the cumulative number of deaths that would have occurred in the absence of any vaccinations. Across all US states, using predicted deaths with and without vaccines, we estimate that COVID-19 vaccinations were associated with 139,393 (95% CI: 13,110, 265,675) averted COVID-19 deaths as of May 9, 2021. Using the same approach, we estimate that the early vaccination campaign was associated with 3.1 million (95% CI: −2.8, 8.9) averted confirmed cases of COVID-19 as of May 9, 2021 (appendix exhibit 4).31 The effect is larger in magnitude, but it is not statistically different from zero (appendix exhibit 5).31
EXHIBIT 3. Estimated COVID-19 deaths averted through vaccination among US adults, by week, 2021.
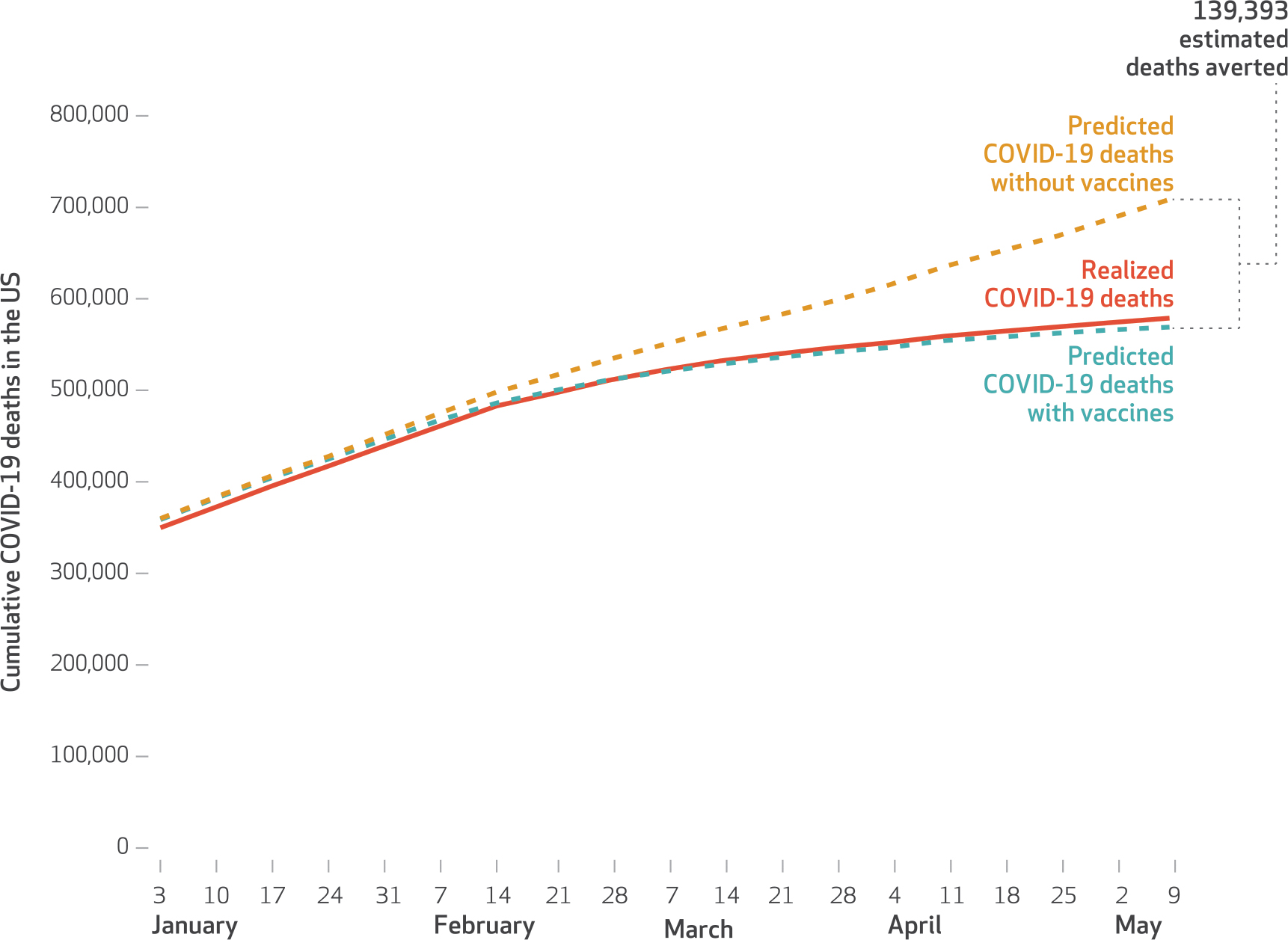
SOURCE Authors’ analysis of state-level vaccine data from the Bloomberg Covid-19 Vaccine Tracker and COVID-19 mortality data from the New York Times Coronavirus (Covid-19) Data in the United States database, December 21, 2020–May 9, 2021. NOTES Averted deaths are the difference between predicted values of deaths at prevailing levels of vaccinations and predicted values of deaths if no vaccinations had occurred during the study period. As described in the Methods section, the models use state fixed effects, week fixed effects, and four-week lags of the vaccination doses per 100 adults (population ages sixteen and older). A similar figure containing analogous results for confirmed cases is in appendix exhibit 4 (see note 30 in text).
