Abstract
A clear understanding of the origin of SARS-CoV-2 is important for future pandemic preparedness. Here, I provided an updated analysis of the type IIS endonuclease maps in genomes of alphacoronavirus, betacoronavirus, and SARS-CoV-2. Scenarios to engineer SARS-CoV-2 in the laboratory and the associated workload was also discussed. The analysis clearly shows that the endonuclease fingerprint does not indicate a synthetic origin of SARS-CoV-2 and engineering a SARS-CoV-2 virus in the laboratory is extremely challenging both scientifically and financially. On the contrary, current scientific evidence does support the animal origin of SARS-CoV-2.
Keywords: Laboratory origin, SARS-CoV-2, Type IIS endonuclease, Genomic analysis, Engineering
1. Introduction
Understanding the origin of SARS-CoV-2 is important for the reconstruction of viral transmission routes and preparing for future zoonotic outbreaks (Bloom et al., 2021; Singh and Yi, 2021; Vineis and Salmaso, 2021). There are two main hypotheses about the origin of SARS-CoV-2: animal origin and laboratory origin. The former states that SARS-CoV-2 emerged from a natural source with zoonotic spillover mostly based on phylogenetic and epidemiological analyses (Andersen et al., 2020; Holmes et al., 2021; Pekar et al., 2022), and the latter is mostly discussed in newspapers and social media and states that the virus is a product of bioengineering and leaked from the laboratory at the Wuhan Institute of Virology (WIV) (Gorski, 2021; Maxmen, 2021; Maxmen and Mallapaty, 2021). Increasing evidence is supporting the zoonotic origin, however, the lab origin hypothesis seems to never go away even no scientific evidence support it. Recently, a preprint paper indicated a synthetic origin of SARS-CoV-2 based on type IIS endonuclease fingerprint analysis of the genome, which quickly revived the laboratory origin hypothesis (Bruttel et al., 2022).
2. Endonuclease fingerprint does not indicate a synthetic origin of SARS-CoV-2
In this preprint paper, Bruttel et al. investigated the pattern of unique restriction endonuclease recognition sites (BsmBI/BsaI) in the genomes of SARS-CoV-2 and 38 other coronaviruses (Bruttel et al., 2022). BsaI and BsmBI are two commonly used type IIS endonucleases for molecular cloning (i.e., Golden gate assembly) and in vitro genome assembly (IVGA), and those sites are typically removed after the genome is assembled (Donaldson et al., 2008; Potapov et al., 2018). The authors found that the restriction map of SARS-CoV-2 is an anomaly in coronaviruses and concluded that SARS-CoV-2 is more likely a synthetic product in the laboratory rather than natural evolution. However, the conclusion can be biased by an analysis of a limited number of coronavirus genomes and does not consider the dynamics of endonuclease recognition sites during viral evolution. Here, I provided a thorough investigation on the BsmBI/BsaI map in betacoronavirus, alphacoronavirus, and SARS-CoV-2 genomes.
I first collected 1316 betacoronavirus genomes sampled before the COVID-19 pandemic (as of 11/30/2019) and analyzed the number and positions of the two restriction enzyme recognition sites in each genome. Restriction enzyme recognition site scanning showed that all the betacoronavirus genomes have at least one BsmBI/BsaI site and exhibit a highly diverse pattern (Fig. 1 A). The number of BsmBI/BsaI sites ranges from 1 to 18 (median 9), leading to 2 to 19 DNA fragments with sizes of 4–29,687 bp after digestion with the two restriction enzymes. More than 80% (80.5%) of betacoronavirus genomes have at least 9 BsmBI/BsaI sites, leading to ≥ 10 fragments after digestion. Using the IVGA fingerprint criteria for a synthetic virus in the paper (Bruttel et al., 2022): digestion with BsmBI/BsaI resulting in 5–8 fragments (i.e., 4–7 BsmBI/BsaI sites) with sticky ends and the largest fragment smaller than 8000 bp, 14 genomes were found to meet their criteria with fragment sizes 200–7923 bp, which is similar to the 645–7578 bp in SARS-CoV-2. Two more genomes were found with six BsmBI/BsaI sites, producing seven 900-8500 bp fragments. The BsmBI/BsaI profiles of all the 16 genomes deviate from the majority of betacoronavirus genomes and were sampled from human, bats, dromedary camels, and wild swine from 1970 to 2019 in China, Nigeria, and the USA (Fig. 1A, bottom).
Fig. 1.
