Abstract
Objective
The purpose of this study was to evaluate the functional and patient-reported outcomes, and their correlation, after percutaneous bone-anchored hearing aid (BAHA) implantation.
Methods
A prospective study was conducted between January 2018 and December 2020 in a tertiary care center. All adult patients who were implanted with a percutaneous BAHA device during this evaluation period were included in the study. Complete auditory function and patients reported outcome measures (PROMs) were assessed in the preoperative period and 6 months after the implant activation. The PROMs included a generic form (Medical Outcome Study 36 Short Form Healthy Survey (MOS SF-36)), and three disease-specific forms (Hearing Handicap Inventory (HHI), Satisfaction with Amplification in Daily Life Scale (SADLS), and Tinnitus Handicap Inventory (THI)).
Results
Twenty-two patients with an average age of 53 years were included in the study. The overall functional gain with the BAHA in sound-field pure tone average (PTA) was 29 dB, with no statistically significant differences according to surgical indication (F(3,18) = 2.319, p = 0.110). The greater the preoperative air-bone gap, the greater the functional gain obtained (r = 0.505, p < 0.05). In the PROMs, we found a significant improvement in HHI scores (p < 0.005) and a significant increase in overall SADLS scores (p < 0.05) with the use of percutaneous BAHA devices. We did not verify any statistically significant correlation between functional and PROMs results.
Conclusions
The BAHA is a safe and effective alternative hearing rehabilitation option in selected patients. The PROMs results prove patient's overall satisfaction.
Keywords: BAHA, Hearing loss, Implant, Hearing results, PROMs
1. Introduction
The bone-anchored hearing aids (BAHA) are devices that work by bone conduction, composed by an osseointegrated bone-anchored titanium implant and an audio processor. Sound vibrations can be conducted directly through the bone to the inner ear, bypassing the external and middle ear (Gardell et al., 2015). There are currently two main categories of BAHA devices: percutaneous devices, whose abutment penetrates the skin and connect directly to the implant, and transcutaneous devices that use magnets to connect with the implant through an intact skin (Iseri et al., 2015). The audio processor has several power levels according to patient's bone conduction threshold.
At our institution, we preferentially use the Baha® Connect system (percutaneous type). Typically, these devices are used by patients who suffer from conductive or mixed hearing loss and are unable to use conventional hearing aids (CHA), or who are contraindicated to using them (as in circumstances involving congenital or acquired malformations of the external ear, recurrent ear infections, and surgically treated patients with altered anatomies, such as mastoid cavities) (Bento et al., 2012; Ellsperman et al., 2021; Fontaine et al., 2014). However, we must always ensure that the patient has a reasonable sensorineural reserve (ideally with a bone conduction threshold <45 dB HL) and that speech discrimination must be at least 60% (Hagr, 2007) (Table 1). This alternative rehabilitation resource has several advantages since it does not obstruct the external auditory canal, thereby reducing the chance of infection, and does not interfere with the local anatomy (Garcier et al., 2021). Moreover, and regarding the percutaneous BAHA, the direct connection of the sound processor to the implant, without skin interposition, offers greater comfort and prevents the signal attenuation that occurs in passive transcutaneous devices (Ellsperman et al., 2021; Ghossaini and Roehm, 2019).
Table 1.
Clinical indications for BAHA placement (adopted by our institution).
| Unilateral or bilateral conductive hearing loss |
|
| Unilateral or bilateral mixed hearing loss |
|
| Single-sided deafness |
|
Legend: AB – Air-bone; HL – Hearing level; AC – Air-conduction; PTA – Pure tone average; BC – Bone-conduction; WRS - Word recognition score.
More recently, they are also used in people with single-sided deafness (SSD), where the sound processor directs the sound transcranially to the healthy contralateral inner ear (with an overall pure tone average (PTA) ≥ 20 dB HL). This allows to reduce the head shadow effect and improve speech discrimination in noisy circumstances (Kim et al., 2017).
Although the hearing rehabilitation benefits and safety of the percutaneous BAHA are well documented, there are still some disadvantages related to the skin penetrating abutment, which include the need for daily care, skin infections, skin overgrowth and hypertrophic scar, defective osseointegration and implant loss with the consequent need for surgical revision (Fontaine et al., 2014; Håkansson et al., 2019; Iseri et al., 2015). The most common complication is skin reactions, which, according to a systematic review (Mohamad et al., 2016), can occur in up to 84% of cases, and are classified according to the Holgers classification system (Holgers et al., 1998), and, more recently, according to the IPS-scale (Kruyt et al., 2017).
Improvement in the hearing handicap, assessed through a complete audiological study, has been demonstrated following BAHA implantation when clinically indicated. Regarding subjective outcomes and patient satisfaction with BAHA, sometimes more critical than the functional gain itself, we consider that the regular use of patient-reported outcome measures (PROMs) is important since they enable us to quantify the perceived benefit and compare it with other treatment options.
The purpose of the present study was to evaluate hearing performance, both functional and patient-reported quality of life and satisfaction measures, and their correlation, in a medium-term follow-up after implantation with a percutaneous BAHA device.
2. Material and methods
2.1. Study population
This study is a prospective, non-randomized,3-years (from January 2018 to December 2020) case series study performed in a single tertiary center that belongs to the Portuguese National Health Service. Following approval by the institutional Ethical Committee, all adult patients (>18 years) who had an indication for a BAHA (Table 1) were prospectively enrolled in the present study. All patients were implanted with the Cochlear™ Baha® Connect System and gave their consent before being included in the study. Baha® 5, Baha® 5 Power, and the Baha® 5 SuperPower audio processors were available, and the choice was determined by the indications (“CochlearTM Baha® 5 System,” 2016). The exclusion criteria were age <18 years and patients with implant loss either due to defective osseointegration or due to trauma.
