Abstract
The population of older individuals is increasing rapidly, but only a small fraction among them is able to experiences a healthy life. Due to lack of physical exercise and oxidative stress, aging leads to sarcopenia and finally end up with frailty. Sarcopenia is a component of the frailty and described as age related degenerative changes in the skeletal muscle mass, strength and quality. Though the loss of muscle strength and mass gradually seem inevitable during aging, it can be partially prevented or overcome by a deeper insight into the pathogenesis. Sirtuin protein leads to longevity across different organisms ranging from worms to mammals. Expression of sirtuin protein increases during physical exercise and thus strengthens muscle mass. Satellite cells leads to muscle repair in a SIRT1 dependent manner. In addition, SIRT1 improves insulin sensitivity and induces autophagy in the aged mice. The current paper discussed the putative role of sirtuins in sarcopenia and frailty. Moreover, it highlighted the pathways by which sirtuins can inhibit ROS production, inflammation and mitochondrial dysfunctions and therefore confers a protective role against frailty and sarcopenia. The critical role of sirtuins in the sarcopenia and frailty pathogenesis can eventually fuel the development of novel interventions by targeting sirtuins.
Keywords: Sirtuins, Frailty, Sarcopenia, Oxidative stress, Aging
Introduction
The phenomenon of aging is essentially associated with the catabolism of muscles that leads to sarcopenia and frailty. In the older population, these two syndromes have emerged as major geriatric giants, and pose a significant burden to our health care system: primarily because of high rate of multisystem decline, leading to falls, fractures, physical disability and mortality. The ICD-10-CM (M62.84) code recognizes sarcopenia as a disease and on the basis of severity, Sarcopenia in Older People by European Working Group (EWGSOP) has categorized three stages of sarcopenia; Pre-sarcopenia, which is associated with low muscle mass and normal muscle strength or physical performance while sarcopenia exhibits both low muscle mass and low muscle strength or physical performance; Severe sarcopenia, the most advanced stage, manifests itself as low muscle mass, low muscle strength, and low physical performance. Physical disability, poor quality of life and death are the detrimental effects of sarcopenia [1]. Sarcopenia finally ends up with frailty and has been identified as a crucial component of frailty in the older people and often leads to cachexia [2]. Frailty is characterised by conditions including exhaustion, weakness, and slowness, whereas sarcopenia refers to the loss of muscle mass. It is noteworthy that frailty is more prevalent in individuals exhibiting lack of physical activity and exercise [3]. Emerging evidence suggests that dietary habits and nutritional status can significantly impact the susceptibility to frailty. In particular, Mediterranean dietary pattern, regular consumption of fruits vegetables and lower consumption of processed food confers protective against the frailty [4-7].
The frail older are more susceptible to outcomes such as falls, increased impairment, hospitalization and mortality [8, 9]. Various definitions have been used to conceptualize and operationalize frailty [10, 11] and the most extensively approved was proposed by Fried et al in 2001 [12]. There are five characteristics of Fried’s criteria; slow motor performance, poor endurance and energy, weakness, shrinking and inadequate physical activity. An individual exhibiting 3 or more criteria out of 5 will be considered as frail.
Physical phenotype of Fried’s criteria, such as lower grip strength and slower gait speed exhibits a significant overlap with the characteristics of sarcopenia. Consequently, sarcopenia and frailty has been regarded as a common geriatric syndrome and are often manifest themselves as adverse health outcome and impaired health-related quality of life. Latest diagnostic tools like Groningen Frailty Indicator and Frailty Index of Rockwood et al, [13, 14] can well distinguish the multiple dimensions of frailty from sarcopenia. Through extensive research and a better understanding of frailty, sarcopenia has been recognized as a crucial component of the frailty [15].
Since frailty is characterised by subtle and subjective clinical features, diagnosis is often difficult, particularly during the early stage. Furthermore, a definitive therapeutic intervention is still lacking, which further highlights the requirement for a reliable biomarker. Increase in the lifespan had simultaneously led to an increase in the incidence of several age-related comorbidities and among them frailty is the most prominent. However, the mechanisms responsible for the onset of frailty are poorly understood. The current situation requires a comprehensive understanding of the underlying pathway and considering the strong association between frailty and senescence, it is imperative to explore the molecules with a strong link with senescence. The current review article describes the putative mechanistic role of an anti-senescence protein sirtuin in the pathogenesis of frailty and sarcopenia.
