Abstract
BACKGROUND
Alpha lipoic acid (ALA) is considered a strong antioxidant with anti-inflammatory properties. Moreover, a number of previous studies have shown its lipid-lowering properties. Therefore, we designed this study to investigate the effects of ALA on lipid profile in patients with metabolic syndrome (MetS), which can lead to an increased risk of cardiovascular disease (CVD) and premature mortality.
METHODS
A total 46 patients with MetS were randomly divided into two groups. They received either 600 mg ALA (n = 23) or 600 mg placebo (n = 23) for 12 weeks. The body weight, height, body mass index (BMI), waist circumference (WC), fasting blood sugar (FBS), hemoglobin A1C (HbA1c), and blood pressure (BP) were assessed at baseline of the study. Physical activity level and dietary intake were assessed at baseline and end of the study. Serum lipid profile including triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and total cholesterol (TC) were measured before and after 12 weeks of intervention.
RESULTS
Baseline characteristics were similar in the ALA and placebo groups (P > 0.05). However, there were statistically significant differences in plasma levels of TG (-36.82 ± 42.48 versus 6.15 ± 25.04 mg/dl, P = 0.001) and TC (-8.91 ± 20.65 versus 10.84 ± 22.97 mg/dl, P = 0.01) after 12 weeks between the ALA group and the placebo group. Yet, there were no statistically significant differences in plasma levels of HDL-C and LDL-C after 12 weeks between the ALA group and the placebo group.
CONCLUSION
The results suggest that daily supplementation of 600 mg ALA for 12 weeks may improve the lipid profile in patients with MetS.
Keywords: Thioctic Acid, Lipoprotein, Metabolic Syndrome, Clinical Trial
Introduction
Metabolic syndrome (MetS) is one of the major health problems with an epidemic throughout the world.1 It is a pathophysiological state characterised by dysglycaemia, insulin resistance, dyslipidemia, central obesity, and hypertension.1 Despite the different definitions of MetS, three or more of the following components are generally necessary to diagnose the condition: high blood pressure (BP), large waist circumference (WC), high triglyceride (TG), low high-density lipoprotein cholesterol (HDL-C), and increased fasting blood sugar (FBS) concentration.2 According to International Diabetes Federation (IDF) estimates, about 25% of the world's population have MetS, which varies across ethnicities and age groups.3 Individuals with MetS have a 5-fold increased risk of type 2 diabetes mellitus (DM) and a 2-3-fold increased risk of cardiovascular diseases (CVDs) compared to the individuals without MetS.4 Since lipid profile imbalance is one of the important factors in the etiology of MetS, adopting different methods to balance lipid profile is one of the first therapeutic strategies for this complication.
Medicine,5 lifestyle modification,6 exercise,7 as well as the use of complementary therapies8 including supplementation with some nutrients9 are among the appropriate solutions to balance the lipid profile in patients with MetS.
In recent years, the lipid-lowering properties of a large number of nutraceuticals have been tested in accessible trials.10 Alpha lipoic acid (ALA) or thioctic acid is a necessary cofactor for mitochondrial a-ketoglutarate dehydrogenase and pyruvate dehydrogenase.11 Both ALA and its active form (dihydrolipoic acid) are considered as strong antioxidants with the ability to regenerate other antioxidants such as vitamin E and ascorbic acid and raise the intracellular glutathione levels.12 In addition, previous studies have reported lipid-lowering properties for ALA by both direct effects and secondary responses related to the anti-obesity properties of this antioxidant.13,14 ALA may protect liver against TG accumulation, as well as increased blood cholesterol levels.15
Several animal and human studies have reported possible effects of ALA on lipid profile, though their results are inconsistent. Carrier et al. showed that ALA could protect Zucker rats fed with a high-fat diet against diet-induced obesity and hypercholesterolemia.15 In another study by Miao et al., similar results were seen in Wister rats.14 Although studying the effects of ALA on lipid profile is not a novel subject, considering that studies done on humans are restricted and ALA may play a major role in improving MetS, we designed this study to assess the effects of ALA on lipid profile in patients with MetS. For this purpose, we determined serum levels of lipid profile before and after the intervention.