2.2. Surgical procedure and follow-up
Since the introduction of the percutaneous BAHA system, the surgical procedure for implant placement has been modified to further improve the results and to shorten the surgery time. In our institution, implant placement is performed in the operating room, under general or local anesthesia. In all cases in this study, the one-step or FAST surgery was performed, according to the surgical instructions recommended (Cochlear, 2015), without soft tissue reduction. The appropriate abutment length was chosen based on the measured tissue thickness before tissue infiltration. Patients were evaluated at weekly outpatient visits to remove dressings and assess wound healing. After the first week, if there was adequate healing, no more dressings were applied, and the patients only kept the protective healing cap daily until the sound processor was fitted.
2.3. Audiometric testing and outcomes evaluation
For this study, we assessed the auditory function with pure-tone average (PTA), speech recognition threshold (SRT) and word recognition score (WRS), and subjective outcomes with PROMs, preoperatively and 6 months after Baha® Connect device use.
Preoperatively and without any amplification device pure-tone audiometry was assessed using the Interacoustics AD629 diagnostic audiometer through air conduction (AC) and bone conduction (BC) in a soundproof cabin, using headphones and an oscillator on the mastoid process respectively, in the frequencies of 250 Hz, 500 Hz, 1000 Hz, 2000 Hz, and 4000 Hz. PTA and air-bone (AB) gap were calculated.
The fitting of the external sound processor took place 3–10 weeks after surgery. After the sound processor was fitted, patients returned for an audiological assessment. Through the brand's software, we obtained the average time of hearing aid use for each patient.
For audiological assessment with the BAHA, we used loudspeakers placed 1m away from the patient in the same soundproof audiometry cabin, and sound-field pure-tone thresholds at 250Hz, 500 Hz, 1000 Hz, 2000 Hz, and 4000 Hz fitted and non-fitted were obtained, calculated both PTA and then hearing aid gain threshold. SRT and WRS were also evaluated with and without the hearing aid. Masking noise was sometimes used in the non-implanted ear to prevent its participation in the test, like in patients with SSD.
In addition to the audiometric evaluation, four different PROMs (Table 2) were completed preoperatively and 6 months after fitting of the percutaneous BAHA sound processor. The measures of health-related quality of life (HRQoL) included one generic form and three disease-specific forms. All these questionnaires are validated for the Portuguese population (Ferreira, 2000; Oliveira and Carmo, 2011; Oliveira and Meneses, 2007; Roque Dos Reis et al., 2017).
Table 2.
Overview of patient-reported outcome measures.
| Questionnaire | Type of instrument | Description | Assessment |
|---|---|---|---|
|
Medical Outcome Study 36 Short Form Healthy Survey (MOS SF-36) (Ferreira, 2000; McHorney et al., 1993) |
Generic | 36 questions to measure eight health concepts: physical function, physical and emotional performance, physical pain, general health, vitality, social function, and mental health. |
|
|
Hearing Handicap Inventory (HHI) (Newman et al., 1990; Oliveira and Carmo, 2011) |
Hearing-specific | 2 subscales: 13-item subscale that explores emotional (E) consequences of hearing loss; 12-item subscale that addresses social (S) and situational effects of hearing loss. |
|
|
Satisfaction with Amplification in Daily Life Scale (SADLS) (Cox and Alexander, 1999; Roque Dos Reis et al., 2017) |
Hearing-specific | 15 questions to assess the degree of satisfaction, hearing benefits and psychosocial disadvantages obtained with the use of hearing aids |
|
|
Tinnitus Handicap Inventory (THI) (Newman et al., 1996; Oliveira and Meneses, 2007) |
Hearing-specific | 25 questions, with the items grouped in 3 subscales: functional (THI-F), emotional (THI-E), and catastrophic (THI-C) subscales, regarding tinnitus. |
|
2.4. Statistical analysis
IBM SPSS® Statistical Package for the Social Sciences, version 26, was used to analyze the data. P values less than 0.05 were considered significant.
Categorical variables were summarized by frequency (N) and percentage. Continuous variables with normal distribution were described as means and standard deviations (SDs). For non-normal data (according to the Shapiro-Wilk test), the median and interquartile ranges (IQRs) were calculated.
The pre and postoperative outcome measures were compared using paired t-test (normal data) or the Wilcoxon's signed-rank test (non-parametric test). Two independent groups were compared using the Mann-WhitneyU test. To determine whether there are any statistically significant differences between three or more groups, the one-way analysis of variance (ANOVA) or Kruskal-Wallis test were employed, respectively. The Spearman correlation coefficient (rs) was used to assess the correlation between objective and subjective outcome measures.
3. Results
3.1. Patients characteristics
Twenty-two adult patients were considered appropriate candidates and were implanted with a percutaneous BAHA device (Table 3). There was a female predominance (81.8%), and the mean age was 53 years (range: 27–70 years). Patients had a previous ipsilateral behind-the-ear CHA in 11 cases (50%). All patients were implanted with a single percutaneous BAHA. The most frequent surgical indication for BAHA was severe conductive hearing loss and chronic otitis media who underwent mastoidectomy but had persistent or recurrent ear discharge with the impossibility to use CHA (54.6%). Most patients (63.6%) use their BAHA for more than 8 h a day and 72.7% for more than 4 h (range: 2–15 h). Patients with previous ipsilateral insufficient gain with CHA are the ones with greater daily use (median 12 h).
Table 3.