SARS-CoV-2 BsmBI/BsaI restriction map is not the only outlier in beta- and alpha-coronavirus genomes. (A–B) BsmBI/BsaI restriction maps for betacoronaviruses before the COVID-19 pandemic (total 1316 genomes as of 11/30/2019) (A) and alphacoronaviruses (total 1378 as of 10/31/2022) (B). Top: Genomes with BsmBI/BsaI sites deviated from the majority were colored. All those genomes have 4–7 BsmBI/BsaI sites. Grey points represent genomes with BsmBI/BsaI map that is unmet with the criteria. Bottom: metadata including accession number, sampling location, sample collection date, host, and fragment size after digestion.
Similar results were also found in alphacoronavirus. There are 1378 genomes published as of 10/31/2022, of which 97.82% genomes (1,348) have 1–19 BsmBI or BsaI restriction sites (median 10). And 28 alphacoronavirus genomes were found to meet the IVGA criteria: 5–8 fragments and maximum size <8000 bp after BsmBI/BsaI digestion. Using a stricter criterion for fragment size 650–8000 bp, there were still 10 genomes sampled from human, bats, wolfs, and cat from 2011 to 2019 in multiple countries (Fig. 1B). Together, those results showed that the pattern of BsamBI/BsaI restriction enzyme recognition sites in alphacoronavirus and betacoronavirus is very diverse, and SARS-CoV-2 is not the only outlier in terms of the number and position of the two endonuclease sites. The identified about 40 coronavirus genomes with 4–7 BsmBI/BsaI restriction sites and fragment sizes <8000 bp serve as the counterexamples for the synthetic origin hypothesis based on endonuclease fingerprints in Bruttel et al.'s paper.
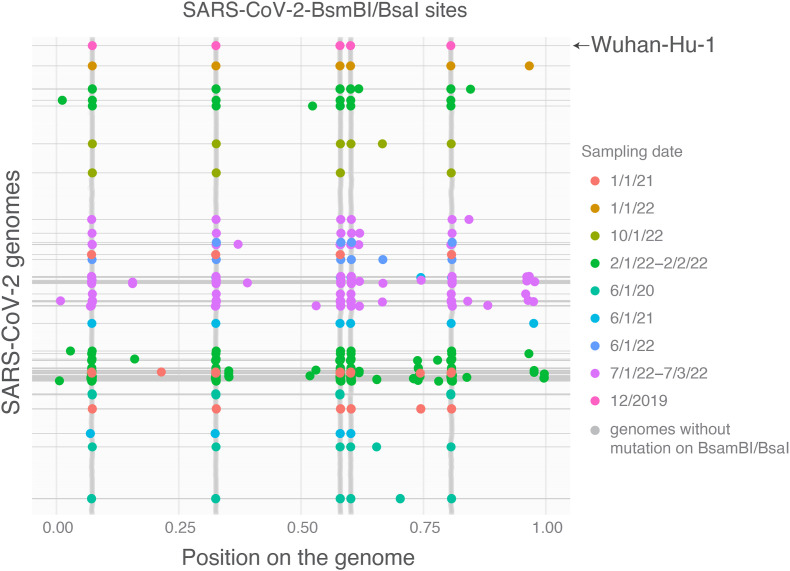
The sequence must be stable to be considered as a fingerprint. To further explore whether endonuclease sites (BsmBI/BsaI) are conserved in SARS-CoV-2 genome, I randomly analyzed 19,250 complete SARS-CoV-2 genomes sampled from 6/1/20 to 10/1/22. As shown in Fig. 2 , the BsmBI/BsaI sites in SARS-CoV-2 genomes are dynamic. New BsmBI/BsaI sites were observed in multiple genomes and distributed throughout the genome. Multiple genomes with missing BsmBI/BsaI sites were also found after removing genomes with ‘N’ in the sequence. These results showed that this endonuclease map is not highly conserved and dynamic in SARS-CoV-2 and reflects its evolutionary diversity in alpha- and betacoronavirus (Fig. 1). Thus, the pattern of BsmBI/BsaI sites is not appropriate to indicate the origin of SARS-CoV-2.
Fig. 2.
Mutations in SARS-CoV-2 result in new or missing BsmBI/BsaI restriction sites throughout the genome. Complete SARS-CoV-2 genomes (19,250) were randomly downloaded from GISAID (https://gisaid.org/) based on the sample collection date from 6/1/2020 to 10/1/2022. Genomes with 6 or <5 BsmBI/BsaI recognition sites were colored based on their sampling dates. The original SARS-CoV-2 Wuhan-Hu-1 genome has 5 BsmBI/BsaI recognition sites. Only 1188 genomes without mutations in BsmBI/BsaI were shown (grey color). Genomes with ‘N’ in the sequence were removed to avoid potential sequencing quality issues for those with less than 5 BsmBI/BsaI recognition sites.