Materials and Methods
Subjects: The size of the sample was determined based on the study by Huerta et al.16 for low-density lipoprotein (LDL). With power of 80% and a = 0.05, the size of the sample was calculated at least 21 patients in each group. For a possible dropout of 10% rate, the sample size was increased to 23 patients in each group. The subjects in this clinical trial were patients diagnosed with MetS. At least three of five factors are required for the diagnosis of MetS based on the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) criteria.2 Diagnosis factors for MetS based on NCEP-ATP III are FBS ≥ 100 mg/dl or on treatment for DM, BP ≥ 130/85 mmHg, WC ≥ 102 cm for men and ≥ 88 cm for women, TG ≥ 150 mg/dl or on treatment, and HDL-C < 40 mg/dl for men and < 50 mg/dl for women. The inclusion criteria were as follows: 1) age range of 18 to 60 years, 2) willingness to continue the study, 3) controlled DM, 4) not having history of myocardial infarction (MI) and brain stroke during the previous year, and 5) not having CVDs and gastrointestinal disorders. The exclusion criteria were: 1) pregnancy and lactating, 2) menopause, 3) MI, 4) brain stroke, 5) diagnosis of uncontrolled DM [hemoglobin A1C (HbA1c) level above 7%] during the study, 6) using any medication that may interfere with the study process (insulin or anti-diabetic drugs, levothyroxine), 7) consumption of less than 90% of the number of supplement or placebo, and 8) involvement in other clinical trial(s) during the past three months.
Experimental design: This study was a randomized double-blind placebo-controlled clinical trial. In total, 46 patients with MetS were enrolled based on inclusion and exclusion criteria and according to the NCEP-ATP III panel whose disorders were diagnosed by an endocrinologist from the Endocrine and Metabolic Center of Shariati Hospital in Tehran, Iran. They were randomy divided into the ALA (n = 23) and placebo (n = 23) groups, and received either one capsule containing 600 mg ALA supplement or placebo (filled with starch) for 12 weeks. Randomization was performed using a computer calculator based on even or odd random numbers by an independent statistician. Both patients and investigators were blinded to randomization. Both supplement and placebo (filled with starch) were prepared from Darou Darman Sepehr Company, Tehran. For blinding the study, the containers (placebo and ALA) were labeled by a person who was not involved in this research. Participants were followed up weekly for any side effects of ALA supplementation. At the end of the study, the remaining capsules were counted to evaluate the participants’ adherence. The participants who had consumed less than 90% of their capsules were excluded from the study.
Demographic information and dietary and physical activity assessment: At the beginning of study, information on age, gender, education, and disease history was collected using a basal characteristic questionnaire with face to face interview. Food intake of the patients was assessed by three 24-hour recalls at the beginning and end of the study and then analyzed using Nutritionist-IV software (First Databank, San Bruno, CA, USA), modified version for Iranian foods. Furthermore, at the baseline and end of the study, physical activity levels of the participants were evaluated using a short form of the International Physical Activity Questionnaire (IPAQ).17
Anthropometric measurements and BP: Body weight was measured while wearing light clothing and without shoes, using a digital scale (Seca, Hamburg, Germany) to the nearest 0.1 kg. Height was also measured using a meter mounted to the wall (Seca, Hamburg, Germany) with 0.1 cm accuracy. Moreover, body mass index (BMI) was calculated via dividing the weight (kg) by the height squared (meters) and WC (cm) was measured midpoint between the iliac crest and the lowest rib. At beginning of the study, BP was measured after 15 minutes of rest with a sphygmomanometer, and the mean of two measurements was reported as individual BP.
