Abstract
Introduction
Many medicinal plants have been introduced in Persian medicine references for various respiratory disorders. Considering the growing interest in herbal medicines, this review aimed to introduce medicinal herbs recommended by Persian Medicine (PM) references for respiratory diseases and to discuss their activity against respiratory viruses.
Methods
The medicinal plants recommended for respiratory disorders were extracted from the main PM textbooks. Subsequently, their activity against respiratory viruses was systematically investigated via queries of scientific databases.
Results
Searching PM references for medicinal plants used in the management of respiratory disorders yielded 45 results. Of them, 18 possess antiviral activity against respiratory viruses. There were 29 in vitro studies (including studies on human cell lines) and 5 in vivo studies.
Conclusion
This research demonstrated that many of the medicinal plants mentioned for the respiratory diseases in PM have considerable activity against respiratory viruses. However, human studies regarding the reported medicinal plants are scarce.
1. Introduction
Viral respiratory infections are one of the most prevalent causes of medical consultations globally [1]. Known for a variety of clinical pictures, from self-limited upper respiratory tract diseases to life-threatening ones [2, 3], these infections deeply influence the quality of life and have a noticeable economic burden [4–6]. Additionally, the World Health Organization reports respiratory infections as the main reason for mortality among all infectious diseases [7]. Respiratory syncytial virus, influenza virus, metapneumovirus, parainfluenza viruses, adenoviruses, bocaviruses, rhinoviruses, and coronaviruses are respiratory viruses that are associated with epidemic or endemic infections in all continents [8]. Moreover, several viruses of the herpesvirus family, including cytomegalovirus, herpes simplex, varicella-zoster virus, human herpesvirus 6, and Epstein-Barr virus, may also be responsible for respiratory disease in immunocompromised individuals [8, 9]. The world is experiencing the third pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the present century [10]. This novel coronavirus disease (COVID-19) is currently the most serious concern for the international community. It is a viral respiratory disease for which no effective treatment has yet been identified [11].
Statistical analysis of studies related to the SARS 2002 outbreak indicated that the integration of traditional Chinese medicine (TCM) with conventional medicine could reduce morbidity and mortality rates as compared with mere conventional therapy [12]. Additionally, various traditional medical systems have brought up the issue of respiratory infections and related treatments [13–17]. Based on the humoral theory, Persian Medicine (PM) is an ancient medical system with multiple options for treating diseases and managing complications [18–21]. Specifically, numerous remedies have been reported in PM references for the treatment of various respiratory disorders, including asthma and pneumonia [22]. Additionally, there are several plausible mechanisms for the antiviral activity of these medicinal plants. Figure 1 shows some of the proposed mechanisms, which may interrupt the coronavirus replication cycle [23–25]. Using herbs rather than contemporary drugs in COVID-19 therapy may have a wide variety of benefits and advantages, from a cheaper price and better worldwide availability to lesser adverse events, a better attitude of the general population towards them, and a decreased demand for conventional drugs and hospitalization [26–28].
Figure 1.
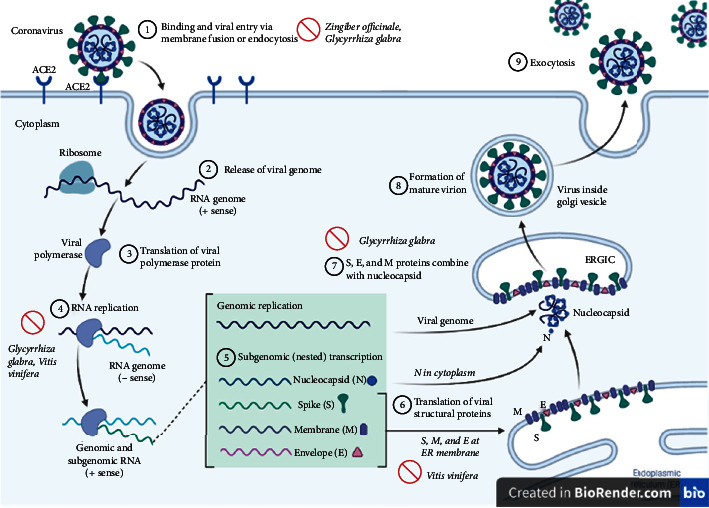
Plausible mechanisms for antiviral activity of medicinal plants, which may interrupt the coronavirus replication cycle.
Considering the global spread of viral respiratory infections, especially COVID-19, and the lack of any proven treatment in many cases, this research aimed to introduce medicinal plants recommended for respiratory diseases in PM and to review their activity against respiratory viruses according to current biomedical literature.
2. Methods
Ketab al-Hawi fi al-Teb (Continens) by Rhazes (9th and 10th centuries), Qanun fi al-Ṭeb (Canon of Medicine) by Avicenna (10th and 11th centuries), Tebb-e Akbari (Akbari's Medicine) by Mohammad Akbar Arzani (18th century), Exir-e Azam (The Great Panacea) by Nazem Jahan (18th and 19th centuries), and Makhzan al-Advieh (Storehouse of Medicaments) by Aghili Shirazi (18th century) are the most important and comprehensive textbooks of PM. They also comprise the references in the Ph.D. program for PM in Iran. Chapters related to respiratory disorders (respiration or tanaffos; lung or shosh, riyah; asthma or rabv; dyspnea with rapid and shallow breathing, cough or sorfeh, sputum or nafth in Persian) were selected and carefully searched for recommended medicinal plants.
The suggested medicinal plants were searched for their scientific and common names in English. Subsequently, medical English and Persian databases including MEDLINE, Scopus, Iranmedex, SID, Magiran, Web of Science, and Google Scholar were systematically searched. Each herb was searched along with keywords including “antivirus,” “coronavirus,” and “COVID-19.”
Two researchers independently screened the articles, reading their abstracts and titles to identify potentially eligible studies. Thereafter, full texts were obtained and read to determine the final included articles. In addition, the references of the retrieved articles were manually searched to identify other potentially eligible studies. Papers published in languages other than English or Persian were excluded. In addition, review articles and conference papers were not included in this systematic review. Moreover, research studies on nonrespiratory viruses and viruses not pathogenic for humans were excluded from the study. Any disagreement was resolved by discussion. The extracted data included plant scientific name, Persian name, English common name, used part of the herb, studied antiviral effect, and study type (including in-vitro, animal, and clinical). It should be noted that each plant's main compounds and route of traditional administration were added based on the PDR for herbal medicines (3rd edition) [29] and Makhzan al-Advieh (Storehouse of Medicaments) by Aghili Shirazi, respectively.