Demographics, BAHA surgical indications, and BAHA usage time of the study sample.
| Gender - % (n) | |
|---|---|
| Male | 18.2 (4) |
| Female | 81.8 (18) |
| Age - years; mean ± SD | 53.0 ± 10.8 |
| Implant side - % (n) | |
| Right | 54.5 (12) |
| Left | 45.5 (10) |
| Sugical Indication - % (n) | |
| COM with insufficient hearing gain with conventional hearing aids | 31.8 (7) |
| COM with recurrent otorrhea | 54.6 (12) |
| SSD | 9.1 (2) |
| External ear malformations | 4.5 (1) |
| Audio Processor Type - % (n) | |
| Baha® 5 | 63.6 (14) |
| Baha® 5 Power | 27.3 (6) |
| Baha® 5 SuperPower | 9.1 (2) |
| Overall daily hours of percutaneous BAHA use – hours; median ± IQR | 9.9 ± 8.6 |
| COM with insufficient hearing gain with conventional hearing aids | 12.0 ± 10.1 |
| COM with recurrent otorrhea | 10.4 ± 3.9 |
| SSD | 4.3 ± 4.7 |
Legend: SD – Standard deviation; COM – Chronic otitis media; SSD – Single-sided deafness; IQR – Interquartile range.
3.2. Functional outcomes
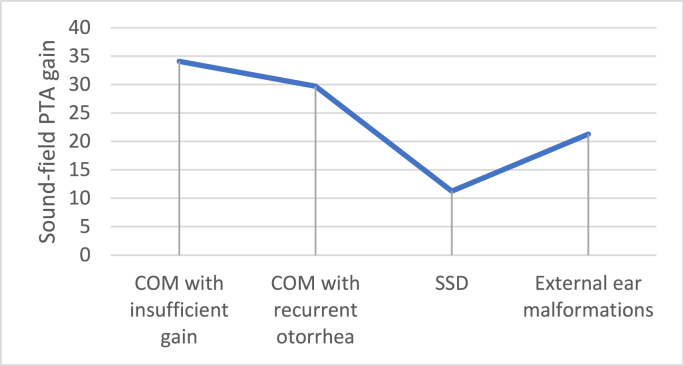
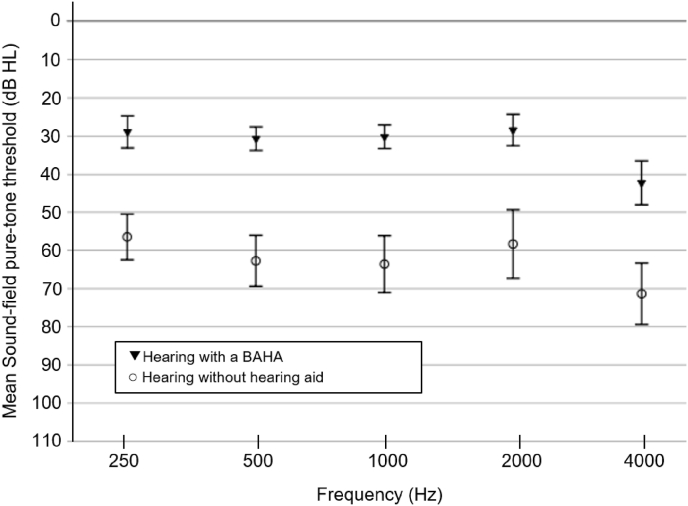
The preoperative audiological assessment and functional gains with the evaluated implantable device are summarized in Table 4, Table 5, respectively, divided by the type of external sound processor used. There was an overall significant improvement in sound-field PTA, SRT and WRS with the percutaneous BAHA. As expected, Baha® 5 SuperPower showed a greater gain, considering PTA evaluation. The overall functional gain with the BAHA in sound-field PTA was 29 dB, with no statistically significant differences according to surgical indication as determined by one-way ANOVA (F(3,18) = 2.319, p = 0.110). The patients who had a BAHA due to a SSD had the lowest functional gain (mean 11.25 dB HL) (Fig. 1). When considering patients with COM or external ear malformations (n = 20), the sound-field hearing thresholds at frequencies of 250 Hz, 500 Hz, 1000Hz, 2000 Hz, and 4000 Hz when hearing with the BAHA were lower by 27.4, 32.1, 32.9, 29.9 and 28.4 dB, respectively, compared to hearing without any hearing aid (Fig. 2). The goal of a sound-field PTA fitting the percutaneous BAHA device that is lower than 30 dB was achieved in 42.9% of the patients with chronic otitis media (COM) and insufficient gain with CHA, in 58.2% of patients with COM who have recurrent otorrhea, and in 100% of patients diagnosed with external ear malformations. Additionally, the greater the preoperative AB gap, the greater the functional PTA gain obtained with BAHA (rs = 0.505, p = 0.016).
Table 4.
Preoperative audiological assessment.
| Preoperative |
||||
|---|---|---|---|---|
| Sound processor | BC PTA (dB HL; mean ± SD) | AB gap (dB; median ± IQR) | SRT (dB HL; median ± IQR) | WRS (%; median ± IQR) |
| Baha® 5 (n = 12) | 22.3 ± 8.1 | 40.0 ± 11.9 | 62.5 ± 27.5 | 95.0 ± 15.0 |
| Baha® 5 Power (n = 6) | 36.0 ± 4.9 | 41.9 ± 11.3 | 85.0 ± 13.8 | 82.5 ± 15.0 |
| Baha® 5 SuperPower (n = 2) | 42.5 ± 1.8 | 38.1b | 82.0b | 80.0b |
| Overall (n = 20)a | 28.48 ± 10.38 | 40 ± 11.88 | 72.5 ± 28.75 | 87.5 ± 15 |
Legend: BC – Bone conduction; PTA – Pure tone average; HL – Hearing level; SD – Standard deviation; AB – Air-bone; IQR – Interquartile range; SRT – Speech recognition threshold; WRS - word recognition score.