Biochemical measurements: At baseline and after 12 weeks of intervention, 10 ml venous blood samples were taken after 12-hour overnight fasting. The serum samples were then frozen and stored at -70 °C after centrifugation. Serum concentrations of total cholesterol (TC), TG, HDL-C, and LDL cholesterol (LDL-C) were measured using an enzymatic method. Moreover at beginning of the study, FBS was measured by enzymatic method and HbA1C was measured using the immunoturbidometric method with HbA1C kit (Roche Company, Tehran, Iran).
Ethical consideration: The present study was approved by the Research Ethics Committee of Islamic Azad University, Science and Research Branch, Tehran (Code of Ethics: IR.IAU.SRB.REC.1396.82). The study protocol was also registered in ClinicalTrials.gov (NCT03589690). Informed consent was submitted by all subjects when they were enrolled.
Statistical analysis: Per-protocol analysis was used to remove data from patients who did not comply with the protocol. All statistical analyses were done by SPSS software (version 24, IBM Corporation, Armonk, NY, USA). Continuous variables were shown as mean and standard deviation (SD), and categorical variables were presented as number and percentage. Normal distribution of the variables was tested using Shapiro-Wilk test. Furthermore, independent samples t-test or Mann-Whitney U test were employed to compare changes of the variables of the anthropometric, biochemical, and dietary intakes between the groups. To compare differences of the biochemical parameters before and after the intervention within the groups, paired samples t-test or Wilcoxon test was used, and to compare categorical variables between the groups, chi-square test (c2 test) was used. To adjust the effects of confounding factors (baseline values of biochemical parameters), analysis of covariance (ANCOVA) was performed. In this study, P < 0.05 was considered statistically significant.
Results
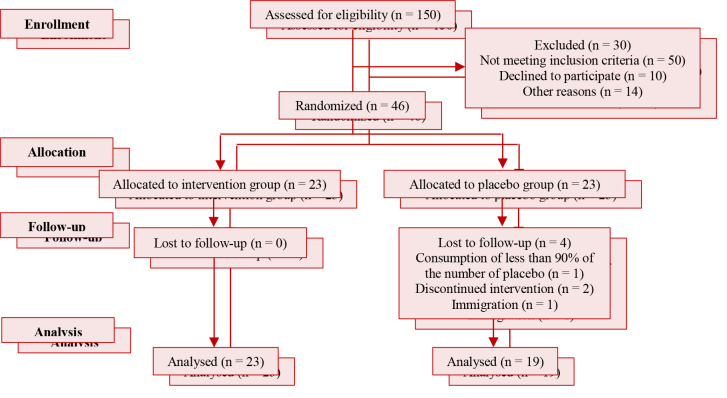
Figure 1 depicts the study flow diagram. Totally, 46 patients with MetS were enrolled in the study. 23 subjects were assigned to the supplement group and 23 subjects were assigned to the placebo group.
Figure 1.
Flow diagram of the study
Four patients in the placebo group were excluded because of poor compliance (n = 1), immigration (n = 1), and discontinued intervention (n = 2) after 12 weeks. Finally, 23 subjects in the supplement group and 19 subjects in the placebo group completed the study.
Baseline characteristics were similar in the study groups (Table 1). There were no statistically significant differences in the age, gender, weight, height, BMI, WC, physical activity level, lipid profile, systolic BP, diastolic BP, FBS, and HbA1C between the two groups before the intervention (P > 0.05).
Table 1.