3. Results
Forty-five medicinal plants recommended for respiratory diseases by PM resources were extracted in the first phase of the study. Overall, eighteen of the herbs recommended by PM resources for respiratory diseases have evidence regarding activity against viruses that can cause infectious respiratory disorders. Most of the research studies in this regard were in vitro studies. In addition, most of the mentioned plants were reported to act against influenza viruses (Table 1).
Table 1.
Medicinal plants recommended by Persian medicine for respiratory diseases with antiviral activity against respiratory viruses.
| No | Scientific name | Persian name | English common name | Main compounds | Route of traditional administration | Used part or ingredient of the herb for biomedical research | Targeted virus | Studied cells/animals/populations | Results | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Portulaca oleracea L. | Khorfeh | Common purslane | Flavonoids alkaloids polysaccharides omega-3 fatty acids | Oral/fresh juice | Aqueous extract | Influenza a virus (IAV) (H1N1) | MDCK and A549 cell lines [30] | IAV infection was inhibited at the entry stage | ||
| 2 | Foeniculum vulgare Mill. | Raziyaneh | Fennel | Transanethole fenchon estragole | Oral/decoction | Ethanol extract | Influenza a virus (H5N1) | MDCK cell line [31] | Plaque reduction (82.8%) in 300 μg/ml of plant extract | ||
| 3 | Cydonia oblonga Mill. | Beh | Quince | Cyanogenic glycosides: amygdalin mucilages fatty oil | Oral/paste | Its fruit extract was used for collecting 3-affeoylquinic acid | Influenza virus | Hemagglutination inhibition [32] | Its fruit extract significantly (p < 0.001) inactivated the virus | ||
| 4 | Glycyrrhiza glabra L. | Shirin-Bayan | Licorice | Triterpene saponins glycyrrhetic acid flavonoids isoflavonoids glycyrol | Oral/decoction | Glycyrrhizin | Corona SARS virus | Vero cells [33] | It inhibited virus replication, adsorption, and penetration | ||
| Glycyrrhizin | Corona SARS virus | Vero cells from ATCC (ATCC CCL81) [29] | It inhibited SARS-CoV replication by changing the interaction pattern of virus to the cell receptor | ||||||||
| Glycyrrhizin | Influenza virus | BALB/c mice (8 weeks) and MDCK cell [34] | It protected mice that exposed to a lethal load of virus. “When mice infected with 20 and 10 LD50s of influenza virus were treated with it, 100 and 70% of the mice, respectively, survived over 21 days.” in addition, it has no inhibitory effect on the virus replication (up to forty-eight hours post-infection) in the in-vitro study | ||||||||
| 5 | Ziziphus jujuba Mill. | Annab | Jujube | Triterpene saponins mucilage tannins | Oral/decoction | Betulinic acid | IAV (PR/8) | A549 cells and C57BL/6 mice (6 to 7 weeks of age) [35] | It showed strong antiviral activity against the virus (about 98%) at the concentration of 50 μM and lesser activity against the virus (about 30%) at the concentration 10 μM. Also, the animal study showed that betulinic acid significantly reduced the virus induced pulmonary pathology | ||
| 6 | Cucurbita pepo L. | Kadoo | Pumpkin | Steroids fatty oil unusual amino acids | Oral/decoction | Oil extracted | Parainfluenza virus type-3 | AGMK and MDBK cell lines [36] | It showed selective inhibitory effect against the virus | ||
|
| |||||||||||
| 7 | Cinnamomum camphora (L.) J.Presl | Kafoor | Camphor | D-camphor linalol cineole | Nasal/inhalant Oral/decoction Topical/boiled | Camphecene (from ethanolamine and camphor) | IAV (H1N1) | MDCK cells (ATCC CCL 34) and BALB/c mice (aged 6 to 8 weeks) [35] | It decreased the number of virions fusing their envelopes with endosomal membranes. Camphecene significantly decreased the viral pathogenicity and attenuated the growth fitness in mice lung tissue | ||
| Camphor | IAV (H1N1) | MDCK cells [34] | Camphor blocked the viral ion channel M2. Then, it prevented the proton flow into the virions and its envelope fusion | ||||||||
| Camphor | IAV (H5N1) | MDCK, and U-87 MG cells [37] | Inhibition of viral hemagglutinin in early stages of virus replication | ||||||||
| Camphor derivative 1,7,7-trimethylbicyclo [2.2.1] heptan-2-ylidene-aminoetha 34nol (camphecene) | Influenza virus | MDCK cells (ATCC # CCL-34) and BALB/c mice (aged 6 to 8 weeks) [38] | Suppression of the virus replication it reduced the infectious titer of the virus in mice lung tissue | ||||||||
| 8 | Vigna radiata (L.) R.Wilczek | Maash | Mung bean | Phenolic acids flavoniods tannins | Oral/decoction | Its sprouts' methanol extract | Respiratory syncytial virus (RSV) | Vero and MRC-5 cell lines [39] | It induced IL-6, IL-1, IFN-β, and TNF-α in the studied cell lines | ||
| 9 | Cicer arietinum L. | Nokhod | Chickpea | Proteins globulins fatty acids | Oral/decoction | Methanol extract | Parainfluenza-3 viruses | Madin-Darby bovine kidney and Vero cell lines [40] | Its extract had cytopathogenic inhibitory effect | ||
| 10 | Cinnamomum verum J. Presl | Darchin | Cinnamon | Cinnamaldehyde weiterhin cinnamylacetate, cinnamyl alcohol tannins | Oral/decoction | Synthesized silver nanoparticles using cinnamon | Influenza virus (H7N3) | Vero cells [41] | It was effective against the viral infection. Its effectiveness increased when a pretreatment (before virus introduction to the cells) by it was added (compared with treatment only after infection) | ||
| Hot water extract of the plant | Human respiratory syncytial virus (HRSV) | Both human upper (HEp-2) and lower (A549) respiratory tract cell lines [42] | It dose-dependently inhibited HRSV-induced plaque formation in both cell lines. In addition its efficacy increased when given before infection. It inhibited “F protein production and syncytium formation to interfere with HRSV spreading” | ||||||||