3. Efforts to engineer a SARS-CoV-2 in the laboratory
Virus engineering has been performed for generating recombinant viruses such as using DNA shuffling for chimeric adeno-associated virus (Li and Samulski, 2020; Maheshri et al., 2006), inserting heterogeneous genes or deleting/mutating viral genes for functional research (Cockrell et al., 2018; Guirakhoo et al., 2000; Zeng et al., 2016), and replacing with counterparts from closely related viruses for gain-of-function study or vaccine development (Chen et al., 2022; Donaldson et al., 2008). All these studies were based on a library of viral strains with known genetic diversity or limited modifications on the viral genome, which is far from engineering a new virus.
The challenge of engineering a new virus is not about the approach or tool for genome assembly but is generating a sizable mutant library and developing an efficient high-throughput screening system to identify the most infectious clones. Taking the RaTG13 as an example, it shares 96.1% homology to SARS-CoV-2 but differs by 1182 nucleotides. It is practically impossible to generate a diverse mutation library with a size of 51,182 (∼10,800) since each locus could have four substitutions and one index (deletion or insertion), under the condition that we have the prior knowledge about the importance of those 1182 loci in a ∼30,000 bp genome. Directed evolution such as DNA shuffling (Lehtonen et al., 2015; Meyer et al., 2014) and random mutagenesis (Copp et al., 2014; Gruet et al., 2012; Iqbal and Sadaf, 2022; Nannemann et al., 2011) is currently the most efficient approach to discover functional variants through gene diversification and screening (Bratulic and Badran, 2017; Iqbal and Sadaf, 2022; Packer and Liu, 2015; Wang et al., 2021), however, the reported mutation library size is typically smaller than 1010 (Bratulic and Badran, 2017; Currin et al., 2015; Patwardhan et al., 2009). Library screening with such a magnitude, even if we assume 99.9% of mutants are deleterious, also requires an efficient screening and validation system (Currin et al., 2015). Development of such an efficient screening system is challenging and highly desired for antiviral drug discovery. Most importantly, we do not have a good understanding of the importance of each nucleotide in the genome to viral infectiousness.
The efforts needed for creating a new virus can also be estimated by comparing SARS-CoV-2 and its variants. There are about 58 amino acid (aa) changes in Omicron compared to the original SARS-CoV-2 strain Wuhan-Hu-1, with 35 aa changes in the spike gene. On the other hand, the Wuhan-Hu-1 strain has 153 aa variations (33 in the spike gene) compared to RaTG13, which is one of the closest relatives of SARS-CoV-2 and discovered by the team at the Wuhan Institute of Virology (Fig. 3 ). The number of aa differences in the spike gene is similar between RaTG13 and Wuhan-Hu-1 compared to that between Omicron BA.1 and Wuhan-Hu-1. However, it has taken about 2 years and 250 million individual infections for the Omicron variant to emerge from the first SARS-CoV-2 strain. The emergence of Omicron variant may be impacted by vaccine-related selection pressure, but this estimation is still reasonable using Alpha variant which emerged in December 2020 when there was no vaccine on the market. There are 10 aa differences in the spike gene between SARS-CoV-2 and Alpha variant, which took about one year and 80 million COVID-19 cases to emerge. This ratio between the number of total cases and aa variations (80 million cases for 10 aa) in the Alpha variant is similar to the ratio (250 million cases for 35 aa differences) in Omicron variant. Thus, it is reasonable to believe that it needs at least similar number of experimental iterations to generate a new virus like SARS-CoV-2 from RaTG13 (Fig. 3).
Fig. 3.
The number of mutations in Omicron BA.1 variant could inform the workload needed for creating SARS-CoV-2 from RaTG13? Wuhan-Hu-1 is the first SARS-CoV-2 genome discovered in December 2019. The y-axis is the number of amino acid differences between Wuhan-Hu-1 and RaTG13, and between Wuhan-Hu-1 and Omicron BA.1 (accession: OM283602). There are 33 aa differences in the spike gene for the former comparison and 35 aa differences for the later comparison, however, it took about 250 million COVID-19 infections and 2 years for Omicron to emerge.
The laboratory consumable cost for a ∼250 million screening and testing experiment would easily exceed 10 billion dollars if assuming $40 per screening including costs for nucleotide synthesis, PCR amplification, purification of bacterial/yeast artificial chromosomes vector, fragment ligation/assembly, recombinant virus transduction, mammalian cell culture, virus rescue and titration for infectivity analysis. The experimentation is laborious and time-consuming and requires sophisticated techniques in molecular biology, cell biology, and virology. As a comparison to this crude estimate ($40 per clone), one recombinant lentivirus production takes at least $350 and another $150 for titration (“Custom Lentivirus Production - Kerafast,“; “Lentivirus Costs,”). The total funding, including public budget, business income, and other sources, that Wuhan Institute of Virology received per year was around 100 million dollars from 2020 to 2022 (“Public information from Wuhan Institute of Virology, CAS.“). In other words, the cost of creating a SARS-CoV-2 would require the institute's full investment for at least 100 years.