Baseline characteristics of participants before intervention
| Variable | Intervention group (n = 23) | Control group (n = 19) | P |
|---|---|---|---|
| Age (year) | 40.39 ± 8.90 | 43.68 ± 3.52 | 0.11a |
| Gender | |||
| Men | 7 (30.00) | 5 (26.00) | 0.76b |
| Women | 16 (70.00) | 14 (74.00) | |
| Weight (kg) | 96.58 ± 30.66 | 93.73 ± 16.81 | 0.74c |
| Height (cm) | 164.76 ± 8.55 | 161.61 ± 9.10 | 0.25a |
| WC (cm) | 113.30 ± 20.40 | 115.63 ± 9.49 | 0.32c |
| BMI (kg/m2) | 34.98 ± 8.26 | 35.35 ± 4.20 | 0.23c |
| FBS (mg/dl) | 107.78 ± 46.04 | 96.84 ± 31.23 | 0.38a |
| SBP (mmHg) | 138.04 ± 17.17 | 129.74 ± 20.30 | 0.15a |
| DBP (mmHg) | 88.70 ± 10.02 | 87.53 ± 10.44 | 0.71a |
| HDL-C (mg/dl) | 37.87 ± 7.18 | 39.47 ± 9.02 | 0.52a |
| LDL-C (mg/dl) | 118.74 ± 28.37 | 120.05 ± 26.23 | 0.87a |
| TC (mg/dl) | 165.00 ± 32.93 | 171.95 ± 33.29 | 0.54a |
| TG (mg/dl) | 187.21 ± 71.07 | 184.31 ± 67.59 | 0.89a |
| HbA1C (%) | 6.12 ± 1.42 | 6.54 ± 1.72 | 0.39a |
| Physical activityd | |||
| Low | 13 (56.52) | 8 (42.10) | 0.63b |
| Moderate | 5 (21.73) | 5 (26.31) | |
| High | 5 (21.73) | 6 (31.57) |
Data are presented as mean ± standard deviation (SD) or number and percentage
P-value calculated using independent samples t-test
P-value calculated using chi-square test
P-value calculated using Mann-Whitney U test
Physical activity levels were classified according to the categorical scoring protocol of the short form of International Physical Activity Questionnaire (IPAQ)
WC: Waist circumference; BMI: Body mass index; FBS: Fasting blood sugar; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; TG: Triglyceride; TC: Total cholesterol; HbA1C: Hemoglobin A1C.
Table 2 shows dietary intake of the study groups (ALA and placebo) before the intervention, which was not significantly different between the two groups.
Table 2.
Dietary intakes of participants before intervention
| Variable | Intervention group (n = 23) | Control group (n = 19) | P |
|---|---|---|---|
| Total energy (Kcal/day) | 2116.00 ± 582.53 | 2026.42 ± 502.83 | 0.59a |
| Carbohydrate (g) | 259.50 ± 86.47 | 288.48 ± 91.20 | 0.29a |
| Protein (g) | 80.12 ± 26.16 | 75.83 ± 21.65 | 0.57a |
| Fat (g) | 80.57 ± 22.53 | 65.90 ± 18.09 | 0.06a |
| MUFA (g) | 22.13 ± 6.90 | 18.50 ± 4.88 | 0.06a |
| PUFA (g) | 28.07 ± 9.33 | 21.98 ± 10.21 | 0.05a |
| Vitamin C (mg/day) | 86.44 ± 41.84 | 100.53 ± 63.00 | 0.65b |
| Vitamin E (g/day) | 2.68 ± 1.88 | 3.46 ± 2.93 | 0.37b |
| Beta carotene (µg/day) | 231.97 ± 173.59 | 611.07 ± 700.37 | 0.13b |
| Selenium (µg/day) | 0.83 ± 0.06 | 0.06 ± 0.02 | 0.34b |
Data are presented as mean ± standard deviation (SD)
P-value calculated using independent samples t-test
P-value calculated using Mann-Whitney U test
MUFA: Monounsaturated fatty acids; PUFA: Polyunsaturated fatty acids
Table 3 shows the mean ± SD of lipid profile before and after intervention in the two groups. According to the within-group analysis, no statistically significant changes were observed in the placebo group (P > 0.05) in terms of lipid profile, while plasma levels of LDL-C (-8.08 ± 14.20 mg/dl, P = 0.012) and TG (-36.82 ± 42.48 mg/dl, P = 0.001) decreased significantly in the ALA group. There was a statistically significant difference between the ALA group and the placebo group in plasma levels of TG after 12 weeks (-36.82 ± 42.48 vs. 6.15 ± 25.04 mg/dl, P = 0.001) (Figure 2).