Sirtuin in aging, sarcopenia, frailty
Sirtuins (silent information regulator) family consists of seven isoforms which are nicotinamide adenine dinucleotide (NAD)-dependent proteins and conserved in all domains of life. Since, last two decades, sirtuins have evolved as a critical epigenetic regulator of aging. It also mediates the consequences of calorie restriction (CR), the only dietary intervention that deaccelerates the process of aging and extends lifespan [16]. Moreover, the beneficial effects of CR get abrogated in global SIRT1 knockout [17] and brain-specific knockout mice [18]. In addition, SIRT5 and SIRT6 overexpress in the animals fed on CR diet [19, 20]. Moreover, SIRT6 overexpression in transgenic mice leads to lifespan extension. SIRT3 also mediates the effect of CR in vivo [21] and gained particular interest due to its localization in the mitochondria and associated with longevity in humans [22]. SIRT1’s role in CR was validated by a clinical study which reported its overexpression in the individuals fed on a CR diet [23]. Furthermore, a previous study made an interesting observation that the expression level SIRT1 and SIRT3 in serum downregulates with age [24, 25].
NAD+, which acts as a cofactor for several vital enzymes like Poly (ADP-ribose) polymerase (PARP), sirtuins, and CD38, decreases with sarcopenia [26]. A reduction in their enzymatic activity impairs mitochondrial function and decreases the muscle strength [27]. Deacetylation of peroxisome proliferator-activated receptor coactivator 1-α (PGC1α) by SIRT1 in vitro as well as in vivo lead to the stabilization of mitochondria in skeletal muscle [28]. During aging, satellite cells plays a vital role in the muscle repair via SIRT1 dependent manner [29]. Several in vivo studies suggests that sarcopenia is characterized by a decrease in the activity and expression of SIRT1 [30-32]. SIRT1 activity also decreases with aging in vivo, which causes PARP-1 hyper-acetylation and NAD+ decrease consequently, which further inhibits activity of SIRT1. PARP-1 acetylation also leads to the stimulation of NF-κB dependent gene expression [32], which leads to increase in inflammation, one of the hallmarks of sarcopenia.
The role of sirtuins in frailty for the first time was determined by Le Couteur et al in 2010 in their landmark study which concluded that there was no significant difference in the level of induced SIRT1 in SK Hep1 cells upon treatment with serum collected from frail and non-frail individuals [33]. Authors also stated the possible existence of reverse association between lower SIRT1 level and the robustness. In a clinical study, Kumar et al. determined the level of different sirtuin in the serum and observed that expression of SIRT1 and SIRT3 decreases with frailty [34].
Surprisingly, Ma et al reported that a higher level of SIRT1 is present in frail individuals, an observation that was in contradiction to the previous study [35]. Another study observed that no significant association exists between SIRT1 single nucleotide polymorphisms (SNPs) and frail status. However, they detected the presence of a weak association between SNPs and conditions such as arthritis, cognitive impairment, and hearing impairment [36]. Overexpression of SIRT6 in vivo can reverse the age-associated decline in physical activity and prevents the onset of frailty at old age [37]. These studies indicate the possible mechanistic association between sirtuins and frailty, although the results are contradictory. Therefore, further studies in multiple cohorts are essential to address these contradictions.
Mechanisms
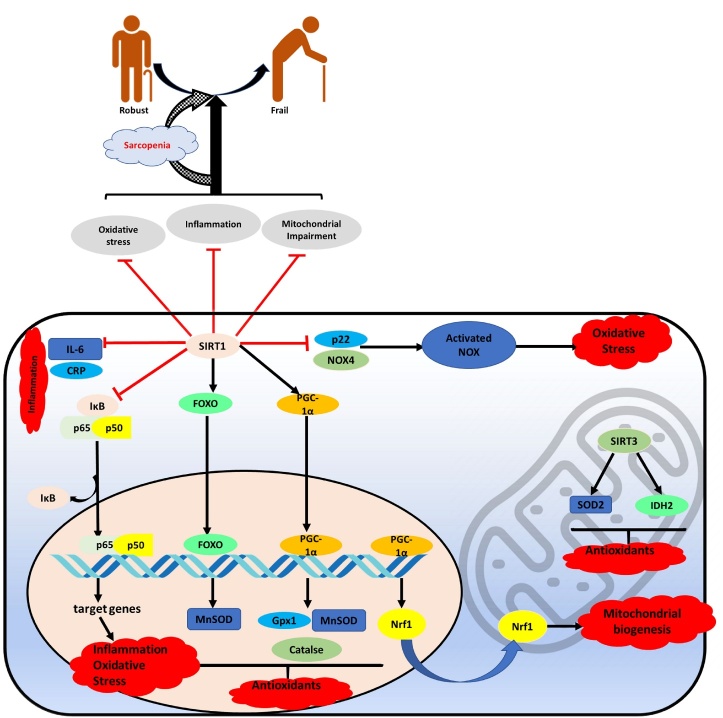
In the previous section, we described the possible association between sirtuins and sarcopenia/frailty, although the mechanisms are still elusive. Based on the currently available evidence, we suspect the following mechanisms are responsible in driving the effects of sirtuins in sarcopenia as well as frailty. Figure 1 illustrated the mechanism suspect of different pathways of SIRT1 and SIRT3 in pathophysiology of sarcopenia that ends up with frailty.