Two cases whose surgical indication was single-sided deafness were not considered in this analysis.
IQR could not be calculated (n = 2).
Table 5.
Postoperative audiological assessment (functional gain).
| Sound-field PTA |
SRT |
WRS |
||||||
|---|---|---|---|---|---|---|---|---|
| Audio processor | With BAHA (dB HL) mean ± SD | Without BAHA (dB HL) mean ± SD | Gain with BAHA (dB HL) mean | With BAHA (dB HL) median±IQ | Without BAHA (dB HL) median±IQR | Gain with BAHA (dB HL) median | With BAHA (%) median ± IQR | Without BAHA (%) median±IQR |
| Baha® 5 (n = 14) | 27.1 ± 6.3 | 55.5 ± 17.2 | 28.4 | 30.0 ± 10.0 | 52.5 ± 30.0 | 27.5 | 97.5 ± 5.0 | 87.5 ± 15.0 |
| Baha® 5 Power (n = 6) | 32.3 ± 6.9 | 61.83 ± 10.4 | 29.5 | 40.0 ± 8.8 | 67.5 ± 27.5 | 27.5 | 95.0 ± 6.3 | 85.0 ± 21.3 |
| Baha® 5 SuperPower (n = 2) | 36.3 ± 1.8 | 68.1 ± 9.7 | 31.8 | 42.5 ∗ | 70.0 ∗ | 27.5 | 90.0 ∗ | 77.5 ∗ |
| Overall (n = 22) | 29.4 ± 6.8 | 58.4 ± 15.2 | 29 ǂ | 40.0 ± 11.3 | 62.5 ± 30.0 | 22.5 Ɨ | 95.0 ± 6.25 Ɨ | 85.0 ± 15.0 Ɨ |
Legend: PTA – Pure tone average; SRT – Speech recognition threshold; WRS - word recognition score; HL – Hearing level; SD – Standard deviation; IQR – Interquartile range.
∗ IQR could not be calculated (n = 2).
ǂ Paired t-test: p = 0.002.
Ɨ Wilcoxon's signed-rank test: p < 0.001.
Fig. 1.
Overall functional gain with the BAHA according to surgical indication.
Fig. 2.
Sound-field pure-tone thresholds (mean ± SD) with and without BAHA, considering patients with COM or external ear malformations.
3.3. Patient-reported outcomes measures
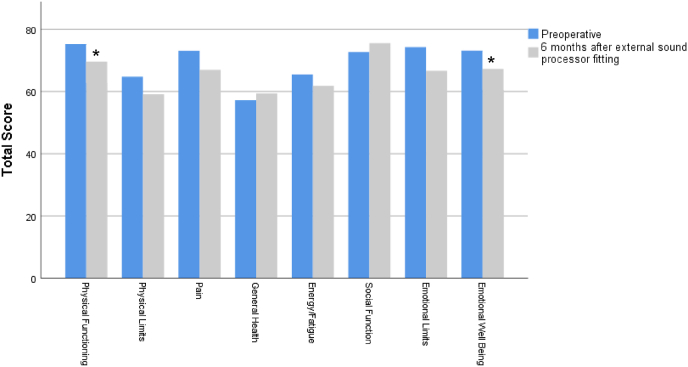
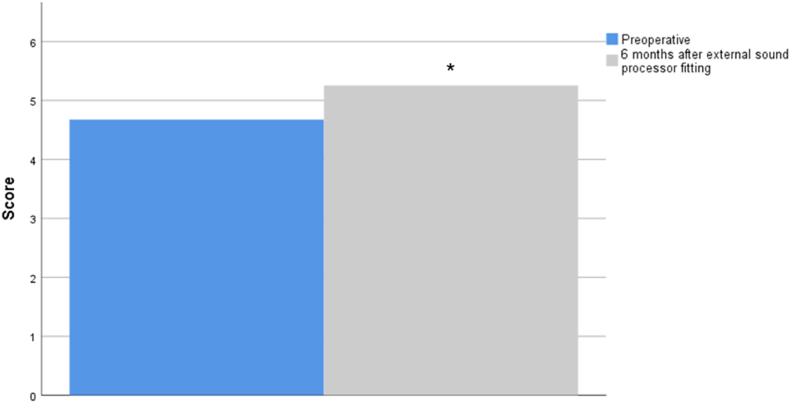
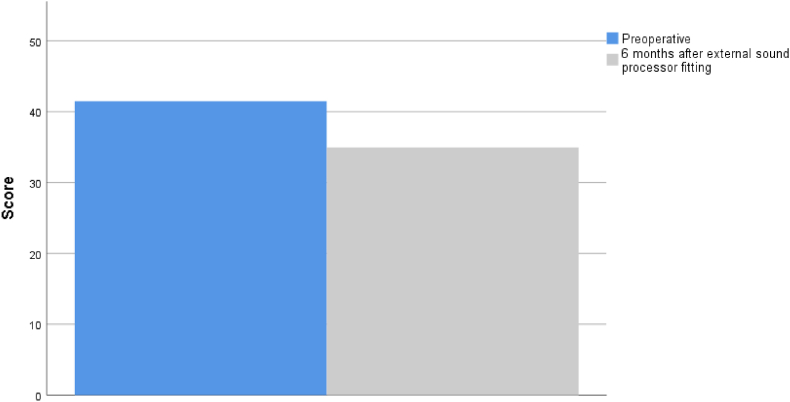
In the self-assessment outcomes, and considering the generic form MOS SF-36, we found a slight but significant decrement in the median physical functioning and emotional well-being (mental health) scores after BAHA implant (Z = −1,971, p = 0.049; Z = −2.002, p = 0.045 respectively) (Fig. 3).