| Essential oils | H1N1 virus | MDCK cells (CCL-34, ATCC) [43] | The essential oil inactivated free-virus particles. It could interfere with virion envelope structures and its entry into the cells | ||||||||
| 11 | Piper nigrum L. | Felfel siah | Black pepper | Sabinene limonene caryophyllene betapinene | Oral/with honey or sugar | Combined methanol/dichloromethane extract of its fruits | Coxsackie virus type B3 (CVB3) | Vascular smooth muscle cells [44] | It had cytopathic inhibition effect | ||
| Chloroform and methanolic extracts | Human para influenza virus (HPIVS) | HeLa cell lines [45] | The extracts had inhibitory effect on the virus | ||||||||
| 12 | Ficus carica L. | Anjir | Fig | Furanocoumarins fruit acids mucilages pectin vitamin B and vitamin C | Oral/boiled with honey | Methanolic, hexanic, ethyl acetate, hexane-ethyl acetate, and chloroformic extracts of the fruit | Echovirus type 11 (ECV-11) and adenovirus (ADV) | ATCC CCL-81 (kidney cells of the African green monkey cercopithecus aethiops) [46] | The hexanic and hexane–ethyl acetate extracts inhibited viruses replication (at 78 mgmL−1.concentration) | ||
|
| |||||||||||
| 13 | Vitis vinifera L. | Maviz | Common grape | Flavonoids anthocyanin vitamin A and vitamin B | Oral/decoction | Tea infusions from grape skins | Influenza virus | MDCK cells [47] | It protected MDCK cells against the virus (at 100 mg/ml concentration) | ||
| Aqueous, ethanol and acetone extracts of grape pomace | Influenza viruses (H5N1) | MDCK cells [48] | Its antiviral effects confirmed using plaque reduction assay | ||||||||
| 14 | Zingiber officinale Roscoe | Zanjebil | Ginger | Zingiberene arcurcumene, β-bisabolene | Oral/decoction, jam | Hot water extracts of fresh and dried ginger fruits | Human respiratory syncytial virus | Human upper (HEp-2) and lower (A549) respiratory tract cell lines [49] | The extract (from fresh fruits) inhibited the virus induced plaque formation in a dose-dependent manner in both cell lines | ||
| 15 | Punica granatum L. | Anar | Pomegranate | Tannins punicalin punicalagin | Oral/boiled with almond oil | Pomegranate polyphenol extract (PPE) | IAV (H3N2) | MDCK cells [50] | It inhibited the virus replication. In addition, “punicalagin blocked replication of the virus RNA, inhibited agglutination of chicken RBC's by the virus and had virucidal effects.” | ||
| Peels' ethyl alcohol extract | IAV | MDCK cells [51] | Its different extract types inhibited thew IAV | ||||||||
| Peels' ethyl alcohol extract | IAV (H1N1; PR8) | MDCK cells [52] | It had inhibitory effects on the adsorption, polymerase activity, RNA replication, and protein expression of the virus | ||||||||
| Peels' ethanol extract | Adenovirus | Hep-2 cell line [53] | The extract was effective against the virus (IC50 of 5.77 mg/mL) | ||||||||
| 16 | Urtica dioica L. | Gazaneh | Common nettle | Histamine serotonin acetylcholine formic acid | Oral/decoction | Urtica dioica agglutinin from its rhizomes | Respiratory syncytial virus (RSV) and IAV | HeLa and MDCK cells [54] | It inhibited the virus-induced cytopathicity | ||
| N-acetylglucosamine-specific stinging nettle lectin | Different SARS-CoV strains | BALB/c mice and Vero cells [55] | Its administration (5 mg/kg) significantly protected animals against a lethal infection. Regarding the in-vitro study, it had inhibitory effects on the virus replication, only if added before its adsorption | ||||||||
| 17 | Mentha × piperita L. | Na'na | Peppermint | Piperitone, β-caryophyllene, germacren D, 1,8-cineole, limonene, diosmin, hesperidin, quercitrin, thymonin, apigenine-7-glucuronide | Nasal/vapor bath oral/decoction | Ethanol extract from its leaves | Respiratory syncytial virus (RSV) | Hep-2 cell line [56] | It had a significant antiviral activity (IC50 of 10.41 lg/mL) | ||
| 18 | Nigella sativa L. | Shooniz | Black seed | Nigellon, thymoquinone, thymohydroquinone, dithymoquinone, thymol, carvacrol, α and β-pinene, d-limonene, d-citronellol, p-cymene | Oral/decoction Topical/oil | Its seeds' ethanolic extract | Corona virus | HeLa-CEACAM1a (HeLa-epithelial carcinoembryonic antigen-related cell adhesion molecule 1a) cells [57] | Its administration had a significant effect on IL-8 level. In addition, it decreased the virus load | ||
Hep-2: human larynx epidermal carcinoma; MDCK: Madin-Darby canine kidney; AGMK: African green monkey kidney, MDBK: Madin-Darby bovine kidney.
4. Discussion
The viral respiratory infection outbreaks promoted the conduct of studies with the purpose of evaluating novel medications, especially natural-based remedies, resulting in the discovery of potential drugs. The effectiveness of various herbs is published as the result of studies designed as case series, clinical trials, and systematic reviews [58–60]. These research studies encouraged further investigations to elucidate the potential of herbal compounds to manage coronavirus infections [61–63].
According to previous records regarding SARS 2002, TCM in combination with routine drugs has been far more effective than conventional therapy alone [12, 60, 61]. TCM physicians prescribed herbal remedies, which are known for their anti-inflammatory, antiviral, and immunomodulatory properties, for better management [12]. Studies have shown that these medicinal plants decrease the mean needed dosage of medications such as corticosteroids in severe cases and also diminish the adverse effects of some drugs. There are some reports that using corticosteroids for managing viral respiratory infections may lead to some adverse events (e.g., the development of fungal infection and femoral head necrosis). According to the results of 24 trials used in a meta-analysis, no long-term side effects due to taking high-dose corticosteroids were reported in integrative treatment (i.e., a combination of herbal drugs and conventional treatment) [61, 64].