Table 3.
Mean and standard deviation (SD) of lipid profile before and after intervention in both groups
| Variable | Intervention group (n = 23) |
Control group (n = 19) |
P | ||||
|---|---|---|---|---|---|---|---|
| Before | After | P | Before | After | P | ||
| HDL-C (mg/dl) | 37.87 ± 7.18 | 38.83 ± 6.54 | 0.390a | 39.47 ± 9.02 | 41.05 ± 8.07 | 0.310a | 0.760c |
| LDL-C (mg/dl) | 118.74 ± 28.37 | 110.65 ± 28.16 | 0.012* | 120.05 ± 26.23 | 123.47 ± 27.48 | 0.400b | 0.070d |
| TG (mg/dl) | 187.21 ± 71.07 | 150.39 ± 58.18 | 0.001* | 184.31 ± 67.59 | 190.47 ± 80.02 | 0.290b | 0.001** |
| TC (mg/dl) | 165.74 ± 32.93 | 156.83 ± 34.15 | 0.060a | 171.95 ± 33.29 | 182.79 ± 35.52 | 0.070a | 0.010** |
P-values obtained from paired samples t-test
P-values obtained from Mann-Whitney U test
P-values calculated using Wilcoxon test
P-values calculated using paired samples t-test
P-value obtained from independent samples t-test
P-value calculated using Mann-Whitney U test
P-value < 0.05 was considered statistically significant
HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; TG: Triglyceride; TC: Total cholesterol
Figure 2.
Triglyceride (TG) in subjects with metabolic syndrome (MetS) at baseline (white bars) and after 12 weeks (purple bars) with intervention or control (* P < 0.05)
Moreover, a statistically significant difference was observed between the ALA group and the placebo group in serum levels of TC after 12 weeks (-8.91 ± 20.65 vs. 10.84 ± 22.97 mg/dl, P = 0.01) (Figure 3).
Figure 3.
Total cholesterol (TC) in subjects with metabolic syndrome (MetS) at baseline (white bars) and after 12 weeks (purple bars) with intervention or control (* P < 0.05)
On the contrary, there were no statistically significant differences between the two groups in HDL-C and LDL-C plasma levels after 12 weeks (Figures 4 and 5).
Figure 4.
Low-density lipoprotein cholesterol (LDL-C) in subjects with metabolic syndrome (MetS) at baseline (white bars) and after 12 weeks (purple bars) with intervention or control (* P < 0.05)
Figure 5.
High-density lipoprotein cholesterol (HDL-C) in subjects with metabolic syndrome (MetS) at baseline (white bars) and after 12 weeks (purple bars) with intervention or control (* P < 0.05)
Discussion
In the current study, we recruited patients with MetS, who were randomly divided into two groups. There were no significant differences in the baseline characteristics and food intakes between the ALA and placebo groups at baseline. The study results indicated a significant decrease in plasma levels of TG and TC. However, no significant differences were observed in plasma levels of HDL-C and LDL-C between the ALA group and the placebo group.
Although the beneficial effects of ALA on some diseases, as well as plasma levels of lipid profile have been previously studied,18,19 to our knowledge, this clinical trial was one of the first studies that investigated the effect of ALA supplementation on serum lipid profile in patients with MetS.
ALA is a strong natural antioxidant produced by animals and humans. This antioxidant plays a major role in lipid metabolism in the liver, kidneys, and blood. It is also an essential cofactor of enzymes in energy metabolism.20 Recently, it has been shown that ALA may affect plasma lipid profile via influencing lipid metabolism.