Fig. 3.
MOS SF-36 score before and after BAHA implant. Higher scores signify more favorable health state. Values are presented as medians. ∗p < 0.05 versus before in the same item, Wilcoxon test.
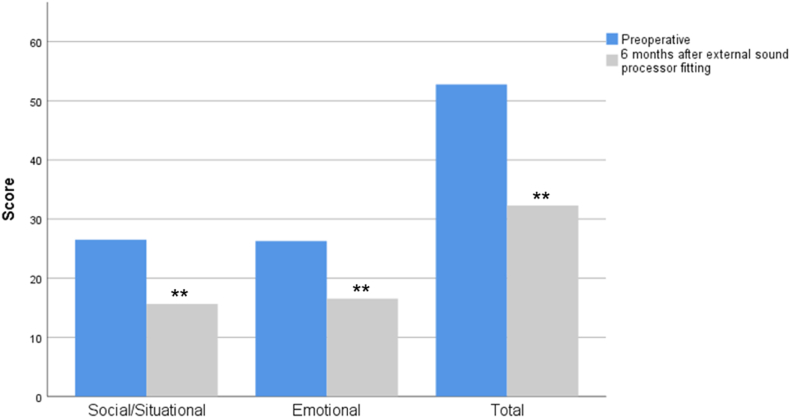
In the disease specific forms, we found a significant improvement in HHI scores (Z = −2.730, p = 0.006), in both emotional and situational parameters, and a significant increase in overall SADLS scores (Z = −2.04, p = 0.041) (Fig. 4, Fig. 5, respectively), and both forms scores are inversely correlated (rs = −0.522, p = 0.922). In fact, after 6 months of using the BAHA, 8 patients (36.4%) did not have any HHI handicap (total score <16). The degree of improvement in the HHI did not have a statistically significant variation when analyzing the different subgroups according to the surgical indication (H(3) = 0.485, p = 0.737).
Fig. 4.
HHI score (subscales and total) before and after BAHA implant. Higher scores indicate a greater degree of disability. Values are presented as medians. ∗∗p < 0.005 versus before in the same item, Wilcoxon test.
Fig. 5.
Total SADLS score before and after BAHA implant. Higher scores indicate a greater satisfaction. Values are presented as medians. ∗p < 0.05 versus before in the same item in patients who previously used conventional hearing aids, Wilcoxon test.
Additionally, in 15 patients with tinnitus, we found that higher levels on the THI correlated with worse scores on the HHI (rs = 0.721, p < 0.005), however, BAHA placement did not significantly improve THI score (Fig. 6), with 6 patients (40%) maintaining a moderate or severe handicap (scores >38).
Fig. 6.
Total THI score before and after BAHA implant. The higher the score, the greater the impairment in daily life. Values are presented as medians.
3.4. Functional outcomes vs PROMs
We assessed whether there was any correlation between the functional outcomes and PROMs and we did not verify any statistically significant correlation between sound-field PTA gain and postactivation HHI and SADLS improvements (rs = 0.118, p = 0.600; rs = 0.318, p = 0.314, respectively).
Similarly, BAHA daily hours of use did not correlate with postactivation sound-field PTA gains (rs = 0.299, p = 0.177), HHI improvement (rs = 0.035, p = 0.877), or SALDS scores (rs = 0.094, p = 0.677). However, we found that patients with a sound-field PTA <30 dB (median 12.5 h vs. 8.2 h) and with a WRS improvement of more than 15% with the BAHA (median 10.8 h vs. 9.7 h) tend to have a longer use of the device, although these differences were not statistically significant (p = 0.664 and p = 0.333, respectively).
4. Discussion
Despite being successfully used for more than 30 years in more than 30000 patients worldwide, since its introduction in clinical practice in Sweden in the 1970s (Fontaine et al., 2014; Sammeth and Cire, 2009), studies of the clinical applicability and functional and quality of life related gains of the BAHA continue to be scarce. This study investigated the real and self-reported benefits of using BAHA.
Most of our patients had chronic discharging ear, that, according to the literature, seems to be the most common indication for BAHA in the adult population (Fontaine et al., 2014; Garcier et al., 2021).
Other studies (Gardell et al., 2015; Saroul et al., 2011) have demonstrated that most BAHA users use their device for over 8 h a day, with almost 80% using it for over 4 h a day, which shows that the BAHA system is comfortable and easy to use. In fact, Saroul et al. (2011) found that 73% of patients found them ‘‘easy’’ or ‘‘very easy’’ to use. Only elderly population seems to be less fitted.
4.1. Functional outcomes
Regarding the functional outcomes, we verified, as expected, that with the BAHA, there was an overall significant improvement in sound-field PTA, SRT, and WRS and that the overall mean improvement of hearing threshold was 29 dB HL, which is slightly better than other published studies (Bruschini et al., 2020; Rahim et al., 2018). Additionally, the degree of improvement was not statistically influenced by the type of hearing loss or indication for implant placement. SSD patients had the lowest functional gains, but the main benefit when fitting the BAHA in patients with SSD is in group conversations where sounds come from different sources and locations: although it does not appear to improve sound localization, the head shadow effect is reduced and sound discrimination in noise is improved (Kim et al., 2017; Saroul et al., 2011).