It should be noted that some ancient medical systems, such as PM, TCM, and Unani medicine, have individualized approaches (phenotype-based personalized medicine) to treatment. Traditional practitioners consider gender, age, season, comorbidities, and many other patient characteristics to diagnose and manage different diseases [12, 65–67].
Human society is currently struggling with the COVID-19 pandemic, and no efficient drugs have been identified as of yet. Therefore, emphasis is placed on preventive measures and symptomatic therapies [68]. Regarding this, numerous research studies have been done to evaluate the safety and efficacy of preventive, therapeutic, supportive, or rehabilitative medicaments recommended in various traditional medical systems [15, 24, 69, 70]. The emphasis of the World Health Organization on the integration of traditional, complementary, and alternative medicine in the conventional health system and the growing interest in natural products for the management of diseases highlight the necessity of studies on different aspects of traditional medicines to reinforce the scientific evidence basis for natural remedies [19, 71].
Several research studies have previously been done to assess the efficacy of medicinal herbs reported in folklore or traditional medicine systems of different countries against various viral or bacterial respiratory infections [14]. A study in Guatemala assessed the antibacterial effect of 68 herbs traditionally applied for respiratory ailments. According to the results, 28 medicinal plants possessed inhibitory effects on one or more gram-positive bacteria, including Streptococcus pneumonia, Staphylococcus aureus, and Streptococcus pyogenes [72]. Another study investigated the antiviral properties of 44 Chinese herbs against the respiratory syncytial virus and demonstrated 27 medicinal plants with antiviral activity against this virus [73].
The current study reviewed the antiviral properties of medicinal plants recommended for respiration disorders in PM. The antiviral effects of 18 (out of 45) herbs have been investigated and confirmed by experimental studies to date. Only some of the studies were performed on humans, while preclinical studies comprised the majority of the reports. The mentioned plants for respiratory disorders have antiviral activity as well. They have several other beneficial effects for patients with viral respiratory infections, including COVID-19 [23, 74–78] (Figure 2).
Figure 2.
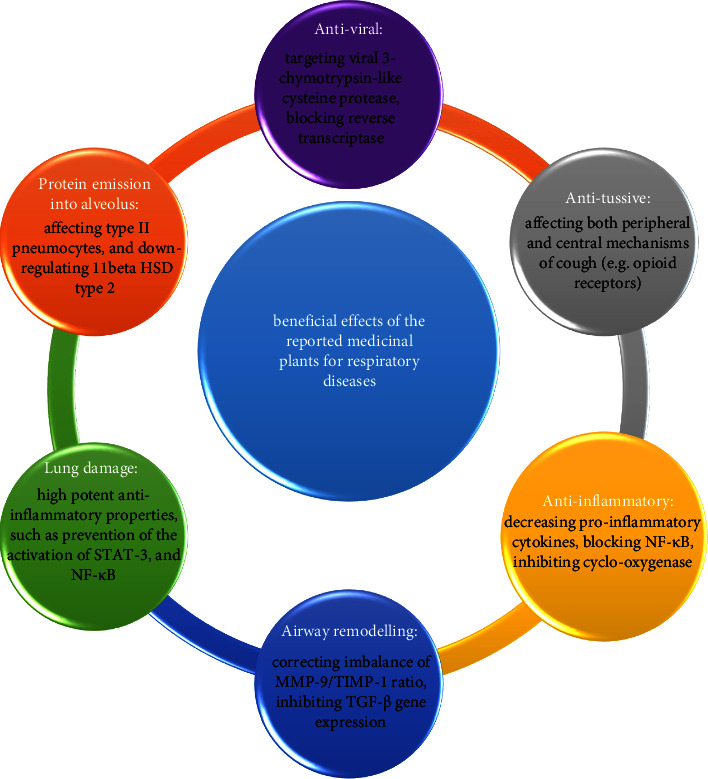
Beneficial effects of medicinal plants recommended by Persian medicine for respiratory disorders and some of their reported mechanisms.
Among these research studies, the efficacy of Glycyrrhiza glabra, Urtica dioica, and Nigella sativa against coronaviruses has been confirmed [33]. These herbs not only possess antiviral activity but can also be used to alleviate symptoms associated with respiratory infections. For example, Glycyrrhizin, an active component (a triterpenoid saponin) of Glycyrrhiza glabra (licorice) root, has shown remarkable antiviral effects against coronavirus isolated from patients with SARS. Virus replication is inhibited when a nitrous oxide donor is added to the culture medium, and it was shown that glycyrrhizin induces nitrous oxide synthase in Vero cells. Also, glycyrrhizin lowers the expression of viral antigens and is able to inhibit the adsorption and penetration of the virus [33]. Moreover, this herb has exhibited antitussive activity on sulphur dioxide-induced cough in experimental rats [79]. In another study, rats with carrageenan-induced paw edema were treated with the hydroalcoholic extract of Glycyrrhiza glabra root. Its potent anti-inflammatory activity has been shown. This extract inhibited the migration of leukocytes dose-dependently, with anti-inflammatory effects comparable to indomethacin [80].
An in vitro study demonstrated that Nigella sativa extract has antiviral action by preventing coronavirus replication [57]. Thymoquinone, an important constituent of Nigella sativa, has been assessed for its antitussive property in guinea pigs. This constituent significantly subsided the cough induced by the nebulized solution of citric acid (20%). Additionally, pretreatment with naloxone leads to suppression of its antitussive effect, indicating stimulation of opium receptors as the mechanism [81]. Furthermore, analgesic and anti-inflammatory activities of the aqueous extract of Nigella sativa have been confirmed in rats via carrageenan-induced paw edema and hot plate reaction time, respectively [82].
Among herbs that have been recommended in PM references for respiratory disorders, the activity of 18 medicinal plants against respiratory viruses has been confirmed to date. Further studies are needed to evaluate whether other suggested medicinal plants have any effect against respiratory viruses or not. Further clinical studies should be considered a very important step towards the utilization of these plants in clinical practice. Also, further studies are necessary to compare the efficiency and safety of these herbs with conventional antiviral drugs. Another limitation of this research was the inclusion of only English and Persian papers.