Our results showed a significant decrease in plasma levels of TG and TC between the groups. Our results are in line with those of Zhang et al. who indicated that 600 mg/day ALA for 2 weeks in obese subjects with impaired glucose tolerance (IGT) led to a significant decrease in the serum levels of TG and TC.21 Furthermore, positive effects of ALA supplementation on TG and TC have been shown in some other studies.14,15 On the contrary, de Oliveira et al. found no improvement in serum TG and TC levels.22
The exact mechanisms through which ALA might affect TG are still unknown. The possible lipid-lowering mechanism of ALA may be associated with increased mitochondrial fatty acid beta-oxidation by the activation of adenosine monophosphate-activated protein kinase (AMPK). The mentioned protein kinase suppresses lipogenesis (inhibition of acetyl-CoA carboxylase activity and fatty acid synthase).23 The next probable mechanism might be related to a decrease in plasma glucose level through ALA24 that helps to increase the level of cyclic adenosine monophosphate (cAMP),25 which in turn reduces serum level of TG.23
Some studies have suggested that ALA might improve TC or LDL through these mechanisms: (i) decreased activity of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase and enhanced activity of lipoprotein lipase, (ii) expression of LDL receptor in the liver which causes the transmission of cholesterol to the liver and enhanced expression of apolipoprotein A,26 and (iii) increased serum levels of adiponectin which improve fatty acid b-oxidation.27 In addition, some studies have also shown that ALA can down-regulate the hepatic expression of lipogenic genes such as carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP1-c).28 In a study by Yang et al., it was demonstrated that fat-lowering effect of ALA might be associated with increased carnitine palmitoyltransferase-1 (CPT-1) and acyl-CoA oxidase (ACOX) expression.29
Our findings indicated no significant differences in plasma levels of HDL-C and LDL-C between the groups. Our results are in line with those of Huerta et al.16 who found no improvement in plasma levels of HDL-C and LDL-C between the groups after 12 weeks. Several studies have also shown similar findings;22,30 however, some previous studies were inconsistent with our results.19,27 Inconsistent results of other studies with those of our study might be justified by less doses of ALA supplementation, shorter duration of intervention, and population diversity.
Our study clearly had some limitations. One of the limitations of our study was inability to measure apolipoproteins. In addition, a healthy control group was not incorporated in our study to compare plasma levels of ALA and lipid profile between the patients with MetS and healthy individuals to achieve more reliable results. Compared to previous studies, however, suitable duration of intervention and being a double-blind and placebo-controlled study were the stenghts of our study.
Conclusion
The results of our study indicated that ALA compared to placebo led to significant differences in plasma levels of TG and TC but not serum HDL-C and LDL-C levels. Therefore, ALA may have positive effects on lipid profile in patients with MetS. Further studies with groups receiving different doses of ALA and large sample sizes are necessary to achieve more reliable results.
Acknowledgments
Hereby, we sincerely thank “Darou Darman Sepehr Company” for providing both supplement and placebo, and the Endocrine and Metabolic Center of Shariati Hospital for collecting samples. Our further gratitute goes to all of the patients who participated in this study.
Conflicts of Interest
Authors have no conflict of interests.
Authors’ Contribution
BA contributed to conception and analysis. BA and MA contributed to the study design and data collection. MA and BA contributed to manuscript draft. BA and SAK contributed to data interpretation and supervised the study. All authors approved the final manuscript for submission.