We also verified that the greater the preoperative AB gap, the greater the functional benefit of BAHA. But more importantly than AB gap, the functional gain achieved by the patients depends mainly on their BC PTA threshold, as shown by the study performed by Hakansson et al. (Håkansson et al., 1990). So, it is currently one of the major criteria to be considered when selecting patients for BAHA placement (ideally, it should be between 0 and 45 dB HL). However, our study showed that it is possible to achieve good functional results even with higher BC thresholds by using an appropriate sound processor, up to a maximum limit generally considered 65 dB.
Additionally, a good functional gain obtained with the percutaneous BAHA could also lead to a longer use, as has been hypothesized by other studies (Bento et al., 2012) that claim that consistent use of the device is highly predictive of patient benefit. We observed that patients with a good functional result (i.e., sound-field PTA <30 dB and WRS improvement >15%) tend to use the device for more hours, although without statistically significant differences. In patients with SSD, BAHA might be most beneficial when they are exposed to noise or participate in group conversations, which can limit their use if they stay more at home and justify the shorter time of use observed (median 4.3 h a day). Additionally, in this study, the assessed period included the Covid-19 pandemic, where people were more isolated. However, more patients and a longer follow-up time will be needed to confirm these results and ascertain the determinants of device use.
4.2. Patient-reported outcomes measures
In the subjective and self-assessment outcomes, we found a significant improvement in median HHI scores, with 36.4% of patients having no HHI handicap after 6 months of using the BAHA. Like the functional results, the PROMs results also did not significantly vary with the medical indication for implant placement. Interestingly, both patients with SSD also demonstrated a total score reduction in the HHI (although without normalization), including both emotional (HHI-E) and social (HHI–S) effects. Patients with SSD implanted with the BAHA might have better hearing discrimination, especially in noisy environments, which could explain improvements in social life and, consequently, emotional well-being. However, further studies with a larger sample of SSD patients will be needed to assess whether these subjective and patient-reported gains are reproducible.
With the SADLS there was a significant improvement in participant satisfaction with amplification with the use of BAHA devices, when compared with the use of CHA. This PROM assesses the global aspects, positives effects, service and cost, negative features, and personal image regarding the use of the evaluated hearing aid.
Several studies proved that the satisfaction obtained with the BAHA is considerable for both conductive hearing-loss and SSD (Saroul et al., 2011). However, there are few studies that use a specific and validated scale such as the SADLS to assess patient satisfaction and no studies, to the best of our knowledge, have compared satisfaction with previous use of CHA. In 91 patients with percutaneous BAHA, Gardell et al. (2015) applied SADLS and concluded that 63% of patients reported that BAHA improved their ability to understand others most of the time and only 28% complained about the whistling from the device, but 52% of the patients were not satisfied with how the device looked. Like the functional results, median SADLS levels were also not correlated with the hours of hearing aid use.
Regarding the THI, BAHA placement did not statistically improve their scores. Studies have already proven that cochlear implant can help to reduce the tinnitus and the tinnitus handicap (Kloostra et al., 2019), which does not seem to happen with the BAHA (Bahmad et al., 2019), although more studies are needed to prove these results. What some authors suggest is that the sound emitted through a bone conduction device has the same potential to mask tinnitus as air-conducted sound (Holgers and Håkansson, 2002). Thus, this may be a strategy to relieve tinnitus in patients with BAHA devices.
In the generic health-related quality of life assessment form MOS SF-36, we did not have improvements but had a slight but significant worsening in physical functioning and mental health. In light of the already established fact that individuals with untreated hearing loss report higher rates of depression, anxiety, paranoia, and less involvement in social activities than those with hearing aids, these results are contradictory to what we would expect (D'Eredità et al., 2012). There is, however, a high degree of variability in HRQoL outcomes across studies, specifically regarding the MOS SF-36 (Sladen et al., 2017). Another explanation may be related with the fact that we are evaluating a small sample size and, especially, the results in a medium-term follow-up. During the initial phase after implantation of the BAHA, patients may feel sicker because they may still not be used to wearing something visible and bigger than a CHA and having a skin penetrating abutment. It may, in this context, require more time of use and habituation to obtain the real subjective benefit. Additionally, as the scale assesses eight basic health concepts, the change/worsening of the patient's general health status may not be entirely due to the placement of the BAHA but due to other life events that may have occurred over the follow-up period that were not controlled in this study. Nevertheless, there are other studies that have evaluated the improvement of patients' HRQoL with BAHA, primarily with the Glasgow Benefit Inventory (GBI), which showed that the BAHA improved the patients' quality of life by improving their wellbeing and social and health status (Hagr, 2007; Rahim et al., 2018).
Considering the relationship between PROMs and functional outcomes, no statistically significant correlations were found in this study. It may be related to the patients' expectations regarding the gain they will have, the time of sound deprivation prior to implant placement or even the daily time of use itself, which may be insufficient to obtain the ideal functional and consequently subjective gain. Probably this could be improved with longer years of use. Another reason may be that we included SSD patients in our analysis. As we have seen, patients who had a BAHA due to a profound unilateral sensorineural hearing loss had, as expected, the lowest functional gain. The objective in these cases is not to restore binaural hearing but to decrease the head shadow effect and improve sound discrimination in noise. Thus, in these cases, despite a little functional gain, there may be a high subjective gain and patient satisfaction, which may contribute to this lack of correlation between PROMs and functional outcomes.