The other approach to create SARS-CoV-2 is by serial passaging using cell culture or animals. This also seems unlikely as discussed in other papers (Garry, 2022a; Holmes et al., 2021). The first challenge is that there was no live and closely-related virus isolated, at least based on published results. The RaTG13 genome was assembled from metagenomic sequencing of a bat fecal swab (Ge et al., 2016; Zhou et al., 2020), and the genome is incomplete, missing the 5′ and 3′ UTRs (untranslated region). Thus, there is no evidence that the sequence of RaTG13 is from one single virus or is biologically functional (Frutos et al., 2022a, 2022b). Second, the hypothesis based on serial passaging is seriously problematic because it assumes that the virus would directly evolve into being more infectious. A more common result is that the virus will be more adapted to the cell line or animals used for passaging and becomes less virulent to the original host (Badgett et al., 2002; Ebert, 1998). Even if we assume that there is an ‘imaginary’ live RaTG13 virus in the WIV laboratory and the evolution goes as we expect, the time for serial passaging to obtain SARS-CoV-2 from RaTG13 is also tremendous. The measured spontaneous mutation rate of SARS-CoV-2 during experimental evolution is 1.3x10−6 per base per infection cycle, which is equivalent to 0.039 mutations per infection cycle (Amicone et al., 2022). One infection cycle in cell culture is typically 1–3 days depending on the inoculated viral MOI (multiplicity of infection) (Amicone et al., 2022; Hasler et al., 2021; Wurtz et al., 2021). So, to generate directed mutations on those 1182 nucleotides in RaTG13, we would need at least 121,230 days (332 years, equals to 1182/0.039*4) by serial cell passaging. The challenge for animal-based serial passaging should be higher than the cell culture-based approach because of the longer viral incubation time and workload related to animal care. Thus, it is unlikely to obtain SARS-CoV-2 from serial passaging.
4. Animal origin of SARS-CoV-2
The animal origin of SARS-CoV-2 has been widely discussed and summarized in detail elsewhere (Andersen et al., 2020; Domingo, 2021, 2022; Holmes et al., 2021; Lytras et al., 2021; Shi, 2021; Wu et al., 2021), and further supported by recent progress. Temmam et al. collected bat samples in northern Laos (Southeast Asia) and discovered coronaviruses including BANAL-52 in Rhinolophus malayanus, which has a higher nucleotide identity (96.8%) with SARS-CoV-2 genome, compared to the 96.1% of RaTG13 (Temmam et al., 2022). Furthermore, the receptor-binding domains of those recently identified bat coronavirus genomes differ from that of SARS-CoV-2 by only 1–2 residues and have high binding affinity to human ACE2 receptor for viral infection. Although no furin cleavage site (FCS) was discovered in those genomes, the sequence recombination analysis did show a mosaic genome of SARS-CoV-2 from multiple donors (Temmam et al., 2022). Further analysis by Robert F. Garry showed that the SARS-CoV-2 FCS is not a product of bioengineering (Garry, 2022b).
A little earlier, four new SARS-CoV-2 related viral genomes, including RpYN06 in R. pusillus sharing 94.48% identity to SARS-CoV-2, were also identified in rhinolophid bats sampled from May 2019 to November 2020 by another research group (Zhou et al., 2021). These independent discoveries of close relatives of SARS-CoV-2 in different geographic locations suggest the high genomic diversity of betacoronavirus, which is also revealed in this study and elsewhere (Anthony et al., 2017; Ar Gouilh et al., 2018; Maganga et al., 2020; Temmam et al., 2022; Zhou et al., 2021). With the increasing discovery of new betacoronaviruses, there may be multiple SARS-CoV linages (SARS-CoV-x) harboring an S1/S2 FCS insertion in the genome.
Most recently, Wang et al. discovered a high frequency of mammalian-associated viral co-infections and identified 12 viruses that are shared among different bat species by meta-transcriptomic analysis of 149 individual bat samples in Yunnan, China (Wang et al., 2022). The authors also found two coronaviruses closely related to SARS-CoV-2 (92%–93% genetic identities), with only five amino acid differences in the receptor-binding domain of one genome compared to the Wuhan-Hu-1 strain. These findings indicate that viral co-infections and spillover are common in bats, which explains the high recombinant events in coronavirus and points to the origin of SARS-CoV-2 from recombinational exchanges among multiple related genomes (Li et al., 2020; Pollett et al., 2021; Temmam et al., 2022; Turakhia et al., 2022).