REFERENCES
- 1.Wang HH, Lee DK, Liu M, Portincasa P, Wang DQ. Novel insights into the pathogenesis and management of the metabolic syndrome. Pediatr Gastroenterol Hepatol Nutr. 2020;23(3):189–230. doi: 10.5223/pghn.2020.23.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zafar U, Khaliq S, Ahmad HU, Manzoor S, Lone KP. Metabolic syndrome: An update on diagnostic criteria, pathogenesis, and genetic links. Hormones (Athens) 2018;17(3):299–313. doi: 10.1007/s42000-018-0051-3. [DOI] [PubMed] [Google Scholar]
- 3.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Tang K, Zhang Q, Peng NC, Zhang M, Xu SJ, Li H, et al. Epidemiology of metabolic syndrome and its components in Chinese patients with a range of thyroid-stimulating hormone concentrations. J Int Med Res. 2020;48(11):300060520966878. doi: 10.1177/0300060520966878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim NH, Han KH, Choi J, Lee J, Kim SG. Use of fenofibrate on cardiovascular outcomes in statin users with metabolic syndrome: Propensity matched cohort study. BMJ. 2019;366:l5125. doi: 10.1136/bmj.l5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe M, Yokotsuka M, Yamaoka K, Adachi M, Nemoto A, Tango T. Effects of a lifestyle modification programme to reduce the number of risk factors for metabolic syndrome: a randomised controlled trial. Public Health Nutr. 2017;20(1):142–53. doi: 10.1017/S1368980016001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morales-Palomo F, Ramirez-Jimenez M, Ortega JF, Mora-Rodriguez R. Effectiveness of aerobic exercise programs for health promotion in metabolic syndrome. Med Sci Sports Exerc. 2019;51(9):1876–83. doi: 10.1249/MSS.0000000000001983. [DOI] [PubMed] [Google Scholar]
- 8.Khoshnaw DM, Ghadge AA. Yoga as a complementary therapy for metabolic syndrome: A narrative review. J Integr Med. 2021;19(1):6–12. doi: 10.1016/j.joim.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Kim HN, Kim SH, Eun YM, Song SW. Effects of zinc, magnesium, and chromium supplementation on cardiometabolic risk in adults with metabolic syndrome: A double-blind, placebo-controlled randomised trial. J Trace Elem Med Biol. 2018;48:166–71. doi: 10.1016/j.jtemb.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Cicero AFG, Colletti A, Bajraktari G, Descamps O, Djuric DM, Ezhov M, et al. Lipid-lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Nutr Rev. 2017;75(9):731–67. doi: 10.1093/nutrit/nux047. [DOI] [PubMed] [Google Scholar]
- 11.Du G, Qiao Y, Zhuo Z, Zhou J, Li X, Liu Z, et al. Lipoic acid rejuvenates aged intestinal stem cells by preventing age-associated endosome reduction. EMBO Rep. 2020;21(8):e49583. doi: 10.15252/embr.201949583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serhiyenko V, Serhiyenko L, Suslik G, et al. Alpha-lipoic acid: Mechanisms of action and beneficial effects in the prevention and treatment of diabetic complications. MOJ Public Health. 2018;7(4):174–8. [Google Scholar]
- 13.Timmers S, de Vogel-van den Bosch J, Towler MC, Schaart G, Moonen-Kornips E, Mensink RP, et al. Prevention of high-fat diet-induced muscular lipid accumulation in rats by alpha lipoic acid is not mediated by AMPK activation. J Lipid Res. 2010;51(2):352–9. doi: 10.1194/jlr.M000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miao Y, Ren J, Jiang L, Liu J, Jiang B, Zhang X. alpha-Lipoic acid attenuates obesity-associated hippocampal neuroinflammation and increases the levels of brain-derived neurotrophic factor in ovariectomized rats fed a high-fat diet. Int J Mol Med. 2013;32(5):1179–86. doi: 10.3892/ijmm.2013.1482. [DOI] [PubMed] [Google Scholar]
- 15.Carrier B, Wen S, Zigouras S, Browne RW, Li Z, Patel MS, et al. Alpha-lipoic acid reduces LDL-particle number and PCSK9 concentrations in high-fat fed obese Zucker rats. PLoS One. 2014;9(3):e90863. doi: 10.1371/journal.pone.0090863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huerta AE, Navas-Carretero S, Prieto-Hontoria PL, Martinez JA, Moreno-Aliaga MJ. Effects of alpha-lipoic acid and eicosapentaenoic acid in overweight and obese women during weight loss. Obesity (Silver Spring) 2015;23(2):313–21. doi: 10.1002/oby.20966. [DOI] [PubMed] [Google Scholar]
- 17.The International Physical Activity Questionnaires (IPAQ) Group. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ) - Short and Long Forms [Online]. 2005. Available from: URL: http://www.ipaq.ki.se/scoring.pdf.