Figure 1.
Mechanisms by which SIRT1 and SIRT3 can influence the pathophysiology of sarcopenia.
Oxidative stress:
Several in vivo studies suggests that oxidative stress plays a key role in the induction of sarcopenia in different experimental models [38, 39]. In aged mice, over-expression of an enzyme glucose-6-phosphate dehydrogenase (G6PD), responsible for reducing the oxidative stress, improved neuromuscular performance [40]. Emerging clinical suggests that the level of oxidative stress is significantly higher in frail individuals in comparison to non-frail [41, 42]. Mice that lacks antioxidant Cu/Zn superoxide dismutase (SOD) exhibits sarcopenia [43]. SIRT1 deacetylate and activate FOXO3A in vitro, which enhances transcription of manganese SOD (Mn-SOD) [44, 45]. SIRT1 deacetylate and activates PGC-1α which enhances the expression of antioxidant likes MnSOD, catalase and glutathione peroxidase (GPx1) [28, 46-48]. Moreover, inhibition of SIRT1 leads to the overexpression of NADPH oxidase (NOX) subunits p22phox and NOX4 and increases the level of ROS production [49]. Deacetylation and activation of endothelial nitric oxide synthase (eNOs) by SIRT1 augment the NO production, which acts as a potential antioxidant [50]. Mounting evidence suggests that SIRT3, a mitochondrial sirtuin, plays a vital role in preventing ROS formation via different mechanisms. It directly deacetylates SOD-2 at two lysine residues and enhances its activity [51-53]. Reduced glutathione, a potent antioxidant compound, generates from oxidized glutathione in a reaction that requires NADPH and thereby validates the role of SIRT3 as an antioxidant molecule [21].
Inflammation:
The first experimental association between frailty and inflammation was well-established by Leng and colleagues, who observed the presence of a higher expression of serum interleukin 6 (IL-6) in frail individuals [54]. Another clinical study indicated that frailty is characterized by an increased level of a C-reactive protein (CRP) and IL-6 [55-57]. Additional inflammatory markers like C-X-C motif chemokine ligand 10 (CXCL10) and neopterin also increases with frailty as per different clinical reports [58-60]. In addition, increase in the levels of IL-6 and CRP enhances the possible risk of sarcopenia [61]. Altogether, these studies point towards the crucial role of inflammation in the onset of sarcopenia and frailty.
Mitochondrial dysfunction:
Emerging evidence suggests that mitochondrial dysfunction leads to sarcopenia and frailty. For example, Andreux et al reported a decrease in the level of proteins involved in the mitochondrial respiratory complex and an impaired phosphocreatine recovery in pre-frail individuals [62]. SIRT1 dependent deacetylation and activation of PGC-1α is a crucial pathway in the biogenesis of mitochondria [47, 63-65]. Activated PGC-1α stimulate the expression of Nuclear Respiratory Factor 1/2 (NRF1/NRF2) and transcription factor A, mitochondrial (TFAM), an essential step in mitochondrial biogenesis [66]. SIRT3 deacetylates several mitochondrial enzymes and regulates ATP production [67].