5. Conclusion
In summary, I analyzed the pattern of Type IIS restriction enzyme recognition sites (BsamBI/BsaI) in all the alphacoronavirus and non-SARS-CoV-2 betacoronavirus. Results showed that the restriction pattern is very diverse for alpha and betacoronaviruses, and the pattern of SARS-CoV-2 is not the only anomaly. Moreover, the BsamBI/BsaI map in SARS-CoV-2 is dynamic and new sites have emerged throughout the genome. Given the number of total genetic variations of SARS-CoV-2 and its closest relatives, it is highly unlikely to create a synthetic virus both scientifically and financially. Although it is still unclear when, where, and how SARS-CoV-2 was transmitted into the human population, current scientific evidence does support the animal origin potentially from genomic recombination events.
Importantly, there is no scientific evidence reported to support the laboratory origin hypothesis except speculation. The conspiracy ‘theories’ on SARS-CoV-2 origin are not unique in the history of virology research, and also happened for other epidemic viruses (Garry, 2022a; Klofstad et al., 2019; Li et al., 2020; Pollett et al., 2021; Ross et al., 2006; Temmam et al., 2022; Turakhia et al., 2022). Although it is understandable for the fear and anxiety of viral epidemics, virologists and virology research are becoming the victims of those rumors and conspiratorial ideation. It is not unwise to acknowledge our limited understanding of the nature, but impatience and a closed mind are.
Credit author statement
Fuqing Wu: Conceptualization, Data analysis, Writing.
Funding sources
This work is supported by funding from the Center of Infectious Diseases at UTHealth, the UT System Rising STARs award, and the Texas Epidemic Public Health Institute (TEPHI).
Declaration of competing interest
The author graduated from Wuhan Institute of Virology in 2012 for the Master of Science degree. Other than that, the author declares no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
I am grateful to colleagues Dr. Lu-Yu Hwang and Dr. Blake Hanson (UTHealth at Houston), and Amy Xiao (Massachusetts Institute of Technology) for their helpful discussion and reading the text.
Handling Editor: Dr Jose L Domingo
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2023.115481.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
The data and code is submitted with this manuscript (Supplemental Material).
References
- Amicone M., Borges V., Alves M.J., Isidro J., Zé-Zé L., Duarte S., Vieira L., Guiomar R., Gomes J.P., Gordo I. Mutation rate of SARS-CoV-2 and emergence of mutators during experimental evolution. Evolution, Medicine, and Public Health. 2022;10:142–155. doi: 10.1093/emph/eoac010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony S.J., Johnson C.K., Greig D.J., Kramer S., Che X., Wells H., Hicks A.L., Joly D.O., Wolfe N.D., Daszak P., Karesh W., Lipkin W.I., Morse S.S., PREDICT Consortium. Mazet J.A.K., Goldstein T. Global patterns in coronavirus diversity. Virus Evol. 2017;3:vex012. doi: 10.1093/ve/vex012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ar Gouilh, M., Puechmaille, S.J., Diancourt, L., Vandenbogaert, M., Serra-Cobo, J., Lopez Roïg, M., Brown, P., Moutou, F., Caro, V., Vabret, A., Manuguerra, J.-C., EPICOREM consortium, 2018. SARS-CoV related Betacoronavirus and diverse Alphacoronavirus members found in western old-world. Virology 517, 88–97. 10.1016/j.virol.2018.01.014. [DOI] [PMC free article] [PubMed]
- Badgett M.R., Auer A., Carmichael L.E., Parrish C.R., Bull J.J. Evolutionary dynamics of viral attenuation. J. Virol. 2002;76:10524–10529. doi: 10.1128/JVI.76.20.10524-10529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom J.D., Chan Y.A., Baric R.S., Bjorkman P.J., Cobey S., Deverman B.E., Fisman D.N., Gupta R., Iwasaki A., Lipsitch M., Medzhitov R., Neher R.A., Nielsen R., Patterson N., Stearns T., van Nimwegen E., Worobey M., Relman D.A. Investigate the origins of COVID-19. Science. 2021;372:694. doi: 10.1126/science.abj0016. 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratulic S., Badran A.H. Modern methods for laboratory diversification of biomolecules. Curr. Opin. Chem. Biol. 2017;41:50–60. doi: 10.1016/j.cbpa.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruttel V., Washburne A., VanDongen A. 2022. Endonuclease Fingerprint Indicates a Synthetic Origin of SARS-CoV-2. [DOI] [Google Scholar]
- Chen D.-Y., Kenney D., Chin C.V., Tavares A.H., Khan N., Conway H.L., Liu G., Choudhary M.C., Gertje H.P., O'Connell A.K., Kotton D.N., Herrmann A., Ensser A., Connor J.H., Bosmann M., Li J.Z., Gack M.U., Baker S.C., Kirchdoerfer R.N., Kataria Y., Crossland N.A., Douam F., Saeed M. 2022. Role of spike in the pathogenic and antigenic behavior of SARS-CoV-2 BA.1 Omicron. bioRxiv 2022.10.13.512134. [DOI] [Google Scholar]
- Cockrell A.S., Johnson J.C., Moore I.N., Liu D.X., Bock K.W., Douglas M.G., Graham R.L., Solomon J., Torzewski L., Bartos C., Hart R., Baric R.S., Johnson R.F. A spike-modified Middle East respiratory syndrome coronavirus (MERS-CoV) infectious clone elicits mild respiratory disease in infected rhesus macaques. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-28900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp J.N., Hanson-Manful P., Ackerley D.F., Patrick W.M. Error-prone PCR and effective generation of gene variant libraries for directed evolution. Methods Mol. Biol. 2014;1179:3–22. doi: 10.1007/978-1-4939-1053-3_1. [DOI] [PubMed] [Google Scholar]
- Currin A., Swainston N., Day J., P., B. Kell, D. Synthetic biology for the directed evolution of protein biocatalysts: navigating sequence space intelligently. Chem. Soc. Rev. 2015;44:1172–1239. doi: 10.1039/C4CS00351A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custom Lentivirus Production - Kerafast https://www.kerafast.com/item/755/custom-lentivirus-production [WWW Document], n.d. URL. accessed 2.7.23.