4.3. Strengths, weaknesses and future research directions
As strengths of this study, we highlight the fact that it is a prospective study where patient analysis, postoperative follow-up, and data collection were homogeneous and standardized. Additionally, besides the audiological assessment, a generic subjective assessment and three disease-specific forms were included in the study, revealing itself as one of the few studies that make a holistic assessment of the effectiveness of percutaneous BAHA. So, in this study, we provide data to help identify which patients are more likely to benefit from the implantation of a BAHA devices, since they are expensive and should be reserved for those who will benefit most from their use, and PROMs measurements bring more information about patients complaints and fears which can help to better elucidate future patients. Despite these aspects, it is a study with a limited number of patients, and the long-term results were not evaluated. Furthermore, SSD patients should have been evaluated with other tools to assess their real functional benefit, like the speech perception in noise (SPIN) test.
Future research, ideally related to the study of the contralateral ear (i.e., if it has associated hearing loss, what type of loss [conduction or sensorineural], and whether it is symmetrical or asymmetrical in relation to the ear to be implanted) and its impact on the success of patients implanted with BAHA, is warranted to address additional controversies related to BAHA.
5. Conclusions
As it proved to be effective, the BAHA offers a feasible and safe alternative hearing rehabilitation option to patients who are unable to use CHA or whose functional gains are insufficient or unsatisfactory. The PROMs results prove patient's overall satisfaction and help us to understand some difficulties of those who use a BAHA device.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The Authors declares that there is no conflict of interest.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Contributor Information
Cátia Azevedo, Email: catiacazevedo17@gmail.com.
Miguel Breda, Email: miguelbreda@gmail.com.
Daniela Ribeiro, Email: dani.ribeiro.correia@gmail.com.
Fernando Milhazes Mar, Email: fernandomilhazesmar@gmail.com.
Sérgio Vilarinho, Email: sergiovilarinho@gmail.com.
Luís Dias, Email: luisdiasorl@gmail.com.
References
- Bahmad F., Cardoso C.C., Caldas F.F., De Souza Chelminski Barreto M.A., Da Silva Hilgenberg A.M., Teixeira M.S., Serra L.S.M. Hearing rehabilitation through bone-conducted sound stimulation: preliminary results. Int. Arch. Otorhinolaryngol. 2019;23:12–17. doi: 10.1055/s-0038-1670694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento R.F., Kiesewetter A., Ikari L.S., Brito R. Bone-anchored hearing aid (BAHA): indications, functional results, and comparison with reconstructive surgery of the ear. Int. Arch. Otorhinolaryngol. 2012;16:400–405. doi: 10.7162/S1809-97772012000300017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruschini L., Canelli R., Morandi A., Cambi C., Fiacchini G., Berrettini S., Forli F. Bone anchored hearing aids for the treatment of asymmetric hearing loss. J. Int. Adv. Otol. 2020;16:313–317. doi: 10.5152/iao.2020.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochlear A bone conduction hearing solution. Surgery Guide. 2015:1–32. [Google Scholar]
- CochlearTM Baha® 5 System Chochlear. 2016. https://mss-p-007-delivery.sitecorecontenthub.cloud/api/public/content/a89feabc2fea49fa957e9ddf33c7dc5d?v=a9aae039&MOD=AJPERES&CONVERT_TO=url&CACHEID=ROOTWORKSPACE-5e7bba46-8d97-48cf-ba29-563cfe5fb9b1-llgpiG7 [WWW Document], URL.
- Cox B.M., Alexander G.C. Measuring satisfaction with amplification in daily life: the SADL scale. Ear Hear. 1999 doi: 10.4135/9781412994248.n350. [DOI] [PubMed] [Google Scholar]
- D'Eredità R., Caroncini M., Saetti R. The new baha implant: a prospective osseointegration study. Otolaryngol. Head Neck Surg. 2012;146:979–983. doi: 10.1177/0194599812438042. [DOI] [PubMed] [Google Scholar]
- Ellsperman S.E., Nairn E.M., Stucken E.Z. Review of bone conduction hearing devices. Audiol. Res. 2021;11:207–219. doi: 10.3390/audiolres11020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira P.L. Criação da versão Portuguesa do MOS SF-36. Parte II--Testes de validação. Acta Med. Port. 2000;13:119–127. [PubMed] [Google Scholar]
- Fontaine N., Hemar P., Schultz P., Charpiot A., Debry C. BAHA implant: implantation technique and complications. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2014;131:69–74. doi: 10.1016/j.anorl.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Garcier M., Lavedrine A., Gagneux C., Eluecque T., Bozorg Grayeli A. Bone-anchored and closed skin bonebridge implant in adults: hearing performances and quality of life. Audiol. Neurotol. 2021;26:310–316. doi: 10.1159/000512496. [DOI] [PubMed] [Google Scholar]
- Gardell I.S.K., Andresen K., Faber C.E., Wanscher J.H. Bone-anchored hearing aids are effective and associated with a high degree of satisfaction. Dan. Med. J. 2015;62:1–5. [PubMed] [Google Scholar]
- Ghossaini S.N., Roehm P.C. Osseointegrated auditory devices: bone-anchored hearing aid and PONTO. Otolaryngol. Clin. 2019;52:243–251. doi: 10.1016/j.otc.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Hagr A. BAHA: bone-anchored hearing aid. Int. J. Health Sci. 2007;1:265–276. [PMC free article] [PubMed] [Google Scholar]