5. Conclusion
Due to challenges with efficacy and safety, high costs, and limited worldwide availability of conventional treatments, the use of herbal medications for the management of viral respiratory infections is increasing. This systematic review showed antiviral activity (especially against influenza viruses and coronaviruses) for a significant portion of the medicinal herbs recommended for respiratory disorders in PM. However, not enough investigations have been conducted to confirm the efficacy of several of these plants on viral respiratory infections. Lack of or scant clinical studies is the main challenge in this regard; more vigorous research is suggested.
Acknowledgments
This study was financially supported by the Shiraz University of Medical Sciences (grant no. 99-01-106-22159).
Contributor Information
Mohammad Javad Raee, Email: raeem@sums.ac.ir.
Mohammad Hashem Hashempur, Email: hashempur@gmail.com.
Data Availability
The data used to support the findings of this study are included within the article.
Ethical Approval
An ethics statement is not applicable because this study is based exclusively on published literature.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Mahdie Hajimonfarednejad was in charge of conceptualization, methodology, investigation, writing the review, and editing; Mohadeseh Ostovar handled methodology, investigation, writing the review, and editing; Fatemeh Sadat Hasheminasab was in charge of methodology, investigation, and writing the original draft; Mohammad Ali Shariati handled investigation, writing the review, editing, and visualization; Muthu Thiruvengadam was in charge of investigation, writing the review, editing, Visualization; Mohammad Javad Raee was in charge of conceptualization, methodology, resources, writing the review, editing, and supervision; and Mohammad Hashem Hashempur handled methodology, investigation, writing the original draft, visualization, and supervision.
References
- 1.Anzueto A., Niederman M. S. Diagnosis and treatment of rhinovirus respiratory infections. Chest . 2003;123(5):1664–1672. doi: 10.1378/chest.123.5.1664. [DOI] [PubMed] [Google Scholar]
- 2.Abed Y., Boivin G. Treatment of respiratory virus infections. Antiviral Research . 2006;70(2):1–16. doi: 10.1016/j.antiviral.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma H., Singh S., Pathak S. Pathogenesis of COVID-19, disease outbreak: a review. Current Pharmaceutical Biotechnology . 2021 doi: 10.2174/1389201022666210127113441. [DOI] [PubMed] [Google Scholar]
- 4.Bertino J. S. Cost burden of viral respiratory infections: issues for formulary decision makers. The American Journal of Medicine . 2002;112(6):42–49. doi: 10.1016/s0002-9343(01)01063-4. [DOI] [PubMed] [Google Scholar]
- 5.Hnyp I. Y. Assessment of functional status and quality of life of students after acute respiratory viral diseases. Pedagogics, psychology, medical-biological problems of physical training and sports . 2015;19(3):10–14. doi: 10.15561/18189172.2015.0302. [DOI] [Google Scholar]
- 6.Kim C.-K., Callaway Z., Gern J. E. Viral infections and associated factors that promote acute exacerbations of asthma. Allergy, asthma & immunology research . 2018;10(1):p. 12. doi: 10.4168/aair.2018.10.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X., Liu Z. Prevention and treatment of viral respiratory infections by traditional Chinese herbs. Chinese Medical Journal . 2014;127(7):1344–1350. [PubMed] [Google Scholar]
- 8.Boncristiani H., Criado M., Arruda E. Respiratory viruses. Encyclopedia of microbiology . 2009:500–518. doi: 10.1016/b978-012373944-5.00314-x. [DOI] [Google Scholar]
- 9.Mackie P. The classification of viruses infecting the respiratory tract. Paediatric Respiratory Reviews . 2003;4(2):84–90. doi: 10.1016/s1526-0542(03)00031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun P., Lu X., Xu C., Sun W., Pan B. Understanding of COVID‐19 based on current evidence. Journal of Medical Virology . 2020;92(6):548–551. doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pascarella G., Strumia A., Piliego C., et al. COVID 19 diagnosis and management: a comprehensive review. Journal of Internal Medicine . 2020;288(2):192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuzimoto A. D., Isidoro C. The antiviral and the coronavirus-host protein pathways inhibiting properties of herbs and natural compounds-Additional weapons in the fight against the COVID-19 pandemic? Journal of Traditional and Complementary Medicine . 2020;10(4):405–419. doi: 10.1016/j.jtcme.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azimi M., Hashemi-Nasab F., Mokaberinejad R., Qaraaty M., Mojahedi M. The prevention and complementary therapy in acute distress syndrome of COVID-19 in the viewpoint of Persian medicine: a narrative review. Journal of Babol University of Medical Sciences . 2021;23 [Google Scholar]
- 14.Azimi M., Mojahedi M., Mokaberinejad R., Hasheminasab F. S. Ethnomedicine knowledge of Iranian traditional healers and the novel coronavirus disease 2019 (COVID-19) Journal of Advances in Medical and Biomedical Research . 2021;29(135):238–245. doi: 10.30699/jambs.29.135.238. [DOI] [Google Scholar]
- 15.Nikhat S., Fazil M. Overview of Covid-19; its prevention and management in the light of Unani medicine. Science of the Total Environment . 2020;728 doi: 10.1016/j.scitotenv.2020.138859.138859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankar A., Dubey A., Saini D., Prasad C. P. Role of complementary and alternative medicine in prevention and treatment of COVID-19: an overhyped hope. Chinese Journal of Integrative Medicine . 2020;26(8):565–567. doi: 10.1007/s11655-020-2851-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adithya J., Nair B., Aishwarya S., Nath L. R. The plausible role of Indian traditional medicine in combating corona virus (SARS-CoV 2): a mini-review. Current Pharmaceutical Biotechnology . 2021;22(7):906–919. doi: 10.2174/18734316mta4hotevx. [DOI] [PubMed] [Google Scholar]