- Domingo J.L. An updated review of the scientific literature on the origin of SARS-CoV-2. Environ. Res. 2022;215 doi: 10.1016/j.envres.2022.114131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo J.L. What we know and what we need to know about the origin of SARS-CoV-2. Environ. Res. 2021;200 doi: 10.1016/j.envres.2021.111785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson E.F., Yount B., Sims A.C., Burkett S., Pickles R.J., Baric R.S. Systematic assembly of a full-length infectious clone of human coronavirus NL63. J. Virol. 2008;82:11948–11957. doi: 10.1128/JVI.01804-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert D. Experimental evolution of parasites. Science. 1998;282:1432–1435. doi: 10.1126/science.282.5393.1432. [DOI] [PubMed] [Google Scholar]
- Frutos R., Javelle E., Barberot C., Gavotte L., Tissot-Dupont H., Devaux C.A. Origin of COVID-19: dismissing the Mojiang mine theory and the laboratory accident narrative. Environ. Res. 2022;204 doi: 10.1016/j.envres.2021.112141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos R., Pliez O., Gavotte L., Devaux C.A. There is no “origin” to SARS-CoV-2. Environ. Res. 2022;207 doi: 10.1016/j.envres.2021.112173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry R.F. The evidence remains clear: SARS-CoV-2 emerged via the wildlife trade. Proc. Natl. Acad. Sci. U. S. A. 2022;119 doi: 10.1073/pnas.2214427119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry R.F. SARS-CoV-2 furin cleavage site was not engineered. Proc. Natl. Acad. Sci. U. S. A. 2022;119 doi: 10.1073/pnas.2211107119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X.-Y., Wang N., Zhang W., Hu B., Li B., Zhang Y.-Z., Zhou J.-H., Luo C.-M., Yang X.-L., Wu L.-J., Wang B., Zhang Y., Li Z.-X., Shi Z.-L. Coexistence of multiple coronaviruses in several bat colonies in an abandoned mineshaft. Virol. Sin. 2016;31:31–40. doi: 10.1007/s12250-016-3713-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski D. 2021. The Rise and Fall of the Lab Leak Hypothesis for the Origin of SARS-CoV-2 | Science-Based Medicine.https://sciencebasedmedicine.org/the-rise-and-fall-of-the-lab-leak-hypothesis-for-the-origin-of-sars-cov-2/ [WWW Document]. URL. accessed 11.27.22. [Google Scholar]
- Gruet A., Longhi S., Bignon C. One-step generation of error-prone PCR libraries using Gateway® technology. Microb. Cell Factories. 2012;11:14. doi: 10.1186/1475-2859-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirakhoo F., Weltzin R., Chambers T.J., Zhang Z.-X., Soike K., Ratterree M., Arroyo J., Georgakopoulos K., Catalan J., Monath T.P. Recombinant chimeric yellow fever-dengue type 2 virus is immunogenic and protective in nonhuman primates. J. Virol. 2000;74:5477–5485. doi: 10.1128/jvi.74.12.5477-5485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler F., Duda A., Kündig T.M., Johansen P. A tissue culture infectious dose-derived protocol for testing of SARS-CoV-2 neutralization of serum antibodies on adherent cells. STAR Protoc. 2021;2 doi: 10.1016/j.xpro.2021.100824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E.C., Goldstein S.A., Rasmussen A.L., Robertson D.L., Crits-Christoph A., Wertheim J.O., Anthony S.J., Barclay W.S., Boni M.F., Doherty P.C., Farrar J., Geoghegan J.L., Jiang X., Leibowitz J.L., Neil S.J.D., Skern T., Weiss S.R., Worobey M., Andersen K.G., Garry R.F., Rambaut A. The origins of SARS-CoV-2: a critical review. Cell. 2021;184:4848–4856. doi: 10.1016/j.cell.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal Z., Sadaf S. Forty years of directed evolution and its continuously evolving technology toolbox: a review of the patent landscape. Biotechnol. Bioeng. 2022;119:693–724. doi: 10.1002/bit.28009. [DOI] [PubMed] [Google Scholar]
- Klofstad C.A., Uscinski J.E., Connolly J.M., West J.P. What drives people to believe in Zika conspiracy theories? Palgrave Commun. 2019;5:1–8. doi: 10.1057/s41599-019-0243-8. [DOI] [Google Scholar]
- Lehtonen S.I., Taskinen B., Ojala E., Kukkurainen S., Rahikainen R., Riihimäki T.A., Laitinen O.H., Kulomaa M.S., Hytönen V.P. Efficient preparation of shuffled DNA libraries through recombination (Gateway) cloning. Protein Eng. Des. Sel. 2015;28:23–28. doi: 10.1093/protein/gzu050. [DOI] [PubMed] [Google Scholar]
- Lentivirus Costs n.d. SPARC drug discovery. https://lab.research.sickkids.ca/sparc-drug-discovery/services/lentivirus/lentivirus-costs/ URL. accessed 2.7.23.