- 18.Mohammadi V, Khorvash F, Feizi A, Askari G. Does alpha-lipoic acid comsumption improve lipid profile in patients with stroke? A randomized, double blind, placebo-controlled clinical trial. Iran Red Crescent Med J. 2017;19(8):E58765. [Google Scholar]
- 19.Elbadawy AM, Abd Elmoniem RO, Elsayed AM. Alpha lipoic acid and diabetes mellitus: potential effects on peripheral neuropathy and different metabolic parameters. Alexandria J Med. 2021;57(1):113–20. [Google Scholar]
- 20.Rochette L, Ghibu S, Muresan A, Vergely C. Alpha-lipoic acid: molecular mechanisms and therapeutic potential in diabetes. Can J Physiol Pharmacol. 2015;93(12):1021–7. doi: 10.1139/cjpp-2014-0353. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Han P, Wu N, He B, Lu Y, Li S, et al. Amelioration of lipid abnormalities by alpha-lipoic acid through antioxidative and anti-inflammatory effects. Obesity (Silver Spring) 2011;19(8):1647–53. doi: 10.1038/oby.2011.121. [DOI] [PubMed] [Google Scholar]
- 22.de Oliveira AM, Rondo PH, Luzia LA, D'Abronzo FH, Illison VK. The effects of lipoic acid and alpha-tocopherol supplementation on the lipid profile and insulin sensitivity of patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Diabetes Res Clin Pract. 2011;92(2):253–60. doi: 10.1016/j.diabres.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Chen WL, Kang CH, Wang SG, Lee HM. alpha-Lipoic acid regulates lipid metabolism through induction of sirtuin 1 (SIRT1) and activation of AMP-activated protein kinase. Diabetologia. 2012;55(6):1824–35. doi: 10.1007/s00125-012-2530-4. [DOI] [PubMed] [Google Scholar]
- 24.Ansar H, Mazloom Z, Kazemi F, Hejazi N. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med J. 2011;32(6):584–8. [PubMed] [Google Scholar]
- 25.Tengholm A, Gylfe E. cAMP signalling in insulin and glucagon secretion. Diabetes Obes Metab. 2017;19(Suppl 1):42–53. doi: 10.1111/dom.12993. [DOI] [PubMed] [Google Scholar]
- 26.Ichikawa T, Liang J, Kitajima S, Koike T, Wang X, Sun H, et al. Macrophage-derived lipoprotein lipase increases aortic atherosclerosis in cholesterol-fed Tg rabbits. Atherosclerosis. 2005;179(1):87–95. doi: 10.1016/j.atherosclerosis.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 27.Vidovic B, Milovanovic S, Stefanovic A, Kotur-Stevuljevic J, Takic M, Debeljak-Martacic J, et al. Effects of Alpha-Lipoic Acid Supplementation on Plasma Adiponectin Levels and Some Metabolic Risk Factors in Patients with Schizophrenia. J Med Food. 2017;20(1):79–85. doi: 10.1089/jmf.2016.0070. [DOI] [PubMed] [Google Scholar]
- 28.Castro MC, Massa ML, Schinella G, Gagliardino JJ, Francini F. Lipoic acid prevents liver metabolic changes induced by administration of a fructose-rich diet. Biochim Biophys Acta. 2013;1830(1):2226–32. doi: 10.1016/j.bbagen.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Yang RL, Li W, Shi YH, Le GW. Lipoic acid prevents high-fat diet-induced dyslipidemia and oxidative stress: A microarray analysis. Nutrition. 2008;24(6):582–8. doi: 10.1016/j.nut.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Salwa K, Mohamed MA, Ahmed MM, El-Arabi GH. a-Lipoic acid ameliorates the oxidative status and serum iron in diabetic patients. J Pharm Biomed Sci. 2011;1(5):97–103. [Google Scholar]