Sirtuin as a marker/therapeutic target
Sirtuins gained significant momentum recently, based on several studies which revealed their potential as a therapeutic target and biomarker. Notably, serum sirtuins can be proposed as a promising marker for sarcopenia and frailty but multiple cohort-based studies are warranted to establish the fact. SIRT1 and SIRT3 [68] displayed a stronger association with frailty and possess the potential to be used as biomarker to prevent the progression to bed-bound phase by detect frailty at an early stage. However, future studies with a larger sample size in multiple cohorts is required to ascertain the role of sirtuins as a marker for the disease onset. Regular exercise and nutritional status had emerged as an essential intervention to prolong lifespan and increase muscle mass. Resveratrol, the activator of SIRT1 improves the effectiveness of exercise on the satellite cell activation in older individuals [46]. A previous study had shown a significant improvement in the state of sarcopenia by the effectiveness of physical activity on mitochondrial enzymes as well as muscle stem cells [69]. Resistance exercise improved muscle strength and mass and proved to be effective in reversing the status of sarcopenia [70-72]. Acute exercise activates SIRT1 and not SIRT3, via phosphorylation of AMPK. Moreover, several sessions of exercise training can lead to activation of both SIRT1 and SIRT3, together with the improvement in mitochondrial oxidative function and biogenesis [73, 74].
Resveratrol can prevent the tumour necrosis factor alpha (TNF-α) induced muscle cells atrophy by restoring Akt/mTOR/S6K and 4E-BP1 signaling in vivo [75]. SRT2104 dependent SIRT1 activation can alleviate the loss of muscle mass in mice [76]. Exercise and resveratrol inhibit age-related changes in the gastrocnemius muscle in mice, via activation of SIRT1, PGC-1 α and 5'AMP-activated protein kinase (AMPK) [77, 78]. Resveratrol improves the forelimb grip strength in aged rats and confers protection to the cultured cells against peroxides [79, 80]. By activating SIRT1, myricanol alleviates dexamethasone-induced skeletal muscle wasting and weakness, which in turn enhances autophagy and promotes mitochondrial biogenesis [81]. Moreover, inhibition of SIRT1 is necessary for Toll-like receptor 9 (TLR9) dependent muscle fibrosis and sarcopenia in aged mice [82]. Resveratrol also protects the mice against negative health consequences of a high-fat diet. Juzentaihoto, a Chinese herbal medicine, prevents muscle atrophy in senescence accelerated mouse (SAMP8) via activation of SIRT1 [83]. Bring together, all these findings suggest that SIRT1 activation plays a pivotal role in the protection against age associated sarcopenia. However, the exact role of sirtuins in frailty is still elusive due to the lack of reliable animal models. Therefore, future studies aimed to develop an appropriate animal model of frailty to identify the exact mechanistic contribution of sirtuin in frailty and exploit them as a therapeutic target. Further longitudinal studies with frail aged individual having several age-related diseases like cognitive impairment, hypertension, diabetes etc are required for future studies
Conclusions
The current review summarized the putative role of sirtuins in sarcopenia and frailty pathogenesis in the older people. This review highlighted the pathways by which sirtuins can influence ROS production, inflammation and mitochondrial dysfunctions to exhibit a protective role against frailty and sarcopenia in the older and its therapeutic intervention in the future. However, a reliable biomarker and efficient therapeutic interventions is still not available for frailty. Sirtuins have unique features including its different complex catalytic mechanism and substrate specificities, which offer great opportunities for the development of drug. Several pharmacological and natural activators of sirtuins, particularly SIRT1 have been under investigation for long and have shown promising results. Although there are promising in vitro studies with convincing results, its potential as therapeutic intervention in vivo and clinical studies remains completely unknown. The previous reports suggests that sirtuins plays a protective role during the onset of frailty by preventing ROS accumulation, inflammation and mitochondrial impairment. However, it is certain that novel modulators targeting SIRT1 and SIRT3 will be explored in the near future, which requires further unravelling the molecular pathway involved in frailty and its component sarcopenia. Moreover, Sirtuins can serve as potential biomarker for early intervention and combat frailty and sarcopenia therapeutically. Based on the previous literature, we suggest that SIRT1 plays a protective role during frailty and its activation can provide a novel therapeutic approach. Moreover, future studies directed towards examining the role of SIRT1 as an early marker for frailty can provide us with an approach to arrest the progression into advanced stages.
Footnotes
Conflicts of interest
No author has conflict of interest and competing financial interests.