- Li C., Samulski R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020;21:255–272. doi: 10.1038/s41576-019-0205-4. [DOI] [PubMed] [Google Scholar]
- Li X., Giorgi E.E., Marichannegowda M.H., Foley B., Xiao C., Kong X.-P., Chen Y., Gnanakaran S., Korber B., Gao F. Emergence of SARS-CoV-2 through recombination and strong purifying selection. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abb9153. eabb9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytras S., Xia W., Hughes J., Jiang X., Robertson D.L. The animal origin of SARS-CoV-2. Science. 2021;373:968–970. doi: 10.1126/science.abh0117. [DOI] [PubMed] [Google Scholar]
- Maganga G.D., Pinto A., Mombo I.M., Madjitobaye M., Mbeang Beyeme A.M., Boundenga L., Ar Gouilh M., N'Dilimabaka N., Drexler J.F., Drosten C., Leroy E.M. Genetic diversity and ecology of coronaviruses hosted by cave-dwelling bats in Gabon. Sci. Rep. 2020;10:7314. doi: 10.1038/s41598-020-64159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshri N., Koerber J.T., Kaspar B.K., Schaffer D.V. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat. Biotechnol. 2006;24:198–204. doi: 10.1038/nbt1182. [DOI] [PubMed] [Google Scholar]
- Maxmen A. Divisive COVID ‘lab leak’ debate prompts dire warnings from researchers. Nature. 2021;594:15–16. doi: 10.1038/d41586-021-01383-3. [DOI] [PubMed] [Google Scholar]
- Maxmen A., Mallapaty S. The COVID lab-leak hypothesis: what scientists do and don't know. Nature. 2021;594:313–315. doi: 10.1038/d41586-021-01529-3. [DOI] [PubMed] [Google Scholar]
- Meyer A.J., Ellefson JaredW., Ellington A.D. Library generation by gene shuffling. Curr. Protoc. Mol. Biol. 2014;105 doi: 10.1002/0471142727.mb1512s105. Unit-15.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nannemann D.P., Birmingham W.R., Scism R.A., Bachmann B.O. Assessing directed evolution methods for the generation of biosynthetic enzymes with potential in drug biosynthesis. Future Med. Chem. 2011;3:809–819. doi: 10.4155/fmc.11.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer M.S., Liu D.R. Methods for the directed evolution of proteins. Nat. Rev. Genet. 2015;16:379–394. doi: 10.1038/nrg3927. [DOI] [PubMed] [Google Scholar]
- Patwardhan R.P., Lee C., Litvin O., Young D.L., Pe’er D., Shendure J. High-resolution analysis of DNA regulatory elements by synthetic saturation mutagenesis. Nat. Biotechnol. 2009;27:1173–1175. doi: 10.1038/nbt.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekar J.E., Magee A., Parker E., Moshiri N., Izhikevich K., Havens J.L., Gangavarapu K., Malpica Serrano L.M., Crits-Christoph A., Matteson N.L., Zeller M., Levy J.I., Wang J.C., Hughes S., Lee J., Park H., Park M.-S., Ching Zi Yan K., Lin R.T.P., Mat Isa M.N., Noor Y.M., Vasylyeva T.I., Garry R.F., Holmes E.C., Rambaut A., Suchard M.A., Andersen K.G., Worobey M., Wertheim J.O. The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2. Science. 2022;377:960–966. doi: 10.1126/science.abp8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollett S., Conte M.A., Sanborn M., Jarman R.G., Lidl G.M., Modjarrad K., Maljkovic Berry I. A comparative recombination analysis of human coronaviruses and implications for the SARS-CoV-2 pandemic. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-96626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapov V., Ong J.L., Kucera R.B., Langhorst B.W., Bilotti K., Pryor J.M., Cantor E.J., Canton B., Knight T.F., Evans T.C., Jr., Lohman G.J.S. Comprehensive profiling of four base overhang ligation fidelity by T4 DNA ligase and application to DNA assembly. ACS Synth. Biol. 2018;7:2665–2674. doi: 10.1021/acssynbio.8b00333. [DOI] [PubMed] [Google Scholar]
- Public information from Wuhan Institute of Virology http://www.whiov.cas.cn/xxgk_160268/xxgkzn_160270/ CAS. [WWW Document], n.d. URL. accessed 11.4.22.