- Håkansson B., Lidén G., Tjellström A., Ringdahl A., Jacobsson M., Carlsson P., Erlandson B.E. Ten years of experience with the Swedish bone-anchored hearing system. Ann. Otol. Rhinol. Laryngol. Suppl. 1990;151:1–16. doi: 10.1177/0003489490099s1001. [DOI] [PubMed] [Google Scholar]
- Håkansson B., Reinfeldt S., Persson A.C., Jansson K.J.F., Rigato C., Hultcrantz M., Eeg-Olofsson M. The bone conduction implant–a review and 1-year follow-up. Int. J. Audiol. 2019;58:945–955. doi: 10.1080/14992027.2019.1657243. [DOI] [PubMed] [Google Scholar]
- Holgers K.M., Håkansson B. Sound stimulation via bone conduction for tinnitus relief: a pilot study. Int. J. Audiol. 2002;41:293–300. doi: 10.3109/14992020209077189. [DOI] [PubMed] [Google Scholar]
- Holgers K.M., Tjellström A., Bjursten L.M., Erlandsson B. Soft tissue reactions around percutaneous implants: a clinical study of soft tissue conditions around skin-penetrating titanium implants for bone-anchored hearing aids. Am. J. Otolaryngol. 1998;9:56–59. [PubMed] [Google Scholar]
- Iseri M., Orhan K.S., Tuncer U., Kara A., Durgut M., Guldiken Y., Surmelioglu O. Transcutaneous bone-anchored hearing aids versus percutaneous ones: multicenter comparative clinical study. Otol. Neurotol. 2015;36:849–853. doi: 10.1097/MAO.0000000000000733. [DOI] [PubMed] [Google Scholar]
- Kim G., Ju H.M., Lee S.H., Kim H.S., Kwon J.A., Seo Y.J. Efficacy of bone-anchored hearing AIDS in single-sided deafness: a systematic review. Otol. Neurotol. 2017;38:473–483. doi: 10.1097/MAO.0000000000001359. [DOI] [PubMed] [Google Scholar]
- Kloostra F.J.J., Verbist J., Hofman R., Free R.H., Arnold R., Van Dijk P. A prospective study of the effect of cochlear implantation on tinnitus. Audiol. Neurotol. 2019;23:356–363. doi: 10.1159/000495132. [DOI] [PubMed] [Google Scholar]
- Kruyt I.J., Nelissen R.C., Johansson M.L., Mylanus E.A.M., Hol M.K.S. The IPS-scale: a new soft tissue assessment scale for percutaneous and transcutaneous implants for bone conduction devices. Clin. Otolaryngol. 2017;42:1410–1413. doi: 10.1111/coa.12922. [DOI] [PubMed] [Google Scholar]
- McCombe A., Baguley D., Coles R., McKenna L., McKinney C., Windle-Taylor P. Guidelines for the grading of tinnitus severity: the results of a working group commissioned by the British Association of Otolaryngologists, Head and Neck Surgeons, 1999. Clin. Otolaryngol. Allied Sci. 2001;26:388–393. doi: 10.1046/j.1365-2273.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- McHorney C., Ware J., Raczek A. The MOS 36-itemshort-form health survey (SF-36): II. Psychometric. Med. Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Mohamad S., Khan I., Hey S.Y., Hussain S.S.M. A systematic review on skin complications of bone-anchored hearing aids in relation to surgical techniques. Eur. Arch. Oto-Rhino-Laryngol. 2016;273:559–565. doi: 10.1007/s00405-014-3436-1. [DOI] [PubMed] [Google Scholar]
- Newman C.W., Jacobson G.P., Spitzer J.B. Development of the tinnitus handicap invntory. Arch. Otolaryngol. Head Neck Surg. 1996;122:143–148. doi: 10.1001/archotol.1996.01890140029007. [DOI] [PubMed] [Google Scholar]
- Newman C.W., Weinstein B.E., Jacobson G.P., Hug G.A. The hearing handicap inventory for adults: psychometric adequacy and audiometric correlates. Ear Hear. 1990;11:430–433. doi: 10.1097/00003446-199012000-00004. [DOI] [PubMed] [Google Scholar]
- Oliveira V., Carmo P.C. Versão portuguesa do hearing handicap inventory for adults - Dados preliminares. VI Encontro nac. das Ciências e Tecnol. da Saúde. 2011 In this issue. [Google Scholar]
- Oliveira V., Meneses R. Vol. 1. Audiologia em revista; 2007. Balanço da utilização da versão Portuguesa do Tinnitus Handicap Inventory (THI) pp. 101–106. [Google Scholar]
- Rahim S.A., Goh B.S., Zainor S., Rahman R.A., Abdullah A. Outcomes of bone anchored hearing aid implant at universiti Kebangsaan Malaysia medical centre (UKMMC) Indian J. Otolaryngol. Head Neck Surg. 2018;70:28–32. doi: 10.1007/s12070-017-1193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque Dos Reis L., Donato M., Sousa R., Escada P. Translation, cultural adaptation and validation of the satisfaction with amplification in daily life scale for European Portuguese. Acta Med. Port. 2017;30:115–121. doi: 10.20344/amp.7794. [DOI] [PubMed] [Google Scholar]
- Sammeth C.A., Cire G. 2009. Effectiveness in Treating Single-Sided Deafness with the Baha System.https://www.hearingreview.com/hearing-products/accessories/components/effectiveness-in-treating-single-sided-deafness-with-the-baha-system [WWW Document]. Hear. Rev. URL. [Google Scholar]
- Saroul N., Gilain L., Montalban A., Giraudet F., Avan P., Mom T. Patient satisfaction and functional results with the bone-anchored hearing aid (BAHA) Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011;128:107–113. doi: 10.1016/j.anorl.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Sladen D.P., Peterson A., Schmitt M., Olund A., Teece K., Dowling B., DeJong M., Breneman A., Beatty C.W., Carlson M.L., Neff B.A., Hughes-Borst B., Driscoll C.L. Health-related quality of life outcomes following adult cochlear implantation: a prospective cohort study. Cochlear Implants Int. 2017;18:130–135. doi: 10.1080/14670100.2017.1293203. [DOI] [PubMed] [Google Scholar]