- 18.Hasheminasab F. S., Hashemi S. M., Dehghan A., et al. Effects of a Plantago ovata-based herbal compound in prevention and treatment of oral mucositis in patients with breast cancer receiving chemotherapy: a double-blind, randomized, controlled crossover trial. Journal of Integrative Medicine . 2020;18(3):214–221. doi: 10.1016/j.joim.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Hasheminasab F. S., Sharififar F., Hashemi S. M., Setayesh M. An evidence-based research on botanical sources for oral mucositis treatment in traditional Persian medicine. Current Drug Discovery Technologies . 2020;18 doi: 10.2174/1570163817666200203110803. [DOI] [PubMed] [Google Scholar]
- 20.Nayebi N., Esteghamati A., Meysamie A., et al. The effects of a Melissa officinalis L. based product on metabolic parameters in patients with type 2 diabetes mellitus: a randomized double-blinded controlled clinical trial. Journal of Complementary and Integrative Medicine . 2019;16(3) doi: 10.1515/jcim-2018-0088. [DOI] [PubMed] [Google Scholar]
- 21.Seyed Hashemi M., Hashempur M. H., Lotfi M. H., et al. The efficacy of asafoetida (Ferula assa-foetidaoleo-gum resin) versus chlorhexidine gluconate mouthwash on dental plaque and gingivitis: a randomized double-blind controlled trial. European Journal of Integrative Medicine . 2019;29 doi: 10.1016/j.eujim.2019.100929.100929 [DOI] [Google Scholar]
- 22.Javadi B., Sahebkar A., Emami S. A. Medicinal plants for the treatment of asthma: a traditional Persian medicine perspective. Current Pharmaceutical Design . 2017;23(11):1623–1632. doi: 10.2174/1381612822666161021143332. [DOI] [PubMed] [Google Scholar]
- 23.Anand A. V., Balamuralikrishnan B., Kaviya M., et al. Medicinal plants, phytochemicals, and herbs to combat viral pathogens including SARS-CoV-2. Molecules . 2021;26(6):p. 1775. doi: 10.3390/molecules26061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahramsoltani R., Rahimi R. An evaluation of traditional Persian medicine for the management of SARS-CoV-2. Frontiers in Pharmacology . 2020;11 doi: 10.3389/fphar.2020.571434.571434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhama K., Karthik K., Khandia R., et al. Medicinal and therapeutic potential of herbs and plant metabolites/extracts countering viral pathogens-current knowledge and future prospects. Current Drug Metabolism . 2018;19(3):236–263. doi: 10.2174/1389200219666180129145252. [DOI] [PubMed] [Google Scholar]
- 26.Silveira D., Prieto-Garcia J. M., Boylan F., et al. COVID-19: is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Frontiers in Pharmacology . 2020;11 doi: 10.3389/fphar.2020.581840.581840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nugraha R. V., Ridwansyah H., Ghozali M., Khairani A. F., Atik N. Traditional herbal medicine candidates as complementary treatments for COVID-19: a review of their mechanisms, pros and cons. Evidence-based Complementary and Alternative Medicine . 2020;2020:12. doi: 10.1155/2020/2560645.2560645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zargaran A., Karimi M., Rezaeizadeh H. Covid 19: natural products and traditional medicines; opportunity or threat? Traditional and Integrative Medicine . 2021;6 [Google Scholar]
- 29.Thomson Healthcare. PDR for Herbal Medicines . Montvale, USA: Thomson Healthcare; 2004. [Google Scholar]
- 30.Li Y.-H., Lai C.-Y., Su M.-C., Cheng J.-C., Chang Y.-S. Antiviral activity of Portulaca oleracea L. against influenza A viruses. Journal of Ethnopharmacology . 2019;241 doi: 10.1016/j.jep.2019.112013.112013 [DOI] [PubMed] [Google Scholar]
- 31.Dorra N., El-Berrawy M., Sallam S., Mahmoud R. Evaluation of antiviral and antioxidant activity of selected herbal extracts. Journal of High Institute of Public Health . 2019;49(1):36–40. doi: 10.21608/jhiph.2019.29464. [DOI] [Google Scholar]
- 32.Hamauzu Y., Yasui H., Inno T., Kume C., Omanyuda M. Phenolic profile, antioxidant property, and anti-influenza viral activity of Chinese quince (Pseudocydonia sinensis Schneid.), quince (Cydonia oblonga Mill.), and apple (Malus domestica Mill.) fruits. Journal of Agricultural and Food Chemistry . 2005;53(4):928–934. doi: 10.1021/jf0494635. [DOI] [PubMed] [Google Scholar]
- 33.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. The Lancet . 2003;361(9374):2045–2046. doi: 10.1016/s0140-6736(03)13615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Utsunomiya T., Kobayashi M., Pollard R. B., Suzuki F. Glycyrrhizin, an active component of licorice roots, reduces morbidity and mortality of mice infected with lethal doses of influenza virus. Antimicrobial Agents and Chemotherapy . 1997;41(3):551–556. doi: 10.1128/aac.41.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong E.-H., Song J. H., Kang K. B., Sung S. H., Ko H.-J., Yang H. Anti-influenza activity of betulinic acid from Zizyphus jujuba on influenza A/PR/8 virus. Biomolecules & therapeutics . 2015;23(4):345–349. doi: 10.4062/biomolther.2015.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sener B., Orhan I., Ozcelik B., Kartal M., Aslan S., Ozbilen G. Antimicrobial and antiviral activities of two seed oil samples of Cucurbita pepo L. and their fatty acid analysis. Natural Product Communications . 2007;2(4) doi: 10.1177/1934578x0700200409.1934578X0700200 [DOI] [Google Scholar]