Discussion
In this study we assessed the association between the early COVID-19 vaccination campaign in the US and COVID-19 deaths. We documented substantial variation in the speed of state-specific vaccination campaigns, and we estimated the association between vaccinations and changes in the number of COVID-19 deaths among US adults. Our estimates suggest that first few months of the vaccination campaign in the US were associated with nearly 140,000 averted COVID-19 deaths.
To place our estimated averted COVID-19 deaths in context, the fitted value from our model implies that by the week ending May 9, 2021, approximately 550,000 people had died of COVID-19 in the US. Using regression models to estimate the counterfactual number of deaths in the absence of the vaccination campaign suggests that the cumulative number of COVID-19 deaths in the US could have reached 709,000. In other words, our study suggests that without the vaccinations of early 2021, the cumulative number of COVID-19 deaths in the US would have been nearly 1.2 times higher than their current level. Previous work suggests that the value per statistical life of COVID-19 deaths ranges from $4.5 million to $10.6 million.36,37 Using these numbers suggests that the value per statistical life of the lives already saved because of the vaccination campaign is in the range of $625 billion to $1.4 trillion.38,39 Other studies estimate that the mortality, health, and economic impacts of the COVID-19 pandemic equate to $16 trillion.6 Through the end of 2020, $13 billion for vaccine development and manufacturing had been allocated in the US by the federal government.40
Establishment of causality is challenging in an observational setting and during a national vaccine rollout; our analytical approach relied on variation in the administration of COVID-19 vaccines across states, as early state campaigns may have been influenced by public health infrastructure. Vaccine administration patterns may be associated with declining mortality because of vaccine prevention of deaths and severe complications as state-level vaccine campaigns allocated initial doses to the highest-risk populations41 with the aim of immediately reducing COVID-19 deaths. Finally, and more important, clinical trial evidence has shown that COVID-19 vaccines have high efficacy.42 Our study provides support for policies that further expand vaccine administration, which will enable larger populations to benefit.
Importantly, several studies have found that racial and ethnic populations and low-income populations have disproportionately borne the health, economic, and social consequences of the COVID-19 pandemic.8–12 At the same time, emerging evidence suggests that these same communities are less likely to receive initial COVID-19 vaccines.27,43 Ensuring that vulnerable patient populations equally share the benefits of COVID-19 vaccination campaigns is critical.44 Although this analysis is beyond the scope of our study, as data become available, it is imperative that researchers continue to evaluate disparities in vaccine access and their consequences in creating further hot spots and heterogeneity in burden.
Despite this study’s limitations, we provide the first assessment of the impacts of state-level vaccination campaigns to address the COVID-19 pandemic. Our results suggest that further efforts to vaccinate populations globally and in a coordinated fashion will be critical to achieving greater control of the COVID-19 pandemic.
Supplementary Material
Acknowledgments
Support was provided by the National Institute on Aging (Grant No. 1K01AG061274 to Christopher Whaley). The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Aaron Kofner and Brenna O’Neill provided helpful data assistance. [Published online August 18, 2021.]
Contributor Information
Sumedha Gupta, Department of Economics, Indiana University–Purdue University Indianapolis, in Indianapolis, Indiana..
Jonathan Cantor, Department of Economics, Sociology, and Statistics, RAND Corporation, in Santa Monica, California..
Kosali I. Simon, Paul H. O’Neill School of Public and Environmental Affairs and associate vice provost for health sciences, Indiana University, in Bloomington, Indiana..
Ana I. Bento, Department of Epidemiology and Biostatistics at the School of Public Health, Indiana University..
Coady Wing, Paul H. O’Neill School of Public and Environmental Affairs, Indiana University..
Christopher M. Whaley, Department of Economics, Sociology, and Statistics, RAND Corporation, in Santa Monica..