References
- [1].Cruz-Jentoft AJ, Sayer AA (2019). Sarcopenia. The Lancet, 393:2636-2646. [DOI] [PubMed] [Google Scholar]
- [2].Gingrich A, Volkert D, Kiesswetter E, Thomanek M, Bach S, Sieber CC, et al. (2019). Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatrics, 19:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].da Silva VD, Tribess S, Meneguci J, Sasaki JE, Garcia-Meneguci CA, Carneiro JAO, et al. (2019). Association between frailty and the combination of physical activity level and sedentary behavior in older adults. BMC Public Health, 19:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ni Lochlainn M, Cox NJ, Wilson T, Hayhoe RPG, Ramsay SE, Granic A, et al. (2021). Nutrition and Frailty: Opportunities for Prevention and Treatment. Nutrients, 13:2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jayanama K, Theou O, Blodgett JM, Cahill L, Rockwood K (2018). Frailty, nutrition-related parameters, and mortality across the adult age spectrum. BMC Medicine, 16:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bonnefoy M, Berrut G, Lesourd B, Ferry M, Gilbert T, Guérin O, et al. (2015). Frailty and nutrition: searching for evidence. J Nutr Health Aging, 19:250-257. [DOI] [PubMed] [Google Scholar]
- [7].Liang H, Li X, Lin X, Ju Y, Leng J (2021). The correlation between nutrition and frailty and the receiver operating characteristic curve of different nutritional indexes for frailty. BMC Geriatrics, 21:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hu K, Zhou Q, Jiang Y, Shang Z, Mei F, Gao Q, et al. (2021). Association between Frailty and Mortality, Falls, and Hospitalization among Patients with Hypertension: A Systematic Review and Meta-Analysis. BioMed Research International, 2021:e2690296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang Q, Zhao X, Liu H, Ding H (2020). Frailty as a predictor of future falls and disability: a four-year follow-up study of Chinese older adults. BMC Geriatrics, 20:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT, Schols JM (2010). Toward a conceptual definition of frail community dwelling older people. Nurs Outlook, 58:76-86. [DOI] [PubMed] [Google Scholar]
- [11].Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A (2011). The identification of frailty: a systematic literature review. J Am Geriatr Soc, 59:2129-2138. [DOI] [PubMed] [Google Scholar]
- [12].Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. (2001). Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci, 56:M146-156. [DOI] [PubMed] [Google Scholar]
- [13].STEVERINK N (2001). Measuring frailty: Developing and testing the GFI (Groningen Frailty Indicator). The Gerontologist, 41:236. [Google Scholar]
- [14].Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. (2005). A global clinical measure of fitness and frailty in elderly people. CMAJ, 173:489-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Greco EA, Pietschmann P, Migliaccio S (2019). Osteoporosis and Sarcopenia Increase Frailty Syndrome in the Elderly. Frontiers in Endocrinology 10:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yu W, Qin J, Chen C, Fu Y, Wang W (2018). Moderate calorie restriction attenuates age-associated alterations and improves cardiac function by increasing SIRT1 and SIRT3 expression. Molecular Medicine Reports, 18:4087-4094. [DOI] [PubMed] [Google Scholar]
- [17].Chen D, Steele AD, Lindquist S, Guarente L (2005). Increase in activity during calorie restriction requires Sirt1. Science, 310:1641. [DOI] [PubMed] [Google Scholar]
- [18].Cohen DE, Supinski AM, Bonkowski MS, Donmez G, Guarente LP (2009). Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev, 23:2812-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Geng Y-Q, Li T-T, Liu X-Y, Li Z-H, Fu Y-C (2011). SIRT1 and SIRT5 activity expression and behavioral responses to calorie restriction. J Cell Biochem, 112:3755-3761. [DOI] [PubMed] [Google Scholar]
- [20].Zhang N, Li Z, Mu W, Li L, Liang Y, Lu M, et al. (2016). Calorie restriction-induced SIRT6 activation delays aging by suppressing NF-κB signaling. Cell Cycle, 15:1009-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, et al. (2010). Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell, 143:802-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hurst LD, Williams EJB, Pál C (2002). Natural selection promotes the conservation of linkage of co-expressed genes. Trends Genet, 18:604-606. [DOI] [PubMed] [Google Scholar]