- 37.Sokolova A. S., Yarovaya ОI., Shernyukov АV., et al. New quaternary ammonium camphor derivatives and their antiviral activity, genotoxic effects and cytotoxicity. Bioorganic & Medicinal Chemistry . 2013;21(21):6690–6698. doi: 10.1016/j.bmc.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zarubaev V., Garshinina A., Tretiak T., et al. Broad range of inhibiting action of novel camphor-based compound with anti-hemagglutinin activity against influenza viruses in vitro and in vivo. Antiviral Research . 2015;120:126–133. doi: 10.1016/j.antiviral.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Hafidh R. R., Abdulamir A. S., Abu Bakar F., Sekawi Z., Jahansheri F., Jalilian F. A. Novel antiviral activity of mung bean sprouts against respiratory syncytial virus and herpes simplex virus− 1: an in vitro study on virally infected Vero and MRC-5 cell lines. BMC Complementary and Alternative Medicine . 2015;15(1):p. 179. doi: 10.1186/s12906-015-0688-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kan A., Özçelik B., Kartal M. In vitro antiviral activities under cytotoxic doses against herpes simples type-1 and parainfluensa-3 viruses of Cicer arietinum L.(Chickpea) African Journal of Pharmacy and Pharmacology . 2009;3(12):627–631. [Google Scholar]
- 41.Fatima M., Zaidi N. U. S. S., Amraiz D., Afzal F. In vitro antiviral activity of Cinnamomum cassia and its nanoparticles against H7N3 influenza a virus. Journal of Microbiology and Biotechnology . 2016;26(1):151–159. doi: 10.4014/jmb.1508.08024. [DOI] [PubMed] [Google Scholar]
- 42.Yeh C. F., Chang J. S., Wang K. C., Shieh D. E., Chiang L. C. Water extract of Cinnamomum cassia Blume inhibited human respiratory syncytial virus by preventing viral attachment, internalization, and syncytium formation. Journal of Ethnopharmacology . 2013;147(2):321–326. doi: 10.1016/j.jep.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Brochot A., Guilbot A., Haddioui L., Roques C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiology (Reading) . 2017;6(4) doi: 10.1002/mbo3.459.e00459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mair C., Liu R., Atanasov A., Schmidtke M., Dirsch V., Rollinger J. Antiviral and anti-proliferative in vitro activities of piperamides from black pepper. Planta Medica . 2016;81(1):S1–S381. doi: 10.1055/s-0036-1596830. [DOI] [Google Scholar]
- 45.Priya N., Kumari P. Antiviral activities and cytotoxicity assay of seed extracts of Piper longum and Piper nigrum on human cell lines. International Journal of Pharmaceutical Sciences Review and Research . 2017;44(1):197–202. [Google Scholar]
- 46.Lazreg Aref H., Gaaliche B., Fekih A., et al. In vitro cytotoxic and antiviral activities of Ficus carica latex extracts. Natural Product Research . 2011;25(3):310–319. doi: 10.1080/14786419.2010.528758. [DOI] [PubMed] [Google Scholar]
- 47.Bekhit A. E.-D. A., Cheng V. J., McConnell M., Zhao J. H., Sedcole R., Harrison R. Antioxidant activities, sensory and anti-influenza activity of grape skin tea infusion. Food Chemistry . 2011;129(3):837–845. doi: 10.1016/j.foodchem.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 48.Gaafar A. A., Asker M. S., Ma A., Salama Z. A. The effectiveness of the functional components of grape (Vitis vinifera) pomace as antioxidant, antimicrobial, and antiviral agents. Jordan Journal of Biological Sciences . 2019;12(5) [Google Scholar]
- 49.Chang J. S., Wang K. C., Yeh C. F., Shieh D. E., Chiang L. C. Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. Journal of Ethnopharmacology . 2013;145(1):146–151. doi: 10.1016/j.jep.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 50.Haidari M., Ali M., Ward Casscells S., Madjid M. Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir. Phytomedicine . 2009;16(12):1127–1136. doi: 10.1016/j.phymed.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 51.Moradi M.-T., Karimi A., Shahrani M., Hashemi L., Ghaffari-Goosheh M.-S. Anti-influenza virus activity and phenolic content of pomegranate (Punica granatum L.) peel extract and fractions. Avicenna Journal of Medical Biotechnology . 2019;11(4):285–291. [PMC free article] [PubMed] [Google Scholar]
- 52.Moradi M.-T., Karimi A., Rafieian-Kopaei M., Rabiei-Faradonbeh M., Momtaz H. Pomegranate peel extract inhibits internalization and replication of the influenza virus: an in vitro study. Avicenna journal of phytomedicine . 2020;10(2):143–151. [PMC free article] [PubMed] [Google Scholar]
- 53.Karimi A., Moradi M.-T., Rabiei M., Alidadi S. In vitro anti-adenoviral activities of ethanol extract, fractions, and main phenolic compounds of pomegranate (Punica granatum L.) peel. Antiviral Chemistry and Chemotherapy . 2020;28 doi: 10.1177/2040206620916571.204020662091657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balzarini J., Neyts J., Schols D., et al. The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antiviral Research . 1992;18(2):191–207. doi: 10.1016/0166-3542(92)90038-7. [DOI] [PubMed] [Google Scholar]
- 55.Kumaki Y., Wandersee M. K., Smith A. J., et al. Inhibition of severe acute respiratory syndrome coronavirus replication in a lethal SARS-CoV BALB/c mouse model by stinging nettle lectin, Urtica dioica agglutinin. Antiviral Research . 2011;90(1):22–32. doi: 10.1016/j.antiviral.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y., Liu Y., Ma A., Bao Y., Wang M., Sun Z. In vitro antiviral, anti-inflammatory, and antioxidant activities of the ethanol extract of Mentha piperita L. Food Science and Biotechnology . 2017;26(6):1675–1683. doi: 10.1007/s10068-017-0217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ulasli M., Gurses S. A., Bayraktar R., et al. The effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) extracts on the replication of coronavirus and the expression of TRP genes family. Molecular Biology Reports . 2014;41(3):1703–1711. doi: 10.1007/s11033-014-3019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu J., Manheimer E., Shi Y., Gluud C. Chinese herbal medicine for severe acute respiratory syndrome: a systematic review and meta-analysis. Journal of Alternative & Complementary Medicine . 2004;10(6):1041–1051. doi: 10.1089/acm.2004.10.1041. [DOI] [PubMed] [Google Scholar]
- 59.Lin L., Han Y., Yang Z. m. Clinical observation on 103 patients of severe acute respiratory syndrome treated by integrative traditional Chinese and Western Medicine. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi= Chinese journal of integrated traditional and Western medicine . 2003;23(6):409–413. [PubMed] [Google Scholar]