- Ross M.W., Essien E.J., Torres I. Conspiracy beliefs about the origin of HIV/AIDS in four racial/ethnic groups. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2006;41:342–344. doi: 10.1097/01.qai.0000209897.59384.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z.-L. Origins of SARS-CoV-2: focusing on science. Infectious Diseases & Immunity. 2021;1:3–4. doi: 10.1097/ID9.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D., Yi S.V. On the origin and evolution of SARS-CoV-2. Exp. Mol. Med. 2021;53:537–547. doi: 10.1038/s12276-021-00604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmam S., Vongphayloth K., Baquero E., Munier S., Bonomi M., Regnault B., Douangboubpha B., Karami Y., Chrétien D., Sanamxay D., Xayaphet V., Paphaphanh P., Lacoste V., Somlor S., Lakeomany K., Phommavanh N., Pérot P., Dehan O., Amara F., Donati F., Bigot T., Nilges M., Rey F.A., van der Werf S., Brey P.T., Eloit M. Bat coronaviruses related to SARS-CoV-2 and infectious for human cells. Nature. 2022;604:330–336. doi: 10.1038/s41586-022-04532-4. [DOI] [PubMed] [Google Scholar]
- Turakhia Y., Thornlow B., Hinrichs A., McBroome J., Ayala N., Ye C., Smith K., De Maio N., Haussler D., Lanfear R., Corbett-Detig R. Pandemic-scale phylogenomics reveals the SARS-CoV-2 recombination landscape. Nature. 2022;609:994–997. doi: 10.1038/s41586-022-05189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vineis P., Salmaso S. The origin of sars-CoV-2: why it matters. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.719914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Jing, Pan Y., Yang L., Yang W., Luo C., Wang Juan, Kuang G., Wu W., Gou Q., Xin G., Li B., Luo H., Chen Y., Shu Y., Guo D., Gao Z.-H., Liang G., Li J., Holmes E.C., Feng Y., Shi M. 2022. Individual Bat Viromes Reveal the Co-infection, Spillover and Emergence Risk of Potential Zoonotic Viruses. [DOI] [Google Scholar]
- Wang Y., Xue P., Cao M., Yu T., Lane S.T., Zhao H. Directed evolution: methodologies and applications. Chem. Rev. 2021;121:12384–12444. doi: 10.1021/acs.chemrev.1c00260. [DOI] [PubMed] [Google Scholar]
- Wu Z., Jin Q., Wu G., Lu J., Li M., Guo D., Lan K., Feng L., Qian Z., Ren L., Tan W., Xu W., Yang W., Wang J., Wang C. SARS-CoV-2’s origin should be investigated worldwide for pandemic prevention. Lancet. 2021;398:1299–1303. doi: 10.1016/S0140-6736(21)02020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz N., Penant G., Jardot P., Duclos N., La Scola B. Culture of SARS-CoV-2 in a panel of laboratory cell lines, permissivity, and differences in growth profile. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:477–484. doi: 10.1007/s10096-020-04106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L.-P., Gao Y.-T., Ge X.-Y., Zhang Q., Peng C., Yang X.-L., Tan B., Chen J., Chmura A.A., Daszak P., Shi Z.-L. Bat severe acute respiratory syndrome-like coronavirus WIV1 encodes an extra accessory protein, ORFX, involved in modulation of the host immune response. J. Virol. 2016;90:6573–6582. doi: 10.1128/JVI.03079-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Ji J., Chen X., Bi Y., Li J., Wang Q., Hu T., Song H., Zhao R., Chen Y., Cui M., Zhang Y., Hughes A.C., Holmes E.C., Shi W. Identification of novel bat coronaviruses sheds light on the evolutionary origins of SARS-CoV-2 and related viruses. Cell. 2021;184:4380–4391.e14. doi: 10.1016/j.cell.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and code is submitted with this manuscript (Supplemental Material).