NOTES
- 1.Coronavirus Resource Center. COVID-19 dashboard [Internet]. Baltimore (MD): Johns Hopkins University and Medicine; [cited 2021 Jul 18]. Available from: https://coronavirus.jhu.edu/map.html [Google Scholar]
- 2.Polyakova M, Udalova V, Kocks G, Genadek K, Finlay K, Finkelstein AN. Racial disparities in excess all-cause mortality during the early COVID-19 pandemic varied substantially across states. Health Aff (Millwood). 2021; 40(2):307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossen LM, Branum AM, Ahmad FB, Sutton P, Anderson RN. Excess deaths associated with COVID-19, by age and race and ethnicity—United States, January 26–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1522–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L, Taylor DDH. Excess deaths from COVID-19 and other causes, March–July 2020. JAMA. 2020;324(15):1562–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S, Montenovo L, Nguyen TD, Rojas FL, Schmutte IM, Simon KI, et al. Effects of social distancing policy on labor market outcomes [Internet]. Cambridge (MA): National Bureau of Economic Research; 2020. May [cited 2021 Jul 7]. (NBER Working Paper No. 27280). Available from: https://www.nber.org/papers/w27280 [Google Scholar]
- 6.Cutler DM, Summers LH. The COVID-19 pandemic and the $16 trillion virus. JAMA. 2020; 324(15):1495–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christakis DA, Van Cleve W, Zimmerman FJ. Estimation of US children’s educational attainment and years of life lost associated with primary school closures during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3(11):e2028786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee FC, Adams L, Graves SJ, Massetti GM, Calanan RM, Penman-Aguilar A, et al. Counties with high COVID-19 incidence and relatively large racial and ethnic minority populations—United States, April 1–December 22, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(13):483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egede LE, Walker RJ. Structural racism, social risk factors, and Covid-19—a dangerous convergence for Black Americans. N Engl J Med. 2020;383(12):e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egede LE, Walker RJ, Garacci E, Raymond JR Sr. Racial/ethnic differences in COVID-19 screening, hospitalization, and mortality in Southeast Wisconsin. Health Aff (Millwood). 2020;39(11):1926–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azar KMJ, Shen Z, Romanelli RJ, Lockhart SH, Smits K, Robinson S, et al. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Aff (Millwood). 2020;39(7):1253–62. [DOI] [PubMed] [Google Scholar]
- 13.Alsan M, Chandra A, Simon KI. The great unequalizer: initial health effects of COVID-19 in the United States [Internet]. Cambridge (MA): National Bureau of Economic Research; 2021. Jun [cited 2021 Jul 7]. (NBER Working Paper No. 28958). Available from: https://www.nber.org/papers/w28958 [Google Scholar]
- 14.Hershbein BJ, Holzer HJ. The COVID-19 pandemic’s evolving impacts on the labor market: who’s been hurt and what we should do [Internet]. Bonn: IZA Institute of Labor Economics; 2021. Feb [cited 2021 Jul 7]. (Report No. 14108). Available from: https://www.iza.org/publications/dp/14108/the-covid-19-pandemics-evolving-impacts-on-the-labor-market-whos-been-hurt-and-what-we-should-do [Google Scholar]
- 15.Gupta S, Nguyen TD, Rojas FL, Raman S, Lee B, Bento A, et al. Tracking public and private responses to the COVID-19 epidemic: evidence from state and local government actions [Internet]. Cambridge (MA): National Bureau of Economic Research; 2020. Apr [cited 2021 Jul 7]. (NBER Working Paper No. 27027). Available from: https://www.nber.org/papers/w27027 [Google Scholar]
- 16.Gupta S, Simon KI, Wing C. Mandated and voluntary social distancing during the COVID-19 epidemic: a review [Internet]. Cambridge (MA): National Bureau of Economic Research; 2020. Nov [cited 2021 Jul 7]. (NBER Working Paper No. 28139). Available from: https://www.nber.org/papers/w28139 [Google Scholar]
- 17.Food and Drug Administration. Emergency use authorization (EUA) for an unapproved product, review memorandum [Internet]. Silver Spring (MD): FDA; 2020. [cited 2021 Jul 7]. Available from: https://www.fda.gov/media/144416/download [Google Scholar]
- 18.Food and Drug Administration [Internet]. Silver Spring (MD): FDA. Press release, FDA takes additional action in fight against COVID-19 by issuing emergency use authorization for second COVID-19 vaccine; 2020. Dec 18 [cited 2021 Jul 7]. Available from: https://www.fda.gov/news-events/press-announcements/fdatakes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid [Google Scholar]
- 19.Food and Drug Administration [Internet]. Silver Spring (MD): FDA. Press release, FDA issues emergency use authorization for third COVID-19 vaccine; 2021. Feb 27 [cited 2021 Jul 7]. Available from: https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine [Google Scholar]