- [23].Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, et al. (2007). Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med, 4:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kumar R, Chaterjee P, Sharma PK, Singh AK, Gupta A, Gill K, et al. (2013). Sirtuin1: a promising serum protein marker for early detection of Alzheimer’s disease. PLoS One, 8:e61560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pradhan R, Kumar R, Shekhar S, Rai N, Ambashtha A, Banerjee J, et al. (2017). Longevity and healthy ageing genes FOXO3A and SIRT3: Serum protein marker and new road map to burst oxidative stress by Withania somnifera. Exp Gerontol, 95:9-15. [DOI] [PubMed] [Google Scholar]
- [26].Migliavacca E, Tay SKH, Patel HP, Sonntag T, Civiletto G, McFarlane C, et al. (2019). Mitochondrial oxidative capacity and NAD+ biosynthesis are reduced in human sarcopenia across ethnicities. Nat Commun, 10:5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Migliavacca E, Tay SKH, Patel HP, Sonntag T, Civiletto G, McFarlane C, et al. (2019). Mitochondrial oxidative capacity and NAD+ biosynthesis are reduced in human sarcopenia across ethnicities. Nat Commun, 10:5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nemoto S, Fergusson MM, Finkel T (2005). SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}. J Biol Chem, 280:16456-16460. [DOI] [PubMed] [Google Scholar]
- [29].Myers MJ, Shepherd DL, Durr AJ, Stanton DS, Mohamed JS, Hollander JM, et al. (2019). The role of SIRT1 in skeletal muscle function and repair of older mice. J Cachexia Sarcopenia Muscle, 10:929-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Koltai E, Hart N, Taylor AW, Goto S, Ngo JK, Davies KJA, et al. (2012). Age-associated declines in mitochondrial biogenesis and protein quality control factors are minimized by exercise training. Am J Physiol Regul Integr Comp Physiol, 303:R127-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ziaaldini MM, Koltai E, Csende Z, Goto S, Boldogh I, Taylor AW, et al. (2015). Exercise training increases anabolic and attenuates catabolic and apoptotic processes in aged skeletal muscle of male rats. Exp Gerontol, 67:9-14. [DOI] [PubMed] [Google Scholar]
- [32].Mohamed JS, Wilson JC, Myers MJ, Sisson KJ, Alway SE (2014). Dysregulation of SIRT-1 in aging mice increases skeletal muscle fatigue by a PARP-1-dependent mechanism. Aging (Albany NY), 6:820-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Le Couteur DG, Benson VL, McMahon AC, Blyth F, Handelsman DJ, Seibel MJ, et al. (2011). Determinants of serum-induced SIRT1 expression in older men: the CHAMP study. J Gerontol A Biol Sci Med Sci, 66:3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kumar R, Mohan N, Upadhyay AD, Singh AP, Sahu V, Dwivedi S, et al. (2014). Identification of serum sirtuins as novel noninvasive protein markers for frailty. Aging Cell, 13:975-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ma L, Niu H, Sha G, Zhang Y, Liu P, Li Y (2019). Serum SIRT1 Is Associated with Frailty and Adipokines in Older Adults. J Nutr Health Aging, 23:246-250. [DOI] [PubMed] [Google Scholar]
- [36].Razi S, Cogger VC, Kennerson M, Benson VL, McMahon AC, Blyth FM, et al. (2017). SIRT1 Polymorphisms and Serum-Induced SIRT1 Protein Expression in Aging and Frailty: The CHAMP Study. J Gerontol A Biol Sci Med Sci, 72:870-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Roichman A, Elhanati S, Aon MA, Abramovich I, Di Francesco A, Shahar Y, et al. (2021). Restoration of energy homeostasis by SIRT6 extends healthy lifespan. Nat Commun, 12:3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Campos F, Abrigo J, Aguirre F, Garcés B, Arrese M, Karpen S, et al. (2018). Sarcopenia in a mice model of chronic liver disease: role of the ubiquitin-proteasome system and oxidative stress. Pflugers Arch, 470:1503-1519. [DOI] [PubMed] [Google Scholar]