- 60.Zhang M.-M., Liu X.-M., He L. Effect of integrated traditional Chinese and Western medicine on SARS: a review of clinical evidence. World Journal of Gastroenterology . 2004;10(23):p. 3500. doi: 10.3748/wjg.v10.i23.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Y., Guo J. J., Healy D. P., Zhan S. Effect of integrated traditional Chinese medicine and western medicine on the treatment of severe acute respiratory syndrome: a meta-analysis. Pharmacy Practice . 2007;5(1):1–9. doi: 10.4321/s1886-36552007000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiu R., Wei X., Zhao M., Zhong C., Zhao C., Hu J. Outcome reporting from protocols of clinical trials of Coronavirus Disease 2019 (COVID-19): a review. medRxiv . 2020 doi: 10.1101/2020.03.04.20031401. [DOI] [Google Scholar]
- 63.Mohammadi Kenari H., Yousefsani B. S., Eghbalian F., Ghobadi A., Jamshidi A., Mahroozade S. Herbal recommendations for treatment of COVID-19 symptoms according to Persian medicine. Journal of Medicinal Plants . 2021;20(77):1–14. doi: 10.52547/jmp.20.77.1. [DOI] [Google Scholar]
- 64.Jia W., Gao W. Is traditional Chinese medicine useful in the treatment of SARS? Phytotherapy Research . 2003;17(7):840–841. doi: 10.1002/ptr.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shirbeigi L., Zarei A., Naghizadeh A., Vaghasloo M. A. The concept of temperaments in traditional Persian medicine. Traditional and Integrative Medicine . 2017:143–156. [Google Scholar]
- 66.Shakeri A., Hashempur M. H., Beigomi A., et al. Strategies in traditional Persian medicine to maintain a healthy life in the elderly. Journal of Complementary and Integrative Medicine . 2020;18(1):29–36. doi: 10.1515/jcim-2019-0273. [DOI] [PubMed] [Google Scholar]
- 67.Rezayat F., Hashempur M. H., Tavahen H., Salmanroghani H., Emtiazy M. The efficacy of Ramak (a traditional herbal medicine preparation) for patients with ulcerative colitis: a pilot, randomized, triple-blinded, placebo-controlled clinical trial. European Journal of Integrative Medicine . 2020;39 doi: 10.1016/j.eujim.2020.101209.101209 [DOI] [Google Scholar]
- 68.Jean S.-S., Lee P.-I., Hsueh P.-R. Treatment options for COVID-19: the reality and challenges. Journal of Microbiology, Immunology, and Infection . 2020;53(3):436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iranzadasl M., Karimi Y., Moadeli F., Pasalar M. Persian medicine recommendations for the prevention of pandemics related to the respiratory system: a narrative literature review. Integrative Medicine Research . 2021;10(1) doi: 10.1016/j.imr.2020.100483.100483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vardanjani H. M., Heydari S. T., Dowran B., Pasalar M. A cross-sectional study of Persian medicine and the COVID-19 pandemic in Iran: rumors and recommendations. Integrative medicine research . 2020;9(3) doi: 10.1016/j.imr.2020.100482.100482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu L., Li J., Zhou Y., Ma H., Hu M. Complementary and alternative medicine for threatened miscarriage: advantages and risks. Evidence-based Complementary and Alternative Medicine . 2021;2021:, 26. doi: 10.1155/2021/5589116.5589116 [DOI] [Google Scholar]
- 72.Caceres A., Alvarez A. V., Ovando A. E., Samayoa B. E. Plants used in Guatemala for the treatment of respiratory diseases. 1. Screening of 68 plants against gram-positive bacteria. Journal of Ethnopharmacology . 1991;31(2):193–208. doi: 10.1016/0378-8741(91)90005-x. [DOI] [PubMed] [Google Scholar]
- 73.Ma S.-C., Du J., But P. P.-H., et al. Antiviral Chinese medicinal herbs against respiratory syncytial virus. Journal of Ethnopharmacology . 2002;79(2):205–211. doi: 10.1016/s0378-8741(01)00389-0. [DOI] [PubMed] [Google Scholar]
- 74.Kuang Y., Li B., Fan J., Qiao X., Ye M. Antitussive and expectorant activities of licorice and its major compounds. Bioorganic & Medicinal Chemistry . 2018;26(1):278–284. doi: 10.1016/j.bmc.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 75.Jezova D., Karailiev P., Karailievova L., Puhova A., Murck H. Food enrichment with Glycyrrhiza glabra extract suppresses ACE2 mRNA and protein expression in rats–possible implications for COVID-19. Nutrients . 2021;13 doi: 10.3390/nu13072321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kianmehr M., Haghmorad D., Nosratabadi R., Rezaei A., Alavinezhad A., Boskabady M. H. The effect of Zataria multiflora on Th1/Th2 and Th17/T regulatory in a mouse model of allergic asthma. Frontiers in Pharmacology . 2017;8:p. 458. doi: 10.3389/fphar.2017.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma X., Ma X., Ma Z., et al. The effects of uygur herb Hyssopus officinalis L. on the process of airway remodeling in asthmatic mice. Evidence-based Complementary and Alternative Medicine . 2014;2014:7. doi: 10.1155/2014/710870.710870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang M., Shen Y., Han N., Chen H., Cai W. Evaluation of lipopolysaccharide-induced acute lung injury attenuation in mice by Glycyrrhiza glabra. Pharmacognosy Magazine . 2020;16(67):p. 92. doi: 10.4103/pm.pm_189_19. [DOI] [Google Scholar]
- 79.Jahan Y., Siddiqui H. Study of antitussive potential of Glycyrrhiza glabra and Adhatoda vasica using a cough model induced by sulphur dioxide gas in mice. International Journal of Pharmaceutical Sciences and Research . 2012;3(6):p. 1668. [Google Scholar]
- 80.Nirmala P., Selvaraj T. Anti-inflammatory and anti-bacterial activities of Glycyrrhiza glabra L. Journal of Agricultural Technology . 2011;7(3):815–823. [Google Scholar]
- 81.Hosseinzadeh H., Eskandari M., Ziaee T. Antitussive effect of thymoquinone, a constituent of Nigella sativa seeds, in Guinea pigs. Pharmacologyonline . 2008;2:480–484. [Google Scholar]
- 82.Al-Ghamdi M. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. Journal of Ethnopharmacology . 2001;76(1):45–48. doi: 10.1016/s0378-8741(01)00216-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