- 20.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, et al. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021;384(19):1824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abu-Raddad LJ, Chemaitelly H, Butt AA, National Study Group for COVID-19 Vaccination. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med. 2021;385(2):187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021. Jul 21. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Creech CB, Walker SC, Samuels RJ. SARS-CoV-2 vaccines. JAMA. 2021;325(13):1318–20. [DOI] [PubMed] [Google Scholar]
- 26.Perniciaro SR, Weinberger DM (Yale University, New Haven, CT). 50 policies, 1 pandemic, 500,000 deaths: associations between state-level COVID-19 testing recommendations, tests per capita, undercounted deaths, vaccination policies, and doses per capita in the United States. medRxiv [serial on the Internet]. 2021. Feb 26 [cited 2021 Jul 7]. Available from: 10.1101/2020.09.04.20188326v3 [DOI]
- 27.Hughes MM, Wang A, Grossman MK, Pun E, Whiteman A, Deng L, et al. County-level COVID-19 vaccination coverage and social vulnerability—United States, December 14, 2020–March 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(12):431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rader B, Astley CM, Sewalk K, Delamater PL, Cordiano K, Wronski L, et al. (Boston Children’s Hospital, Boston, MA). Spatial accessibility modeling of vaccine deserts as barriers to controlling SARS-CoV-2. medRxiv [serial on the Internet]. 2021. Jun 12 [cited 2021 Jul 7]. Available from: 10.1101/2021.06.09.21252858v1.full [DOI] [PMC free article] [PubMed]
- 29.Centers for Disease Control and Prevention. COVID data tracker [Internet]. Atlanta (GA): CDC; 2020. [cited 2021 Jul 18]. Available from: https://covid.cdc.gov/covid-data-tracker [Google Scholar]
- 30.Randall T, Armstrong D, Tartar A. Covid-19 vaccine tracker frequently asked questions. Bloomberg [serial on the Internet]. [last updated 2021 Jan 29; cited 2021 Jul 7]. Available from: https://www.bloomberg.com/graphics/covid-vaccine-tracker-global-distribution/faq.html
- 31.To access the appendix, click on the Details tab of the article online.
- 32.Surveillance, Epidemiology, and End Results Program. SEER incidence data, 1975–2018 [Internet]. Bethesda (MD): National Cancer Institute, SEER; [cited 2021 Jul 7]. Available from: https://seer.cancer.gov/data/ [Google Scholar]
- 33.New York Times. Coronavirus (Covid-19) data in the United States. GitHub; [serial on the Internet]. 2021. [cited 2021 Jul 7]. Available from: https://github.com/nytimes/covid-19-data [Google Scholar]
- 34.Kim M-C, Cui C, Shin K-R, Bae J-Y, Kweon O-J, Lee M-K, et al. Duration of culturable SARS-CoV-2 in hospitalized patients with Covid-19. N Engl J Med. 2021;384(7):671–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Machhi J, Herskovitz J, Senan AM, Dutta D, Nath B, Oleynikov MD, et al. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J Neuroimmune Pharmacol. 2020;15(3):359–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson LA, Sullivan R, Shogren JF. Do the benefits of COVID-19 policies exceed the costs? Exploring uncertainties in the age-VSL relationship. Risk Anal. 2020;41(5):761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ash T, Bento AM, Kaffine D, Rao A, Bento AI (University of Southern California, Los Angeles, CA). Disease-economy trade-offs under alternative pandemic control strategies. medRxiv [serial on the Internet]. 2021. Feb 13 [cited 2021 Jul 7]. Available from: 10.1101/2021.02.12.21251599v1 [DOI]
- 38.Viscusi WK, Aldy JE. The value of a statistical life: a critical review of market estimates throughout the world. J Risk Uncertain. 2003;27(1):5–76. [Google Scholar]
- 39.Cutler DM, Rosen AB, Vijan S. The value of medical spending in the United States, 1960–2000. N Engl J Med. 2006;355(9):920–7. [DOI] [PubMed] [Google Scholar]
- 40.Government Accountability Office. Operation Warp Speed: accelerated COVID-19 vaccine development status and efforts to address manufacturing challenges [Internet]. Washington (DC): GAO; 2021. Feb 11 [cited 2021 Jul 7]. (Report No. GAO-21–319). p. 2. Available from: https://www.gao.gov/products/gao-21-319 [Google Scholar]
- 41.Dooling K, Marin M, Wallace M, McClung N, Chamberland M, Lee GM, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69(5152):1657–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin EJ, Longo DL. SARS-CoV-2 vaccination—an ounce (actually, much less) of prevention. N Engl J Med. 2020;383(27):2677–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen KH, Srivastav A, Razzaghi H, Williams W, Lindley MC, Jorgensen C, et al. COVID-19 vaccination intent, perceptions, and reasons for not vaccinating among groups prioritized for early vaccination—United States, September and December 2020. MMWR Morb Mortal Wkly Rep. 2021;70(6):217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jean-Jacques M, Bauchner H. Vaccine distribution—equity left behind? JAMA. 2021;325(9):829–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