- [39].Higashihara T, Nishi H, Takemura K, Watanabe H, Maruyama T, Inagi R, et al. (2021). β2-adrenergic receptor agonist counteracts skeletal muscle atrophy and oxidative stress in uremic mice. Sci Rep, 11:9130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Arc-Chagnaud C, Salvador-Pascual A, Garcia-Dominguez E, Olaso-Gonzalez G, Correas AG, Serna E, et al. (2021). Glucose 6-P dehydrogenase delays the onset of frailty by protecting against muscle damage. Journal of Cachexia, Sarcopenia and Muscle, 12:1879-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Álvarez-Satta M, Berna-Erro A, Carrasco-Garcia E, Alberro A, Saenz-Antoñanzas A, Vergara I, et al. (2020). Relevance of oxidative stress and inflammation in frailty based on human studies and mouse models. Aging (Albany NY), 12:9982-9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liu CK, Lyass A, Larson MG, Massaro JM, Wang N, D’Agostino RB, et al. (2016). Biomarkers of oxidative stress are associated with frailty: the Framingham Offspring Study. Age (Dordr), 38:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Deepa SS, Van Remmen H, Brooks SV, Faulkner JA, Larkin L, McArdle A, et al. (2019). Accelerated sarcopenia in Cu/Zn superoxide dismutase knockout mice. Free Radic Biol Med, 132:19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. (2004). Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science, 303:2011-2015. [DOI] [PubMed] [Google Scholar]
- [45].Kops GJPL, Dansen TB, Polderman PE, Saarloos I, Wirtz KWA, Coffer PJ, et al. (2002). Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature, 419:316-321. [DOI] [PubMed] [Google Scholar]
- [46].Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. (2006). Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell, 127:1109-1122. [DOI] [PubMed] [Google Scholar]
- [47].Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim S-H, Mostoslavsky R, et al. (2007). Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J, 26:1913-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, et al. (2006). Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell, 127:397-408. [DOI] [PubMed] [Google Scholar]
- [49].Zarzuelo MJ, López-Sepúlveda R, Sánchez M, Romero M, Gómez-Guzmán M, Ungvary Z, et al. (2013). SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol, 85:1288-1296. [DOI] [PubMed] [Google Scholar]
- [50].Mattagajasingh I, Kim C-S, Naqvi A, Yamamori T, Hoffman TA, Jung S-B, et al. (2007). SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A, 104:14855-14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Qiu X, Brown K, Hirschey MD, Verdin E, Chen D (2010). Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab, 12:662-667. [DOI] [PubMed] [Google Scholar]
- [52].Chen Y, Zhang J, Lin Y, Lei Q, Guan K-L, Zhao S, et al. (2011). Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep, 12:534-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Xu H, Gan C, Gao Z, Huang Y, Wu S, Zhang D, et al. (2020). Caffeine Targets SIRT3 to Enhance SOD2 Activity in Mitochondria. Front Cell Dev Biol, 8:822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Leng S, Chaves P, Koenig K, Walston J (2002). Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc, 50:1268-1271. [DOI] [PubMed] [Google Scholar]
- [55].Pothier K, Gana W, Bailly N, Fougère B (2022). Associations Between Frailty and Inflammation, Physical, and Psycho-Social Health in Older Adults: A Systematic Review. Frontiers in Psychology 13:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Marcos-Pérez D, Sánchez-Flores M, Maseda A, Lorenzo-López L, Millán-Calenti JC, Gostner JM, et al. (2018). Frailty in Older Adults Is Associated With Plasma Concentrations of Inflammatory Mediators but Not With Lymphocyte Subpopulations. Frontiers in Immunology 9:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ferrucci L, Fabbri E (2018). Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol, 15:505-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C, et al. (2012). Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev, 133:456-466. [DOI] [PubMed] [Google Scholar]
- [59].Qu T, Yang H, Walston JD, Fedarko NS, Leng SX (2009). Upregulated monocytic expression of CXC chemokine ligand 10 (CXCL-10) and its relationship with serum interleukin-6 levels in the syndrome of frailty. Cytokine, 46:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Leng SX, Tian X, Matteini A, Li H, Hughes J, Jain A, et al. (2011). IL-6-independent association of elevated serum neopterin levels with prevalent frailty in community-dwelling older adults. Age Ageing, 40:475-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Schaap LA, Pluijm SMF, Deeg DJH, Visser M (2006). Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med, 119:526.e9-17. [DOI] [PubMed] [Google Scholar]
- [62].Andreux PA, van Diemen MPJ, Heezen MR, Auwerx J, Rinsch C, Groeneveld GJ, et al. (2018). Mitochondrial function is impaired in the skeletal muscle of pre-frail elderly. Sci Rep, 8:8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Scarpulla RC (2011). Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta, 1813:1269-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Guo P, Pi H, Xu S, Zhang L, Li Y, Li M, et al. (2014). Melatonin Improves mitochondrial function by promoting MT1/SIRT1/PGC-1 alpha-dependent mitochondrial biogenesis in cadmium-induced hepatotoxicity in vitro. Toxicol Sci, 142:182-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Gurd BJ (2011). Deacetylation of PGC-1α by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl Physiol Nutr Metab, 36:589-597. [DOI] [PubMed] [Google Scholar]
- [66].Gureev AP, Shaforostova EA, Popov VN (2019). Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front Genet, 10:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Meng H, Yan W-Y, Lei Y-H, Wan Z, Hou Y-Y, Sun L-K, et al. (2019). SIRT3 Regulation of Mitochondrial Quality Control in Neurodegenerative Diseases. Front Aging Neurosci, 11:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Rahul Kumar, Rashmita Pradhan, Akash Kumar Ambashtha, Swapnil Jathar, Dey AB, Sharmistha Dey (2016). COMPARATIVE EVALUATION OF SEVEN ISOFORMS OF SERUM SIRTUINS AS PROTEIN MARKER FOR FRAILTY. Journal of Proteins and Proteomics, 7:101-106. [Google Scholar]
- [69].Anwar M, Mallick S, Paliwal D, Shekhar S, Panda SK, Dey S, et al. (2021). Impact of physical activity on mitochondrial enzymes, muscle stem cell and anti-oxidant protein Sestrins in Sarcopenic mice. Exp Gerontol, 150:111358. [DOI] [PubMed] [Google Scholar]
- [70].Dent E, Morley JE, Cruz-Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, et al. (2018). International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J Nutr Health Aging, 22:1148-1161. [DOI] [PubMed] [Google Scholar]
- [71].Peterson MD, Sen A, Gordon PM (2011). Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc, 43:249-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Peterson MD, Rhea MR, Sen A, Gordon PM (2010). Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev, 9:226-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Vargas-Ortiz K, Pérez-Vázquez V, Macías-Cervantes MH (2019). Exercise and Sirtuins: A Way to Mitochondrial Health in Skeletal Muscle. Int J Mol Sci, 20:E2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Radak Z, Suzuki K, Posa A, Petrovszky Z, Koltai E, Boldogh I (2020). The systemic role of SIRT1 in exercise mediated adaptation. Redox Biol, 35:101467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wang D-T, Yin Y, Yang Y-J, Lv P-J, Shi Y, Lu L, et al. (2014). Resveratrol prevents TNF-α-induced muscle atrophy via regulation of Akt/mTOR/FoxO1 signaling in C2C12 myotubes. Int Immunopharmacol, 19:206-213. [DOI] [PubMed] [Google Scholar]
- [76].Mercken EM, Mitchell SJ, Martin-Montalvo A, Minor RK, Almeida M, Gomes AP, et al. (2014). SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell, 13:787-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Liao Z-Y, Chen J-L, Xiao M-H, Sun Y, Zhao Y-X, Pu D, et al. (2017). The effect of exercise, resveratrol or their combination on Sarcopenia in aged rats via regulation of AMPK/Sirt1 pathway. Exp Gerontol, 98:177-183. [DOI] [PubMed] [Google Scholar]
- [78].Li F-H, Yu H-T, Xiao L, Liu Y-Y (2016). Response of BAX, Bcl-2 Proteins, and SIRT1/PGC-1α mRNA Expression to 8-Week Treadmill Running in the Aging Rat Skeletal Muscle. Adv Exp Med Biol, 923:283-289. [DOI] [PubMed] [Google Scholar]
- [79].Liao Z-Y, Zhao K-X, Xiao Q (2017). [Effect of resveratrol on forelimb grip strength and myofibril structure in aged rats]. Nan Fang Yi Ke Da Xue Xue Bao, 37:1405-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Haramizu S, Asano S, Butler DC, Stanton DA, Hajira A, Mohamed JS, et al. (2017). Dietary resveratrol confers apoptotic resistance to oxidative stress in myoblasts. J Nutr Biochem, 50:103-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Shen S, Liao Q, Liu J, Pan R, Lee SM-Y, Lin L (2019). Myricanol rescues dexamethasone-induced muscle dysfunction via a sirtuin 1-dependent mechanism. J Cachexia Sarcopenia Muscle, 10:429-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lyu A-K, Zhu S-Y, Chen J-L, Zhao Y-X, Pu D, Luo C, et al. (2019). Inhibition of TLR9 attenuates skeletal muscle fibrosis in aged sarcopenic mice via the p53/SIRT1 pathway. Exp Gerontol, 122:25-33. [DOI] [PubMed] [Google Scholar]
- [83].Morita Y, Ishida T, Morisawa S, Jobu K, Ou Y, Fujita H, et al. (2021). Juzentaihoto Suppresses Muscle Atrophy and Decreased Motor Function in SAMP8 Mice. Biol Pharm Bull, 44:32-38. [DOI] [PubMed] [Google Scholar]